Abstract
The enzyme indoleamine 2, 3-dioxygenase (IDO) catalyzes degradation of tryptophan, an essential amino acid required for lymphocyte activation and proliferation. Many tumors express IDO which implied that it acts as a mechanism to evade T cell-mediated immune attack, and also to establish an immunosuppressive tumor microenvironment. The purpose of this study was to determine whether primary and metastatic uveal melanoma expressed the IDO gene and whether uveal melanoma cells could deplete tryptophan. In situ expression of IDO in primary uveal melanoma from tumor bearing eyes and metastatic uveal melanoma liver tissues was determined by immunohistostaining with IDO-specific antibody. Reverse transcription PCR was used to assess IDO gene transcription by primary and metastatic uveal melanoma cell lines. IDO protein expression was determined by Western blot of uveal melanoma cell protein lysate. IDO catalytic activity was assessed by measuring the presence of kynurenine, a product generated by tryptophan degradation, in uveal melanoma culture supernatants.
Primary uveal melanoma from tumor-bearing eyes and metastatic uveal melanoma from the liver did not express IDO in situ. IDO was not constitutively expressed in either primary or metastatic uveal melanoma cell lines. However, stimulation of primary and metastatic uveal melanoma cell cultures with interferon-gamma (IFN-γ) universally upregulated both IDO gene and protein expression. Culture supernatants from IFN-γ treated primary and metastatic uveal melanoma cell cultures contained elevated levels of kynurenine. Addition of the IDO inhibitor 1-methyl DL-tryptophan significantly diminished kynurenine levels in IFN-γ treated uveal melanoma cell cultures. The results from this study suggest that IFN-γ inducible IDO upregulation by primary and metastatic uveal melanoma may generate a local immune privileged microenvironment to promote escape from T cell-mediated immune surveillance.
1. INTRODUCTION
The enzyme indoleamine 2, 3-dioxygenase (IDO) provides the initial rate-limiting step for tryptophan catabolism in the kynurenine pathway. T cells require tryptophan for clonal expansion and proliferation, therefore, tryptophan depletion by IDO curtails T cell-mediated immune responses (Munn et al., 1999; Terness et al., 2002; Frumento et al., 2002; Mellor et al., 2002). Upregulation of IDO expression is mediated by proinflammatory signals provided by microbial endotoxin and by the cytokine interferon gamma (IFN-γ) (Takikawa et al., 1991). Immunoregulation and immunosuppression provided by IDO plays a role in: 1) controlling microbial infections (Daubener et al., 1999), 2) prolonging survival of transplanted allografts (Hainz et al., 2007), 3) mitigating autoimmune inflammation (Alexander et al., 2002; Hayashi et al., 2004; Platten et al., 2005), and 4) supporting an immune privileged environment at the maternal/fetal interface during pregnancy (Munn et al., 1998). Fetal trophoblast cells and antigen presenting cells (macrophages and dendritic cells) produce IDO, which prevents maternal T cells from rejecting the allogeneic fetus (Baban et al., 2004; Honig et al., 2004; Munn et al., 1999). Administration of a pharmacologic inhibitor of IDO, 1-methyl DL-tryptophan (1-MT), results in T cell-mediated rejection of allogeneic murine concepti (Munn et al., 1998). Interestingly, the eye and the fetus employ similar strategies to support immune privilege and avoid immune-mediated injury. These include: 1) absence of MHC Class Ia expression and compensatory expression of class Ib molecules, 2) expression of cell membrane-bound molecules that protect the eye and fetus from immune-mediated injury including TRAIL, FasL, and complement regulatory proteins, and 3) immune deviation induced by overexpression of TGF-β in the fetus and in the anterior chamber (Niederkorn, 2006). Therefore, expression of IDO by ocular cells may play a role in maintaining ocular immune privilege.
Early studies determined that IDO expression in the eye was localized the retina, iris/ciliary body, lens, and cornea (Malina and Martin, 1993) and played two important roles in protecting the eye against ultraviolet light-mediated damage. First, IDO functioned as an antioxidant enzyme that limited intracellular reactive oxygen species formation (Malina and Martin, 1996; Bodaghi et al., 1999), and second, IDO was responsible for producing tryptophan-based UV filters that protected the cornea, lens, and retina from UV-induced photo-oxidative damage (Takikawa et al., 1999). It was hypothesized that IDO expression by corneal endothelial cells would extend survival of corneal allografts by inhibiting T cell mediated rejection. However, IDO upregulation by IFN-γ stimulated corneal endothelial cells was insufficient to inhibit T cell proliferation and failed to prolong corneal graft survival. Interestingly, IDO transfected corneal endothelial cells overexpressing IDO suppressed T cell proliferation and significantly extended the survival of allografts, suggesting that IDO expression by ocular tissues may function as a novel mechanism that blunts T cell immune responses in the eye. (Beutelspacher et al., 2006).
Tumor expression of IDO may contribute to tumor escape from immune-mediated rejection. IDO expression by immunogenic murine tumors inhibited tumor rejection by preventing the establishment of tumor-specific T cell expansion and differentiation (Uyttenhove et al., 2003). Tumor rejection was partially restored when mice were treated with the IDO inhibitor 1-methyl DL-tryptophan. Moreover, IDO expression has been implicated in contributing to tumor evasion of T cell-mediated immune surveillance in a number of human tumors including lung (Astigiano et al., 2005), colon (Brandacher et al., 2006), liver (Ishio et al., 2004), breast, (Basu et al., 2006), and endometrial (Ino et al., 2006) carcinomas, and skin melanoma (Weinlich et al., 2007). Unlike skin melanoma cells, which are excellent in eliciting allogeneic T cell responses (Haanen, J.B., 2006), uveal melanoma cells, both primary and metastatic, are poor stimulators of allogeneic lymphocyte proliferation (Verbik et al., 1997), suggesting that uveal melanoma cells generate a local microenvironment that cannot support expansion of tumor-specific T cells. Therefore, IDO expression by uveal melanoma may profoundly affect T cell and IFN-γ based immunotherapeutic strategies. We hypothesize that IDO is upregulated by uveal melanoma which provides an immune escape mechanism for evading immune surveillance. The present study investigated whether primary and metastatic uveal melanoma expressed IDO, and whether IDO expression is upregulated by IFN-γ.
