Abstract
With the looming expansion of the elderly population of the US, a thorough understanding of “normal” aging-related changes on the respiratory system is paramount. The respiratory system undergoes various anatomical, physiological and immunological changes with age. The structural changes include chest wall and thoracic spine deformities which impairs the total respiratory system compliance leading to increase work of breathing. The lung parenchyma loses its supporting structure causing dilation of air spaces: “senile emphysema”. Respiratory muscle strength decreases with age and can impair effective cough, which is important for airway clearance. The lung matures by age 20–25 years, and thereafter aging is associated with progressive decline in lung function. The alveolar dead space increases with age, affecting arterial oxygen without impairing the carbon dioxide elimination. The airways receptors undergo functional changes with age and are less likely to respond to drugs used in younger counterparts to treat the same disorders. Older adults have decreased sensation of dyspnea and diminished ventilatory response to hypoxia and hypercapnia, making them more vulnerable to ventilatory failure during high demand states (ie, heart failure, pneumonia, etc) and possible poor outcomes.
Keywords: older adults, lung function, immunology, respiratory mechanics
Introduction
Since the 1950s the median age of the US population increased by 20 years. Increased life expectancy reflects, in part, a better understanding of the disease states, newer interventions, and the success of public health programs. The number of persons aged 65 and over is projected to increase from 35 million in 2000 to an estimated 71 million in 2030, with the largest increase in individuals age 80 and above (US Census 2005). Differentiating a disease state from “normal” aging is vital when encountering this population in the clinical setting. Respiratory system undergoes various structural, physiological, and immunological changes with age. There is a large variation in different physiologic measures among older adults, making it difficult to construct “normal” limits to differentiate a disease from a normal state. The current review explores age-related structural, physiological, and immunological changes of the respiratory system. These changes may in part explain older individuals’ clinical presentation and/or aberrant diagnostic studies, avoiding unnecessary interventions, and how these changes may predispose these vulnerable individuals to increased risk for respiratory diseases. Moreover, these changes may in part be responsible for variable response to currently available drugs to treat pulmonary diseases in older adults.
Respiratory system mechanics
The respiratory system comprises primarily the thoracic cage, lungs, and diaphragm. Total respiratory system compliance includes lung and chest wall compliance. Compliance is change in volume relative to change in pressure. Lung compliance determines the rate and force of expiration and the thoracic compliance determines the elastic load during inspiration. With aging there are structural changes to the thoracic cage causing reduction in chest wall compliance. Age-related osteoporosis results in reduced height of the thoracic vertebrae. Stiffening of the thoracic cage from calcification of the rib cage and age-related kyphosis from osteoporosis reduces the ability of the thoracic cage to expand during inspiration and places the diaphragm at a mechanical disadvantage to generate effective contraction. Mittman et al (1965) studied total respiratory compliance in 42 healthy male subjects, ages 24 to 78 yrs, with 5 subjects being age ≥70 years. Lung compliance was similar, but the chest wall compliance was lower in older subjects. Moreover, subjects with lower chest wall compliance had higher residual volume (RV), suggesting an impediment to complete emptying of the lungs from stiff chest wall (Mittman et al 1965).
Respiratory muscle function
The diaphragm is the most important respiratory muscle and plays an essential role during inspiration. Exact measurement of diaphragmatic strength can only be done in vivo. There is very little information on the effect of aging on the contractile properties of the diaphragm. Respiratory muscle strength can be measured by transdiaphragmatic pressure (Pdi), maximum voluntary ventilation (MVV), and maximum inspiratory pressure (MIP). MIP is an index of strength of the diaphragm measured using a mechanical pressure gauge with a closed valve at the mouth during an inspiration. MIP is an indicator of inspiratory muscle strength and a determinant of vital capacity. Decline in MIP can lead to inadequate ventilation and impaired clearance of airway secretions, as seen in patients with neuromuscular disease. Studies measuring MIP have been cross-sectional, and their primary objective has been to establish reference values rather than to evaluate the longitudinal impact of age on diaphragmatic function. MIP is 30% higher in men compared with women at all age groups and decreases by 0.8 cm to 2.7 cm of H20/year between the ages of 65 and 85 years, with larger age-related declines seen in men (Enright et al 1994). Tolep et al (1995) showed a 25% lower Pdi measured using Mueller maneuver in healthy older subjects (age 65–75, n=10) compared with young healthy adults (age 19–28, n=9). Similar results were seen in a study by Polkey et al (1997). The degree of reduction in Pdi in healthy older compared with healthy young controls was less (13%) in the latter study, partly explained by different measurement techniques (Table 1). Maximum voluntary ventilation is also reduced with age, and one longitudinal study showed a 12% decline over 6 years in older trained athletes (McClaran et al 1995). Likely explanation for reduced diaphragmatic strength with age is related to muscle atrophy and age-related decrease in fast twitch fibers, responsible for generating higher peak tensions. This age-related decline in diaphragmatic strength may predispose older individuals to diaphragmatic fatigue and ventilatory failure during increased ventilatory load on the respiratory system.
