Abstract
Before life could start on earth, it was important that the amino acid building blocks be present in a predominant handedness called the L configuration and that the ribose of RNA be predominantly in the D configuration. Because ordinary chemical processes would produce them in equal L and D amounts, it has long been a puzzle how the needed selectivities could have arisen. Carbonaceous chondrites such as the Murchison meteorite, which landed in Australia in 1969, brought some unusual amino acids with a methyl group replacing their α hydrogen. They cannot racemize and have a small but real excess of those with the L configuration. We have shown that they can partake in a synthesis of normal L amino acids under credible prebiotic conditions. We and others showed that small preferences can be amplified into solutions with very high dominance of the L amino acids because of the higher solubility of the pure L form than of the more stable DL racemic compound crystal. Here, we show that such solubility-based amplification of small excesses of three D nucleosides, uridine, adenosine, and cytidine, can also occur to form solutions with very high D dominance under credible prebiotic conditions. Guanosine crystallizes as a conglomerate and does not amplify in this way. However, under prebiotic conditions it could have been formed from homochiral D ribose from the hydrolysis of amplified adenosine or cytidine.
Keywords: chiral amplification, Murchison meteorite, transamination, water solubilities
Ever since the discovery of chirality there has been speculation about why and how our protein amino acids have the L configuration and the ribose and deoxyribose in nucleic acids have the D configuration (1, 2). It is generally understood that such homochirality was needed in prebiotic times so proteins could have their well defined structures, impossible with a random mixture of the L and D amino acid enantiomers (but possible if all of the amino acids were D, as may have happened in other parts of the universe). Similarly, ribose must have been in its homochiral D form so the RNA of the prebiotic RNA world and the DNA and RNA of our world could have well defined structures such as those in their helices, impossible with a random mixture of D and L ribose. Because chemical reactions to synthesize these amino acids and ribose would normally form a DL equal mixture in the absence of some chiral influence, scientists have proposed various ideas about how the compounds could have been formed in the prebiotic world with the complete homochirality we see in proteins and nucleic acids (1). However, as Rikken and Raupach (3) commented, “Clearly the question of the origin of the homochirality of life is far from answered.”
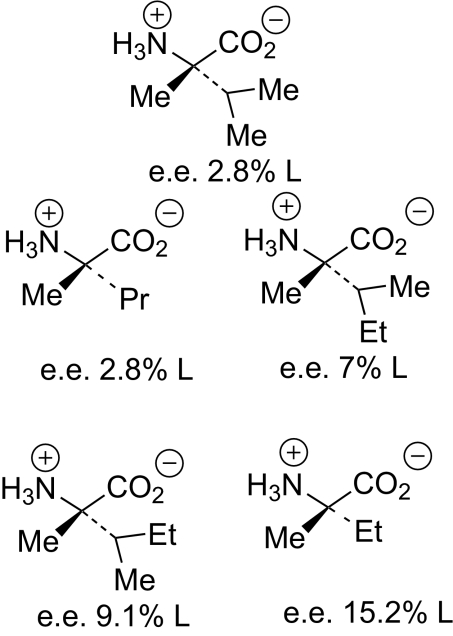
An important piece of experimental evidence on this question arrived on earth in 1969: the Murchison meteorite that landed in Australia carried a number of α-methyl amino acids that all show small excesses of the L forms (Fig. 1), named L with methyl groups replacing the α protons of L amino acids (4, 5). They have the high levels of 13C and of deuterium characteristic of compounds formed in space, where the isotope effects at 10 K are smaller than those on earth (4). They are also not found as part of our normal terrestrial chemistry. The Murchison chondrite was a member of the class of carbonaceous chondritic meteorites, essentially a piece of rock with carbon compounds in its interstices. Because the rock is not combustible and is a thermal insulator, the carbon compounds found in its crash site were able to survive entry into our atmosphere. Normal amino acids are also found in meteorites, but as racemates. If they had once been formed with small enantioexcesses as well, as seems likely (see next paragraph), they could racemize on heating. The α-methyl analogs cannot racemize.
Fig. 1.
