Abstract
Neospora caninum is an intracellular parasite that causes major economic impact on cattle raising farms, and infects a wide range of warm-blooded hosts worldwide. Innate immune mechanisms that lead to protection against this parasite are still unknown. In order to investigate whether myeloid differentiation factor 88 (MyD88) is required for resistance against N. caninum, genetically deficient mice (MyD88−/−) and wild type littermates were infected with live tachyzoites and the resistance to infection was evaluated. We found that sub-lethal tachyzoite doses induced acute mortality of MyD88−/− mice, which succumbed to infection due to uncontrolled parasite replication. Higher parasitism in MyD88−/− mice was associated with the lack of IL-12 production by dendritic cells, delayed IFN-γ responses by NKT, CD4+ and CD8+ T lymphocytes, and production of high levels of IL-10. MyD88−/− mice replenished with IL-12 and IFN-γ abolished susceptibility as the animals survived throughout the experimental period. We conclude that protective IFN-γ-mediated immunity to N. caninum is dependent on initial MyD88 signaling, in a mechanism triggered by production of IL-12 by dendritic cells. Further knowledge on Toll-like receptor recognition of N. caninum antigens is encouraged, since it could generate new prophylactic and therapeutic tools to control parasite burden.
Keywords: Neospora caninum, innate immunity, MyD88, IL-12, IFN-γ
1. Introduction
Neospora caninum is an intracellular parasite that causes major reproductive losses in bovines, due to abortions and stillbirths caused by mid-gestational exposure or recrudescence of latent infections [15, 29]. Some research groups have attempted to estimate the economic impact of neosporosis in cattle and they have suggested that several million dollars are lost per year with miscarriages and veterinary medical assistance [3, 28]. Dogs and coyotes are N. caninum definitive hosts, once oocysts are shed after oral challenge with different parasite-harboring tissues [7, 9, 16]. Canine neosporosis occurs mostly in puppies, which are infected in utero after recrudescence of a maternal latent infection, and its main clinical features are neuromuscular disorders [6, 17]. Also, there are unanswered questions that have been raised as if this parasite is able to infect humans, considering that serological evidence is found in different populations worldwide [14, 21, 27].
Innate immunity plays an important role in protection and pathogenesis of protozoan infections. In addition to conferring resistance to initial parasite replication, innate immunity activation is essential for the establishment of adaptive Th1 cellular responses, in order to control active infections and consequently overcome re-exposures [4, 19]. Parasite control is mainly based on an early IFN-γ production, dependent on lymphocyte priming by antigen presenting cells (APC) and IL-12 [2, 8]. APC, which are crucial for early parasite recognition and control, distinguish pathogens through Toll-like receptors (TLR). Stimulation of TLR induces immediate protective responses through the production of diverse antimicrobial peptides and cytokines [1]. Myeloid differentiation factor 88 (MyD88) is a critical signaling element after microbial recognition by TLR, once it represents a common cytoplasmatic adaptor protein to most of these receptors and it is responsible for pro-inflammatory cytokine synthesis, leading to the elimination of intracellular pathogens [26]. In the last decade, MyD88 was found as crucial for resistance to almost 40 different pathogens in experimental models, including viruses, bacteria, fungi and protozoa [30].
In this sense, the present work was aimed at determining the requirement of TLR-associated adaptor protein MyD88 in the control of infection by N. caninum. Infection kinetics were analyzed in adaptor protein deficient mice and special emphasis was given to host parasitism, inflammatory cell migration and main Th1 cytokine production. Our results showed that MyD88 is responsible for IL-12 production and consequent early acute IFN-γ response, resulting in infection control and host survival.
2. Materials and methods
2.1. Animals
Six- to eight-week old wild type (WT) C57BL/6 background mice, along with animals deficient in adaptor protein MyD88 (MyD88−/−), IL-12p40 (IL-12p40−/−) and IFN-γ (IFN-γ−/−) deficient mice were bred and maintained at the institutions’ animal facilities, Department of Biochemistry and Immunology, School of Medicine of Ribeirão Preto, USP (Ribeirão Preto, Brazil), with food and water ad libitum. IL-12p40−/− and IFN-γ−/− mice, along with WT littermates, were acquired at a commercial supplier (The Jackson Laboratory, Bar Harbor, ME, USA). MyD88−/− mice were originally donated from Dr Shizuo Akira (Research Institute for Microbial Disease, Immunology Frontier Research Center, Osaka University, Japan). The genetically deficient mice were screened for their respective gene depletion and mated along with deficient siblings to preserve their lineage characteristics. Maintenance and care of these animals complied with the guidelines of the Laboratory Animal Ethics Committee from the FMRP/USP. Animal euthanasia was performed in accordance with international welfare grounds, according to the American Veterinary Medical Association Guidelines on Euthanasia (2007).
2.2. In vitro N. caninum maintenance
N. caninum tachyzoites of the NC-1 isolate were propagated in African green monkey kidney (Vero) cells in RPMI medium supplemented with 2 mM L-glutamine, 100 U penicillin, and 100 μg of streptomycin (Invitrogen, Carlsbad, CA, USA). Parasites were harvested after 80% lysis of host cell monolayer, by mechanical disruption, and used to infect new culture flasks. To prepare the inocula, parasite suspensions were submitted to repeated passages through a syringe and needle, with decreasing gauges, until complete host cell disruption. The next step consisted of a centrifugation at 1 000 × g for 10 min at 4 °C, in RPMI medium, and parasite number was adjusted to 1 × 106 of viable tachyzoites/mice, confirmed by Trypan blue staining.
