Abstract
Hyaluronan (HA), a long-chain polysaccharide, is currently being evaluated as a potential therapeutic agent for a number of inflammatory disorders. The effect of HA on inflammation appears to be related to its molecular size, with larger polysaccharide chains having anti-inflammatory activity and smaller ones having proinflammatory properties. This dichotomous behavior is particularly relevant to the work of our laboratory on an aerosolized preparation of HA to treat pulmonary emphysema. The breakdown of inhaled HA into smaller fragments could possibly induce an inflammatory reaction in the lung that counteracts any beneficial effect. Consequently, the proposed therapeutic use of HA will require development of treatment strategies aimed at minimizing its proinflammatory activity.
Keywords: hyaluronan, emphysema, asthma, elastin, elastase, endotoxin
Introduction
In terms of understanding the pathogenesis of pulmonary emphysema, the use of papain to experimentally induce the disease represented an initial breakthrough (Gross et al 1964). Originally intended as a possible treatment for interstitial pulmonary fibrosis, intratracheal instillment of papain instead produced emphysematous changes in the lung. This finding had added significance because it came at a time when the relationship between alpha-1-antiproteinase deficiency and pulmonary emphysema was just being recognized (Laurell and Eriksson 1963). Both observations emphasized the importance of proteolysis as a cause of the disease. An imbalance between lung proteases and their inhibitors was hypothesized to be responsible for the airspace enlargement that characterizes pulmonary emphysema (Janoff 1985; Senior and Kuhn 1988). It was proposed that excess elastase activity caused damage to the elastic fiber network of the lung, leading to dilatation and rupture of alveoli, reduced gas-exchange, and eventual respiratory failure.
The proteinase-antiproteinase concept of pulmonary emphysema served to focus research on the role of elastases with the hope that inhibiting the activity of these enzymes would prevent lung injury. This treatment strategy assumed, however, that emphysema was caused by a single abnormality; namely, excess elastase activity. If the disease represented a more general response of the lung to a variety of insults, then enzyme inhibition might have only limited efficacy.
With this in mind, our laboratory has studied the possible use of aerosolized hyaluronan (HA) to directly protect lung elastic fibers from injury (Cantor et al 1998; Cantor et al 2000, 2005). HA, a long-chain polysaccharide, has been shown to preferentially bind to elastic fibers, prevent elastolysis, and limit air-space enlargement in experimental models of emphysema induced by either porcine pancreatic elastase, human neutrophil elastase, or chronic exposure to cigarette smoke (Figure 1) (Cantor et al 1998, 2000, 2005). Since elastic fiber breakdown may be a final common pathway in pulmonary emphysema, this form of treatment might be effective against a number of agents capable of causing the disease, including various oxidants present in air pollutants and cigarette smoke.
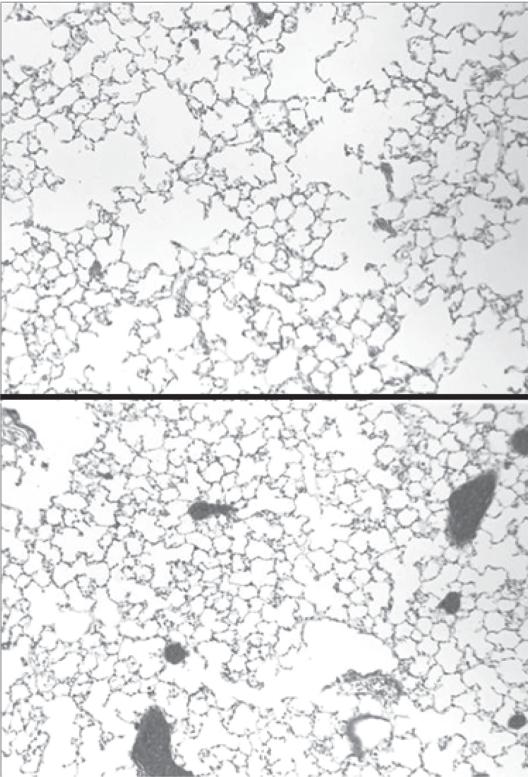
Figure 1.
(Upper) Mouse lung exposed to cigarette smoke for 3 months shows significant airspace enlargement. (Lower) Mouse lung treated with aerosolized HA during the same 3-month period of smoke exposure shows only minimal airspace enlargement.
