Abstract
Co-morbid depression is common in patients with diabetes mellitus and has a negative impact on diabetes self-care, adherence to treatment and the development of complications. Effective treatment of depression has been associated with improvement in metabolic parameters. We evaluated the feasibility of a two question screen for co-morbid depression in diabetic patients and studied the effect of the serotonin norepinephrine reuptake inhibitor antidepressant, milnacipran, on metabolic and psychological parameters in 64 type 2 diabetic patients with co-morbid depression. The severity of depression was evaluated using the Beck Depression Inventory (BDI). Patients received milnacipran, and diabetes was treated according to the guidelines of the Austrian Diabetes Association in a 6-month open label study. Metabolic parameters and BDI were measured at baseline and after 1, 3 and 6 months. 46 patients satisfied the criteria for an antidepressant response (reduction of baseline BDI score of at least 50%). Hemoglobin A1c, fasting blood glucose, body mass index, total and LDL-cholesterol and serum triglyceride levels were all significantly decreased in these patients at the end of the study whereas in antidepressant non-responders these parameters were not significantly changed. Diagnosis and treatment of depression is an important factor for the improvement of metabolic control in patients with type 2 diabetes and co-morbid depression.
Keywords: depression, diabetes, milnacipran, metabolic control, metformin
Introduction
Depression is common in patients with diabetes mellitus.1 The prevalence of any depression (major depression or minor forms) is significantly higher in patients with type 2 diabetes than in those without diabetes, 17.6% versus 9.8% respectively.2 In addition, the co-morbidity of diabetes and depression is associated with a significantly increased risk of death from all causes, beyond that due to having either diabetes or depression alone.3 There is a synergistic interaction between diabetes and depression, resulting in decreased metabolic control, a higher incidence of micro- and macro-angiopathic diabetic late complications and decreased quality of life.3–6 In spite of the importance of co-morbid depression for the prognosis of diabetic patients, typically there is little diagnostic focus on mood disorders and depression frequently goes undetected.
The pathophysiological relationship between co-morbid depression and diabetes is poorly understood. As with other severe chronic illnesses, psychological factors associated with the hardship of diabetes may trigger or enhance depressive symptoms.7 There is, however, evidence to suggest that depression may precede and act as a causal factor for weight gain and diabetes.8 A relation between obesity and depression has been described in several studies.9,10 Approximately 80% of diabetic patients are obese or overweight and therefore obesity may also play a role in the development of co-morbid depression.11,12
Data on the impact of antidepressant therapy on metabolic and anthropometric parameters in diabetic patients with co-morbid depression are still scarce.13,14 The aim of the present study was thus to evaluate the efficacy of long-term treatment with the antidepressant, milnacipran, in type 2 diabetic patients with co-morbid depression by measuring in parallel effects on depressive symptoms and metabolic parameters. In addition we examined the practicability of using a simple two-question screening tool for depression in the non-psychiatric setting of diabetes outpatient departments.
Patients and methods
All patients fulfilled the inclusion criteria of a diagnosis of diabetes mellitus type 2 and a depressive disorder. Diabetes mellitus was defined according to the diagnostic criteria of the American Diabetes Association.15 Depression was detected by a simple two-question screen16 (Question 1. During the past month, have you often been bothered by feeling down, depressed, or hopeless? Question 2: During the past month, have you often been bothered by little interest or pleasure in doing things?). In the case of a positive answer to both questions, the diagnosis of depression was subsequently confirmed using the 12-item Major Depression Inventory (MDI) questionnaire according to ICD-10 criteria for depressive episode.17 Exclusion criteria were contra-indications for either metformin or milnacipran, treatment with an antidepressant drug within the last 6 months or significant suicidal ideation.
The following parameters were measured at baseline and after 6 months’ treatment: fasting blood glucose (fBG), hemoglobin A1c (HbA1c), total cholesterol, low-density lipoprotein cholesterol (LDL-cholesterol), high-density lipoprotein cholesterol (HDL-cholesterol), serum triglycerides, blood pressure and weight. Height was measured at baseline. Blood samples were taken after an overnight fast of 12 hours. Body mass index (BMI, kg/m2) was calculated using body weight measured to the nearest kilogram and height measured to the nearest cm. Some additional metabolic parameters were also measured at 1 and 3 months (data not shown).
