Abstract
Zonisamide (ZNS) efficacy and safety in epilepsy have been demonstrated in four double-blind, placebo-controlled studies. In the present article, we examined all long-term studies performed with this drug. Nine open-label studies, in which ZNS had been administered as an add-on or as monotherapy to epileptic patients for at least 6 months, were selected for our analysis. Four outcome measures were searched. Retention of this drug after 1 year varied between 45% and 65%. The percentages of patients achieving a ≥50% seizure reduction, with respect to baseline, ranged between 37% and 65%. In patients with drug-resistant forms of epilepsy, the percentage of patients reaching a 6-month seizure freedom period was 9%. The percentages of patients who discontinued the experimental drug due to adverse effects ranged between 4% and 24%. Somnolence and dizziness were the most frequently reported adverse effects. Long-term studies demonstrate that ZNS has a good efficacy and tolerability profile, and support its use as adjunctive therapy for epileptic patients.
Keywords: antiepileptic drugs, zonisamide, long-term treatment, review, epilepsy
Introduction
Zonisamide (ZNS) is a new antiepileptic drug (AED) that has been approved for broad use in Japan, while regulatory agencies in the USA and Europe have approved it only as an adjunctive therapy for treatment of partial seizures in adults.
This drug is a benzisoxazole derivative, first synthesized in 1972, and is structurally different from all other AEDs.1 Its anticonvulsant activity, which was fortuitously discovered, has been proven in a number of experimental seizure models.1 Current evidence suggests that ZNS has multiple modes of action. It exerts its pharmacological effects by blockade of neuronal voltage-gated sodium channels and low-voltage-activated (T-type) calcium channels.2 A modest inhibitory effect on carbonic anhydrase has been reported, which is thought to be 100 to 200 times less potent than that of acetazolamide. This mechanism is not believed to significantly contribute to the antiepileptic actions of the drug.3 ZNS has also some effects on the synthesis, release, and degradation of a number of different neurotransmitters, including glutamate, GABA, dopamine, serotonin, and acetylcholine which may lead to enhancement of synaptic inhibition.4
Several in vitro experiments have shown that ZNS has some neuroprotective effects that may suggest a potential use in treating subjects with traumatic brain injury.5
ZNS has a favorable pharmacokinetic profile. It is rapidly absorbed from the gastrointestinal tract, and peak plasma concentrations are achieved within 2 to 5 hours after oral dosing. Food can reduce the rate, but not the extent, of absorption. Oral bioavailability approaches 100%, and the kinetics are linear after the administration of a single dose of 100 to 800 mg.6
Although inactivation of ZNS occurs predominantly by hepatic metabolism involving CYP3A4-mediated reduction, this drug neither induces nor inhibits the hepatic cythochrome P450 isoenzymes that are implicated in the metabolism of several AEDs.7 ZNS clearance increases to about 50% when administered in combination with other AEDs that are known inducers of CYP3A4, such as phenobarbital (PB), carbamazepine (CBZ), or phenytoin (PHT). However, since the half-life of ZNS is 50 to 70 hours in non-induced subjects,8,9 a slight increase of ZNS metabolism is not expected to have any significant clinical effect. Nevertheless, if discontinuation of a co-administered, inducing AED is planned, the dose of ZNS should be adapted appropriately.
Finally, experimental data revealed only modest differences in the most relevant kinetic ZNS parameters between adults and special populations of young or old healthy volunteers.9,10
ZNS efficacy and safety have been demonstrated in several pre-registration, randomized, double-blind, placebo-controlled studies in which this drug was administered as adjunctive therapy in drug-resistant epileptic patients.11–14 Results of these studies have been the object of several reviews and meta-analyses.15,16
The transferability of these findings to clinical practice, however, is limited mainly because of the short duration of double-blind studies, which precludes any assessment of long-term efficacy and chronic adverse effects. Furthermore, doses used in regulatory trials do not always reflect those actually used in clinical practice, and the efficacy and safety profiles of patients enrolled in randomized trials may differ strikingly from those of patients treated in the daily practice.
Several years ago, we compiled a review of the literature on long-term, open studies with gabapentin (GBP), lamotrigine (LTG), levetiracetam (LEV), and topiramate (TPM), when used as add-on in drug-resistant epileptic patients.17 In that study, we searched for three robust markers of efficacy or tolerability: the percentage of patients attaining a 6-month, seizure-free period; the percentage of patients withdrawing because of adverse effects; and the percentage of patients continuing treatment. Clear differences between these four drugs were observed.
Here, we analyze long-term studies performed with ZNS. The markers that we chose for this analysis were: the percentage of patients continuing treatment (retention in the study), the percentage of responder patients (those patients achieving a ≥50% seizure reduction), the percentage of patients attaining seizure freedom for a defined time (at least 6 months), and percentage of patients withdrawing from the study because of adverse effects. While the first parameter is a mixed measure of efficacy and tolerability, the second and third are pure measures of efficacy, and the fourth is a pure measure of tolerability. The heterogeneity of the selected studies precluded any sophisticated analysis of the above-mentioned markers of efficacy or tolerability.
Methods
In December 2008, a computer search of Medline (PubMed version) was conducted for all articles on ZNS using the following Boolean keyword syntax: zonisamide AND epilepsy. The Medline search produced 430 articles. Other studies were found by manual searching.
We found 41 open studies documenting the clinical evaluation of ZNS in epilepsy. From these articles we selected trials that met the following criteria: 1) open-label studies performed in epileptic patients to whom ZNS was administered as monotherapy or add-on therapy at standard dosages, 2) the duration of the study was at least 6 months, 3) at least one of the outcome measures (see below) had to be reported, 4) populations of adults and/or children were included. Studies specifically aimed at recruiting special populations (such as the elderly, intellectually disabled, patients affected by specific epileptic syndromes) were excluded.