Special properties of HA
As a constituent of the extracellular matrix, HA acts to stabilize proteoglycans and also contributes to tissue growth and repair (Toole 1981; Heinegard and Paulsson 1984). In particular, HA has been shown to be a critical component in amphibian limb regeneration, strongly influencing the initial stages of the process (Toole and Gross 1971). One of the main functions of HA may be to reduce cellular cohesion, thereby facilitating the restructuring of tissues (Toole and Gross 1971).
Production of HA is greatly elevated following experimental induction of both emphysema and interstitial fibrosis (Karlinsky 1982; LaFuma et al 1985; Bray et al 1991). The increases observed in both diseases occur shortly after initiation of lung injury. In the case of interstitial fibrosis, damage to alveolar lining cells results in marked epithelial (type II cell) hyperplasia and subsequent lung remodeling, both of which are associated with HA synthesis (Cantor et al 1989).
Since cell proliferation is not characteristic of pulmonary emphysema, the role of HA in this disease may depend more upon its interactions with other matrix components than its capacity to enhance tissue growth. Several studies suggest that HA and other glycosaminoglycans form a network which surrounds elastic fibers (Baccarani-Contri et al 1990; Bray et al 1994). Thus, the increased HA synthesis following emphysematous injury could prevent further damage to elastic fibers and perhaps facilitate their repair.
More recently, HA has been found to play an important role in inflammation. The inflammatory activity of HA is related to its molecular weight, which can vary in tissues from thousands to millions of daltons. Low molecular weight fragments accumulate at sites of inflammation, and are known to stimulate the production of a variety of cytokines (McKee et al 1996; Horton et al 1999; Mascarenhas et al 2004). Conversely, high molecular weight HA fragments possess anti-inflammatory properties, and may also promote repair of tissues (Moreland 2003; Noble and Jiang 2006).
The proinflammatory potential of HA may be related to its affinity for the CD44 receptor, an 85 kDa cell surface glycoprotein that is expressed by a variety of cell types. Interactions between endothelial cell HA and leukocyte CD44 receptors facilitate the extravasation of inflammatory cells at sites of injury (Calkins et al 2001; Wang et al 2002; Kipnis et al 2003; Khan et al 2004). Furthermore, the binding of HA to macrophage CD44 receptors induces transcription of proinflammatory genes (McKee et al 1996).
The anti-inflammatory activity of HA may be equally potent, but is less well understood. The mechanisms responsible for the mitigation of inflammation by HA may depend not only on direct interaction with inflammatory cells, but on the physical properties of the molecule itself. By virtue of its strongly anionic nature (due to negatively charged carboxyl groups), HA can retain relatively large amounts of water.
The ability of HA to expand its domain in a fluid environment may have a number of consequences for tissue reactions. By creating a more viscous milieu, HA may impede the movement of cells and molecules through the extracellular matrix, thereby reducing the activity of leukocytes and their proinflammatory products. The consequences of this “physical barrier” effect of HA have been explored in our laboratory, where introduction of exogenous HA into the lung has been shown to slow the progression of lung injury in several animal models of pulmonary emphysema.
Aerosolized HA in experimental lung injury
Pulmonary emphysema
The conceptual basis for using nebulized HA to treat pulmonary emphysema developed from a series of animal experiments designed to determine whether agents other than elastases were capable of inducing or enhancing pulmonary emphysema. A nonelastolytic enzyme, hyaluronidase, was shown to produce pulmonary airspace enlargement in hamsters when administered in conjunction with 60 percent oxygen (Cantor et al 1993). Damage to elastic fibers occurred only when both agents were given concomitantly, suggesting the possibility that hyaluronidase may facilitate the breakdown of these fibers by making them more accessible to injury. This hypothesis was also tested in studies demonstrating that hyaluronidase enhances airspace enlargement in a well-established model of emphysema induced by intratracheal administration of elastase (Cantor et al 1995).
