Narrow band imaging (NBI), a technology initially produced by Olympus Medical Systems of Tokyo, Japan, is a relatively new and well-recognized advancement in endoscopic imaging. Other manufacturers have variations of NBI that are similar but not as widely used. The present article will use NBI as a reference point; however, it is recognized that other manufacturers have similar systems with different names that will not be addressed further in the present discussion. The main goal of NBI technology is the ability to predict pathology in real time, based on the mucosal and vascular enhancement offered by endoscopes that have this capability. The purpose of the present paper is to discuss the clinical situations in which NBI technology is used for the detection of neoplastic lesions of the colon as well as the current evidence for or against these practices.

Dr Mitchell Lee
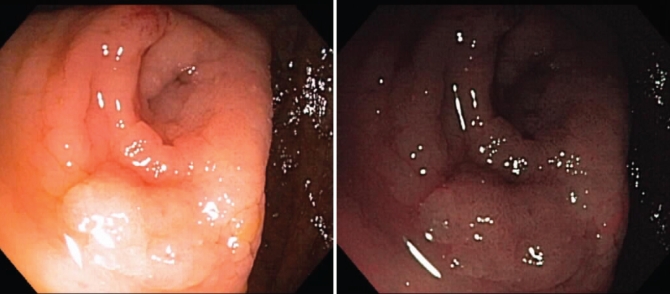
Although conventional white-light (CWL) imaging uses the entire spectrum of visible light (400 nm to 700 nm), NBI technology is based on the use of optic filters to isolate two specific bands of light: 415 nm (blue) and 540 nm (green) (1–3). By using the different absorptive and reflective properties of these wavelengthts of light on mucosa, an image that enhances the visualization of superficial vascular structures (blue: superficial capillary; green: subepithelial vessels) (1–3) is created. The NBI mode on an endoscope, which can be activated or deactivated by an endoscopist with a control button on the endoscope, typically darkens the appearance of the vessels. Examples of NBI images taken at the St Paul’s Hospital, Vancouver, British Columbia, are shown in Figure 1.
Figure 1).
Representative photographs of narrow band imaging (NBI) with the endoscope NBI mode deactivated (left panel) and NBI activated (right panel)
NBI is often referred to as ‘digital chromoendoscopy’ (4), because it was developed as an alternative method of enhancing the mucosa and vasculature similar to that seen in chromoendoscopy, a technique in which the mucosa is sprayed with a dye (ie, indigo carmine) during the endoscopy procedure. In chromoendoscopy, the absorptive property of the dye, rather than the properties of the light shining onto the surface of the mucosa, is used to enhance the image. The images produced by chromoendoscopy are very similar to the images produced by NBI, with minor differences (5). Although chromoendoscopy is frequently used in Japan, it has not received the same popularity in North America because it is believed by many to be more time consuming to apply the dye, and may require specialized training to perform properly.
In the upper gastrointestinal tract, NBI has been used for various disorders such as gastroesophageal reflux disease, Barrett’s esophagitis and gastric neoplasia (3,6–9). In the lower gastrointestinal tract, NBI has been used to detect and assess colon polyps (particularly those that are flat), and for surveillance colonoscopy in patients with ulcerative colitis (UC) and hereditary nonpolyposis colon cancer (HNPCC) (10–13). NBI has also been used in a variety of applications outside of gastroenterology such as laryngoscopy (14) and cystoscopy (15).
NBI for detecting colon polyps
Colonoscopy is regarded as the gold standard diagnostic test for the surveillance of neoplastic lesions of the colon. In addition to the ability to obtain tissue for diagnostic biopsies of suspicious lesions, colonoscopy also has a therapeutic role in the removal of polyps. Screening colonoscopies have a significant adenoma miss rate of approximately 20% for small adenomas (16–19). Despite the miss rate, studies (20,21) have consistently shown that approximately 80% of colon cancers are prevented as a result of this screening measure. The current procedure with conventional colonoscopy in North America involves removing all polyps seen during the procedure and submitting them for histopathological diagnosis. NBI is used in the setting of colonoscopy to help the endoscopist visualize and characterize more polyps to determine in vivo tissue type and manage accordingly. With NBI technology, it is hypothesized that a trained endoscopist could sample fewer areas by selectively taking more selected biopsies, resulting in less risk to the patient, saving time during the procedure and decreasing overall cost to the health care system. To help the endoscopist predict the pathology of colon polyps in vivo from NBI, the ‘pit pattern’ classification, initially described by Kudo et al (22–24) for chromoendoscopy, has been widely adopted for use in NBI due to the similarities of the images produced with the two modalities, but has yet to be formally validated for use in use in NBI.