The severity of depression at baseline and after 1, 3 and 6 months treatment was evaluated using the Beck Depression Inventory (BDI).18 An antidepressant response was defined as a reduction of at least 50% in the baseline BDI score.
Spontaneously reported adverse events were recorded at each visit.
This observational study was conducted at seven investigational sites (diabetes outpatient departments) across Austria in an open longitudinal manner. As a non-interventional observational study, ethics committee was not required by Austrian legislation. Patient recruitment started in February 2006 and the last visit of the last patient was documented in November 2007.
Diabetes therapy was performed according to the Guidelines of the Austrian Diabetes Association,19 starting with metformin at 500 to 2000 mg/day as monotherapy after lifestyle adjustment failure. Antidepressant treatment was initiated with milnacipran at 25 or 50 mg/day. The initial dose and subsequent dose adjustment was at the clinicians’ discretion and based on clinical response and the patient’s tolerance of the drug.
Statistical analysis
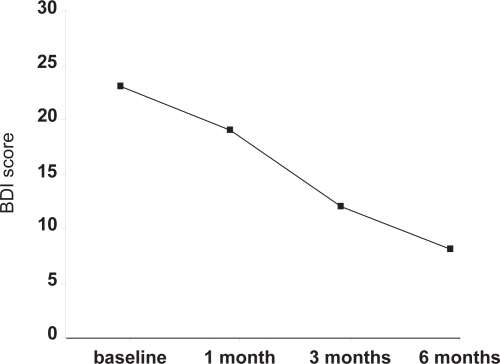
Depression data (Figure 1) were analyzed on an intention to treat basis. Other analyses, especially comparative analyses, are based on those patients for whom data were available at baseline and end-point (observed cases technique). All significance values are calculated as two-tailed.
Figure 1.
Evolution of Beck depression inventory scores throughout the study.
Note: Values are medians of all 64 patients who started the study (ITT analysis).
Abbreviation: BDI, Beck Depression Inventory.
Results
Sixty-four patients were recruited into the study and had a full baseline examination. Six patients dropped out of the study, including one because of an adverse event (the patient reported nausea after 3 months). Other withdrawals were due to lack of compliance (1), patients’ decision (2) and lost to follow-up (2). Fifty-eight patients finished the study.
Table 1 shows the baseline demographic and clinical characteristics of the 58 patients who completed the trial. Fifty-two patients (90%) of the patients had never taken antidepressant medication and 47 patients (81%) were anti-diabetes drug naive and on lifestyle adjustment therapy only. Patients were generally obese and their glycemic control was rated as moderate or poor. Metabolic parameters at baseline and after 6 months treatment (endpoint) are shown in Table 2. Over the duration of the study median values showed a statistically significant improvement for fBG levels, HbA1c, body weight, BMI, total cholesterol, LDL-cholesterol and serum triglycerides. The proportion of patients with HbA1c <7% increased significantly from 5.2% at baseline to 51.7% after 6 months treatment (p < 0.001) while the proportion of patients with HbA1c >8% decreased significantly from 46.6% at baseline to 6.9% at endpoint.
Table 1.
Characteristics at baseline of patients completing the study
| Patients (n) | 58 |
|---|---|
| Sex distribution – m/f (%) | 23/35 (40%/60%) |
| Median age – years (range) | 61 (39–85) |
| Naïve to depression treatment | 52 (90%) |
| Naïve to diabetes drug treatment | 47 (81%) |
Table 2.
