Abstract
Influenza is a major respiratory pathogen, which exerts a huge human and economic toll on society. Influenza is a vaccine preventable disease, however, the vaccine strains must be annually updated due to the continuous antigenic changes in the virus. Inactivated influenza vaccines have been used for over 50 years and have an excellent safety record. Annual vaccination is therefore recommended for all individuals with serious medical conditions, like COPD, and protects the vaccinee against influenza illness and also against hospitalization and death. In COPD patients, influenza infection can lead to exacerbations resulting in reduced quality of life, hospitalization and death in the most severe cases. Although there is only limited literature on the use of influenza vaccination solely in COPD patients, there is clearly enough evidence to recommend annual vaccination in this group. This review will focus on influenza virus and prophylaxis with inactivated influenza vaccines in COPD patients and other “at risk” groups to reduce morbidity, save lives, and reduce health care costs.
Keywords: influenza, vaccine, immune response, efficacy, COPD
Introduction
Influenza virus is one of the leading causes of viral disease resulting in widespread morbidity, a large number of lives lost and substantial economic loss. During annual influenza outbreaks, an estimated 5%–15% of the world’s population is infected causing an estimated one million deaths every year (WHO 2002; Yewdell and Garcia-Sastre 2002). Moreover, we are currently facing a potential new pandemic that could cause unprecedented levels of global morbidity and mortality, particularly in the developing countries but also in the industrialized world. Today’s inactivated influenza vaccines provide satisfactory protection against seasonal influenza in healthy adults (Beyer et al 2002; WHO 2005) and annual vaccination is cost effective (Nichol et al 1995). However, there is still room for improvement in current vaccines, especially in the elderly “at risk” groups where vaccine efficacy is not optimal. Two classes of antiviral agents are also licensed for therapeutic and prophylactic use against influenza, complementing but not replacing, the use of influenza vaccines (Reviewed in Oxford et al 2003).
In man, influenza virus is transmitted via droplets expelled upon coughing and sneezing and normally infects the epithelial cells of the upper respiratory tract (Nicholson 1998). The incubation period is usually 2–3 days, but can be as long as 7 days. The patient is generally contagious during the febrile phase, but cases of viral spread have been observed prior to the onset of symptoms (Nicholson 1998). The duration of illness is usually one week and is normally accompanied by high fever, headache, myalgia, sore throat and rhinitis. People with low levels of viral shedding often have fewer clinical symptoms or are asymptomatic (Murphy et al 1973; Murphy and Webster 1996).
Healthy people usually recover within one week of illness without requiring any medical intervention. In the very young, the elderly and people with underlying medical problems (eg, chronic obstructive pulmonary disease (COPD), diabetes, cancer, heart disease) influenza poses a serious risk, and infection may lead to hospitalization and in some cases death (Nguyen-Van-Tam 1998). Frequently, the cause of hospitalization or death is viral pneumonia or secondary bacterial pneumonia. Substantial levels of community morbidity occur during an influenza outbreak, resulting in a significant strain on the health care system. The economic burden of influenza on society can also be significant. In the USA alone, a conservative estimate predicted an annual loss of 12–17 billion dollars (Williams et al 1988; WHO 2005).
Respiratory tract infections such as influenza often lead to exacerbation of disease in COPD patients. Influenza infection in such patients requires increased medical intervention and serious complications can result in hospitalization and death (Yap et al 2004). Each exacerbation in COPD patients leads to a progressive irreversible decrease in lung function, the frequency and severity of the exacerbations increases as the disease worsens (Niewoehner 2006). The patient’s quality of life therefore deteriorates with an increased need for hospitalization. Importantly, influenza is a vaccine preventable disease and this review will focus on influenza virus and prophylaxis with inactivated influenza vaccines in “at risk” groups, herein COPD patients.
The influenza virus and its life cycle
Influenza belongs to the family of Orthomyxoviridae (Fauquet et al 2004). There are three types of influenza, A, B and C, which are classified on the basis of antigenic differences in the internal proteins (nucleoprotein (NP) and matrix (M1) protein). Influenza A and B viruses are important human pathogens, whereas influenza C infection results only in a mild respiratory infection in man and will not be discussed further in this review. The influenza A genus is further subdivided based on the antigenic properties of its surface glycoproteins, the haemagglutinin (HA or H) and the neuraminidase (NA or N). Currently, there are 16 HA and 9 NA subtypes recognized by the WHO (WHO 2005) and of these the H3N2 and H1N1 subtypes are circulating widely in man today.
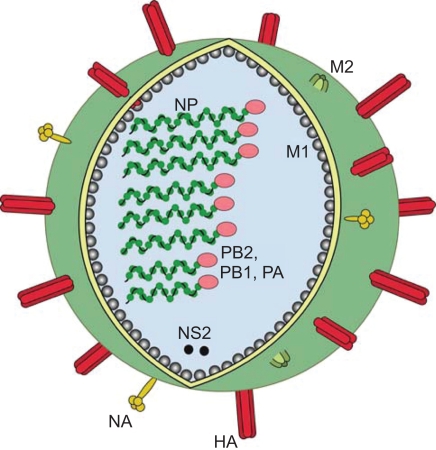
Influenza virus has a negative sense, segmented, single stranded (ss) RNA genome. The genome of influenza A virus has 8 segments, each coding for one or two proteins, in all a total of 11 proteins (Table 1). Each segment is encapsulated by the NP to form a ribonucleoprotein complex (RNP). Bound to each RNP, is the viral RNA polymerase complex, consisting of the three viral gene products (PB1, PB2, PA) (Figure 1). Three viral proteins are found in the viral envelope of influenza A virus; the HA, NA and the ion channel protein M2. The M1 protein lines the viral envelope in close proximity to the RNP and is hypothesized to interact with the cytoplasmic tails of the surface glycoproteins (Lamb and Krug 1996). The virion is pleomorphic in structure, the main form is a spherical particle (80–120 nm in diameter), but also filamentous and bean-like structures are found.
Table 1.
Influenza A proteins
| Protein | Function |
|---|---|
| HA | Receptor binding and release of viral genome. Most important antigenic protein. Trimeric structure. |
| NA | Second most important antigenic determinant. Tetrameric enzymatic protein and target for antiviral treatment. |
| M2 | Tetrameric H+ ion channel and target for antiviral treatment |
| PB1, PB2, PA | Viral polymerase complex |
| NP | Nucleoprotein |
| M1 | Structural matrix protein |
| NS1, NS2, PB1-F2 | Regulatory proteins and RNA transport |
Figure 1.
Schematic figure of influenza virus. On the surface of the virus there are three viral proteins, haemagglutinin (HA), neuraminidase (NA) and the matrix 2 protein (M2). Underlining the viral envelope is the matrix 1 protein (M1), the nucleoprotein (NP) encapsidates the genome segments with one complex of the polymerase attached (PB1, PB2 and PA). The non-structural protein 2 (NS2) is also contained in the virion in low numbers.
The HA and NA are the major antigenic proteins of the virus. Most antibodies produced are directed against these proteins and they will be briefly discussed here. The HA is a trimeric protein, composed of three identical monomers, which must be post-translationally cleaved by cellular proteases in order to be functional (Colman 1994). The distal tip of HA contains the receptor binding sites and the transmembrane stalk attaches the HA to the viral envelope (Figure 1). NA is a tetramer with a mushroom shape and contains the viral enzyme (neuraminidase), which is responsible for release of newly assembled virus from the cell. The antiviral drugs Oseltamivir and Zanamivir bind to the enzymatic site of NA, reducing or hindering the release of new virus.
Influenza virus replicates in the epithelial cells lining the respiratory tract. The enzymatic activity of NA helps the virus in navigating through the mucus layer and upon cell contact allows the HA to attach to the sialic acid containing host cell receptor. The virus particle is then engulfed and taken up into the cell in a vesicle (endosome) in a process called receptor-mediated endocytosis (Lamb and Krug 1996). The low pH initiates a conformational change in the HA molecule facilitating a fusion with the endosomal membrane. At the same time the M2 ion channel protein lowers the pH inside the virion, so that the RNPs become disassociated from M1, allowing the RNPs to enter the cytosol. The M2 protein is the target for the influenza antiviral drugs Amantadine and imantadine (Oxford et al 2003).
The RNPs migrate to the nucleus and the viral polymerase complex transcribes and replicates the viral RNA to form new viral RNA and mRNA. The NP and the M1 are translated in the cytosol, before they migrate to the nucleus to take part in the RNP assembly and transport. Viral proteins, possibly NP, may regulate the switch between transcription and replication (Portela and Digard 2002). New viral genomes are encapsidated by NP and migrate to the cytosol. The HA, NA and the M2 proteins are translated on the endoplasmatic reticulum and transported to the cell membrane. M1 and RNPs interact with the cytoplasmic tails of HA and NA at the cell membrane. The M1 protein further interacts with cellular proteins and is involved in determining both the virion shape and size, as well as being involved in the viral budding process (Lamb and Krug 1996; Hui et al 2003). The virus is then released by budding from the cell surface membrane, which is facilitated by the NA.