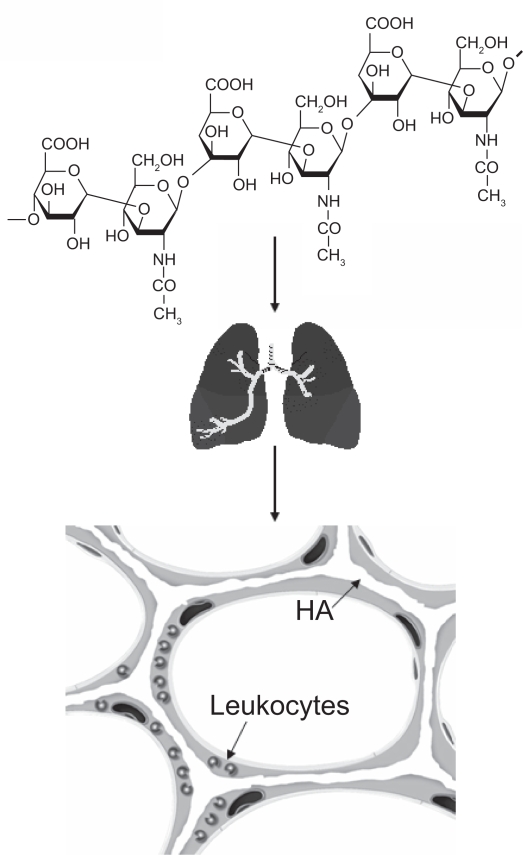
Experiments were then undertaken to examine the effect of low molecular weight (150 kDa) HA itself on this model. Animals treated with aerosolized HA prior to instillation of elastase showed significantly less airspace enlargement than controls treated with elastase alone (Cantor et al 1998, 2000). The exogenous HA preferentially adhered to elastic fibers, suggesting that it may protect these fibers from enzymatic breakdown (Figure 2) (Cantor et al 1998, 2000).
Figure 2.
HA is a long-chain polysaccharide composed of repeating disaccharide units of glucuronic acid and n-acetylglucosamine. When administered intratracheally, it binds to alveolar septal elastic fibers and may prevent their degradation by elastases released from macrophages and neutrophils.
To determine if HA could actually prevent elastolysis, radiolabeled elastic fibers, derived from rat pleural mesothelial cells, were used as a test substrate (Cantor et al 2000). Coating the fibers with HA significantly decreased elastolysis induced by several different types of elastase, including human neutrophil elastase and human metalloproteinase.
In addition to protecting elastic fibers from enzymatic breakdown, exogenously administered HA may also prevent air-space enlargement by increasing the water content of the extracellular matrix. Since the distensibility of elastic fibers is dependent on interactions with water molecules, addition of HA may improve the mechanical properties of these fibers, thereby preventing alveolar dilatation and rupture (Wasserman and Salemme 1990; Mariani et al 1997; Li et al 2001; Cantor et al 2004).
Airway hyperreactivity
While our laboratory has concentrated on the use of HA to treat emphysematous lung injury, other investigators have demonstrated a beneficial effect of this agent in animal models of airway hyperreactivity. When aerosolized HA was given to sheep, it significantly reduced bonchoconstriction due to inhalation of either neutrophil or pancreatic elastase (Scuri et al 2001; Scuri and Abraham 2003). In both cases, increasing the molecular weight of HA enhanced its effectiveness.
The mechanism of action of HA in preventing bronchoconstriction may involve an affinity for tissue kallikrein, which could reduce kinin-mediated inflammatory reactions in airway walls (Forteza et al 1999). The increased ability of higher molecular weight HA to prevent bronchoconstriction might therefore reflect a greater capacity for binding kallikrein.
More recently, aerosolized HA was shown to prevent exercise-induced bronchoconstriction in asthmatic patients (Petrigni and Allegra 2006). Here again, the molecular weight of HA may play a critical role, as evidenced by the fact that another study using smaller-sized HA showed no protective effect (Kunz et al 2006).
Acute lung injury
Although previous studies from this laboratory indicated that administration of our preparation of HA was not associated with pulmonary inflammation, other investigators have found that low-molecular weight HA may be proinflammatory (McKee et al 1996; Horton et al 1999; Mascarenhas 2004). This prompted us to determine if aerosolized HA could possibly enhance inflammation in a more fulminant type of lung injury.
Using an experimental model involving intratracheal instillation of endotoxin (Yasui et al 1995), we tested the effects of HA on the acute inflammatory response (Nadkarni et al 2005). Animals were given aerosolized HA either immediately before or after administration of endotoxin, and studied over the next 24-hours. The results indicate that treatment with HA following endotoxin instillation enhanced lung inflammation whereas pretreatment had the opposite effect.