Metabolic parameters at baseline and after 6 months’ treatment with milnacipran of patients completing the study
| Parameter | Baseline | 6 months | p |
|---|---|---|---|
| fBG (mg/dL) | 155.0 | 113.0 | <0.0001 |
| HbA1c (% of total Hb) | 7.9 | 6.9 | <0.0001 |
| Body weight (kg) | 90.5 | 84 | <0.001 |
| BMI (kg/m2) | 30.4 | 28 | <0.001 |
| Total chol (mg/dL) | 199.5 | 178 | <0.0001 |
| LDL-chol (mg/dL) | 117 | 108 | <0.001 |
| HDL-chol (mg/dL) | 47.0 | 48.0 | NS |
| Triglyc (mg/dL) | 190 | 157 | <0.001 |
Notes: All values are given as the median. p = significance of the difference between baseline and 6 month values.
Abbreviations: HbA1c, hemoglobin A1c; fBG, fasting blood glucose; BMI, body mass index; Total chol, total cholesterol; LDL-chol, LDL-cholesterol; HDL-chol, HDL-cholesterol; Triglyc, serum triglycerides.
Figure 1 shows the improvement in BDI scores over the duration of the study. After 1 month of treatment 20.3% of patients had responded to antidepressant treatment, 51.6% after 3 months and 71.9% after 6 months. There was no difference between responders and non-responders concerning age, severity of depression, metabolic control or BMI at baseline. Mean dose of milnacipran administered during the final three months was significantly higher in responder patients than non-responder patients (p < 0.05). Metformin doses were similar for responders and non-responders (Table 3).
Table 3.
Mean doses of drugs administered throughout the study
|
Months 0–1 |
Months 2–3 |
Months 4–6 |
||||
|---|---|---|---|---|---|---|
| Miln (mg/day) | Met (mg/day) | Miln (mg/day) | Met (mg/day) | Miln (mg/day) | Met (mg/day) | |
| Responders (n = 46) | 43 | 1264 | 72 | 1674 | 81 | 1737 |
| Non-responders (n = 12) | 46 | 1563 | 56 | 1700 | 58* | 1742 |
p < 0.05.
Abbreviations: Miln, milnacipran; Met, metformin.
As shown in Table 4, antidepressant-responder patients had significant improvements in almost all metabolic and anthropometric parameters while non-responders showed no significant improvement.
Table 4.
Change of metabolic and anthropometric parameters in depression responders and non-responders during milnacipran treatment
|
Responders n = 46 |
Non-responders n = 12 |
|||||
|---|---|---|---|---|---|---|
| baseline | 6 months | Δ | baseline | 6 months | Δ | |
| fBG | 153 | 111*** | –42 | 157 | 131 | –26 |
| HbA1c | 8.1 | 6.9*** | –1.2 | 7.7 | 7.1 | –0.6 |
| Bodyweight | 92.0 | 84.0* | –8.0 | 76.0 | 76.5 | +0.5 |
| BMI | 31.3 | 28.7* | –2.6 | 28.0 | 28.4 | +0.4 |
| Total cholest | 199.5 | 178.0*** | –21.5 | 198.5 | 182.0 | –16.5 |
| LDL | 119.0 | 109.0** | –10.0 | 110.5 | 95.0 | –15.5 |
| HDL | 47.0 | 47.0 | 0.0 | 47.0 | 49.0 | +2.0 |
| Triglyc | 196.0 | 159.0* | –37.0 | 151.0 | 146.0 | –5.0 |
Notes: All values are given as the median. Units are the same as those given in Table 2.
p > 0.05;
p > 0.01;
p > 0.001 for the difference between baseline and 6 months.
Abbreviations: fBG, fasting blood glucose; HbA1c, hemoglobin A1c; BMI, body mass index; Total cholest, total cholesterol; LDL, low density lipoprotein bound cholesterol; HDL, high density lipoprotein bound cholesterols; Triglyc, serum triglycerides.
Discussion
Diabetic patients with severe depressive symptoms frequently have insufficient metabolic control and poorer diet and medication regimen adherence, than patients with less severe or no depressive symptoms.20,21 Several studies have shown that depression is directly associated with an increased risk of diabetic complications, especially retinopathy and macrovascular complications.5,6
The management of depression with behavioral therapy has been shown to be useful and effective in diabetic patients with co-morbid depression.22,23 In Austria, however, access to psychotherapy is not widespread because of the relatively high cost and the need for long-term treatment in most cases. The principal treatment of depression in diabetic patients is, therefore, oral antidepressant medication.