Outcome measures
Our analysis included those trials that reported at least one of the following parameters:
Number and/or percentage of patients continuing the experimental treatment for the indicated period of time (6 months or longer).
Number and/or percentage of responders (a responder is a patient who has a ≥50% seizure reduction, with respect to a baseline seizure frequency). Although some studies reported the percentage of patients acquiring ≥25%, and ≥75% seizure reduction, for our analysis, we considered only the first outcome measure.
Number and/or percentage of patients who were seizure-free for a specific period of time (at least 6 months).
Number and/or percentage of patients who withdrew from the study because of adverse effects.
Statistical analyses
The 95% confidence intervals (CI) were calculated to estimate the statistical variations of our end-points. Standard methods were used to calculate 95% CI of the mean. In the case of proportions, the calculation was based on the equations described by Fleiss.18
Results
Thirty-two studies were excluded from our analysis because they reported on specific epileptic syndromes of infancy, had insufficient or unspecified durations, did not use our outcome measures for evaluation or for miscellaneous causes (such as duplication of data from other studies, utilization of unusual dosages, not published in English, French or Italian).
Nine articles were selected for the analysis.19–27 Their characteristics and results are summarized in Table 1.
Table 1.
Selected long-term clinical studies on zonisamide (ZNS)
| Study | Characteristics of the study and number of patients included | Duration of the study and ZNS dose or levels | Retention number (%) | Efficacy measures number (%) | AEW pts number (%) |
|---|---|---|---|---|---|
| Leppik et al19 | Prospective, add-on
Long-term data from a short open study Pts included in open study: 167 drug-resistant, adults Pts included in long-term study: 113 |
Long-term study duration: 16 mo
ZNS dose range: 50–1100 mg/day |
81/167 (48.5%) after 16 mo | Data not reported | 37/167 (22.1%) 21 in the open study, 16 in the long-term study |
| Study 922 Ext (US) from Leppik20 | Prospective, add-on
Long-term data from a double-blind clinical study Pts included in the open phase: 145 drug-resistant, adults |
Study duration: 24 mo (mean)
Mean ZNS levels: 21.7 ± 14.1 ug/mL |
Data not reported | Responders: 63/145 (43.2%)
Seizure-free: not reported |
Data not reported |
| Study 912 Ext (US) from Leppik20 | Prospective, add-on
Long-term data from a double-blind study Pts included in the open phase: 123 drug-resistant, adults |
Study duration: 14 mo (mean)
ZNS dose: 500 mg/day |
Data not reported | Responders: 45/123 (37%)
Seizure-free: not reported |
Data not reported |
| Study 912–39 Ext (US) from Leppik20 | Prospective, add-on
Long-term data from a double-blind study Pts included in the open phase: 137 drug-resistant, adults |
Study duration: 14 mo (mean)
ZNS dose: 600 mg/day |
Data not reported | Responders: 57/137 (42%)
Seizure-free: not reported |
Data not reported |
| Pooled analysis of data from double-blind studies Leppik20 | Prospective, add-on
Long-term data from 4 placebo-controlled and open label studies Pts included: 1207 drug-resistant, adults |
Study duration: up to 48 mo
ZNS dose: different drug dosages |
Data not reported | Analytical description of efficacy data given in previous studies | 263/1207 (21.8%) |
| Wroe et al21 | Prospective, add-on
Long-term data from a double blind study Pts included: 317 drug-resistant, adults (228 received ZNS during double blind period, 89 pts initially allocated to placebo) |
Study duration: up to 36 months
ZNS dose: 100, 300 or 500 mg/day during double-blind study Adjusted in the open phase with a dose range: 100–600 mg/day |
135/207 (65,3%) after 12 mo
62/139 (44.5%) after 24 mo 26/91 (28.8%) after 36 mo |
Responders: 45% at each time point (LOCF analysis)
Seizure-free: 29/317 pts (9.1%) any 6-mo period 21/317 (6.6%) any 12-mo period. 6/317 (2%) and 1/317 (0.3%) last 6 or 12 mo, respectively |
Tolerability data have been included in the pooled analysis |
| Shinnar et al22 | Prospective, add-on
Pts included: 109 (mean age 7.6 ± 3.5 yrs), drug-resistant, children |
Study duration: 15 mo
ZNS dose: standard titration Adjusted, maximum dose: 12 mg/kg/day |
52/109 after 15 mo | Responders: data not reported
Seizure-free: data not reported |
10/109 (9%) |
| Coppola et al23 | Prospective, add-on Pts included: 82 (age range 3–34 yrs) drug-resistant, adults and children | Study duration: mean follow up 11.9 mo (range 2–64)
ZNS dose: standard titration. Adjusted, maximum dose: 12 mg/kg/day. |
Data not reported | Responders:
Total number: 40/82 (48.8%) Pts with generalized epilepsies: 13/35 (37.1%) Pts with partial epilepsies: 27/47 (57.4%) Seizure-free: data not reported |
1/82 (1.2%) |
| Iinuma and Haginoya26 | Prospective. Add-on and monotherapy.
