Abstract
Chronically SIVagm-infected African green monkeys (AGMs) have a remarkably stable nonpathogenic disease course, with levels of immune activation in chronic SIVagm infection similar to those observed in uninfected monkeys and with stable viral loads for long periods of time. In vivo administration of LPS or an IL-2/diphtheria toxin fusion protein (Ontak) to chronically SIVagm-infected AGMs triggered increases in immune activation and subsequently of viral replication and depletion of intestinal CD4+ T cells. Our study indicates that circulating microbial products can increase viral replication by inducing immune activation and increasing the number of viral target cells, thus demonstrating that immune activation and T cell proliferation are key factors in AIDS pathogenesis.
Comparative studies of pathogenic HIV/SIV infection in humans and rhesus macaques (Rh) 3 and SIV infection in African nonhuman primate natural hosts that generally do not progress to AIDS have resulted in the emergence of a new paradigm of AIDS pathogenesis in which immune activation is a central factor underlying disease progression. According to this model, viral replication during acute infection results in rapid, massive mucosal CD4+ T cell depletion in both progressive and nonprogressive SIV infections (1–3). During pathogenic HIV/SIV infection, immunologic and structural damage to the mucosal barrier results in microbial translocation from the gut lumen into the systemic circulation, which contributes to chronic immune activation and progression to AIDS (4). It was reported that the degree of immune activation in HIV-infected patients is a better predictor of disease progression rate than plasma viral load (VL) (5). Natural hosts (such as African green monkeys (AGMs), mandrills, and sooty mangabeys) generally do not progress to AIDS when infected with SIV and are able to maintain their intestinal barrier integrity (1, 3), perhaps through the suppression of inflammation (6) and lack of enteropathy despite significant mucosal CD4+ T cell depletion (1, 3). Consequently, natural hosts maintain normal levels of T cell activation, proliferation, and apoptosis and show significant recovery of mucosal CD4+ T cells during chronic infection despite high levels of viral replication (1, 3, 7). A direct causal relationship between immune activation, viral replication, and CD4+ T cell depletion has not yet been established in natural hosts, and no successful attempt to experimentally induce immune activation was reported thus far. We addressed the question of whether immune activation can be experimentally induced in AGMs and whether it results in increased VL and CD4+ T cell depletion.
Materials and Methods
Animals, infection, treatments, and samples
Ten Caribbean AGMs (Chlorocebus sabaeus) were included. They received plasma equivalent to 300 50% tissue culture-infective doses (TCID50) of the African green monkey SIV (SIVagm) strain SIVagm.sab92018 (7). Animals were housed and handled at the Tulane National Primate Research Center (Covington, LA) in accordance with guidelines for the care and use of laboratory animals from the U.S. Public Health Service, the American Association for Accreditation of Laboratory Animal Care, and the Animal Welfare Act. The Tulane University Institutional Animal Care and Use Committee approved all protocols and procedures.
Acute infection follow-up was done on blood samples collected as described (3, 8). During chronic infection the AGMs were split into two groups; monkeys in the first group (n = 6) received i.v. 20 U/kg LPS (Escherichia coli lot G; U.S. Pharmacopeia, Rockville, MD). After treatment, blood samples were collected every 2 h for 6 h and then daily for the first 4 days. Additional samples were collected at days 7 and 14 after LPS treatment. To evaluate whether the observed effect was not due to repeated sampling and anesthesia episodes, four of the six AGMs received a control inoculation consisting of saline in the same volume as the LPS administration 1 mo after the last blood draw. The same sampling schedule as for the LPS study was used during saline administration.
Monkeys in the second group (n = 4) were treated with Ontak (Ligand Pharmaceutical). Three Ontak treatments consisting of daily administration (15 mg/kg) for 5 consecutive days every 2 mo were administered to each monkey in this study. Blood samples were collected before Ontak administration and then at days 4, 8, 12, 15, and 18 after treatment initiation.
Tissue sampling
Blood samples were collected as reported (3, 7) and plasma and mononuclear cells were isolated as described (3, 7) for VL quantification, cytokine determination, and flow cytometry.
Viral quantification
Plasma VLs were quantified as described (6, 9). Assay sensitivity was 100 copies/ml.