An important issue to consider with the use of NBI endoscopes is the darker visual field compared with conventional endoscopes; as a result, this requires the endoscope to be closer to the mucosal surface. In addition, some centres use NBI with magnification to investigate smaller details with regard to a point of interest.
NBI in UC surveillance
In addition to the use of NBI for the detection and characterization of colon polyps, it has also been used for surveillance of patients with UC or Crohn’s colitis. The current protocol for surveillance colonoscopy recommends having four-quadrant random biopsies performed every 10 cm from the cecum to the rectum (25) coupled with sampling of other areas of suspicion. This results in many biopsy tissue samples being taken for pathological examination, increased time and cost for the procedure and low risks for the patient. It has been proposed that targeted rather than random biopsies could be taken with NBI for colitis surveillance. The first case report describing the use of NBI for distinguishing dysplastic mucosa in patients with UC was conducted by East et al (12). Since this initial report, a recent prospective, randomized, crossover study by Dekker et al (10) compared NBI with random biopsies in the surveillance of 42 patients with UC. The study found that the sensitivity of NBI in detecting neoplasia in patients was similar to that seen with conventional colonoscopy; however, twice as many suspicious lesions were detected with NBI, and as a result, more biopsies were taken in the NBI group versus the conventional endoscopy group (148 versus 85, respectively). There were no statistically significant differences in the adenoma detection rates or procedure times between the two procedures. Presently, the use of NBI for UC or other inflammatory bowel diseases remains an active area of research that is likely to expand our understanding of this difficult area.
NBI in HNPCC surveillance
NBI has also been used in the surveillance of patients with HNPCC. Because patients with HNPCC have an approximately 80% to 85% lifetime chance of developing colon cancer, they are closely surveyed by optical colonoscopy every one to two years from the age of 25 years (26–29). In a recent study (11) of 62 HNPCC patients who underwent back-to-back surveillance colonoscopy with conventional colonoscopy and NBI, the total number of adenomas detected increased from 25 to 46 in the NBI group (P <0.001), and the proportion of flat adenomas detected was significantly higher in the NBI pass than with the CWL imaging pass (45% versus 12%; P=0.03). It has been suggested that the increased detection rate of flat adenomas may represent a population of polyps that are difficult to detect with conventional colonoscopy. Although there are few studies that have investigated the issue of HNPCC surveillance with NBI, the results appear promising. Further studies are required to determine the effectiveness of using NBI for HNPCC surveillance are required, particularly as it relates to long-term outcome measures such as the development of colorectal cancer.
Is NBI more effective than standard colonoscopy? A review of the literature
Because NBI is a relatively new diagnostic modality in endoscopy, few studies have been performed to evaluate NBI versus standard colonoscopy and chromoendoscopy. However, the available results appear promising for NBI. The first study that examined the use of NBI for detecting colorectal lesions was from Japan (30). Thirty-four patients were examined with NBI, chromoendoscopy and/or conventional endoscopy and the results of the three modalities were compared. They found no statistically significant difference between NBI and chromoendoscopy in differentiating neoplastic versus non-neoplastic lesions (sensitivity 100%, specificity 75%), both of which were better than conventional colonoscopy (sensitivity 83%, specificity 44%; P<0.05 for specificity).
Since the initial study from Machida et al (30) in 2004, there have been two other prospective studies that examined NBI versus conventional colonoscopy and chromoendoscopy. The results of these studies are summarized in Table 1. All three studies showed that the diagnostic accuracy of NBI was better than conventional colonoscopy, and not statistical different from chromoendoscopy.
TABLE 1.
