Abstract
Wine chemical compositions, which result from a complex interplay between environmental factors, genetic factors, and viticultural practices, have mostly been studied using targeted analyses of selected families of metabolites. Detailed studies have particularly concerned volatile and polyphenolic compounds because of their acknowledged roles in the organoleptic and therapeutic properties. However, we show that an unprecedented chemical diversity of wine composition can be unraveled through a nontargeted approach by ultrahigh-resolution mass spectrometry, which provides an instantaneous image of complex interacting processes, not easily or possibly resolvable into their unambiguous individual contributions. In particular, the statistical analysis of a series of barrel-aged wines revealed that 10-year-old wines still express a metabologeographic signature of the forest location where oaks of the barrel in which they were aged have grown.
Keywords: diagenesis, Fourier transform, ion cyclotron resonance, metabolite, mass spectrometry
Considerable progress has been made in recent years in the characterization of grape and wine metabolites, which have been shown to be responsible for therapeutic (1, 2) or organoleptic properties (3, 4). Metabolic changes occur throughout the growth and maturation of grape berries, and at harvest time the berries contain the major grapevine compounds contributing to the body and flavor of the wine (5). During winemaking processes and in particular during fermentation, these compounds act as a carbon, nitrogen, and element source for yeasts, and are either further metabolized, chemically transformed, or directly transferred to the wine. In traditional winemaking practices, several processes can then subtly modulate the characteristics of wine, and in most cases these modulations involve trace amounts and interplay of metabolites within a complex matrix that appear to be a unique beverage (6). As a consequence, it is likely that deeper understanding of organoleptic or therapeutic activities of wine will rely on its consideration as a complex blend of wine active compounds.
Most of the current analyses of wine with classical analytical technologies contribute to a targeted metabolite profiling in oenology. Here, we report the nontargeted metabolite analysis of a set of wine samples that can be characterized as an “oenolomic” approach of wine, which we define in accordance with the “metabolomics” definition (7) as the quantitative description of all low molecular weight metabolites in a specified biological sample (vine grapes, yeast, or wood). This approach reveals the extremely high yet unknown chemical diversity of wine metabolites. In particular, we concentrated our analysis on a set of wines that were initially part of a full-scale study involving 9 French forests, designed to evaluate the influence of the geographic origin and the species of oak wood on the quality of wines matured in oak barrels (8).
Results and Discussion
Describing the Chemical Spaces of Wine.
A holistic nontargeted approach that enables an instant molecular picture of wines requires both the mass resolving power and the mass accuracy of high-field ion cyclotron resonance-Fourier transform mass spectrometry (ICR-FT/MS), up to considerable mass ranges. We recorded electrospray ionization (ESI) ICR-FT/MS mass spectra of samples representative of distinct steps of the elaboration of wine, proceeding from vine grape extracts to fully aged wines (Fig. 1A). Within the mass range explored (150–2,000 m/z), the spectra exhibit several thousand peaks, which correspond to metabolites that can be ionized under the selected experimental electrospray conditions (9). Data reduction was followed according to elemental composition assignments using isotopic abundance patterns (10) before any further data treatments (11). For example, the spectrum of a red wine from Burgundy (i.e., Vosne Romanée, 1995) can lead up to 17,400 peaks at a signal-to-noise ratio of 2, (115,000 at a signal-to-noise ratio of 1), which can be unambiguously attributed to 1,180 unique elemental CHONS compositions (Fig. S1) with 200 ppb tolerance and confirmation with 13C-signal (3,890 compositions at 500 ppb tolerance), from which only a few hundred may correspond to masses of metabolites, which have already been observed in model solutions or in wines through targeted analyses.
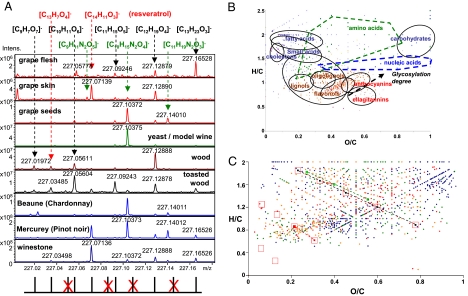
Fig. 1.
Sorting out individual masses in chemical spaces. (A) Detail on nominal mass 227 with all major signals and their attributions to CHON elemental compositions from the grape berry to the wine. Bottom lines show the 11 theoretical hits within this nominal mass in the CHO chemical space, with the 4 absent hits (red crossed). (B) van Krevelen representation showing the positioning of various classes of molecules. The glycosylation line represents the virtual line along which the (O/C, H/C) values would move on the diagram when following successive glycosylations (i.e., anthocyanins and their corresponding mono and di-glycosides). (C) Visualisation in van Krevelen diagram (H/C vs. O/C atomic ratios) of the 3 series of elemental compositions from (A) in the light of the chemical space of over 2,000 cumulated elemental compositions found in the Beaune and Mercurey wines (color code: CHO, blue; CHOS, green; CHON, red; CHONS, orange). Red open squares indicate the 11 theoretical hits from A.