2.3. Study design
WT and MyD88−/− mice were infected through intraperitoneal route and sacrificed at 0, 3, 7, and 10 days post-infection (p.i.). Serum samples and sections of liver, lung and heart were harvested and fixed in phosphate-buffered formaldehyde (10%) and freeze-mounting media (Sakura Finetek, Torrance, CA, USA). Additionally, the peritoneal cavity of each mouse was washed with 1 mL of RPMI medium in order to determine the cell influx phenotype and to measure the levels of local cytokine production. Parasitism was determined in peritoneal exudate cells (PEC) by analyzing the percentage of cells with the presence of parasitophorous vacuoles by light microscopy (Olympus, Tokyo, Japan), after cytospin and Diff-Quick staining. Each group/date contained three WT and MyD88−/− mice for the above-described experiments. Experimental results were confirmed by independent groups from different sets of experiments. In order to observe mortality rates to N. caninum infection, additional groups of WT, MyD88−/−, IL-12p40−/−, and IFN-γ−/− mice were infected, using the same parasite load, and consisting of six animals/group. MyD88−/− animals were left untreated or treated with recombinant murine IL-12p70 (rIL-12p70 – 250 ng/animal for 10 days – BD, USA) and IFN-γ (rIFN-γ – 1 000 U/animal for 10 days – Sigma-Aldrich, St. Louis, MO, USA). Survival experiments with genetically deficient mice were performed at least twice in order to observe reproducibility of the experimental data.
2.4. Histological analysis
Liver, lung and heart obtained from uninfected and infected WT and MyD88−/− mice were embedded in paraffin, sectioned, stained with hematoxylin-eosin, and examined by light microscopy (Olympus). Quantification of inflammatory cell infiltrates were performed by ImageJ software (NIH, Bethesda, MD, USA), in 40 microscopic fields per histological section (2 sections/mouse/group/date), with 400 times magnification. Two sections from each mouse and three mice per group were used in these experiments. The results are expressed as inflammatory index, determined by a ratio between the mean number of cell nuclei present in infected animals and the mean value obtained from uninfected animals for each period of infection.
2.5. Cellular phenotyping and intracellular cytokine production
Differential PEC influx was determined through fluorescent staining, performed with monoclonal antibodies directed to specific cell surface markers and conjugated to fluorescein isothiocyanate (FITC) and Phycoerythrin (PE) (R&D systems, Minneapolis, MN, USA; Ebiosciense, San Diego, CA, USA). Assays to determine the proportion of macrophages (MΦ – CD11b+/CD11c−), dendritic cells (DCs - CD11b+/CD11c+), B cells (CD19+/CD3−), neutrophils (Gr-1+/MHCII-), NK (CD56+/CD3−), NKT (CD56+/CD3+), TCD4+ (CD3+/CD4+), and TCD8+ (CD3+/CD8+) cells were performed following a previous description [5]. Monoclonal antibodies against IL-12p40 and IFN-γ conjugated to biotin and streptotavidin-PE-Cy5, were used to detect intracellular cytokine production, together with a commercially available permeabilization kit, which was used according to the manufacturer’s instructions (Becton Dickinson, Franklin Lakes, NJ, USA). Cell viability was confirmed by negative staining with Trypan blue, and 200 000 events were acquired per tube/animal/group/date. Data acquisition was performed using a FACscan flow cytometer (Becton Dickinson). Appropriated isotype-matched rat IgG was used as negative controls. Data analysis was carried out with FlowJo 8.7 software (Tree Star, Ashland, Oregon, USA). Cell counts were obtained by calculation of the percentage obtained for each cell type by flow cytometry analysis from the total number of leukocytes present in the peritoneal cavity of each animal/group/date. Total number of leukocytes was acquired through a Neubauer hemocytometer. Intracellular cytokine production was analyzed by the difference between the mean intensity of fluorescence (MIF) of uninfected and infected mice at 3 days p.i. (ΔMIF).
2.6. Parasite detection in tissue samples
Fluorescent staining of the parasites was performed in slides containing frozen tissue sections, previously standardized through block titration of the reagents and control sera. Slides were probed with polyclonal antibodies against N. caninum (1:100) produced in an experimentally infected calf, which was seronegative to related pathogens, as described previously [7]. Anti-bovine IgG labeled with FITC (Sigma-Aldrich) were used as secondary antibodies, according to the manufacturer’s instructions (1:128). The slides were mounted on carbonate-buffered glycerin (pH 9.0) and analyzed under fluorescence microscopy (Olympus). Samples were considered positive when bright stains were observed in the tissues analyzed, indicating parasite clusters. Uninfected controls were assayed in parallel to assure test specificity. Tissue parasite burden was also determined by quantitative real-time PCR (qPCR) as previously described [24], using primer pairs (sense 3’-GCTGAACACCGTATGTCGTAAA-5’; antisense 3’-AGAGGAATGCCACATAGAAGC -5’) designed to detect the N. caninum Nc-5 sequence through the SYBR green system (Invitrogen). DNA extraction was performed from 20 mg of each analyzed tissue using a commercially available kit (Promega Co., Madison, WI, USA), following the manufacturer’s instructions. DNA concentration was set by UV spectrophotometry (260 nm) and adjusted to 100 ng/μL with sterile DNAse free water. Assays to determine N. caninum tachyzoite loads were performed through real-time PCR (Applied Biosystems, Foster City, CA, USA) and parasite counts were calculated from a standard curve (10 to 0.0000001 ng) with DNA equivalents extracted from 107 NC-1 tachyzoites, which were included in each run.
