Abstract
Hypoxia and endothelial dysfunction play a central role in the development of pulmonary hypertension. Cor pulmonale is a maladaptive response to pulmonary hypertension. The presence of peripheral edema in cor pulmonale is almost invariably associated with hypercapnia. Correction of abnormalities of gas exchange and ventilation can ameliorate pulmonary hypertension and improve survival. This review focuses on new information about the pathogenesis and treatment of pulmonary hypertension in COPD including information derived from lung volume reduction surgery, the role of brain natriuretic peptide, exhaled nitric oxide for diagnosis, and the treatment of cor pulmonale with recently available specific pulmonary vasodilators.
Keywords: cor pulmonale, chronic obstructive pulmonary disease, pulmonary hypertension, brain natriuretic peptide, nitric oxide, phlebotomy
Introduction
Cor pulmonale was classically defined as “hypertrophy of the right ventricle resulting from diseases affecting the function and/or structure of the lungs except when these pulmonary alterations are the result of diseases that primarily affect the left side of the heart” (WHO expert committee report 1963). Since this definition does not indicate the presence of right heart failure, and since the presence of edema does not always imply underlying right heart failure in stable COPD patients, the terms cor pulmonale and right heart failure are not synonymous. Pulmonary hypertension (PH) however is always the underlying pathologic mechanism for right venrticular hypertrophy in cor pulmonale.
Pulmonary hypertension in COPD is placed in group 3 of the 2003 WHO classification of PH (Rubin 2004), ie, PH associated with disorders of the respiratory system and/or hypoxemia. PH associated with lung disease is defined as resting mean PAP (mPAP) greater than 20 mm Hg (Weitzenblum 2003), which is different from the definition of primary pulmonary hypertension (mPAP >25 mm Hg).
Prevalence
The exact prevalence of PH and cor pulmonale in COPD is unknown because it is not feasible to perform right heart catheterization on a large scale. The reported prevalence varies considerably from 20%–91% (Burrows et al 1972; Weitzenblum et al 1984; Oswald-Mammosser et al 1991; Scharf et al 2002; Thabut et al 2005) depending on the definition of pulmonary hypertension, the severity of lung disease in the group studied and the method of measuring the PAP. In a large series of patients with advanced COPD referred for lung volume reduction surgery the prevalence of pulmonary hypertension is reported as just over 50% (Thabut et al 2005).
In severe COPD patients with or without resting PH, steady-state exercise may raise PAP to about twice the level of its resting value because PVR fails to decrease (Weitzenblum 2003). In severe COPD activities of daily living such as climbing stairs or walking can induce transient PH.
During an exacerbation of COPD, PAP may rise by as much as 20 mm Hg and return to its baseline after recovery (Abraham et al 1969; Weitzenblum et al 1979). In patients with advanced COPD, oxygen saturation may fall during REM sleep by 20%–30% (Catterall et al 1983; Fletcher et al 1984) and PAP may rise by as much as 20 mm Hg (Coccagna and Lugaresi 1978). It remains unproven that nocturnal desaturation in COPD leads to the development of pulmonary hypertension and cor pulmonale (Fletcher et al 1992; Chaouat et al 2001).
Pathophysiology of pulmonary hypertension and cor pulmonale in COPD
Traditionally, PH in COPD has been considered to be the result of hypoxic pulmonary vasoconstriction, polycythemia and destruction of the pulmonary vascular bed by emphysema. Recently, it has been recognized that hyperinflation and endothelial dysfunction also play a role in the pathogenesis of PH.
Hypoxic pulmonary vasoconstriction (HPV) is an adaptive response to divert blood away from the poorly ventilated alveoli to maintain ventilation-perfusion balance and a normal PaO2. Classic studies show that a decreased hydrogen ion concentration augments hypoxic pulmonary vasoconstriction (Enson 1964).
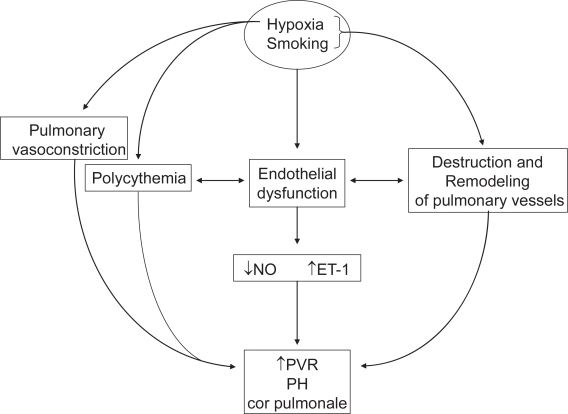
Acute hypoxia inhibits specific voltage-gated potassium (Kv) channels and induces influx of cytosolic calcium, which triggers membrane depolarization in pulmonary artery smooth muscle cells (PASMCs) and leads to pulmonary vasoconstriction (Weir EK et al 2005). Chronic hypoxia selectively inhibits messenger RNA and protein expression of the Kv channel pore forming α-subunits and decreases the number of functional Kv channels in PASMCs. The consequent reduction in potassium currents through the Kv channels depolarizes PASMCs, raises cytosolic calcium, stimulates cell proliferation (Sweeney and Yuan 2000) and inhibits apoptosis. The resultant vascular remodeling is characterized by intimal fibrosis and proliferation of longitudinal smooth muscle, neomuscularization of pulmonary arterioles and medial hypertrophy of small pulmonary arteries (Wilkinson et al 1988) (Figure 1). After remodeling occurs, the narrower, thicker and more muscular pulmonary arteries are less compliant and offer higher resistance to flow.
Figure 1.
Pathophysiology of cor pulmonale in COPD.
Abbreviations: NO, nitric oxide; ET-1, endothelin-1; PVR, pulmonary vascular resistance; PH, pulmonary hypertension.
Destruction of the pulmonary vascular bed in emphysema reduces the total cross-sectional area of the pulmonary circulation and increases the total pulmonary vascular resistance when the remaining capacitance vessels are abnormal and unable to accommodate the increased diverted pulmonary blood flow.
The inverse relationship between FEV1 and pulmonary hypertension has been reported (Oswald-Mammoser et al 1991) yet pulmonary hypertension can develop in those without significant resting hypoxemia. Severe emphysema with air-trapping and hyperinflation is associated with intrinsic positive end-expiratory pressure of 5–7.5 cm H2O (Tschernko et al 1998). The positive alveolar pressure throughout respiration contributes to the high pulmonary vascular resistance and PH. This mechanism may assume a more important role in development of PH in patients with severe emphysema who are not hypoxemic and also perhaps during exercise in some patients. In a hemodynamic study of 120 non-hypoxemic patients evaluated for lung volume reduction surgery (Scharf et al 2002), a mPAP >20 mm Hg occurred in 90.8%. In this study however wedge pressure was greater than 12 mmHg in over 60%. It is unclear whether the reduction in hyperinflation following LVRS significantly ameliorates PH independently of improved arterial oxygen tension.