This dichotomous response was reflected by measurement of several parameters of lung injury, including histogical evidence of inflammation, bronchoalveolar (BAL) cell content, TNFR1 expression on BAL macrophages, and lung apoptosis. Administration of aerosolized HA immediately before instillation of endotoxin significantly reduced all of these inflammatory markers compared to controls treated with endotoxin alone. Conversely, treatment with HA following endotoxin instillation significantly increased all of the markers compared to endotoxin administration alone.
The proinflammatory effects of HA following endotoxin instillation may possibly involve alterations in the rate of leukocyte migration into the lung. By binding to CD44 receptors on the surface of neutrophils, the exogenous HA may reduce the interaction of these cells with the surrounding extracellular matrix and accelerate their influx into airspaces (Calkins et al 2001; Wang et al 2002; Kipnis et al 2003; Khan et al 2004). Furthermore, increased expression of TNFR1 on macrophages may enhance TNF-alpha-induced secretion of enzymes that degrade HA into smaller fragments, thereby triggering the release of cytokines that amplify lung injury (McKee et al 1996). Enhanced expression of TNFR1 may also account for the increased apoptosis seen in the lungs of animals post-treated with HA (Alikhani et al 2004).
In contrast, pre-exposure to HA may decrease endotoxin-induced inflammation by limiting cell-cytokine interactions. For example, HA could bind to macrophage CD44 receptors, coat the cell surface, and block the attachment of TNF-alpha, thus preventing activation of various proinflammatory genes (Figure 3).
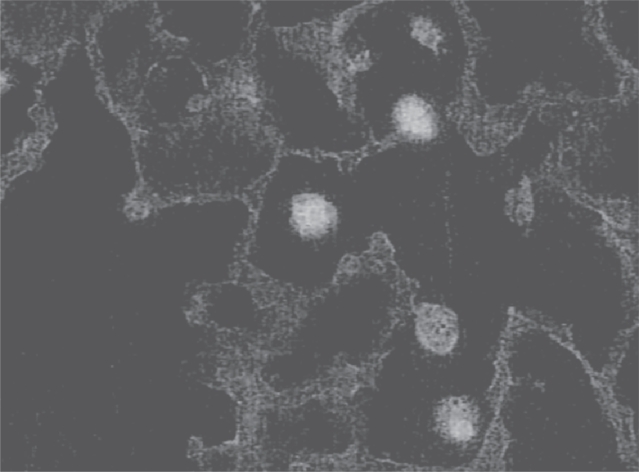
Figure 3.
Photomicrograph showing attachment of fluorescein-labeled HA to alveolar macrophages. By coating the cell surface, HA could block interactions with various cytokines.
Although the HA used in our experiments had a relatively low molecular weight, it nevertheless exhibited antiinflammatory activity when administered prior to endotoxin. This could possibly be explained by the self-aggregating properties of this molecule (Scott et al 1991). Aerosolized HA deposited in the lung interstitium prior to endotoxin instillation may form larger complexes that exhibit the same anti-inflammatory activity as high-molecular weight HA. When administered after endotoxin administration, however, HA might immediately be broken down and never form such complexes.
Maximizing the therapeutic potential of HA
The results of the experiments with endotoxin indicate that HA may be a double-edged sword with regard to its therapeutic efficacy. In the absence of underlying inflammation, HA may exert a beneficial effect, as both an anti-inflammatory agent and a protective barrier against the degradative activities of various inflammatory products. Conversely, the presence of a pre-existing inflammatory milieu has the potential to convert HA into a proinflammatory mediator and thereby counteract its therapeutic activity.
Further clinical testing of aerosolized HA should therefore involve frequent measurement of inflammatory markers. These might include differential cell counts in bronchoalveolar lavage fluid, sputum and plasma levels of proinflammatory cytokines, and markers of proteinase activity, such as plasma fibronogen fragments (Stolk et al 2005). The results of these studies could then be used to determine a dosing schedule for HA that minimizes the risk of an inflammatory response.
Since HA can break down into smaller, proinflammatory fragments, increasing its initial molecular weight might also reduce the possibility of further lung injury. The use of 500 kDa HA, instead of a 150 kDa preparation, could limit the formation of proinflammatory, low-molecular weight fragments.
However, any increase in molecular weight would have to be weighed against possible changes in therapeutic efficacy. Large molecules such as HA do not readily penetrate the alveolar epithelial barrier. Consequently, the dosage size and frequency of administration of HA will need to be carefully titrated to prevent its accumulation in alveolar spaces, where it can be broken down into smaller fragments. Measuring the clearance rate of HA from the lung could help determine the optimum dosing schedule.