2.7. Cytokine quantification by ELISA
The concentrations of IL-12p40, IFN-γ, IL-10 and IL-17 in serum samples and peritoneal exudate were measured by commercial ELISA kit (Becton Dickinson – IL-12p40, IFN-γ and IL-10; R&D systems – IL-17). The assays were performed according to the manufacturer’s instructions. The reaction was revealed with peroxidase-streptavidin conjugate, followed by the addition of substrate containing hydrogen peroxide and TMB (BioRad, Hercules, CA, USA) as a chromogen. Microplates were read in an ELISA reader (Molecular Devices, Sunnyvale, CA, USA) at 450 nm. Cytokine concentrations were calculated from standard curves of murine recombinant cytokines. The detection limits for each ELISA were the following: IL-12p40, IFN-γ and IL-10 = 2 000 to 31.3 pg/mL; IL-17 = 1 000 to 15.625 pg/mL.
2.8. Statistical analysis
Two-way ANOVA followed by Bonferroni post-tests were applied to compare WT and MyD88−/− groups in relation to tissue parasitism and inflammation, peritoneal cell parasitism and phenotype of cellular influx, as well as IL-12p40, IFN-γ, IL-10 and IL-17 production. Differences in survival rates between groups were compared using Kaplan-Meier survival analysis, through a log-rank Mantel-Cox test. In all measurements, differences were considered significant when p < 0.05. Statistical analysis of the data obtained was carried out using GraphPad Prism software (GraphPad, La Jolla, CA, USA).
3. Results
3.1. MyD88−/− mice are highly susceptible to acute N. caninum infection
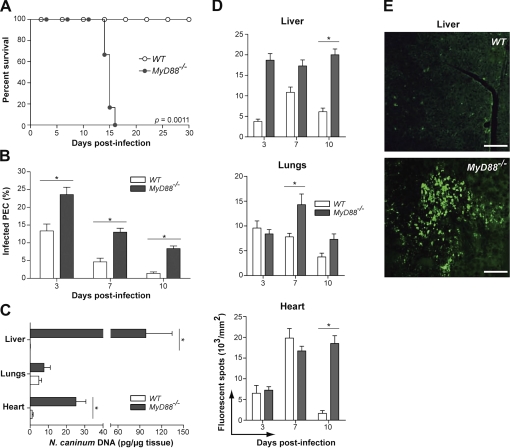
Experimental infections with sub-lethal doses of N. caninum tachyzoites induced severe clinical signs in MyD88−/− mice as huddling, rough coats, reduced motility, apathy and/or uncoordinated movements, evolving to ataxia. All MyD88−/− mice were euthanized between 14 and 16 days p.i. and Kaplan-Meyer survival curves presented significant differences (p < 0.05) when deficient mice were compared with WT littermates (Fig. 1A). WT mice presented only slight clinical alterations at the initial stage of infection, while most MyD88−/− mice presented ascites at the time of death, as well as macroscopic lesions in the liver, lungs and heart. Microscopically, a higher percentage of infected cells (p < 0.05) were observed in the peritoneal fluid of MyD88−/− compared to WT mice, during the whole experimental period (Fig. 1B). qPCR demonstrated that livers and hearts from deficient animals presented significantly higher parasite DNA at 10 days p.i. (p < 0.05) (Fig. 1C). Livers from MyD88−/− mice presented over 100 times more detectable DNA than those extracted from WT animals. Parasite-specific fluorescent staining demonstrated that the observed tissues presented significant differences in parasite burden throughout the experimental time, confirming that MyD88−/− mice were unable to control parasite replication (Fig. 1D). WT mice reduced parasite loads significantly by 10 days p.i. Also, the liver was the tissue that harbored the highest parasite replication in MyD88−/− mice (Fig. 1E).
Figure 1.
MyD88 is required for parasite replication control and host survival to N. caninum infection. (A) Groups of WT and MyD88−/− mice (n = 6 mice/group) were infected at the same time with sub-lethal parasite doses and survival was observed through a 30-day period. Data are representative of five independent experiments. (B) Freshly collected PEC (n = 3 mice/group/date) were analyzed for the presence of parasitophorous vacuoles through Diff-Quick staining. Data are representative of three independent experiments. (C) qPCR analysis of tissues collected from both mice groups (n = 3 mice/group) after 10 days p.i. with N. caninum tachyzoites. (D) Quantification kinetics of tissue parasite burden of WT and MyD88−/− mice detected by parasite-specific immunofluorescent staining. (E) Representative images of tissues obtained from the different mice groups at 10 days p.i. after specific immunostaining for N. caninum (n = 3 mice/group). Histological images are representative of 10 days p.i. and magnification scale is set to 5 μm (200×). * Indicates significant statistical differences (mean ± SEM, p < 0.05).
3.2. MyD88-independent inflammatory cell migration to parasitized tissues
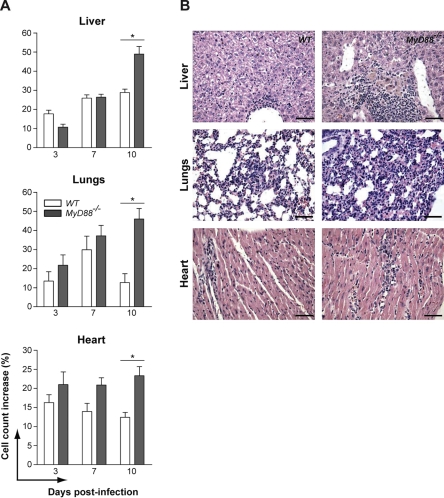
Microscopic analysis of target tissues showed that inflammatory cells migrated to the tissues of both groups of mice, indicating that MyD88 is not required for cellular recruitment to N. caninum infected organs. Interestingly, cell counts presented comparable increases in liver, lungs and heart of WT and MyD88−/− mice until 7 days p.i. (Fig. 2A). At day 10 p.i., MyD88−/− mice presented significantly higher inflammatory influx, in a similar pattern as that observed for parasite burden (Fig. 1D). The most pronounced differences in inflammatory infiltrate size were observed in the livers and lungs of WT and MyD88−/− mice. Both mice groups presented similar increasing cell influx to hepatic and pulmonary tissues until 7 days p.i. However, while WT mice maintained similar cell counts at 10 days p.i. MyD88−/− mice presented a sharp increase in inflammatory cell infiltrate (p < 0.05), reaching nearly a 50% raise in cell numbers of both tissues, when compared to uninfected controls (Fig. 2B).
