Abstract
Multidetector-row computed tomography (MDCT) can be used to quantify morphological features and investigate structure/function relationship in COPD. This approach allows a phenotypical definition of COPD patients, and might improve our understanding of disease pathogenesis and suggest new therapeutical options. In recent years, magnetic resonance imaging (MRI) has also become potentially suitable for the assessment of ventilation, perfusion and respiratory mechanics. This review focuses on the established clinical applications of CT, and novel CT and MRI techniques, which may prove valuable in evaluating the structural and functional damage in COPD.
Keywords: emphysema, computed tomography, magnetic resonance, airways, functional imaging
Introduction
Chronic obstructive pulmonary disease (COPD), which encompasses both chronic bronchitis and emphysema, is one of the commonest respiratory conditions of adults in the developed world. Despite being defined by the presence of abnormal pulmonary function, namely a largely irreversible airflow obstruction, COPD patients also exhibit specific morphologic changes in the central and peripheral airways, lung parenchyma, and pulmonary vasculature. Those changes are well depicted by computed tomography (CT), whereas magnetic resonance imaging (MRI) has brought a new dimension to studies of COPD. Both techniques have much improved our understanding of the pathophysiology of this disorder.
CT indices of emphysema reflect lung anatomy and represent the best way to assess emphysema severity in life (Newell et al 2004). The introduction of multidetector-row CT (MDCT) scanners has provided powerful tools to evaluate changes in both small and large airways. In addition, dynamic imaging via MDCT allows the assessment of perfusion and ventilation in the lung parenchyma. Therefore, CT is able to precisely define the pathological process by providing accurate anatomic informations as well as functional data from the area of interest.
The increasing interest in the definition of the functional damage in COPD patients has also motivated investigation in MRI. Improved imaging protocols are now available on every MR system for the morphologic evaluation of the lung. Several investigators have also demonstrated that, within the same examination, MRI has potentials to provide clinically relevant information on lung function.
This review focuses on the definition by CT of a number of different phenotypes observed in COPD. In addition, it summarizes the main CT quantification systems of morphological abnormalities and the potential role of CT in evaluating COPD-related functional abnormalities. Finally, it presents an overview of some of the recent MR techniques for measuring lung function, which in future might become routinely available for the assessment of COPD patients.
COPD phenotypes according to CT findings: a practical approach
Although the diagnostic criteria established by the Global Strategy for the Diagnosis, Management and Prevention of Chronic Obstructive Lung Disease (GOLD [Global Initiative for Chronic Obstructive Lung Disease]) do not currently include CT findings (Pauwels et al 2001), a consistent body of literature suggests that CT represents an important tool in the clinical setting. COPD in primary care is a heterogeneous condition (O’Brien et al 2000) and the challenge for the clinicians is to identify what causes the airflow limitation and to separate reversible from irreversible components of such limitation. In this regard, CT can reveal what both clinical examinations and pulmonary function tests (PFTs) often cannot, namely the lesions that may mimic COPD and the predominance as well as the interplay between emphysema and airways disease.
A broad spectrum of disorders, including bronchiectasis, upper airway lesions, bronchiolar diseases, and interstitial lung diseases, are often associated with chronic airflow obstruction. CT, and in particular HRCT, can discriminate between different causes of airflow obstruction. In order to assess the diagnostic utility of HRCT in diagnosing COPD, Kurashima et al (2005) showed in a cohort of 516 consecutive patients whose FEV1/FVC was less than 70% after inhalation of bronchodilator, that HRCT was essential in revealing that 12.7% of them had pulmonary diseases other COPD, including sarcoidosis, diffuse panbronchiolitis and pneumoconiosis. Undoubtedly, a better characterization of the underlying disease is crucial in order to reduce the variability of response to treatment in COPD patients. The variable proportion of emphysema, bronchial wall thickening and air-trapping – all common features of COPD – can be morphologically characterized by HRCT.
HRCT scan may reveal evidence of pulmonary emphysema even in asymptomatic smokers with a normal lung function. Recent data from our laboratory on 43 cases classified according to the GOLD criteria, demonstrated that 13/18 subjects with Stage 0 had emphysema on HRCT. Although it has been demonstrated that in patients with stage 0 symptoms of chronic bronchitis do not increase the risk of progression in COPD, no longitudinal study has clarified the role of emphysema at that stage. Recent studies highlight the importance of identifying and quantifying emphysema in patients with COPD as the emphysematous phenotype is associated with more severe disease (Boschetto et al 2003, 2006). COPD patients with emphysema on HRCT display an higher BODE index (body mass index, airflow obstruction, dyspnoea, exercise performance) and a lower inspiratory capacity to total lung capacity ratio (IC/TLC) than those without emphysema, suggesting a more important systemic involvement in emphysematous patients (Boschetto et al 2006a). Nevertheless, it has been also shown that the distribution of emphysema on CT predicts mortality, with an improved survival in patients with more extensive emphysema in the upper zones compared with the lower zones of the lung (Martinez et al 2006). The authors speculated that lower-lobe emphysema may be either a marker of increased disease severity or, alternatively, a phenotypic or pathobiologic variant (Martinez et al 2006). However, the same authors suggested that their findings may not be applicable to all patients with COPD, since their subjects were part of the National Emphysema Treatment Trial (NETT), and exhibited specific features (Martinez et al 2006).
Emphysema and airway-related COPD are distinct phenotypes and have different relationships with smoking (Patel et al 2006). Airway and parenchymal phenotypes show familial concordance. Moreover, differences in the extent of emphysema are not explained by cumulative smoking, suggesting a complex gene–environment interaction (Patel et al 2006). The ability to separate airway-predominant from parenchymal-predominant pathology in COPD may prove useful in applying specific therapies designed to prevent airway remodelling and parenchymal destruction (Grenier 2005). Measurements of lung physiology do not always discriminate between the abnormalities resulting from predominant emphysema from those resulting from predominant small airways disease. As discussed later, advances in quantitative CT scanning have the potential separate and quantify both emphysema and airway component in COPD patients. However, a simple visual assessment may already provide important informations. Despite an inherent subjectivity in the visual evaluation, many variables (particularly the analysis of lung texture) do not lend themselves to quantification using usual automated methods. The insight that some patients given the label of COPD have “pure” airways diseases or emphysema has been provided by HRCT. Furthermore, “naked eye” has additional advantages in detecting heterogeneity and co-morbidities on HRCT scans of COPD patients. Radiologists can often distinguish between a smoking disease phenotype (usually characterized by centrilobular emphysema) and lesions associated with alpha1-proteinase inhibitor (α1-antitrypsin) deficiency (Figures 1 and 2). In addition, HRCT can suggest the coexistence of panlobular and centrilobular emphysema in the same individual, as some patients with α1-antitrypsin deficiency are also smokers (Copley et al 2002). Nevertheless, it should be also appreciated that the lung surrounding the emphysematous lesions does not always appear normal as smoking-induced infiltrative lung diseases may coexist (Hansell et al 2005).
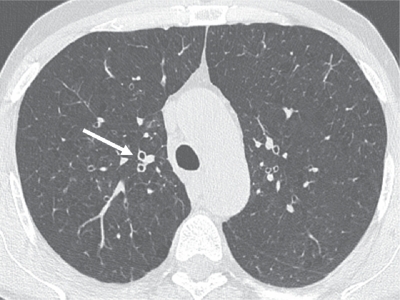
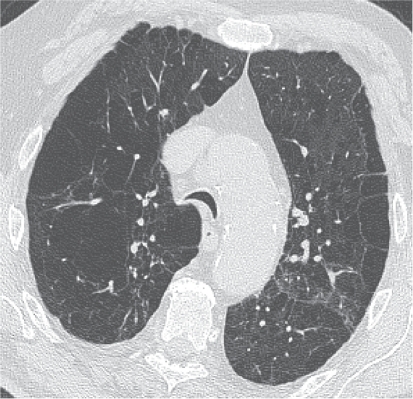
Figure 1.
Thin-section CT scan of a smoker with chronic cough associated with chronic obstructive pulmonary disease. The segmental bronchial walls (white arrow) in the upper lobes are thickened. Early centrilobular emphysema is also present.