2. MATERIALS AND METHODS
2.1 Human Uveal Melanoma Cell Lines
Six primary uveal melanoma cell lines designated OCM 1, OCM 3, Mel 202, Mel 270, Mel 290, and OM 431, and two cell lines isolated from metastatic lesions designated OMM 2.3, and OMM 2.6 were used. OCM 1 and OCM 3 were kindly provided by June Kan-Mitchell (University of California, San Diego, CA). Mel 202, Mel 270, Mel 290, OMM 2.3, and OMM 2.6 were kindly provided by Bruce Ksander (Schepens Eye Research Institute, Boston, MA). Primary uveal melanoma cell line OM 431 was graciously given by Daniel Albert (University of Wisconsin, Madison, WI). All cell lines were maintained in complete RPMI (BioWhittaker, Walkersville, MD), containing 10% fetal bovine serum (Hyclone, Logan, UT). The establishment of the human uveal melanoma cell lines, and all research performed in this study adhered to the tenets of the Declaration of Helsinki.
2.2 Cytokines, Reagents, and Antibodies
Human recombinant interferon-γ (IFN-γ), 1-methyl DL-tryptophan, and rabbit IgG were purchased from Sigma-Aldrich, Inc. (St. Louis, MO). Rabbit anti-indoleamine 2, 3 dioxygenase (IDO) polyclonal antibody (AB5968) was purchased from Chemicon International Inc. (Temecula, CA). Mouse anti-Human IDO monoclonal antibody (05–840) was purchased from Upstate USA Inc. (Charlottesville, VA).
2.3 Immunohistochemistry on Primary and Metastatic Uveal Melanoma Samples
Cytoplasmic expression of IDO in six primary and one metastatic uveal melanoma tissue samples was determined by immunohistostaining with rabbit anti-human IDO polyclonal antibody. Tumor-bearing eyes from uveal primary melanoma patients and liver biopsies from metastatic uveal melanoma patients were embedded in paraffin and cut into 4μm sections. Tissue sections were incubated with 5μg anti-IDO (Chemicon) or 5μg mouse IgG1 isotype control using the Vectastain Elite ABC system (Vector Laboratories, Burlingame, CA), and counterstained with methyl green. Positive IDO staining of the retina served as an internal positive control.
2.4 Isolation of RNA
Total RNA was isolated from primary and metastatic uveal melanoma cells using the RNAqueous RNA extraction kit (Ambion, Austin, TX). Briefly, uveal melanoma cells were trypsinized and washed 3x with PBS. Lysates containing guanidinium isothiocyanate were prepared from the cells, and RNA collected on cellulose filters. RNA was washed and eluted from the filters with 60μl sodium citrate. To eliminate possible DNA contamination, RNA solutions were mixed with 30μl 2.5M LiCr, incubated 1 hour at −20°C, and centrifuged at 15,000 x g for 30 min. at 4°C. RNA pellets were washed 1x with ice-cold 95% ethanol and dissolved with 20μl 1mM sodium citrate. Total RNA was analyzed for concentration and purity by spectrophotometry at A260 and A280 using a DTX 880 multiplate spectrophotometer (Beckman Coulter, Fullerton, CA).
2.5 Reverse Transcription PCR
Total cellular RNA was prepared from lysed tumor cells using a RNAqueous RNA isolation kit (Ambion, Austin, TX). Complimentary DNA was generated using an iScript cDNA Synthesis Kit (Bio-Rad, Hercules, CA). Reverse transcription was performed at 42ºC for 30 min, followed by inactivation of the reverse transcriptase at 85ºC for 5 min. using a MJ Systems PTC-200 thermocycler (Bio-Rad). Each PCR reaction contained a 1μl sample of cDNA supplemented with 10μl of 10x PCR buffer containing MgCl2, 84.5μl of nuclease-free H2O, and 0.5μl of 5 U/ml Taq polymerase (Roche Diagnostics, Indianapolis, IN). Intron-spanning IDO-specific primers were added to the cDNA samples at a concentration of 20pM, making the total volume of each PCR reaction 100μl. Amplification was performed using a MJ Systems PTC-200 thermocycler for 35 cycles (1 min. at 95ºC, 1 min. at 59.5ºC, and 1 min. at 72ºC) with a final extension step at 72°C for 5 minutes. Oligonucleotide primers specific for human IDO were synthesized according to the previously reported sequences (Sedlmayr et al., 2002). The primer sequences used in our studies were: (sense) 5′ GCA AAT GCA AGA ACG GGA CAC T 3′, and (anti-sense) 5′ TCA GGG AGA CCA GAG CTT TCA CAC 3′. PCR of uveal melanoma cDNA with GAPDH (glyceraldehyde 3-phosphate dehydrogenase) primers (sense) 5′ ACC ACA GTC CAT GCC ATC AC 3′, (anti-sense) 5′ TCC ACC ACC CTG TTC CTG TA 3′ was performed using identical PCR conditions and served as an internal control. PCR amplification products were separated by electrophoresis using 3% agarose gels (Bio-Rad) prestained with 1x GelStar nucleic acid stain (Cambrex Bioscience Rockland Inc., Rockland, ME). PCR samples (5μl), were added to each lane and run in an electrophoresis apparatus at 80V for 90 min (BioRad). PCR products were identified by analysis using a Typhoon 9410 imager (GE Healthcare, Piscataway, NJ).
2.6 Western Blot Analysis
Uveal melanoma protein lysates were prepared using a 1X RIPA buffer (Upstate) supplemented with 1mM PMSF (phenylmethylsulfonyl fluoride, Sigma), 5μl/ml aprotonin (Sigma), 5μl/ml leupeptin (Sigma) and 5μl/ml pepstatin (Sigma) protease inhibitors. Protein concentration was determined by a DC Protein Assay Kit (BioRad). Ten micrograms of protein from each sample were loaded in wells of commercially prepared 10% Ready Gel precast SDS-PAGE gels (Bio-Rad) and electrophoresed for 35 min. at 200V. Kaleidoscope protein standards (216kDa – 7.6kDa, Bio-Rad) were separated in parallel to identify protein size. Separated proteins were transferred to PVDF membranes (Bio-Rad) by wet electophoretic transfer for 1 hour at 70V. Membranes were washed 2x with PBS containing 0.1% Tween-20 (PBST) and then incubated with 5% blocking buffer for 1 hour. PVDF membranes were incubated with either mouse anti-IDO antibody (Upstate) or mouse anti-α-actin antibody (Chemicon) for a minimum of 1 hour, then washed 3x with PBST. Protein detection was achieved using an Amplified Opti-4CN kit (Bio-Rad). Briefly, PVDF membranes were incubated in 5% blocking buffer containing a 1:3000 dilution of goat anti-mouse-HRP (Bio-Rad) antibody for 1 hour, washed 3x with PBST, then incubated with an amplification solution for 10 minutes. Membranes were washed 3x with 10% DMSO-PBST wash buffer, 3x with PBST, then incubated with a 1:2500 dilution of streptavidin-HRP (Bio-Rad) antibody for 30 minutes. Membranes were washed 4x with PBST, and protein bands were visualized by incubating membranes in substrate solution for 30 min., followed by 2x wash with distilled water. Membranes were allowed to air dry, and protein bands were documented by digital scanning.