The diversity of chemical spaces of wine can already be observed in the mass distributions within the 200 millimass range of a single nominal mass (see Fig. 1A); for example, when considering only the compositions based on C, H, and O (CHO chemical space in Fig. 1A), 7 out of a total of 11 theoretically possible combinations appear in the different spectra within the given 140-mDa range (12). Of those, 2 series of CHO molecules (black and red) and another series of CHNO molecules (green) vary by a formal exchange of O against CH4. The peak at m/z 227.0713, which is present in the spectrum of the grape skin extract but absent from the spectrum of the grape flesh extract, corresponds to the [M-H]− ion with absolute mass formula [C14H11O3]− and can most likely be assigned to resveratrol isomers. This attribution is further supported by the presence of an analogous mass peak in the spectrum of the Mercurey red wine, whereas it is absent from the spectrum of the Beaune white wine (see Fig. 1A). Interestingly, Fig. 1A shows reveratrol along with many other metabolites (see the corresponding full spectrum of the wine in Fig. S1) in tartar precipitates that may appear in bottles upon aging. Finally, Fig. 1A shows representatively for all of the mass range that nitrogen-containing molecules, such as for m/z 227.1037, are a signature of grape seeds and yeast metabolites.
An initial interpretation of such compilations of masses is made following assignment of elemental compositions with 2-dimensional van Krevelen diagrams (11, 13), which sort each elemental composition onto 2 axes according to its H/C and O/C atomic ratios. Using a home-compiled database of compounds that can exist in model wine solutions or that have been actually observed in wines enables a similar representation of the specific contributions of phenolics, peptides, polysaccharides, nucleotides, and any other classes of compounds present in wines, which can be positively or negatively ionized (Fig. 1B). Unprecedented graphical representations of the various chemical spaces (CHO, CHOS, CHON, CHONS) of wines are then obtained, which visually highlight specific cluster series of elemental compositions observed within nominal masses (Fig. 1C). It must be noted, however, that many of the compounds responsible for the aroma of wines, which exhibit m/z values below 150, are not detected under our experimental conditions.
From Oenolomics to Systems Oenology.
When a wine's spectrum is transposed into van Krevelen diagrams, the result does not simply reflect the superposition of all separate diagrams that can be assembled from each separate step of its elaboration. Instead, it provides an instantaneous metabolite picture of a complex biological system (super organism approach), which encompasses all of the initial contributions of genetic factors modulated by constantly evolving environmental factors. When analyzed separately, each of these steps can be characterized by the potential release into the wine of thousands of compounds of extensive molecular diversity (Fig. S2). It must be borne in mind that ICR-FT/MS alone does not allow one to distinguish isomers; therefore, it is likely that in any of the observed chemical spaces, the actual chemical diversity is considerably higher than that derived from mass peaks alone (12).
In the context thus described, a metabolomics approach in oenology would require the analysis of countless samples to gather a comprehensive description of wine metabolites. Yet, it would have to integrate the decisive—but so versatile—“human” factor, because in essence, wine producers are providing a sensory experience to the consumer (14). Alternatively, the nontargeted metabonomics approach (15), which combines multivariate statistics with high-dimensional unannotated variables, offers the possibility to integrate all of the history of time-related metabolic changes of wine throughout its elaboration process. We thus define for a given grape genotype, and following Nicholson and colleagues (7, 15), “systems oenology” as the sums, products, and interactions of the individual compartments/metabolomes in a complex organism (here the global system equals wine). The systems oenology goal is thus a description of the qualitative and quantitative dynamic and multiparametric metabolic response of wine to environmental modifications.
When Systems Oenology Witnesses the Story That a Wine Tells.