α-Methyl amino acids from the Murchison meteorite.
As described in refs. 6 and 7, it seems likely that the amino acids with and without the α-methyl group were formed as racemates by Strecker reactions of carbonyl compounds, ammonia, HCN, and H2O on solid particles, such as asteroids, in our solar system. Then the right circularly polarized light that has been detected by astronomers in our region of space (8), and that may be generated by synchrotron or cyclotron processes in neutron stars, can partially destroy the D forms of the amino acids (Bonner and colleagues had shown this in the case of normal amino acids (1, 2), in which right circularly polarized light selectively destroyed the D enantiomers and afforded small excesses of the L amino acids, similar to what is seen in the α-methyl meteoritic amino acids). This could lead to the small but real excesses seen in the α-methyl amino acids deposited by meteorites. Our atmosphere now or in prebiotic times would prevent high energy light from penetrating to earth, so the processes invoked can happen only outside our planet. In other parts of the universe, the mirror image D amino acids could have been preferred.
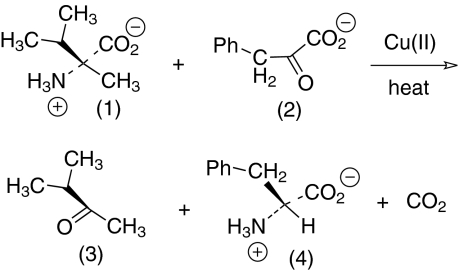
We have shown that reaction of L α-methyl amino acids with α-keto acids under Cu(II) catalysis can produce normal amino acids with significant transfer of chirality, as molecular models suggested, and under credible prebiotic conditions (Fig. 2) (7).
Fig. 2.
L-α-Methylvaline (1) heated with sodium phenylpyruvate (2) in the presence of cupric sulfate at 160 °C afforded 3-methyl-2-butanone (3) and L-phenylalanine (4) with 37% transfer of chirality.
We also showed that the small resulting enantioexcesses in the product amino acids could be amplified in water solution under credible prebiotic conditions (6). The thermodynamic process was analogous to that reported by Klussman et al. (9) at the same time. We also showed that under kinetic control, we could see even higher amplification-concentration in a solution with the equilibrium enantioexcess of L-tryptophan (7).
The thermodynamic amplification of the enantioexcesses that are dissolved in water solution involves the greater solubility of the homochiral crystals than of the racemic crystals, and the effects can be striking. The phenomenon has been known for a long time, but the first description of how it could have been used on prebiotic earth was that of Morowitz (10), many years before our work and that of Blackmond.
In brief, as Morowitz described, the solubility of homochiral crystals is compared with the solubility product of the racemic crystals. Starting with a small excess of an L amino acid, the final ratio of [L] to [D] is expressed in Eq. 1, provided the amount of water is small enough that both crystals are dissolved to saturation (10).
where the L enantiomer is in excess, SL is the solubility of L, and SDL is the solubility product of a racemic crystal of DL, both in pure water.
By this equation, even if the homochiral crystals were only twice as soluble as the racemic crystals, the final ratio of [L] to [D] would be 16/1, a solution with 94% of the L and 6% of the D enantiomer. This is a bit less than the 19/1 ratio we observed for phenylalanine. The large excess of the dissolved L enantiomer decreases the solubility of the racemate relative to its solubility in pure water.
It is important to note that this final ratio can in principle be achieved even starting from extremely small original enantioexcesses. The only requirement is that the amount of water be small enough that a saturated solution of the small homochiral excess, and of the racemate, be achieved.
In our work, we simply dissolved the entire mixture in water, starting with as little as a 1% excess of an L amino acid, and slowly evaporated the water until the maximum enantioexcess was seen in solution, often >90% for various amino acids (6, 7). Klussman et al. (9) and Noorduin et al. (11) have reported a more extensive studies, with similar results for the cases we examined, by simply suspending the mixtures in small amounts of water (9, 11).