Table 1.
Aging and respiratory muscle strength
| Study | Technique | Pdi (cm of H20) | Reduction | p-value | |
|---|---|---|---|---|---|
| Young | Elderly | ||||
| Tolep et al 1995 | Mueller | 171±8 | 128±9 | 25% | <0.003 |
| Polkey et al 1997 | Sniff | 136±17 | 119±22 | 13% | 0.05 |
Abbreviations: Pdi, transdiaphragmatic pressure.
Anatomical changes
Senile hyperinflation of the lungs is a well known entity in the medical literature. It is not clear whether it reflects aging-associated destruction of lung parenchyma or loss of supporting structures within the lung parenchyma. Gillooly and Lamb (1993) studied 38 (age ≥65 yrs, n=14) autopsy or surgical lung resection specimens in lifelong nonsmokers and showed increase in airspace size with advancing age. There is homogeneous degeneration of the elastic fibers around the alveolar duct starting around 50 years of age resulting in enlargement of airspaces. Reduction in supporting tissue results in premature closure of small airways during normal breathing and can potentially cause air trapping and hyperinflation, hence “senile emphysema”.
Immunologic changes
Bronchoalveolar lavage (BAL) fluids in healthy older subjects have consistently shown an increased proportion of neutrophils and lower percentage of macrophages compared with younger adults. There is age-associated increase in immunoglobins IgA and IgM in the BAL fluid. Ratio of CD4+/CD8+ lymphocyte increases with age in the BAL fluid, suggesting the presence of primed T-cell from repeated antigenic stimuli of the lower respiratory tract mucosa (Meyer et al 1996). Moreover, there is increased ability of alveolar macrophages to release superoxide anion in response to stimuli in the elderly. These changes likely represent the combined affect of repetitive antigenic stimuli from environmental exposure and age-related decline in down regulatory response to antigenic exposure. Persistent low grade inflammation in the lower respiratory tract can cause proteolytic and oxidant-mediated injury to the lung matrix resulting in loss of alveolar unit and impaired gas exchange across the alveolar membrane seen with aging. The clinical implication of immune dysregulation with age is yet to be determined.
The cellular profile of epithelial lining fluid (ELF) changes with age. The ELF is rich in antioxidant defenses and minimizes oxidative injury to the respiratory epithelium following toxic exposure. ELF is rich in superoxide dismutase, catalase, metal binding proteins, glutathione, and vitamins C and E. In vitro and in vivo studies showed reduction in antioxidant levels in the ELF on exposure to ozone, nitrous oxide, and particulate matter. Composition of ELF changes with age, increasing susceptibility of older individuals to environmental toxic exposure (Kelly et al 2003) (Figure 1).
Figure 1.
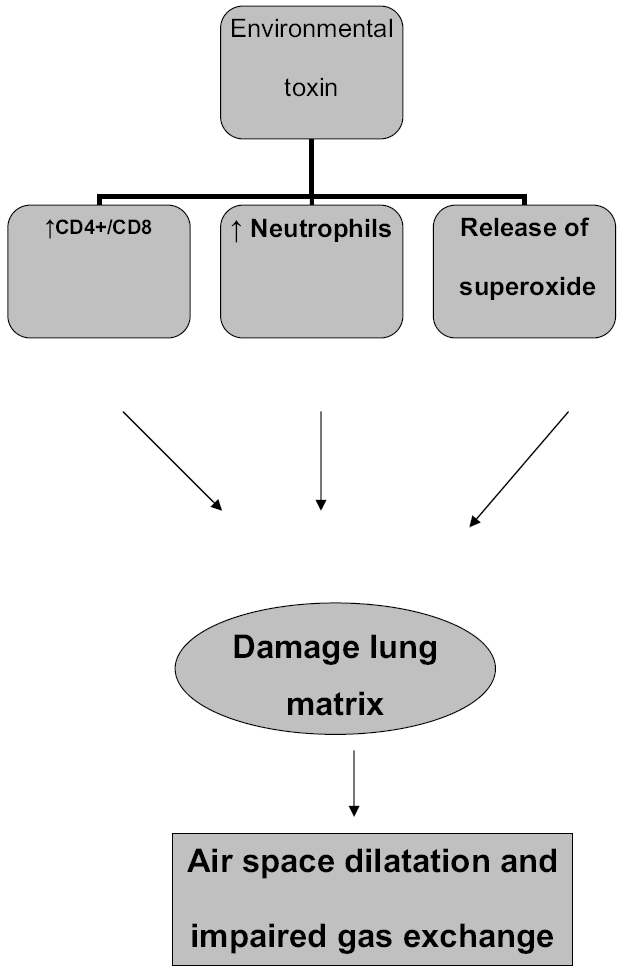
Effect of environmental exposure on airways.