Figure 2.
The inflammatory influx to target tissues in response to N. caninum infection is MyD88-independent. (A) Quantification kinetics of the relative cell counts in livers, lungs and hearts of WT and MyD88−/− mice (n = 3 mice/group/date) experimentally infected with N. caninum. (B) Representative histological images of the liver, lungs and heart obtained form WT and MyD88−/− mice before and after 10 days p.i. Magnification scale is set to 10 μm (400×). * Indicates significant statistical differences (mean ± SEM, p < 0.05).
3.3. MyD88 induces pro-inflammatory cytokine production during N. caninum infection
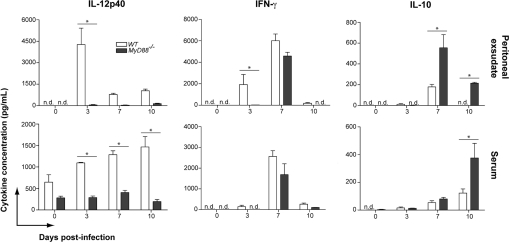
To understand the mechanism that leads to parasite clearance and host survival, we next determined the production of IL-12, IFN-γ, IL-10, and IL-17 in the infected mice (Fig. 3). We found that IL-12p40 levels increased sharply in peritoneal exsudate of WT mice, with peak levels at 3 days p.i., and basal levels of this cytokine were found in samples from MyD88−/− mice. Systemic production of IL-12p40 also remained unaltered in MyD88−/− mice in response to N. caninum infection, while WT mice presented increasing serum cytokine levels (p < 0.05) throughout the experimental period. WT mice presented increasing IFN-γ production until 7 days p.i. with N. caninum in the peritoneal cavity and serum, which decreased to low or undetectable levels at 10 days p.i. MyD88−/− mice also presented a similar IFN-γ peak at 7 days p.i., followed by a sharp decrease in cytokine production. However, IFN-γ response to tachyzoite infection in MyD88−/− mice was delayed in comparison to WT littermates. ELISA detection of IL-10 demonstrated that MyD88−/− mice produced significantly higher quantities of this cytokine at 7 and 10 days p.i. (p < 0.05), leading to a low IFN-γ:IL-10 ratio when compared to WT mice. IL-17 production was not detected in peritoneal exudate and serum samples obtained from WT and MyD88−/− mice throughout the experimental period.
Figure 3.
MyD88 is responsible for the early induction of Th1-related cytokines after N. caninum infection. The presence of IL-12p40, IFN-γ, and IL-10 was evaluated by ELISA in the peritoneal exudate and serum samples of uninfected, and infected WT and MyD88−/− mice at 3, 7, and 10 days p.i. (n = 3 mice/group/date). Data are representative of two independent experiments. * Indicates significant statistical differences (mean ± SEM, p < 0.05).
3.4. N. caninum induces MyD88-dependent IL-12 production by DC
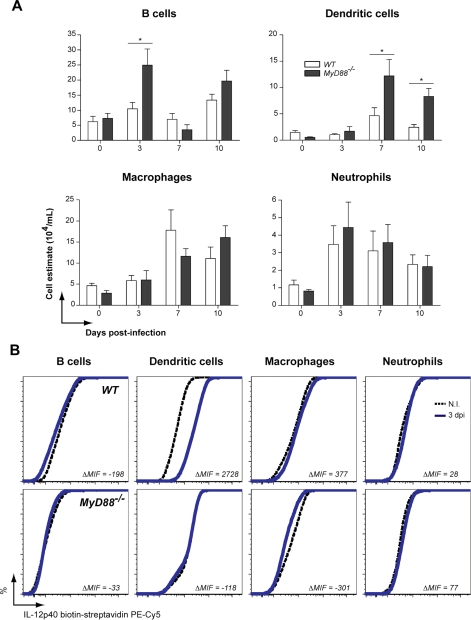
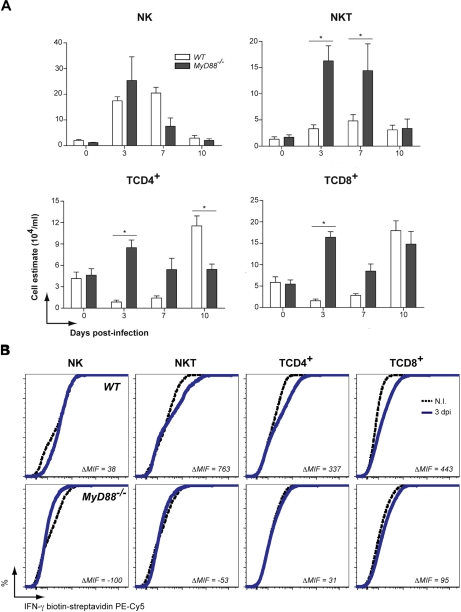
Migration of B cells, DC, MΦ and neutrophils to the peritoneal cavity demonstrated similar patterns in WT and MyD88−/− mice after infection by N. caninum. Moreover, significantly higher influx of B cells at 3 days p.i. (p < 0.05), and DC at 7 and 10 days p.i. (p < 0.05) was observed in genetically deficient mice (Fig. 4A). Intracellular cytokine staining demonstrated that DC and, in a minor scale, MΦ, were responsible to peak IL-12p40 production present in the peritoneal cavity of WT mice at 3 days p.i., as determined by ELISA. Both DC and MΦ obtained from MyD88−/− mice did not present distinct intracellular cytokine profiles between uninfected and mice at 3 days p.i. with N. caninum (Fig. 4B). Accordingly, we also observed that IFN-γ+ cells (Fig. 5A) migrated to the peritoneal cavity of infected mice despite MyD88 depletion, differently from WT littermates (Fig. 5A). Significantly higher cell migration (p < 0.05) was observed for NKT, TCD4+ and TCD8+ lymphocytes, especially at 3 and 7 days p.i. in MyD88−/− mice. Intracellular staining showed that the early acute IFN-γ response by PEC was dependent on the production of NKT, TCD4+, and TCD8+ cells in WT animals, while the production of this key cytokine was completely abrogated in cells from MyD88−/− mice (Fig. 5B).