Abs and flow cytometry
Whole blood was stained for flow cytometry as described (3, 7). mAbs used were as follows: CD3- FITC or CD3-PerCP; CD20-PE; CD8-PerCP or CD8-PE; CD4-allophycocyanin or CD4-PerCP; HLA-DR-PerCP; CD95-FITC or CD95-allophycocyanin; CD28-allophycocyanin or CD28-PE; and Ki-67-FITC (BD Biosciences). All Abs were validated and titrated using AGM and Rh PBMC. Data were acquired with a FACSCalibur flow cytometer (BD Immunocytometry Systems) and analyzed with CellQuest software (BD Biosciences). CD4+ and CD8+ T cell percentages were obtained by first gating on lymphocytes and then on CD3+ T cells. Memory, activation, and proliferation markers were determined by gating on lymphocytes, then on CD3+ T cells, and finally on CD4+CD3+ or CD8+CD3+ T cells.
Cytokine determination
Cytokine testing in plasma was done using a sandwich immunoassay-based protein array system, the human cytokine 25-Plex (BioSource International), as instructed by the manufacturer and read by the Bio-Plex array reader (Bio-Rad Laboratories), which uses fluorescent bead-based technology from Luminex.
Soluble CD14 (sCD14) measurement
Plasma sCD14 levels were measured using a commercially available ELISA (R&D Systems). Plasma was diluted 1/300 and assay was performed in duplicate according to the manufacturer’s protocol.
Statistics
Paired t test was used to analyze data when we only had one series of measurements (LPS experiments and RT-PCR data). Linear mixed effects models were used to analyze the Ontak data with three series of measurements using the data of the first 10 days after administration. Monkeys were considered random effects; time and Ontak treatment were used as additive fixed covariates. Calculations were done in Excel (Microsoft) and the software package R.
Results and Discussion
We previously reported that, in chronically SIVagm-infected AGMs, normal levels of immune activation are associated with normal levels of T cell apoptosis and proliferation, as well as a lack of enteropathy and plasma LPS levels that are similar to those in uninfected monkeys (3). To provide proof-of-concept data showing that release of bacterial components into the systemic circulation results in increased immune activation and viral replication, we initially administered a single dose of LPS to six chronically SIVagm-infected AGMs. This resulted in a transient increase in the frequency of activated CD4+ T cells (Fig. 1a) (p = 0.02) and was accompanied by a transient but very rapid increase in VL (Fig. 1b). One of the animals had VL comparable to that observed at the peak of viral replication during acute infection (Fig. 1b). Moreover, the LPS was bioactive in vivo as indicated by increases in plasma levels of sCD14 (Fig. 1c). To confirm that these increases in immune activation and viral replication were specifically induced by the LPS administration, we then treated four of the animals with saline alone and observed no effect (Fig. 1, a and b).
FIGURE 1.
In vivo LPS and saline administration in chronically SIVagm-infected AGMs. A single dose of LPS induces a transient but significant increase in the activation of CD4+ T cells (a), a transient increase in the VL (b), and an increase in plasma sCD14 (c). In FV75, VL reaches the levels of virus replication from the primary infection. Saline administration in the same AGMs did not modify the steady state of the chronic SIVagm infection (a and b).
A second mechanism that may explain the lack of immune activation in natural SIV infections involves regulatory T cells (Tregs) (6). Previously we determined that the ratio between the total number of Tregs and conventional CD4+ T cells is higher in uninfected AGMs compared with uninfected Rh in both the inductive and effector lymphoid sites of the intestine (10). Furthermore, the level of FoxP3 in CD4+ T cells sorted from intestine was higher in AGMs compared with Rh (10). Additionally, we reported differences in the dynamics of Tregs in SIVagm-infected AGMs compared with Rh infected with SIV of macaques (SIVmac), such that FoxP3 expression was increased at day 1 after SIVagm infection in AGMs but at a later time point in SIV-mac-infected Rh (6). The same applies to cytokines associated with Tregs in that TGFβ and IL-10 are increased very early in SIVagm infection but only late in SIVmac infection (6). Thus, the mobilization of Tregs may be of insufficient rapidity and quantity to be effective in controlling immune activation in SIVmac-infected Rh.