The physical form in which HA is administered might also affect its therapeutic efficacy. The use of a dry powder instead of a solution, or addition of a carrier molecule, might limit the breakdown of HA in the lung. Alternatively, it may be possible to modify the molecular structure of HA to reduce its proinflammatory potential while mainintaining its protective effects.
Conclusion
The use of nebulized HA as a potential treatment for pulmonary emphysema represents a different approach to treating disease – one that is directed toward the extracellular matrix rather than intracellular processes. The proposed mechanisms of action of HA discussed in this paper may have broad applicability to a number of other conditions involving injury to extracellular matrix components, including osteoarthritis (which is already being treated with exogenous HA), vascular aneurysms, and skin aging (Moreland 2003; Gandhi et al 1994; Fisher et al 1997). However, exploiting the therapeutic potential of HA will require a greater understanding of the complex relationship between this molecule and the inflammatory process. Such insight may not only advance the use of HA as a treatment for a variety of diseases, but also facilitate the development of a whole new class of agents designed to protect the extracellular matrix against the damaging effects of inflammation.
Acknowledgments
The work of the author was supported in part by grants from the US National Institutes of Health (NHLBI HL68383) and the Alpha-1 Foundation.
References
- Alikhani M, Alikhani Z, Graves DT. Apoptotic effects of LPS on fibroblasts are indirectly mediated through TNFR1. J Dent Res. 2004;83:671–6. doi: 10.1177/154405910408300903. [DOI] [PubMed] [Google Scholar]
- Baccarani-Contri M, Vincenzi D, Cicchetti F, et al. Immunocytochemical localization of proteoglycans within normal elastin fibers. Eur J Cell Biol. 1990;53:305–12. [PubMed] [Google Scholar]
- Bray BA, Hsu W, Turino GM. Lung hyaluronan as assayed with a biotinylated hyaluronan-binding protein. Exp Lung Res. 1994;20:317–30. doi: 10.3109/01902149409064390. [DOI] [PubMed] [Google Scholar]
- Bray BA, Sampson PM, Osman M, et al. Early changes in lung tissue hyaluronan (hyaluronic acid) and hyaluronidase in bleomycin-induced alveolitis in hamsters. Am Rev Respir Dis. 1991;143:284–8. doi: 10.1164/ajrccm/143.2.284. [DOI] [PubMed] [Google Scholar]
- Calkins CM, Heimbach JK, Bensard DD, et al. TNF receptor I mediates chemokine production and neutrophil accumulation in the lung following systemic lipopolysaccharide. J Surg Res. 2001;101:232–7. doi: 10.1006/jsre.2001.6274. [DOI] [PubMed] [Google Scholar]
- Cantor JO. Bleomycin-induced pulmonary fibrosis. In: Cantor JO, editor. Handbook of animal models of pulmonary disease, vol 1. Boca Raton, FL: CRC Press; 1989. pp. 117–29. [Google Scholar]
- Cantor JO, Cerreta JM, Armand G, et al. Pulmonary air-space enlargement induced by intratracheal instillment of hyaluronidase and concomitant exposure to 60% oxygen. Exp Lung Res. 1993;19:177–92. doi: 10.3109/01902149309031718. [DOI] [PubMed] [Google Scholar]
- Cantor JO, Cerreta JM, Keller S, et al. Modulation of airspace enlargement in elastase-induced emphysema by intratracheal instillment of hyaluronidase and hyaluronic acid. Exp Lung Res. 1995;21:423–36. doi: 10.3109/01902149509023717. [DOI] [PubMed] [Google Scholar]
- Cantor JO, Cerreta JM, Armand G, et al. Aerosolized hyaluronic acid decreases alveolar injury induced by human neutrophil elastase. Proc Soc Exp Biol Med. 1998;217:471–5. doi: 10.3181/00379727-217-44260. [DOI] [PubMed] [Google Scholar]
- Cantor JO, Shteyngart B, Cerreta JM, et al. The effect of hyaluronan on elastic fiber injury in vitro and elastase-induced airspace enlargement in vivo. Proc Soc Exp Biol Med. 2000;225:65–71. doi: 10.1046/j.1525-1373.2000.22508.x. [DOI] [PubMed] [Google Scholar]