Summary of results from recent studies
| Reference | Conventional colonoscopy accuracy (%) | Conventional colonoscopy SE, SP (%) | Chromoendoscopy accuracy (%) | Chromoendoscopy SE, SP (%) | NBI accuracy | NBI SE, SP (%) |
|---|---|---|---|---|---|---|
| Machida et al (30) | 79.1 | 85.3, 44.4 | 93.4 | 100, 75 | 93.4 | 100, 75 |
| Su et al (31) | 81.8 | 82.9, 80 | 92.7 | 95.7, 87.5 | 92.7 | 95.7, 87.5 |
| Chiu et al (32)* | 67.2 to 68.3 | 62.1 to 65.2, 74.4 to 85.4 | 78.9 to 85 | 78.7 to 85.1, 79.5 to 84.6 | 80.6 to 82.4 | 82.3 to 86.5, 59 to 82.7 |
Values for low-magnification chromoendoscopy and narrow band imaging (NBI) are listed; SE Sensitivity; SP Specificity
Further studies have shown an improvement in the adenoma detection rate with NBI compared with conventional colonoscopy (33–35). However, there have been studies in which the benefits of either technique were not statistically significant or there was no difference in detection rates between the two groups (36–38). One of these studies was a randomized controlled study by Rex et al (38) that compared high-definition wide-angle colonoscopy with NBI for detecting adenomas. In this study, all examinations were performed by the same endoscopist with a documented high detection rate of adenomas. It was discovered that there was no difference in adenoma detection rates between the CWL and NBI groups. The authors suggested that NBI may improve detection rates of adenomas by examiners who experience low adenoma detection rates using CWL endoscopy.
In regard to the question as to whether NBI causes a prolongation of endoscope withdrawal time, the results in the literature have been mixed. Studies have shown that there is no significant difference between withdrawal times in NBI and conventional colonoscopy (33,37). In contrast, there have been studies that have shown a statistically significant difference in withdrawal times (36,38), which is often attributed to the darker field of view with NBI that requires closer examination of the mucosa in locations where the lumen is larger (ie, rectum and ascending colon). In one of the studies with a statistically significant difference in withdrawal time (38), the withdrawal time for the CWL group was 7.3 min, whereas the withdrawal time in the NBI group was 7.7 min (P=0.003). The second study with a statistically significant difference (36) showed that the withdrawal time in the CWL group was 7.9 min, versus 8.5 min in the NBI group. Considering the more recent emphasis on ‘slowing’ the withdrawal speed to enhance sensitivity of the test, these minor differences in duration likely should not dissuade us from considering NBI more often.
Finally, because NBI is a new technology, the issue of training endoscopists to use NBI effectively and its effect on results has been brought forth in the literature. A study by Rogart et al (35) incorporated training and feedback into their trial of conventional colonoscopy versus NBI. The initial diagnostic accuracies were 80% with NBI and 77% with CWL (P=0.35); however, after appropriate training and feedback with pre- and post-tests, the second half of the study showed that NBI accuracy significantly improved to 87% while CWL had an accuracy of 79% (P<0.05). Furthermore, it has been suggested in a study by Adler et al (37) that NBI may help endoscopists improve diagnostic accuracy with CWL endoscopy. The study investigated the two techniques back-to-back in consecutive subgroups of 100 patients, and found that the adenoma detection rates in the NBI group remained stable (approximately 25%), whereas the adenoma detection rates in the conventional colonoscopy group increased with each subgroup (8%, 15%, 17%, and 26.5%, respectively). Although this explanation is speculative, it would be interesting to follow-up these results with further studies to see if they hold true.
Limitations
There are many other issues that may determine whether NBI is used on a daily clinical basis. As mentioned previously, the addition of yet another instrument to view the gastrointestinal tract may add more time to an already ‘time constrained’ unit. Although studies are conflicting regarding how much extra time it takes, there is little doubt that at least some extra time (compared with CWL) is required to use NBI. Additionally, the instrumentation is not available on all endoscopes through all manufacturers. Because most endoscopy units gradually replace old endoscopes with new, more capable endoscopes (such as NBI), many units (such as ours at the St Paul’s Hospital) presently have a mixture of NBI-capable and NBI-incapable endoscopes. Therefore, if an endoscopist wants to use an NBI-capable endoscope, a request is required before the day of the procedure to ensure the correct endoscope is available for a specific procedure.