Studies with antidepressants have shown variable effects on metabolic control.13,14,24–26 In a study with sertraline, HbA1c levels were reduced during treatment but did not differ between the sertraline and placebo groups.13 In another study no significant reduction in HbA1c levels was observed in patients treated with fluoxetine or paroxetine although depressive symptoms were significantly improved.14,24 Similarly, treatment with escitalopram resulted in a significant reduction of depression ratings but only a modest, non-significant reduction in fBG levels and HbA1c levels.25 With bupropion, BMI and HbA1c levels decreased significantly over an acute treatment phase with the reduction of depression severity associated with lower HbA1c levels.26
Diabetes is usually diagnosed when the disease has already been present for at least 7 years and the patient is already showing signs of micro and/or macro-vascular complications, sexual dysfunction and non-alcoholic steatohepatitis. The choice of which antidepressant to associate must take into account the presence of these symptoms. Ideally, the antidepressant should have no influence on body weight, cause no interactions with the numerous medications that the patient is likely to be taking, produce neither hepatotoxicity nor cardiovascular or blood pressure effects. It should also cause minimal sedation and minimal (or no) sexual dysfunction.
For this reason, in the present study, we chose to use the antidepressant, milnacipran. In addition to its good overall tolerability,27 it has been demonstrated to be weight neutral, to be free of cytochrome P450 drug interactions and cardio-toxicity and to produce minimal sexual dysfunction.
The reduction in depressive symptoms throughout the study was similar to that seen with milnacipran in other studies of major depression.28–30 By the end of the study over 70% of the patients fulfilled the criteria for an “antidepressant response”. A dose-response relationship for the antidepressant effect of milnacipran has been reported.31 In the present study “antidepressant-responder patients” were treated with higher doses than non-responders (Table 3) especially during the second half of the treatment period. Dose-escalation was at the initiative of the treating physician. Whether a lack of dose increase resulted from satisfaction with the effect of the lower dose or a worry of intolerance of higher doses was not recorded. It is, thus, impossible to judge whether the higher dose levels were responsible for their improved depressive symptoms of the “responder patients”.
Over the duration of the study all of the measured metabolic and anthropometric parameters (with the exception of HDL cholesterol) improved significantly (Table 2). In particular, the number of patients with HbA1c >8%, a common benchmark for the need for intensive therapeutic intervention, decreased dramatically.
Separate analysis of the “antidepressant responder” and “antidepressant non-responder” groups showed that none of the metabolic and anthropometric parameters were significantly improved in the non-responder group (Table 4).
These results extend earlier reports that improvement of depressive symptoms can lead to sustained improvement in HbA1c levels.23–26 Significant reductions of serum triglyceride levels and body weight were also seen in antidepressant-responder patients.
A weakness of the comparison of these two groups is the small size of the non-responder group which may have prevented certain effects from reaching statistical significance. Interestingly the non-responder group had a median BMI which was numerically considerably smaller than the responder group although the difference was not statistically significant. It may be interesting to examine the relationship between baseline BMI and response to an antidepressant in a larger cohort of diabetic patients with co-morbid depression.