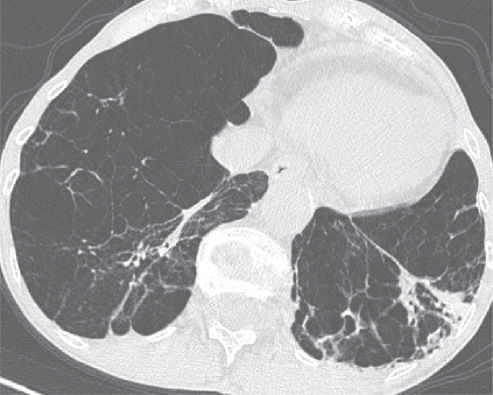
Figure 2.
α1-Antitrypsin deficiency associated panlobular emphysema. There is a generalized decreased attenuation of the lung parenchyma and a striking paucity of pulmonary vasculature. Bronchiectasis are more prominent in the left lower lobe and coexist with patchy consolidation (probable infection). It is worth keeping in mind that panlobular emphysema shows an increased prevalence and extent of long lines in comparison to patients with obliterative bronchiolitis.
It has been recently shown that COPD patients with chronic bronchitis display an increased bronchial wall thickening compared to patients without chronic bronchitis (Orlandi et al 2005). Despite being a non specific sign, it looks worth relating bronchial wall thickening at segmental level (eg, the trunk of the apical bronchus of the right upper lobe, which is usually sliced in cross section and easily identified on CT scans) with emphysema in some individuals (Figure 1). The wall thickening in large or intermediate airways provides only a rough estimate of small airways disease, whereas air trapping on HRCT in current smokers or ex-smokers correlates with the inflammatory infiltration of peripheral airways (Berger et al 2003). Accordingly, air-trapping on CT could mirror the peripheral airway obstruction, which is hardly detectable with conventional lung function tests. However, a “mosaic” pattern representing small airway disease is difficult, if not impossible, to identify on HRCT if a background of widespread emphysema co-exists (Aziz et al 2005). In COPD patients, areas of decreased attenuation of the lung parenchyma can be seen together with bronchiectasis. In this context, the mosaic pattern is likely to reflect the accompanying obliteration observed in small airway disease (Kang et al 1995).
Bronchiectasis in COPD patients has been sparsely reported. The presence of bronchiectasis on CT may provide a means of identifying those patients with COPD who are at risk of more severe disease exacerbations (Figure 2). One study reported that 29% of COPD patients who developed disease exacerbations displayed bronchiectatic changes on HRCT (O’Brien et al 2000). In a study by Patel et al (2004), the extent of bronchiectasis in the lower lobes was related to colonization by a potential pathogen in the lower airways, increased airway inflammatory markers, and longer time to symptom recovery after exacerbation. However, no correlation was observed with frequency of exacerbations (Patel et al 2004). It is unclear whether COPD patients with bronchiectasis should be regarded as a specific phenotype or whether bronchiectasis simply represents a concomitant disease. It is possible that multiple patho-physiologic alterations – including damaged muco-ciliary transport, localized or diffuse peripheral obliteration of the bronchial tree or lung tissue scarring act in concert in COPD – produce the typical structural abnormalities seen on HRCT. Longitudinal studies are required in order to clarify the significance of the bronchiectatic structural changes observed in COPD, and their influence on the natural history of this condition (Patel et al 2004).
The last decade has seen much progress toward understanding of the larger airway diseases via MDCT. Small pits can be often detected along the inner surfaces of the large bronchi, using volumetric thin-section CT (Zompatori et al 2006). It has been suggested that such pits (or diverticula) may correspond to dilated bronchial gland ducts opening into the airway lumen (Grenier et al 2004; Zompatori et al 2006). Zompatori et al reported that diverticula can be often seen in patients with chronic bronchitis (Figure 3) together with abnormalities typical of chronic bronchitis, such as erythema, mucosal irregularities, secretions, and friability (Zompatori et al 2006). Since even physician-confirmed self-reported diagnosis of chronic bronchitis rarely satisfies the criteria for chronic bronchitis, the evaluation of the bronchial pits might support the clinical diagnosis if they reveal (through pathologicradiologic correlations) signs of chronic bronchitis.
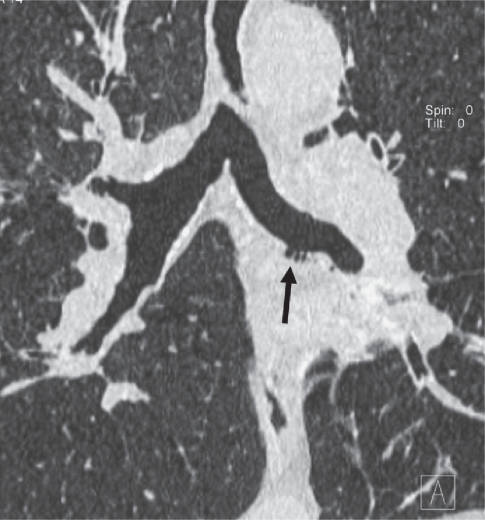
Figure 3.
CT coronal reformation of a patient with symptoms of chronic bronchitis. Small bronchial diverticula (arrow) seen as outpouchings of the bronchial lumen visible along the left main bronchus.
CT quantification emphysema
CT has been used to detect and characterize emphysema and grade its severity. Emphysema is evident on CT scans performed with 5–10 mm thick sections in the vast majority of cases. Thinner sections reconstructed by using an edge enhancing algorithm (high resolution CT, HRCT) improve the definition of emphysematous changes in the lung. On the other hand, MDCT allows a volumetric evaluation of the chest with 1 mm collimation (volumetric HRCT). The most prominent features of the MDCT technology are represented by an increased speed and post-processing algorithms. When using up-to-date scanner technology, even patients with severe COPD are able to hold their breath both in inspiration and expiration (Zaporozhan et al 2005). The HRCT discriminates between various types of emphysema (Figures 1 and 2). In particular, the use of minimum intensity projection (MinIP) images allows the detection of subtle emphysema (Remy-Jardin et al 1996). Thin-section volumetric acquisitions provide an isotropic data set, which can be rearranged in any plane. As a result, these multiplanar reformations allow to easily evaluating the distribution of the emphysematous areas.
Methods for quantifying emphysema on CT include visual estimation and automated techniques, both of which have their proponents (Desai et al 2006). Both methods demonstrated good correlation with pathological assessment of emphysema severity (Bergin et al 1986; Muller et al 1988; Kuwano et al 1990; Gevenois et al 1995; Gevenois, De Vuyst 1996). The extent of the disease may be visually graded according to a categorical scale, which can be confined to selected levels (eg, three or five) (Bergin et al 1986; Mishima, Itoh et al 1999). A potential drawback of visual estimation is measurement of “noise” due to inter-observer variation, although observer disagreement in scoring the extent of emphysema has not been a major factor in previous studies (Cleverley et al 2000; Desai et al 2006). The potential interobserver variation is balanced by the speed and the simplicity of the technique.
The density mask method represents a cornerstone of CT lung assessment for emphysema (Muller et al 1988; Gevenois, De Vuyst et al 1996). It applies a density threshold (HU) below which all voxels are considered to be emphysema (Muller et al 1988). Using precise morphometric measurements of resected lung tissue, Bankier et al (1999) showed that observers, regardless of their experience, tend to overestimate the extent of emphysema on CT, whereas CT densitometry correlates better with the morphometric reference. The density mask is particularly useful in characterizing mild/moderate and severe emphysema and has been used in the NETT to identify subgroups of patients who show benefit from lung volume reduction surgery (LVRS) (Fishman et al 2003). The percentage of emphysema quantified by density mask is also predictive of survival in α1-antitrypsin–induced emphysema (Dawkins et al 2003).
With the software available on most MDCT scanners, computer-aided recognition and quantification of emphysema is now even faster than human evaluation. In addition, it is possible to apply techniques measuring lung density to volumetric data. The resolution achieved in thoracic CT allows the application of high-precision 3D image analysis tools to CT data (Kuhnigk et al 2005) (Figure 4). The analyses allow a convenient, regional (lobar or segmental) assessment of CT parameters of the lung, such as total volume, mean density, pixel index, and emphysema type (Kuhnigk et al 2005). The regional distribution of emphysema as it may influence the mortality of COPD patients (Martinez et al 2006). Therefore, its objective assessment might prove crucial. However, a density-masking approach alone is not sufficient to accurately distinguish between normal and diseased lung, especially in the case of early or mixed pathologic processes (Uppaluri et al 1997, 1999; Hoffman et al 2006). Further, CT densitometry may be influenced by several factors (eg, age, weight, beam hardening from adjacent ribs etc.) and calibration must be performed in order to obtain reliable densitometry results (Shaker et al 2004; Stoel et al 2004; Coxson and Rogers 2005; Gevenois, Scillia et al 1996). Neither visual nor pure densitometric approaches to CT quantification of emphysema are, therefore, perfect.
