Abstract
Most of our knowledge regarding the process of protein import into mitochondria has come from research employing Saccharomyces cerevisiae as a model system. Recently, several mammalian homologues of the mitochondrial motor proteins were identified. Of particular interest for us is the human Tim14/Pam18-Tim16/Pam16 complex. We chose a structural approach in order to examine the evolutionary conservation between yeast Tim14/Pam18-Tim16/Pam16 proteins and their human homologues. For this purpose, we examined the structural properties of the purified human proteins and their interaction with their yeast homologues, in vitro. Our results show that the soluble domains of the human Tim14/Pam18 and Tim16/Pam16 proteins interact with their yeast counterparts, forming heterodimeric complexes and that these complexes interact with yeast mtHsp70.
Keywords: Tim14 (also known as Pam18), Tim16 (also known as Pam16), mtHsp70, translocation motor
1. Introduction
Mitochondria are vital organelles for eukaryotes because they serve as a site for many essential processes such as respiration, lipid metabolism, heme metabolism, synthesis of metabolites and metal homeostasis. Moreover, in higher eukaryotes, mitochondria participate in calcium signaling and in mediation of apoptosis. It is estimated that ~800–1,000 proteins are involved in these and other mitochondrial functions. However, only a very small fraction of the mitochondrial proteins is actually produced in situ (eight in S. cerevisiae). The rest are encoded by nuclear genes, synthesized in the cytosol and then delivered to one of the four mitochondrial compartments: the outer membrane, inner membrane, the intermembrane space and the matrix. Consequently, functional import systems for nuclear encoded proteins are indispensable for the biogenesis of mitochondria and accordingly for the viability of eukaryotic cells. The import of nuclear-encoded proteins into the mitochondria is a multistep process mediated by the coordinated action of translocation machineries localized in both the outer and inner mitochondrial membranes [1–3].
In the outer membrane, the multimeric TOM complex serves as both a receptor for recognition of mitochondrial precursor proteins and a main portal of protein entry into mitochondria [1–4]. The TOM complex is composed of the primary receptors, Tom20 and Tom70, and the subunits Tom40, Tom22, Tom7, Tom6, and Tom5 that together form the stable core of the complex. Tom40 forms the protein-conducting channel, providing a route for precursor proteins to cross the outer membrane. On their way to the matrix, proteins that contain cleavable amino-terminal targeting signals are transferred from the TOM complex to the TIM23 preprotein translocase. This complex is composed of a core of two multispanning integral inner membrane proteins Tim17 and Tim23. The latter forms a channel which allows proteins to integrate into, or to cross, the inner membrane. A third protein, Tim50, seems to serve as a sorting receptor in the mitochondrial intermembrane space and maintains the permeability barrier that is formed by Tim23 [5].
The final steps of translocation across the inner membrane are mediated by the mitochondrial translocation motor (Presequence translocase-Associated protein import Motor, PAM). The translocation motor itself is a multisubunit complex, which contains the ATP-hydrolyzing 70 kDa heat-shock protein, mtHsp70, as its central component. Precursor proteins emerging from the matrix side of the TIM23 channel bind to mtHsp70, with whose help the protein unfolds and completes its translocation into the mitochondrial matrix [2,6–9]. In order to mediate protein import, mtHsp70 must anchor to the TIM23 import channel at certain stages of the import process. This is accomplished by another component of the translocation motor, Tim44, a peripheral membrane protein that binds simultaneously to mtHsp70 and the TIM23 complex [10–13]. MtHsp70 undergoes conformational changes that are controlled by ATP hydrolysis upon binding, unfolding and release of precursor proteins.
Additional components of the motor are suggested to play a regulatory role in either the function or stability of the motor. These include accessory proteins that directly regulate the function of mtHsp70 [7]. For example, Mge1 acts as a nucleotide-exchange factor and promotes the release of imported precursor proteins from mtHsp70. J-domain containing proteins, DnaJs, usually enhance the ATPase activity of Hsp70 chaperones. Such enhancement of the ATPase activity is required for promoting the tight binding of unfolded substrate proteins to the peptide-binding pocket of mtHsp70. The latter role is played by the membrane-bound J-domain-containing protein named Tim14/Pam18 [11,14,15]. Biochemical and in-organelle studies has shown that Tim14/Pam18 forms a stable complex with another protein, Tim16/Pam18 [16–18] which acts as an antagonist to the Tim14/Pam18 ATPase enhancement [16,19]. High resolution structure of the complex has been published recently and provided valuable mechanistic insight into the function of the yeast Tim14/Pam18-Tim16/Pam16 heterdimer [20].