Pulmonary vascular endothelial dysfunction is a major factor in the pathogenesis of pulmonary hypertension. The balance between constriction and dilatation is maintained by a number of small molecular mediators one of the more important of which is nitric oxide (NO). Nitric oxide (NO) has vasodilator and anti-proliferative properties is produced by endothelial NO synthase (eNOS). Prostacyclin, another vasodilator that also protects against vascular remodeling, is produced by the activity of prostacyclin synthase. Countering vasodilation is endothelium-derived endothelin-1 (ET-1).
An imbalance of vasoconstriction opposed to dilatation is likely to be involved in the pathogenesis of pulmonary hypertension in COPD. In patients with COPD and PH there is a reduction in the synthesis and/or release of NO from the lung (Dinh-Xuan et al 1991; Clini et al 2000; Clini et al 2002). In COPD there is a reduction in the expression of prostacyclin synthase mRNA (Lee et al 2005), and an excessive expression of endothelin-1 (ET-1) (Giaid et al 1993) in the pulmonary arteries of patients with secondary pulmonary hypertension. Arterial ET1 increases shortly after episodes of nocturnal oxygen desaturation in patients with COPD and remains higher during the day in these subjects (Spiropoulos et al 2003).
The pulmonary vascular response to hypoxia is genetically determined. Serotonin (5-hydroxytryptamine, 5-HT) and its transporter (5-HTT) play a role in PASMC proliferation and vascular remodeling. In animal studies hypoxia is a strong inducer of 5-hydroxytryptamine transporter (5-HTT) expression, which leads to a greater mitogenic response of PASMC to 5HT (Eddahibi et al 1999). The severity of PH in hypoxic COPD patients depends upon 5-HTT gene polymorphism. PH is most severe in patients carrying the LL genotype, which is associated with higher levels of 5-HTT expression in PASMCs (Eddahibi et al 2003).
Angiotensin converting enzyme (ACE) in the lung converts Angiotensin 1 to A2, a vasoconstrictor and mediator of PASMC proliferation. ACE is present in very high concentrations in the lungs and its activity is further increased by hypoxia (King et al 1989). The ACE gene contains a polymorphism based on the presence (insertion [I]) or absence (deletion [D]) of a nonsense DNA domain, resulting in three genotypes (DD, DI or II) (Rigat et al 1992). The ACE DD genotype is associated with increased circulating and tissue concentrations of ACE. Moreover, the ACE DD genotype is associated with exaggerated PH during exercise in COPD patients (Kanazawa et al 2000). Future studies could be designed to enroll populations with COPD genetically susceptible to PH for new therapeutic trials.
Right and left ventricular function in cor pulmonale
Classic hemodynamic studies have shown the existence of two patterns of cardiovascular abnormalities in COPD patients with high pulmonary vascular resistance. Patients with predominant “emphysema” have mild hypoxia, mild pulmonary hypertension at rest, and low cardiac index with dyspnea (“the pink puffer”). On the other hand, patients with predominant “chronic bronchitis” have more severe hypoxia in association with hypercapnia, more severe pulmonary hypertension peripheral edema and a normal cardiac index (“the blue bloater”) (Khaja and Parker 1971; Burrows et al 1972). These distinctions have become less useful conceptually as it is increasingly recognized that many patients show features of both types. In addition later work has indicated that the classic definition may not hold. For example, cardiac output in the “pink puffer type” has been found to be normal (Oswald Mammoser et al 1991).
In response to the increased pulmonary vascular resistance (PVR) the RV gradually undergoes hypertrophy and dilatation (cor pulmonale). This increase in end-diastolic volume ie, pre-load, to maintain a normal stroke volume accounts for the reduced right ventricular ejection fraction. The right ventricular stroke work index probably remains normal even during exercise and explained by greater pressure work and the expense of RV output (Khaja and Parker 1971). However, in severe emphysema hyperinflation, the low elastic recoil of the lungs and less negative intrathoracic pressure has the effect of compressing the two ventricles into each other (Sciurba et al 1996). The RV is therefore unable to dilate and end-diastolic volume does not increase. This decreases RV preload and results in lower cardiac output. A decrease in intrathoracic pressure as may occur after lung volume reduction surgery, redistributes the blood volume to the thoracic compartment and increases RV and LV preload and cardiac function (Jorgenen et al 2003).
Changes in RV output must invariably alter LV preload, because the two ventricles are serially linked through the pulmonary vasculature. LV preload can also be directly altered by changes in RVEDV by the mechanism of ventricular interdependence. The increased RVEDV in cor pulmonale induces a shift of the interventricular septum into the LV and decreases LV diastolic compliance but this does not adversely affect LV output because the increased RV systolic pressure in cor pulmonale also pushes the septum into the LV towards its free wall to empty the LV. This “help” from the RV in systole tends to preserve LV ejection fraction (LVEF) in emphysematous patients with severe RV hypertrophy (Dong et al 1995; Vonk Noordegraaf et al 1997).
RV contractility, as assessed by end systolic pressure-volume relation, is normal in stable COPD patients and the RV operates on an extension of the normal RV function Frank-Starling curve. During an exacerbation, the RV end-diastolic pressure and volume rise and RV ejection fraction falls, resulting in peripheral edema and systemic congestion (MacNee et al 1988; Weitzenblum et al 1994). These changes may not be associated with a rise in PAP suggesting other factors reduce RV contractility (MacNee et al 1988).
Diagnosis of cor pulmonale in COPD
Clinical features
The clinical exam lacks sensitivity and specificity. Hyperinflation reduces the yield of cardiac auscultation for the classic signs of PH and CP ie, loud P2, S3 gallop, the systolic murmur of tricuspid regurgitation. Peripheral edema can be present in the absence of right heart failure in COPD (Weitzenblum et al 1994) and is not diagnostic of cor pulmonale. The pathogenesis of edema formation in COPD is complex. Renal blood flow is reduced, the renin-angiotensin system is activated, renal dopamine output is reduced and plasma ANP level is elevated leading to increase in proximal renal tubular sodium reabsorption (Skwarski et al 1998; de Leeuw and Dees 2003). Sodium retention is enhanced by hypercapnia and ameliorated by long-term oxygen therapy in hypoxemic patients (Bratel et al 2003). True right heart failure is characterized by raised jugular venous pressures, congestive hepatomegaly as well as peripheral edema.