Pts included: 759 (age range: less than 1 yr to 15 yrs). ZNS administered on monotherapy in 245/401 (61%) intellectually normal and 46/328 (14%) disabled pts Study duration was between 6 m. and 5 yrs) Thirty pts eliminated from the analysis because lost to follow up (also due to adverse effects). |
Study duration: range between 6 mo and 60 mo
ZNS dose: not specified |
Data not reported | Responders:
Total number: 455/729 (62.4%) Intellectually normal with generalized 55/67 (82%) and with partial 256/331 (77%) epilepsies Intellectually disabled with generalized 54/152 (36%) and with partial 85/170 (50%) epilepsies Total responders, ITT population: 455/759 (59.9%) Seizure-free: not reported |
Data not clearly specified |
| Chung et al27 | Retrospective. Add-on and monotherapy study (presumably, the majority of pts were drug-resistant)
Several AEDs tested. Pts taking ZNS included: 128. Age not specified |
Study duration: 24 mo
ZNS dose: not specified |
84/128 (65.6%) after 6 mo
78/128 (61%) after 12 mo 77/128 (60.2%) after 24 mo |
Not reported | 30/128 (23.4%) |
| Fukushima and Seino24 | Retrospective monotherapy
Pts included: 77 (mean age: 13.9 ± 1.3) This population included 18 newly diagnosed pts and 59 pts already being treated with AEDs |
Duration: more than 6 mo
ZNS dose: 233 ± 111mg/day. |
38/77 (49.3%) for. more than 6 m | Responders
Total number: 49/77 (64%) Newly diagnosed: 14/18 (77%) From previous AEDs: 35/59 (59%) Seizure-free at least 6 m Total number: 18/77 (24%). Newly diagnosed 4/18 (22%). Previously treated 14/59 (24%) |
3/77 (3.9%) Early: 1/77 (1.3%) Late 2/77 (2.6%) |
| Newmark and Dubinsky25 | Retrospective, monotherapy
Pts included: 54 (age range: 12–72 yrs). This population included 15 newly diagnosed pts and 39 pts previously exposed to other AEDs |
Study duration: up to 18 mo
ZNS dose: mean dose of 193 mg/day and 218 mg/day for newly diagnosed and previously treated pts, respectively. |
Data not clearly specified | Responders: data not reported
Seizure-free for more than 6 m Total n: 14/54 (25.9%) Newly diagnosed 4/15 (26.6%). Previously treated 10/39 (25.6%) Seizure-free for more than 12 m: 9/54 (16.6%) |
5/54 (9%) |
With fixed ZNS doses (100, 300, 500 mg/day).11
Notes: Retention: number and percentage of patients who continue to take the experimental drug at specified time points. Responders: the number and percentage of patients achieving a ≥50% seizure reduction with respect to a baseline seizure frequency. Seizure-free patients: the number and percentage of patients achieving seizure freedom for a specified duration of time. Only data from those patients who achieved seizure freedom for six months or more is included. AEW (antiepileptic withdrawing) pts: the number of patients withdrawing from the study because of adverse effects.
One of the chosen articles was a review20 of long-term data from patients who had been recruited in the four key ZNS double-blind studies.11–14 From this review, we drew information concerning three extension studies (studies 922 ext, 912 ext, 912–39 ext, all conducted in US). In each study, the number of patients included in the open phase was reported. Data from the fourth long-term extension study included in Leppick’s review has been recently published in extenso.21 Therefore, for this study we selected data from the last publication only. While it was possible to report efficacy data for any single extension study, the percentage of patients withdrawing for adverse effects was available only as cumulative data from all of the double-blind, and some open, long-term studies.20 We considered each of these evaluations as a separate study. Therefore, the total number of clinical studies actually included in our analysis was 12. Nine studies were prospective,19–23,26 and three were retrospective.24,25,27
The main difference among the selected studies was the population of patients recruited. The first group of eight studies19–23 was performed in adult, drug-resistant patients, mainly with localization-related epilepsies, and in whom ZNS was added to a previous AED treatment. Two retrospective studies included patients treated with ZNS as monotherapy.24,25 However, in both studies, the patient population was heterogeneous, consisting of newly diagnosed patients and patients who were being treated with another AED that was later withdrawn due to its ineffectiveness or lack of tolerance.
One prospective study26 included a mixed population of pediatric patients treated with ZNS, as an add-on or monotherapy. Finally, the last study was retrospective, and the percentage of patients on monotherapy or polytherapy was not specified.27
Retention of patients in the trial
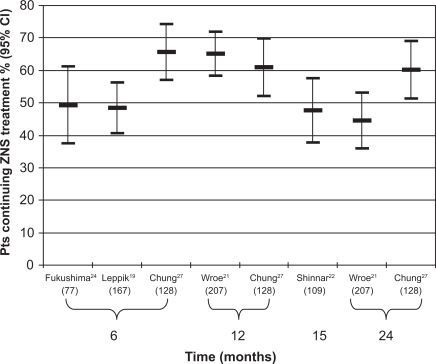
Five of the studies provided this information,19–22,24,27 and these data, calculated at different time points, are reported in Figure 1. In all of these studies, ZNS was added in lieu of an unsatisfactory AED treatment. We observed that the percentages of patients who continued to use this drug after 1 year ranged between 50% and 60%, with a modest drop-off over time.
Figure 1.
Percentage of patients continuing treatment with zonisamide (ZNS) after 6, 12, 15, and 24 months. For explanation see text. On the horizontal axis is the name of the first author of each clinical study which reported this information. The number of patients recruited in each study are given in parentheses.
Percentage of responders
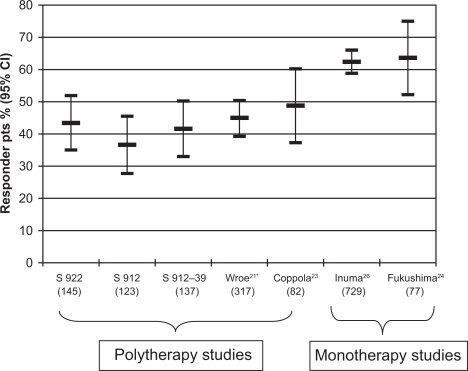
The percentages of patients achieving a ≥50% seizure reduction, with respect to a baseline seizure frequency, was calculated for each of the seven studies,20,21,23,24,26 and is represented in Figure 2. We observed that, in those studies where ZNS was added to a previous therapy,20,21,23 percentages of responders were between 37% and 50%. In those studies performed on mixed populations of patients24,26 (patients mainly on monotherapy with presumably less severe forms of epilepsy), percentages of responders were higher (between 60% and 65%).