Homologues of yeast Tim14/Pam18 and Tim16/Pam16 were also identified in humans (DNAJC19 and Magmas respectively). DNAJC19 and Magmas are of great interest since they are associated with several human disorders. A novel autosomal recessive disorder called “DCMA syndrome” has already been associated with a mutation in the DNAJC19 protein [21], while Magmas is suspected to be involved in increased rates of anaerobic metabolism, resistance to apoptosis and altered growth-factor sensitivity, characteristic of cancer cells [22–25]. In this study, we used recombinantly purified proteins to investigate the ability of human Tim14/Pam18 and Tim16/Pam16 to form hetero-oligomers with each other and with their yeast homologues. Our results suggest that there is structural conservation between the yeast and human homologues.
2. Results and Discussion
The aim of this work was to study in vitro, for the first time, the structural properties of two human disease-associated proteins, Tim14/Pam18 and Tim16/Pam16 (also named DNAJC19 and Magmas, respectively). It is noteworthy that, while the yeast proteins have been studied extensively both in vivo and in vitro, little has been done to study the human proteins. Even complex formation between human Tim14/Pam18 and Tim16/Pam16 has never been demonstrated. In particular, we wanted to examine the following aspects. i) Do human Tim14/Pam18 and Tim16/Pam16, similar to their yeast homologues, interact to form a stable complex? ii) Which forces stabilizes the human Tim14/Tim16 complex? iii) Do the human Tim14/Pam18 and Tim16/Pam16 proteins form stable complexes with their yeast counterparts? Answers to these questions will provide important information on the evolutionary conservation of these complexes.
2.1. Purification of a Tim14/Pam18 - Tim16/Pam16 complex
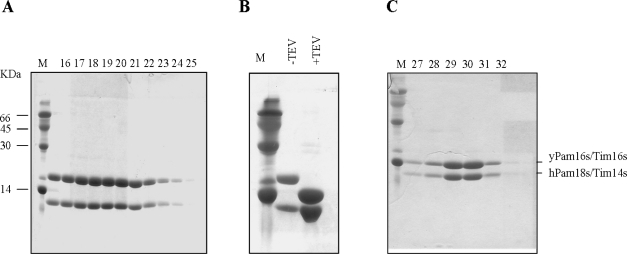
It was demonstrated that in solubilized mitochondria yeast Tim14/Pam18 and Tim16/Pam18 form a stable hetero-oligomeric complex with a reduced ability to stimulate the ATPase activity of mtHsp70, compared to Tim14/Pam18 alone. Additionally, the formation of the Tim14/Pam18-Tim16/Pam16 complex is essential for the correct function of both proteins in vivo [16]. Previous studies have also shown that the J-domains alone of Pam18/Tim14 and Pam16/Tim16 are able to form a complex [16, 20,26]. Therefore, in order to test the ability of human Tim14/Pam18 to interact with yeast Tim16/Pam16 and to form a complex, we cloned the soluble J domains of both proteins (a.a 24–116 and a.a 25–130, respectively) and co-expressed them in bacteria. Since only the yeast Tim16/Pam16s (a.a 25–130 of Tim16/Pam16) contains an octahistidine tag, the efficient purification of both proteins during all steps of isolation indicates that a complex is indeed formed between hTim14/Pam18s and yeast yTim16/Pam16s (figure 1). The proof of concept for this strategy, using the homologous yeast proteins, was published previously [26]. As shown in Figure 1, human Tim14/Pam18 was able to form a complex with yeast Tim16/Pam16. Using the same strategy, we were also able to demonstrate complex formation between yeast Tim14/Pam18 and human Tim16/Pam16 (not shown) and between human Tim14/Pam18 and human Tim16/Pam16 (not shown). We conclude that the putative human Tim14/Pam18 and Tim16/Pam16 do interact, in vitro, to form heter-oligomers and that they can also form heterologous complexes with their yeast counterparts.
Figure 1.
SDS–PAGE analysis of the purified recombinant hTim14/Pam18s-yTim16/Pam16s complex. Human Tim14/Pam18s and yeast Tim16/Pam16s were co-expressed in bacteria. Only the latter contained an octahistidine tag. Purified protein was analyzed using 16% SDS–PAGE, and stained with Coomassie blue. A) Ni-agarose column. B) Cleavage of the octahistidine tag with TEV protease. C) Gel filtration column. The fraction number is indicated on top of the gel. M: molecular weight markers (a similar approach has been used to isolate the yeast Tim14/Pam18-Tim16/Pam18 complex [26]).