Pulmonary function tests
The Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines does not recommend the routine measurement of the PAP in patients with COPD (workshop report 2005). Early studies indicate that, with the exception of low oxygen tensions during exercise and resting hypercapnia, pulmonary function tests are poor predictors of the severity of pulmonary hypertension in COPD (Keller et al 1986). However in a selected group of patients undergoing LVRS the level of PAP varies inversely with the FEV1 (Scharf et al 2002; Thabut et al 2005). Similarly studies of COPD patients without severe hypoxia (PaO2 > 55 mm Hg) have also shown that mPAP better correlates with the FEV1 than with the resting PaO2 (Oswald-Mammosser et al 1991; Doi et al 2003). It is also notable that these studies indicating a closer FEV1 PAP relationship were conducted in patients with severe hyperinflation which itself is likely to predispose to PH.
Chest radiography
An increase in the diameter of the right descending pulmonary artery to more than 16 mm on the postero-anterior projection combined with a diameter of the left descending pulmonary artery of more than 18 mm on the left lateral projection can identify PH with 98% sensitivity (Matthay et al 1981).
EKG
The sensitivity for right ventricular hypertrophy is only 25%–40%. Presence of S1S2S3 or right atrial overload pattern ie, P wave axis of +90° or more, implies a poor prognosis (Incalzi et al 1999).
Echocardiography
Hyperinflation precludes optimal visualization of the heart. In a cohort of lung transplant candidates estimation of systolic PAP (sPAP) was possible in only 38% of the 253 patients with COPD. Hyperinflation with a residual volume of greater than 150% lessened the likelihood of sPAP estimation. Sensitivity, specificity, negative predictive value and positive predictive value of sPAP estimated by echocardiography for the diagnosis of PH were 76, 65, 93, and 32 per cent, respectively (Arcasoy et al 2003). In the absence of sPAP estimation, figures for right ventricular abnormalities were 84, 56, 96, and 22 per cent respectively. Although the negative predictive value is high enough to exclude PH, the presence of a high sPAP or RV abnormalities requires confirmation by right heart catheterization.
Magnetic resonance imaging
In COPD, the echocardiographic window might be obscured by hyperinflation. Contrast MRI provides excellent images of right ventricular structure and function. Right ventricular wall thickness has a high correlation with the mean PAP (r = 0.9) (Saito et al 1992). Readers are referred to McGoon et al (2004) for further details.
Right heart catheterization
Right heart catheterization is the gold standard to determine the exact PAPs. It also allows measurement of the transpulmonary gradient, measurement of cardiac output, with calculation of the pulmonary vascular resistance and determination of reversibility with a vasodilator. Exercise induced pulmonary hypertension can also be evaluated.
The degree of PH in stable COPD is usually mild to moderate (mPAP <35 mm Hg). A mPAP >40 mm Hg is rare in COPD and should initiate a search for an additional cause of PH eg, left heart disease, sleep apnea syndrome, pulmonary embolism. Rarely a COPD patient may present with severe PH (Chaouat et al 2005; Thabut et al 2005). They are characterized by mild to moderate airway obstruction, severe hypoxemia, hypocapnia and a very low diffusing capacity (Table 1). They also suffer from more severe exertional dyspnea and have a shorter survival (Chaouat et al 2005). These patients may have an exaggerated response to chronic hypoxemia as may be seen in certain high altitude dwellers (Maggiorini et al 2003).
Table 1.
Comparison of COPD patients without other causes of pulmonary hypertension
| Severe PH group (without additional causes of PH & mPAP ≥40 mm Hg) (N = 11) | Control group with less severe PH (mPAP ≥ 20 mm Hg) (N = 16) | p value | |
|---|---|---|---|
| FEV1 (% predicted) | 50 (44–56) | 27 (23–34) | <0.01 |
| DLCO (ml/min/mm Hg) | 4.6 (4.2–6.7) | 10.3 (8.9–12.8) | <0.01 |
| PaO2 (mm Hg) | 46 (41–53) | 56 (54–64) | <0.01 |
| PaCO2 (mm Hg) | 32 (28–37) | 47 (44–49) | <0.01 |
| RAP (mm Hg) | 7 (5–9) | 3 (1.3–4) | <0.01 |
| mPAP (mm Hg) | 48 (46–50) | 25 (22–27) | <0.01 |
| PCWP (mm Hg) | 6 (4–7) | 7 (6.5–7.5) | NS |
| CI (L/min/m2) | 2.3 (1.8–2.5) | 2.8 (2.4–3.1) | <0.01 |
| TPR (IU/m2) | 21.3 (17.6–26.6) | 9 (7.4–9.9) | <0.01 |
Adapted with permission from Chaouat A, Bugnet A, Kadaoui N, et al. 2005. Severe pulmonary hypertension and chronic obstructive pulmonary disease. Am J Respir Crit Care Med, 172:189–94. Official Journal of the American Thoracic Society. © American Thoracic Society.
Abbreviations: FEV1, forced expiratory volume in the first second; DLCO, diffusing capacity; RAP, right atrial pressure; mPAP, mean pulmonary artery pressure; PCWP, pulmonary capillary wedge pressure; CI, cardiac index; TPR, total pulmonary resistance; IU, international units.
Brain natriuretic peptide
Plasma plasma brain natriuretic peptide (BNP) may provide a reliable and accurate diagnostic test for PH. BNP is a cardiac hormone, which is synthesized by the ventricle and secreted into the circulation in response to increased wall stretch and tension during elevations in end-diastolic pressure (Yamamoto et al 1996). BNP seems promising as a marker for pulmonary hypertension and right ventricular dysfunction. One study of 38 patients with stable COPD, 20 of whom had clinical cor pulmonale, found significant correlation between BNP and echocardiographically determined PASP (r = 0.68, p < 0.001) (Bozkanat et al 2005). Increased BNP also correlated with lower arterial oxygen tensions suggesting that BNP can also be released in response to hypoxia. More recently, BNP has been reported to provide prognostic information in patients with chronic lung disease (Leuchte et al 2006). Given the limitations of these small studies larger scale studies are required to determine if BNP offers specific diagnostic and prognostic information especially in the presence of left heart disease and/or chronic hypoxemia.
Exhaled nitric oxide
Clini et al measured levels of exhaled nitric oxide (eNO) in COPD patients including those with cor pulmonale. First they studied 34 consecutive patients with stable COPD and found that using a sPAP of 35 mm Hg as cut-off, patients with cor pulmonale showed lower values of eNO compared to those with normal resting sPAP (Clini et al 2000). The study was limited by the use of echocardiography to measure PAP but reduced exhaled NO in COPD patients with cor pulmonale compare to those without is a plausible finding.