Influenza ecology
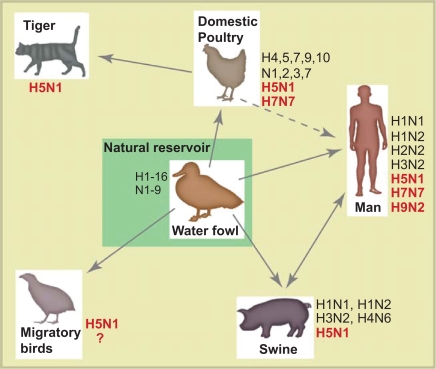
Influenza A viruses infect a wide range of species such as birds, seals, horses, man and pigs (Murphy and Webster 1996). In contrast, influenza B viruses are mainly found in man. The natural reservoir for influenza A viruses is birds, with waterfowl being the most important host and will, for the foreseeable future, continue to be a threat to human health (Webster et al 1992; Murphy and Webster 1996). The virus may spread from this reservoir to other species including man (Figure 2). Normally, avian influenza is a mild or asymptomatic intestinal infection in birds and the virus may be secreted in high titers through the cloacae for a period up to 30 days (Hinshaw et al 1980). The practice in many parts of the world of keeping free-ranging poultry close to the family dwelling, allowing both exchange of avian influenza with wild birds as well as facilitating zoonoses, is considered to be unsafe (reviewed in Webster and Bean 1998).
Figure 2.
A simplified overview of the ecology of influenza A virus. The subtypes that have been detected in each species are shown and the subtypes marked in bold are the highly pathogenic avian influenza subtypes that have caused illness in humans. The main reservoir of influenza A viruses is waterfowl, which may carry highly pathogenic subtypes of influenza without visible illness. The H5N1 has also been detected in domestic cats, however, little is known about their role in H5N1 epidemiology. Currently, we do not have enough knowledge about the importance of migratory birds in the spread of influenza virus (hence the question mark). Interested readers can consult the reviews by Webster et al 1992 or Murphy et al 1996 for further information.
The receptor binding specificity of the surface glycoprotein HA is an important determinant of host range. Only one amino acid substitution in HA (226Leu→Gln) may change the receptor specificity of HA (Eisen et al 1997), shifting from human (SAα-(2, 6)Gal) to avian (SAα-(2, 3)Gal) receptor preference. Humans also express a low level of the avian receptor (SAα-(2, 3)Gal) in the lower respiratory tract, which may explain why avian influenza virus only rarely infects man (Shinya et al 2006). In addition to the HA gene, the pathogenicity of influenza is also determined by a number of gene segments including the polymerase complex, the NS1 and the NA (Goto and Kawaoka 1998; Basler et al 2001; Taubenberger et al 2005).
Influenza epidemiology
The annual influenza outbreaks usually start during the winter months in temperate climates. In contrast, in the tropics and subtropics influenza virus may be isolated throughout the year. The number of suspected influenza cases, designated influenza like illness (ILI), is a frequently used as a measure of epidemic activity (Fleming et al 2000; Stephenson and Zambon 2002). However, there is more than one case definition of ILI used. The WHO defines ILI as a sudden onset of fever (>38 °C), cough or sore throat in the absence of other diagnoses (WHO 1999), whereas others have used a sudden onset of fever, cough and fatigue (Thursky et al 2003), or fever as well as least two of the following symptoms: headache, cough, sore throat and myalgia (Boivin et al 2000). Generally, influenza A H3N2 normally results in the most serious illness, B viruses intermediate and influenza A H1N1 cases present with the mildest manifestations (Monto et al 1985). Influenza A viruses are normally responsible for four-fold more hospitalizations than influenza B viruses (Murphy and Webster 1996).
Influenza related deaths are frequently under-reported because influenza often exacerbates underlying disease, which may be recorded as the primary cause of death (Nicholson 1998). The number of influenza related deaths is therefore often monitored as the number of excess deaths compared to a period without (known) influenza activity. Influenza mortality usually occurs in the “at risk” groups; people with underlying chronic medical conditions like COPD, diabetes, cancer and heart disease as well as in the elderly. Influenza related deaths worldwide are estimated to be about 1 million people each year (WHO 2002; Yewdell and Garcia-Sastre 2002) and in the USA about 60%–70% of deaths occur in people over 65 years old (Perrotta et al 1985). However, the true number of influenza related deaths worldwide is difficult to estimate, since most epidemiological data are derived from industrialized countries in the temperate zone (WHO 2005).
Antigenic drift of influenza
Influenza is an RNA virus and as such has a high frequency of copy errors during replication due to a lack of proof-reading by the polymerase. This results in substitutions in the genome at a rate that is many-fold higher than that found for DNA viruses (Drake et al 1998).
The accumulation of mutations, particularly in the HA, may lead to changes in the antigenic signature of the virus allowing the virus to escape herd immunity and cause new outbreaks, a process called antigenic drift (Murphy and Webster 1996). Influenza A viruses mutate more frequently than influenza B viruses and hence influenza B is more antigenically stable (Yamashita et al 1988). The slower evolution of influenza B viruses may be attributed to a longer co-evolution in man and host specific adaptations (Webster et al 1992). The risk of reassorting with avian subtypes makes influenza A virus a particularly dangerous infectious agent for man.
Antigenic shift of influenza
During the 20th century there were three pandemics, namely in 1918, 1957 and 1968 (WHO 2005). The term pandemic is only used when an antigenic shift occurs and the novel influenza A subtype infects humans causing global widespread outbreaks resulting in substantial morbidity and mortality. The 1918 pandemic had the highest mortality rates of the 20th century pandemics, causing approximately 40 million deaths worldwide, with an unprecedented number of deaths in young adults (review in Reid et al 2001).
Antigenic shift may occur after reassortment of viral genome segments from two different influenza A subtypes. When two viruses co-infect the same cell and exchange segments, a novel reassorted virus with new combinations of HA and NA surface glycoproteins may be generated. When a virus with a novel HA (and NA) spreads efficiently in man, the virus may cause a pandemic (Webster et al 1992). These novel influenza A viruses cross the species barrier from the large avian reservoir and can strike at unpredictable intervals. Pigs, having receptors for both the human and avian influenza viruses, and are thought to play a role as a “mixing vessel” producing new reassortments of influenza A virus (see Figure 2). The pandemics of 1957 and 1968 were the result of a reassortment between avian and human influenza viruses, possibly in pigs (Webster et al 1992).
An antigenic shift can also occur after direct transfer of an avian virus into man. The H1N1 virus responsible for the 1918 pandemic was not a reassortant, but was transmitted to man in toto from an avian source (Taubenberger et al 2005). The zoonotic cases of avian H5N1 in Hong Kong in 1997 and the ongoing zoonosis of H5N1 demonstrate that avian viruses can directly infect man and do not necessarily require a “mixing vessel” (Subbarao et al 1998; Chotpitayasunondh et al 2005). In fact, the genetic reassortment between avian and human subtypes could well take place in man. However, to date there has not been an adaptation of this novel H5N1 subtype to allow a sustained human-to-human spread.
Vaccines and immunity
The two main types of influenza vaccine are inactivated virus, which is by far the most commonly used, and live virus vaccines. Inactivated vaccines are normally administered parenterally. In contrast, live virus vaccines are administered intranasally with an attenuated virus to produce a limited upper respiratory tract infection without causing any overt clinical illness. However, live influenza vaccines are not licensed outside Russia and the USA. In the USA, live vaccines are so far only recommended for healthy subjects 5–49 years old, thus excluding their use in COPD patients (Harper et al 2005).
Current inactivated influenza vaccines are trivalent containing strains from two influenza A subtypes (H1N1 and H3N2) and one influenza B variant. The vaccine is normally administered during October and November in the northern hemisphere. A single-dose regime is mostly used, but for preschool children two doses are recommended at one-months intervals. The vaccine strains need to be epidemiologically relevant, and the WHO updates their strain recommendation on an annual basis for each hemisphere. The selected strains are either reassorted with a high-growth laboratory strain or adapted to give better yields in embryonated eggs. The reassorted strain expresses the desired HA and NA of the field virus, while maintaing the high growth potential in eggs (Kilbourne 1969). Embryonated hens’ eggs are the most commonly used vaccine substrate. However, some vaccine manufacturers are now licensing their cell culture based vaccines. This will give the vaccine industry greater flexibility and allow scaling up their production if a sudden surge in demand should occur.