- Cantor JO, Turino GM. Can exogenously administered hyaluronan improve respiratory function in patients with pulmonary emphysema. Chest. 2004;125:288–92. doi: 10.1378/chest.125.1.288. [DOI] [PubMed] [Google Scholar]
- Cantor JO, Cerreta JM, Ochoa M, et al. Aerosolized hyaluronan limits airspace enlargement in a mouse model of cigarette smoke-induced pulmonary emphysema. Exper Lung Res. 2005;31:417–30. doi: 10.1080/01902140590918669. [DOI] [PubMed] [Google Scholar]
- Fisher GJ, Wang ZQ, Datta SC, et al. Pathophysiology of premature aging induced by ultraviolet light. N Engl J Med. 1997;337:1419–28. doi: 10.1056/NEJM199711133372003. [DOI] [PubMed] [Google Scholar]
- Forteza R, Lauredo I, Abraham WM, et al. Bronchial tissue kallikrein activity is regulated by hyaluronic acid binding. Am J Respir Cell Mol Biol. 1999;21:666–74. doi: 10.1165/ajrcmb.21.6.3651. [DOI] [PubMed] [Google Scholar]
- Gandhi RH, Irizarry E, Cantor JO, et al. Analysis of elastin cross-linking and the connective tissue matrix of abdominal aortic aneurysms. Surgery. 1994;115:617–20. [PubMed] [Google Scholar]
- Gross P, Babyak MA, Tolker E, et al. Enzymatically produced pulmonary emphysema: a preliminary report. J Occup Med. 1964;6:481–4. [PubMed] [Google Scholar]
- Heinegard D, Paulsson M. Structure and metabolism of proteoglycans. In: Piez KA, Reddi AH, editors. Extracelular matrix biochemistry. New York: Elsevier; 1984. pp. 277–328. [Google Scholar]
- Horton MR, Shapiro S, Bao C, et al. Induction and regulation of macrophage metalloelastase by hyaluronan fragements in mouse macrophages. J Immunol. 1999;162:4171–6. [PubMed] [Google Scholar]
- Janoff A. Elastases and emphysema: current assessment of the protease-antiprotease hypothesis. Am Rev Respir Dis. 1985;132:417–33. doi: 10.1164/arrd.1985.132.2.417. [DOI] [PubMed] [Google Scholar]
- Karlinsky JB. Glycosaminoglycans in emphysematous and fibrotic hamster lungs. Am Rev Respir Dis. 1982;125:85–8. doi: 10.1164/arrd.1982.125.1.85. [DOI] [PubMed] [Google Scholar]
- Khan AI, Kerfoot SM, Heit B, et al. Role of CD44 and hyaluronan in neutrophil recruitment. J Immunol. 2004;173:7594–601. doi: 10.4049/jimmunol.173.12.7594. [DOI] [PubMed] [Google Scholar]
- Kipnis A, Basaraba RJ, Turner J, et al. Increased neutrophil influx but no impairment of protective immunity to tuberculosis in mice lacking the CD44 molecule. J Leukoc Biol. 2003;74:992–7. doi: 10.1189/jlb.0603301. [DOI] [PubMed] [Google Scholar]
- Kunz LI, van Rensen EL, Sterk PJ. Inhaled hyaluronic acid against exercise-induced bronchoconstriction in asthma. Pulm Pharmacol Ther. 2006;19:286–91. doi: 10.1016/j.pupt.2005.04.011. [DOI] [PubMed] [Google Scholar]
- LaFuma C, Moczar M, Lange F, et al. Biosynthesis of hyaluronic acid, heparan sulfate and structural glycoproteins in hamster lung explants during elastase induced emphysema. Connect Tissue Res. 1985;13:169–79. doi: 10.3109/03008208509152395. [DOI] [PubMed] [Google Scholar]
- Laurell C-B, Eriksson S. The electrophoretic alpha1-globulin pattern of serum in alpha1-antitrypsin deficiency. Scand J Clin Lab Invest. 1963;15:132–40. [Google Scholar]
- Li B, Alonso DO, Bennion BJ, Daggett V. Hydrophobic hydration is an important source of elasticity in elastin-based biopolymers. J Am Chem Soc. 2001;123:11991–8. doi: 10.1021/ja010363e. [DOI] [PubMed] [Google Scholar]
- Mariani TJ, Sandefur S, Pierce RA. Elastin in lung development. Exp Lung Res. 1997;23:131–45. doi: 10.3109/01902149709074026. [DOI] [PubMed] [Google Scholar]