Presently, we use the NBI endoscope on a regular basis; however, it is used for selected cases in which specific lesions are being sought. For the lower gastrointestinal tract, it tends to be for difficult polyp syndromes (ie, HNPCC) and dysplasia assessment (ie, chronic colitis). It is therefore not used in the majority of cases.
CONCLUSION
NBI is a new and promising advance in the field of endoscopy that uses two specific wavelengths of light to enhance visualization of vascular and mucosal patterns seen in lesions of the gastrointestinal tract. We have discussed the applications of this technology with emphasis on its use in the detection of colonic lesions.
As more endoscopists become acquainted with the new technology and gain more experience in using it, NBI will aid in the detection of colon polyps. However, before guidelines can be established for the use of NBI in everyday practice, further studies are necessary to develop a standardized system for classifying the different patterns seen on NBI. In addition, further studies investigating the cost-effectiveness of this new technology as well as further studies to determine the diagnostic accuracy are required before NBI can replace conventional colonoscopy in colon cancer screening.
REFERENCES
- 1.ASGE Technology Committee. Song LM, Adler DG, et al. Narrow band imaging and multiband imaging. Gastrointest Endosc. 2008;67:581–9. doi: 10.1016/j.gie.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 2.Gono K, Obi T, Yamaguchi M, et al. Appearance of enhanced tissue features in narrow-band endoscopic imaging. J Biomed Opt. 2004;9:568–77. doi: 10.1117/1.1695563. [DOI] [PubMed] [Google Scholar]
- 3.Yoshida T, Inoue H, Usui S, et al. Narrow-band imaging system with magnifying endoscopy for superficial esophageal lesions. Gastrointest Endosc. 2004;59:288–95. doi: 10.1016/s0016-5107(03)02532-x. [DOI] [PubMed] [Google Scholar]
- 4.Sano Y, Muto M, Tajiri H, et al. Optical/digital chromoendoscopy during colonoscopy using narrow-band imaging system. Dig Endosc. 2005:S43–48. [Google Scholar]
- 5.East JE, Suzuki N, Saunders BP. Comparison of magnified pit pattern interpretation with narrow band imaging versus chromoendoscopy for diminutive colonic polyps: A pilot study. Gastrointest Endosc. 2007;66:310–16. doi: 10.1016/j.gie.2007.02.026. [DOI] [PubMed] [Google Scholar]
- 6.Hamamoto Y, Endo T, Nosho K, et al. Usefulness of narrow-band imaging endoscopy for diagnosis of Barrett’s esophagus. J Gastroenterol. 2004;39:14–20. doi: 10.1007/s00535-003-1239-z. [DOI] [PubMed] [Google Scholar]
- 7.Larghi A, Lecca PG, Costamagna G. High-resolution narrow band imaging endoscopy. Gut. 2008;57:976–86. doi: 10.1136/gut.2007.127845. [DOI] [PubMed] [Google Scholar]
- 8.Sharma P, Wani S, Bansal A, et al. A feasibility trial of narrow band imaging endoscopy in patients with gastroesophageal reflux disease. Gastroenterology. 2007;133:454–64. doi: 10.1053/j.gastro.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 9.Uedo N, Ishihara R, Iishi H, et al. A new method of diagnosing gastric intestinal metaplasia: Narrow-band imaging with magnifying endoscopy. Endoscopy. 2006;38:819–24. doi: 10.1055/s-2006-944632. [DOI] [PubMed] [Google Scholar]
- 10.Dekker E, van der Broek FJ, Reitsma JB, et al. Narrow-band imaging compared with conventional colonoscopy for the detection of dysplasia in patients with longstanding ulcerative colitis. Endoscopy. 2007;39:216–21. doi: 10.1055/s-2007-966214. [DOI] [PubMed] [Google Scholar]