To examine Treg involvement in the suppression of immune activation in AGMs, we conducted an in vivo Treg-depletion study in SIVagm-infected AGMs. We used Ontak (denileukin diftitox), a U.S. Federal Drug Administration-approved drug that depletes CD25+ cells in humans. Ontak is a fusion protein that combines a rIL-2 and a cytocidal diphtheria toxin moiety. Three Ontak treatments (of five daily injections) were administered at 2-mo intervals to chronically SIV-infected AGMs. Ontak did not change the total numbers of CD4+ CD25+ T cells (p = 0.79) (Fig. 2a), but it reduced the percentage of these cells (p = 0.0001) (supplementary figure 1 and supplemental table I).4 The absolute numbers (p = 0.28) and percentages (p = 0.48) of Tregs defined by FoxP3 expression were also not decreased; however, a transient increase in FoxP3 expression was observed by quantitative PCR (p = 0.006 at day 4) during On-tak administration (supplemental figure 2). Ontak also failed to induce a decrease in Treg function, as sorted Tregs from treated AGMs were as capable of inhibiting the cell proliferation of conventional CD4+ CD25− T cells as Tregs purified from nontreated AGMs (supplemental figure 3).
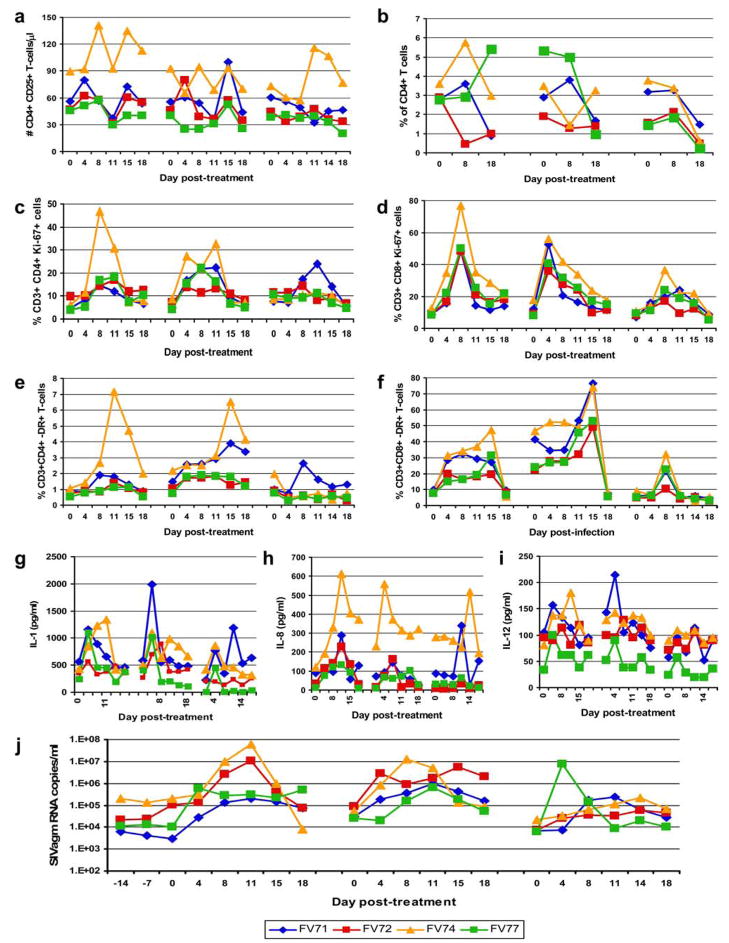
FIGURE 2.
Impact of Ontak administration in chronically SIVagm-infected AGMs. Although Ontak did not induce significant depletion of CD25+CD4+ T cells (Tregs) (a), at the end of the treatment mucosal CD4+ T cells were significantly depleted (b). Significant increases in the cell proliferation of CD4+ (c) and CD8+ T cells (d), as well as immune activation of CD4+ (e) and CD8+ T cells (f), were observed after Ontak administration. This increase in immune activation was confirmed by the dynamics of proinflammatory cytokines IL-1 (g), IL-8 (h), and IL-12 (i) and resulted in a significant increase in VLs (j).