In 1998, a full-scale integrated study involving 9 French forests and 4 sets of French wines was designed to evaluate the influence of the geographic origin and the species of oak wood on the quality of wines matured in oak barrels (8). Each of these 4 sets corresponded to 12 repetitions of the same wine, which only differed by the oak wood species and origin of the trees used for the elaboration of barrels in which they were aged. We hypothesized that such sets of samples would represent unique panels of wine compositions with subtle variations, and as such, would be ideal candidates for the assessment of a systems approach in oenology. It must be noted that to date, no other analytical or sensory analysis of these wines was able to significantly discriminate them according to the species or the origin of oaks used for the barrels, regardless of the grape variety and the color. This situation can be explained by the high interindividual variability of chemical compositions among trees encountered in targeted analyses (16, 17), the fact that sensory analyses of wines often conclude to preferences according to the forest origin that are different for white and red wines (18), and above all, by the fact that so far correlations between sensory descriptors and related compounds have only been reported for the abundant species-related oak cis-whisky lactone (17), whereas direct organoleptic effects of abundant nonvolatile ellagitanins on the wine flavor are, at best, subtle (19, 20).
We recorded the negative- and positive-ion ESI mode ICR-FT/MS mass spectra of each of 60 wines (see SI Text), and these data were further statistically processed to identify possible discriminations among wines (only the negative-ion electrospray data are shown here; positive-ion data showed the same differentiations). Partial least-square regression discriminant analysis (PLS-DA) score plots of wines according to their color provided an illustration of the diversity of metabolites that could basically lead to significant discriminations, regardless of the species and origin of oak (Fig. 2A). The 2 predictive components of the PLS-DA model, R2(Y) = 0.99 and the prediction accuracy Q2(cum) = 0.96, were obtained through a typical 7-fold cross-validation and guaranteed that this model is satisfactory. For example, within the 150 to 2,000 m/z range explored, 356 signals corresponding to unique CHO formulae were found representative for red wines, whereas 281 signals were found representative for the Chardonnay white wine. From the 2-dimensional van Krevelen representation (Fig. S3) of the corresponding signals, it can be seen that polyphenolic compounds (O/C region between 0.4 and 0.6, and H/C region around 1.0) obviously discriminate red wines. However, if unambiguous identifications would necessarily rely on separation methodologies, such procedure would be tedious and for many of the discriminant metabolites here, highly inefficient because of their very low concentrations. Alternatively, with such high-resolution mass data, reliable structural assumptions can be drawn from the questioning of topic-related available databases, such as KEGG, accessible with the MassTRIX interface (21). Hence, when all of the exact masses that discriminate red wines are processed through MassTRIX, 35 distinct formulas are found that can be associated with 66 possible metabolites from the different annotated Vitis vinifera organism pathways (Fig. 3A and Table S1). Consistently with the van Krevelen diagram (see Fig. S3), 46 out of the 66 possible metabolites arise from polypenolic related patways: that is, phenylpropanoid biosynthesis, flavonoid biosynthesis, and flavone and flavonol biosynthesis (see Fig. 3A). Many of these metabolites (among which for example are isomers of quercetin, catechin and myricetin) are known to exist in red wines and are therefore a reliable validation of such database treatment of our raw sets of masses. Likewise, the peak at m/z 243.0662 corresponding to the [M-H]− ion with absolute mass formula [C14H11O4]−, and which is found in the phenylpropanoid pathway as the antioxidant tetrahydroxystilbene piceatannol (see Table S1), a parent molecule of resveratrol, is known to be efficiently ionized in negative mode ESI (22). Finally, the observation of peaks, such as the one at m/z 273.0768, corresponding to the [M-H]− ion with absolute mass formula [C15H13O5]− and the possible attribution to afzelechin (flavonoid biosynthesis pathway), known as a secondary metabolite in grape leaves (23), would witness the presence of yet unreported antioxidant compounds in red wines. Applying a similar MassTRIX treatment to masses that discriminate the white wine leads to the identification of 21 distinct formulas that can be associated with 31 possible metabolites from the different annotated Vitis vinifera organism pathways (see Fig. 3A and Table S1). In a consistent contrast with red wines, only one possible metabolite arises from the phenylpropanoid biosynthesis pathway, which corresponds to coniferyl alcohol, a common lignin moiety, suggesting that in white wines the latter could remain in the bottle after extraction from the barrel without any further reactions. However, as for red wines, possible identifications (of N-containing molecules) agree with expected compounds in oak-aged Chardonnay wines such as tryptophol (24), whereas other identifications also suggest less common compounds, such as pyridoxal or pyridoxo-lactone, which are derivatives of the B6 vitamin. As a whole, this MassTRIX treatment of compounds that discriminates red and white wines not only illustrates the possibility to consistently propose structural identifications of some of the compounds, but most importantly points to the molecular diagenesis that occurs upon aging, because for both red and white wines less than 10% of the discriminant masses can actually find hits in the different Vitis vinifera pathways. Moreover, only less than 20% of all of the found signals can be assigned structures from existing related databases, showing the importance of the structurally unresolved chemistry of wine. This is all the more important because, as shown in van Krevelen diagrams (see Fig. S3), such diagenesis not only concerns the mostly studied polyphenolic compounds but also several other signals in the regions of fatty acids, amino acids, or carbohydrates.