Figure 4.
Antigen presenting cells from MyD88−/− mice are unable to produce IL-12, despite their intact migration pattern. (A) Immunophenotyping of potential IL-12+ cells to the peritoneal cavity at 3, 7, and 10 days p.i. (n = 3 mice/group/date), determined by flow cytometry analysis of cell surface markers. Data are representative of two independent experiments. (B) Intracellular IL-12 staining of immunophenotyped APC present in the peritoneal cavity of uninfected WT and MyD88−/− and after 3 days p.i. The results show one representative animal of a total from three of each group/date. * Indicates significant statistical differences (mean ± SEM, p < 0.05).
Figure 5.
Lymphocytes from genetically deficient mice in MyD88 are not able to produce acute IFN-γ after N. caninum infection. (A) Immunophenotyping of potential IFN-γ+ cells to the peritoneal cavity at 3, 7, and 10 days p.i. (n = 3 mice/group/date), determined by flow cytometry analysis of cell surface markers. Data are representative of two independent experiments. (B) Intracellular IFN-γ staining of immunophenotyped lymphocytes present in the peritoneal cavity of uninfected WT and MyD88−/− and after 3 days p.i. The results show one representative animal of a total from three of each group/date. * Indicates significant statistical differences (mean ± SEM, p < 0.05).
3.5. Treatment of MyD88−/− mice with IL-12 and IFN-γ confers resistance to acute infection
To establish the importance of MyD88-dependent IL-12 and IFN-γ production to control N. caninum acute infection, survival curves of IFN-γ−/−, IL-12p40−/−, and MyD88−/− mice treated or untreated with recombinant IFN-γ and IL-12p70 were determined. Interestingly, MyD88−/− mice treated with rIL-12p70 survived throughout the experimental infection, differently than untreated deficient mice (p = 0.0021, Fig. 6A). Furthermore, IL-12p40−/− mice presented similar survival kinetics to MyD88−/− mice (p = 0.4454). Comparatively, IFN-γ−/− mice demonstrated to be more susceptible to acute infection by N. caninum than MyD88 and IL-12p40 deficient mice (p = 0.0001), once all animals were euthanized until 11 days p.i. (Fig. 6B). Replenishment of MyD88−/− mice with rIFN-γ induced resistance through acute N. caninum infection in the same manner as IL-12p70 treatment.
Figure 6.
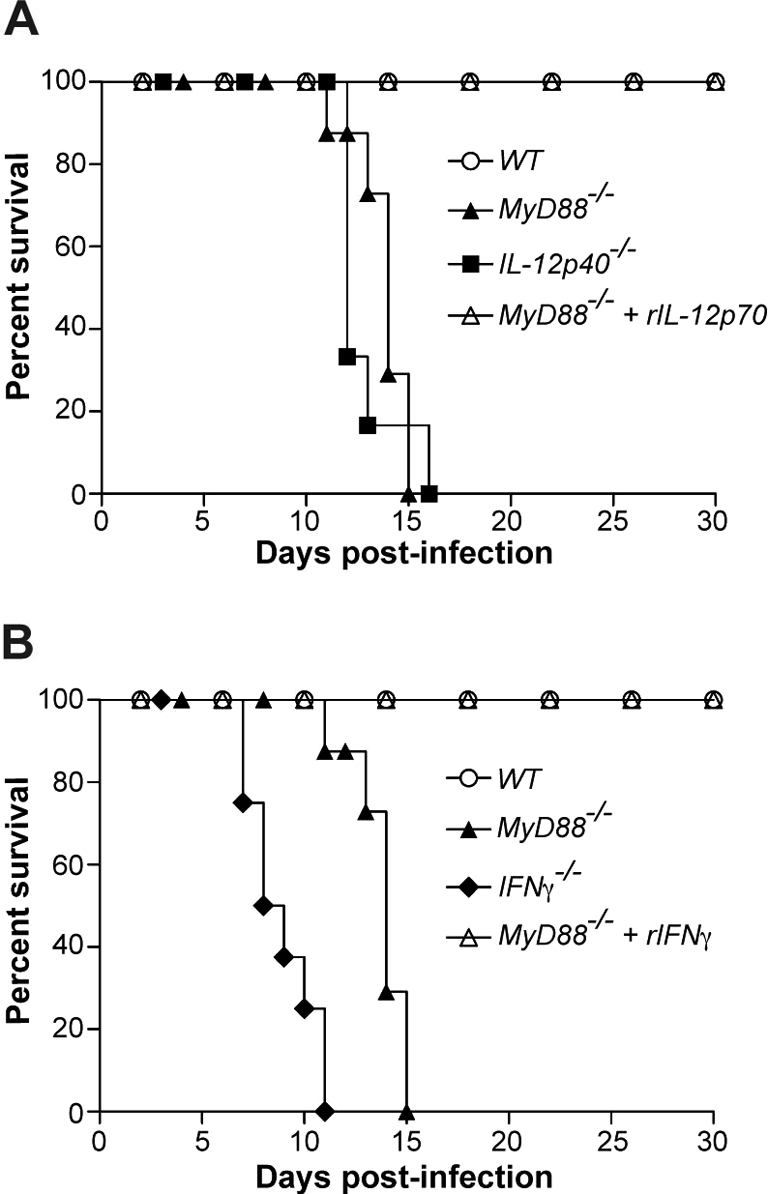
Replenishment of IL-12 and IFN-γ reverts susceptibility of MyD88−/− mice to acute N. caninum infection. Survival curves of (A) WT, IL-12p40−/−, MyD88−/− and MyD88−/− mice treated with rIL-12p70 or (B) WT, IFNγ−/−, MyD88−/− and MyD88−/− mice treated with rIFNγ (n = 6 mice/group), infected at the same time with sub-lethal parasite doses. Survival was observed through a 30-day period. Data are representative of two independent experiments.