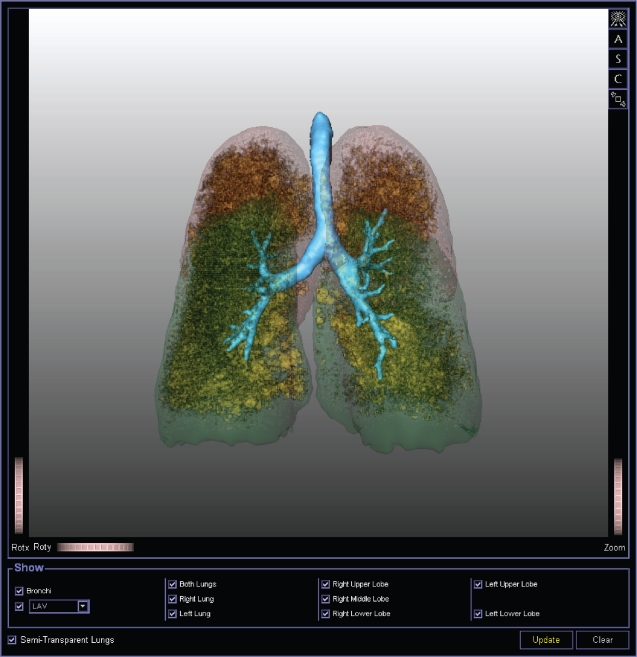
Figure 4.
3D rendering of thin-section CT (postero-anterior view) scan with tracheobronchial tree (azure-blue) and both lower lobes (green) and upper lobes (red). Inside lobes all pixels of −950 HU or less are highlighted (yellow), identifying areas of emphysema. Image generated using MeVisPULMO software (www.mevis.de). (Courtesy of Jan-Martin Kuhnigk, Bremen, Germany).
Beside a pure analysis of the percentage of voxels below a certain threshold, CT scan data can be approached by a more sophisticated analysis tools. Contiguous emphysema areas can be clustered to obtain the volumes for small-sized, medium-sized, and large-sized emphysematous areas (Blechschmidt et al 2001; Zaporozhan et al 2005). The cluster distribution was reported to be useful in revealing the pattern of progression of emphysema (Mishima, Hirai et al 1999). Furthermore, the cluster analysis could be performed on scans obtained by scanners of different manufacturers. Uppaluri et al (1997, 1999) examined multiple features of the CT images and X-ray attenuation values to describe the lung and developed the Adaptive Multiple-Feature Method (AMFM), which can assess as many as 22 independent textural features from HRCT scans to classify a tissue pattern. This “texture” is very sensitive for lung analysis and can distinguish smokers from non-smokers in the absence of other disease (Hoffman et al 2006). Nonetheless, this technique has not been validated with pathological correlation and is limited to the single scanner it has been trained for.
CT quantification of small airways
Several studies confirmed that airways smaller than 2 mm in internal diameter represent the major site of airway obstruction in patients with COPD (Hogg et al 2004). The 2 mm airways are located between the 4th and the 14th generation of tracheobronchial tree branching. Recent histologic evaluation of a large group of cases has shown that airflow limitation strongly correlated with severity of the lumen occlusion by inflammatory exudates and wall thickening of the small airways by a repair and remodeling process (Hogg 2006).
A number of CT scoring systems have been developed that allow an assessment of the extent and distribution of airway abnormalities. Air-trapping is a key finding for depicting small airways obstruction on HRCT scans. Berger et al (Berger et al 2003) showed that air trapping on HRCT in current smokers or ex-smokers correlated with inflammatory infiltration of peripheral airways. The areas of decreased attenuation may not be visible on inspiratory CT. A dynamic acquisition by MDCT, using low dose over the entire thorax during continuous forced expiratory maneuver, provides an exhaustive assessment of air-trapping and airway collapse (Grenier et al 2002, 2005). Quantification of air-trapping extent is obtained by semiquantitative visual score that is, however, not always straightforward in COPD patients. More sophisticated approaches to the quantification of obstructive lung diseases include evaluation of texture characteristics of the lung parenchyma, which is able to discriminate between apparently non-specific areas of decreased attenuation (Chabat et al 2003).
It has been shown that the size of the large and intermediate airways reflect airway dimensions in the smaller airways (Nakano et al 2005). These data suggest that, in COPD, measuring airway dimensions in the larger bronchi, which are accurately assessed by CT, can provide an estimate of small airway remodeling (Figure 1). There has been considerable interest in the development of objective measurements to determine airway wall dimensions; this is largely due to the interobserver disagreement in the visual interpretation of bronchial wall thickness on CT scans (Muller and Coxson 2002). The most commonly reported method for measuring the airway lumen and wall areas relies on the “full-width-at-half-maximum” (or “half-max”) technique, in which the inner and outer airway wall boundaries are defined as the point corresponding to the half maximal intensity of the airway wall voxels (Nakano et al 2000; de Jong et al 2005). Using the half-max method, Nakano and colleagues (2000) showed that an increased thickness of the apical segmental bronchus in the right upper lobe, as assessed by HRCT, correlated with the severity of airflow obstruction and gas trapping (FEV1, FVC, and FEV1/FVC) in smokers with COPD independently of the degree of emphysema measured on the same HRCT scans. However, Nakano, Whithall et al (2002) have also shown that the half-max method results in very large fractional errors in the measurements of small bronchi. Indeed, measurements of airway lumen and wall area depends on the lung volume and angle between the airway central axis and the plane of section (Grenier et al 2004). This has led to efforts to develop alternative computational methods to overcome limitations related to the use of two-dimensional planar data for airway analysis.
The advent of MDCT has changed the approach to airways measurements. Hasegawa et al (2006) developed a new software, which uses curved multiplanar reformations for depicting a three-dimensional airway skeleton and measures luminal areas of airways and wall areas (based on the full width at half-maximum principle). Using phantoms, they demonstrated that it is possible to obtain accurate short images with an inner diameter as small as 2 mm and a wall thickness of 1 mm, and that the short axis images obtained are precisely perpendicular to the long axis even with a sigmoid-shaped phantom (Hasegawa et al 2006). The same authors also demonstrated in patients with COPD that FEV1 (% predicted) correlates strongly with airway luminal area and, to a lesser extent, with wall thickening from the third to the sixth generation of both apical and basal bronchus, and that the correlation coefficients improves as airway size decreases (Hasegawa et al 2006). Newer airway segmentation methods have been also shown to be robust in the presence of mixed diseases (including emphysema, fibrosis and bronchiectasis) and when applied to images acquired using low-dose scanning protocols (Hoffman et al 2006). These methods may also provide reliable comparisons of airway dimensions between individuals or in the same individual over time (Hoffman et al 2006).
CT functional imaging
Lung structure and function can be linked by CT. The term functional imaging is open to two broad interpretations: one is the idea that an understanding of functional abnormalities can be obtained by correlation with objective quantification of morphological abnormalities seen on detailed images of the lung (Hansell 2004). These correlations are complex in COPD patients. The chronic airflow limitation is attributed to the narrowing of the small airway lumen secondary to morphological changes and to a decrease in lung elastic recoil. Several studies have shown the interdependence of airway remodeling and emphysema in their contribution to the degree of airflow obstruction (Gurney et al 1992; Gelb et al 1993; Nakano, Muller et al 2002; Aziz et al 2005). In COPD, the extent of centrilobular emphysema is quite variable for a given level of airflow limitation, confirming that the extent of emphysema is not the only morphologic abnormality causing airflow obstruction. In the HRCT scan studies of structure-function, correlation between the degree of emphysema and FEV1 has been extremely variable; usually, correlation coefficient (rho) values are in the range of −0.7, indicating that only about half of the variability of the population has been accounted for (Cosio and Snider 2001). The CT evaluation of the airway diseases improves the embracement of those functional-structural correlations.