Lung function
Lung function can be divided into three categories: spirometry to assess the dynamic flow rates: forced expiratory volume in one second (FEV1), forced vital capacity (FVC), and FEV-1/FVC ratio. The dynamic flow rates are dependant on lung volumes (Table 2). The static lung volumes include total lung capacity (TLC), vital capacity (VC), residual volume (RV), and functional residual capacity (FRC) (Figure 3). The gas exchange across alveolar capillary membrane is measured using diffusion capacity for carbon monoxide (DLCO). Pulmonary function tests are reported as % predicted compared with individuals of same age, sex, and height.
Table 2.
Static lung volumes
| Tidal volume (TV) | Volume of air inspired or expired during quiet breathing |
| Inspiratory reserve volume (IRV) | Maximum volume of air inspired above the tidal volume |
| Expiratory reserve volume (ERV) | Maximum volume of air expired below the tidal volume |
| Residual volume (RV) | Amount of air in the lungs after maximum expiration |
Figure 3.

Age-related decline in forced expiratory volume in one second (FEV1)% predicted plotted as % of maximal at age 20 years against age.
Note: Adapted from Ware JH, Dockery DW, Louis TA, et al. 1990. Longitudinal and cross-sectional estimates of pulmonary function decline in never-smoking adults. Am J Epidemiol, 132:685-700. Copyright © 2004. Reprinted with permission from Oxford University Press.
The lungs undergo a phase of growth and maturation during the first two decades of life and achieve maximal lung function around age 20 years in females and 25 years in males. Lung function remains steady with very minimal change from age 20 to 35 years and starts declining thereafter (Figure 3). The decline in pulmonary function tests depends on peak lung function achieved during adulthood, the duration of the plateau phase, and rate of lung function decline. Studies on lung function are done either to establish the reference values for the pulmonary function laboratories or to determine the age-related decline (Knudson 1981; Janssens et al 1999; Zeleznik 2003).
The variability in similar physiologic measurements is much greater among healthy older individuals compared with younger individuals, making it problematical to establish a “normal” range for the older adults. Most prediction equations are derived from cross-sectional studies with under representation from older adults and ethnic minorities. The cohort and period effect unique to the cross sectional studies can under- or overestimate the lung function. Environmental exposures, nutritional deficiencies, or childhood infection common to individuals born within a defined time period that may not be present in successive generations explains why younger individuals now have a higher lung function compared with 50 years ago. The cumulative cohort effect is estimated to be 5 ml/yr. This means that each former 25 year old generation had a vital capacity which is lower by 125 ml compared with the present generation (Xu et al 1995).
On the contrary, period effects include changes in techniques, equipment, and “learning effect” from repeated measures that can all improve the lung function measurement on subsequent studies. Studies estimating the period effect on lung function showed an average FEV1 increase by 250 ml in men and 219 ml in women during two different survey periods (from 1973–1978 and 1985–1990) (Xu et al 1995). Most cross-sectional studies showed linear decline in FEV1 with age and, underestimated the rate of decline. Longitudinal studies showed a nonlinear decline with age. The estimated rate of decline in FEV1 is 25–30 ml/yr starting at age 35–40 years and can double to 60ml/yr after age 70 years. Precise rate of decline is difficult to assess as inter-individual variability (up to 125 ml) far surpasses the estimated annual decline by various regression equations (Morris et al 1971; Crapo et al 1981; Knudson et al 1983; Dockery et al 1985; Burrows et al 1986; Ware et al 1990; Roberts et al 1991; Enright et al 1993; Kerstjens et al 1997) (Table 4).
Table 4.