Formulation of inactivated vaccines
There are three main formulations of inactivated vaccines (extensively reviewed in Furminger 1998). Whole virus vaccine is inactivated by chemical agents (eg, formaldehyde or β-propiolactone) in a procedure that does not destroy the viral envelope (Goldstein and Tauraso 1970). This type of vaccine was widely utilized until the end of the 1970s, but its use was largely discontinued due to a somewhat higher frequency of side reactions (Barry et al 1976; Boyer et al 1977; Gross et al 1977; Hehme et al 2002, 2004). A split virus vaccine is produced using chemical agents (eg, ether or tributyl phosphate) to disrupt the viral envelope (Davenport et al 1960; Barry et al 1976). Immunization with split virus vaccine produces fewer side reactions (ie, has a lower reactogenicity), but has also a somewhat reduced immunogenicity compared to whole virus vaccine (Barry et al 1976; Gross et al 1977). A third vaccine formulation, the subunit type, consists of highly purified surface antigens, HA and NA, and is the least reactogenic influenza vaccine on the market today (Potter et al 1975). All inactivated vaccines are administered parenterally, either intramuscularly or deep subcutaneously. However, more recent studies using highly purified virus have found that split and whole virus vaccine induce similar levels of side reactions (Hehme et al 2002; Hehme et al 2004).
The normal adult human dose is standardized to a concentration of 15 μg HA per strain, and thus the trivalent vaccine contains a total of 45 μg of HA. Current influenza vaccines are not adjuvanted, the use of adjuvants may be necessary for pandemic vaccines based on avian influenza viruses where the vaccine recipient would be immunologically naïve (Stephenson et al 2004).
Immunity after vaccination
Humans have generally experienced a number of influenza infections, which prime the immune system and create an immunological memory (Smith 1977; Palladino et al 1995; Tamura and Kurata 2004). Upon vaccination with inactivated vaccine, reactivation of immunological memory results in production of mainly IgG as well as some IgA antibodies (Brokstad et al 1995; Brokstad et al 2002; Couch 2003; Guthrie et al 2004). In contrast, after a natural infection the immune response will elicit more IgA antibodies and a cellular T-cell response (Beyer et al 2002). If there is no pre-existing immunity (for example in children), two doses of vaccine are recommended given at least one month apart to achieve a satisfactory immune response (Harper et al 2005). It is likely that a two dose regime will be required for a pandemic vaccine containing a novel influenza subtype, in addition to the use of an adjuvanted formulation (Stephenson et al 2003; Hehme et al 2004).
The systemic response after vaccination is rapid in healthy subjects with the antibody secreting cell response peaking after one week, while the serum antibody continues to increase up to 2–3 weeks after vaccination (Cox et al 1994). The concentration of influenza-specific serum antibody then wanes over time, but remains elevated at least 8 months post vaccination (Clark et al 1983). The importance of serum antibody is shown by the fact that the higher the serum antibody level, the less likely the person is to experience clinical illness upon subsequent infection (Hobson et al 1972; Kendal et al 1982). Passively derived serum IgG probably leaks through the infected epithelial cell layer and neutralizes the virus and thus prevents binding to the cellular receptor (Tamura and Kurata 2004). IgG diffuses more readily across the alveolar wall in the lower respiratory tract than across the epithelial cells in the upper airways (Murphy 2005), which makes IgG antibodies particularly important in avoiding the most serious complications of infection.
COPD patients have a damaged epithelial cell layer and it has been shown in experimental models for asthma and chronic bronchitis, a lasting change in the airway epithelium and smooth muscle behavior after viral infection (Holtzman et al 2005). These authors have hypothesized a viral cause of both acute and chronic manifestations of asthma and chronic bronchitis. Studies in healthy individuals and animal models have shown apoptosis of cells in the epithelial cell layer and the resulting inflammation also attract effector cells to the site of infection causing further cell death (Brydon et al 2005). This could have consequences also for the immune response in the damaged lungs of COPD sufferers, but no study to date has adequately addressed this issue. A thorough review of the aetiology of exacerbations in COPD sufferers has recently been published (Sapey and Stockley 2006).
Immunity after influenza infection
Initially, influenza virus replicates in the epithelial cells of the respiratory tract and this is also an important site for the immune response (Tamura and Kurata 2004). IgA is actively transported in its secretory form, S-IgA, across the epithelial surfaces of the respiratory tract and neutralizes virus by binding to its surface proteins. Once the infection is established, a cytotoxic response is induced, often against the internal viral proteins, and is involved in viral clearance and recovery from infection (Sambhara et al 2001; Takada et al 2003). The internal influenza proteins are more conserved than HA and NA, thus memory T-cells may be more cross-reactive against drifted viruses and possibly across influenza A subtypes.
Infection-induced immunity is thought to be long-lived, but will more or less become redundant due to the continuous antigenic changes of the virus. A special case was demonstrated when the H1N1 virus re-appeared in 1977, which had circulated 20 years earlier (Dowdle 1999). Before the first H1N1 wave, antibodies were only detected in people that were in their childhood in the 1950s and not in older or younger individuals (Haaheim 2003). The long-lived immune response to influenza virus experienced in early childhood is often referred to as ‘Antigenic Sin’ (Francis et al 1953). During the first wave, people under the age of 20 were almost exclusively infected (Potter 1998) and they also had a marked lower immune response to inactivated vaccines containing H1N1, suggesting that they did not have any immunological memory against this virus (Smith 1977). The 1977 virus was antigenically very similar to a 1950 isolate and was possibly inadvertently released into the human population (Palese 2004).
Target groups for vaccination
The WHO has issued a priority list of groups, which should be annually vaccinated (Listed in full in Table 2 (WHO 2005)). These groups are: (1) Residents of institutions for the elderly or the disabled; (2) Elderly non-institutionalized individuals with certain chronic medical conditions; (3) Other individuals in the community with certain chronic medical conditions; (4) Individuals who are above a nationally defined age limit; and (5) Other groups defined on the basis of national data such as those with frequent contact with high-risk persons, health care workers, pregnant women and children age 6–23 months old. The WHO has to date not issued any recommendations on the use of live influenza vaccines (WHO 2005).
Table 2.
Target groups for vaccination (WHO 2005)
| 1 | Residents of institutions for the elderly or the disabled; |
| 2 | Elderly non-institutionalised individuals with 1 or more of the following chronic conditions, chronic cardiovascular, pulmonary, metabolic or renal disease, or who are immunocompromised; |
| 3 | Other individuals (adults and children aged >6 months) in the community who have chronic cardiovascular, pulmonary, metabolic or renal disease, or are immunocompromised; |
| 4 | Individuals who are above a nationally defined age limit irrespective of their medical risk status (most countries define the limit of age >65 years); |
| 5 | Other groups defined on the basis of national data such as those with frequent contact with high-risk persons, health care workers, pregnant women and children 6–23 months old. |
Since influenza strains are prone to drift antigenically from one influenza season to the next, annual vaccination is recommended. Repeated influenza vaccination does not compromise the immune response, and should therefore not be used as an argument against annual vaccination (Keitel et al 1997; Beyer et al 1999).
Safety of inactivated influenza vaccines
More than 300 million doses of influenza vaccines are administered each year and the vaccine has an excellent safety record (Beyer et al 2002). Local side reactions usually occur in 15%–20% of the vaccinees, most commonly pain and redness at the injection site (WHO 2005). Transient and mild systemic reactions can also occur and include low-grade fever and headache, especially in children, but generally do not interfere with daily activities (Wiselka 1998).
Most people can be vaccinated without any complications. However, children younger than 6 months and people with known allergies to egg protein are advised against vaccination, whereas persons with an acute febrile illness should just postpone their vaccination until they have recovered (Wiselka 1998; WHO 2005). However, a mild febrile illness is not considered a contraindication to vaccination (Harper et al 2005).
Influenza vaccination is recommended for a number of groups with underlying medical conditions and there is considerable information on the vaccine’s safety in these patients. A good safety record has been observed using inactivated influenza vaccines in patients with COPD (Howells and Tyler 1961; Poole et al 2000; Wongsurakiat et al 2004) In adults with asthma there is a very low incidence of clinically significant adverse reactions, for example, out of 40 million vaccine doses administered to such patients, only 5 cases of asthma exacerbations were reported over a ten-year period (Palache and van der Velden 1992). In asthmatic patients on oral steroid therapy, no serious local or systemic side reactions were reported after vaccination with an inactivated influenza vaccine (Park et al 1996).