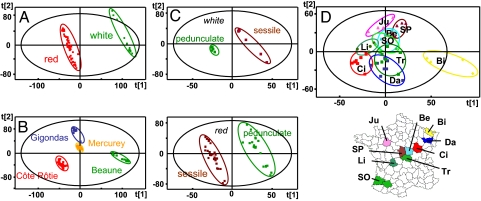
Fig. 2.
Statistical discriminations of wines from the “Tonnellerie 2000” experiment. Classes of PLS-DA score plots are (A) white (green) and red (red) wines; (B) Gigondas (blue), Mercurey (yellow), Beaune (green), and Côte Rôtie (red) wines; (C) white and red wines aged in Sessile (brown) and Pedunculate (green) barrels; (D) wines sorted according to forests of origin of oaks of barrels they were aged in, regardless of the species: (Ju) Jupilles, (SP) Saint Palais, (Be) Bertrange, (Li) Limousin, (SO) Sud Ouest, (Tr) Tronçais, (Ci) Citeaux, (Da) Darney, (Bi) Bitche, along with their location on the map of France.
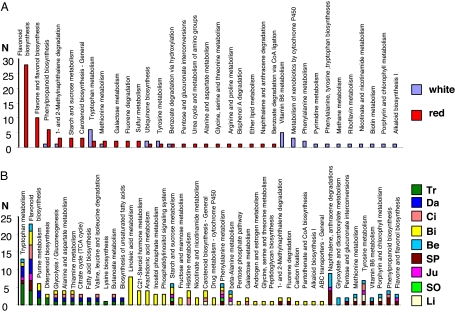
Fig. 3.
Histogram plots of the number (N) of metabolites in pathways of the Vitis vinifera organism as annotated from ICR-FT/MS data with the Masstrix translator into pathways for (A) white wines versus red wines and (B) wines as a function of the forest from which the oak of the barrel originates.
Similarly, several hundred peaks were selectively observed in each of the 4 wines of this study, which led to a clear discrimination of the wines according to their geographical origin, and in our case, to the variety of the grape from which they were elaborated (Fig. 2B). Among the possible metabolites identified in Vitis vinifera pathways, pentose sugars, such as lyxose, xylose, or arabinose would discriminate the southern (thus from the warmer region) Gigondas samples, whereas Mercurey and Côte-Rotie wines would be both discriminated by N- and S-containing molecules, such as the cystathionine amino acid for the former and the antioxidant gluthatione tripeptide for the latter (see Table S1).
The possibility to discriminate wines according to the oak species of the barrels in which they were aged has already been demonstrated, with the particular identification of significantly higher amounts of aromatic whisky lactones in wines aged in European sessile or American white oak barrels (25). However, the PLS-DA score plots for both red and white wines provide an enhanced representation of how wines aged in barrels from a given wood species are grouped together (Fig. 2C). Most interestingly, these results show a significantly narrower distribution among white wines aged in pedunculate barrels than among those aged in sessile barrels, whereas no such difference in distribution is observed for red wines. For white wines, these findings corroborate the previously observed narrower distribution among pedunculate oak wood extracts than among sessile oak extracts (16). In contrast, the broader distribution among red wines aged in pedunculate barrels witnesses to the multiple—yet to be discovered—products of the possible reactions between ellagitannins and wine nucleophiles, such as polyphenolic compounds characteristic of red wines (26).