2.7 Treatment of Uveal Melanoma with 1-methyl DL-tryptophan (1-MT)
Uveal melanoma cells (5×105/well) were cultured in 6-well plates for 72 hours in 5ml complete RPMI 1640 supplemented with 10% FBS in the presence or absence of IFN-γ (100U/ml). To block IDO function, the IDO inhibitor, 1-methyl-DL-tryptophan (5 mM; Sigma), was added to the uveal melanoma cells cultured in complete RPMI 1640 containing 2.5μM tryptophan and supplemented with 10% FBS in the presence or absence of IFN-γ (100U/ml). Culture supernatants were collected, centrifuged (1200 rpm/5 min.), and transferred to fresh 15ml tubes. All supernatants were stored at −20°C until use.
2.8 IDO Enzymatic Assay
IDO activity by uveal melanoma was assessed by colorimetric detection of kynurenine in uveal melanoma culture supernatants using a modified assay by Kudo and Boyd. (2000). Briefly, 5×105 uveal melanoma cells were cultured in duplicate 6-well plates containing either 5ml of 10% complete RPMI 1640 medium containing 2.5μM tryptophan or in 5ml of 10% complete RPMI 1640 medium containing 2.5μM tryptophan and 100U/ml IFN-γ. Culture supernatants were collected 72 hours later, and an equal volume of 0.2% TCA (Sigma) was added to precipitate proteins. Supernatants were centrifuged at 15,000 x g for 10 min. and transferred to new 1.5ml tubes. Supernatants were mixed in 1:1 vol/vol with a 1.2% p-dimethylaminobenzaldehyde (Sigma)/acetic acid solution in 96-well plates and incubated at ambient temperature for 10 minutes. Kynurenine was detected by colorimetric analysis using a spectrophotometer at a wavelength of A490 (Beckman Coulter). Kynurenine concentration in uveal melanoma culture supernatants were determined by standard regression analysis derived from colorimetric analysis of kynurenine (Sigma) concentration standards (500μM - 0μM).
3. RESULTS
3.1 Indoleamine 2, 3 deoxygenase gene transcription by primary and metastatic uveal melanoma cells
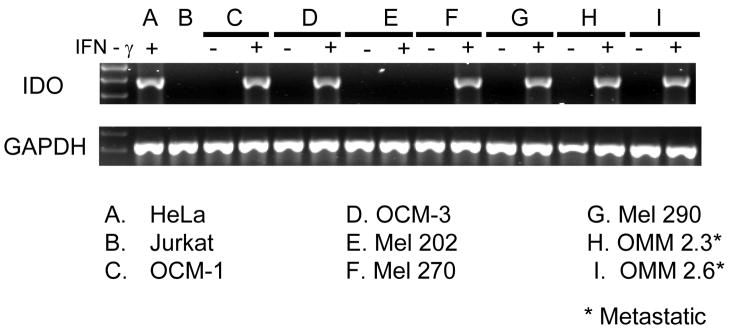
We used reverse transcription PCR to examine whether primary and/or metastatic uveal melanoma expressed the IDO gene. Total RNA from primary and metastatic uveal melanoma cells was isolated and purified by LiCr precipitation. Total RNA was reverse transcribed to cDNA and assessed for IDO expression by PCR amplification using IDO-specific primers. IDO gene expression was confirmed with our positive control (HeLa + IFN-γ), which produced a predicted PCR product band of 460bp. (Figure 1). Jurkat cells, which do not express IDO, served as a negative control. Our results demonstrated that all primary uveal melanoma cells tested did not constitutively transcribe the IDO gene. Similarly, all metastatic uveal melanoma cell lines did not express IDO. By contrast, primary or metastatic uveal melanoma cells cultured in the presence of 100U/ml IFN-γ upregulated IDO gene expression in all but one primary cell line (Mel 202). Together, our data indicated that the IDO gene was not constitutively expressed in all primary and metastatic uveal melanoma cell lines, but could potentially express IDO upon in vitro stimulation with IFN-γ.
Figure 1. Transcription of the IDO gene by primary uveal melanoma and metastatic uveal melanoma cells.
Total cellular RNA was prepared from untreated and IFN-γ treated tumor cells, and used to generate cDNA. Intron-spanning IDO-specific primers were used in a PCR assay to amplify IDO message from cDNA. PCR using GAPDH specific primers was used as an internal control. Products from PCR amplification were separated by electrophoresis in a 3% agarose gel containing GelStar nucleic acid stain and visualized using a Typhoon 9410 imager. IDO specific RT-PCR on primary and metastatic uveal melanoma cell cDNA was performed 4 separate times.
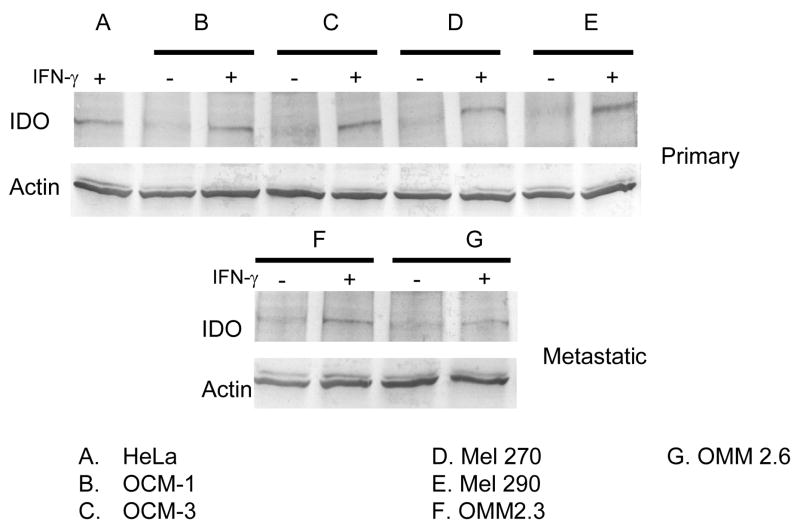
3.2 Indoleamine 2, 3 deoxygenase protein expression by primary and metastatic uveal melanoma cells
To determine whether IFN-γ treated primary and metastatic uveal melanoma cells expressed the IDO enzyme, we performed Western blot analysis on protein lysates from primary and metastatic uveal melanoma cells. Protein lysates were generated from primary and metastatic uveal melanoma cell lines cultured in the presence or absence of IFN-γ. Proteins were separated by electrophoresis on SDS-PAGE gels, transferred to PVDF membranes, and probed with either anti-IDO antibody or anti α-actin which served as a protein loading control. As expected, primary and metastatic uveal melanoma cells did not constitutively express IDO protein, whereas all IFN-γ treated primary and metastatic uveal melanoma cells upregulated IDO protein expression. Alpha actin expression was similar in all protein lysate samples demonstrating that an equal amount of protein was loaded for each protein lysate sample.