2.2. The oligomeric state of recombinant Tim14/Pam18s-Tim16/Tim16s complexes
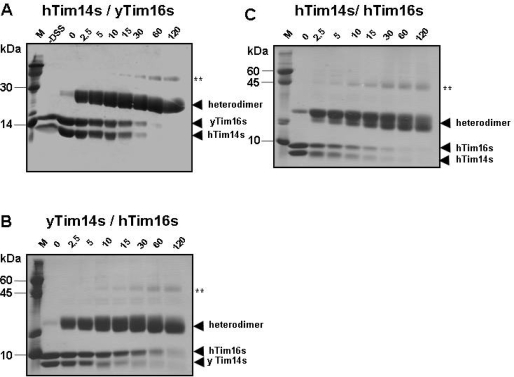
Previous studies showed that yTim14/Pam18s and yTim16/Pam16s assemble into hetero-dimers in solution, [16,19,26]. We used cross-linking to examine the oligomeric state of the three complexes purified in this study (one homologous human hetero-dimer and two heterologous human/yeast hetero-dimers). The purified complexes were cross-linked using DSS and the cross-linking products were analyzed using SDS-PAGE. The results presented in figure 2 show clearly that upon exposure to the cross-linker, bands representing the monomeric proteins weaken while at the same time hetero-dimer cross-linking products appear, in all three complexes examined. A similar pattern, indicative of dimeric molecules, was observed previously for the homologous yeast complex [26]. The similarity in the cross-linking pattern of the three complexes examined in this study, together with the previously described cross-linking pattern for the yeast Tim14/Pam18-Tim16/Pam16 complex, supports the idea that the interaction between the yeast and human Tim14/Pam18-Tim16/Pam16 complex is evolutionarily conserved. This suggests that their function is also most likely conserved.
Figure 2.
Cross-linking experiments of the different Tim14/Pam18s-Tim16/Pam16s complexes. A) Cross-linking of the hTim14/Pam18s-yTim16/Pam16 complex. B) Cross-linking of the yTim14/Pam18s-hTim16/Pam16s complex. C) Cross-linking products of the hTim14/Pam18s-hTim16/Pam16s complex. Cross-linking was carried out with 1 mM DSS at room temperature in a buffer containing 20 mM Na-Hepes pH 7.4, 200 mM NaCl, 100 mM KCl and 1 mM MgCl2, at a protein concentration of 15 μM. The cross-linking reactions were stopped at different times by addition of 10 μl SDS sample buffer and further boiling for 5 minutes. The cross-linking products (15 μl) were analyzed using 16% acrylamide gels. **minor amount of higher oligomeric cross-linked forms, presumably tetramers, of Tim14/Pam18s-Tim16/Pam16s. M: molecular weight markers. The Mw is indicated to the left of the gel.
2.3. The folding and thermal stability of the purified complexes
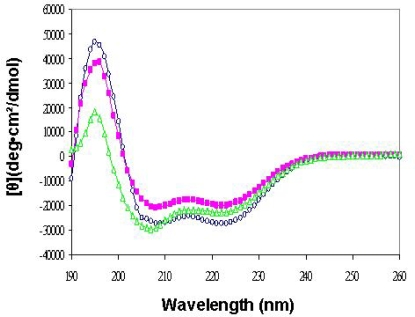
Yeast Tim14/Pam18 and Tim16/Pam16 are members of the J and J-like protein families, respectively. These proteins are characterized by a large domain composed of three α helices. As such, they exhibit distinct CD spectra [26]. Since hTim14/Pam18 and hTim16s/Pam16 are homologues of these yeast proteins, oligomers containing them should display a similar CD spectrum, assuming they fold into similar structures. To determine whether this is indeed the case, we carried out a CD analysis to determine the secondary structure of the purified constructs of hTim14/Pam18s (not shown), hTim16/Pam16s (figure 3), a complex of yTim14/Pam18s-hTim16/Pam16s (figure 3) and a complex of hTim14/Pam18s-hTim16/Pam16s (figure 3). A similar CD spectrum was obtained for all proteins tested, which was characterized by two minima at 222 nm and at 208 nm, typical of α-helical proteins (Figure 3). The results indicate that the human and yeast purified proteins are similarly folded and contain essentially α-helical structures.
Figure 3.
Represenatative Circular Dichroism (CD) analysis of some of the purified proteins. Spectra were obtained at 4° C in PBS buffer (pH=7.4). (
 ) CD spectra of hTim16/Pam16s (
) CD spectra of hTim16/Pam16s (
 ) CD spectra of yTim14/Pam18s-hTim16/Pam16s complex (
) CD spectra of yTim14/Pam18s-hTim16/Pam16s complex (
 ) CD spectra of hTim14/Pam18s/-hTim16/Pam16s complex. Similar spectra were obtained for all constructs presented in Table 1.
) CD spectra of hTim14/Pam18s/-hTim16/Pam16s complex. Similar spectra were obtained for all constructs presented in Table 1.