Influenza vaccination will of course only protect against illness caused by influenza virus and not by other acute respiratory infections as shown in a recent study where the incidences of respiratory illnesses over a year was not affected by influenza vaccination (Wongsurakiat et al 2004). This is to be expected as, at least in the temperate zone, influenza normally is only epidemic for a few months each year. About 30% of exacerbations of COPD are caused by viral pathogens, and of these less than half are caused by influenza (Johnson and Stevenson 2002; Cameron et al 2006). Even so, influenza vaccination clearly reduced the frequency of exacerbations in COPD cohorts (Poole et al 2000). Some COPD patients occasionally report a worsening of their condition immediately following vaccination. However, in a recent study no increased usage of corticosteroids was found after vaccination (Tata et al 2003). People with asthma and COPD are considered to be one of the groups that benefit most from influenza vaccination, consequently they are included in the “at risk” groups by the WHO (WHO 2000, 2005).
Vaccine efficacy: measurement and contributing factors
The protection elicited by influenza vaccine is notoriously difficult to quantify. The term vaccine efficacy refers to well-controlled experiments with a placebo control group (Hannoun et al 2004) and sometimes involves young study subjects with a better than average health. Vaccine effectiveness is used to describe the protective effect of the vaccine when used as a part of a public health scheme and can be considerably lower than the efficacy. Both vaccine effectiveness and efficacy are highly dependent upon the specific outcome being measured, the degree of antigenic match between epidemic virus in circulation and the vaccine strain, as well as the severity of the epidemic. However, the vaccine efficacy, especially when measured by serological correlates of protection, may be an underestimate of the true effect of vaccination, partly because little data is available on the number of vaccinees who undergo a sub-clinical influenza infection (Palache 1997).
Vaccine efficacy is commonly measured using surrogate correlates of protection. The serum antibody response is often tested by the haemagglutination inhibition (HI) assay (Hobson et al 1972; Kendal et al 1982). An HI titer ≥40 indicates a 50% protective level against influenza (Hobson et al 1972; Kendal et al 1982). The level of seroprotection, ie, the percentage of vaccinees that have an HI titer after vaccination of at least 40, was found in a meta-study of mainly young health subjects under 65, to be 80%–90% (Table 3) (Beyer et al 2002). Vaccination with inactivated influenza vaccine has been shown to prevent laboratory confirmed influenza in 70%–90% of healthy adults (Ruben et al 1973; Wilde et al 1999; Bridges et al 2000; Beyer et al 2002; Kawai et al 2003; Harper et al 2005; WHO 2005). The vaccine efficacy is reduced if a more general clinical outcome is measured, for instance one study showed only a 25% reduction in upper respiratory tract illness and a 43% reduction in absenteeism from work (Nichol et al 1995).
Table 3.
The efficacy of influenza vaccine in different population groups from representative studies
| Serum HI | Laboratory confirmed influenza | Hospitalization P&I | Death | References | |
|---|---|---|---|---|---|
| Adults | 83–90% | 73–78% | Beyer 2002 | ||
| Elderly, no other risk | 37–86% | 30–81% | 33% | 50–75% | Goevert 1994, Fleming 1995,Vu 2002, McElhaney 2005 |
| Elderly, institution and non-institution | 53% | 50% | 68% | Gross 1995 | |
| Elderly, chronic lung diseases | 52% | 45–70% | Nichol 1999 | ||
| COPD patients | 45–87% | 45% | Wang 2006, Wongsurakiat, 2004 |
A significant factor when evaluating the efficacy of the vaccine is the antigenic match between the vaccine strains and the circulating influenza viruses in the community. Normally, the antigenic difference between them is trivial, but occasionally there is a mismatch. Due to the poorer immune response in the elderly, this may be more important in this group than in healthy adults (de Jong et al 2000). In healthy subjects, however, the vaccine is effective (49%–53%) in preventing illness also in years with a sub-optimal match between the vaccine and circulating influenza strains (Pyhälä et al 2001; Ritzwoller et al 2005). The level of circulating influenza in the community also complicates the calculation of the effectiveness of the vaccine. In years of high influenza activity and widespread outbreaks, the vaccine will show a higher efficacy (Jefferson et al 2005) and better cost/benefit ratio (Nichol et al 2005; Turner et al 2005).
Efficacy and effectiveness in “at risk” populations
The immune response elicited after influenza vaccination in the elderly is poorer than in younger subjects (Table 3) (Palache et al 1993), which is important for COPD patients as they are often at an advanced age (Vilkman et al 1996). This is reflected in the influenza vaccine licensing criteria in the EU, which have less stringent requirements for the vaccine’s immunogenicity in subjects over 60 years of age (CHMP 1997). In older patients (>65), a lower number of subjects elicits an increase in serum antibody after vaccination than in healthy adults, and the seroprotection rate was generally 40%–70% (Gross et al 1987; McElhaney et al 1993; Palache et al 1993; Govaert et al 1994; McElhaney et al 2005). Similar findings were observed in a trial with COPD patients with 45%–87% achieving a protective HI titer (Wongsurakiat et al 2004). However, institutionalized elderly and infirm subjects often have a particularly poor response after vaccination (Gross et al 1989). For ethical reasons few case-control studies have been undertaken investigating the efficacy of inactivated vaccines in the “at risk” groups.
A much used marker for measuring vaccine efficacy is the prevention of ILI. Some ILI cases are not due to influenza virus, but other respiratory pathogens and the vaccine efficacy in the elderly and probably also COPD sufferers against ILI is relatively low (35%) (Vu et al 2002). The positive predictive value of ILI actually being due to influenza is 23%–60%, and is very dependent on the case definition (Thursky et al 2003). The study conducted by Wongsurakiat et al showed a significant reduction (66%) in the number of ILI cases between vaccinated and unvaccinated COPD patients, despite a small patient group size (Wongsurakiat et al 2004). Using a more specific influenza diagnosis, namely laboratory confirmed influenza, the vaccine had a 58% efficacy in healthy elderly subjects (Govaert et al 1994). This is similar to the reduction of laboratory confirmed influenza observed in adults 16–64 years old (Kawai et al 2003).
During the influenza season there is an increase of hospitalization of COPD patients, clearly demonstrating the impact influenza illness can have on this group of patients (Yap et al 2004). Influenza vaccination, however, only partly protects this group against hospitalization for pneumonia and influenza (P&I) as there are several causes not related to influenza for P&I. Several studies in the elderly population, found the reduction in hospitalization for P&I to be significant after influenza vaccination (33%–52%) (Gross et al 1995; Nichol et al 1996; Vu et al 2002). There is an rapid deterioration in the quality of life with increasing number of COPD exacerbations (Niewoehner 2006) and influenza vaccine has been shown to have a 75%–80% effectiveness in reduction of acute respiratory illnesses, independently of the severity of COPD (mild, moderate and severe) (Howells and Tyler 1961; Wongsurakiat et al 2004).
Older people may not fully recover after an influenza infection and thus one significant consequence of hospitalization may be permanent disability (McElhaney 2005). Influenza vaccination reduced the hospitalization rate by 52% in elderly patients with chronic lung disease (Nichol et al 1999). An additional strategy for protecting these residents is therefore vaccination of the nursing-home staff caring for them (Potter et al 1997). Vaccination of family members and other close contacts will therefore indirectly protect the “at risk” groups (Piedra et al 2005) and is a policy that is advocated by the WHO (WHO 2005).
The efficacy of inactivated influenza vaccines in preventing influenza related deaths is 50%–75% in elderly “at risk” groups (Fleming et al 1995; Gross et al 1995; Nichol et al 1996; Jefferson et al 2005). This has been verified by trials in non-institutionalized elderly (Vu et al 2002) and in elderly with pulmonary or heart diseases (Nichol 1999). A large recent study involving over 100,000 people in Taiwan investigating the impact of influenza vaccination on mortality, also demonstrated a 45% reduction in mortality of COPD patients following influenza vaccination and the vaccination was strongly correlated with prevention of death from lung disease in general (Wang et al 2007).
The assumption that influenza vaccination also appears to protect the vaccinee from all causes of death and not only influenza related deaths, is controversial (Simonsen et al 2005) and underlines the difficulty in determining the vaccines effectiveness in large cohort studies. The efficacy of influenza vaccines to prevent serious influenza-related complications in the elderly, can be increased by using both influenza and pneumococcal vaccines and results in a reduction of the number of hospitalizations. Both vaccines are safe and can be co-administered without impairing the antibody response to either vaccine (CDC 1997).