Lung function studies
| Study | Time | Cross-sectional (C)/Longitudinal (L) | Age range | N | Age ≥65 years | Decline |
|---|---|---|---|---|---|---|
| Morris et al | 1969–70 | C | 20–84 | 988 | 79 | Linear |
| Crapo et al | 1979–80 | C | 18–91 | 251 | 68 | Linear |
| Knudson et al | 1972–75 | C | 6–88 | 421 | - | Linear |
| Dockery et al | 1974–76 | C | 25–74 | 2454 | 430 | Nonlinear |
| Burrows et al | 1972–84 | L | 20–72 | 466 | - | Nonlinear |
| Ware et al | 1974–83 | L | 25–74 | 2454 | 430 | Nonlinear |
| Enright et al | 1989–90 | C | 65–85 | 777 | 777 | Linear |
Note: Adapted from Kerstjens HAM, Rijcken B, Schouten JP, et al. 1997. Decline of FEV by age and smoking status: facts, figures, and fallacies. Thorax, 52:820-7. Copyright © 1997. Reproduced with permission from BMJ.
Lung volumes depend on body size, especially height. Total lung capacity (TLC) corrected for age remains unchanged throughout life. Functional residual capacity and residual volume increase with age, resulting in a lower vital capacity.
Gas exchange in the lungs occurs across the alveolar capillary membrane. It is measured by diffusing capacity of carbon monoxide (DLCO). The DLCO is dependant upon lung volume (TLC) and alveolar ventilation. Diffusion across the alveolar–capillary interface is directly proportional to the alveolar surface and inversely proportional to the alveolar–capillary membrane thickness. Stam et al (1994) studied effect of age on diffusion capacity in 55 healthy subjects (age ≥70 yrs, n=3) and showed a decline in DLCO with age corrected for alveolar volume. This suggests alteration in the alveolar–capillary membrane as the potential mechanism, though not proven.
Dyspnea and response to hypoxia and hypercapnia
Minute ventilation, a product of volume inhaled per breath and respiratory rate over one minute, is identical in younger and older individuals. There is no change in tidal volume with age, and older individuals maintain the required minute ventilation by increasing the respiratory rate. The ventilatory response to lower oxygen tension or raised carbon dioxide tension is markedly impaired in older adults. Kronenberg and Drage (1973) studied 8 healthy young and 8 older subjects and noted a 50% reduction in response to hypoxia and 40% reduction in response to hypercarbia. These results were confirmed by Peterson et al (1981) in a study of older healthy adults. Aging is associated with loss of potentially protective mechanisms and this patient population is more likely to be exposed to these states, making them more vulnerable. The likely explanation of reduced response is age-related decline in efferent neural output to respiratory muscles during hypoxic or hypercarbic states, supported by the fact that older adults generate lower occlusion pressure compared with younger individuals during these states (Peterson et al 1981).
Bronchial hyper-responsiveness and age-related pulmonary receptor changes
Age had a significant effect on airway reactivity and response to bronchodilator therapy. Airway reactivity is studied using methacholine challenge in the pulmonary function laboratory. Most individuals will experience bronchospasm to inhaled methacholine, and the dose required to cause significant reduction in FEV1 (ie, 20% reduction from the baseline) determines the bronchial reactivity. Lower doses signify increased bronchial reactivity and higher likelihood of asthma. Hopp et al (1985) retrospectively studied the cumulative methacholine dose required to produce a 20% decline in FEV1 in 148 healthy subjects (age ≥70, n=7) and showed a hyperbolic curve, suggesting that younger and older individuals required lesser methacholine doses to cause significant bronchoconstriction compared with middle-aged individuals. Not only do older individuals require a lower cumulative dose to cause bronchoconstriction, they take longer to recover post β-adrenergic agonist therapy (Connolly et al 1995). The exact mechanisms of these findings are unclear. It is speculated that this is likely due to age-related β-adrenergic receptor dysfunction.
β-adrenoreceptor
β-adrenoreceptor stimulation by agonist or antagonist is mediated through cyclic adenosine monophosphate (AMP) production. Adenosine triphosphate (ATP) is converted to cyclic AMP by catalytic unit of adenylate cyclase in the presence of a ligand. The β-adrenoreceptor can exist in two physiological states: high or low affinity. Cyclic AMP production is reduced during agonist binding to a low affinity state. The β-receptor density remains unchanged during lifetime, but receptor affinity is reduced (Abrass and Scarface 1982). This reduction in high affinity receptor represents functional uncoupling of the receptor from the adenyl cyclase complex and impaired receptor-mediated adenyl cyclase activity (Feldman et al 1984; Connolly 1993) (Figure 4).