The ratio of inspiratory to expiratory CT density may represent an easy index, which allows to evaluate the contribution of small airways (Eda et al 1997). In particular, the expiratory scans provide critical insights into the relationship between structure and function. Expiratory HRCT measurements correlate best with pulmonary function tests (PFTs), particularly with measures of air trapping (O’Donnell et al 2004; Zaporozhan et al 2005). Further, O’Donnell et al (2004) showed that the ratio of the mean lung density (MLD) on expiration to MLD on inspiration (E/I) is more effective in distinguishing smokers with and without airflow obstruction than inspiratory HRCT, carbon monoxide transfer factor (TLco) or carbon monoxide transfer coefficient (Kco). Interestingly, the same authors showed that the sputum inflammatory cell count strongly correlated with measurements of peripheral airway obstruction and gas trapping (RV/TLC ratio), MLD on expiration and the E/I ratio; on the other hand, no correlation was observed with either inspiratory percentage of emphysema or TLco, both indicators of emphysema. Thereby, they speculated that increased sputum neutrophil counts is likely to reflect the more distal neutrophilic inflammation that is associated with bronchiolitis and/or destruction of peripheral airway alveolar attachments (O’Donnell et al 2004).
The anatomical site of the emphysema determines the degree of impairment of lung function. This issue has been addressed in a number of studies showing that the extent of emphysema on HRCT in the upper zones correlates with functional abnormalities less strongly than the extent of emphysema in the lower zones (Parr et al 2004). Furthermore, the radial distribution of emphysema may have functional consequences. Emphysema located in the lung periphery has less effect on DLco than more central emphysema (Nakano et al 1999).
While most functional-morphologic correlations between extent of emphysema and various functional parameters have provided a somehow contradictory array of data, stepwise analysis has been usefully applied in an opposite manner. Desai et al (2006) constructed a composite physiologic index (CPI) against the morphologic extent of disease in order to calibrate the quantification of emphysema using pulmonary function tests in isolation. FEV1 and Kco levels were the only retained variables by the stepwise technique; interestingly, the equation explanatory power was higher when using visual score (r2 = 0.57) than when quantification of emphysema was automated (r2 = 0.48). However, it has to be emphasised that the composite index does not replace the morphological information provided by CT but does add precision to evaluation of functional severity (Desai et al 2006).
In terms of airflow limitation secondary to emphysema, the contribution of large airways diseases should not be over-looked. An association between tracheobronchomalacia and COPD has been also suggested (Heussel et al 2004), although prevalence data are limited. Because tracheobronchomalacia escapes detection on routine end-inspiratory imaging studies, it is widely considered an under-diagnosed condition. However, cine MDCT during coughing has been showed to be a powerful tool for the diagnosis of tracheomalacia (Boiselle et al 2006) (Figure 5). Correlation with clinical symptoms may also be helpful in determining the clinical significance of patients who show tracheal collapse between 50% and 69% during coughing (Boiselle et al 2006) (Figure 5). In this regard, it is difficult to accurately identify the relative contributions of tracheomalacia to airflow obstruction.
Figure 5.
Tracheomalacia elicited by coughing maneuver in 65-year-old man. CT scan shows near complete collapse of airway lumen, consistent with tracheomalacia. Advanced centrilobular and paraseptal emphysema also coexist.
The second interpretation of functional imaging is based on the idea of quantifying specific functional aspects of the lungs (both pulmonary ventilation and perfusion) and depicting the data pictorially (for example a color-coded map of regional ventilation) (Hansell 2004) (Figure 6).
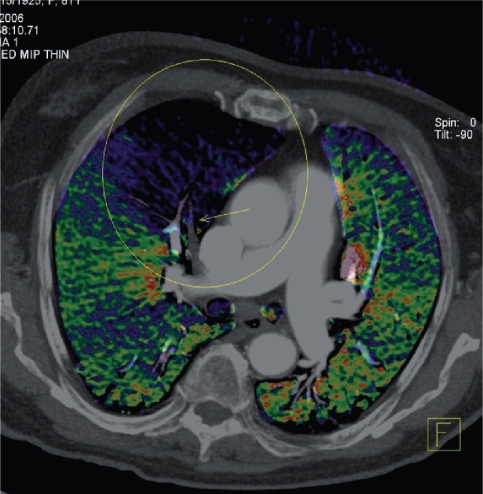
Figure 6.
Example of a reconstruction from dual-energy CT in a patient presenting with an exacerbation of chronic obstructive pulmonary disease. Axial image with superimposed color-coded iodine distribution shows the lung perfusion and a defect (blue-black) caused by an occluding embolus in the right descending artery (arrow). (Courtesy of Christian Fink, MD, Munich, Germany).
Ventilation assessed by CT
The technique of Xe-CT was recognised over 30-years ago (Foley et al 1978). Stable (non-radioactive) xenon gas has a K edge similar to that of iodine and is, therefore, a potent X-ray attenuator, which provides good contrast enhancement when used in conjunction with CT scanning. Regional ventilation is measured from time course of CT density change during a multi-breath wash-in and wash-out of radiodense Xe gas (Hoffman and Chon 2005). The enhancement due to xenon yields direct assessments of well-ventilated versus poorly ventilated lung regions. More recently, Xe has been used in an animal experiment involving monochromatic synchrotron radiation CT (Bayat et al 2001). By obtaining simultaneous images at two different levels, absolute Xe concentrations can be calculated via a subtraction mechanism. Furthermore, the spatial distribution of Xe concentrations in the airspaces, the dynamic of airspace filling as well as images of airways with a diameter in the range of 1 mm could be obtained (Bayat et al 2001). With the introduction of dual energy CT scanners, single-breath Xe (without previous unenhanced acquisitions) technique is currently experiencing new interest (Figure 6). However, the reliability and clinical diagnostic value of these applications needs to be confirmed in future studies (Johnson et al 2006).
Perfusion assessed by CT
Various approaches for determining pulmonary perfusion have been described. After the injection of an iodinated contrast material, an ECG-gated dynamic MDCT scanning can be performed (Hoffman et al 2006). Lung segmentation and post-processing analysis are used to determine the mean transit time and pulmonary blood flow normalized to regional air content (Schoepf et al 2004) (Figure 6). These measurements may be potentially used for detecting the early micro-vascular changes in the lung parenchyma associated with emphysema. Furthermore, Hoffman and co-workers (2006) have recently proposed an attractive hypothesis regarding the pathogenesis of emphysema. Regional alterations in blood flow parameters may not only serve as an early marker of inflammatory processes but may also cause the pathologic process itself, leading to emphysema in a subset of smokers (not all smokers have emphysema) (Hoffman et al 2006). An inherent loss of the ability to blunt regional hypoxic pulmonary vasoconstriction (HPV), when the regional hypoxia is due to inflammatory events, may set the individual up for the evolution of the tissue destruction, which is a hallmark of emphysema (Hoffman et al 2006). Accordingly, Remy-Jardin and colleagues (2002) have demonstrated that, in a subset of smokers who show decline over time in pulmonary function tests, this decline correlates with the development of emphysema in regions showing signs of ground glass and micro-nodules at an earlier time point. However, not all smokers with ground glass and micronodules developed emphysema in these regions but these subjects also did not show a decline in pulmonary function tests (Remy-Jardin et al 2002). Although shunting blood away from regions of poor ventilation is commonly believed to be an advantageous and protective mechanism, when the poor ventilation is secondary to inflammatory processes (ie, micronodules and ground glass found by Remy-Jardin and colleagues 2002), maintaining an adequate blood supply to that area can be beneficial for the resolution of the inflammation (Hoffman et al 2006).
Functional evaluation of the lung with MRI
Magnetic resonance imaging of the lung is notoriously challenging (Kauczor and Kreitner 1999). The low proton density of lung parenchyma usually leads to poor signal using standard MRI techniques. Susceptibility artifacts caused by multiple air-tissue interfaces, and motion artifacts due to cardiac and respiratory function (Kauczor and Kreitner 1999) further contribute in degrading image quality. Recent technical advances in MR pulse sequences and hardware design have improved the visualization of lung parenchyma. The possibility to administer contrast agents via inhalation or venous injection has further enhanced the potential capabilities of MRI for the clinical evaluation of lung morphology and function (Hatabu et al 1999; Kauczor and Kreitner 1999; Biederer et al 2003).