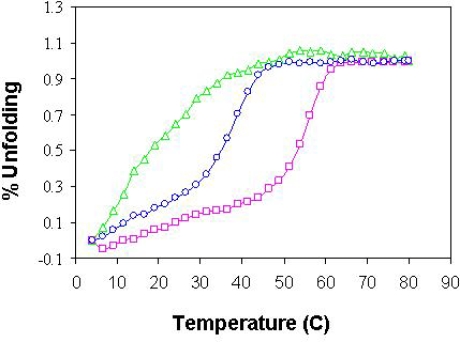
We reported previously that yTim14/Pam18s and yTim16/Pam16s are only marginally stable proteins that undergo unfolding at very low temperatures (Tm values for the individual proteins of 16.5°C and 29°C, respectively) [26]. Upon mixing the purified proteins, or when both proteins are co-expressed in bacteria, yTim14/Pam18s and yTim16/Pam16s form a hetero-dimer that is thermally more stable (Tm of ~40° C) compared to the individual proteins. Consequently, we proposed that any dissociation of the yeast Tim14/Pam18-Tim16/Pam16 hetero-dimer complex in vivo would theoretically lead to denaturation of these essential import components. Therefore, it was speculated that the formation of a stable Tim14/Pam18-Tim16/Pam16 complex is favored in vivo and the regulation of their function on the translocation motor is exerted through conformational changes [26].
We determined unfolding midpoints (Tm) by monitoring changes in the secondary structure content, detected by CD spectroscopy at 222 nm. The unfolding midpoints of individual Tim/Pam proteins and their complexes are summarized in Table 1. The results indicate that while the three proteins yTim14/Pam18, yTim16/Pam16s and hTim16/Pam16s are essentially unstable proteins (Tm of 16.5, 29, 22.5° C, respectively) the human Tim14/Pam18s is significantly much more stable (Tm of ~45). Complex formation between the yeast proteins, yTim14/Pam18 and yTim16/Pam16s, and subsequent conformational changes lead to the stabilization of their complex [26]. Interestingly, when hTim16/Pam16s interacts with hTim14/Pam18s, the stability of the complex is similar to that of the latter protein. Thus, two different factors contribute to the stability of yeast and human Tim/Pam proteins. In the case of the yeast proteins, the individual proteins are significantly less stable than their complex. Thus, complex formation between them probably increases their folding which in its turn increases complex stability (41° C compared to 16.5° C and 29° C for the individual proteins). In the case of the human proteins, the Tm of their complex is very close to that of hTim14/Pam18s. In general, when we formed a complex that contained one of the human Tim/Pam proteins, the stability of the complex was not increased further than the stability of either of the individual proteins. We conclude that the thermal stability of human Tim14/Pam18s determines the stability of the full human complex.
Table 1.
Tm values of Tim14/Pam18 and Tim16/Pam16 constructs. Tm values were extracted from experiments carried out as described in figure 4.
| Name of construct | Tm (°C) |
|---|---|
| yTim14/Pam18s | 16.5 |
| yTim16/Pam16s | 29 |
| yTim14/Pam18-yTim16/Pam16 | 41 |
| hTim14/Pam18s | 45 |
| hTim16/Pam16 | 22.5 |
| hTim14/Pam18s-yTim16/Pam16s | 52 |
| yTim14/Pam18s-hTim16/Pam16s | 35 |
| hTim14/Pam18s-hTim16/Pam18s | 49 |
2.4. The effect of the y Tim14/Pam18-hTim16/Pam16 complex on the ATPase activity of yeast mtHsp70
As mentioned above, mtHsp70 serves as the core of the mitochondrial protein import motor [9,27,28]. This chaperone drives insertion of unfolded precursors into the matrix in an ATP-dependent manner. In vivo, mtHsp70 functions with the aid of several co-chaperones that regulate its ATPase activity. The first is the nucleotide exchange factor, Mge1, which enables ADP/ATP exchange and recycling of mtHsp70 [29–31]. The second co-chaperone is the yeast Tim14/Pam18, known to have a major role in stimulating the ATPase activity of mtHsp70 [11,14,15]. The third component, yeast Tim16/Pam16, antagonizes the function of Tim14/Pam18. Tim16/Pam16 specifically inhibits the Tim14/Pam18-induced ATPase stimulation of mtHsp70 [16,19]. We wanted to examine whether the human Tim/Pam homologues can affect the ATPase activity of yeast mtHsp70. An effect on ATP hydrolysis will indicate that not only the structure of the human Tim/Pam proteins is conserved, but also their function. To this end, we studied the ATPase activity of yeast mtHsp70 in the presence of the various co-chaperones (Figure 5).