There is evidence that depression is under-recognized in patients with diabetes.32 The unfavorable impact of untreated depression on diabetes care and prognosis has been clearly documented.32,33 In particular the incidence of late diabetic complications and mortality risk are both elevated in patients with co-morbid depression.3,5,6,20,21 The present results are consistent with other data and suggest that successful treatment of depression results in a parallel improvement of diabetic symptoms.13,14,23–26 Diabetes is one of the most psychologically and behaviorally demanding of the chronic medical illnesses. At least 90% of diabetes management is conducted by the patient himself. The presence of co-morbid depression can reduce motivation for self-care resulting in an even more unfavorable or even potentially fatal course of diabetes.34 Although most physicians are aware of the importance of detecting co-morbid depression in patients with diabetes many of them find it difficult in practice. Most diagnostic tools for screening depression are complicated, time-consuming and often require at least some psychiatric training. A number of questionnaires have been developed to help identify depression in the primary care setting. These are frequently time consuming and therefore not widely used in clinical practice. With this limitation in mind, a simple two-question screen has been developed.16 It is based on the essential criteria of a depressive episode, a period of at least 2 weeks during which there is either depressed mood and/or the loss of interest or pleasure in nearly all activities. This two-question screen (Question 1. During the past month, have you often been bothered by feeling down, depressed, or hopeless? Question 2: During the past month, have you often been bothered by little interest or pleasure in doing things?) has been found to be an effective means of identifying subjects with depression. A “no” response to both of the two questions made depression highly unlikely. For patients who answer yes to either of the questions, other symptoms should be elicited, to confirm diagnosis of depression. Our use of this instrument in a non-psychiatric setting, diabetes departments of general hospitals, confirmed its simplicity of use and its ready acceptance by the medical personnel. Of the patients screened, there were no “false positives”, patients identified as possibly depressive who were subsequently found not to have depressive symptoms. The use of this simple instrument should be encouraged.
The study made an in depth investigation of a large number of parameters of a relatively long period of time. The study, however, has some methodological weaknesses. It was not a randomized, double-blind design and the cohort was relatively small especially the non-responder group. In addition the dose of milnacipran was not closely controlled and an effect of different doses cannot be completely ruled out. Nevertheless the fact that (with the single exception of HDL cholesterol) all of the parameters were improved in the anti-depressant responder group whereas none of the parameters were improved in the non-responder group suggests that the findings are probably qualitatively reliable. Clearly a larger, randomized trial is warranted.
Long-term treatment of type 2 diabetic patients with co-morbid depression with milnacipran results in a clear improvement in both depressive symptoms and metabolic parameters. Furthermore, patients who did not respond to the antidepressant showed no improvement in metabolic parameters despite receiving diabetes treatment as recommended by international guidelines. Although the present results are insufficient to conclude on the necessity of an improvement of depressive symptoms in order for metabolic parameters to improve this remains a viable working hypothesis. Our results thus underline the importance of screening ALL diabetic patients for depression with, for example, the simple two-question screen. For patients with co-morbid depression treatment with an effective antidepressant drug such as milnacipran, should be immediately initiated in parallel with diabetes treatment as recommended by international guidelines.
Acknowledgments
We gratefully acknowledge those who contributed to planning, performance and evaluation of this study namely: Franz Michael Auhser MD, Tulln, Austria. Mike Briley, PhD, Castres, France. Ljiljana Durovic MD, Wiener Neustadt, Austria. Evelyn Kunschitz, MD, Vienna, Austria. Johann Loipl, MD, Rohrbach, Austria. Anita Luiskandl, MD, Vienna Austria. Karl Nekrep, PhD, Vienna Austria. Kurt Neumann, MS, Vienna Austria. Susanne Maria Pusarnig, MD, Vienna Austria.
Footnotes
Disclosures
The authors declare no conflicts of interest.