The major outcome of this nontargeted approach is the previously unavailable opportunity to discriminate wines according to the forest origin of the oaks used for barrel aging of these wines, and to provide a significance in terms of related chemical spaces (Fig. 3B), regardless of the color, the origin of production (and grape variety), and the barrel oak species. The 2-dimensional van Krevelen diagram (see Fig. S3) clearly shows that in the CHO chemical space, compounds responsible for the discrimination not only include polyphenolic-related species but also span from saturated weakly oxygenated molecules to unsaturated highly oxygenated ones. Such discrimination is necessarily based either on wood extractables or on related products of the molecular diagenesis that could have occurred upon aging. Although no metabolic pathways for the Quercus organism are available, but bearing in mind that oak and vine are both ligneous plants, we have also processed these discriminant masses through the MassTRIX interface by questioning the Vitis vinifera pathways (see Table S1). Fig. 3B, which gathers the different hits in a histogram plot, provides a remarkable illustration of the distribution of possible molecular identifications within 3 subgroups of forests: the forests of Citeaux (Ci), Darney (Da), and Tronçais (Tr), the forests of Jupilles (Ju), Saint-Palais (SP), and Bertranges (Be), and the forest of Bitche (Bi), whereas the 2 forests typical of the pedunculate species [Limousin (Li) and Sud-Ouest (SO)] display nearly no hits (see Table S1). It is worth noting that such discrimination of forests observed in wines is correlated to observed physical parameters of the corresponding woods (27), in particular concerning the wood grain, the width of the final wood (considered to be under environmental control), and the number of rows of large vessels, all of which had minimum values for Bi wood and maximum values for Li and SO woods, the remaining forests having intermediate values. When considering oak species separately, we have also checked the similar differentiations between sessile and pedunculate forests (results not shown). Among possible molecular identifications (see Fig. 3B and Table S1), all of the forests would be discriminated in wines by phenolic compounds, such as isomers of liquiritigenin or dihydromyricetin for Ci, Da, and Tr, quercetin for Be, Ju, and SP, and the eriodyctiol flavanone for Bi. But the octadecenoic fatty acid for Da and Tr, or amino acids such as thiamin for Bi, would also contribute to forest differentiations, indicating that various chemical families are involved. Except for quercetin, which has been shown to be an oak-aged related metabolite (28), a thorough survey of the literature using SciFinder Scolar (29) indicates that liquiritigenin, dihydromyricetin, or eriodyctiol (or isomers) have not been reported as oak extractables so far.
We paid particular attention to wines aged in oaks from the Bitche forest, which are significantly discriminated from others (Fig. 2D). It has been shown that oaks from this forest are little subject to frost-crack, despite chemically poor soils, which led to the hypothesis that this forest could be a unique sessile oak ecotype (30). In this ecosystem, considered to be “extreme” for the production of cooperage wood, oaks are mixed with the frugal Scots pines and grow on dry acidic soils that result from the decomposition of sandstone from the Vosges. These conditions explain the slow growth of these oaks and the corresponding low values for the physical parameters mentioned above (27), which are in turn likely correlated to some of the various metabolites that discriminate this forest from others in wines. Another remarkable characteristic of this forest is the presence of an exceptional biodiversity of epiphytic lichens, some of them being very rare, which is a marker of high ecological quality of the forest (31). The [C19H17O8]− absolute ionic formula, to which is unambiguously assigned the discriminant signal at m/z 373.0929 for this forest, finds 2 hits relevant to oak-aged wines in SciFinder Scholar: the hydroxy-tetramethoxy flavan-dione oxydation product of quercetin, observed in model conditions (32) or, more remarkably, the known bitter Atranorin lichen product, supposed to be transferable from the lichen to its oak carrier (33). In such a case, a lichen metabolite would be a marker of cooperage oaks from the Bitche forest in wines. It must be noted though, that possible off-flavor consequences associated with this bitterness have not been detected since preliminary sensory analyses showed a preference for wines (Beaune and Côte-Rotie) aged in Bi barrels (18). Interestingly, for forests typical of the ellagitannin-richer pedunculate species (SO and Li), very few hits are found in the database (see Table S1), which is consistent with previous observations that ellagitannins in oak-aged wines are not correlated to the amount that is potentially extractable (34), therefore emphasizing again the molecular diagenesis that occurs in wine. However, the ionic formula [C27H19O18]− likely represents the vescalin/castalin ellagitannin (35), which consistently discriminates wines aged in Li barrels. Under our experimental conditions, none of the known flavanol-ellagitannin condensation products (26) were detected.
Hence, our approach not only allows us to integrate the intrinsic cooperage variability arising from the fact that all of the staves and barrels of the Tonnellerie 2000 experiment did not undergo the same drying procedure and were not made by the same cooper, but also illustrates that the different steps of elaboration of barrels can complement the chemical signature of a given forest without necessarily erasing it (34). As such, our systems oenology approach provides an unprecedented example of metabologeography (36) translated into chemical representations of the way such noble nectar can shape on the papillas of the wine taster some of the outlines of the scene of its birth.
Materials and Methods
Barrel Elaboration.
The detailed procedure followed to select trees of the Tonnellerie 2000 experiment has already been described elsewhere (37). In brief, 12 lots (5 pedunculate and 7 sessile) of 24 trees were selected from 9 French forests. One lot of 24 trees corresponded to 1 barrel. Each barrel has thus been assembled from 24 trees that stood each for one-twenty-fourth of the toasted surface (body) and one-twenty-fourth of the untoasted surface (head and bottom). For additional details, see SI Text.
Wine Elaboration.