However, T cell functional measurements revealed increased proliferation of conventional CD4+ T cells in the Ontak-treated AGMs, which was confirmed by significant increases in the frequency of T cells expressing the proliferation marker Ki-67 (p = 0.003 for CD4+ and p = 0.0002 for CD8+; Fig. 2, c and d). Over the 5 days of Ontak administration we also found significant increases of both the percentages (p < 0.00005) and the absolute numbers (p = 0.02) of peripheral CD4+ T cells (supplemental figure 4), as well of effector memory CD4+ T cells (p = 0.006), which are preferentially depleted in SIV/HIV infections (supplemental figure 5). It is therefore possible that this increase in overall T cell proliferation masked any partial depletion of both CD4+ CD25+ T cells and Tregs. Importantly, Ontak administration induced increases in T cell immune activation as measured by HLA-DR expression on both CD4+ (p = 0.07) and CD8+ T cells (p = 0.002) (Fig. 2, e and f), as well as by the dynamics of the proinflammatory cytokines IL-1 (p = 0.004), IL-8 (p = 0.02), and IL-12 (p = 0.01) (Fig. 2, g–i). As can be seen in the figures, these had different dynamics; IL-1 and IL-12 showed an early increase (<5 days), whereas DR expression and IL-8 had a slower increase (over the first 8 –11 days). The levels of CCR5 by RT-PCR were also increased (p = 0.005 at day 4), similar to primary infection, confirming an increase of immune activation after the Ontak treatment (supplemental figure 6). Ontak administration resulted in significant up-regulation of genes associated with immune activation such as CD80, HLA-DR, IL-3, IL-4, Toll-like receptors 4 and 6, IL-12R, and IL-17R as well as down-regulation of TGF-β1, CD3z, CD3d, CD3g, and T-box21 genes (data not shown). Finally, this marked increase in immune activation resulted in a significant increase in viral replication in all treated chronically SIVagm-infected AGMs (p = 0.0001; Fig. 2j) These increases in viral replication were up to the levels of the peak VLs. Moreover, in two animals increased viral replication was maintained between the first two treatments. Consequently, at the end of the Ontak treatment CD4+ T cells were significantly depleted in the intestine (p = 0.0001; Fig. 2b).
Taken together, our data provide proof-of-concept that experimental induction of immune activation in natural hosts of SIV infection drives increases in viral replication and mucosal CD4+ T cell depletion. In the first set of the experiments we observed that a single low-dose LPS administration results in increased immune activation and a consequent increase in viral replication, thus adding weight to the concept that microbial translocation contributes to systemic immune activation and disease progression. In the second set of experiments, a more persistent and significant increase in immune activation, and consequently a more robust and sustained increase in VL and significant depletion of mucosal CD4+ T cells, was obtained after Ontak administration in chronically SIVagm-infected AGMs. Although Ontak did not induce significant Treg depletion, the drug has potential to induce immune activation as it contains an IL-2 moiety combined with a TLR ligand (the diphtheria toxin) that interacts with the innate immune system. The increases in immune activation in both experiments through TLR ligand stimulation demonstrate that interaction between innate and adaptive immune systems is functional in the natural hosts and can induce significant increase of viral replication and CD4+ T cell depletion in this animal model that otherwise is characterized by very stable chronic lentiviral infection. Our results are in agreement with a recent study showing augmentation of viral replication at mucosal sites during early SIVmac infection of Rh due to increased immune activation driven by CTLA-4 blockade (11) and with data reporting that the levels of VL in sooty mangabeys depends on the availability of activated CD4+ T cells (12).
Therefore, our results demonstrate that immune activation and proliferation of the target cells for the virus are key factors in AIDS pathogenesis. These data suggest that therapeutic strategies to reduce immune activation should be explored, in addition to the classic antiretroviral therapies, in preventing progression to AIDS in chronically HIV-infected individuals.
Acknowledgments
We thank Donald Sodora and Preston Marx for helpful discussion, the Division of Veterinary Medicine of Tulane National Primate Research Center for animal care, and Robin Rodriguez for help in preparing the figures.
Footnotes
This work was supported by National Institutes of Health/National Institute of Allergy and Infectious Diseases/National Center for Research Resources Grants R01 AI064066 and R21AI069935 (to I.P.), R01 AI065325 (to C.A.), and RR-00168 (to Tulane National Primate Research Center).
Abbreviations used in this paper: Rh, rhesus macaque; AGM, African green monkey; sCD14, soluble CD14; SIVagm, African green monkey SIV; SIVmac, macaque SIV; Treg, T regulatory cell; VL, viral load.
The online version of this article contains supplemental material.
Disclosures
The authors have no financial conflict of interest.