Figure 2.
Percentage of responders: patients achieving a ≥50% seizure reduction during treatment with zonisamide in studies which reported this information. For explanation see text. On the horizontal axis is the name of the study or the first author of each study. The number of patients recruited is given in parentheses.
*LOCF analysis.
Patients who were seizure-free
The percentage of patients reaching seizure freedom was given in three studies.21,24,25 However, one study calculated this parameter in a manner that was different to the other two. The first study,21 conducted on a population of drug-resistant patients, considered those patients who did not have seizures for a period of 6 or 12 months during the study, and at the end of the observation period (terminal seizure freedom) (Table 1). The two remaining studies,24,25 in which ZNS was given as a monotherapy, considered those patients who had been free of seizures for at least 6 months, to be 6-months seizure-free.
Patients who discontinued the study due to adverse effects
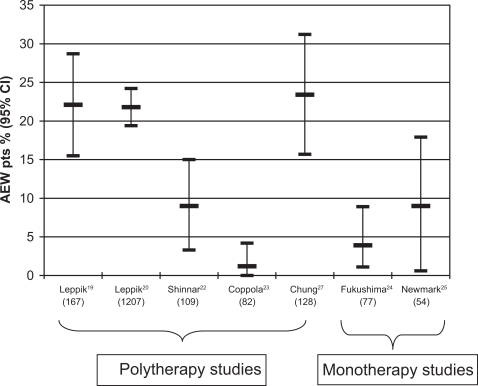
The percentage of patients who discontinued the experimental drug because of adverse effects were reported in seven clinical studies19,20,22–25,27 and were between 4% and 25% (see Figure 3). It was evident that these seven studies, which were long-term extensions of double-blind studies, had dropout rates that were generally higher than prospective-open studies.
Figure 3.
Percentage of patients withdrawing from zonisamide for adverse effects (AEW pts). For explanation see text. On the horizontal axis is the name of the first author of each clinical study which reported this information. The number of patients recruited in each study is given in parentheses.
Discussion
Efficacy and tolerability of ZNS have been demonstrated in four double-blind, placebo-controlled, multicenter trials.11–14 In these studies, about 800 patients with drug-resistant forms of epilepsy received ZNS for a period of ≤6 months. The overall results of these studies showed that ZNS was effective at doses of 300 to 500 mg/day, although it was suggested that lower doses may be beneficial for some patients.28 Treatment-related adverse events were mostly mild to moderate in severity and resulted in few discontinuations. In fact, only 19.3% of patients withdrew due to adverse side effects, compared with 8.6% of placebo-treated patients.28
However, the transferability of findings obtained from these double-blind studies to clinical practice, is limited by several factors. First, the doses used did not reflect the actual ones used in clinical practice. For example, in two studies, ZNS doses were fixed and some patients were randomized to a dose, which were ineffective.11 This may have led to an apparently low overall efficacy.16 Second, the efficacy and safety profiles in patients enrolled in randomized trials may differ from those of patients treated in everyday practice. Finally, the follow up period of these double-blind studies was short, being ≤6 months.
Since epilepsy is a chronic disease, in the majority of cases AEDs must be taken for years and often for the life of a patient; thus, further studies are necessary to ascertain whether a treatment will maintain its efficacy and tolerability over the long-term.
The analysis of long-term, open-label studies is a useful way to provide this crucial information.
In our review, we included studies performed in different populations of patients. It has been reported that the range of patients with newly diagnosed epilepsies and whose seizures are completely controlled by a first AED, is 50% to 60%. In those patients who continue to have seizures despite multiple, appropriate drug treatments, the chance of becoming seizure-free when a new AED is added is lower than 5%.29 For this reason, clinical data from add-on studies must be discussed apart from those where this drug was used as monotherapy.
Long-term, add-on, studies performed in patients with drug-resistant epilepsies
These studies were all prospective and were often open, long-term extension studies of previous double-blind studies.20,21 Three studies19,22,23 were not extensions of controlled studies, but were each performed in similar populations of drug-resistant patients. A retrospective study was performed, at least in part, in a presumably similar population of patients.27
Retention data, available only for some of these studies,19,21,22,27 showed that the percentages of patients continuing treatment with the experimental drug after 1 year were between 60% and 70%, with only a modest decrease over time. Data from other AEDs,17 in similar populations of patients, showed that after 1 year, retention of GBP was very low, at 25%, while LTG had retention values between 40% and 60%, TPM retention was around 55%, and LEV, which had the best scores, had retention values between 60% and 80%. Therefore ZNS, in regard to this mixed measure of efficacy and tolerability, had a performance similar to that of most effective AEDs.
The purest measure of efficacy is the percentage of patients who reached a seizure-free state for a certain period of time. In a previous analysis,17 we considered those patients who did not have seizures for 6 months to be seizure-free; this evaluation, was reported in three studies (Table 1).21,24,25 One of these studies, however, included a population of patients who were not controlled with the appropriate AED treatment. In this study,21 about 9% of patients acquired a 6-month period of seizure freedom, but only 2% of patients were seizure-free in the last 6 months (terminal seizure freedom). Data obtained from a study of other AEDs,17 reported that GBP and LTG were associated with 6-month seizure-freedom rates below 4%, while TPM had seizure-free rates around 8% and LEV about 13%. However, the method used for calculating seizure freedom may influence the result. In fact, as the duration of a study increases, so does the probability of acquiring seizure freedom for the specified period of 6 months; and the probability of attaining terminal 6-month seizure freedom is stable.