References
- 1.Katon WJ. The comorbidity of diabetes mellitus and depression. Am J Med. 2008;121(11 Suppl 2):S8–S15. doi: 10.1016/j.amjmed.2008.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ali S, Stone MA, Peters JL, et al. The prevalence of co-morbid depression in adults with type 2 diabetes: a systematic review and meta-analysis. Diabet Med. 2006;23:1165–1173. doi: 10.1111/j.1464-5491.2006.01943.x. [DOI] [PubMed] [Google Scholar]
- 3.Egede LE, Nietert PJ, Zheng D. Depression and All-Cause and coronary heart disease mortality among adults with and without diabetes. Diabetes Care. 2005;28:1339–1345. doi: 10.2337/diacare.28.6.1339. [DOI] [PubMed] [Google Scholar]
- 4.Egede LE, Ellis C. The effects of depression on diabetes knowledge, diabetes self-management, and perceived control in indigent patients with type 2 diabetes. Diabetes Technol Ther. 2008;10:213–219. doi: 10.1089/dia.2007.0278. [DOI] [PubMed] [Google Scholar]
- 5.Katon WJ, Russo JE, Von Korff M, et al. Long-term effects on medical costs of improving depression outcomes in patients with depression and diabetes. Diabetes Care. 2008;31:1155–1159. doi: 10.2337/dc08-0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DeGroot M, Anderson R, Freedland KE, et al. Association of depression and diabetes complications: A meta-analysis. Psychosom Med. 2001;63:619–630. doi: 10.1097/00006842-200107000-00015. [DOI] [PubMed] [Google Scholar]
- 7.Talbot F, Nouwen A. A review of the relationship between depression and diabetes in adults: is there a link. Diabetes Care. 2000;23:1556–1562. doi: 10.2337/diacare.23.10.1556. [DOI] [PubMed] [Google Scholar]
- 8.Knol MJ, Twisk JW, Beekman AT, et al. Depression as a risk factor for the onset of type 2 diabetes mellitus. A meta-analysis. Diabetologia. 2006;49:837–845. doi: 10.1007/s00125-006-0159-x. [DOI] [PubMed] [Google Scholar]
- 9.Dixon JB, Hayden MJ, Lambert GW, et al. Raised CRP levels in obese patients: symptoms of depression have an independent positive association Obesity (Silver Spring) 200822(Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- 10.Dong C, Sanchez LE, Price RA. Relationship of obesity to depression: a family-based study. Int J Obesity. 2004;28:790–795. doi: 10.1038/sj.ijo.0802626. [DOI] [PubMed] [Google Scholar]
- 11.Russell-Jones D, Khan R. Insulin-associated weight gain in diabetes-causes, effects and coping strategies. Diabetes Obes Metab. 2007;9:799–812. doi: 10.1111/j.1463-1326.2006.00686.x. [DOI] [PubMed] [Google Scholar]
- 12.Onyike CU, Crum RM, Lee HB, et al. Is obesity associated with major depression? Results from the third national health and nutrition examination survey. Am J Epidemiol. 2003;158:1139–1147. doi: 10.1093/aje/kwg275. [DOI] [PubMed] [Google Scholar]
- 13.Lustmann PJ, Clouse RE, Nix BD, et al. Sertraline for prevention of depression recurrence in diabetes mellitus. Arch Gen Psychiatry. 2006;63:521–529. doi: 10.1001/archpsyc.63.5.521. [DOI] [PubMed] [Google Scholar]
- 14.Gülseren L, Gülseren S, Hekimsoy Z, et al. Comparison of fluoxetine and paroxetine in type 2 diabetes mellitus patients. Arch Med Res. 2005;36:159–165. doi: 10.1016/j.arcmed.2004.12.018. [DOI] [PubMed] [Google Scholar]
- 15.Diagnosis and classification of diabetes mellitus, American Diabetes Association. Diabetes Care. 2006;29(Suppl 1):S43–S48. [PubMed] [Google Scholar]
- 16.Whooley MA, Avins AL, Miranda J, et al. Case-Finding Instrument for Depression. J Gen Intern Med. 1997;12:439–445. doi: 10.1046/j.1525-1497.1997.00076.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beck AT, Beamesderfer A. Assessment of depression: the depression inventory. Mod Probl Pharmacopsychiatry. 1974;7:151–169. doi: 10.1159/000395074. [DOI] [PubMed] [Google Scholar]