A considerable contribution in the understanding of structure-function relationships in the lung has been brought by the use of non-radioactive noble gas isotopes in MRI (ie, 3-He and 129-Xe) (Albert et al 1994; Kauczor et al 1996; Salerno et al 2001; van Beek et al 2004). Although the first hyperpolarized gas MR images of the lung were obtained with 129-Xe (Albert et al 1994), much of the recent clinical research has been focused on 3-He MRI. Following the exposure to polarized laser light, 3-He increases its spin polarization and can be used as inhaled MR contrast agent. The signal of hyperpolarized 3-He is detected by MR coils tuned to resonance frequency of the gas. The signal enhancement produced by hyperpolarized 3-He is measured with high temporal and spatial resolution three-dimensional imaging techniques. This enables the dynamic assessment of gas flow into the airways (ie, spirometry) and pulmonary airspace (ie, ventilation) (Gast et al 2002). Within the same protocol, 3-He density images can be obtained, demonstrating the homogeneity of ventilation. Diffusion weighted imaging are also used to measure the degree of restriction of the Brownian motion of 3-He atoms, reflecting lung microstructure (Morbach et al 2005). Additionally, as the decay of 3-He hyperpolarization is dependent on the alveolar concentration of oxygen, the local intrapulmonary PO2 can be measured (Wild et al 2005), providing indirect assessment of pulmonary perfusion, ventilation/perfusion ratio and oxygen uptake. Potential applications of this imaging tool have been shown in recent studies (Ley et al 2004; Woodhouse et al 2005; Woods et al 2006). In the functional characterization of patients with COPD, in smokers, in asthmatics and in a subject with allergies, ventilation defects can be accurately depicted. Diffusion weighted images demonstrate quantitatively the emphysematous changes in the pulmonary structure. Good agreement with lung function tests in the characterization of emphysema (ie, hyperinflation, large and small airway disease) has been demonstrated (Ley et al 2004).
Similarly to hyperpolarized 3-He, molecular oxygen can be used as an inhaled MR contrast agent (Loffler et al 2000; Mai et al 2000). In oxygen-enhanced MRI, however, the signal intensity of the lung increases by different mechanism. Following 100%-oxygen inhalation, the paramagnetic effect of this gas on proton spins of blood water causes a T1-shortening effect in the lung. Signal enhancement is generated after molecular oxygen diffuses through the alveolar-capillary barrier and dissolves into the capillary bloodstream. Because diffusion is part of this process, oxygen-enhanced MRI may be used as a method to estimate the local transfer of oxygen in the lung. The use of standard proton MR equipment also makes oxygen-enhanced MRI less expensive and more keen to a clinical application. However, some technical difficulties essentially related to the weak paramagnetic effect of oxygen have delayed the assessment of the clinical relevance of this imaging modality. In fact, the inhalation of 100% oxygen produces only a limited signal intensity change in the lung. To demonstrate this effect, artificial signal variations caused by cardiac pulsatility and respiration must be minimized and post-processing of imaging data is also required. For the same reason, the total imaging time may suffer from the need to acquire numerous images during room air and 100% oxygen inhalation. Additionally, the process causing the signal intensity increase in the lung is still under investigation. Over the years, clinical applications of oxygen-enhanced MRI have been successfully demonstrated (Ohno et al 2001, 2005). The development of new MR protocols for multislice image acquisition have also increased the potential applicability of this technique in a clinical setting (Dietrich et al 2005).
The administration of blood contrast media has been primarily used in lung MRI to demonstrate the pulmonary vessels. Dynamic contrast-enhanced MR angiography of the lung has been indicated as a potential alternative, with comparable accuracy, to digital subtraction and CT angiography (Pedersen et al 2006). The implementation of newer techniques for data acquisition, such as parallel imaging and view sharing, on clinical systems have increased the time resolution of the MRI protocols (Fink et al 2004). Combined with larger anatomical coverage and improved spatial resolution, such three-dimensional techniques can easily and accurately visualize the transit of the contrast bolus in the pulmonary vasculature. Because the temporal resolution can be increased to less than 1 second, dynamic three-dimensional contrast-enhanced MRI is very sensitive to the capillary transit of the contrast medium (ie, perfusion). The signal of the lung during dynamic contrast-enhanced MRI can be also analyzed mathematically to obtain quantitative parameters, such as pulmonary blood flow, blood volume and mean transit time (Fink et al 2004; Ohno et al 2004). Quantitative maps of lung perfusion, obtained by those parameters, have been compared with perfusion scintigraphy, and the results of such studies look promising (Yilmaz et al 2005; Molinari et al 2006). In patients with emphysema, the typical diffuse abnormalities of the peripheral pulmonary vasculature have been demonstrated (Morino et al 2006). Because quantification of pulmonary blood flow is also potentially feasible, dynamic contrast-enhanced MRI has been proposed for the evaluation and the monitoring of the severity of the disease (Sergiacomi et al 2003; Morino et al 2006). In patients with extensive emphysematous changes in the lung, however, the quantitative assessment of perfusion may be influenced by the increased air content and reduced proton density of lung parenchyma (Molinari et al 2006). The feasibility of perfusion MRI in COPD patients has been demonstrated (Amundsen et al 2000). Clinical applications, such as the estimation of residual lung function in patients undergoing surgery, using perfusion MRI have been also proposed (Johkoh et al 2000).
Finally, MRI has been also used for the assessment of lung volumes and respiratory mechanics (Qanadli et al 1999; Suga et al 2000; Plathow et al 2004; Voorhees et al 2005; Chu et al 2006; Swift et al 2007). Continuous dynamic acquisition of MR images without contrast administration has gained considerable temporal resolution to accurately depict the movements of the chest wall and diaphragm during the respiratory cycle (Plathow et al 2004). Quantitative parameters derived from MRI measurements have been compared to pulmonary functional tests assessing static and dynamic lung volumes (Plathow et al 2004; Swift et al 2007). Dynamic MRI during respiration has been also proposed to assess noninvasively and directly the impaired respiratory mechanics associated with abnormal ventilation in pulmonary emphysema (Suga et al 2000).
Conclusion
Advances in CT technology suggest that this technique can be used in big-time studies in COPD. On the other hand, MDCT imaging of the lung is sufficiently developed to justify its use for assessing both the natural history of emphysema and the impact of surgical and medical interventions. We believe that MDCT may potentially represent the gold standard against which MRI quantitative measures can be refined. In addition, it seems reasonable to hypothesize that the clinical use of lung MRI will further increase in the near future, improving the evaluation of the functional damage associated with COPD.
Acknowledgments
The authors are deeply grateful to Dr Christian Fink (University of Munich, Germany) for critical review of the manuscript.