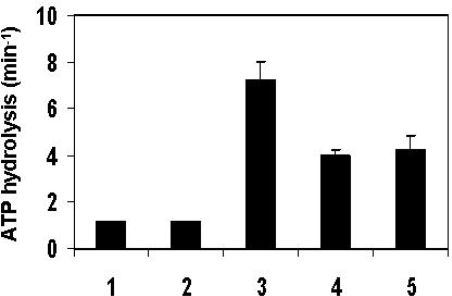
Figure 5.
The effect of human Tim/Pam proteins on the ATPase activity of yeast mtHsp70.
The ATPase assay was carried using the pyruvate kinase/lactate dehydrogenase-coupled assay as described previously [33]. The reaction mixture (total volume of 300 μl) contained the following components: 50 mM Tris-HCl pH=7.4, 50 mM K-Acetate pH=7.4, 10 mM Mg-Acetate pH=7.4, 0.3 mM NADH, 0.2 mM phosphoenolpyruvate, 20 units of pyruvate kinase, 10 units of lactate dehydrogenase and the indicated combinations of the following proteins mtHsp70 (2.5 μM), Mge1 (5 μM), full length Tim14/Pam18 and the soluble domain of Tim16/Pam16 from either Saccharomyces cerevisiae or human (2.5 μM). The reaction was initiated by the addition of 2 mM ATP. Rates were extracted from the linear phase of the reaction. Each column represents at least four independent repeats of the experiment. 1) mtHsp70. 2) mtHsp70+Mge1. 3) mtHsp70+Mge1+yTim14/Pam18 (full length). 4) mtHsp70+Mge1+yTim14/Pam18 (full length)+yTim16/Pam16s. 5) mtHsp70+Mge1+yTim14/Pam18 (full length)+hTim16/Pam16s.
In the absence of any additional components or in the presence of Mge1, mtHsp70 displays a low basal ATPase activity (1 turnover/min). Further addition of the purified yTim14/Pam18, containing a J-domain, significantly increases the rate of hydrolysis. Upon addition of the yTim16/Pam16s, there is an evident decrease in the ATPase activity [19,32], approximately 65% compared to the maximal hydrolysis activity, obtained for mtHsp70 in the presence of Mge1 and yTim14. These results demonstrate that the J domain of Tim14/Pam18 is less active in stimulating mtHsp70’s ATPase activity when in complex with the soluble domain of Tim16/Pam16. This is in agreement with previous reports [19,32]. A similar effect was observed using human Tim16/Pam16s, which inhibited the increase in the ATPase activity of mtHsp70 (obtained due to the presence of yTim14/Pam18) by almost 50%. The latter result indicates that hTim16/Pam16s can replace its yeast Tim16/Pam18s homologue, in vitro, and can act as a negative regulator of yTim14/Pam18. Based on the ATP hydrolysis experiments, we conclude that the function of Tim/Pam proteins is conserved between yeast and humans.
3. Experimental Section
3.1. Cloning and Purification of the proteins used in this study
Human Tim14/Pam18 and human Tim16/Pam16 were isolated by PCR from a human cDNA library and cloned as individual proteins or complexes into a modified version of the bacterial expression vector pET21d, in which a TEV protease site was inserted between the histidine tag and the N-terminus of the protein. The proteins used in this study are detailed in Table 2. The purification of the constructs 1–2 in Table 2 was carried out as described previously for the soluble domains (constructs 3–4) of yeast Tim14s/Pam18s and Tim16s/Pam16s [26]. Constructs 5–7 were purified as described previously for construct 8 [26]. Constructs 9–10 were purified carrying an octa-histidine tag on Ni-agarose following manufacture's protocol. In constructs 1–8, the hisitidine tag was removed from the final purified protein by proteolysis with TEV protease. In constructs 9–10, the hisitidine tag was not removed from the final purified protein.
Table 2.
Constructs used in this study and their abbreviations.
| Description | Amino acids included in construct | Abbreviated name | |
|---|---|---|---|
| 1 | soluble domain of human Tim14/Pam18 | 24–116 | hTim14s/Pam18s |
| 2 | soluble domain of human Tim16/Pam16 | 24–125 | hTim16s/Pam16s |
| 3 | soluble domain of yeast Tim14/Pam18* | 84–168 | yTim14s |
| 4 | soluble domain of yeast Tim16/Pam16* | 25–130 | yTim16s/Pam16s |
| 5 | yeast Tim14s/Pam18s in complex with human Tim16s/Pam16s | 84–168 | yTim14s/Pam18s-hTim16s/Pam16s |
| 24–125 | |||
| 6 | Human Tim14s/Pam18s in complex with yeast Tim16s/Pam16s | 24–116 | hTim14s/Pam18s-yTim16s/Pam16s |
| 25–130 | |||
| 7 | human Tim14/Pam18 in complex with human Tim16s/Pam16s* | 24–116 | hTim14s/Pam18s-hTim16s/Pam16s |
| 24–125 | |||
| 8 | yeast Tim14s/Pam18s in complex with yeast Tim16s/Pam16 | 84–168 | yTim14s/Pam18s-yTim16s/Pam16s |
| 25–130 | |||
| 9 | full length yeast Tim14 | 1–168 | yTim14/Pam18 |
| 10 | yeast Tim14/Pam18 in complex with yeast Tim16/Pam16 (both full length) | 1–168 | yTim14s/Pam18s-yTim16s/Pam16s |
| 1–149 | |||
The purification of these constructs was reported previously.