- 11.East JE, Suzuki N, Stavrinidis M, et al. Narrow band imaging for colonoscopic surveillance in hereditary non-polyposis colorectal cancer. Gut. 2008;57:65–70. doi: 10.1136/gut.2007.128926. [DOI] [PubMed] [Google Scholar]
- 12.East JE, Suzuki N, von Herbay A, et al. Narrow band imaging with magnification for dysplasia detection and pit pattern assessment in ulcerative colitis surveillance: A case with multiple dysplasia associated lesions or masses. Gut. 2006;55:1432–35. doi: 10.1136/gut.2005.087171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thorlacius H, Toth E. Role of chromoendoscopy in colon cancer surveillance in inflammatory bowel disease. Inflamm Bowel Dis. 2007;13:911–17. doi: 10.1002/ibd.20118. [DOI] [PubMed] [Google Scholar]
- 14.Watanabe A, Taniguchi M, Tsujie H, et al. The value of narrow band imaging for early detection of laryngeal cancer. Eur Arch Otorhinolaryngol. 2008 doi: 10.1007/s00405-008-0835-1. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 15.Herr HW, Donat SM. A comparison of white-light cystoscopy and narrow-band imaging cystoscopy to detect bladder tumour recurrences. BJU Int. 2008;102:1111–4. doi: 10.1111/j.1464-410X.2008.07846.x. [DOI] [PubMed] [Google Scholar]
- 16.Bensen S, Mott LA, Dain B, et al. The colonoscopic miss rate and true one-year recurrence of colorectal neoplastic polyps. Polyp Prevention Study Group. Am J Gastroenterol. 1999;94:194–9. doi: 10.1111/j.1572-0241.1999.00796.x. [DOI] [PubMed] [Google Scholar]
- 17.Hixson LJ, Fennerty MB, Sampliner RE, et al. Prospective blinded trial of the colonoscopic miss rate of large colorectal polyps. Gastrointest Endosc. 1991;37:125–7. doi: 10.1016/s0016-5107(91)70668-8. [DOI] [PubMed] [Google Scholar]
- 18.Rex DK, Cutler CS, Lemmel GT, et al. Colonoscopic miss rates of adenomas determined by back-to-back colonoscopies. Gastroenterology. 1997;112:24–8. doi: 10.1016/s0016-5085(97)70214-2. [DOI] [PubMed] [Google Scholar]
- 19.van Rijn JC, Reitsma JB, Stoker J, et al. Polyp miss rate determined by tandem colonoscopy: A systematic review. Am J Gastroenterol. 2006;101:343–50. doi: 10.1111/j.1572-0241.2006.00390.x. [DOI] [PubMed] [Google Scholar]
- 20.Winawer SJ, Zauber AG, Ho MN, et al. Prevention of colorectal cancer by colonoscopic polypectomy. The National Polyp Study Workgroup. N Engl J Med. 1993;329:1977–81. doi: 10.1056/NEJM199312303292701. [DOI] [PubMed] [Google Scholar]
- 21.Rex DK. Maximizing detection of adenomas and cancers during colonoscopy. Am J Gastroenterol. 2006;101:2866–77. doi: 10.1111/j.1572-0241.2006.00905.x. [DOI] [PubMed] [Google Scholar]
- 22.Kudo S, Hirota S, Nakajima T, et al. Colorectal tumours and pit pattern. J Clin Pathol. 1994;47:880–5. doi: 10.1136/jcp.47.10.880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kudo S, Tamura S, Nakajima T, et al. Diagnosis of colorectal tumorous lesions by magnifying endoscopy. Gastrointest Endosc. 1996;44:8–14. doi: 10.1016/s0016-5107(96)70222-5. [DOI] [PubMed] [Google Scholar]
- 24.Kudo S, Rubio CA, Teixeira CR, et al. Pit pattern in colorectal neoplasia: Endoscopic magnifying view. Endoscopy. 2001;33:367–73. doi: 10.1055/s-2004-826104. [DOI] [PubMed] [Google Scholar]
- 25.The role of colonoscopy in the management of patients with inflammatory bowel disease. American Society for Gastrointestinal Endoscopy. Gastrointest Endosc. 1998;48:689–90. doi: 10.1016/s0016-5107(98)70062-8. [DOI] [PubMed] [Google Scholar]