References
- 1.Gordon S, Klatt NR, Milush JM, Engram J, Dunham RM, Paiardini M, Strobert EA, Apetrei C, Pandrea I, Staprans S, et al. Severe depletion of mucosal CD4+ T cells in AIDS-free SIV-infected sooty mangabeys. J Immunol. 2007;179:3026–3034. doi: 10.4049/jimmunol.179.5.3026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grossman Z, Meier-Schellersheim M, Paul WE, Picker LJ. Pathogenesis of HIV infection: what the virus spares is as important as what it destroys. Nat Med. 2006;12:289–295. doi: 10.1038/nm1380. [DOI] [PubMed] [Google Scholar]
- 3.Pandrea I, Gautam R, Ribeiro R, Brenchley JM, Butler IF, Pattison M, Rasmussen T, Marx PA, Silvestri G, Lackner AA, et al. Acute loss of intestinal CD4+ T cells is not predictive of SIV virulence. J Immunol. 2007;179:3035–3046. doi: 10.4049/jimmunol.179.5.3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brenchley JM, Price DA, Schacker TW, Asher TE, Silvestri G, Rao S, Kazzaz Z, Lambotte O, Altmann D, Blazar BR, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006;12:1365–1371. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- 5.Giorgi JV, Hultin LE, McKeating JA, Johnson TD, Owens B, Jacobson LP, Shih R, Lewis J, Wiley DJ, Phair JP, et al. Shorter survival in advanced human immunodeficiency virus type 1 infection is more closely associated with T lymphocyte activation than with plasma virus burden or virus chemokine coreceptor usage. J Infect Dis. 1999;179:859–870. doi: 10.1086/314660. [DOI] [PubMed] [Google Scholar]
- 6.Kornfeld C, Ploquin MJ, Pandrea I, Faye A, Onanga R, Apetrei C, Poaty-Mavoungou V, Rouquet P, Estaquier J, Mortara L, et al. Antiinflammatory profiles during primary SIV infection in African green monkeys are associated with protection against AIDS. J Clin Invest. 2005;115:1082–1091. doi: 10.1172/JCI23006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pandrea I, Apetrei C, Dufour J, Dillon N, Barbercheck J, Metzger M, Jacquelin B, Bohm R, Marx PA, Barre-Sinoussi F, et al. Simian immunodeficiency virus (SIV) SIVagm.sab infection of Caribbean African green monkeys: new model of the study of SIV pathogenesis in natural hosts. J Virol. 2006;80:4858 – 4867. doi: 10.1128/JVI.80.10.4858-4867.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pandrea I, Ribeiro RM, Gautam R, Gaufin T, Pattison M, Barnes M, Monjure C, Stoulig C, Silvestri G, Miller M, et al. Simian immunodeficiency virus SIVagm dynamics in African green monkeys. J Virol. 2008;82:3713–3724. doi: 10.1128/JVI.02402-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pandrea I, Kornfeld C, Ploquin MJI, Apetrei C, Faye A, Rouquet P, Roques P, Simon F, Barré-Sinoussi F, Müller-Trutwin MC, Diop OM. Impact of viral factors on very early in vivo replication profiles in SIVagm-infected African green monkeys. J Virol. 2005;79:6249–6259. doi: 10.1128/JVI.79.10.6249-6259.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coleman CA, Muller-Trutwin MC, Apetrei C, Pandrea I. T regulatory cells: aid or hindrance in the clearance of disease? J Cell Mol Med. 2007;11:1291–1325. doi: 10.1111/j.1582-4934.2007.00087.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cecchinato V, Tryniszewska E, Ma ZM, Vaccari M, Boasso A, Tsai WP, Petrovas C, Fuchs D, Heraud JM, Venzon D, et al. Immune activation driven by CTLA-4 blockade augments viral replication at mucosal sites in simian immunodeficiency virus infection. J Immunol. 2008;180:5439–5447. doi: 10.4049/jimmunol.180.8.5439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klatt NR, Villinger F, Bostik P, Gordon SN, Pereira L, Engram JC, Mayne A, Dunham RM, Lawson B, Ratcliffe SJ, et al. Availability of activated CD4+ T cells dictates the level of viremia in naturally SIV-infected sooty mangabeys. J Clin Invest. 2008;118:2039–2049. doi: 10.1172/JCI33814. [DOI] [PMC free article] [PubMed] [Google Scholar]