3.3 Indoleamine 2, 3 deoxygenase expression in primary uveal melanoma and metastatic uveal melanoma tissue samples
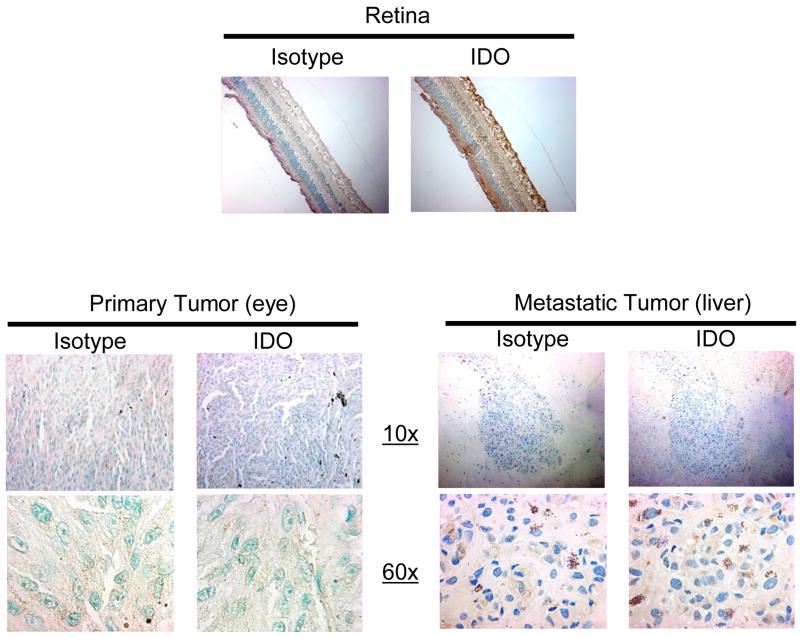
The results, showing that primary and metastatic uveal melanoma cell lines do not constitutively express IDO, do not necessarily mean that primary and metastatic uveal melanoma do not express IDO in situ. Therefore, we investigated IDO expression in primary uveal melanoma in tumor-bearing eyes and in metastatic uveal melanoma from the liver by IDO specific immunohistostaining of paraffin embedded tissues from six primary uveal melanoma samples and one metastatic uveal melanoma sample. None of the primary uveal melanomas in tumor-bearing eyes expressed IDO. A representative primary uveal melanoma sample is shown in Figure 3. Similarly, metastatic uveal melanoma in the liver tissues did not express IDO in situ. By contrast, all of the cellular components of the retina, including the ganglion cell layer, photoreceptors, and retinal pigment epithelium served as an internal positive control for IDO expression.
Figure 3. Primary uveal melanoma and metastatic uveal melanoma do not express IDO in situ.
Paraffin embedded samples of six tumor bearing eyes with primary uveal melanoma, one liver biopsy from metastatic uveal melanoma sample and a section from a normal eye were cut into 4μm sections, incubated with anti-IDO or mouse IgG1 isotype control antibody and counterstained with methyl green. Visualization of IDO positive cells was performed using 10x and 60x objectives. Positive staining of the retina served as a positive IDO control. IDO expression on the primary uveal melanoma sample in this figure is representative of IDO expression in all primary uveal melanoma sections.
3.4 Indoleamine 2, 3 deoxygenase produced by primary and metastatic uveal melanoma cells catabolize tryptophan
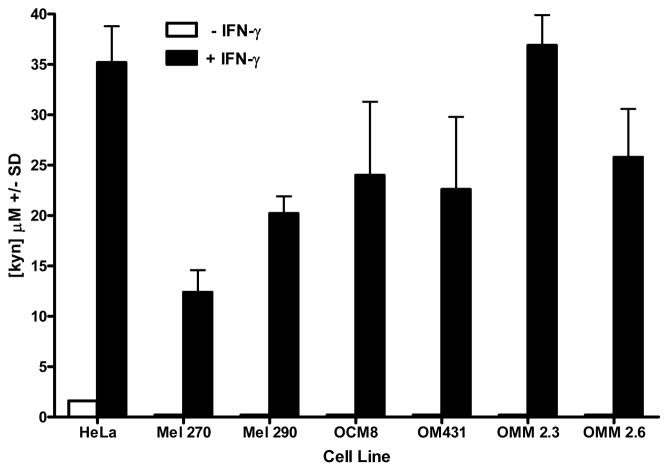
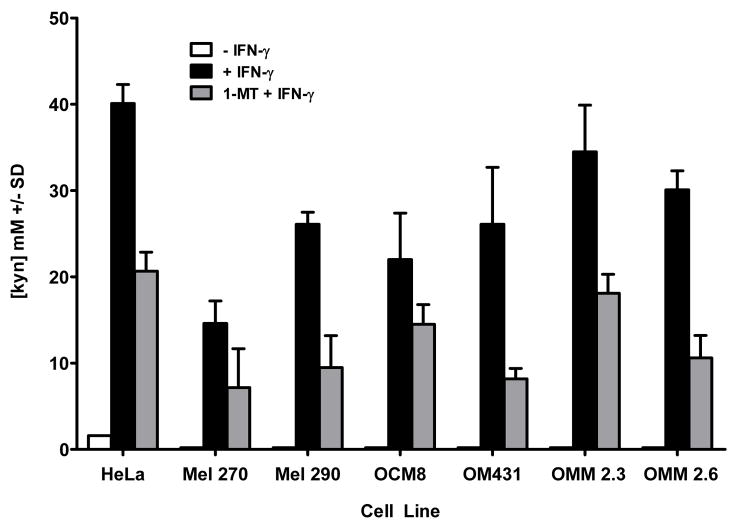
IDO expression in cells facilitates tryptophan degradation to kynurenine. We tested whether IDO expressed by uveal melanoma cells was enzymatically active by detection of kynurenine in primary and metastatic uveal melanoma culture supernatants. Primary and metastatic uveal melanoma cells were cultured in medium containing 2.5 μM tryptophan for 72 hours in the presence or absence of IFN-γ. Culture supernatants were assessed for kynurenine by a modified procedure of Kudo and Boyd (2000). Culture supernatants from primary and metastatic uveal melanoma cells cultured in the absence of IFN-γ do not contain kynurenine (Figure 4). By contrast, kynurenine was detected in culture supernatants of primary and metastatic uveal melanoma cell lines cultured with IFN-γ. Addition of the IDO inhibitor 1-MT to primary and metastatic uveal melanoma cell cultures blocks IDO catabolism of tryptophan to kynurenine. Culture supernatants from primary and metastatic uveal melanoma cells incubated in the presence of IFN-γ and 1-MT universally demonstrated reduced kynurenine concentrations (Figure 5). By contrast, culture supernatants of primary and metastatic uveal melanoma cell lines cultured in IFN-γ-free medium and 1-MT did not contain kynurenine (data not shown).