In this review we have also considered another, less robust, measure of efficacy: percentage of patients achieving a reduction in seizure frequency of ≥50%, with respect to baseline. Findings from studies on drug-resistant patients who reported this outcome measure20,21,23 (Figure 2) to be between 35% and 50%, allowed comparison with double-blind studies where similar parameters were evaluated. In a review of all double-blind studies,28 similar values were observed when patients were randomized to a ZNS dose of 300 to 500 mg/day. This finding confirmed data from controlled studies and indicated that ZNS maintained its efficacy over time.
With regards to tolerability in populations of patients with drug-resistant epilepsies, we found (Figure 3) that in some studies,19,20,22,23,27 the percentage of patients withdrawing from the experimental drug was around 25%, while other studies reported lower percentages. We can speculate that, at least in some cases, this difference may be a consequence of the titration scheme. In fact, the long-term studies which were extensions of previous double-blind studies20 (in double-blind studies a fixed-titration scheme and a high maintenance dose has to be reached) had higher withdrawal rates than those studies in which a low standard initial dose was used, and where titration speed and final doses were individually adjusted. In two of these studies,22,23 fewer than 10% of patients withdrew because of adverse effects. Similar studies performed with other AEDs found percentages around 28% for TPM, and lower for GBP, LTG and LEV (18%, 15%, and 13% respectively).17
Long-term studies performed in patients on monotherapy
Data about efficacy and tolerability of ZNS administered as monotherapy was derived from four studies. Due to heterogeneity of patients recruited and treatment administered, only some of the data could be used in our study. Two retrospective studies24,25 included patients with newly diagnosed epilepsies and patients who discontinued treatment with a previously unsatisfactory AED (for inefficacy or lack of tolerability). It was reported that the percentage of patients with newly diagnosed epilepsies, whose seizures were completely controlled by the first AED used, ranged between 50% and 60%.When the first AED was ineffective, the chance of a second AED being fully effective was about 10%.29,30 Two other studies26,27 included patients treated with ZNS as an add-on and monotherapy. The studies did not provide separate analyses.
Data about retention was reported in one study only.27 This included patients on both monotherapy and polytherapy, with unknown percentages, and was a retrospective mono-therapy study. Results, already discussed in the previous section, did not give information on ZNS effectiveness when this drug was administered as a monotherapy. The percentage of patients on monotherapy who acquired seizure freedom for at least 6 months was reported in two studies.24,25 In both cases, they (between 24% and 26%) were higher than that usually observed in drug-resistant patients, but much lower than that found when a new drug was administered to newly diagnosed patients.29,30 The result probably reflects the heterogeneity of the studied population.
Responder rates were calculated in two studies24,26 and ranged between 60% and 65%. This, too, may reflect the heterogeneity of the populations included in these studies. Finally, the percentage of patients withdrawing because of adverse effects was low in both monotherapy studies,24,25 but was higher in one study with a mixed population of patients on add-on or monotherapy ZNS treatment. The limited amount, and the heterogeneity, of data did not allow us to draw definitive conclusions on efficacy and tolerability of ZNS administered as monotherapy.
Long-term adverse effects of zonisamide
From the analysis of double-blind controlled studies emerges a favorable ZNS tolerability profile.16 In a meta-analysis of all adverse effects observed with new AEDs in key, placebo-controlled clinical studies, ZNS and GBP significantly associated with two adverse effects (somnolence and dizziness), while LEV associated with one adverse effect; all other AEDs significantly associated with three or four adverse effects.31
In Table 2, the adverse effects most frequently observed in some long-term studies are reported.19,20,21,22 Somnolence and dizziness were the most frequently observed adverse effects.
Table 2.
Selection of the most important adverse effects in some clinical studies
| Adverse effect | Leppik20* | Wroe et al21* | Leppik et al19*,& | Shinnar et al22** |
|---|---|---|---|---|
| Number of patients included in the study | N = 1207 | N = 318 | N = 113 | N = 109 |
| Drowsiness or somnolence | 263 (21.8%) | 47 (14.8%) | 15 (13.3%) | 22 (20.2%) |
| Dizziness | 247 (20.5%) | 54 (17%) | 18 (15.9%) | 4 (3.7%) |
| Anorexia or decreased appetite | 201 (16.7%) | 17 (15%) | 20 (18.3%) | |
| Fatigue or tiredness or asthenia | 182 (15.1%) | 39 (12.3%) | 10 (8.8%) | 5 (4.6%) |
| Nausea | 159 (13.2%) | 35 (11%) | 17 (15%) | |
| Headache | 145 (12%) | 74 (23.3%) | 18 (15.9%) | |
| Disturbance in attention or confusional states | 145 (12%) | 12 (10.6%) | ||
| Mental slowing | 138 (11.4%) | |||
| Thinking abnormal | 11 (9.7%) | 5 (4.6%) | ||
| Behavioral changes or epileptic psychosis, irritability, agitation, nervousness, | 133 (11%) | 10 (8.8%) | 9 (8.3%) | |
| Hostility | 9 (8.3%) | |||
| Emotional lability | 6 (5.5%) | |||
| Diplopia | 131 (10.9%) | |||
| Difficulty with memory | 125 (10.4%) | |||
| Weight loss | 43 (13.5%) | |||
| Diarrhea | 33 (10.4%) | |||
| Ataxia | 12 (10.6%) | |||
| Dysarthria | 10 (8.8%) | |||
| Decreased sweating | 8 (7.3%) |
Adverse events reported by ≥10% of patients.