References
- Albert MS, Cates GD, Driehuys B, et al. Biological magnetic resonance imaging using laserpolarized 129Xe. Nature. 1994;370:199–201. doi: 10.1038/370199a0. [DOI] [PubMed] [Google Scholar]
- Amundsen T, Torheim G, Waage A, et al. Perfusion magnetic resonance imaging of the lung: characterization of pneumonia and chronic obstructive pulmonary disease. A feasibility study. J Magn Reson Imaging. 2000;12:224–31. doi: 10.1002/1522-2586(200008)12:2<224::aid-jmri3>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Aziz ZA, Wells AU, Desai SR, et al. Functional impairment in emphysema: contribution of airway abnormalities and distribution of parenchymal disease. AJR Am J Roentgenol. 2005;185:1509–15. doi: 10.2214/AJR.04.1578. [DOI] [PubMed] [Google Scholar]
- Bankier AA, De Maertelar V, Keyzer C, et al. Pulmonary emphysema: subjective visual grading versus objective quantification with macroscopic morphometry and thin-section CT densitometry. Radiology. 1999;211:851–8. doi: 10.1148/radiology.211.3.r99jn05851. [DOI] [PubMed] [Google Scholar]
- Bayat S, Le DG, Porra L, et al. Quantitative functional lung imaging with synchrotron radiation using inhaled xenon as contrast agent. Phys Med Biol. 2001;46:3287–99. doi: 10.1088/0031-9155/46/12/315. [DOI] [PubMed] [Google Scholar]
- Berger P, Laurent F, Begueret H, et al. Structure and function of small airways in smokers: relationship between air trapping at CT and airway inflammation. Radiology. 2003;228:85–94. doi: 10.1148/radiol.2281020187. [DOI] [PubMed] [Google Scholar]
- Bergin C, Muller N, Nichols DM, et al. The diagnosis of emphysema. A computed tomographic-pathologic correlation. Am Rev Respir Dis. 1986;133:541–6. doi: 10.1164/arrd.1986.133.4.541. [DOI] [PubMed] [Google Scholar]
- Biederer J, Both M, Graessner J, et al. Lung morphology: fast MR imaging assessment with a volumetric interpolated breath-hold technique: initial experience with patients. Radiology. 2003;226:242–9. doi: 10.1148/radiol.2261011974. [DOI] [PubMed] [Google Scholar]
- Blechschmidt RA, Werthschutzky R, Lorcher U. Automated CT image evaluation of the lung: a morphology-based concept. IEEE Trans Med Imaging. 2001;20:434–42. doi: 10.1109/42.925296. [DOI] [PubMed] [Google Scholar]
- Boiselle PM, Lee KS, Lin S, et al. Cine CT during coughing for assessment of tracheomalacia: preliminary experience with 64-MDCT. AJR Am J Roentgenol. 2006;187:175–7. doi: 10.2214/AJR.05.1456. [DOI] [PubMed] [Google Scholar]
- Boschetto P, Miniati M, Miotto D, et al. Predominant emphysema phenotype in chronic obstructive pulmonary. Eur Respir J. 2003;21:450–4. doi: 10.1183/09031936.03.00048703. [DOI] [PubMed] [Google Scholar]
- Boschetto P, Quintavalle S, Zeni E, et al. Association between markers of emphysema and more severe chronic obstructive pulmonary disease. Thorax. 2006;61:1037–42. doi: 10.1136/thx.2006.058321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chabat F, Yang GZ, Hansell DM. Obstructive lung diseases: texture classification for differentiation at CT. Radiology. 2003;228:871–7. doi: 10.1148/radiol.2283020505. [DOI] [PubMed] [Google Scholar]
- Chu WC, Li AM, Ng BK, et al. Dynamic magnetic resonance imaging in assessing lung volumes, chest wall, and diaphragm motions in adolescent idiopathic scoliosis versus normal controls. Spine. 2006;31:2243–9. doi: 10.1097/01.brs.0000232822.74349.32. [DOI] [PubMed] [Google Scholar]
- Cleverley JR, Desai SR, Wells AU, et al. Evaluation of patients undergoing lung volume reduction surgery: ancillary information available from computed tomography. Clin Radiol. 2000;55:45–50. doi: 10.1053/crad.1999.0326. [DOI] [PubMed] [Google Scholar]
- Copley SJ, Wells AU, Muller NL, et al. Thin-section CT in obstructive pulmonary disease: discriminatory value. Radiology. 2002;223:812–19. doi: 10.1148/radiol.2233010760. [DOI] [PubMed] [Google Scholar]
- Cosio MG, Snider Chest computed tomography: is it ready for major studies of chronic obstructive pulmonary disease? Eur Respir J. 2001;17:1062–4. doi: 10.1183/09031936.01.00225201. [DOI] [PubMed] [Google Scholar]
- Coxson HO, Rogers RM. Quantitative computed tomography of chronic obstructive pulmonary disease. Acad Radiol. 2005;12:1457–63. doi: 10.1016/j.acra.2005.08.013. [DOI] [PubMed] [Google Scholar]
- Dawkins PA, Dowson LJ, Guest PJ, et al. Predictors of mortality in alpha1-antitrypsin deficiency. Thorax. 2003;58:1020–6. doi: 10.1136/thorax.58.12.1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong PA, Muller NL, et al. Computed tomographic imaging of the airways: relationship to structure and function. Eur Respir J. 2005;26:140–52. doi: 10.1183/09031936.05.00007105. [DOI] [PubMed] [Google Scholar]
- Desai SR, Hansell DM, Walker A, et al. Quantification of emphysema: a composite physiologic index derived from CT estimation of disease extent. Eur Radiol. 2006:29. doi: 10.1007/s00330-006-0369-0. [DOI] [PubMed] [Google Scholar]
- Dietrich O, Losert C, Attenberger U, et al. Fast oxygen-enhanced multislice imaging of the lung using parallel acquisition techniques. Magn Reson Med. 2005;53:1317–25. doi: 10.1002/mrm.20495. [DOI] [PubMed] [Google Scholar]
- Eda S, Kubo K, Fujimoto K, et al. The relations between expiratory chest CT using helical CT and pulmonary function tests in emphysema. Am J Respir Crit Care Med. 1997;155:1290–4. doi: 10.1164/ajrccm.155.4.9105069. [DOI] [PubMed] [Google Scholar]
- Fink C, Puderbach M, Bock M, et al. Regional lung perfusion: assessment with partially parallel three-dimensional MR imaging. Radiology. 2004;231:175–84. doi: 10.1148/radiol.2311030193. [DOI] [PubMed] [Google Scholar]
- Fishman A, Martinez F, Naunheim K, et al. A randomized trial comparing lung-volume reduction surgery with medical therapy for severe emphysema. N Engl J Med. 2003;348:2059–73. doi: 10.1056/NEJMoa030287. [DOI] [PubMed] [Google Scholar]
- Foley WD, Haughton VM, Schmidt J, et al. Xenon contrast enhancement in computed body tomography. Radiology. 1978;129:219–20. doi: 10.1148/129.1.219. [DOI] [PubMed] [Google Scholar]
- Gast KK, Puderbach MU, Rodriguez I, et al. Dynamic ventilation (3)He-magnetic resonance imaging with lung motion correction: gas flow distribution analysis. Invest Radiol. 2002;37:126–34. doi: 10.1097/00004424-200203000-00005. [DOI] [PubMed] [Google Scholar]
- Gelb AF, Schein M, Kuei J, et al. Limited contribution of emphysema in advanced chronic obstructive pulmonary disease. Am Rev Respir Dis. 1993;147:1157–61. doi: 10.1164/ajrccm/147.5.1157. [DOI] [PubMed] [Google Scholar]
- Gevenois PA, de Maertelaer V, De Vuyst P, et al. Comparison of computed density and macroscopic morphometry in pulmonary emphysema. Am J Respir Crit Care Med. 1995;152:653–7. doi: 10.1164/ajrccm.152.2.7633722. [DOI] [PubMed] [Google Scholar]
- Gevenois PA, De Vuyst P, de Maertelaer V, et al. Comparison of computed density and microscopic morphometry in pulmonary emphysema. Am J Respir Crit Care Med. 1996;154:187–92. doi: 10.1164/ajrccm.154.1.8680679. [DOI] [PubMed] [Google Scholar]
- Gevenois PA, Scillia P, De Maertelaer V, et al. The effects of age, sex, lung size, and hyperinflation on CT lung densitometry. AJR Am J Roentgenol. 1996;167:1169–73. doi: 10.2214/ajr.167.5.8911175. [DOI] [PubMed] [Google Scholar]
- Grenier PA. Detection of altered lung physiology. Eur Radiol. 2005;15:42–7. doi: 10.1007/s10406-005-0126-5. [DOI] [PubMed] [Google Scholar]
- Grenier PA, Beigelman-Aubry C, Fetita C, et al. Large airways at CT: Bronchiectasis, Asthma and COPD. In: Kauczor HU, editor. Functional imaging of the chest. Heidelberg: Springer; 2004. pp. 39–55. [Google Scholar]
- Grenier PA, Beigelman-Aubry C, Fetita C, et al. New frontiers in CT imaging of airway disease. Eur Radiol. 2002;12:1022–44. doi: 10.1007/s00330-002-1342-1. [DOI] [PubMed] [Google Scholar]
- Gurney JW, Jones KK, Robbins RA, et al. Regional distribution of emphysema: correlation of high-resolution CT with pulmonary function tests in unselected smokers. Radiology. 1992;183:457–63. doi: 10.1148/radiology.183.2.1561350. [DOI] [PubMed] [Google Scholar]