3.2. Circular dichroism (CD)
All CD measurements were performed with an Aviv CD spectrometer, as described previously [26].
3.3. Cross-linking experiments
Cross-linking of the proteins was carried out at room temperature in 20 mM of Na-HEPES (pH 7.4), containing 100 mM of KCl, with 1 mM DSS. A protein concentration of 0.5 mg/mL was used. The cross-linking reaction was stopped at different times by the addition of SDS-containing sample buffer and boiling for 5 min. The cross-linking products (20 μL) were analyzed by 16% SDS-PAGE [26].
3.4. Miscellaneous
mtHsp70 was purified as described in [34]. ATPase assays were carried out as described in [33]. The concentrations of proteins were determined with the Bicinchoninic Acid Protein Assay (Sigma; Cat. no.B9643) using BSA as a standard [33].
4. Conclusions
The major goal of this study was to characterize the structure of human Tim14/Pam18 and Tim16/Pam16 (individually and in complex) and to show that their function is evolutionarily conserved with their yeast homologues. Human Tim14/Pam18 and Tim16/Pam16 (originally known as DNAJC19 and Magmas, respectively), have recently been identified [21,22,25]. So far, very limited in vitro data has been accumulated regarding these two proteins. The results presented in this study show clearly that the human proteins associate and form a complex in vitro. The formed complex has similar properties to those of a complex formed by yeast Tim14/Pam18 and Tim16/Pam16: i). Both complexes assemble into hetero-dimers containing one copy of each subunit. ii) Both complexes exhibit CD spectra typical of proteins with α helical structures. Interestingly, we found that factors contributing to the stability of the yeast complexes against heat denaturation are distinct from those that stabilize the human proteins. In the case of the yeast complex, stabilization is achieved by simultaneous conformational changes on both proteins, Tim14/Pam18 and Tim16/Pam16. In contrast, stabilization of the folded state of the human complex is governed by the human Tim14/Pam18 protein, which is more stable to heat denaturation than the other individual proteins. The conservation of the structural properties between the yeast and human proteins (including their ability to form heterologous complexes) together with the ability of human Tim16/Pam16 to affect the ATPase activity of the yeast mitochondrial Hsp70 chaperone suggest that the function of both complexes is evolutionary conserved from yeast to humans.
Figure 4.
Thermal denaturation of purified proteins and complexes, as obtained from CD spectroscopy: Representative experiments. Similar experiments were carried out to extract the data presented in table 1. The fraction of denatured protein (% unfolding) was obtained by following changes in the ellipticity at 222 nm at various temperatures. A) (
 ) 0.1 mg/mL hTim16/Pam16s. B) (
) 0.1 mg/mL hTim16/Pam16s. B) (
 ) 0.1 mg/mL yTim14/Pam16s-hTim16/Pam16s. C) (
) 0.1 mg/mL yTim14/Pam16s-hTim16/Pam16s. C) (
 ) 0.1 mg/mL hTim14/Pam18-hTim16/Pam16s.
) 0.1 mg/mL hTim14/Pam18-hTim16/Pam16s.
Acknowledgments
This work was supported by the German-Israeli Foundation for Scientific Research and Development (GIF) (Grant no. 753/181) and German-Israeli Project Cooperation (DIP) (grant no. F5.1). We thank Dr. Celeste Weiss-Katz for critically reading this manuscript.