4. Discussion
The major aim of the present work was to observe survival, parasite control, cellular migration and cytokine responses of WT and MyD88−/− mice after N. caninum acute infection. Although mice are naturally resistant to N. caninum [23], they were not able to overcome parasite exposure in the absence of MyD88. Such a phenomenon was also observed by the depletion of other crucial immune components, since IL-12 and IFN-γ, cytokines that combined lead to infection control. Experiments herein conducted demonstrated that MyD88 is required for IL-12 synthesis by DC, which induce an early acute IFN-γ production. Sequentially, effector Th1 responses mediated by an elevated IFN-γ:IL-10 ratio confers control of parasite replication and consequent host survival. To our knowledge, this is the first report that demonstrates the mechanisms that underlie the production of these key cytokines during N. caninum infection.
Comparatively, related protozoan T. gondii is recognized by MyD88-dependent TLR2, TLR9, and TLR11 [18, 20, 31]. In [24], MyD88−/− mice were unable to control parasite replication and succumbed to T. gondii after infection with low numbers of tachyzoites similarly to the results shown here. The pro-inflammatory environment required for host resistance to intracellular pathogens is manly generated by pathogen recognition by APC, which release chemical mediators that activate and direct effector cell phenotypes. IL-12 is a key cytokine involved in NK activation and commitment of naïve CD4+ lymphocytes to the Th1 subset. Mice treated with anti-IL-12 monoclonal antibodies presented higher morbidity/mortality to N. caninum acute infection, demonstrating the key role of this cytokine in host protection, similarly to the results presented here [11]. Our results clearly demonstrated that the lack of IL-12 production in MyD88−/− mice after N. caninum infection reinforces the vital role of this cytokine, which has its production triggered by pathogen innate recognition, and inducing posterior protective immune responses. Thus, these results are in agreement to additional observations showing similar survival kinetics between IL-12 and MyD88 deficient mice.
Seemingly, IFN-γ has a pivotal role in host resistance to N. caninum, inducing effector mechanisms like apoptosis of infected cells and NO production, as reviewed elsewhere [10]. Likewise, IFN-γ was shown to be more relevant for the protection of N. caninum infection than MyD88 and IL-12, once IFN-γ−/− mice succumbed to acute infection earlier than the other genetically deficient mice analyzed. IL-12 stimulus after N. caninum infection triggers effector IFN-γ production and consequent infection control as shown previously [11, 22]. In addition to the higher relevance of IFN-γ in N. caninum infection, induction of this key cytokine seems to present MyD88-independent pathways, once MyD88−/− mice were able to produce late IFN-γ responses together with high levels of IL-10, in compliance with data recently published for T. gondii [25]. Additionally, it is also important to highlight that, although IL-12p40 may also indicate IL-23 activity, treatment with the IL-12 active subunit (p70) conferred protective immunity to MyD88−/− mice, suggesting that Th17 responses were not involved in the mechanisms proposed here for N. caninum infection in mice. Accordingly, IL-17 was not detected in serum samples or peritoneal wash of WT and MyD88 deficient mice during the experimental observation.
The absence of IL-12 production leading to the absence of early IFN-γ response was not a consequence of impaired migration of producer cells to the site of infection, since a high influx of different cell lines with potential to produce both key cytokines was observed in the peritoneal cavity of MyD88−/− mice. Moreover, inflammatory migration to parasitized tissues was clearly MyD88-independent, once cell infiltrate in deficient mice was higher than in WT littermates at 10 days p.i. This enhanced cell migration might have occurred in response to higher parasite burden observed in MyD88−/− mice. Conversely, WT mice possessed active cytokine-producing DC and lymphocytes at early acute infection, while MyD88-deficient cell lines were anergic to parasite stimuli. Corroborating with the results obtained in the present study, T. gondii induces a MyD88-independent, vigorous and long-lasting chemokine response in infected MΦ dependent on phosphoinositide 3-kinase signaling pathways [12], in contrast to pro-inflammatory cytokine production, which presents major participation of MyD88 after T. gondii infection [13, 18].