The first set of 2 experiments was designed during the 1998 harvest; one with the appellation “Mercurey rouge 1er cru” (12 lots times 2 repeats), and the other with the appellation “Beaune 1er cru” (12 lots times 2 repeats). The second set of 2 experiments was similarly designed during the 2002 harvest, one with the appellation “Côtes Rotie” (12 lots times 2 repeats), and the other with the appellation “Gigondas” (12 lots times 2 repeats). For additional details, see SI Text.
Wine Sample Preparation.
Wines were sampled directly from the bottles through the cork using a Hamilton needle with side hole. Only 20 μl of wine was diluted into 1-ml methanol from which only 50 μl was used for 1 experiment and to reach the spectral quality presented herein.
ICR-FT/MS Analysis.
High-resolution mass spectra for molecular formula assignment were acquired on a Bruker APEX Qe ICR-FT/MS equipped with a 12-Tesla superconducting magnet and a APOLO II ESI source in the negative ionization mode. Samples were introduced into the microelectrospray source at a flow rate of 120 μl/h, with a nebuliser gas pressure of 20 psi and a drying gas pressure of 15 psi (200 °C). Spectra were externally calibrated on clusters of arginine (10 mg/l in methanol) and accuracy reached values lower than 0.1 ppm in day-to-day measurements. Further internal calibration was done for each sample using fatty acids and accuracy reached values lower than 0.05 ppm. The spectra were acquired with a time domain of 1 megaword (4 megaword for selected samples) with a mass range of 150 to 2,000 m/z. The spectra were zero filled to a processing size of 2 megawords and an average resolution of 250.000 was reached at m/z 200 (100.000 at respectively m/z 600) in full scan. Before Fourier transformation of the time-domain transient, a sine apodization was performed. The ion accumulation time in the ion source was set to 0.2 s for each scan. The number of scans accumulated per samples was 1,024.
Statistical Analyses.
Raw data (mass spectra) were normalized, and then transformed to log(X + 0.00001). The constant 0.00001 was added to provide nondetectable components with a small nonzero value (38). Transformed variables were then mean centered and Pareto scaled and represented as an X matrix (39). The sample classification and the prior information about the sample were done using the Hierarchical clustering analysis-unsupervised method. PLS-DA, performed with SIMCA 11.5, was used to discover characteristic biomarkers (40). This multivariate procedure provided bioinformatic clues for the selection of a limited number of masses most effective in discriminating different species and forests.
Supplementary Material
Acknowledgments.
We thank the United Nations Educational Scientific and Cultural Organization chair “Culture et Traditions du Vin” from the Université de Bourgogne and all of the partners involved in the “Tonnellerie 2000” experiment: Institut Universitaire de la Vigne et du Vin, Ecole Nationale Supérieure de Biologie Appliquée a la Nutrition et a l'Alimentation, Office National des Forets, Ecole Nationale du Génie Rural et des Eaux et Forets, Institut National de la Recherche Agronomique (Nancy, Montpellier), Institut Technique de la Vigne et du Vin Beaune, Bureau Interprofessionnel des Vins de Bourgogne, Professionnels de Bourgogne et de Côte du Rhône, Direction de l'Espace Rural et de la Foret, Inter-Rhône, Conseil Régional de Bourgogne, Office National Interprofessonnel des Vins, Syndicat des tonneliers de Bourgogne, Fédération Française de Tonnellerie. J. Caseiro is thanked for donating the Vosne Romanée wine. V. David is thanked for the preparation of the Saccharomyces cerevisae culture media.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0901100106/DCSupplemental.