References and Notes
- 1.Rapaport D. How does the TOM complex mediate insertion of precursor proteins into the mitochondrial outer membrane? J. Cell Biol. 2005;171:419–423. doi: 10.1083/jcb.200507147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mokranjac D, Neupert W. Protein import into mitochondria. Biochem. Soc. Trans. 2005;33:1019–1023. doi: 10.1042/BST20051019. [DOI] [PubMed] [Google Scholar]
- 3.Pfanner N, Chacinska A. The mitochondrial import machinery: preprotein-conducting channels with binding sites for presequences. Biochim. Biophys. Acta. 2002;1592:15–24. doi: 10.1016/s0167-4889(02)00260-4. [DOI] [PubMed] [Google Scholar]
- 4.Koehler CM. New developments in mitochondrial assembly. Annu. Rev. Cell Dev. Biol. 2004;20:309–335. doi: 10.1146/annurev.cellbio.20.010403.105057. [DOI] [PubMed] [Google Scholar]
- 5.Meinecke M, Wagner R, Kovermann P, Guiard B, Mick DU, Hutu DP, Voos W, Truscott KN, Chacinska A, Pfanner N, Rehling P. Tim50 maintains the permeability barrier of the mitochondrial inner membrane. Science. 2006;312:1523–1526. doi: 10.1126/science.1127628. [DOI] [PubMed] [Google Scholar]
- 6.van der Laan M, Rissler M, Rehling P. Mitochondrial preprotein translocases as dynamic molecular machines. FEMS Yeast Res. 2006;6:849–861. doi: 10.1111/j.1567-1364.2006.00134.x. [DOI] [PubMed] [Google Scholar]
- 7.Stojanovski D, Rissler M, Pfanner N, Meisinger C. Mitochondrial morphology and protein import-A tight connection? Biochim. Biophys. Acta. 2006;1763:414–421. doi: 10.1016/j.bbamcr.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 8.Matouschek A, Pfanner N, Voos W. Protein unfolding by mitochondria. The Hsp70 import motor. EMBO Rep. 2000;1:404–410. doi: 10.1093/embo-reports/kvd093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Horst M, Azem A, Schatz G, Glick BS. What is the driving force for protein import into mitochondria? Biochim. Biophys. Acta. 1997;1318:71–78. doi: 10.1016/s0005-2728(96)00131-4. [DOI] [PubMed] [Google Scholar]
- 10.Bomer U, Meijer M, Maarse AC, Honlinger A, Dekker PJ, Pfanner N, Rassow J. Multiple interactions of components mediating preprotein translocation across the inner mitochondrial membrane. EMBO J. 1997;16:2205–2216. doi: 10.1093/emboj/16.9.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Truscott KN, Voos W, Frazier AE, Lind M, Li Y, Geissler A, Dudek J, Muller H, Sickmann A, Meyer HE, Meisinger C, Guiard B, Rehling P, Pfanner N. A J-protein is an essential subunit of the presequence translocase-associated protein import motor of mitochondria. J. Cell Biol. 2003;163:707–713. doi: 10.1083/jcb.200308004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moro F, Sirrenberg C, Schneider HC, Neupert W, Brunner M. The TIM17.23 preprotein translocase of mitochondria: composition and function in protein transport into the matrix. EMBO J. 1999;18:3667–3675. doi: 10.1093/emboj/18.13.3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Slutsky-Leiderman O, Marom M, Iosefson O, Levy R, Maoz S, Azem A. The interplay between components of the mitochondrial protein translocation motor studied using purified components. J. Biol. Chem. 2007;282:33935–33942. doi: 10.1074/jbc.M704435200. [DOI] [PubMed] [Google Scholar]
- 14.D’Silva PD, Schilke B, Walter W, Andrew A, Craig EA. J protein cochaperone of the mitochondrial inner membrane required for protein import into the mitochondrial matrix. Proc. Nat. Acad. Sci. USA. 2003;100:13839–13844. doi: 10.1073/pnas.1936150100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mokranjac D, Sichting M, Neupert W, Hell K. Tim14, a novel key component of the import motor of the TIM23 protein translocase of mitochondria. EMBO J. 2003;22:4945–4956. doi: 10.1093/emboj/cdg485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.D’Silva PR, Schilke B, Walter W, Craig EA. Role of Pam16’s degenerate J domain in protein import across the mitochondrial inner membrane. Proc. Natl. Acad. Sci. USA. 2005;102:12419–12424. doi: 10.1073/pnas.0505969102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frazier AE, Dudek J, Guiard B, Voos W, Li Y, Lind M, Meisinger C, Geissler A, Sickmann A, Meyer HE, Bilanchone V, Cumsky MG, Truscott KN, Pfanner N, Rehling P. Pam16 has an essential role in the mitochondrial protein import motor. Nat. Struct. Mol. Biol. 2004;11:226–233. doi: 10.1038/nsmb735. [DOI] [PubMed] [Google Scholar]