- Hansell DM. General role of imaging in the evaluation of diffuse infiltrative and airways diseases. In: Kauczor HU, editor. Functional imaging of the chest. Heidelberg: Springer; 2004. pp. 1–19. [Google Scholar]
- Hansell DM, Armstrong P, Lynch DA, et al. Imaging of diseases of the chest. Philadelphia: Elsevier Mosby; 2005. [Google Scholar]
- Hasegawa M, Nasuhara Y, Onodera Y, et al. Airflow limitation and airway dimensions in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2006;173:1309–15. doi: 10.1164/rccm.200601-037OC. [DOI] [PubMed] [Google Scholar]
- Hatabu H, Chen Q, Stock KW, et al. Fast magnetic resonance imaging of the lung. Eur J Radiol. 1999;29:114–32. doi: 10.1016/s0720-048x(98)00174-0. [DOI] [PubMed] [Google Scholar]
- Heussel CP, Ley S, Biedermann A, et al. Respiratory lumenal change of the pharynx and trachea in normal subjects and COPD patients: assessment by cine-MRI. Eur Radiol. 2004;14:2188–97. doi: 10.1007/s00330-004-2461-7. [DOI] [PubMed] [Google Scholar]
- Hoffman EA, Chon D. Computed tomography studies of lung ventilation and perfusion. Proc Am Thorac Soc. 2005;2:492–8. 506. doi: 10.1513/pats.200509-099DS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman EA, Simon BA, McLennan G. State of the art. A structural and functional assessment of the lung via multidetector-row computed tomography: phenotyping chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2006;3:519–32. doi: 10.1513/pats.200603-086MS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogg JC. State of the art. Bronchiolitis in chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2006;3:489–93. doi: 10.1513/pats.200603-065MS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogg JC, Chu F, Utokaparch S, et al. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med. 2004;350:2645–53. doi: 10.1056/NEJMoa032158. [DOI] [PubMed] [Google Scholar]
- Johkoh T, Muller NL, Kavanagh PV, et al. Scintigraphic and MR perfusion imaging in preoperative evaluation for lung volume reduction surgery: pilot study results. Radiat Med. 2000;18:277–81. [PubMed] [Google Scholar]
- Johnson TR, Krauss B, Sedlmair M, et al. Material differentiation by dual energy CT: initial experience. Eur Radiol. 2006:7. doi: 10.1007/s00330-006-0517-6. [DOI] [PubMed] [Google Scholar]
- Kang EY, Miller RR, Muller NL. Bronchiectasis: comparison of preoperative thin-section CT and pathologic findings in resected specimens. Radiology. 1995;195:649–54. doi: 10.1148/radiology.195.3.7753989. [DOI] [PubMed] [Google Scholar]
- Kauczor HU, Hofmann D, Kreitner KF, et al. Normal and abnormal pulmonary ventilation: visualization at hyperpolarized He-3 MR imaging. Radiology. 1996;201:564–8. doi: 10.1148/radiology.201.2.8888259. [DOI] [PubMed] [Google Scholar]
- Kauczor HU, Kreitner KF. MRI of the pulmonary parenchyma. Eur Radiol. 1999;9:1755–64. doi: 10.1007/s003300050919. [DOI] [PubMed] [Google Scholar]
- Kitaguchi Y, Fujimoto K, Kubo K, et al. Characteristics of COPD phenotypes classified according to the findings of HRCT. Respir Med. 2006;100:1742–52. doi: 10.1016/j.rmed.2006.02.003. [DOI] [PubMed] [Google Scholar]
- Kuhnigk JM, Dicken V, Zidowitz S, et al. Informatics in radiology (infoRAD): new tools for computer assistance in thoracic CT. Part 1. Functional analysis of lungs, lung lobes, and bronchopulmonary segments. Radiographics. 2005;25:525–36. doi: 10.1148/rg.252045070. [DOI] [PubMed] [Google Scholar]
- Kurashima K, Takayanagi N, Sato N, et al. High resolution CT and bronchial reversibility test for diagnosing COPD. Respirology. 2005;10:316–22. doi: 10.1111/j.1440-1843.2005.00701.x. [DOI] [PubMed] [Google Scholar]
- Kuwano K, Matsuba K, Ikeda T, et al. The diagnosis of mild emphysema. Correlation of computed tomography and pathology scores. Am Rev Respir Dis. 1990;141:169–78. doi: 10.1164/ajrccm/141.1.169. [DOI] [PubMed] [Google Scholar]
- Ley S, Zaporozhan J, Morbach A, et al. Functional evaluation of emphysema using diffusionweighted 3Helium-magnetic resonance imaging, high-resolution computed tomography, and lung function tests. Invest Radiol. 2004;39:427–34. doi: 10.1097/01.rli.0000129468.79005.1d. [DOI] [PubMed] [Google Scholar]
- Loffler R, Muller CJ, Peller M, et al. Optimization and evaluation of the signal intensity change in multisection oxygen-enhanced MR lung imaging. Magn Reson Med. 2000;43:860–6. doi: 10.1002/1522-2594(200006)43:6<860::aid-mrm12>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Mai VM, Chen Q, Bankier AA, et al. Multiple inversion recovery MR subtraction imaging of human ventilation from inhalation of room air and pure oxygen. Magn Reson Med. 2000;43:913–16. doi: 10.1002/1522-2594(200006)43:6<913::aid-mrm21>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Martinez FJ, Foster G, Curtis JL, et al. Predictors of mortality in patients with emphysema and severe airflow obstruction. Am J Respir Crit Care Med. 2006;173:1326–34. doi: 10.1164/rccm.200510-1677OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishima M, Itoh H, Sakai H, et al. Optimized scanning conditions of high resolution CT in the follow-up of pulmonary emphysema. J Comput Assist Tomogr. 1999;23:380–4. doi: 10.1097/00004728-199905000-00011. [DOI] [PubMed] [Google Scholar]
- Mishima M, Hirai T, Itoh H. Complexity of terminal airspace geometry assessed by lung computed tomography in normal subjects and patients with chronic obstructive pulmonary disease. Proc Natl Acad Sci USA. 1999;96:8829–34. doi: 10.1073/pnas.96.16.8829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molinari F, Fink C, Risse F, et al. Assessment of differential pulmonary blood flow using perfusion magnetic resonance imaging: comparison with radionuclide perfusion scintigraphy. Invest Radiol. 2006;41:624–30. doi: 10.1097/01.rli.0000225399.65609.45. [DOI] [PubMed] [Google Scholar]
- Morbach AE, Gast KK, Schmiedeskamp J, et al. Diffusion-weighted MRI of the lung with hyperpolarized helium-3: a study of reproducibility. J Magn Reson Imaging. 2005;21:765–74. doi: 10.1002/jmri.20300. [DOI] [PubMed] [Google Scholar]
- Morino S, Toba T, Araki M, et al. Noninvasive assessment of pulmonary emphysema using dynamic contrast-enhanced magnetic resonance imaging. Exp Lung Res. 2006;32:55–67. doi: 10.1080/01902140600691548. [DOI] [PubMed] [Google Scholar]
- Muller NL, Coxson H. Chronic obstructive pulmonary disease. 4: imaging the lungs in patients with chronic obstructive pulmonary disease. Thorax. 2002;57:982–5. doi: 10.1136/thorax.57.11.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller NL, Staples CA, Miller RR, et al. “Density mask”. An objective method to quantitate emphysema using computed tomography. Chest. 1988;94:782–7. doi: 10.1378/chest.94.4.782. [DOI] [PubMed] [Google Scholar]
- Nakano Y, Muller NL, King GG, et al. Quantitative assessment of airway remodeling using high-resolution CT. Chest. 2002;122:271S–275S. [PubMed] [Google Scholar]
- Nakano Y, Muro S, Sakai H, et al. Computed tomographic measurements of airway dimensions and emphysema in smokers. Correlation with lung function. Am J Respir Crit Care Med. 2000;162:1102–8. doi: 10.1164/ajrccm.162.3.9907120. [DOI] [PubMed] [Google Scholar]
- Nakano Y, Sakai H, Muro S, et al. Comparison of low attenuation areas on computed tomographic scans between inner and outer segments of the lung in patients with chronic obstructive pulmonary disease: incidence and contribution to lung function. Thorax. 1999;54:384–9. doi: 10.1136/thx.54.5.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano Y, Whithall KP, Kalloger SE, et al. Development and validation of human airway analysis algorithm using multidetector row CT. Proceedings of SPIE. 2002;4683:460–9. [Google Scholar]
- Nakano Y, Wong JC, de Jong PA, et al. The prediction of small airway dimensions using computed tomography. Am J Respir Crit Care Med. 2005;171:142–6. doi: 10.1164/rccm.200407-874OC. [DOI] [PubMed] [Google Scholar]