Two years later the same group published the results of changes in eNO in a prospective, controlled study of 47 stable COPD patients who underwent a multi-disciplinary pulmonary rehabilitation program for 8–10 weeks. They found that both peak workload and eNO increased significantly and independent of the severity of airway obstruction, in all the patients except the 7 with cor pulmonale on LTOT (Clini et al 2002). The authors did not indicate whether the group with cor pulmonale received oxygen during rehabilitation. At this time the role of the test to diagnose PH in COPD and monitor the response to therapy remains uncertain.
Therapy of cor pulmonale
Although pulmonary hypertension in COPD is usually mild (mPAP 20–35 mm Hg), it may increase markedly during exercise (Horsfield et al 1968; Weitzenblum 2003), sleep (Coccagna et al 1978; Catterall et al 1983; Fletcher et al 1984), and exacerbations (Weitzenblum et al 1994). Frequent exacerbations can promote the development of right heart failure and this should be preventable by general management measures recommended for COPD.
Oxygen is the best pulmonary vasodilator in COPD patients with cor pulmonale but not all patients benefit from it. Calcium channel blockers, β2-agonists, nitrates, angiotensin converting enzyme inhibitors, theophylline, and α1-antagonists have also been used. (MacNee 2004). Most of these cause a modest fall in PH accompanied by a rise in cardiac output and a reduced pulmonary vascular resistance. Most of them are also associated with systemic hypotension and with worsening of ventilation-perfusion mismatch that may or may not be offset by the improvement in cardiac output. None has been studied long enough to determine any survival benefit (MacNee 2004).
Oxygen
Although acute oxygen therapy may not improve hemodynamics significantly (MacNee et al 1988), two landmark trials conducted more than twenty years ago by the US National Institute of Health and the UK Medical Research Council showed that continuous long-term oxygen therapy (LTOT) improves survival and prevents progression of PH (nocturnal oxygen therapy trial group 1980; MRC working party 1981). Follow up of hypoxemic COPD patients on LTOT for 6 years demonstrated a reversal but not normalization of PH (Weitzenblum et al 1985). Another long term study confirmed this finding (Zielinski et al 1998). Reduced patient compliance with oxygen therapy may be an issue limiting the effectiveness of this treatment.
Unfortunately not all the patients with COPD who meet criteria for LTOT benefit from it. Patients who exhibit a significant drop in mean PAP of more than 5 mm Hg after acute oxygen therapy (28% for 24 hours) have an 88% 2 year survival compared to 22% in non-responders when both groups of patients are subsequently treated with continuous LTOT (Ashutosh et al 1983). Similarly in the nocturnal oxygen therapy trial continuous LTOT resulted in an improved survival only in patients whose baseline pulmonary vascular resistance was not greater than 400 dyne.s.cm−5 (Timms et al 1985). Pulmonary vascular remodeling may limit the effectiveness of oxygen induced vasodilation in some patients. Oxygen therapy during exercise and sleep ameliorates the rise in mean PAP (Horsfield et al 1968; Keisaku et al 2002; Raeside et al 2002) in moderate to severe COPD. Whether such therapy prevents the eventual development of pulmonary hypertension in susceptible subjects is unknown.
Pulmonary vasodilators
The limitations of LTOT in improving PAP provide an impetus for the development of additional therapies.
Inhaled nitric oxide
Inhaled nitric oxide (iNO) is a more potent vasodilator than oxygen. However, when used alone iNO worsens ventilation-perfusion imbalance. In a randomized controlled trial of 40 patients with severe COPD receiving LTOT, pulsed iNO was delivered with oxygen for 3 months (Vonbank et al 2003). There was a significant improvement in mPAP, pulmonary vascular resistance, and cardiac output (Table 2). Systemic hemodynamics and left heart function remained unchanged. PaCO2 decreased significantly in the treatment group, suggesting improved perfusion of the better ventilated areas. Although the delivery of nitric oxide as a practical approach to treatment still has significant obstacles, particularly cost, this study shows a promising role for selective pulmonary vasodilators in COPD.
Table 2.
Comparison of patients treated with oxygen alone versus oxygen and inhaled nitric oxide
|
Oxygen alone |
Oxygen + iNO |
||||
|---|---|---|---|---|---|
| Baseline | After 3 months | Baseline | After 3 months | p value | |
| mPAP (mm Hg) | 24 ± 5 | 25 ± 6 | 27 ± 4 | 20 ± 5 | <0.001 |
| PVR (dyn × s/cm5) | 259 ± 101 | 264 ± 109 | 276 ± 96 | 173 ± 87 | 0.001 |
| CO (l/min) | 5.5 ± 1.3 | 5.3 ± 1.3 | 5.6 ± 1.3 | 6.1 ± 1.0 | 0.025 |
| VO2 (ml/min) | 883 ± 303 | 842 ± 232 | 896 ± 406 | 1005 ± 326 | NS |
Adapted from Chaouat et al (2001).
Abbreviations: mPAP, mean pulmonary artery pressure; PVR, pulmonary vascular resistance; CO, cardiac output; VO2, oxygen consumption.
Sildenafil
The phosphodiesterase 5 inhibitor sildenafil is a specific pulmonary vasodilator and increases the effects of iNO through the cGMP pathway. It relaxes human PASMCs by inhibiting PDE 5 thereby activating large conductance calcium activated potassium channels (Michelakis et al 2003). It has been used successfully in primary PH (Michelakis et al 2003), scleroderma (Ghofrani et al 2002), and chronic thromboembolic disease (Ghofrani et al 2003). However, it has been studied in COPD patients in only one small open label trial of sildenafil 50 mg orally twice daily for 3 months (Alp et al 2005). The mPAP and PVR decreased significantly and the 6 minute walk distance (6 MWD) improved from 351 ± 49 to 433 ± 52 m (p < 0.05). This 82 m increase in 6 MWD is greater than that seen in patients with idiopathic PH treated with other pulmonary vasodilators bosentan and iloprost (Alp et al 2005). However without further studies in COPD, the effectiveness of sildenafil is speculative.
Reduction in hematocrit
Polycythemia increases the viscosity of blood, the resistance to blood flow through the pulmonary circulation and augments hypoxic pulmonary vasoconstriction by causing a local deficiency of nitric oxide (NO) (Deem et al 1998; Azarov et al 2005). Phlebotomy is indicated in patients with a severe elevation in hematocrit not responding to LTOT, yet its effects may be short lived. After repeated phlebotomy followed by volume replacement over 3 months, Borst et al (1999) showed improvement in exercise tolerance with a fall in mean PAP. The mean Hct in this group of 7 was 53, lower than is commonly associated with the need for phlebotomy.