- 18.Kozany C, Mokranjac D, Sichting M, Neupert W, Hell K. The J domain-related cochaperone Tim16 is a constituent of the mitochondrial TIM23 preprotein translocase. Nat. Struct. Mol. Biol. 2004;11:234–241. doi: 10.1038/nsmb734. [DOI] [PubMed] [Google Scholar]
- 19.Li Y, Dudek J, Guiard B, Pfanner N, Rehling P, Voos W. The presequence translocaseassociated protein import motor of mitochondria. Pam16 functions in an antagonistic manner to Pam18. J. Biol. Chem. 2004;279:38047–38054. doi: 10.1074/jbc.M404319200. [DOI] [PubMed] [Google Scholar]
- 20.Mokranjac D, Bourenkov G, Hell K, Neupert W, Groll M. Structure and function of Tim14 and Tim16, the J and J-like components of the mitochondrial protein import motor. EMBO J. 2006;25:4675–4685. doi: 10.1038/sj.emboj.7601334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Davey KM, Parboosingh JS, McLeod DR, Chan A, Casey R, Ferreira P, Snyder FF, Bridge PJ, Bernier FP. Mutation of DNAJC19, a human homologue of yeast inner mitochondrial membrane co-chaperones, causes DCMA syndrome, a novel autosomal recessive Barth syndrome-like condition. J. Med. Genet. 2006;43:385–393. doi: 10.1136/jmg.2005.036657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jubinsky PT, Messer A, Bender J, Morris RE, Ciraolo GM, Witte DP, Hawley RG, Short MK. Identification and characterization of Magmas, a novel mitochondria-associated protein involved in granulocyte-macrophage colony-stimulating factor signal transduction. Exp. Hematol. 2001;29:1392–1402. doi: 10.1016/s0301-472x(01)00749-4. [DOI] [PubMed] [Google Scholar]
- 23.Jubinsky PT, Short MK, Mutema G, Morris RE, Ciraolo GM, Li M. Magmas expression in neoplastic human prostate. J. Mol. Histol. 2005;36:69–75. doi: 10.1007/s10735-004-3840-8. [DOI] [PubMed] [Google Scholar]
- 24.Jubinsky PT, Short MK, Mutema G, Witte DP. Developmental expression of Magmas in murine tissues and its co-expression with the GM-CSF receptor. J. Histochem. Cytochem. 2003;51:585–596. doi: 10.1177/002215540305100504. [DOI] [PubMed] [Google Scholar]
- 25.Peng J, Huang CH, Short MK, Jubinsky PT. Magmas gene structure and evolution. Silico Biol. 2005;5:251–263. [PubMed] [Google Scholar]
- 26.Iosefson O, Levy R, Marom M, Slutsky-Leiderman O, Azem A. The Pam18/Tim14-Pam16/Tim16 complex of the mitochondrial translocation motor: The formation of a stable complex from marginally stable proteins. Protein Sci. 2007;16:316–322. doi: 10.1110/ps.062459607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Neupert W, Herrmann JM. Translocation of proteins into mitochondria. Annu. Rev. Biochem. 2007;76:723–749. doi: 10.1146/annurev.biochem.76.052705.163409. [DOI] [PubMed] [Google Scholar]
- 28.Kang PJ, Ostermann J, Shilling J, Neupert W, Craig EA, Pfanner N. Requirement for hsp70 in the mitochondrial matrix for translocation and folding of precursor proteins. Nature. 1990;348:137–143. doi: 10.1038/348137a0. [DOI] [PubMed] [Google Scholar]
- 29.Westermann B, Prip-Buus C, Neupert W, Schwarz E. The role of the GrpE homologue, Mge1p, in mediating protein import and protein folding in mitochondria. EMBO J. 1995;14:3452–3460. doi: 10.1002/j.1460-2075.1995.tb07351.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Laloraya S, Gambill BD, Craig EA. A role for a eukaryotic GrpE-related protein, Mge1p, in protein translocation. Proc. Natl. Acad. Sci. USA. 1994;91:6481–6485. doi: 10.1073/pnas.91.14.6481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bolliger L, Deloche O, Glick BS, Georgopoulos C, Jeno P, Kronidou N, Horst M, Morishima N, Schatz G. A mitochondrial homolog of bacterial GrpE interacts with mitochondrial hsp70 and is essential for viability. EMBO J. 1994;13:1998–2006. doi: 10.1002/j.1460-2075.1994.tb06469.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mokranjac D, Sichting M, Popov-Celeketic D, Berg A, Hell K, Neupert W. The import motor of the yeast mitochondrial TIM23 preprotein translocase contains two different J proteins, Tim14 and Mdj2. J. Biol. Chem. 2005;280:31608–31614. doi: 10.1074/jbc.M502397200. [DOI] [PubMed] [Google Scholar]
- 33.Bonshtien AL, Weiss C, Vitlin A, Niv A, Lorimer GH, Azem A. Significance of the Nterminal domain for the function of chloroplast cpn20 chaperonin. J. Biol. Chem. 2007;282:4463–4469. doi: 10.1074/jbc.M606433200. [DOI] [PubMed] [Google Scholar]
- 34.Weiss C, Niv A, Azem A. Two-step purification of mitochondrial Hsp70, Ssc1p, using Mge1(His)(6) immobilized on Ni-agarose. Protein Expr. Purif. 2002;24:268–273. doi: 10.1006/prep.2001.1563. [DOI] [PubMed] [Google Scholar]