Figure 4. IDO expressed by IFN-γ-stimulated primary and metastatic uveal melanoma degrade tryptophan and produce kynurenine.
IDO activity by uveal melanoma was assessed by colorimetric detection of kynurenine in uveal melanoma culture supernatants using a modified assay by Kudo and Boyd (2000). Uveal melanoma cells were cultured in either 2.5uM tryptophan medium, with or without IFN-γ for 72 hours. Supernatants were collected, treated with TCA to precipitate proteins, and mixed with p-dimethylaminobenzaldehyde to stabilize kynurenine in the samples. Kynurenine was detected by colorimetric analysis using a spectrophotometer at a wavelength of A490. Kynurenine concentration in uveal melanoma culture supernatants was determined by standard regression analysis derived from colorimetric analysis of kynurenine concentration standards. The results are representative of 4 separate experiments.
Figure 5. Inhibition of IDO by 1-methyl DL-tryptophan prevents tryptophan catabolism.
Uveal melanoma cells were cultured in the presence or absence of IFN-γ and also in the presence or absence of 1-methyl DL tryptophan for 72 hours. Supernatants were collected and treated with TCA to precipitate proteins, and mixed with p-dimethylaminobenzaldehyde to stabilize kynurenine in the samples. Kynurenine was detected by colorimetric analysis using a spectrophotometer at a wavelength of A490. Kynurenine concentration in uveal melanoma culture supernatants was determined by standard regression analysis derived from colorimetric analysis of kynurenine concentration standards. The results are representative of 3 separate experiments.
DISCUSSION
Uveal melanoma have the capacity to produce and express the same factors the eye employs to maintain immune privilege, which may explain why it is difficult to develop successful immunotherapy against this tumor. Some of these factors include complement regulatory proteins that could protect tumor cells from lysis by complement fixing antibodies (Goslings et al., 1996), the secretion of transforming growth factor-β (TGF-β) (Jampel et al., 1990; Cousins et al., 1991) and tumor production macrophage migration inhibitory factor (MIF) (Clemente et al., 1996; Apte et al., 1997; Apte and Niederkorn, 1996; Apte et al., 1998), which can inhibit natural killer (NK) cell-mediated cytolysis (Apte and Niederkorn, 1996; Apte et al., 1998; Rook et al., 1986). Expression of IDO by ocular tissues is one of many mechanisms the eye employs to maintain its immune privileged status. Therefore, we investigated whether primary and metastatic uveal melanoma cells utilized IDO expression in their development from primary to metastatic disease to escape immune-mediated rejection. Interestingly, we did not detect IDO expression in primary uveal melanoma tissue samples, or in primary melanoma cell lines. Since the ocular environment employs multiple immunosuppressive mechanisms, including IDO expression by ocular tissues, it is conceivable that primary uveal melanomas residing in an immune privileged environment would not be required to express IDO in situ. In addition, we also did not detect IDO expression in metastatic uveal melanoma tissues, or in metastatic uveal melanoma cell lines. The liver is also an immune privileged site since the introduction of antigens through the hepatic portal vein or orthotopic liver transplantation results in tolerance (Crispe et al., 2006). Hepatic cells express the liver-specific enzyme tryptophan pyrrolase, also known as tryptophan 2, 3 deoxygenase, that catabolizes tryptophan to produce kynurenine (Dick et al., 2001). Thus, metastatic uveal melanomas residing in the liver enjoy an immunosuppressive environment that could protect the tumor from T cell-mediated elimination in a microenvironment that resembles the immune privileged environment found in the eye. Interestingly, both primary and metastatic uveal melanoma cells upregulate IDO expression when they encounter the Th1-associated cytokine IFN-γ which could allow them to deplete the local environment of tryptophan required for T cell expansion and survival.
There is evidence that IDO expression is crucial in maintaining the immune privileged environment in the eye. Foreign antigens introduced into the anterior chamber induce the generation of regulatory T cells that abrogate Th1 T cell-mediated immune responses in a phenomenon known as ACAID - anterior chamber-associated immune deviation (Niederkorn, 2002). Studies by Chen et al., (2006) demonstrated that ACAID induction by intracameral injection of OVA-induced upregulation of IDO and IL-4 expression in splenocytes, suppression of IFN-γ production by lymph node cells, and suppression of DTH responses. By contrast, mice intracamerally challenged with OVA and received injections of the IDO inhibitor 1-MT, suppressed splenocyte IDO expression and IL-4 secretion, restored expression of IFN-γ by lymph node cells, and restored OVA-specific DTH responses, suggesting that IDO expression by ocular tissues supports ACAID and ocular immune privilege. IDO expression also plays a role in mitigating sight-threatening inflammation in the eye. Intraocular inflammation associated with experimental autoimmune uveoretinitis (EAU) terminates ocular immune privilege by degeneration of the blood-ocular barrier resulting in the loss of the immunosuppressive ocular environment (Ohta et al., 1999). Treatment of EAU-positive mice with agonistic anti-4-1BB antibody (CD137) suppressed intraocular inflammation by inducing rapid clonal expansion of CD11c+ CD8+ T cells that produced IFN-γ. Stimulation of CD11c+ dendritic cells by IFN-γ upregulated IDO expression resulting in depletion of tryptophan in the local environment and apoptosis of CD4+ T cells responsible for mediating EAU. (Choi et al., 2006). Interestingly, 4-1BB-mediated suppression of EAU was reversed when the IDO inhibitor 1-MT was administered to animals prior to EAU induction. Together, these two studies exemplify the role IDO plays in maintaining the immunosuppressive ocular environment when the eye encounters an antigenic insult.
Uveal melanoma is the most common primary intraocular tumor in adults with a frequency of six to seven cases per one million adults (Egan et al., 1988). While the incidence of developing primary disease is infrequent compared to skin melanoma, over 50% of patients who develop primary uveal melanoma subsequently develop metastasis primarily in the liver (Singh et al., 2006). Prognosis of patients with metastatic uveal melanoma is poor since this disease is resistant to most conventional therapies, including proton beam therapy, chemotherapy, and antiangiogenic agent therapy (Bedikian, 1990). Moreover, current therapeutic regimens are only palliative, increasing the median survival of patients from 2 months to 5 months (Bedikian, 1990). Novel strategies being developed to treat uveal melanoma include the use of tumor antigen–based immunotherapy. Tumor vaccines against uveal melanoma have demonstrated some success in generating tumor-specific T cells that eliminate ocular tumors in a transgenic mouse model (Sutmuller et al., 2000). Additionally, immunization of a single uveal melanoma patient with a vaccine against multiple melanoma antigens (tyrosinase, Melan-A/MART-1, and NY-ESO1) also successfully stimulated antigen-specific cytotoxic CD8+ T cells that recognized tumor antigen peptide pulsed targets (Valmori et al, 2003). However, CD8+ T cell responses declined rapidly after the initial immunization indicating that the feasibility of immunotherapy against uveal melanomas, while encouraging, is still in its nascent stages.