Activation of the renin-angiotensin system may contribute to polycythemia in COPD (Vlahakos et al 1999). Plasma renin and aldosterone levels are increased in such patients when matched with controls for hypoxemia. The mechanism of action is serum erythropoietin independent. In a small study, losartan was used in weekly escalating doses to a maximum of 100 mg daily for 4 weeks in 9 stable severe COPD patients with polycythemia (hematocrit >52%). The regimen caused a significant reduction in the hematocrit of all patients from 56 ± 0.9% to 46 ± 0.7% (p < 0.001). The higher the baseline value, the greater the reduction in hematocrit (r = 0.7085; p < 0.05) (Vlahakos et al 2001). At 3 months after discontinuation of losartan the hematocrit increased to 50 ± 0.7%. This ‘bloodless phlebotomy’ appears to be a promising therapy. In a randomized controlled study in 60 COPD patients irbesartan induced a significant reduction in hematocrit (Andreas et al 2006).
Diuretics
Diuretics reduce right ventricular dilatation and improve its contractility and also reduce extravascular lung water (Turino et al 1970). They should be used cautiously as they can cause intravascular volume depletion that may deprive the right ventricle of adequate pre-load to maintain a normal stroke volume. Moreover, accumulation of bicarbonate from diuretic therapy can worsen alveolar hypoventilation and hypercapnia. The latter can result fluid retention despite diuretic therapy. Treatment of chronic hypercapnia may therefore be as important as diuretics in ameliorating sodium retention.
Lung volume reduction surgery (LVRS)
LVRS provides a model to study the effects of reduction in hyperinflation and improved gas exchange on pulmonary hypertension. In a small study of in 9 patients who underwent LVRS resting mPAP remained unchanged whereas exercising mPAP decreased slightly but not significantly. However, the improvement in arterial oxygenation during exercise was closely correlated with the improvement in exercise mPAP, whereas it was unrelated to changes in FEV1 (Oswald-Mammoser et al 1998). It is possible that LVRS results in better distribution of pulmonary blood flow and ventilationperfusion matching. In another small study (Weg et al 1999) however mean PAP in 9 subjects rose from 26.5 to 31.8 mm Hg without change in pulmonary artery occlusion pressure 3 months after surgery. In 3 patients pulmonary artery systolic pressure rose to 60 mm Hg or greater. In retrospect of these subjects may have been unsuitable candidates for LVRS by current selection criteria.
Prognosis
In the era before the widespread availability of LTOT the presence of PH doubled the mortality in COPD (Oswald-Mammosser et al 1995). Studies showed an increase in PAP between 1.5–2.8 mm Hg/year (MRC trial 1981; Weitzenblum et al 1985). However, even on LTOT the 5-year survival rate is only 36% in those whose initial mPAP is >25 mm Hg compared to 66% in those whose initial mPAP is <25 mm Hg (Oswald-Mammosser et al 1995). LTOT appears to stabilize but not reduce PAP indicating that other factors responsible for prognosis in these patients (Zielinski et al 1998) Those who develop severe PH (mPAP >40 mm Hg) suffer an extremely poor prognosis (5-year survival ~15% vs~55% in those with less severe PH (mPAP 20–40) (Chaouat et al 2005).
In those with mild or moderate hypoxemia (>65 mm Hg), PH develops in 25% over a 6-year follow up but is usually mild (Kessler et al 2001). These patients are characterized by worsening hypoxemia and hypercapnia Those with PH during exercise at initial catheterization have a greater risk of progression (Kessler et al 2001; Weitzenblum et al 1984). It may be necessary to follow such patients with periodic arterial blood gases and BNP.
Only continuous LTOT has been shown to improve survival in cor pulmonale. It is unclear if this is the result of improved oxygen delivery to vital organs, reduction of systemic inflammation associated with COPD or of the reversal or stabilization of PH.
Conclusion
New insights into the central role of endothelial dysfunction and the availability of specific pulmonary vasodilators should renew enthusiasm in the study and treatment of PH in COPD. Together these have the potential to further improve survival in patients with COPD who develop cor pulmonale.
Acknowledgments
The authors thank Professor Jahar Bhattacharya at Columbia University for his suggestions.
References
- Abraham AS, Cole RB, Green ID. Factors contributing to the reversible pulmonary hypertension in patients with acute respiratory failure studied by serial observations during recovery. Circ Res. 1969;24:51–60. doi: 10.1161/01.res.24.1.51. [DOI] [PubMed] [Google Scholar]
- Alp S, Skrygan M, Scmidt WE, et al. Sildenafil improves hemodynamic parameters in COPD- an investigation of six patients. Pulm Pharmacol Ther. 2006;19:386–90. doi: 10.1016/j.pupt.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Andreas S, Hermann-Lingen C, Raupach T, et al. Angiotensin II blockers in obstructive pulmonary disease. A randomized, controlled trial. Eur Respir J. 2006;27:972–9. doi: 10.1183/09031936.06.00098105. [DOI] [PubMed] [Google Scholar]
- Arcasoy SM, Christie JD, Ferrari VA, et al. Echocardiographic assessment of pulmonary hypertension in patients with advanced lung disease. Am J Respir Crit Care Med. 2003;167:735–40. doi: 10.1164/rccm.200210-1130OC. [DOI] [PubMed] [Google Scholar]
- Ashutosh K, Mead G, Dunsky M. Early effects of oxygen administration and prognosis in chronic obstructive pulmonary disease and cor pulmonale. Am Rev Respir Dis. 1983;127:399–404. doi: 10.1164/arrd.1983.127.4.399. [DOI] [PubMed] [Google Scholar]
- Azarov I, Huang KT, Basu, et al. Nitric oxide scavenging by red blood cells as a function of hematocrit and oxygenation. J Biol Chem. 2005;280:39024–32. doi: 10.1074/jbc.M509045200. [DOI] [PubMed] [Google Scholar]