Adverse events observed during long-term phase only in patients enrolled in the long-term study.
Adverse events reported by ≥2% of patients.
Adverse effects characteristic of ZNS and TPM, included anorexia and/or decreased appetite. These effects generally caused a small amount of weight loss, which did not appear to be progressive and stabilized over period of 2 years.20 In some controlled studies,12,13 a small percentage of patients developed cognitive problems during short-term ZNS treatment. Table 2 shows that adverse effects were also observed during long-term treatment. These effects were usually related to dose and titration speed.20,32 To specifically address long-term effects of different doses of ZNS on cognition, an assessment of several cognitive functions was performed in epileptic patients before, and after 1 year of ZNS treatment. It was observed that some cognitive functions, such as delayed word recall and verbal fluency, were adversely affected by ZNS after 1 year of treatment, and that the effect was more pronounced at higher doses.33
Kidney stones, a relatively rare complication of ZNS, were reported in about 4% of chronically treated patients.34 In Leppik’s study,19 which is the oldest long-term study, kidney stones were observed in 3.5% of their patients. Other less frequently reported, and often idiosyncratic, adverse effects do not always emerge from the analysis of clinical studies. Oligohydrosis was reported in one study only,22 and may be rarely caused by ZNS.1,5,8 The suggested mechanism may be the weak inhibition of carbonic anhydrase by ZNS. In a small percentage of cases (between 1.4% in USA and Europe, and 2% in Japan), a cutaneous rash, leading to drug discontinuation, was associated with ZNS treatment. Interestingly, this rash was more frequently observed in patients on monotherapy than in patients on polytherapy.8,35. Low incidences of serious skin reactions, such as Stevens-Johnson syndrome and toxic epidermal necrolysis, have been reported by post-marketing experiences from Japan.20,36
In conclusion, ZNS is a new AED, which has been approved in the USA and Europe as adjunctive therapy for epileptic patients who are refractory to monotherapy. Among all other new AEDs which have been approved for this indication, ZNS should be considered because it has a predictable pharmacokinetic profile, allowing once- or twice-daily dosing, and no clinically relevant interactions with other first-line AEDs. Also, this drug has multiple mechanisms of action, which beget two important advantages: a broad spectrum of action in the field of epilepsy and possible effects on other neurological or psychiatric diseases. Uncontrolled data suggest that beneficial effects extend across a range of seizure types and syndromes including generalized epilepsy,37 juvenile myoclonic epilepsy, and progressive myoclonic epilepsy.36
ZNS is also under evaluation for the treatment of several other diseases. To date, controlled studies have shown that at doses of 25 to 100 mg/day, it is an effective adjunctive treatment in patients with Parkinson disease.38 ZNS is also being tested for treatment of essential tremors.39,40 Since ZNS, like TPM, is associated with weight loss, trials have also been performed in obese patients41 and patients with binge eating disorder, with interesting results.42,43 Preliminary data suggest that ZNS may be effective for prophylactic treatment in patients with bipolar disorders.44 Finally, one open study showed that this drug may be a safe and effective adjunctive agent for migraine prevention,45 while one controlled study failed to show clinically significant results in patients with painful, diabetic neuropathies.46
Epilepsy is a chronic condition that may be associated with several other diseases.47 In patients who display other diseases in association with epilepsy, the availability of a drug that exerts a positive effect on the comorbid disease may be of strategic importance for optimizing therapy on an individual basis.48
Our review of long-term studies shows that ZNS maintains its efficacy and has good tolerability over time. It may therefore be a good choice as an add-on treatment in epileptic patients where other AEDs have produced unsatisfactory results.
Footnotes
Disclosures
The authors disclose no conflicts of interest.
References
- 1.Baulac M. Introduction to zonisamide. Epilepsy Res. 2006;68(Suppl 2):S3–S9. doi: 10.1016/j.eplepsyres.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 2.Rogawski MA, Lösher W. The neurobiology of antiepileptic drugs. Nature Rev Neuroscience. 2004;5:553–564. doi: 10.1038/nrn1430. [DOI] [PubMed] [Google Scholar]
- 3.Masuda Y, Karasawa T. Inhibitory effect of zonisamide on human carbonic anhydrase in vitro. Arzneimittel-Forschung. 1993;43:416–417. [PubMed] [Google Scholar]
- 4.Biton V. Clinical pharmacology and mechanism of action of zonisamide. Clin Neuropharmacol. 2007;30:230–240. doi: 10.1097/wnf.0b013e3180413d7d. [DOI] [PubMed] [Google Scholar]