Figure 4.
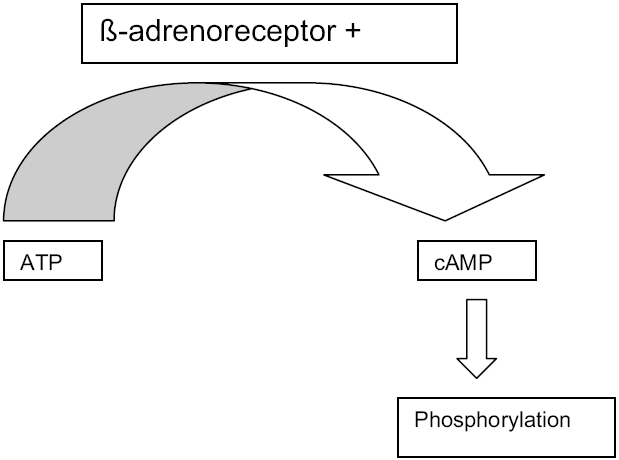
Mechanism of action of beta agonist.
Abbreviations: ATP, adenosine triphosphate; cAMP, cyclic adenosine monophosphate.
Muscarinic receptor
The data on age-related changes in pulmonary muscarinic receptor in humans is limited. A study on age-related changes in muscarinic receptors in guinea pigs showed no change in the receptor density but noticed a significant reduction in high affinity agonist binding sites in old tissues compared with young tissues. There was a change in the muscuranic receptor subtypes and receptor coupling to G proteins with senescence (Willis-Karp 1993). Clinical significance of these findings in terms of variation in anticholinergic responsiveness in older adults is yet to be determined.
Cysteinyl-Leukotriene (CysLT1) receptor
The stimulation of cysteinyl-leukotriene (CysLT1) receptor located on the airway smooth muscles induces bronchoconstriction. CysLT1 antagonists, namely zafrilukast and montelukast, have shown promise in the treatment of asthma in younger adults. There are no in vivo or in vitro studies looking at the effect of age on CysLT1 receptors. Nonetheless, clinical studies of leukotriene receptor antagonist in the treatment of asthma showed minimal improvement in FEV1 in older adults with asthma. Meta-analysis of five randomized, double-blind studies of over 1900 patients showed treatment with zafrileukast in older adults with asthma showed no improvement in lung function; on the contrary, it increases asthma exacerbations. The latter observation is of particular concern as older adults have reduced perception of dyspnea which may cause delay in getting medical attention (Cuttitta et al 2001). Baseline airway reversibility was similar across all age groups, and the lack of improvement in lung function are unlikely to be explained by “fixed airway disease” secondary to airway remodeling in older adults (Creticos et al 2002). Similar results were noticed in an open label trial of over 3700 patients on zafrileukast (Korenblatt et al 2000).
In summary, the clinical significance of age-related β-adrenoreceptor and muscuranic receptor changes are not well studied, but the role of CysLT1 receptor antagonist in older adults remains controversial.
Exercise capacity
The effect of aging on exercise capacity is highly variable and depends upon individual fitness and regular physical activity. Maximum oxygen consumption (VO2 max), an objective surrogate of fitness, peaks between 20 to 30 years of age, then declines by a rate of about 1% per year depending upon individual level of physical activity (declining more in sedentary compared with physically active adults). McClaran et al (1995) studied the longitudinal effects of aging on lung function at rest and during exercise in healthy older adults and showed an 11% reduction in VO2 max as a group over a 6 year period. All participants were highly trained in aerobic fitness, and their VO2 max was still twice the age predicted maximum. The ventilatory response for a given CO2 production is higher in older adults compared with younger individuals, suggesting a possible increase in dead space ventilation (Vd/Vt) (Table 5). Tenney and Miller (1956) studied 18 healthy males between the ages of 68 and 89 years and showed that Vd/Vt is a disproportionately large fraction of total minute ventilation in older individuals (235±67.5 ml). Dead space ventilation increases by 55% compared with that of younger adults (average Vd/Vt in younger adults 150 ml). A 5 mm Hg increase in A-a gradient raises dead space ventilation by about 40 ml. Increase in Vd/Vt with age reflects disparity in the distribution of ventilation and perfusion with age. Additional age-related changes including reduced heart rate response, cardiac output, and peripheral muscle mass may account for the decline in VO2 max with age rather than reduced lung function alone.