- Borst MM, Leschke M, Konig U, et al. Repetitive hemodilution in chronic obstructive pulmonary disease and pulmonary hypertension: effects on pulmonary hemodynamics, gas exchange, and exercise capacity. Respiration. 1999;66:225–32. doi: 10.1159/000029382. [DOI] [PubMed] [Google Scholar]
- Bozkanat E, Tozkoparan E, Baysan O, et al. The significance of elevated brain natriuretic peptide levels in chronic obstructive pulmonary disease. J Intern Med Res. 2005;33:537–44. doi: 10.1177/147323000503300509. [DOI] [PubMed] [Google Scholar]
- Bratel T, Ljungman S, Runold M, et al. Renal function in hypoxemic chronic obstructive pulmonary disease: effects of long-term oxygen treatment. Respir Med. 2003;97:308–16. doi: 10.1053/rmed.2002.1401. [DOI] [PubMed] [Google Scholar]
- Burrows B, Kettel LJ, Niden AH, et al. Patterns of cardiovascular dysfunction in chronic obstructive lung disease. N Engl J Med. 1972;286:912–17. doi: 10.1056/NEJM197204272861703. [DOI] [PubMed] [Google Scholar]
- Catterall JR, Douglas NJ, Calverley PM, et al. Transient hypoxemia during sleep in chronic obstructive pulmonary disease is not a sleep apnea syndrome. Am Rev Respir Dis. 1983;128:24–9. doi: 10.1164/arrd.1983.128.1.24. [DOI] [PubMed] [Google Scholar]
- Chaouat A, Weitzenblum E, Kessler R, et al. Outcome of COPD patients with mild daytime hypoxaemia with or without sleep related oxygen desaturation. Eur Resp J. 2001;17:848–55. doi: 10.1183/09031936.01.17508480. [DOI] [PubMed] [Google Scholar]
- Chaouat A, Bugnet A, Kadaoui N, et al. Severe pulmonary hypertension and chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2005;172:189–94. doi: 10.1164/rccm.200401-006OC. [DOI] [PubMed] [Google Scholar]
- Clini E, Cremona G, Campana M, et al. Production of endogenous nitric oxide in chronic obstructive pulmonary disease and patients with cor pulmonale. Correlates with Echo-Doppler assessment. Am J Respir Crit Care Med. 2000;162:446–50. doi: 10.1164/ajrccm.162.2.9909105. [DOI] [PubMed] [Google Scholar]
- Clini E, Bianchi L, Foglio K, et al. Exhaled nitric oxide and exercise tolerance in severe COPD patients. Respir Med. 2002;96:312–16. doi: 10.1053/rmed.2001.1286. [DOI] [PubMed] [Google Scholar]
- Coccagna G, Lugaresi E. Arterial blood gases and pulmonary and systemic arterial pressure during sleep in chronic obstructive pulmonary disease. Sleep. 1978;1:117–24. doi: 10.1093/sleep/1.2.117. [DOI] [PubMed] [Google Scholar]
- de Leeuw PW, Dees A. Fluid homeostasis in chronic obstructive pulmonary disease. Eur Respir J. 2003;22(suppl 46):33S–40S. doi: 10.1183/09031936.03.00000603a. [DOI] [PubMed] [Google Scholar]
- Deem S, Swenson ER, Alberts MK, et al. Red-blood cell augmentation of hypoxic pulmonary vasoconstriction. Hematocrit dependence and the importance of nitric oxide. Am J Respir Crit Care Med. 1998;157:1181–6. doi: 10.1164/ajrccm.157.4.9707165. [DOI] [PubMed] [Google Scholar]
- Dinh-Xuan AT, Higenbottam TW, Clelland CA, et al. Impairment of endothelium-dependent pulmonary artery relaxation in chronic obstructive lung disease. N Engl J Med. 1991;324:1539–47. doi: 10.1056/NEJM199105303242203. [DOI] [PubMed] [Google Scholar]
- Doi M, Nakano K, Hiramoto T, et al. Significance of PAP in emphysema patients with mild-to-moderate hypoxemia. Respir Med. 2003;97:915–20. doi: 10.1016/s0954-6111(03)00115-x. [DOI] [PubMed] [Google Scholar]
- Dong SJ, Crawley AP, MacGregor JH, et al. Regional left ventricular systolic function in relation to the cavity geometry in patients with chronic right ventricular pressure overload. A three-dimensional tagged magnetic resonance imaging study. Circulation. 1995;91:2359–70. doi: 10.1161/01.cir.91.9.2359. [DOI] [PubMed] [Google Scholar]
- Eddahibi S, Fabre V, Boni C, et al. Induction of serotonin transporter by hypoxia in pulmonary vascular smooth muscle cells. Relationship with the mitogenic action of serotonin. Circ Res. 1999;84:329–36. doi: 10.1161/01.res.84.3.329. [DOI] [PubMed] [Google Scholar]
- Eddahibi S, Chaouat A, Morrell N, et al. Polymorphism of the serotonin transporter gene and pulmonary hypertension in chronic obstructive pulmonary disease. Circulation. 2003;108:1839–44. doi: 10.1161/01.CIR.0000091409.53101.E8. [DOI] [PubMed] [Google Scholar]
- Enson Y, Giuntini C, Lewis ML, et al. The influence of Hydrogen Ion Concentration and Hypoxia on the Pulmonary Circulation. J Clin Invest. 1964;43(6):1146–62. doi: 10.1172/JCI104999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher EC, Levin DC. Cardiopulmonary hemodynamics during sleep in subjects with chronic obstructive pulmonary disease: the effect of short and long-term oxygen. Chest. 1984;85:6–14. doi: 10.1378/chest.85.1.6. [DOI] [PubMed] [Google Scholar]
- Fletcher EC, Donner CF, Midgren B, et al. Survival in COPD patients with daytime PaO2 of greater than 60 mm Hg with and without nocturnal oxyhemaglobin desaturation. Chest. 1992;101:649–55. doi: 10.1378/chest.101.3.649. [DOI] [PubMed] [Google Scholar]
- Ghofrani HA, Wiedemann R, Rose F, et al. Sildenafil for treatment of lung fibrosis and pulmonary hypertension: a randomized controlled trial. Lancet. 2002;360:895–900. doi: 10.1016/S0140-6736(02)11024-5. [DOI] [PubMed] [Google Scholar]
- Ghofrani HA, Schermuly RT, Wiedemann R, et al. Sildenafil for long-term treatment of non-operable chronic thromboembolic pulmonary hypertension. AM J Respir Crit Care Med. 2003;167:1139–41. doi: 10.1164/rccm.200210-1157BC. [DOI] [PubMed] [Google Scholar]
- Giaid A, Yanagisawa M, Langleben D, et al. Expression of endothelin- 1 in the lungs of patients with pulmonary hypertension. N Engl J Med. 1993;328:1732–9. doi: 10.1056/NEJM199306173282402. [DOI] [PubMed] [Google Scholar]
- Horsfield K, Segal N, Bishop JM. The pulmonary circulation in chronic bronchitis at rest and during exercise breathing air and 80% oxygen. Clin Sci. 1968;43:473–83. [PubMed] [Google Scholar]