The presence of tumor infiltrating lymphocytes (TIL) in cutaneous melanoma is commonly associated with a more favorable prognosis and longer median survival (Haanen J.B. et al., 2006). In addition, cutaneous melanoma cells are capable of inducing allogeneic T cell expansion (Gervois et al., 2000). By contrast, the presence of TIL in uveal melanomas is associated with an unfavorable prognosis and an increased risk of metastatic disease (de al Cruz et al., 1990; Whelchel et al., 1993; Staibano et al., 2006). Uveal melanoma cells are poor stimulators of allogeneic T cells. Studies by Verbik et al, (1997), demonstrated that primary uveal melanoma cells are unable to directly stimulate allogeneic PBL in vitro. Moreover, addition of regulator uveal melanoma cells to allogeneic MLR cultures suppressed responder cell proliferation. Interestingly, we demonstrate in this report that IFN-γ stimulated primary uveal melanoma cell lines (Mel 290 and Mel 270) and metastatic uveal melanoma cell lines (OMM 2.3 and OMM 2.6) used in this study upregulate IDO expression upon IFN-γ stimulation, resulting in kynurenine production and tryptophan depletion. It is conceivable that uveal melanoma cells, acting as regulators, upregulate IDO expression in response to IFN-γ produced by MLR responder lymphocytes culminating in tryptophan-depleted medium and feeble lymphocyte proliferation due to tryptophan deficiency. Thus, metastasizing uveal melanoma cells improve their probability of survival when traversing non-immune privileged sites by directly affecting T cell-mediated immune surveillance through the expression of IDO and the establishment of a local immune privileged microenvironment.
Figure 2. IDO protein is expressed by IFN-γ treated primary and metastatic uveal melanoma cells.
Uveal melanoma protein lysates were prepared and 10μg of protein per sample which were fractionated by electrophoresis. Separated proteins were transferred to PVDF membranes, washed and probed using IDO or α-actin specific antibodies and visualized by HRP colorimetric assay. This assay was performed on 3 separate occasions.
Acknowledgments
This work supported by NIH grants EY05631, CA30276, and an unrestricted grant from Research to Prevent Blindness, Inc., New York, NY
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alexander AM, Crawford M, Bertera S, Rudert WA, Takikawa O, Robbins PD, Trucco M. Indoleamine 2, 3-dioxygenase expression in transplanted NOD Islets prolongs graft survival after adoptive transfer of diabetogenic splenocytes. Diabetes. 2002;51:356–365. doi: 10.2337/diabetes.51.2.356. [DOI] [PubMed] [Google Scholar]
- Amberger A. Prognostic value of indoleamine 2, 3-dioxygenase expression in colorectal cancer: effect on tumor-infiltrating T cells. Clin Cancer Res. 2006;12:1144–1151. doi: 10.1158/1078-0432.CCR-05-1966. [DOI] [PubMed] [Google Scholar]
- Apte RS, Niederkorn JY. Isolation and characterization of a unique natural killer cell inhibitory factor present in the anterior chamber of the eye. J Immunol. 1996;156:2667–2673. [PubMed] [Google Scholar]
- Apte RS, Mayhew E, Niederkorn JY. Local inhibition of natural killer cell activity promotes the progressive growth of intraocular tumors. Invest Ophthalmol Vis Sci. 1997;38:1277–1282. [PubMed] [Google Scholar]
- Apte RS, Sinha D, Mayhew E, Wistow GJ, Niederkorn JY. Cutting edge: role of macrophage migration inhibitory factor in inhibiting NK cell activity and preserving immune privilege. J Immunol. 1998;160:5693–5696. [PubMed] [Google Scholar]
- Astigiano S, Morandi B, Costa R, Mastracci L, D’Agostino A, Ratto GB, Melioli G, Frumento G. Eosinophil granulocytes account for indoleamine 2, 3-dioxygenase-mediated immune escape in human non-small cell lung cancer. Neoplasia. 2005;7:390–396. doi: 10.1593/neo.04658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baban B, Chandler P, McCool D, Marshall B, Munn DH, Mellor AL. Indoleamine 2, 3-dioxygenase expression is restricted to fetal trophoblast giant cells during murine gestation and is maternal genome specific. J Reprod Immunol. 2004;61:67–77. doi: 10.1016/j.jri.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Basu GD, Tinder TL, Bradley JM, Tu T, Hattrup CL, Pockaj BA, Mukherjee P. Cyclooxygenase-2 inhibitor enhances the efficacy of a breast cancer vaccine: role of IDO. J Immunol. 2006;177:2391–2402. doi: 10.4049/jimmunol.177.4.2391. [DOI] [PubMed] [Google Scholar]
- Bedikian AY. Metastatic uveal melanoma therapy: current options. Int Ophthalmol Clin. 2006;46:151–166. doi: 10.1097/01.iio.0000195852.08453.de. [DOI] [PubMed] [Google Scholar]
- Beutelspacher SC, Pillai R, Watson MP, Tan PH, Tsang J, McClure MO, George AJ, Larkin DF. Function of indoleamine 2, 3-dioxygenase in corneal allograft rejection and prolongation of allograft survival by over-expression. Eur J Immunol. 2006;36:690–700. doi: 10.1002/eji.200535238. [DOI] [PubMed] [Google Scholar]
- Bodaghi B, Goureau O, Zipeto D, Laurent L, Virelizier JL, Michelson S. Role of IFN-gamma-induced indoleamine 2, 3 dioxygenase and inducible nitric oxide synthase in the replication of human cytomegalovirus in retinal pigment epithelial cells. J Immunol. 1999;162:957–964. [PubMed] [Google Scholar]
- Brandacher G, Perathoner A, Ladurner R, Schneeberger S, Obrist P, Winkler C, Werner ER, Werner-Felmayer G, Weiss HG, Gobel G, Margreiter R, Konigsrainer A, Fuchs D, Amberger A. Prognostic value of indoleamine 2, 3-dioxygenase expression in colorectal cancer: effect on tumor-infiltrating T cells. Clin Cancer Res. 2006;12:1144–1151. doi: 10.1158/1078-0432.CCR-05-1966. [DOI] [PubMed] [Google Scholar]