Results and Discussion
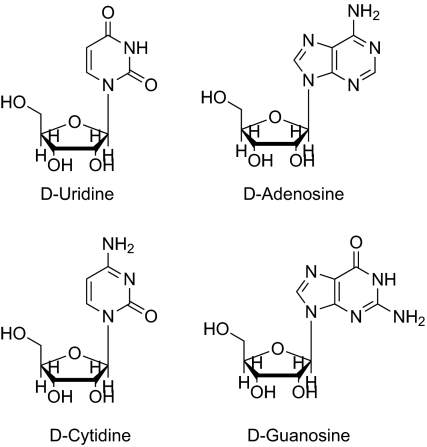
The other important homochiral prebiotic material needed before life could start was D-ribose. Ribose can be credibly derived from formaldehyde by the formose reaction, in which formaldehyde forms glycolaldehyde and then further reactions with formaldehyde afford sugars by aldol condensations (12–14). Catalysis of these aldol condensations by single enantiomers of amino acids could in principle generate an excess of the D enantiomer of ribose under prebiotic conditions (15) (a question we are studying.) We have now shown that the solution amplification seen with amino acids in water can also occur with nucleosides, producing solutions with very high ratios of D to L nucleosides that would permit the RNA world to begin.
The critical question was whether a DL mixture would form a racemic DL compound crystal or simply a conglomerate, a mixture of D and L crystals. If a DL racemate were formed, would it be less soluble than the D crystal so that amplification could occur? We found that a 1:1 mixture of D-ribose and L-ribose—synthesized from L arabinose by a known procedure (16, 17)—did not have the higher melting point, relative to the homochiral crystals, that signals a racemate with lower solubility. In fact, the DL mixture and the D ribose had identical melting points. This indicates that they form crystals composed of a solid solution of the D and L riboses, an example of the common sort classified as Type I by Rozeboom (18). In such crystals, the unit cells can contain either a D or L species in any overall ratio with no significant effect on crystal properties such as solubility or melting points.
We then examined ribonucleosides, the next stage in the prebiotic synthesis of RNA. (Fig. 3). We prepared the DL ribonucleosides by using the commercial D ribonucleosides and the L ribonucleosides that we synthesized (16, 17) from L-ribose, prepared by a known procedure (16) from L-arabinose. We crystallized both the DL mixture and the pure D commercial sample from ethanol and dried them in vacuo with heating. We then examined the melting points and solubilities of the pure D ribonucleosides and of their DL 1:1 mixtures. As hoped, DL-uridine had a melting point of 176 ± 1 °C, 21 °C higher than that for D-uridine (which we measured as 155 °C). More important, the solubility of D-uridine in water at 22°C was 454 mg/mL whereas that of the DL racemate was 87 mg/mL (5.2-fold smaller). From Eq. 1, this would predict a [D]/[L] ratio of 108/1 in a solution saturated in both crystals.
Fig. 3.
The D ribonucleosides.
We examined such a doubly saturated water solution of D-uridine and DL-uridine by HPLC on a Chiralpak AD column and observed that the actual [D]/[L] ratio was 96/4, smaller than the prediction from Eq. 1 but still quite high. In the real life situation, contact of the D-uridine crystals with a solution containing some L-uridine apparently diminished their solubility, an effect not envisioned in the theory behind Eq. 1.
We also synthesized L-adenosine and compared the DL racemate with the pure D enantiomer. The melting point of the DL-adenosine was 243 ± 1 °C whereas that of D-adenosine was only 231 ± 1 °C. Thus, DL-adenosine is a racemic compound with one D and one L together in the unit cell of the crystal, not a conglomerate of D and L crystals or a solid solution. We found that the solubility of D-adenosine in water at 22 °C was 5.2 mg/mL whereas that of the racemic DL crystal was only 0.8 mg/mL a factor of 6.3 smaller. From Eq. 1 this would predict a [D]/[L] ratio in a solution saturated in both crystals to be 160/1. By HPLC we observed a ratio of 99/1, again smaller than that from Eq. 1 but still very large. To repeat, this final solution of 99% D-adenosine and 1% L-adenosine can be obtained from any original, even small, excess of the D enantiomer provided it and the DL crystals are in equilibrium with a saturated solution.