- Incalzi RA, Fuso L, De Rosa M, et al. Electrocardiographic signs of chronic cor pulmonale. A negative prognostic finding in chronic obstructive pulmonary disease. Circulation. 1999;99:1600–5. doi: 10.1161/01.cir.99.12.1600. [DOI] [PubMed] [Google Scholar]
- Jorgenen K, Houltz E, Wastfelt U, et al. Effects of lung volume reduction surgery on left ventricular diastolic filling and dimensions in patients with severe emphysema. Chest. 2003;124:1863–70. doi: 10.1378/chest.124.5.1863. [DOI] [PubMed] [Google Scholar]
- Kanazawa H, Okamoto T, Hirata K, et al. Deletion polymorphisms in the angiotensin converting enzyme gene are associated with pulmonary hypertension evoked by exercise challenge in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2000;162:1235–8. doi: 10.1164/ajrccm.162.4.9909120. [DOI] [PubMed] [Google Scholar]
- Keisaku F, Matsusawa Y, Yamaguchi S, et al. Benefits of oxygen on exercise performance and pulmonary hemodynamics in patients with COPD with mild hypoxemia. Chest. 2002;122:457–63. doi: 10.1378/chest.122.2.457. [DOI] [PubMed] [Google Scholar]
- Keller CA, Shepard JW, Jr, Chun DS, Vasquez P, et al. Pulmonary hypertension in chronic obstructive pulmonary disease. Multivariate analysis. Chest. 1986;90:185–92. doi: 10.1378/chest.90.2.185. [DOI] [PubMed] [Google Scholar]
- Kessler R, Faller M, Weitzenblum E, et al. “Natural history” of pulmonary hypertension in series of 131 patients with chronic obstructive lung disease. Am J Respir Crit Care Med. 2001;164:219–24. doi: 10.1164/ajrccm.164.2.2006129. [DOI] [PubMed] [Google Scholar]
- Khaja F, Parker JO. Right and left ventricular performance in chronic obstructive lung disease. Am Heart J. 1971;82:319–27. doi: 10.1016/0002-8703(71)90298-5. [DOI] [PubMed] [Google Scholar]
- King SJ, Booyse FM, Lin P, et al. Hypoxia stimulates endothelial cell angiotensin-converting enzyme antigen synthesis. Am J Physiol. 1989;256:C1231–8. doi: 10.1152/ajpcell.1989.256.6.C1231. [DOI] [PubMed] [Google Scholar]
- Lee DJ, Taraseviciene-Stewart L, Keith R, et al. The expression of prostacyclin synthase is decreased in the small pulmonary arteries from patients with emphysema. Chest. 2005;128:575S. doi: 10.1378/chest.128.6_suppl.575S. [DOI] [PubMed] [Google Scholar]
- Leuchte HH, Baumgartner RA, Nounou ME, et al. Brain natriuretic peptide is a prognostic parameter in chronic lung disease. Am J Respir Crit Care Med. 2006;173:744–50. doi: 10.1164/rccm.200510-1545OC. [DOI] [PubMed] [Google Scholar]
- MacNee W, Wathen CG, Flenley DC, et al. The effects of controlled oxygen therapy on ventricular function in patients with stable and decompensated cor pulmonale. Am Rev Respir Dis. 1988;137:1289–95. doi: 10.1164/ajrccm/137.6.1289. [DOI] [PubMed] [Google Scholar]
- MacNee W. An integrated approach to the treatment of pulmonary hypertension due to hypoxic lung disease. In: Peacock AJ, Rubin LJ, editors. Pulmonary Circulation: diseases and their treatment. Second edition. London: Arnold; 2004. pp. 398–409. [Google Scholar]
- Maggiorini M, Leon-Velarde F. High altitude pulmonary hypertension: a pathophysiological entity to different diseass. Eur Respir J. 2003;22:1019–25. doi: 10.1183/09031936.03.00052403. [DOI] [PubMed] [Google Scholar]
- Matthay RA, Schwarz MI, Ellis JH, Jr, et al. Pulmonary artery hypertension in chronic obstructive pulmonary disease: determination by chest radiography. Invest Radiol. 1981;16:95–100. doi: 10.1097/00004424-198103000-00003. [DOI] [PubMed] [Google Scholar]
- McGoon M, Gutterman D, Steen V, et al. Screening, early detection and diagnosis of pulmonary arterial hypertension. ACCP evidence based practice guidelines. Chest. 2004;126:14S–34S. doi: 10.1378/chest.126.1_suppl.14S. [DOI] [PubMed] [Google Scholar]
- Medical Research Council Working Party Long term domiciliary oxygen therapy in chronic hypoxic cor pulmonale complicating chronic bronchitis and emphysema. Lancet. 1981;i:681–6. [PubMed] [Google Scholar]
- Michelakis ED, Tymchak W, Noga M. Long-term treatment with oral sildenafil is safe and improves functional capacity and hemodynamics in patients with pulmonary arterial hypertension. Circulation. 2003;108:2066–9. doi: 10.1161/01.CIR.0000099502.17776.C2. [DOI] [PubMed] [Google Scholar]
- Nocturnal Oxygen Therapy Trial Group Continuous or nocturnal oxygen therapy in hypoxemic chronic obstructive lung disease: a clinical trial. Ann Intern Med. 1980;93:391–8. doi: 10.7326/0003-4819-93-3-391. [DOI] [PubMed] [Google Scholar]
- Oswald-Mammosser M, Apprill M, Bachez P, et al. Pulmonary hemodynamics in chronic obstructive pulmonary disease of the emphysematous type. Respiration. 1991;58:304–10. doi: 10.1159/000195950. [DOI] [PubMed] [Google Scholar]
- Oswald-Mammosser M, Weitzenblum E, Quoix E, et al. Prognostic factors in COPD patients receiving long-term oxygen therapy. Chest. 1995;107:1193–8. doi: 10.1378/chest.107.5.1193. [DOI] [PubMed] [Google Scholar]
- Oswald-mammosser M, Kessler R, Massard G, et al. Effect of lung volume reduction surgery on gas exchange and pulmonary hemodynamics at rest and during exercise. Am J Respir Crit Care Med. 1998;158:1020–5. doi: 10.1164/ajrccm.158.4.9710057. [DOI] [PubMed] [Google Scholar]
- Raeside DA, Brown A, Patel KR, et al. Ambulatory PAP monitoring during sleep and exercise in normal individuals and patients with COPD. Thorax. 2002;57:1050–3. doi: 10.1136/thorax.57.12.1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigat B, Hubert C, Corvol P, et al. PCR detection of the insertion/deletion polymorphism of the human angiotensin converting enzyme (DCP1) Nucl Acids Res. 1992;20:1433. doi: 10.1093/nar/20.6.1433-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin LJ. Diagnosis and management of pulmonary arterial hypertension: ACCP evidence-based clinical practice guidelines. Chest. 2004;126:7S–10S. doi: 10.1378/chest.126.1_suppl.7S. [DOI] [PubMed] [Google Scholar]