- Chen X, Liu L, Yang P, Wu C, Jin H, Xing L, Li B, Zhou H, Huang X, Zhu L. Indoleamine 2, 3-dioxygenase (IDO) is involved in promoting the development of anterior chamber-associated immune deviation. Immunol Lett. 2006;107:140–147. doi: 10.1016/j.imlet.2006.08.006. [DOI] [PubMed] [Google Scholar]
- Choi BK, Asai T, Vinay DS, Kim YH, Kwon BS. 4-1BB-mediated amelioration of experimental autoimmune uveoretinitis is caused by indoleamine 2, 3-dioxygenase-dependent mechanisms. Cytokine. 2006;34:233–242. doi: 10.1016/j.cyto.2006.04.008. [DOI] [PubMed] [Google Scholar]
- Clemente CG, Mihm MC, Jr, Bufalino R, Zurrida S, Collini P, Cascinelli N. Prognostic value of tumor infiltrating lymphocytes in the vertical growth phase of primary cutaneous melanoma. Cancer. 1996;77:1303–1310. doi: 10.1002/(SICI)1097-0142(19960401)77:7<1303::AID-CNCR12>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Cordiali-Fei P, Mottolese M, Tecce R, Natali P, Ferrone S. Accessory cell function of human melanoma cells in mitogen-induced T cell proliferation. Cell Immunol. 1988;116:149–162. doi: 10.1016/0008-8749(88)90217-1. [DOI] [PubMed] [Google Scholar]
- Cousins SW, McCabe MM, Danielpour D, Streilein JW. Identification of transforming growth factor-beta as an immunosuppressive factor in aqueous humor. Invest Ophthalmol Vis Sci. 1991;32:2201–2211. [PubMed] [Google Scholar]
- Crispe IN, Giannandrea M, Klein I, John B, Sampson B, Wuensch S. Cellular and molecular mechanisms of liver tolerance. Immunol Rev. 2006;213:101–118. doi: 10.1111/j.1600-065X.2006.00435.x. [DOI] [PubMed] [Google Scholar]
- Daubener W, MacKenzie CR. IFN-gamma activated indoleamine 2, 3-dioxygenase activity in human cells is an antiparasitic and an antibacterial effector mechanism. Adv Exp Med Biol. 1999;467:517–524. doi: 10.1007/978-1-4615-4709-9_64. [DOI] [PubMed] [Google Scholar]
- de la Cruz PO, Jr, Specht CS, McLean IW. Lymphocytic infiltration in uveal malignant melanoma. Cancer. 1990;65:112–115. doi: 10.1002/1097-0142(19900101)65:1<112::aid-cncr2820650123>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Dick R, Murray BP, Reid MJ, Correia MA. Structure-function relationships of rat hepatic tryptophan 2,3-dioxygenase: identification of the putative heme-ligating histidine residues. Arch Biochem Biophys. 2001;392:71–78. doi: 10.1006/abbi.2001.2420. [DOI] [PubMed] [Google Scholar]
- Egan KM, Seddon JM, Glynn RJ, Gragoudas ES, Albert DM. Epidemiologic aspects of uveal melanoma. Surv Ophthalmol. 1988;32:239–251. doi: 10.1016/0039-6257(88)90173-7. [DOI] [PubMed] [Google Scholar]
- Frumento G, Rotondo R, Tonetti M, Damonte G, Benatti U, Ferrara GB. Tryptophan-derived catabolites are responsible for inhibition of T and natural killer cell proliferation induced by indoleamine 2, 3-dioxygenase. J Exp Med. 2002;196:459–468. doi: 10.1084/jem.20020121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gervois N, Labarriere N, Le Guiner S, Pandolfino MC, Fonteneau JF, Guilloux Y, Diez E, Dreno B, Jotereau F. High avidity melanoma-reactive cytotoxic T lymphocytes are efficiently induced from peripheral blood lymphocytes on stimulation by peptide-pulsed melanoma cells. Clin Cancer Res. 2000;6:1459–1467. [PubMed] [Google Scholar]
- Haanen JB, Baars A, Gomez R, Weder P, Smits M, de Gruijl TD, von Blomberg BM, Bloemena E, Scheper RJ, van Ham SM, Pinedo HM, van den Eertwegh AJ. Melanoma-specific tumor-infiltrating lymphocytes but not circulating melanoma-specific T cells may predict survival in resected advanced-stage melanoma patients. Cancer Immunol Immunother. 2006;55:451–458. doi: 10.1007/s00262-005-0018-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hainz U, Jurgens B, Heitger A. The role of indoleamine 2, 3-dioxygenase in transplantation. Transplant Int. 2007;20:118–127. doi: 10.1111/j.1432-2277.2006.00370.x. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Beck L, Rossetto C, Gong X, Takikawa O, Takabayashi K, Broide DH, Carson DA, Raz E. Inhibition of experimental asthma by indoleamine 2, 3-dioxygenase. J Clin Invest. 2004;114:270–279. doi: 10.1172/JCI21275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honig A, Rieger L, Kapp M, Sutterlin M, Dietl J, Kammerer U. Indoleamine 2, 3-dioxygenase (IDO) expression in invasive extravillous trophoblast supports role of the enzyme for materno-fetal tolerance. J Reprod Immunol. 2004;61:79–86. doi: 10.1016/j.jri.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Ino K, Yoshida N, Kajiyama H, Shibata K, Yamamoto E, Kidokoro K, Takahashi N, Terauchi M, Nawa A, Nomura S, Nagasaka T, Takikawa O, Kikkawa F. Indoleamine 2, 3-dioxygenase is a novel prognostic indicator for endometrial cancer. Br J Cancer. 2006;95:1555–1561. doi: 10.1038/sj.bjc.6603477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishio T, Goto S, Tahara K, Tone S, Kawano K, Kitano S. Immunoactivative role of indoleamine 2, 3-dioxygenase in human hepatocellular carcinoma. J Gastroenterol Hepatol. 2004;19:319–326. doi: 10.1111/j.1440-1746.2003.03259.x. [DOI] [PubMed] [Google Scholar]
- Jampel HD, Roche N, Stark WJ, Roberts AB. Transforming growth factor-beta in human aqueous humor. Curr Eye Res. 1990;9:963–969. doi: 10.3109/02713689009069932. [DOI] [PubMed] [Google Scholar]
- Kudo Y, Boyd CA. Human placental indoleamine 2, 3-dioxygenase: cellular localization and characterization of an enzyme preventing fetal rejection. Biochimica Biophysica Acta. 2000;1500:119–124. doi: 10.1016/s0925-4439(99)00096-4. [DOI] [PubMed] [Google Scholar]