References
- 1.Jang M, et al. Cancer chemoprotective activity of resveratrol, a natural product derived from grapes. Science. 1997;275:218–220. doi: 10.1126/science.275.5297.218. [DOI] [PubMed] [Google Scholar]
- 2.Corder R, et al. Red wine procyanidins and vascular health. Nature. 2006;444:566. doi: 10.1038/444566a. [DOI] [PubMed] [Google Scholar]
- 3.Lee S-J, Noble AC. Characterization of odor-active compounds in Californian Chardonnay wines using GC-olfactometry and GC-mass spectrometry. J Agric Food Chem. 2003;51:8036–8044. doi: 10.1021/jf034747v. [DOI] [PubMed] [Google Scholar]
- 4.Cheynier V, et al. Structure and properties of wine pigments and tannins. Am J Enol Vitic. 2006;57:298–305. [Google Scholar]
- 5.Lund ST, Bohlmann J. The molecular basis for wine grape quality—A volatile subject. Science. 2006;311:804–805. doi: 10.1126/science.1118962. [DOI] [PubMed] [Google Scholar]
- 6.Burns J, Crozier A, Lean ME. Alcohol consumption and mortality: is wine different from other alcoholic beverages ? Nutr Metab Cardiovasc Dis. 2001;11:249–258. [PubMed] [Google Scholar]
- 7.Lindon JC, Nicholson JK, Holmes E, eds . The Handbook of Metabonomics and Metabolomics. Amsterdam: Elsevier; 2007. [Google Scholar]
- 8.Feuillat M, et al. Variability of stave-wood: to suffer or to act … (Translated from French) Rev Oenologues. 2003;109(S):19–22. [Google Scholar]
- 9.Cooper HJ, Marshall AG. Electrospray ionization Fourier transform mass spectrometric analysis of wine. J Agric Food Chem. 2001;49:5710–5718. doi: 10.1021/jf0108516. [DOI] [PubMed] [Google Scholar]
- 10.Kind T, Fiehn O. Metabolomic database annotations via query of elemental compositions: Mass accuracy is insufficient even at less than 1 ppm. BMC Bioinf. 2006;7:234–244. doi: 10.1186/1471-2105-7-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rossello-Mora R, et al. Metabolic evidences of biogeographic isolation of the extremophilic bacterium Salinibacter ruber. Nature ISME Journal. 2008;2:242–253. doi: 10.1038/ismej.2007.93. [DOI] [PubMed] [Google Scholar]
- 12.Hertkorn N, et al. Natural organic matter and the event horizon of mass spectrometry. Anal Chem. 2008;80:8908–8919. doi: 10.1021/ac800464g. [DOI] [PubMed] [Google Scholar]
- 13.Wu Z, Rodgers RP, Marshall AG. Two-and three-dimensional van Krevelen diagrams: A graphical analysis complementary to the Kendrick mass plot for sorting elemental compositions of complex organic mixtures based on ultrahigh-resolution broadband Fourier transform ion cyclotron resonance mass measurements. Anal Chem. 2004;76:2511–2516. doi: 10.1021/ac0355449. [DOI] [PubMed] [Google Scholar]
- 14.Bisson LF, Waterhouse AL, Ebeler SE, Andrew Walker M, Lapsley JT. The present and future of the international wine industry. Nature. 2002;418:696–699. doi: 10.1038/nature01018. [DOI] [PubMed] [Google Scholar]
- 15.Nicholson JK, Lindon JC, Holmes E. “Metabonomics”: understanding the metabolic responses of living systems to pathophysiological stimuli via multivariate statistical analysis of biological NMR spectroscopic data. Xenobiotica. 1999;29:1181–1189. doi: 10.1080/004982599238047. [DOI] [PubMed] [Google Scholar]
- 16.Gougeon RD, et al. Expressing forest origins in the chemical composition of cooperage oak woods and corresponding wines by FTICR-MS. Chem Eur J. 2009;15:600–611. doi: 10.1002/chem.200801181. [DOI] [PubMed] [Google Scholar]
- 17.Sauvageot F, Feuillat F. The influence of oak wood (Quercus robur L., Quercus petraea Liebl) on the flavor of Burgundy Pinot noir. An examination of variation among individual trees. Am J Enol Vitic. 1999;50:447–455. [Google Scholar]
- 18.Lacroix J-P. Cooperage Wood, From the Forest to the Vine and Wine. 2006 (Translated from French). (Editions du Gerfaut) [Google Scholar]
- 19.Puech J-L, Feuillat F, Mosedale JR. The tannins of oak heartwood: Structure, properties, and their influence on wine flavor. Am J Enol Vitic. 1999;50:469–478. [Google Scholar]
- 20.Glabasnia A, Hofmann T. Sensory-directed identification of taste-active ellagitannins in American (Quercus alba L.) and European oak wood (Quercus robur L.) and quantitative analysis in Bourbon whiskey and oak-matured red wines. J Agric Food Chem. 2006;54:3380–3390. doi: 10.1021/jf052617b. [DOI] [PubMed] [Google Scholar]