- Mascarenhas MM, Day RM, Ochoa CD, et al. Low molecular weight hyaluronan from stretched lung enhances interleukin-8 expression. Am J Respir Cell Mol Biol. 2004;30:51–60. doi: 10.1165/rcmb.2002-0167OC. [DOI] [PubMed] [Google Scholar]
- McKee CM, Penno MB, Cowman M, et al. Hyaluronan (HA) fragments induce chemokine gene expression in alveolar macrophages. The role of HA size and CD44. J Clin Invest. 1996;98:2403–13. doi: 10.1172/JCI119054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreland LW. Intra-articular hyaluronan (hyaluronic acid) and hylans for the treatment of osteoarthritis: mechanisms of action. Arthritis Res Ther. 2003;5:54–67. doi: 10.1186/ar623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadkarni PP, Kulkarni GS, Cerreta JM, Ma S, et al. Dichotomous effect of aerosolized hyaluronan in a hamster model of endotoxin-induced lung injury. Exper Lung Res. 2005;31:807–18. doi: 10.1080/01902140600574942. [DOI] [PubMed] [Google Scholar]
- Noble PW, Jiang D. Matrix regulation of lung injury, inflammation, and repair: The role of innate immunity. Proc Am Thorac Soc. 2006;3:401–4. doi: 10.1513/pats.200604-097AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrigni G, Allegra L. Aerosolised hyaluronic acid prevents exercise-induced bronchoconstriction, suggesting novel hypotheses on the correction of matrix defects in asthma. Pulm Pharmacol Ther. 2006;19:166–71. doi: 10.1016/j.pupt.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Scott JE, Cummings C, Brass A, et al. Secondary and tertiary structures of hyaluronan in aqueous solution, investigated by rotary shadowing-electron microscopy and computer simulation. Hyaluronan is a very efficient network-forming polymer. Biochem J. 1991;274:699–705. doi: 10.1042/bj2740699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scuri M, Abraham WM, Botvinnikova Y, et al. Hyaluronic acid blocks porcine pancreatic elastase (PPE) - induced bronchoconstriction in sheep. Am J Respir Crit Care Med. 2001;164:1855–9. doi: 10.1164/ajrccm.164.10.2011115. [DOI] [PubMed] [Google Scholar]
- Scuri M, Abraham WM. Hyaluronan blocks human neutrophil elastase (HNE) - induced airway responses in sheep. Pulm Pharmacol Ther. 2003;16:335–40. doi: 10.1016/S1094-5539(03)00089-0. [DOI] [PubMed] [Google Scholar]
- Senior RM, Kuhn C., III . The pathogenesis of emphysema. In: Fishman AP, editor. Pulmonary diseases and disorders. 2nd ed. New York: McGraw-Hill; 1988. pp. 1209–18. [Google Scholar]
- Stolk J, Veldhuisen B, Annovazzi L, et al. Short-term variability of biomarkers of proteinase activity in patients with emphysema associated with type Z alpha-1-antitrypsin deficiency. Respir Res. 2005;6:47. doi: 10.1186/1465-9921-6-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toole BP, Gross J. The extracellular matrix of regenerating newt limb: synthesis and removal of hyaluronate prior to differentiation. Dev Biol. 1971;25:57–77. doi: 10.1016/0012-1606(71)90019-4. [DOI] [PubMed] [Google Scholar]
- Toole BP. Glycosaminoglycans and morphogenesis. In: Hay ED, editor. Cell biology of extracellular matrix. New York: Plenum; 1981. pp. 259–94. [Google Scholar]
- Wang Q, Teder P, Judd NP, et al. CD44 deficiency leads to enhanced neutrophil migration and lung injury in Escherichia coli pneumonia in mice. Am J Pathol. 2002;161:2219–28. doi: 10.1016/S0002-9440(10)64498-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasserman ZR, Salemme FR. A molecular dynamics investigation of the elastomeric restoring force in elastin. Biopolymers. 1990;29:1613–31. doi: 10.1002/bip.360291211. [DOI] [PubMed] [Google Scholar]
- Yasui S, Nagai A, Aoshiba K, et al. A specific neutrophil elastase inhibitor (ONO-5046.Na) attenuates LPS-induced acute lung inflammation in the hamster. Eur Respir J. 1995;8:1293–9. doi: 10.1183/09031936.95.08081293. [DOI] [PubMed] [Google Scholar]