We prepared L-cytidine from our synthetic L-ribose and determined the properties of its 1:1 DL mixtures relative to the properties of the pure D nucleoside. (We observed decomposition rather than clean melting for both crystals, so their melting points could not be compared.) The solubility of D-cytidine in water at 22 °C was 192 mg/mL whereas that of the DL crystal was 24.3 mg/mL. Thus, DL-cytidine forms a racemic compound crystal. From Eq. 1, the [D]/[L] ratio should be 250/1. By direct measurement, a solution saturated in both crystals had a [D]/[L] ratio of 99.5/0.5, again extremely high but less than the theoretical value.
The fourth nucleoside, guanosine, was a surprise. We prepared L-guanosine from our L-ribose and compared the properties of the D crystals with those of the crystals from the 1:1 DL mixture. (We observed decomposition rather than clean melting for both crystals, so their melting points could not be compared.). The solubilities were 0.46 mg/mL for D-guanosine and 0.84 mg/mL for DL-guanosine. In contrast with the other nucleosides, guanosine is more soluble as the racemate than as the pure enantiomer.
The DL mixture of guanosine apparently crystallized as a conglomerate of separate D and L crystals, related to the D and L crystals of sodium ammonium tartrate that Pasteur separated manually. Thus, it cannot be amplified in the way the other 3 nucleosides can. Of course under prebiotic conditions, the amplified D-adenosine or D-cytidine could hydrolyze to form essentially homochiral D-ribose. This D-ribose could then be used to form D-guanosine, as well as taking part in other reactions related to the roles that D-ribose plays in metabolism.
Conclusion
As this work shows, the solution amplification of both D-cytidine and D-adenosine, in a mixture with the DL racemates, is very large, enough to produce solutions under prebiotic conditions with at least 99% enantioexcesses of the D enantiomers. The 96% excess of D-uridine could also be enough to permit biological selection for the D component in solution. Again we emphasize that these final concentrations will be obtained in solution starting with even quite small initial ratios of [D] to [L] providing the amount of water in contact with them is small enough that the solution is saturated in both D and DL. DL-Ribose itself forms a solid solution whereas DL-guanosine is a conglomerate. Neither of these can be amplified in water solution, but homochiral ribose and then homochiral guanosine can be obtained from the hydrolysis of the homochiral adenosine or cytidine.
Biology could then start with a selective preference for using the enantiomers that are in large excess, so that the final successful biological systems will have the pure enantiomers.
More work is needed to show that ribose can indeed be formed with some preference for the D enantiomer under credible prebiotic conditions, with chiral catalysis by amino acids. However, the very high amplifications that we have seen indicate that when such a preference was incorporated into adenosine, cytidine, and uridine, it could be amplified to establish a prebiotic source of predominantly homochiral D nucleic acids and the derived D-ribose.
Materials and Methods
L-Ribose, L-uridine, L-adenosine, L-cytidine, and L-guanosine were prepared according to literature methods, and their spectroscopic data were identical with those reported (16, 17). At a ratio of 1:1, mixtures of the D and L components were dissolved in ethanol, and the DL crystals were collected after partial evaporation of solvent. They were then dried in vacuo with heating. The commercial D nucleosides were also recrystalized from ethanol and vacuum dried.
Melting points were measured on a Thomas-Hoover melting point apparatus, UV experiments were conducted on a Cary-100 UV-visible spectrophotometer, and HPLC was run on a Waters 600 HPLC system with a Waters 996 photodiode array spectrophotometer. A Chiralpak AD column was used for chiral analyses, with a hexane/ethanol gradient. Ethanol and hexane solvents were HPLC grade from Fisher Scientific. Under our conditions, the retention time for D-uridine was 16.5 min and that for L-uridine was 25.4 min. For L-adenosine, it was 17.4 min, and for D-adenosine, it was 22.6 min. For L-cytidine, it was 31 min, and for D-cytidine, it was 40 min.
Saturated solutions of the D nucleosides and of their DL mixtures (pure and in combination) were allowed to equilibrate over 2 days, the solids were then removed by centrifugation, and the solutions were passed through a 0.1-μm syringe filter. The filtrates were diluted to appropriate concentrations for UV and HPLC analyses.