Table 5.
Ventilatory response to hypoxia and hypercapnia
| Young (22–30 years) | Elderly (64–79 years) | p-value | |
|---|---|---|---|
| VE (Minute ventilation) in litres/min | |||
| Kronenberg et al 1973 | |||
| PaO2 40 mm Hga | 40.1±4.7 | 10.2±1.2 | <0.001 |
| PaCO2 (L/min/mm Hg)b | 3.4±0.5 | 2.0±0.02 | <0.025 |
| Peterson et al 1981 | |||
| SpO2 75%c | 30.3±2.9 L/min | 20.9±2.3 L/min | <0.01 |
| PaCO2 60 mm Hgd | 48.7±4.3 L/min | 34.6±2.9 L/min | |
Note: aChange in minute ventilation from baseline to during hypoxia (arterial oxygen tension of 40 mm of Hg);
bChange in minute ventilation per unit change in arterial carbondioxide tension from baseline;
cminute ventilation at arterial oxygen saturation of 75%;
dminute ventilation at arterial carbondioxide tension of 60 mm Hg.
Summary
There is marked variation in the effect of aging on lung function. Aging is associated with reduction in chest wall compliance and increased air trapping. The decline in FEV1 with age likely has a nonlinear phase with acceleration in rate of decline after age 70 years. There is an increase in airspace size with aging resulting from loss of supporting tissue. Respiratory muscle strength decreases with age and much more so in men than in women (Table 6). Despite these changes the respiratory system is capable of maintaining adequate oxygenation and ventilation during the entire life span. However, the respiratory system reserve is limited with age, and diminished ventilatory response to hypoxia and hypercapnia makes it more vulnerable to ventilatory failure during high demand states (ie, heart failure, pneumonia, etc) and possible poor outcomes. Moreover, the reduced perception of bronchial constriction may result in delayed medical attention. Sustained inflammation of the lower respiratory tract may predispose older adults to increased susceptibility to toxic environmental exposure and accelerated lung function decline. As Americans are getting older, future studies need to address the clinical implication of “normal” age-related changes of the respiratory system.
Table 6.
Anatomical and physiological changes of respiratory system with aging
| Anatomical | |
| Air space size | Increased |
| Compliance | |
| Chest wall compliance | Decreased |
| Lung compliance | Increased to unchanged |
| Total respiratory system compliance | Decreased |
| Muscle strength | |
| Maximal inspiratory pressure (MIP) | Decreased |
| Trans diaphragmatic pressure (Pdi) | Decreased |
| Maximum voluntary ventilation (MVV) | Decreased |
| Lung function | |
| FEV1 | Decreased |
| FVC | Decreased |
| TLC | Unchanged |
| Vital capacity | Decreased |
| Functional residual capacity | Increased |
| Residual Volume | Increased |
| DLCO/VA | Decreased |
| Exercise capacity | |
| VO2 max | Decreased |
| Dead space ventilation | Increased |
| Immunology | |
| Bronchial fluid | |
| Neutrophils % | Increased |
| Ratio of CD4+/CD8+ cells | Increased |
| Epithelial lining fluid antioxidants | Decreased |
Abbreviations: DLCO, diffusing capacity of carbon monoxide; FEV1, forced expiratory volume in one second; FVC, forced vital capacity; TLC, total lung capacity; VO2, oxygen consumption; VA, alveolar volume.
Figure 2.

Relationship between lung volumes and lung capacities.
Abbreviations: ERV, expiratory reserve volume; FRC, functional residual capacity; IC, inspiratory capacity; IRV, inspiratory reserve volume; RV, residual volume; TLC, total lung capacity; TV, total volume; VC, vital capacity.
Table 3.
Lung capacities are sum of two or more static lung volumes
| Inspiratory capacity (IC) | TV + IRV |
| Functional residual capacity (FRC) | RV + ERV |
| Vital capacity (VC) | TV + IRV + ERV |
| Total lung capacity (TLC) | TV + IRV + ERV + RV |
Abbreviations: ERV, expiratory reserve volume; IRV, inspiratory reserve volume; RV, residual volume; TV, tidal volume.