Future challenges in the use of influenza vaccines
One of the most critical challenges is to increase the coverage rate of current influenza vaccines among the “at risk” groups, and many developed countries have adopted a policy to increase vaccine uptake. The World Health Assembly has set a goal of annual immunization of at least 75% of people over 65 years of age by 2010 (WHO 2005). In recent years, some countries have had vaccine supply problems, which can only be rectified by increasing vaccine production and encouraging more manufacturers to produce influenza vaccine. This will benefit not only COPD sufferers, but also the general public, as the industry’s production capacity will be better placed to meet the considerable demand for vaccine when a new pandemic strikes (Wood 2001). Most influenza vaccine manufacturers use embryonated hens’ eggs as the vaccine substrate. Quality-assured eggs cannot be delivered at short notice, as the manufacturers need to plan their production a year in advance. The use of cell culture systems that are more easily scaled up provides more flexibility to accommodate the increasing demand for influenza vaccines and improves pandemic preparedness.
Currently used inactivated influenza vaccines are safe, but the immunogenicity, especially in elderly, is suboptimal. There are a number of different approaches being employed to improve the vaccine efficacy. One option is to return to the use of the more immunogenic whole virus vaccine formulation, which in recent trials has also been shown to have an acceptable reactogenicity profile (Hehme et al 2002) or alternatively, to use a virosomal influenza vaccine, which has the viral surface antigens in a reconstituted viral envelope (Huckriede et al 2005). The vaccine immunogenicity can also be increased by adjuvanting the vaccine. This raises new safety issues, especially in patients on medication. However, one of these adjuvants, MF59, has been shown not to cause more side reactions in COPD patients on steroid therapy than healthy subjects (de Roux et al 2005). Several trials have investigated the combined effect of both live and inactivated influenza vaccination, this is however, not considered as a practical routine procedure (Harper et al 2005). To date, no beneficial effect of vaccination with both live and inactivated influenza vaccines has been found in older patients with COPD (Gorse et al 2003; Gorse et al 2004).
Conclusion
Influenza remains today an important cause of morbidity and mortality, especially in groups with underlying medical conditions like COPD. Inactivated influenza vaccines have been used for many years with hundreds of millions of doses administered and have an excellent safety record in all patients groups. There have been relatively few studies based solely on COPD patients, but nonetheless the conclusion is that there is enough evidence to recommend annual vaccination in this group (Poole et al 2000; Baydur 2004; Wongsurakiat et al 2004). Even if the efficacy of current influenza vaccine is not optimal, there is no doubt that its use in COPD sufferers will continue to reduce morbidity, save lives, and reduce health care costs.
References
- Barry DW, Mayner RE, Staton E, et al. Comparative trial of influenza vaccines. I. Immunogenicity of whole virus and split product vaccines in man. Am J Epidemiol. 1976;104:34–46. doi: 10.1093/oxfordjournals.aje.a112272. [DOI] [PubMed] [Google Scholar]
- Basler CF, Reid AH, Dybing JK, et al. Sequence of the 1918 pandemic influenza virus nonstructural gene (NS) segment and characterization of recombinant viruses bearing the 1918 NS genes. Proc Natl Acad Sci USA. 2001;98:2746–51. doi: 10.1073/pnas.031575198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baydur A. Influenza vaccination in vulnerable populations. Chest. 2004;125:1971–2. doi: 10.1378/chest.125.6.1971. [DOI] [PubMed] [Google Scholar]
- Beyer WE, de Bruijn IA, Palache AM, et al. Protection against influenza after annually repeated vaccination: a meta-analysis of serologic and field studies. Arch Intern Med. 1999;159:182–8. doi: 10.1001/archinte.159.2.182. [DOI] [PubMed] [Google Scholar]
- Beyer WE, Palache AM, de Jong JC, et al. Cold-adapted live influenza vaccine versus inactivated vaccine: systemic vaccine reactions, local and systemic antibody response, and vaccine efficacy. A meta-analysis. Vaccine. 2002;20:1340–53. doi: 10.1016/s0264-410x(01)00471-6. [DOI] [PubMed] [Google Scholar]
- Boivin G, Hardy I, Tellier G, et al. Predicting influenza infections during epidemics with use of a clinical case definition. Clin Infect Dis. 2000;31:1166–9. doi: 10.1086/317425. [DOI] [PubMed] [Google Scholar]
- Boyer KM, Cherry JD, Welliver RC, et al. IgM and IgG antibody responses after immunization of children with inactivated monovalent (A/New Jersey/76) and bivalent (A/New Jersey/76-A/Victoria/75) influenza virus vaccines. J Infect Dis. 1977;136(Suppl):S665–71. doi: 10.1093/infdis/136.supplement_3.s665. [DOI] [PubMed] [Google Scholar]
- Bridges CB, Thompson WW, Meltzer MI, et al. Effectiveness and cost-benefit of influenza vaccination of healthy working adults: A randomized controlled trial. Jama. 2000;284:1655–63. doi: 10.1001/jama.284.13.1655. [DOI] [PubMed] [Google Scholar]
- Brokstad KA, Cox RJ, Olofsson J, et al. Parenteral influenza vaccination induces a rapid systemic and local immune response. J Infect Dis. 1995;171:198–203. doi: 10.1093/infdis/171.1.198. [DOI] [PubMed] [Google Scholar]
- Brokstad KA, Eriksson JC, Cox RJ, et al. Parenteral vaccination against influenza does not induce a local antigen-specific immune response in the nasal mucosa. J Infect Dis. 2002;185:878–84. doi: 10.1086/339710. [DOI] [PubMed] [Google Scholar]
- Brydon EW, Morris SJ, Sweet C. Role of apoptosis and cytokines in influenza virus morbidity. FEMS Microbiol Rev. 2005;29:837–50. doi: 10.1016/j.femsre.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Cameron RJ, de Wit D, Welsh TN, et al. Virus infection in exacerbations of chronic obstructive pulmonary disease requiring ventilation. Intensive Care Med. 2006;32:1022–9. doi: 10.1007/s00134-006-0202-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC Prevention of pneumococcal disease: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 1997;46(RR-8):1–24. [PubMed] [Google Scholar]
- CHMP (1997). Committee for Proprietary Medicinal Products. Note for guidance on harmonization of requirements for influenza vaccines. CPMP/BWP/214/96, Circular No. 96-0666:1–22.
- Chotpitayasunondh T, Ungchusak K, Hanshaoworakul W, et al. Human disease from influenza A (H5N1), Thailand, 2004. Emerg Infect Dis. 2005;11:201–9. doi: 10.3201/eid1102.041061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark A, Potter CW, Jennings R, et al. A comparison of live and inactivated influenza A (H1N1) virus vaccines. 2. Long-term immunity. J Hyg(Lond) 1983;90:361–70. doi: 10.1017/s0022172400028990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colman PM. Influenza virus neuraminidase: structure, antibodies, and inhibitors. Protein Sci. 1994;3:1687–96. doi: 10.1002/pro.5560031007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couch RB. An overview of serum antibody responses to influenza virus antigens. Dev Biol(Basel) 2003;115:25–30. [PubMed] [Google Scholar]
- Cox RJ, Brokstad KA, Zuckerman MA, et al. An early humoral immune response in peripheral blood following parenteral inactivated influenza vaccination. Vaccine. 1994;12:993–9. doi: 10.1016/0264-410x(94)90334-4. [DOI] [PubMed] [Google Scholar]
- Davenport FM, Rott R, Schaefer W. Physical and biological properties of influenza virus components obtained after ether treatment. J Exp Med. 1960;112:765–82. doi: 10.1084/jem.112.5.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong JC, Beyer WE, Palache AM, et al. Mismatch between the 1997/1998 influenza vaccine and the major epidemic A(H3N2) virus strain as the cause of an inadequate vaccine-induced antibody response to this strain in the elderly. J Med Virol. 2000;61:94–9. [PubMed] [Google Scholar]