- 5.Komatsu M, Hiramatsu M, Willmore LJ. Zonisamide reduces the increase in 8-hydroxyl-2-deoxyguanosine levels formed during iron-induced epileptogenesis in the brain of rats. Epilepsia. 2000;41:1091–1094. doi: 10.1111/j.1528-1157.2000.tb00312.x. [DOI] [PubMed] [Google Scholar]
- 6.Shah J, Shellenberger K, Canfax DM. Zonisamide Chemistry, biotransformation, and pharmacokinetics. In: Levy RH, Mattson RH, Meldrum BS, Perucca E, editors. Antiepileptic drugs. 5th edition. Philadelphia: Lippincott, Williams and Wilkins; 2002. pp. 873–879. [Google Scholar]
- 7.Mimaki T. Clinical pharmacology and therapeutic drug monitoring of zonisamide. Ther Drug Monit. 1998;20:593–597. doi: 10.1097/00007691-199812000-00001. [DOI] [PubMed] [Google Scholar]
- 8.Kothare SV, Kaleyias J. Zonisamide: review of pharmacology, clinical efficacy, tolerability, and safety. Expert Opin Drug Metab Toxicol. 2008;4:493–506. doi: 10.1517/17425255.4.4.493. [DOI] [PubMed] [Google Scholar]
- 9.Sills GJ, Brodie MJ. Pharmacokinetics and drug interactions with Zonisamide. Epilepsia. 2007;48(3):435–441. doi: 10.1111/j.1528-1167.2007.00983.x. [DOI] [PubMed] [Google Scholar]
- 10.Wallace J, Shellenberger K, Groves L. Pharmacokinetics of zonisamide in young and elderly subjects. Epilepsia. 1998;39(Suppl 6):190–191. [Google Scholar]
- 11.Brodie MJ, Duncan R, Vespignani H, Solyom A, Bitenskyy V, Lucas C. Dose-dependent safety and efficacy of zonisamide: a randomized, double-blind, placebo-controlled study in patients with refractory partial seizures. Epilepsia. 2005;46(1):31–41. doi: 10.1111/j.0013-9580.2005.14704.x. [DOI] [PubMed] [Google Scholar]
- 12.Sackellares JC, Ramsay RE, Wilder BJ, Browne TR, 3rd, Shellenberger MK. Randomized, controlled clinical trial of zonisamide as adjunctive treatment for refractory partial seizures. Epilepsia. 2004;45(6):610–617. doi: 10.1111/j.0013-9580.2004.11403.x. [DOI] [PubMed] [Google Scholar]
- 13.Faught E, Ayala R, Montouris GG, Leppik IE, Zonisamide 922 Trial Group Randomized controlled trial of zonisamide for the treatment of refractory partial-onset seizures. Neurology. 2001;57(10):1774–1779. doi: 10.1212/wnl.57.10.1774. [DOI] [PubMed] [Google Scholar]
- 14.Schmidt D, Jacob R, Loiseau P, et al. Zonisamide for add-on treatment of refractory partial epilepsy: a European double-blind trial. Epilepsy Res. 1993;15(1):67–73. doi: 10.1016/0920-1211(93)90011-u. [DOI] [PubMed] [Google Scholar]
- 15.Otoul C, Arrigo C, van Rijckevorsel K, French JA. Meta-analysis and indirect comparisons of levetiracetam with other second-generation antiepileptic drugs in partial epilepsy. Clin Neuropharmacol. 2005;28:72–78. doi: 10.1097/01.wnf.0000159956.87511.67. [DOI] [PubMed] [Google Scholar]
- 16.Chadwick DW, Marson AG. Zonisamide add-on for drug-resistant partial epilepsy. Cochrane Database Syst Rev. 2005;19(4):CD001416. doi: 10.1002/14651858.CD001416.pub2. [DOI] [PubMed] [Google Scholar]
- 17.Zaccara G, Messori A, Cincotta M, Burchini G. Comparison of the efficacy and tolerability of new antiepileptic drugs: what can we learn from long-term studies. Acta Neurol Scand. 2006;114:157–168. doi: 10.1111/j.1600-0404.2006.00705.x. [DOI] [PubMed] [Google Scholar]
- 18.Fleiss JL. Statistical methods for rates and proportions. New York: John Wiley and Sons; 1981. pp. 13–17. [Google Scholar]
- 19.Leppik IE, Willmore LJ, Homan RW, et al. Efficacy and safety of zonisamide: results of a multicenter study. Epilepsy Res. 1993;14:165–173. doi: 10.1016/0920-1211(93)90021-x. [DOI] [PubMed] [Google Scholar]
- 20.Leppik IE. Practical prescribing and long-term efficacy and safety of zonisamide. Epilepsy Res. 2006;68S:S17–S24. doi: 10.1016/j.eplepsyres.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 21.Wroe SJ, Yeates AB, Marshall A. Long-term safety and efficacy of zonisamide in patients with refractory partial-onset epilepsy. Acta Neurol Scand. 2008;118(2):87–93. doi: 10.1111/j.1600-0404.2008.01055.x. [DOI] [PubMed] [Google Scholar]
- 22.Shinnar S, Pellock JM, Conry JA. Open-label, long-term safety study of zonisamide administered to children and adolescents with epilepsy. Eur J Paediatr Neurol. 2009;13(1):3–9. doi: 10.1016/j.ejpn.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 23.Coppola G, Grosso S, Verrotti A, et al. Zonisamide in children and young adults with refractory epilepsy: An open label, multicenter Italian study Epilepsy Res 2008. doi:10.1016/j.epilepsyres.2008.10.012 [DOI] [PubMed] [Google Scholar]
- 24.Fukushima K, Seino M. A long-term follow-up of zonisamide monotherapy. Epilepsia. 2006;47:1860–1864. doi: 10.1111/j.1528-1167.2006.00831.x. [DOI] [PubMed] [Google Scholar]
- 25.Newmark ME, Dubinsky S. Zonisamide monotherapy in a multi-group clinic. Seizure. 2004;13(4):223–225. doi: 10.1016/S1059-1311(03)00150-X. [DOI] [PubMed] [Google Scholar]