- Malina HZ, Martin XD. Indoleamine 2, 3-dioxygenase activity in the aqueous humor, iris/ciliary body, and retina of the bovine eye. Graefes Arch Clin Exp Ophthalmol. 1993;231:482–486. doi: 10.1007/BF02044236. [DOI] [PubMed] [Google Scholar]
- Malina HZ, Martin XD. Indoleamine 2, 3-dioxygenase: antioxidant enzyme in the human eye. Graefes Arch Clin Exp Ophthalmol. 1996;234:457–462. doi: 10.1007/BF02539413. [DOI] [PubMed] [Google Scholar]
- Mellor AL, Keskin DB, Johnson T, Chandler P, Munn DH. Cells expressing indoleamine 2, 3-dioxygenase inhibit T cell responses. J Immunol. 2002;168:3771–3776. doi: 10.4049/jimmunol.168.8.3771. [DOI] [PubMed] [Google Scholar]
- Munn DH, Zhou M, Attwood JT, Bondarev I, Conway SJ, Marshall B, Brown C, Mellor AL. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science. 1998;281:1191–1193. doi: 10.1126/science.281.5380.1191. [DOI] [PubMed] [Google Scholar]
- Munn DH, Shafizadeh E, Attwood JT, Bondarev I, Pashine A, Mellor AL. Inhibition of T cell proliferation by macrophage tryptophan catabolism. J Exp Med. 1999;189:1363–1372. doi: 10.1084/jem.189.9.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niederkorn JY. Immune privilege in the anterior chamber of the eye. Crit Rev Immunol. 2002;22:13–46. [PubMed] [Google Scholar]
- Niederkorn JY. See no evil, hear no evil, do no evil: the lessons of immune privilege. Nat Immunol. 2006;7:354–359. doi: 10.1038/ni1328. [DOI] [PubMed] [Google Scholar]
- Ohta K, Wiggert B, Taylor AW, Streilein JW. Effects of experimental ocular inflammation on ocular immune privilege. Invest Ophthalmol Vis Sci. 1999;40:2010–2018. [PubMed] [Google Scholar]
- Platten M, Ho PP, Youssef S, Fontoura P, Garren H, Hur EM, Gupta R, Lee LY, Kidd BA, Robinson WH, Sobel RA, Selley ML, Steinman L. Treatment of autoimmune neuroinflammation with a synthetic tryptophan metabolite. Science. 2005;310:850–855. doi: 10.1126/science.1117634. [DOI] [PubMed] [Google Scholar]
- Rook AH, Kehrl JH, Wakefield LM, Roberts AB, Sporn MB, Burlington DB, Lane HC, Fauci AS. Effects of transforming growth factor beta on the functions of natural killer cells: depressed cytolytic activity and blunting of interferon responsiveness. J Immunol. 1986;136:3916–3920. [PubMed] [Google Scholar]
- Sedlmayr P, Blaschitz A, Wintersteiger R, Semlitsch M, Hammer A, MacKenzie CR, Walcher W, Reich O, Takikawa O, Dohr G. Localization of indoleamine 2, 3-dioxygenase in human female reproductive organs and the placenta. Mol Hum Reprod. 2002;8:385–391. doi: 10.1093/molehr/8.4.385. [DOI] [PubMed] [Google Scholar]
- Singh AD, Bergman L, Seregard S. Uveal melanoma: epidemiologic aspects. Ophthalmol Clin North Am. 2006;18:75–84. doi: 10.1016/j.ohc.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Staibano S, Mascolo M, Tranfa F, Salvatore G, Mignogna C, Bufo P, Nugnes L, Bonavolonta G, De Rosa G. Tumor infiltrating lymphocytes in uveal melanoma: a link with clinical behavior? Int J Immunopathol Pharmacol. 2006;19:171–179. [PubMed] [Google Scholar]
- Sutmuller RP, Schurmans LR, van Duivenvoorde LM, Tine JA, van Der Voort EI, Toes RE, Melief CJ, Jager MJ, Offringa R. Adoptive T cell immunotherapy of human uveal melanoma targeting gp100. J Immunol. 2000;165:7308–1735. doi: 10.4049/jimmunol.165.12.7308. [DOI] [PubMed] [Google Scholar]
- Takikawa O, Habara-Ohkubo A, Yoshida R. Induction of indoleamine 2, 3-dioxygenase in tumor cells transplanted into allogeneic mouse: interferon-gamma is the inducer. Adv Exp Med Biol. 1991;294:437–444. doi: 10.1007/978-1-4684-5952-4_40. [DOI] [PubMed] [Google Scholar]
- Takikawa O, Littlejohn T, Jamie JF, Walker MJ, Truscott RJ. Regulation of indoleamine 2, 3-dioxygenase, the first enzyme in UV filter biosynthesis in the human lens. Relevance for senile nuclear cataract. Adv Exp Med Biol. 1999;467:241–245. doi: 10.1007/978-1-4615-4709-9_31. [DOI] [PubMed] [Google Scholar]
- Terness P, Bauer TM, Rose L, Dufter C, Watzlik A, Simon H, Opelz G. Inhibition of allogeneic T cell proliferation by indoleamine 2, 3-dioxygenase-expressing dendritic cells: mediation of suppression by tryptophan metabolites. J Exp Med. 2002;196:447–457. doi: 10.1084/jem.20020052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uyttenhove C, Pilotte L, Theate I, Stroobant V, Colau D, Parmentier N, Boon T, Van den Eynde BJ. Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2, 3-dioxygenase. Nat Med. 2003;9:269–274. doi: 10.1038/nm934. [DOI] [PubMed] [Google Scholar]
- Valmori D, Dutoit V, Ayyoub M, Rimoldi D, Guillaume P, Lienard D, Lejeune F, Cerottini JC, Romero P, Speiser DE. Simultaneous CD8+ T cell responses to multiple tumor antigen epitopes in a multipeptide melanoma vaccine. Cancer Immun. 2003;3:15–28. [PubMed] [Google Scholar]
- Verbik DJ, Murray TG, Tran JM, Ksander BR. Melanomas that develop within the eye inhibit lymphocyte proliferation. Int J Cancer. 1997;73:470–478. doi: 10.1002/(sici)1097-0215(19971114)73:4<470::aid-ijc3>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Weinlich G, Murr C, Richardsen L, Winkler C, Fuchs D. Decreased serum tryptophan concentration predicts poor prognosis in malignant melanoma patients. Dermatol. 2007;214:8–14. doi: 10.1159/000096906. [DOI] [PubMed] [Google Scholar]
- Whelchel JC, Farah SE, McLean IW, Burnier MN. Immunohistochemistry of infiltrating lymphocytes in uveal malignant melanoma. Invest Ophthalmol Vis Sci. 1993;34:2603–2606. [PubMed] [Google Scholar]