- 26.Lynch HT, de la Chapelle A. Hereditary colorectal cancer. N Engl J Med. 2003;348:919–32. doi: 10.1056/NEJMra012242. [DOI] [PubMed] [Google Scholar]
- 27.Jarvinen HJ, Aarnio M, Mustonen H, et al. Controlled 15-year trial on screening for colorectal cancer in families with hereditary nonpolyposis colorectal cancer. Gastroenterology. 2000;118:829–34. doi: 10.1016/s0016-5085(00)70168-5. [DOI] [PubMed] [Google Scholar]
- 28.Dunlop MG. Guidance on gastrointestinal surveillance for hereditary nonpolyposis colorectal cancer, familial adenomatous polypolis, juvenile Polyposis, and Peutz-Jeghers syndrome. Gut. 2002;51:V21–7. doi: 10.1136/gut.51.suppl_5.v21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Winawer S, Fletcher R, Rex D, et al. Colorectal cancer screening and surveillance: Clinical guidelines and rationale – update based on new evidence. Gastroenterology. 2003;124:544–60. doi: 10.1053/gast.2003.50044. [DOI] [PubMed] [Google Scholar]
- 30.Machida H, Sano Y, Hamamoto Y. Narrow-Band Imaging in the Diagnosis of Colorectal Mucosal Lesions: A Pilot Study. Endoscopy. 2004;36:1094–8. doi: 10.1055/s-2004-826040. [DOI] [PubMed] [Google Scholar]
- 31.Chiu HM, Chang CY, Chen CC, et al. A prospective comparative study of narrow-band imaging, chromoendoscopy, and conventional colonoscopy in the diagnosis of colorectal neoplasia. Gut. 2007;56:373–9. doi: 10.1136/gut.2006.099614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Su MY, Hsu CM, Ho YP, et al. Comparative study of conventional colonoscopy, chromoendoscopy, and narrow-band imaging systems in differential diagnosis of neoplastic and nonneoplastic colonic polyps. Am J Gastroenterol. 2006;101:2711–6. doi: 10.1111/j.1572-0241.2006.00932.x. [DOI] [PubMed] [Google Scholar]
- 33.Inoue T, Murano M, Murano N, et al. Comparative study of conventional colonoscopy and pan-colonic narrow-band imaging system in the detection of neoplastic colonic polyps: A randomized, controlled trial. J Gastroenterol. 2008;43:45–50. doi: 10.1007/s00535-007-2125-x. [DOI] [PubMed] [Google Scholar]
- 34.Sikka S, Ringold DA, Jonnalagadda S, et al. Comparison of white light and narrow band high definition images in predicting colon polyp histology, using standard colonoscopes without optical magnification. Endoscopy. 2008;40:818–22. doi: 10.1055/s-2008-1077437. [DOI] [PubMed] [Google Scholar]
- 35.Rogart JN, Jain D, Siddiqui UD, et al. Narrow-band imaging without high magnification to differentiate polyps during real-time colonscopy: Improvement with experience. Gastrointest Endosc. 2008;68:1136–45. doi: 10.1016/j.gie.2008.04.035. [DOI] [PubMed] [Google Scholar]
- 36.Achenbeck J, Adler A, Yenerim T, et al. Narrow-band versus white-light HDTV endoscopic imaging for screening colonoscopy: A prospective randomized trial Gastroenterology 2008 [Epub ahead of print] doi: 10.1053/j.gastro.2008.10.022. [DOI] [PubMed] [Google Scholar]
- 37.Adler A, Pohl H, Papanikolaou IS, et al. A prospective randomised study on narrow-band imaging versus conventional colonoscopy for adenoma detection: Does narrow-band imaging induce a learning effect? Gut. 2008;57:59–64. doi: 10.1136/gut.2007.123539. [DOI] [PubMed] [Google Scholar]
- 38.Rex DK, Helbig CC. High yields of small and flat adenomas with high-definition colonoscopes using either white light or narrow band imaging. Gastroenterology. 2007;133:42–7. doi: 10.1053/j.gastro.2007.04.029. [DOI] [PubMed] [Google Scholar]