References
- Abrass IB, Scarface PJ. Catalytic unit of adenylate cyclase: reduced activity in aged-human lymphocytes. J Clin Endocr Metab. 1982;55:1026–8. doi: 10.1210/jcem-55-5-1026. [DOI] [PubMed] [Google Scholar]
- Burrows B, Lebowitz MD, Camilli AE, et al. Longitudinal changes in forced expiratory volume in one second in adults. Am Rev Respir Dis. 1986;133:974–80. doi: 10.1164/arrd.1986.133.6.974. [DOI] [PubMed] [Google Scholar]
- Connolly MJ. Aging, late-onset asthma and the beta-adrenoceptor. Pharm Therapeutics. 1993;60:389–404. doi: 10.1016/0163-7258(93)90029-d. [DOI] [PubMed] [Google Scholar]
- Connolly MJ, Crowley JJ, Charan NB, et al. Impaired bronchodilator response to albuterol in healthy elderly men and women. Chest. 1995;108:401–6. doi: 10.1378/chest.108.2.401. [DOI] [PubMed] [Google Scholar]
- Crapo RO, Morris AH, Gardner RM. Reference spirometric values using techniques and equipment that meets ATS recommendations. Am Rev Respir Dis. 1981;123:659–64. doi: 10.1164/arrd.1981.123.6.659. [DOI] [PubMed] [Google Scholar]
- Creticos P, Knobil K, Edwards LD, et al. Loss of response to treatment with leukotriene receptor antagonists but not inhale corticosteroids in patients over 50 years of age. Ann Allergy Asthma Immunol. 2002;88:401–9. doi: 10.1016/S1081-1206(10)62372-1. [DOI] [PubMed] [Google Scholar]
- Cuttitta G, Cibella F, Bellia V, et al. Changes in FVC during methacholine-induced bronchoconstriction in elderly patients with asthma: bronchial hyper responsiveness and aging. Chest. 2001;119:1685–90. doi: 10.1378/chest.119.6.1685. [DOI] [PubMed] [Google Scholar]
- Dockery DW, Ware JH, Ferris BG, et al. Distribution of forced expiratory volume in one second and forced vital capacity in healthy, white, adult, never smokers in six U.S. cities. Am Rev Respir Dis. 1985;131:511–20. doi: 10.1164/arrd.1985.131.4.511. [DOI] [PubMed] [Google Scholar]
- Enright PL, Kronmal RA, Higgens M, et al. Spirometry reference values for women and men 65 to 85 years of age. Cardiovascular Health Study. Am Rev Respir Dis. 1993;147:125–33. doi: 10.1164/ajrccm/147.1.125. [DOI] [PubMed] [Google Scholar]
- Enright PL, Kronmal RA, Manolio TA, et al. Respiratory muscle strength in the elderly. Am J Respir Crit Care Med. 1994;149:430–8. doi: 10.1164/ajrccm.149.2.8306041. [DOI] [PubMed] [Google Scholar]
- Feldman RD, Limbird LE, Nadeau J, et al. Alterations in leukocyte β-adrenergic affinity with aging. A potential explanation of altered β-adrenergic sensitivity in the elderly. N Engl J Med. 1984;310:815–19. doi: 10.1056/NEJM198403293101303. [DOI] [PubMed] [Google Scholar]
- Gillooly M, Lamb D. Airspace size in lungs of lifelong non-smokers: effect of age and sex. Thorax. 1993;48:39–43. doi: 10.1136/thx.48.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopp RJ, Bewtra A, Nair NM, et al. The effect of age on methacholine response. J Allergy Clin Immunol. 1985;76:609–13. doi: 10.1016/0091-6749(85)90783-3. [DOI] [PubMed] [Google Scholar]
- Janssens JP, Pache JC, Nicod LP. Physiological changes in respiratory function associated with aging. Eur Respir J. 1999;13:197–205. doi: 10.1034/j.1399-3003.1999.13a36.x. [DOI] [PubMed] [Google Scholar]
- Kelly FJ, Dunster C, Mudway I. Air pollution and the elderly: oxidant/antioxidant issues with consideration. Eur Respir J Suppl. 2003;40:70–75s. doi: 10.1183/09031936.03.00402903. [DOI] [PubMed] [Google Scholar]
- Kerstjens HAM, Rijcken B, Schouten JP, et al. Decline of FEV by age and smoking status: facts, figures, and fallacies. Thorax. 1997;52:820–7. doi: 10.1136/thx.52.9.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudson RJ. How aging affects the normal lung. J Respir Dis. 1981;2:74–84. [Google Scholar]