- 21.Suhre K, Schmitt-Kopplin P. Mass TRIX: Mass translator into pathways. Nucleic Acids Res. 2008;36:W481–W484. doi: 10.1093/nar/gkn194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Buiarelli F, Coccioli F, Jasionowska R, Merolle M, Terracciano A. Analysis of some stilbenes in Italian wines by liquid chromatography/tandem mass spectrometry. Rapid Commun Mass Spectrom. 2007;21:2955–2964. doi: 10.1002/rcm.3174. [DOI] [PubMed] [Google Scholar]
- 23.Pfeiffer J, et al. Biosynthesis of flavan 3-ols by leucoanthocyanidin 4-reductases and anthocyanidin reductases in leaves of grape (Vitis vinifera L.), apple (Malus x domestica Borkh.) and other crops. Plant Physiol Biochem. 2006;44:323–334. doi: 10.1016/j.plaphy.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 24.Gonzales-Marco A, Jiménez-Moreno N, Ancin Azpilicueta C. Concentration of volatile compounds in Chardonnay wine fermented in stainless steel tanks and oak barrels. Food Chem. 2008;108:213–219. [Google Scholar]
- 25.Waterhouse AL, Towey JP. Oak lactone isomer ratio distinguishes between wine fermented in American and French oak barrels. J Agric Food Chem. 1994;42:1971–1974. [Google Scholar]
- 26.Quideau S, et al. The chemistry of wine polyphenolic C-glycosidic ellagitannins targeting human topoisomerase II. Chem Eur J. 2005;11:6503–6513. doi: 10.1002/chem.200500428. [DOI] [PubMed] [Google Scholar]
- 27.Bakour R. Influence of the Species and the Origin of the Two Major French Oaks (Quercus robur L., Quercus petraea Liebl.) on the Anatomical Structure and the Physical Properties of Stave-Wood. Nancy, France: Thesis, Université Henri Poincaré; 2003. pp. 149–221. (Translated from French) [Google Scholar]
- 28.Hernandez T, Estrella I, Duenas M, Fernandez de Simon B, Cadahia E. Influence of wood origin in the polyphenolic composition of a Spanish red wine aging in bottle, after storage in barrels of Spanish, French and American oak wood. Eur Food Res Technol. 2007;224:695–705. [Google Scholar]
- 29. [Accessed January 5, 2009];SciFinder Scholar. http://www.cas.org/SCIFINDER/SCHOLAR/. (Chemical Abstract Services, American Chemical Society)
- 30.Le Tacon F, Mormiche A. About the ecology of stave-wood oak in Lorraine. Bulletin Technique de l'ONF. 1974;6:44–55. Translated from French. [Google Scholar]
- 31.Signoret J, Diederich P. Inventory of lichenized and lichenicolous fungi from the nature reserve of rocks and peat bogs of the Bitche region (Translated from French) Ann Sci Rés Bios Trans Vosges du Nord. 2003;11:193–222. [Google Scholar]
- 32.Ohashi H, Kyogoku T, Ishikawa T, Kawase S-I, Kawai S. Antioxidative activity of tree phenolic constituents I: Radical-capturing reaction of flavon-3-ols with radical initiator. J Wood Sci. 1999;45:53–63. [Google Scholar]
- 33.Bourgeois G, Suire C, Vivas N, Benoist F, Vitry C. Atraric acid, a marker for epiphytic lichens in the wood used in cooperage: Identification and quantification by GC/MS/(MS) Analusis. 1999;27:281–283. [Google Scholar]
- 34.Mosedale JR, Puech J-L, Feuillat F. The influence on wine flavor of the oak species and natural variation of heartwood components. Am J Enol Vitic. 1999;50:503–512. [Google Scholar]
- 35.Prida A, Puech J-L. Influence of geographical origin and botanical species on the content of extractives in American, French, and East European oak woods. J Agric Food Chem. 2006;54:8115–8126. doi: 10.1021/jf0616098. [DOI] [PubMed] [Google Scholar]
- 36.Green JL, Bohannan BJM, Whitaker RJ. Microbial biogeography: From taxonomy to traits. Science. 2008;320:1039–1043. doi: 10.1126/science.1153475. [DOI] [PubMed] [Google Scholar]
- 37.Feuillat F, Keller R, Sauvageot F, Puech J-L. Characterization of French oak cooperage (Quercus robur L., Quercus petraea Liebl) research of the Study Group on Barrel-Aging Burgundy Wines. Am J Enol Vitic. 1999;50:513–518. [Google Scholar]
- 38.Sjödin K, Schroeder LM, Eidmann HH, Norin T, Wold S. Attack rates of scolytids and composition of volatile wood constituents in healthy and mechanically weakened pine trees. Scand J Forest Res. 1989;4:379–391. [Google Scholar]
- 39.Eriksson L, Johansson E, Kettaneh-Wold N, Wold S. Multi- and Megavariate Data Analysis−Principles and Applications. Umeå: Umetrics AB; 2001. [Google Scholar]
- 40.Wold S, Sjöström M, Eriksson L. In: The Encyclopaedia of Computational Chemistry. Schleyer P v R, et al., editors. Chichester: Wiley; 1999. pp. 2006–2020. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.