- de Roux A, Marx A, Burkhardt O, et al. Impact of corticosteroids on the immune response to a MF59-adjuvanted influenza vaccine in elderly COPD-patients. Vaccine. 2005 doi: 10.1016/j.vaccine.2005.10.007. [DOI] [PubMed] [Google Scholar]
- Dowdle WR. Influenza A virus recycling revisited. Bull World Health Organ. 1999;77:820–8. [PMC free article] [PubMed] [Google Scholar]
- Drake JW, Charlesworth B, Charlesworth D, et al. Rates of spontaneous mutation. Genetics. 1998;148:1667–86. doi: 10.1093/genetics/148.4.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen MB, Sabesan S, Skehel JJ, et al. Binding of the influenza A virus to cell-surface receptors: structures of five hemagglutinin-sialyloligosaccharide complexes determined by X-ray crystallography. Virology. 1997;232:19–31. doi: 10.1006/viro.1997.8526. [DOI] [PubMed] [Google Scholar]
- Fauquet CM, Mayo MA, Maniloff J, et al., editors. Eight report of the International Committee on Taxonomy of Viruses. San Diego, Wien, New York: Academic Press, Elsevier; 2004. [Google Scholar]
- Fleming DM, Watson JM, Nicholas S, et al. Study of the effectiveness of influenza vaccination in the elderly in the epidemic of 1989–90 using a general practice database. Epidemiol Infect. 1995;115:581–9. doi: 10.1017/s095026880005874x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming DM, Zambon M, Bartelds AI. Population estimates of persons presenting to general practitioners with influenza-like illness, 1987–96: a study of the demography of influenza-like illness in sentinel practice networks in England and Wales, and in The Netherlands. Epidemiol Infect. 2000;124:245–53. doi: 10.1017/s0950268899003660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis T, Jr, Davenport FM, Hennessy AV. A serological recapitulation of human infection with different strains of influenza virus. Trans Assoc Am Physicians. 1953;66:231–9. [PubMed] [Google Scholar]
- Furminger IGS. Vaccine production. In: Nicholson KG, Webster RG, Hay AJ, editors. Textbook of Influenza. Oxford: Blackwell Science; 1998. pp. 324–32. [Google Scholar]
- Goldstein MA, Tauraso NM. Effect of formalin, beta-propiolactone, merthiolate, and ultraviolet light upon influenza virus infectivity chicken cell agglutination, hemagglutination, and antigenicity. Appl Microbiol. 1970;19:290–4. doi: 10.1128/am.19.2.290-294.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorse GJ, O’Connor TZ, Newman FK, et al. Immunity to influenza in older adults with chronic obstructive pulmonary disease. J Infect Dis. 2004;190:11–19. doi: 10.1086/421121. [DOI] [PubMed] [Google Scholar]
- Gorse GJ, O’Connor TZ, Young SL, et al. Efficacy trial of live, cold-adapted and inactivated influenza virus vaccines in older adults with chronic obstructive pulmonary disease: a VA cooperative study. Vaccine. 2003;21:2133–44. doi: 10.1016/s0264-410x(02)00748-x. [DOI] [PubMed] [Google Scholar]
- Goto H, Kawaoka Y. A novel mechanism for the acquisition of virulence by a human influenza A virus. Proc Natl Acad Sci USA. 1998;95:10224–8. doi: 10.1073/pnas.95.17.10224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govaert TM, Sprenger MJ, Dinant GJ, et al. Immune response to influenza vaccination of elderly people. A randomized double-blind placebo-controlled trial. Vaccine. 1994;12:1185–9. doi: 10.1016/0264-410x(94)90241-0. [DOI] [PubMed] [Google Scholar]
- Govaert TM, Thijs CT, Masurel N, et al. The efficacy of influenza vaccination in elderly individuals. A randomized double-blind placebo-controlled trial. Jama. 1994;272:1661–5. [PubMed] [Google Scholar]
- Gross PA, Ennis FA, Gaerlan PF, et al. A controlled double-blind comparison of reactogenicity, immunogenicity, and protective efficacy of whole-virus and split-product influenza vaccines in children. J Infect Dis. 1977;136:623–32. doi: 10.1093/infdis/136.5.623. [DOI] [PubMed] [Google Scholar]
- Gross PA, Hermogenes AW, Sacks HS, et al. The efficacy of influenza vaccine in elderly persons. A meta-analysis and review of the literature. Ann Intern Med. 1995;123:518–27. doi: 10.7326/0003-4819-123-7-199510010-00008. [DOI] [PubMed] [Google Scholar]
- Gross PA, Quinnan GV, Jr, Weksler ME, et al. Relation of chronic disease and immune response to influenza vaccine in the elderly. Vaccine. 1989;7:303–8. doi: 10.1016/0264-410x(89)90190-4. [DOI] [PubMed] [Google Scholar]
- Gross PA, Weksler ME, Quinnan GV, Jr, et al. Immunization of elderly people with two doses of influenza vaccine. J Clin Microbiol. 1987;25:1763–5. doi: 10.1128/jcm.25.9.1763-1765.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guthrie T, Hobbs CG, Davenport V, et al. Parenteral influenza vaccination influences mucosal and systemic T cell-mediated immunity in healthy adults. J Infect Dis. 2004;190:1927–35. doi: 10.1086/425517. [DOI] [PubMed] [Google Scholar]
- Hannoun C, Megas F, Piercy J. Immunogenicity and protective efficacy of influenza vaccination. Virus Res. 2004;103:133–8. doi: 10.1016/j.virusres.2004.02.025. [DOI] [PubMed] [Google Scholar]
- Harper SA, Fukuda K, Uyeki TM, et al. Prevention and control of influenza. Recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2005;54(RR-8):1–40. [PubMed] [Google Scholar]
- Hehme N, Engelmann H, Kuenzel W, et al. Immunogenicity of a monovalent, aluminum-adjuvanted influenza whole virus vaccine for pandemic use. Virus Res. 2004;103:163–71. doi: 10.1016/j.virusres.2004.02.029. [DOI] [PubMed] [Google Scholar]
- Hehme N, Engelmann H, Kunzel W, et al. Pandemic preparedness: lessons learnt from H2N2 and H9N2 candidate vaccines. Med Microbiol Immunol(Berl) 2002;191:203–8. doi: 10.1007/s00430-002-0147-9. [DOI] [PubMed] [Google Scholar]
- Hinshaw VS, Bean WJ, Webster RG, et al. Genetic reassortment of influenza A viruses in the intestinal tract of ducks. Virology. 1980;102:412–9. doi: 10.1016/0042-6822(80)90108-7. [DOI] [PubMed] [Google Scholar]
- Hobson D, Curry RL, Beare AS, et al. The role of serum haemagglutination-inhibiting antibody in protection against challenge infection with influenza A2 and B viruses. J Hyg (Lond) 1972;70:767–77. doi: 10.1017/s0022172400022610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzman MJ, Tyner JW, Kim EY, et al. Acute and chronic airway responses to viral infection: implications for asthma and chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2005;2:132–40. doi: 10.1513/pats.200502-015AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howells CH, Tyler LE. Prophylactic use of influenza vaccine in patients with chronic bronchitis. A pilot trial. Lancet. 1961;2:1428–32. doi: 10.1016/s0140-6736(61)91248-x. [DOI] [PubMed] [Google Scholar]
- Huckriede A, Bungener L, Stegmann T, et al. The virosome concept for influenza vaccines. Vaccine. 2005;23(Suppl 1):S26–38. doi: 10.1016/j.vaccine.2005.04.026. [DOI] [PubMed] [Google Scholar]
- Hui EK, Barman S, Yang TY, et al. Basic residues of the helix six domain of influenza virus M1 involved in nuclear translocation of M1 can be replaced by PTAP and YPDL late assembly domain motifs. J Virol. 2003;77:7078–92. doi: 10.1128/JVI.77.12.7078-7092.2003. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Haaheim LR. Original antigenic sin. A confounding issue? Dev Biol (Basel) 2003;115:49–53. [PubMed] [Google Scholar]
- Jefferson T, Rivetti D, Rivetti A, et al. Efficacy and effectiveness of influenza vaccines in elderly people: a systematic review. Lancet. 2005;366:1165–74. doi: 10.1016/S0140-6736(05)67339-4. [DOI] [PubMed] [Google Scholar]
- Johnson MK, Stevenson RD. Management of an acute exacerbation of copd: are we ignoring the evidence? Thorax. 2002;57(Suppl 2):II15–II23. [PMC free article] [PubMed] [Google Scholar]