- Knudson RJ, Lebowitz MD, Holberg CJ, et al. Changes in the normal maximal expiratory flow-volume curve with growth and aging. Am Rev Respir Dis. 1983;127:725–34. doi: 10.1164/arrd.1983.127.6.725. [DOI] [PubMed] [Google Scholar]
- Korenblatt PE, Kemp JP, Scherger JE, et al. Effect of age on response to zafirlucast in patients with asthma in the Accolate clinical experience pharmacoepidemiology trial (ACCEPT) Ann Allergy Asthma Immunol. 2000;84:217–25. doi: 10.1016/S1081-1206(10)62759-7. [DOI] [PubMed] [Google Scholar]
- Kronenberg RS, Drage CW. Attenuation of the ventilatory and heart rate responses to hypoxia and hypercapnea with aging in normal men. J Clin Invest. 1973;52:1912–19. doi: 10.1172/JCI107363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClaran SR, Babcock MA, Pagelow DF, et al. Longitudinal effects of aging on lung function at rest and exercise in healthy active fit elderly adults. J Appl Physiol. 1995;78:1957–68. doi: 10.1152/jappl.1995.78.5.1957. [DOI] [PubMed] [Google Scholar]
- Meyer KC, Ershler W, Rosenthal NS, et al. Immune dysregulation in the aging human lung. Am J Respir Crit Care Med. 1996;153:1072–9. doi: 10.1164/ajrccm.153.3.8630547. [DOI] [PubMed] [Google Scholar]
- Mittman C, Edelman NH, Norris AH, et al. Relationship between chest wall and pulmonary compliance with age. J Appl Physiol. 1965;20:1211–16. [Google Scholar]
- Morris JF, Koski A, Johnson LC. Spirometric standards for health non-smoking adults. Am Rev Respir Dis. 1971;103:57–67. doi: 10.1164/arrd.1971.103.1.57. [DOI] [PubMed] [Google Scholar]
- Peterson DD, Pack AI, Silage DA, et al. Effects of aging on ventilatory and occlusion pressure responses to hypoxia and hypercapnia. Am Rev Respir Dis. 1981;124:387–91. doi: 10.1164/arrd.1981.124.4.387. [DOI] [PubMed] [Google Scholar]
- Polkey MI, Harris ML, Hughes PD, et al. The contractile properties of the elderly human diaphragm. Am J Respir Crit Care Med. 1997;155:1560–4. doi: 10.1164/ajrccm.155.5.9154857. [DOI] [PubMed] [Google Scholar]
- Roberts CM, MacRae KD, Winning AJ, et al. Reference values and prediction equations for normal lung function in a non-smoking white urban population. Thorax. 1991;46:643–50. doi: 10.1136/thx.46.9.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stam H, Hrachovina V, Stijnen T, et al. Diffusing capacity dependent on lung volume and age in normal subjects. J Appl Physiol. 1994;76:2356–63. doi: 10.1152/jappl.1994.76.6.2356. [DOI] [PubMed] [Google Scholar]
- Tenney SM, Miller RM. Dead space ventilation in old age. J Appl Physiol. 1956;9:321–7. doi: 10.1152/jappl.1956.9.3.321. [DOI] [PubMed] [Google Scholar]
- Tolep K, Higgins N, Muza S, et al. Comparison of diaphragm strength between healthy adult elderly and young men. Am J Respir Crit Care Med. 1995;152:677–82. doi: 10.1164/ajrccm.152.2.7633725. [DOI] [PubMed] [Google Scholar]
- US Census 2005US Census Bureau [online]Accessed 23 March 2005. URL: http://www.census.gov/
- Ware JH, Dockery DW, Louis TA, et al. Longitudinal and cross-sectional estimates of pulmonary function decline in never-smoking adults. Am J Epidemiol. 1990;132:685–700. doi: 10.1093/oxfordjournals.aje.a115710. [DOI] [PubMed] [Google Scholar]
- Willis-Karp M. Age-related changes in pulmonary muscarinic receptor binding properties. Am J Physiol. 1993;265:L103–9. doi: 10.1152/ajplung.1993.265.2.L103. [DOI] [PubMed] [Google Scholar]
- Xu X, Laird N, Dockery DW, et al. Age, period, and cohort effects on pulmonary function in a 24-year longitudinal study. Am J Epidemiol. 1995;141:554–66. doi: 10.1093/oxfordjournals.aje.a117471. [DOI] [PubMed] [Google Scholar]
- Zeleznik J. Normative aging of the respiratory system. Clin Geriatr Med. 2003;19:1–18. doi: 10.1016/s0749-0690(02)00063-0. [DOI] [PubMed] [Google Scholar]