- Newell JD, Jr, Hogg JC, Snider GL. Report of a workshop: quantitative computed tomography scanning in longitudinal studies of emphysema. Eur Respir J. 2004;23:769–75. doi: 10.1183/09031936.04.00026504. [DOI] [PubMed] [Google Scholar]
- O’Brien C, Guest PJ, Hill SL, et al. Physiological and radiological characterisation of patients diagnosed with chronic obstructive pulmonary disease in primary care. Thorax. 2000;55:635–42. doi: 10.1136/thorax.55.8.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell RA, Peebles C, Ward JA, et al. Relationship between peripheral airway dysfunction, airway obstruction, and neutrophilic inflammation in COPD. Thorax. 2004;59:837–42. doi: 10.1136/thx.2003.019349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno Y, Hatabu H, Higashino T, et al. Oxygen-enhanced MR imaging: correlation with postsurgical lung function in patients with lung cancer. Radiology. 2005;236:704–11. doi: 10.1148/radiol.2361040005. [DOI] [PubMed] [Google Scholar]
- Ohno Y, Hatabu H, Murase K, et al. Quantitative assessment of regional pulmonary perfusion in the entire lung using three-dimensional ultrafast dynamic contrast-enhanced magnetic resonance imaging: preliminary experience in 40 subjects. J Magn Reson Imaging. 2004;20:353–65. doi: 10.1002/jmri.20137. [DOI] [PubMed] [Google Scholar]
- Ohno Y, Hatabu H, Takenaka D, et al. Oxygen-enhanced MR ventilation imaging of the lung: preliminary clinical experience in 25 subjects. AJR Am J Roentgenol. 2001;177:185–94. doi: 10.2214/ajr.177.1.1770185. [DOI] [PubMed] [Google Scholar]
- Orlandi I, Moroni C, Camiciottoli G, et al. Chronic obstructive pulmonary disease: thinsection CT measurement of airway wall thickness and lung attenuation. Radiology. 2005;234:604–10. doi: 10.1148/radiol.2342040013. [DOI] [PubMed] [Google Scholar]
- Parr DG, Stoel BC, Stolk J, et al. Pattern of emphysema distribution in alpha1 antitrypsin deficiency influences lung function impairment. Am J Respir Crit Care Med. 2004;170:1172–8. doi: 10.1164/rccm.200406-761OC. [DOI] [PubMed] [Google Scholar]
- Patel B, Make B, Coxson HO, et al. Airway and parenchymal disease in chronic obstructive pulmonary disease are distinct phenotypes. Proc Am Thorac Soc. 2006;3:533. doi: 10.1513/pats.200603-087MS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel IS, Vlahos I, Wilkinson TM, et al. Bronchiectasis, exacerbation indices, and inflammation in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2004;170:400–7. doi: 10.1164/rccm.200305-648OC. [DOI] [PubMed] [Google Scholar]
- Pauwels RA, Buist AS, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) Workshop summary. Am J Respir Crit Care Med. 2001;163:1256–76. doi: 10.1164/ajrccm.163.5.2101039. [DOI] [PubMed] [Google Scholar]
- Pedersen MR, Fisher MT, van Beek EJ. MR imaging of the pulmonary vasculature – an update. Eur Radiol. 2006;16:1374–86. doi: 10.1007/s00330-005-0109-x. [DOI] [PubMed] [Google Scholar]
- Plathow C, Ley S, Fink C, et al. Evaluation of chest motion and volumetry during the breathing cycle by dynamic MRI in healthy subjects: comparison with pulmonary function tests. Invest Radiol. 2004;39:202–9. doi: 10.1097/01.rli.0000113795.93565.c3. [DOI] [PubMed] [Google Scholar]
- Qanadli SD, Orvoen-Frija E, Lacombe P, et al. Estimation of gas and tissue lung volumes by MRI: functional approach of lung imaging. J Comput Assist Tomogr. 1999;23:743–8. doi: 10.1097/00004728-199909000-00020. [DOI] [PubMed] [Google Scholar]
- Remy-Jardin M, Edme JL, Boulenguez C, et al. Longitudinal follow-up study of smoker’s lung with thin-section CT in correlation with pulmonary function tests. Radiology. 2002;222:261–70. doi: 10.1148/radiol.2221001154. [DOI] [PubMed] [Google Scholar]
- Remy-Jardin M, Remy J, Gosselin B, et al. Sliding thin slab, minimum intensity projection technique in the diagnosis of emphysema: histopathologic-CT correlation. Radiology. 1996;200:665–71. doi: 10.1148/radiology.200.3.8756912. [DOI] [PubMed] [Google Scholar]
- Salerno M, Altes TA, Brookeman JR, et al. Dynamic spiral MRI of pulmonary gas flow using hyperpolarized (3)He: preliminary studies in healthy and diseased lungs. Magn Reson Med. 2001;46:667–77. doi: 10.1002/mrm.1244. [DOI] [PubMed] [Google Scholar]
- Schoepf UJ, Wildberger JE, Niethammer M. Pulmonary perfusion. In: Kauczor HU, editor. Functional imaging of the chest. Heidelberg: Springer; 2004. pp. 175–201. [Google Scholar]
- Sergiacomi G, Sodani G, Fabiano S, et al. MRI lung perfusion 2D dynamic breath-hold technique in patients with severe emphysema. In Vivo. 2003;17:319–24. [PubMed] [Google Scholar]
- Shaker SB, Dirksen A, Laursen LC, et al. Volume adjustment of lung density by computed tomography scans in patients with emphysema. Acta Radiol. 2004;45:417–23. doi: 10.1080/02841850410005525. [DOI] [PubMed] [Google Scholar]
- Stoel BC, Bakker ME, Stolk J, et al. Comparison of the sensitivities of 5 different computed tomography scanners for the assessment of the progression of pulmonary emphysema: a phantom study. Invest Radiol. 2004;39:1–7. doi: 10.1097/01.rli.0000091842.82062.a3. [DOI] [PubMed] [Google Scholar]
- Suga K, Tsukuda T, Awaya H, et al. Interactions of regional respiratory mechanics and pulmonary ventilatory impairment in pulmonary emphysema: assessment with dynamic MRI and xenon-133 single-photon emission CT. Chest. 2000;117:1646–55. doi: 10.1378/chest.117.6.1646. [DOI] [PubMed] [Google Scholar]
- Swift AJ, Woodhouse N, Fichele S, et al. Rapid lung volumetry using ultrafast dynamic magnetic resonance imaging during forced vital capacity maneuver: correlation with spirometry. Invest Radiol. 2007;42:37–41. doi: 10.1097/01.rli.0000250735.92266.6b. [DOI] [PubMed] [Google Scholar]
- Uppaluri R, Hoffman EA, Sonka M, et al. Computer recognition of regional lung disease patterns. Am J Respir Crit Care Med. 1999;160:648–54. doi: 10.1164/ajrccm.160.2.9804094. [DOI] [PubMed] [Google Scholar]
- Uppaluri R, Mitsa T, Sonka M, et al. Quantification of pulmonary emphysema from lung computed tomography images. Am J Respir Crit Care Med. 1997;156:248–54. doi: 10.1164/ajrccm.156.1.9606093. [DOI] [PubMed] [Google Scholar]
- van Beek EJ, Wild JM, Kauczor HU, et al. Functional MRI of the lung using hyperpolarized 3-helium gas. J Magn Reson Imaging. 2004;20:540–54. doi: 10.1002/jmri.20154. [DOI] [PubMed] [Google Scholar]
- Voorhees A, An J, Berger KI, et al. Magnetic resonance imaging-based spirometry for regional assessment of pulmonary function. Magn Reson Med. 2005;54:1146–54. doi: 10.1002/mrm.20682. [DOI] [PubMed] [Google Scholar]
- Wild JM, Fichele S, Woodhouse N, et al. 3D volume-localized PO2 measurement in the human lung with 3He MRI. Magn Reson Med. 2005;53:1055–64. doi: 10.1002/mrm.20423. [DOI] [PubMed] [Google Scholar]
- Woodhouse N, Wild JM, Paley MN, et al. Combined helium-3/proton magnetic resonance imaging measurement of ventilated lung volumes in smokers compared to never-smokers. J Magn Reson Imaging. 2005;21:365–9. doi: 10.1002/jmri.20290. [DOI] [PubMed] [Google Scholar]
- Woods JC, Choong CK, Yablonskiy DA, et al. Hyperpolarized 3He diffusion MRI and histology in pulmonary emphysema. Magn Reson Med. 2006;56:1293–300. doi: 10.1002/mrm.21076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yilmaz E, Akkoclu A, Degirmenci B, et al. Accuracy and feasibility of dynamic contrast-enhanced 3D MR imaging in the assessment of lung perfusion: comparison with Tc-99 MAA perfusion scintigraphy. Clin Radiol. 2005;60:905–13. doi: 10.1016/j.crad.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Zaporozhan J, Ley S, Eberhardt R, et al. Paired inspiratory/expiratory volumetric thin-slice CT scan for emphysema analysis: comparison of different quantitative evaluations and pulmonary function test. Chest. 2005;128:3212–20. doi: 10.1378/chest.128.5.3212. [DOI] [PubMed] [Google Scholar]
- Zompatori M, Sverzellati N, Gentile T, et al. Imaging of the patient with chronic bronchitis: an overview of old and new signs. Radiol Med. 2006;111:634–9. doi: 10.1007/s11547-006-0061-0. [DOI] [PubMed] [Google Scholar]