Acknowledgments.
This work was supported by the National Science Foundation.
Footnotes
The authors declare no conflict of interest.
References
- 1.Bonner W. The origin and amplification of biomolecular chirality. Orig Life Evol Biosphere. 1991;21:59–111. doi: 10.1007/BF01809580. [DOI] [PubMed] [Google Scholar]
- 2.Bonner W, Greenberg J, Rubenstein E. The extraterrestrial origin of the homochirality of biomolecules – rebuttal to a critique. Orig Life Evol Biosphere. 1999;29:215–219. doi: 10.1023/a:1006544203107. [DOI] [PubMed] [Google Scholar]
- 3.Rikken G, Raupach E. Thermodynamic control of asymmetric amplification in amino acid catalysis. Nature. 2000;405:932–935. [Google Scholar]
- 4.Cronin J, Pizzarello S. Enantiomeric excesses in meteoritic amino acids. Science. 1997;275:951–955. doi: 10.1126/science.275.5302.951. [DOI] [PubMed] [Google Scholar]
- 5.Pizzarello S. The chemistry of life's origin: A carbonaceous meteorite perspective. Acc Chem Res. 2006;39:231–237. doi: 10.1021/ar050049f. [DOI] [PubMed] [Google Scholar]
- 6.Breslow R, Levine M. Amplification of enantiomeric concentrations under credible prebiotic conditions. Proc Natl Acad Sci USA. 2006;103:12979–12980. doi: 10.1073/pnas.0605863103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Levine M, Kenesky C, Mazori D, Breslow R. Enantioselective synthesis and enantiomeric amplification of amino acids under prebiotic conditions. Org Lett. 2008;10:2433–2436. doi: 10.1021/ol8007099. [DOI] [PubMed] [Google Scholar]
- 8.Bailey J. Astronomical sources of circularly polarized light and the origin of homochirality. Orig Life Evol Biosphere. 2001;31:167–183. doi: 10.1023/a:1006751425919. [DOI] [PubMed] [Google Scholar]
- 9.Klussman M, et al. Thermodynamic control of asymmetric amplification in amino acid catalysis. Nature. 2006:621–623. doi: 10.1038/nature04780. [DOI] [PubMed] [Google Scholar]
- 10.Morowitz H. A mechanism for the amplification of fluctuations in racemic mixtures. J Theor Biol. 1969;25:491–494. doi: 10.1016/s0022-5193(69)80035-4. [DOI] [PubMed] [Google Scholar]
- 11.Noorduin W, et al. Emergence of a single solid chiral state from a nearly racemic amino acid derivative. J Am Chem Soc. 2008;130:1158–1159. doi: 10.1021/ja7106349. [DOI] [PubMed] [Google Scholar]
- 12.Langenbeck W. Organic catalysts. Course of development of organic catalysts. Tetrahedron. 1958;3:185–196. [Google Scholar]
- 13.Breslow R. On the mechanism of the formose reaction. Tetrahedron Lett. 1959;21:22–26. [Google Scholar]
- 14.Ricardo A, et al. 2-Hydroxymethylboronate as a reagent to detect carbohydrates: Application to the analysis of the formose reaction. J Org Chem. 2006;71:9503–9505. doi: 10.1021/jo061770h. [DOI] [PubMed] [Google Scholar]
- 15.Weber A. The sugar model: Catalysis by amines and amino acid products. Orig Life Evol Biosphere. 2001;31:71–86. doi: 10.1023/a:1006750423942. [DOI] [PubMed] [Google Scholar]
- 16.Chang J, et al. A solid-phase approach to novel purine and nucleoside analogs. Bioorg Med Chem. 2005;13:4760–4766. doi: 10.1016/j.bmc.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 17.Moyroud E, Strazewski P. L-Ribonucleosides from L-xylose. Tetrahedron. 1999;55:1277–1284. [Google Scholar]
- 18.Jacques J, Collet A, Wilen S. Enantiomers, Racemates, and Resolutions. Melbourne, FL: Krieger; 1994. [Google Scholar]