- Kawai N, Ikematsu H, Iwaki N, et al. A prospective, Internet-based study of the effectiveness and safety of influenza vaccination in the 2001–2002 influenza season. Vaccine. 2003;21:4507–13. doi: 10.1016/s0264-410x(03)00508-5. [DOI] [PubMed] [Google Scholar]
- Keitel WA, Cate TR, Couch RB, et al. Efficacy of repeated annual immunization with inactivated influenza virus vaccines over a five year period. Vaccine. 1997;15:1114–22. doi: 10.1016/s0264-410x(97)00003-0. [DOI] [PubMed] [Google Scholar]
- Kendal AP, Pereira MS, Skehel J.1982Concepts and procedures for laboratory-based influenza surveillancePublication no. B17–35.Centers for Disease Control; Atlanta, Ga [Google Scholar]
- Kilbourne ED. Future influenza vaccines and the use of genetic recombinants. Bull World Health Organ. 1969;41:643–5. [PMC free article] [PubMed] [Google Scholar]
- Lamb RA, Krug RM. Ortomyxoviridae: the viruses and their replication. In: Fields BN, Knipe DM, Howley PM, editors. Fields Virology. 3rd edition. Phiadelphia: Lippincott-Raven Publishers; 1996. pp. 1353–95. [Google Scholar]
- McElhaney JE. The unmet need in the elderly: designing new influenza vaccines for older adults. Vaccine. 2005;23(Suppl 1):S10–25. doi: 10.1016/j.vaccine.2005.04.019. [DOI] [PubMed] [Google Scholar]
- McElhaney JE, Hooton JW, Hooton N, et al. Comparison of single versus booster dose of influenza vaccination on humoral and cellular immune responses in older adults. Vaccine. 2005;23:3294–300. doi: 10.1016/j.vaccine.2005.01.080. [DOI] [PubMed] [Google Scholar]
- McElhaney JE, Meneilly GS, Lechelt KE, et al. Antibody response to whole-virus and split-virus influenza vaccines in successful ageing. Vaccine. 1993;11:1055–60. doi: 10.1016/0264-410x(93)90133-i. [DOI] [PubMed] [Google Scholar]
- Monto AS, Koopman JS, Longini IM., Jr Tecumseh study of illness. XIII. Influenza infection and disease, 1976–1981. Am J Epidemiol. 1985;121:811–22. doi: 10.1093/oxfordjournals.aje.a114052. [DOI] [PubMed] [Google Scholar]
- Murphy BR. Mucosal immunity to viruses. In: Mestecky J, Lamm ME, McGhee JR, et al., editors. Mucosal Immunology. London: Elsevier Academic Press; 2005. pp. 799–813. [Google Scholar]
- Murphy BR, Chalhub EG, Nusinoff SR, et al. Temperature-sensitive mutants of influenza virus. 3. Further characterization of the ts-1(E) influenza A recombinant (H3N2) virus in man. J Infect Dis. 1973;128:479–87. doi: 10.1093/infdis/128.4.479. [DOI] [PubMed] [Google Scholar]
- Murphy BR, Webster RG. Orthomyxoviruses. In: Fields BN, Knipe DM, Howley PM, editors. Fields Virology. 3rd edition. Philadelphia: Lippincott-Raven Publishers; 1996. pp. 1397–445. [Google Scholar]
- Nguyen-Van-Tam JS. Epidemiology of influenza. In: Nicholson KG, Webster RG, Hay AJ, editors. Textbook of Influenza. Oxford: Blackwell Science; 1998. pp. 181–206. [Google Scholar]
- Nichol KL. Complications of influenza and benefits of vaccination. Vaccine. 1999;17(Suppl 1):S47–52. doi: 10.1016/s0264-410x(99)00105-x. [DOI] [PubMed] [Google Scholar]
- Nichol KL, Baken L, Nelson A. Relation between influenza vaccination and outpatient visits, hospitalization, and mortality in elderly persons with chronic lung disease. Ann Intern Med. 1999;130:397–403. doi: 10.7326/0003-4819-130-5-199903020-00003. [DOI] [PubMed] [Google Scholar]
- Nichol KL, Lind A, Margolis KL, et al. The effectiveness of vaccination against influenza in healthy, working adults. N Engl J Med. 1995;333(14):889–93. doi: 10.1056/NEJM199510053331401. [DOI] [PubMed] [Google Scholar]
- Nichol KL, Margolis KL, Wouremna J, et al. Effectiveness of influenza vaccine in the elderly. Gerontology. 1996;42:274–9. doi: 10.1159/000213803. [DOI] [PubMed] [Google Scholar]
- Nichol KL, Nordin J, Mullooly J. Influence of clinical outcome and outcome period definitions on estimates of absolute clinical and economic benefits of influenza vaccination in community dwelling elderly persons. Vaccine. 2005 doi: 10.1016/j.vaccine.2005.10.004. [DOI] [PubMed] [Google Scholar]
- Nicholson KG. Human influenza. In: Nicholson KG, Webster RG, Hay AJ, editors. Textbook of Influenza. Oxford: Blackwell Science; 1998. pp. 219–64. [Google Scholar]
- Niewoehner DE. The impact of severe exacerbations on quality of life and the clinical course of chronic obstructive pulmonary disease. Am J Med. 2006;119(10 Suppl 1):38–45. doi: 10.1016/j.amjmed.2006.08.006. [DOI] [PubMed] [Google Scholar]
- Oxford JS, Bossuyt S, Balasingam S, et al. Treatment of epidemic and pandemic influenza with neuraminidase and M2 proton channel inhibitors. Clin Microbiol Infect. 2003;9:1–14. doi: 10.1046/j.1469-0691.2003.00564.x. [DOI] [PubMed] [Google Scholar]
- Palache AM. Influenza vaccines. A reappraisal of their use. Drugs. 1997;54:841–56. doi: 10.2165/00003495-199754060-00004. [DOI] [PubMed] [Google Scholar]
- Palache AM, Beyer WE, Sprenger MJ, et al. Antibody response after influenza immunization with various vaccine doses: a double-blind, placebo-controlled, multi-centre, dose-response study in elderly nursing-home residents and young volunteers. Vaccine. 1993;11:3–9. doi: 10.1016/0264-410x(93)90333-s. [DOI] [PubMed] [Google Scholar]
- Palache AM, van der Velden JW. Influenza vaccination in asthma. Lancet. 1992;339:741. doi: 10.1016/0140-6736(92)90637-i. [DOI] [PubMed] [Google Scholar]
- Palese P. Influenza: old and new threats. Nat Med. 2004;10(12 Suppl):S82–7. doi: 10.1038/nm1141. [DOI] [PubMed] [Google Scholar]
- Palladino G, Mozdzanowska K, Washko G, et al. Virus-neutralizing antibodies of immunoglobulin G (IgG) but not of IgM or IgA isotypes can cure influenza virus pneumonia in SCID mice. J Virol. 1995;69:2075–81. doi: 10.1128/jvi.69.4.2075-2081.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park CL, Frank AL, Sullivan M, et al. 1996Influenza vaccination of children during acute asthma exacerbation and concurrent prednisone therapy Pediatrics 98(2 Pt 1)196–200. [PubMed] [Google Scholar]
- Perrotta DM, Decker M, Glezen WP. Acute respiratory disease hospitalizations as a measure of impact of epidemic influenza. Am J Epidemiol. 1985;122:468–76. doi: 10.1093/oxfordjournals.aje.a114128. [DOI] [PubMed] [Google Scholar]
- Piedra PA, Gaglani MJ, Kozinetz CA, et al. Herd immunity in adults against influenza-related illnesses with use of the trivalent-live attenuated influenza vaccine (CAIV-T) in children. Vaccine. 2005;23:1540–8. doi: 10.1016/j.vaccine.2004.09.025. [DOI] [PubMed] [Google Scholar]
- Poole P, Chacko E, Wood-Baker R, et al. 2000Influenza vaccine for patients with chronic obstructive pulmonary diseaseThe cochrane database of systematic reviews (3). [DOI] [PubMed] [Google Scholar]
- Portela A, Digard P.2002The influenza virus nucleoprotein: a multifunctional RNA-binding protein pivotal to virus replication J Gen Virol 83(Pt 4)723–34. [DOI] [PubMed] [Google Scholar]
- Potter CW. Chronicle of Influenza Pandemics. In: Nicholson KG, Webster RG, Hay AJ, editors. Textbook of Influenza. Oxford: Blackwell Science; 1998. pp. 3–18. [Google Scholar]
- Potter CW, Jennings R, McLaren C, et al. A new surface-antigen-adsorbed influenza virus vaccine. II. Studies in a volunteer group. J Hyg(Lond) 1975;75:353–62. doi: 10.1017/s0022172400024414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter J, Stott DJ, Roberts MA, et al. Influenza vaccination of health care workers in long-term-care hospitals reduces the mortality of elderly patients. J Infect Dis. 1997;175:1–6. doi: 10.1093/infdis/175.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyhälä R, Haanpaa M, Kleemola M, et al. Acceptable protective efficacy of influenza vaccination in young military conscripts under circumstances of incomplete antigenic and genetic match. Vaccine. 2001;19:3253–60. doi: 10.1016/s0264-410x(01)00010-x. [DOI] [PubMed] [Google Scholar]
- Reid AH, Taubenberger JK, Fanning TG. The 1918 Spanish influenza: integrating history and biology. Microbes Infect. 2001;3:81–7. doi: 10.1016/s1286-4579(00)01351-4. [DOI] [PubMed] [Google Scholar]