In conclusion, we have shown that DC are the main producers of MyD88-dependent IL-12 during N. caninum infection in mice and, in the absence of the TLR adaptor protein, effector lymphocytes are not primed to produce early IFN-γ that is crucial to control infection. It is now necessary to establish which MyD88-associated TLR are relevant in Th1 activation after infection by this parasite. Moreover, the mechanisms that underlie TLR recognition of N. caninum leading to the induction of an effector immune response against the parasite are yet to be determined. We believe that further knowledge on N. caninum specific TLR agonists and their mechanisms of action may lead to prophylactic and therapeutic strategies against neosporosis.
Acknowledgments
The authors would like to thank Cristiane M. Milanezi and Walter M. Turato for their technical assistance. This work was financially supported by Brazilian finding agencies CNPq (473178/2007-9) and FAPESP (2006/06803-4).
References
- 1.Akira S.. Toll-like receptor signaling. J. Biol. Chem. 2003;278:38105–38108. doi: 10.1074/jbc.R300028200. [DOI] [PubMed] [Google Scholar]
- 2.Aliberti J.C., Cardoso M.A., Martins G.A., Gazzinelli R.T., Vieira L.Q., Silva J.S.. Interleukin-12 mediates resistance to Trypanosoma cruzi in mice and is produced by murine macrophages in response to live trypomastigotes. Infect. Immun. 1996;64:1961–1967. doi: 10.1128/iai.64.6.1961-1967.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bartels C.J., Arnaiz-Seco J.I., Ruiz-Santa-Quitera A., Björkman C., Frössling J., von Blumröder D.. et al. Supranational comparison of Neospora caninum seroprevalences in cattle in Germany, The Netherlands, Spain and Sweden. Vet. Parasitol. 2006;137:17–27. doi: 10.1016/j.vetpar.2005.12.016. [DOI] [PubMed] [Google Scholar]
- 4.Biron C.A., Gazzinelli R.T.. Effects of IL-12 on immune responses to microbial infections: a key mediator in regulating disease outcome. Curr. Opin. Immunol. 1995;7:485–496. doi: 10.1016/0952-7915(95)80093-x. [DOI] [PubMed] [Google Scholar]
- 5.Cavassani K.A., Campanelli A.P., Moreira A.P., Vancim J.O., Vitali L.H., Mamede R.C.. et al. Systemic and local characterization of regulatory T cells in a chronic fungal infection in humans. J. Immunol. 2006;177:5811–5818. doi: 10.4049/jimmunol.177.9.5811. [DOI] [PubMed] [Google Scholar]
- 6.Dubey J.P., Vianna M.C., Kwok O.C., Hill D.E., Miska K.B., Tuo W.. et al. Neosporosis in Beagle dogs: clinical signs, diagnosis, treatment, isolation and genetic characterization of Neospora caninum. Vet. Parasitol. 2007;149:158–166. doi: 10.1016/j.vetpar.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 7.Furuta P.I., Mineo T.W.P., Carrasco A.O.T., Godoy G.S., Pinto A.A., Machado R.Z.. Neospora caninum infection in birds: experimental infections in chicken and embryonated eggs. Parasitology. 2007;134:1931–1939. doi: 10.1017/S0031182007003344. [DOI] [PubMed] [Google Scholar]
- 8.Gazzinelli R.T., Wysocka M., Hayashi S., Denkers E.Y., Hieny S., Caspar P.. et al. Parasite-induced IL-12 stimulates early IFN-gamma synthesis and resistance during acute infection with Toxoplasma gondii. J. Immunol. 1994;153:2533–2543. [PubMed] [Google Scholar]
- 9.Gondim L.F., McAllister M.M., Pitt W.C., Zemlicka D.E.. Coyotes (Canis latrans) are definitive hosts of Neospora caninum. Int. J. Parasitol. 2004;34:159–161. doi: 10.1016/j.ijpara.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 10.Hemphill A., Vonlaufen N., Naguleswaran A.. Cellular and immunological basis of the host-parasite relationship during infection with Neospora caninum. Parasitology. 2006;133:261–278. doi: 10.1017/S0031182006000485. [DOI] [PubMed] [Google Scholar]
- 11.Khan I.A., Schwartzman J.D., Fonseka S., Kasper L.H.. Neospora caninum: role for immune cytokines in host immunity. Exp. Parasitol. 1997;85:24–34. doi: 10.1006/expr.1996.4110. [DOI] [PubMed] [Google Scholar]
- 12.Lee C.W., Sukhumavasi W., Denkers E.Y.. Phosphoinositide-3-kinase-dependent, MyD88-independent induction of CC-type chemokines characterizes the macrophage response to Toxoplasma gondii strains with high virulence. Infect. Immun. 2007;75:5788–5797. doi: 10.1128/IAI.00821-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu C.H., Fan Y.T., Dias A., Esper L., Corn R.A., Bafica A.. et al. Cutting edge: dendritic cells are essential for in vivo IL-12 production and development of resistance against Toxoplasma gondii infection in mice. J. Immunol. 2006;177:31–35. doi: 10.4049/jimmunol.177.1.31. [DOI] [PubMed] [Google Scholar]