- Saito H, Dambara T, Alba M, et al. Evaluation of cor pulmonale on a modified short-axis section of the heart by magnetic resonance imaging. Am Rev Respir Dis. 1992;146:1576–81. doi: 10.1164/ajrccm/146.6.1576. [DOI] [PubMed] [Google Scholar]
- Scharf SM, Iqbal M, Keller C, et al. National Emphysema Treatment Trial (NETT) Group Hemodynamic characterization of patients with severe emphysema. Am J Respir Crit Care Med. 2002;166:314–22. doi: 10.1164/rccm.2107027. [DOI] [PubMed] [Google Scholar]
- Sciurba FC, Rogers RM, Keenan RJ, et al. Improvement in pulmonary function and elastic recoil after lung volume reduction surgery for diffuse emphysema. N Engl J Med. 1996;334:1095–9. doi: 10.1056/NEJM199604253341704. [DOI] [PubMed] [Google Scholar]
- Skwarski KM, Morrison D, Barratt A, et al. Effects of hypoxia on renal hormonal balance in normal subjects and in patients with COPD. Respir Med. 1998;92:1331–6. doi: 10.1016/s0954-6111(98)90138-x. [DOI] [PubMed] [Google Scholar]
- Spiropoulos K, Trakada G, Nikolaou E, et al. Endothelin-1 levels in the pathophysiology of chronic obstructive pulmonary disease and bronchial asthma. Respir Med. 2003;97:983–9. doi: 10.1016/s0954-6111(03)00129-x. [DOI] [PubMed] [Google Scholar]
- Sweeney M, Yuan JXJ. Hypoxic pulmonary vasoconstriction: role of voltage-gated potassium channels. Respiratory Research. 2000;1:40–8. doi: 10.1186/rr11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thabut G, Dauriat G, Stern JB, et al. Pulmonary hemodynamics in advanced COPD candidates for lung volume reduction surgery or lung transplantation. Chest. 2005;127:1531–6. doi: 10.1378/chest.127.5.1531. [DOI] [PubMed] [Google Scholar]
- Timms RM, Khaja FU, Williams GW, the NOTT group Hemodynamic response to oxygen therapy in chronic obstructive pulmonary disease. Ann Intern Med. 1985;102:29–36. doi: 10.7326/0003-4819-102-1-29. [DOI] [PubMed] [Google Scholar]
- Tschernko EM, Gruber EM, Jaksch P, et al. Ventilatory function and mechanics during exercise before and after lung volume reduction surgery. Am J Respir Crit Care Med. 1998;158:1424–31. doi: 10.1164/ajrccm.158.5.9702086. [DOI] [PubMed] [Google Scholar]
- Turino GM, Goldring RM, Heineman MD. Water, electrolytes and Acid Based Relationships in Chronic Cor Pulmonale. Progress in Cardiovascular Disease. 1970;12:467–83. [Google Scholar]
- Vlahakos DV, Kosmas EN, Dimopoulo I, et al. Association between activation of rennin-angiotensin system and secondary erythrocytosis in patients with chronic obstructive pulmonary disease. Am J Med. 1999;106:158–64. doi: 10.1016/s0002-9343(98)00390-8. [DOI] [PubMed] [Google Scholar]
- Vlahakos DV, Kosmas EN. Losartan reduces hematocrit in patients with chronic obstructive pulmonary disease and secondary erythrocytosis. Ann Intern Med. 2001;134:426–7. doi: 10.7326/0003-4819-134-5-200103060-00022. [DOI] [PubMed] [Google Scholar]
- Vonbank K, Ziesche R, Higenbottam TW, et al. Controlled prospective randomized trial on the effects on pulmonary hemodynamics of the ambulatory long term use of nitric oxide and oxygen in patients with severe COPD. Thorax. 2003;58:289–93. doi: 10.1136/thorax.58.4.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vonk Noordegraaf A, Marcus JT, et al. The effect of right ventricular hypertrophy on left ventricular ejection fraction in pulmonary emphysema. Chest. 1997;112:640–5. doi: 10.1378/chest.112.3.640. [DOI] [PubMed] [Google Scholar]
- Weg IL, Rossoff L, McKeon K, et al. Development of pulmonary hypertension after lung volume reduction surgery. Am J Respir Crit Care Med. 1999;159:552–6. doi: 10.1164/ajrccm.159.2.9802056. [DOI] [PubMed] [Google Scholar]
- Weir EK, Lopez-Barneo J, Buckler KJ, et al. Acute oxygen sensing mechanisms. New Engl J Med. 2005;353:2042–53. doi: 10.1056/NEJMra050002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weitzenblum E, Loiseau A, Hirth C, et al. Course of pulmonary hemodynamics in patients with chronic obstructive pulmonary disease. Chest. 1979;75:656–62. doi: 10.1378/chest.75.6.656. [DOI] [PubMed] [Google Scholar]
- Weitzenblum E, Sautegeau A, Ehrhart M, et al. Long term course of pulmonary arterial pressure in chronic obstructive pulmonary disease. Am Rev Respir Dis. 1984;130:993–8. doi: 10.1164/arrd.1984.130.6.993. [DOI] [PubMed] [Google Scholar]
- Weitzenblum E, Sautegeau A, Ehrhart M, et al. Long-term oxygen therapy can reverse the progression of pulmonary hypertension in patients with chronic obstructive pulmonary disease. Am Rev Respir Dis. 1985;131:493–8. doi: 10.1164/arrd.1985.131.4.493. [DOI] [PubMed] [Google Scholar]
- Weitzenblum E, Apprill M, Oswald-Mammosser M, et al. Pulmonary hemodynamics in patients with chronic obstructive pulmonary disease before and during an episode of peripheral edema. Chest. 1994;105:1377–82. doi: 10.1378/chest.105.5.1377. [DOI] [PubMed] [Google Scholar]
- Weitzenblum E. Chronic cor pulmonale. Heart. 2003;89:225–30. doi: 10.1136/heart.89.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson M, Langhorn CA, Heath D, et al. A pathological study of 10 cases of hypoxic cor pulmonale. Q J Med. 1988;66:65–85. [PubMed] [Google Scholar]
- Workshop report: global strategy for the diagnosis, management, and prevention of COPD. Updated 2005.http://www.goldcopd.com
- World Health Organization Chronic cor pulmonale. A report of the expert committee. Circulation. 1963;27:594–8. [Google Scholar]
- Yamamoto K, Burnett JC, Jr, Jougasaki M, et al. Superiority of brain natriuretic peptide as a hormonal marker of ventricular systolic and diastolic dysfunction and ventricular hypertrophy. Hypertension. 1996;28:988–94. doi: 10.1161/01.hyp.28.6.988. [DOI] [PubMed] [Google Scholar]
- Zielinski J, Tobiasz M, Hawrylkiewicz I, et al. Effects of long term oxygen therapy on pulmonary hemodynamics in COPD patients. A 6 year prospective study. Chest. 1998;113:65–70. doi: 10.1378/chest.113.1.65. [DOI] [PubMed] [Google Scholar]