- 26.Iinuma K, Haginoya K. Clinical efficacy of zonisamide in childhood epilepsy after long-term treatment:a postmarketing, multi-institutional survey. Seizure. 2004;13(Suppl 1):S34–S39. doi: 10.1016/j.seizure.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 27.Chung S, Wang N, Hank N. Comparative retention rates and long-term tolerability of new antiepileptic drugs. Seizure. 2007;16:296–304. doi: 10.1016/j.seizure.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 28.Brodie MJ. Zonisamide as adjunctive therapy for refractory partial seizures. Epilepsy Res. 2006;68(Suppl 2):S11–S16. doi: 10.1016/j.eplepsyres.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 29.Mohanraj R, Brodie MJ. Outcomes in newly diagnosed localization-related epilepsies. Seizure. 2005;14(5):318–323. doi: 10.1016/j.seizure.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 30.Mohanraj R, Brodie MJ. Diagnosing refractory epilepsy: response to sequential treatment schedules. Eur J Neurol. 2006;13(3):277–282. doi: 10.1111/j.1468-1331.2006.01215.x. [DOI] [PubMed] [Google Scholar]
- 31.Zaccara G, Gangemi PF, Cincotta M. Central nervous system adverse effects of new antiepileptic drugs: a meta-analysis of placebo-controlled studies. Seizure. 2008;17:405–421. doi: 10.1016/j.seizure.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 32.Berent S, Sackellares JC, Giordani B, Wagner JD, Donofrio PD, Abou-Khalil B. Zonisamide (CI-912) and cognition: results from preliminary study. Epilepsia. 1987;28:61–67. doi: 10.1111/j.1528-1157.1987.tb03624.x. [DOI] [PubMed] [Google Scholar]
- 33.Park SP, Hwang YH, Lee HW, Suh CK, Kwon SH, Lee BI. Long-term cognitive and mood effects of zonisamide monotherapy in epilepsy patients. Epilepsy Behav. 2008;12(1):102–108. doi: 10.1016/j.yebeh.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 34.Wroe S. Zonisamide and renal calculi in patients with epilepsy: how big an issue. Curr Med Res Opin. 2007;23:17–73. doi: 10.1185/030079907X210499. [DOI] [PubMed] [Google Scholar]
- 35.Ohtahara S, Yamatogi Y. Safety of zonisamide therapy. Prospective follow up survey. Seizure. 2004;13(Suppl 1):S50–S55. doi: 10.1016/j.seizure.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 36.Baulac M, Leppik IE. Efficacy and safety of agjunctive zonisamide therapy for refractory partial epilepsy. Seizure. 2007;75:75–83. doi: 10.1016/j.eplepsyres.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 37.Thomas G, McCabe PH.Clinical use of zonisamide in generalsed seizure disorders Epilepsia 200546Suppl 7abstract 2.294. [Google Scholar]
- 38.Murata M, Hasegawa K, Kanazawa I, The Japan Zonisamide on PD Study Group Zonisamide improves motor function in Parkinson disease: a randomized, double-blind study. Neurology. 2007;68(1):45–50. doi: 10.1212/01.wnl.0000250236.75053.16. [DOI] [PubMed] [Google Scholar]
- 39.Morita S, Miwa H, Kondo T. Effect of zonisamide on essential tremor: a pilot crossover study in comparison with arotinolol. Parkinsonism Relat Disord. 2005;11(2):101–103. doi: 10.1016/j.parkreldis.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 40.Zesiewicz TA, Ward CL, Hauser RA, Sanchez-Ramos J, Staffetti JF, Sullivan KL. A double-blind placebo-controlled trial of zonisamide (zonegran) in the treatment of essential tremor. Mov Disord. 2007;22(2):279–282. doi: 10.1002/mds.21282. [DOI] [PubMed] [Google Scholar]
- 41.Gadde KM, Franciscy DM, Wagner HR, 2nd, Krishnan KR. Zonisamide for weight loss in obese adults: a randomized controlled trial. JAMA. 2003;289(14):1820–1825. doi: 10.1001/jama.289.14.1820. [DOI] [PubMed] [Google Scholar]
- 42.McElroy SL, Kotwal R, Guerdjikova AI, et al. Zonisamide in the treatment of binge eating disorder with obesity: a randomized controlled trial. J Clin Psychiatry. 2006;67(12):1897–1906. doi: 10.4088/jcp.v67n1209. [DOI] [PubMed] [Google Scholar]
- 43.Gadde KM, Yonish GM, Foust MS, Wagner HR. Combination therapy of zonisamide and bupropion for weight reduction in obese women: a preliminary, randomized, open-label study. J Clin Psychiatry. 2007;68(8):1226–1229. doi: 10.4088/jcp.v68n0809. [DOI] [PubMed] [Google Scholar]
- 44.Ghaemi SN, Shirzadi AA, Klugman J, Berv DA, Pardo TB, Filkowski MM. Is adjunctive open-label zonisamide effective for bipolar disorder. J Affect Disord. 2008;105:1–3. 311–314. doi: 10.1016/j.jad.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 45.Drake ME, Jr, Greathouse NI, Renner JB, Armentbright AD. Open-label zonisamide for refractory migraine. Clin Neuropharmacol. 2004;27(6):278–280. doi: 10.1097/01.wnf.0000150866.98887.77. [DOI] [PubMed] [Google Scholar]
- 46.Atli A, Dogra S. Zonisamide in the treatment of painful diabetic neuropathy: a randomized, double-blind, placebo-controlled pilot study. Pain Med. 2005;6(3):225–234. doi: 10.1111/j.1526-4637.2005.05035.x. [DOI] [PubMed] [Google Scholar]
- 47.Nuyen J, Schellevis FG, Satariano WA, et al. Comorbidity was associated with neurologic and psychiatric diseases: a general practice-based controlled study. J Clin Epidemiol. 2006;59:1274–1284. doi: 10.1016/j.jclinepi.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 48.Zaccara G.Neurological comorbidity and epilepsy: the implications for treatment Acta Neurologica Scand 2009. In press. [DOI] [PubMed] [Google Scholar]