- 14.Lobato J., Silva D.A.O., Mineo T.W.P., Amaral J.D., Segundo G.R., Costa-Cruz J.M.. et al. Detection of immunoglobulin G antibodies to Neospora caninum in humans: high seropositivity rates in patients who are infected by human immunodeficiency virus or have neurological disorders. Clin. Vaccine Immunol. 2006;13:84–89. doi: 10.1128/CVI.13.1.84-89.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Long M.T., Baszler T.V.. Neutralization of maternal IL-4 modulates congenital protozoal transmission: comparison of innate versus acquired immune responses. J. Immunol. 2000;164:4768–4774. doi: 10.4049/jimmunol.164.9.4768. [DOI] [PubMed] [Google Scholar]
- 16.McAllister M.M., Dubey J.P., Lindsay D.S., Jolley W.R., Wills R.A., McGuire A.M.. Dogs are definitive hosts of Neospora caninum. Int. J. Parasitol. 1998;28:1473–1478. [PubMed] [Google Scholar]
- 17.Mineo T.W.P., Silva D.A.O., Costa G.H.N., Von Ancken A.C., Kasper L.H., Souza M.A.. et al. Detection of IgG antibodies to Neospora caninum and Toxoplasma gondii in dogs examined in a veterinary hospital from Brazil. Vet. Parasitol. 2001;98:239–245. doi: 10.1016/s0304-4017(01)00441-1. [DOI] [PubMed] [Google Scholar]
- 18.Minns L.A., Menard L.C., Foureau D.M., Darche S., Ronet C., Mielcarz D.W.. et al. TLR9 is required for the gut-associated lymphoid tissue response following oral infection of Toxoplasma gondii. J. Immunol. 2006;176:7589–7597. doi: 10.4049/jimmunol.176.12.7589. [DOI] [PubMed] [Google Scholar]
- 19.Müller K., Van Zandbergen G., Hansen B., Laufs H., Jahnke N., Solbach W., Laskay T.. Chemokinesnatural killer cells and granulocytes in the early course of Leishmania major infection in mice. Med. Microbiol. Immunol. 2001;190:73–76. doi: 10.1007/s004300100084. [DOI] [PubMed] [Google Scholar]
- 20.Mun H.S., Aosai F., Norose K., Chen M., Piao L.X., Takeuchi O.. et al. TLR2 as an essential molecule for protective immunity against Toxoplasma gondii infection. Int. Immunol. 2003;15:1081–1087. doi: 10.1093/intimm/dxg108. [DOI] [PubMed] [Google Scholar]
- 21.Nam H.W., Kang S.W., Choi W.Y.. Antibody reaction of human anti-Toxoplasma gondii positive and negative sera with Neospora caninum antigens. Korean J. Parasitol. 1998;36:269–275. doi: 10.3347/kjp.1998.36.4.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ramamoorthy S., Sanakkayala N., Vemulapalli R., Duncan R.B., Lindsay D.S., Schurig G.S.. et al. Prevention of lethal experimental infection of C57BL/6 mice by vaccination with Brucella abortus strain RB51 expressing Neospora caninum antigens. Int. J. Parasitol. 2007;37:1521–1529. doi: 10.1016/j.ijpara.2007.04.020. [DOI] [PubMed] [Google Scholar]
- 23.Scanga C.A., Aliberti J., Jankovic D., Tilloy F., Bennouna S., Denkers E.Y.. et al. Cutting edge: MyD88 is required for resistance to Toxoplasma gondii infection and regulates parasite-induced IL-12 production by dendritic cells. J. Immunol. 2002;168:5997–6000. doi: 10.4049/jimmunol.168.12.5997. [DOI] [PubMed] [Google Scholar]
- 24.Srinivasan S., Muller J., Suana A., Hemphill A.. Vaccination with microneme protein NcMIC4 increases mortality in mice inoculated with Neospora caninum. J. Parasitol. 2007;93:1046–1055. doi: 10.1645/GE-1181R1.1. [DOI] [PubMed] [Google Scholar]
- 25.Sukhumavasi W., Egan C.E., Warren A.L., Taylor G.A., Fox B.A., Bzik D.J., Denkers E.Y.. TLR adaptor MyD88 is essential for pathogen control during oral toxoplasma gondii infection but not adaptive immunity induced by a vaccine strain of the parasite. J. Immunol. 2008;181:3464–3473. doi: 10.4049/jimmunol.181.5.3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takeda K., Akira S.. Toll-like receptors in innate immunity. Int. Immunol. 2005;17:1–14. doi: 10.1093/intimm/dxh186. [DOI] [PubMed] [Google Scholar]
- 27.Tranas J., Heinzen R.A., Weiss L.M., McAllister M.M.. Serological evidence of human infection with the protozoan Neospora caninum. Clin. Diagn. Lab. Immunol. 1999;6:765–767. doi: 10.1128/cdli.6.5.765-767.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Trees A.J., Davison H.C., Innes E.A., Wastling J.M.. Towards evaluating the economic impact of bovine neosporosis. Int. J. Parasitol. 1999;29:1195–1200. doi: 10.1016/s0020-7519(99)00093-4. [DOI] [PubMed] [Google Scholar]
- 29.Trees A.J., Williams D.J.. Endogenous and exogenous transplacental infection in Neospora caninum and Toxoplasma gondii. Trends Parasitol. 2005;21:558–561. doi: 10.1016/j.pt.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 30.Von Bernuth H., Picard C., Jin Z., Pankla R., Xiao H., Ku C.L.. et al. Pyogenic bacterial infections in humans with MyD88 deficiency. Science. 2008;321:691–696. doi: 10.1126/science.1158298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yarovinsky F., Zhang D., Andersen J.F., Bannenberg G.L., Serhan C.N., Hayden M.S.. et al. TLR11 activation of dendritic cells by a protozoan profilin-like protein. Science. 2005;308:1626–1629. doi: 10.1126/science.1109893. [DOI] [PubMed] [Google Scholar]