- Ritzwoller DP, Bridges CB, Shetterly S, et al. Effectiveness of the 2003–2004 influenza vaccine among children 6 months to 8 years of age, with 1 vs 2 doses. Pediatrics. 2005;116:153–9. doi: 10.1542/peds.2005-0049. [DOI] [PubMed] [Google Scholar]
- Ruben FL, Akers LW, Stanley ED, et al. Protection with split and whole virus vaccines against influenza. Arch Intern Med. 1973;132:568–71. [PubMed] [Google Scholar]
- Sambhara S, Kurichh A, Miranda R, et al. Heterosubtypic immunity against human influenza A viruses, including recently emerged avian H5 and H9 viruses, induced by FLU-ISCOM vaccine in mice requires both cytotoxic T-lymphocyte and macrophage function. Cell Immunol. 2001;211:143–53. doi: 10.1006/cimm.2001.1835. [DOI] [PubMed] [Google Scholar]
- Sapey E, Stockley RA. COPD exacerbations. 2: aetiology. Thorax. 2006;61:250–8. doi: 10.1136/thx.2005.041822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinya K, Ebina M, Yamada S, et al. Avian flu: influenza virus receptors in the human airway. Nature. 2006;440:435–6. doi: 10.1038/440435a. [DOI] [PubMed] [Google Scholar]
- Simonsen L, Viboud C, Taylor R. Influenza vaccination in elderly people. Lancet. 2005;366:2086. doi: 10.1016/S0140-6736(05)67884-1. [DOI] [PubMed] [Google Scholar]
- Smith JWG. Antibody responses and reactogenicity of graded doses of inactivated influenza A/New Jersey/76 whole-virus vaccine in humans. J Infect Dis. 1977;136(Suppl):S475–83. doi: 10.1093/infdis/136.supplement_3.s475. [DOI] [PubMed] [Google Scholar]
- Stephenson I, Nicholson KG, Colegate A, et al. Boosting immunity to influenza H5N1 with MF59-adjuvanted H5N3 A/Duck/Singapore/97 vaccine in a primed human population. Vaccine. 2003;21:1687–93. doi: 10.1016/s0264-410x(02)00632-1. [DOI] [PubMed] [Google Scholar]
- Stephenson I, Nicholson KG, Gluck R, et al. Safety and antigenicity of whole virus and subunit influenza A/Hong Kong/1073/99 (H9N2) vaccine in healthy adults: phase I randomised trial. Lancet. 2003;362:1959–66. doi: 10.1016/S0140-6736(03)15014-3. [DOI] [PubMed] [Google Scholar]
- Stephenson I, Nicholson KG, Wood JM, et al. Confronting the avian influenza threat: vaccine development for a potential pandemic. Lancet Infect Dis. 2004;4:499–509. doi: 10.1016/S1473-3099(04)01105-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson I, Zambon M. The epidemiology of influenza. Occup Med (Lond) 2002;52:241–7. doi: 10.1093/occmed/52.5.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subbarao K, Klimov A, Katz J, et al. Characterization of an avian influenza A (H5N1) virus isolated from a child with a fatal respiratory illness. Science. 1998;279:393–6. doi: 10.1126/science.279.5349.393. [DOI] [PubMed] [Google Scholar]
- Takada A, Matsushita S, Ninomiya A, et al. Intranasal immunization with formalin-inactivated virus vaccine induces a broad spectrum of heterosubtypic immunity against influenza A virus infection in mice. Vaccine. 2003;21:3212–8. doi: 10.1016/s0264-410x(03)00234-2. [DOI] [PubMed] [Google Scholar]
- Tamura S, Kurata T. Defense mechanisms against influenza virus infection in the respiratory tract mucosa. Jpn J Infect Dis. 2004;57:236–47. [PubMed] [Google Scholar]
- Tata LJ, West J, Harrison T, et al. Does influenza vaccination increase consultations, corticosteroid prescriptions, or exacerbations in subjects with asthma or chronic obstructive pulmonary disease? Thorax. 2003;58:835–9. doi: 10.1136/thorax.58.10.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taubenberger JK, Reid AH, Lourens RM, et al. Characterization of the 1918 influenza virus polymerase genes. Nature. 2005;437:889–93. doi: 10.1038/nature04230. [DOI] [PubMed] [Google Scholar]
- Thursky K, Cordova SP, Smith D, et al. Working towards a simple case definition for influenza surveillance. J Clin Virol. 2003;27:170–9. doi: 10.1016/s1386-6532(02)00172-5. [DOI] [PubMed] [Google Scholar]
- Turner DA, Wailoo AJ, Cooper NJ, et al. The cost-effectiveness of influenza vaccination of healthy adults 50–64 years of age. Vaccine. 2005 doi: 10.1016/j.vaccine.2004.12.033. [DOI] [PubMed] [Google Scholar]
- Vilkman S, Keistinen T, Tuuponen T, et al. Age distribution of patients treated in hospital for chronic obstructive pulmonary disease. Age Ageing. 1996;25:109–12. doi: 10.1093/ageing/25.2.109. [DOI] [PubMed] [Google Scholar]
- Vu T, Farish S, Jenkins M, et al. A meta-analysis of effectiveness of influenza vaccine in persons aged 65 years and over living in the community. Vaccine. 2002;20:1831–6. doi: 10.1016/s0264-410x(02)00041-5. [DOI] [PubMed] [Google Scholar]
- Wang CS, Wang ST, Lai CT, et al. Impact of influenza vaccination on major cause-specific mortality. Vaccine. 2007;25:1196–203. doi: 10.1016/j.vaccine.2006.10.015. [DOI] [PubMed] [Google Scholar]
- Webster RG, Bean WJ. Evolution and ecology of influenza viruses: interspecies transmission. In: Nicholson KG, Webster RG, Hay AJ, editors. Textbook of influenza. Oxford: Blackwell Science; 1998. pp. 109–19. [Google Scholar]
- Webster RG, Bean WJ, Gorman OT, et al. Evolution and ecology of influenza A viruses. Microbiol Rev. 1992;56:152–79. doi: 10.1128/mr.56.1.152-179.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO 1999WHO recommended surveillance standards Geneva: WHO/CDS/CSR/ISR/99.2, WHO; 1–116. [Google Scholar]
- WHO Influenza vaccines. Recommendations for the use of inactivated influenza vaccines and other preventive measures. Wkly Epidemiol Rec. 2000;75:281–88. [Google Scholar]
- WHO Draft WHO guidelines on the use of vaccines and antivirals during influenza pandemics. Wkly Epidemiol Rec. 2002;77:394–404. [PubMed] [Google Scholar]
- WHO 2005. Influenza pandemic preparedness and response. Geneva, Executive Board rapport - EB115/44.
- WHO Influenza vaccines -WHO position paper. Wkly Epidemiol Rec. 2005;80:279–87. [Google Scholar]
- Wilde JA, McMillan JA, Serwint J, et al. Effectiveness of influenza vaccine in health care professionals: a randomized trial. Jama. 1999;281:908–13. doi: 10.1001/jama.281.10.908. [DOI] [PubMed] [Google Scholar]
- Williams WW, Hickson MA, Kane MA, et al. Immunization policies and vaccine coverage among adults. The risk for missed opportunities. Ann Intern Med. 1988;108:616–25. doi: 10.7326/0003-4819-108-4-616. [DOI] [PubMed] [Google Scholar]
- Wiselka MJ. Vaccine safety. In: Nicholson KG, Webster RG, Hay AJ, editors. Textbook of Influenza. Oxford: Blackwell Science; 1998. pp. 346–57. [Google Scholar]
- Wongsurakiat P, Maranetra KN, Gulprasutdilog P, et al. Adverse effects associated with influenza vaccination in patients with COPD: a randomized controlled study. Respirology. 2004;9:550–6. doi: 10.1111/j.1440-1843.2004.00616.x. [DOI] [PubMed] [Google Scholar]
- Wongsurakiat P, Maranetra KN, Wasi C, et al. Acute respiratory illness in patients with COPD and the effectiveness of influenza vaccination: a randomized controlled study. Chest. 2004;125:2011–20. doi: 10.1378/chest.125.6.2011. [DOI] [PubMed] [Google Scholar]
- Wood JM. Developing vaccines against pandemic influenza. Philos Trans R Soc Lond B Biol Sci. 2001;356:1953–60. doi: 10.1098/rstb.2001.0981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita M, Krystal M, Fitch WM, et al. Influenza B virus evolution: co-circulating lineages and comparison of evolutionary pattern with those of influenza A and C viruses. Virology. 1988;163:112–22. doi: 10.1016/0042-6822(88)90238-3. [DOI] [PubMed] [Google Scholar]
- Yap FH, Ho PL, Lam KF, et al. Excess hospital admissions for pneumonia, chronic obstructive pulmonary disease, and heart failure during influenza seasons in Hong Kong. J Med Virol. 2004;73:617–23. doi: 10.1002/jmv.20135. [DOI] [PubMed] [Google Scholar]
- Yewdell J, Garcia-Sastre A. Influenza virus still surprises. Curr Opin Microbiol. 2002;5:414–18. doi: 10.1016/s1369-5274(02)00346-6. [DOI] [PubMed] [Google Scholar]