- 18.Bech P, Rasmussen NA, Olsen LR, et al. The Sensivity and Specifity of the Major Depression Inventory, using the present State Examination as the index of diagnostic validity. J Affect Dis. 2001;88:159–164. doi: 10.1016/s0165-0327(00)00309-8. [DOI] [PubMed] [Google Scholar]
- 19.Austrian Diabetes Association Therapeutic guidelines of the Austrian Diabetes Association. http://www.oedg.org/pdf/Leitlinien_2007.pdf
- 20.Rush WA, Whitebird RR, Rush MR, et al. Depression in patients with diabetes: does it impact clinical goals. J Am Board Fam Med. 2008;21:392–397. doi: 10.3122/jabfm.2008.05.070101. [DOI] [PubMed] [Google Scholar]
- 21.Gonzalez JS, Safren StA, Cagliero E, et al. Depression, self-care and medication adherence in type 2 diabetes. Diabetes Care. 2007;30:2222–2227. doi: 10.2337/dc07-0158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lustmann PJ, Griffith LS, Freedland KE, et al. Cognitive behavioural therapy for depression in type 2 diabetes mellitus. Ann Intern Med. 1998;129:613–621. doi: 10.7326/0003-4819-129-8-199810150-00005. [DOI] [PubMed] [Google Scholar]
- 23.Peyrot M, Rubin RR. Behavioral and psychosocial interventions in diabetes: A conceptual review. Diabetes Care. 2007;30:2433–2440. doi: 10.2337/dc07-1222. [DOI] [PubMed] [Google Scholar]
- 24.Lustmann PJ, Griffiti LS, Freedland KE, et al. Fluoxetine for depression in diabetes. Diabetes Care. 2000;23:618–623. doi: 10.2337/diacare.23.5.618. [DOI] [PubMed] [Google Scholar]
- 25.Amsterdam JD, Shults J, Rutherford N, et al. Safety and efficacy of s-citalopram in patients with comorbid major depression and diabetes mellitus. Neuropsychobiology. 2007;54:208–214. doi: 10.1159/000100369. [DOI] [PubMed] [Google Scholar]
- 26.Lustmann PJ, Williams MW, Sayur GS, et al. Factors influencing glycemic control in type 2 diabetes during acute- and maintenance-phase treatment of major depressive disorder with bupropion. Diabetes Care. 2007;30:459–466. doi: 10.2337/dc06-1769. [DOI] [PubMed] [Google Scholar]
- 27.Montgomery SA.Tolerability of serotonin norepinephrine reuptake inhibitor antidepressants CNS Spectr 2008137(Suppl 11):27–33. [DOI] [PubMed] [Google Scholar]
- 28.Lopez-Ibor J, Guelfi JD, Pletan Y, et al. Milnacipran and selective serotonin reuptake inhibitors in major depression. Int clin Psychophamacol. 1996;11(Suppl 4):41–46. doi: 10.1097/00004850-199609004-00006. [DOI] [PubMed] [Google Scholar]
- 29.Clerc G, Milnacipran/Fluvoxamine Study Group Antidepressant efficacy and tolerability of milnacipran, a dual serotonin and noradrenaline reuptake inhibitor: a comparison with fluvoxamine. Int Clin Psychopharmacol. 2001;16:145–151. doi: 10.1097/00004850-200105000-00003. [DOI] [PubMed] [Google Scholar]
- 30.Sechter D, Vandel P, Weiller E, et al. A comparative study of milnacipran and paroxetine in outpatients with major depression. J Affect Disord. 2004;83:233–236. doi: 10.1016/j.jad.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 31.Okumura K, Furukawa TA. Remission rates with milnacipran 100mg/day and 150 mg/day in the long-term treatment of major depression. Clin Drug Invest. 2006;26:135–142. doi: 10.2165/00044011-200626030-00003. [DOI] [PubMed] [Google Scholar]
- 32.Ciechanowski PS, Katon WJ, Russo JE. Depression and diabetes. Impact of depressive symptoms on adherence, functions and costs. Arch Intern Med. 2000;160:3278–3285. doi: 10.1001/archinte.160.21.3278. [DOI] [PubMed] [Google Scholar]
- 33.Paile-Hyvärinen M, Wahlbeck K, Eriksson JG. Quality of life and metabolic status in mildly depressed women with type 2 diabetes treated with paroxetine: a single-blind randomised placebo controlled trial. BMC Fam Pract. 2003;4:7. doi: 10.1186/1471-2296-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gonzalez JS, Peyrot M, McCarl LA, et al. Depression and diabetes treatment non-adherence: A meta-analysis. Diabetes Care. 2008;31:2398–2403. doi: 10.2337/dc08-1341. [DOI] [PMC free article] [PubMed] [Google Scholar]