Abstract
Mitochondrial dysfunction is a hallmark of almost all diseases. Acquired or inherited mutations of the mitochondrial genome DNA may give rise to mitochondrial diseases. Another class of disorders, in which mitochondrial impairments are initiated by extramitochondrial factors, includes neurodegenerative diseases and syndromes resulting from typical pathological processes, such as hypoxia/ischemia, inflammation, intoxications, and carcinogenesis. Both classes of diseases lead to cellular energetic depression (CED), which is characterized by decreased cytosolic phosphorylation potential that suppresses the cell’s ability to do work and control the intracellular Ca2+ homeostasis and its redox state. If progressing, CED leads to cell death, whose type is linked to the functional status of the mitochondria. In the case of limited deterioration, when some amounts of ATP can still be generated due to oxidative phosphorylation (OXPHOS), mitochondria launch the apoptotic cell death program by release of cytochrome c. Following pronounced CED, cytoplasmic ATP levels fall below the thresholds required for processing the ATP-dependent apoptotic cascade and the cell dies from necrosis. Both types of death can be grouped together as a mitochondrial cell death (MCD). However, there exist multiple adaptive reactions aimed at protecting cells against CED. In this context, a metabolic shift characterized by suppression of OXPHOS combined with activation of aerobic glycolysis as the main pathway for ATP synthesis (Warburg effect) is of central importance. Whereas this type of adaptation is sufficiently effective to avoid CED and to control the cellular redox state, thereby ensuring the cell survival, it also favors the avoidance of apoptotic cell death. This scenario may underlie uncontrolled cellular proliferation and growth, eventually resulting in carcinogenesis.
Keywords: mitochondria, energy depression, mitochondrial cell death, neurodegenerative diseases, inflammation, hypoxia, cancer
1. Introduction
The importance of impaired mitochondrial function in cellular pathophysiology was first recognized by Otto Warburg, who proposed that development of cancer is causally related to an altered energy metabolism due to suppression of OXPHOS and activation of glycolysis [1]. Later it was shown that clinically expressed hypermetabolic state also resulted from dysfunction of mitochondria [2] and that defects in mitochondrial metabolism due to mutations in mitochondrial DNA (mtDNA) and nuclear DNA (nDNA) underlie various encephalomyopathic syndromes (Table 1) [3–6]. Based on these studies, the concept of mitochondrial diseases [7] was introduced, which considers these diseases to be manifested in many different tissues, but primarily caused by defects in OXPHOS due to mutations of mitochondrial proteins (Table 1).
Table 1.
Hereditary mitochondrial diseases
| Type of mutation of mtDNA | Large scale deletions: KSS, Pearson’s syndrom, PEO |
| Point mutations: MELAS, MERRF, NARP, LHON | |
| Mutations in nuclear genes controlling the stability of mtDNA | ANT1, Twinkle, POLGI1, TP, TH2, DGUOK, deoxynucleotide carrier |
| Mutations in nuclear genes encoding the respiratory chain proteins | Complex I, NDUFS1, NDUFS2, NDUSFS4, NDUFS8, NDUFV1 |
| Complex II, SDHA, SDHB, SDHC, SDHD | |
| Complex III UQCRB, subunit VII | |
| Mutations in nuclear genes indirectly involved in repiratory chain | FRDA1 (Friedreich’s ataxia) gene |
| Genes responsible for X-linked deafness ataxia and sideroblastic anaemia | |
| Genes for hereditary spastic paraplegia | |
| Genes for X-linked deafness-dystonia syndrome | |
| Genes for autosomal dominant optic atrophy | |
| Genes responsible for deficiency of coenzyme Q and cardiolipin |
The concept of mitochondrial medicine was successful in promoting a massive search for new DNA mutations underlying the pathogenesis of different diseases (see http://www.mitomap.org and recent reviews [8–11]). At the same time, genetic approaches have suffered from serious limitations in disclosing the underlying pathomechanisms.
Firstly, they do not provide an explanation for enormous phenotypic variety of clinical manifestations of mitochondrial dysfunction [12], partially because in most cases disorders of mitochondria either are not primarily causal for the disease, or their disease-specific role has not been revealed [13–26]. Secondly, the underlying genetic defects are still unknown for about 50% of the adult patients and for 80 – 90% of sick children [10].
Thirdly, the gene-based concepts of disease largely ignore the complex physiological properties of mitochondria (impermeable membranes and transmembrane gradients for many compounds [27], specific osmotic behavior, and the fission – fusion equilibrium [28]) that underlie a large network of relations between mitochondria and cells. Different alterations in these networks may lead to variable phenotypic presentations of the disease states.
Fourthly, gene-function relationships can not be fully assessed because the function of some 160 out of 1,200 mitochondrial proteins is still unknown [29].
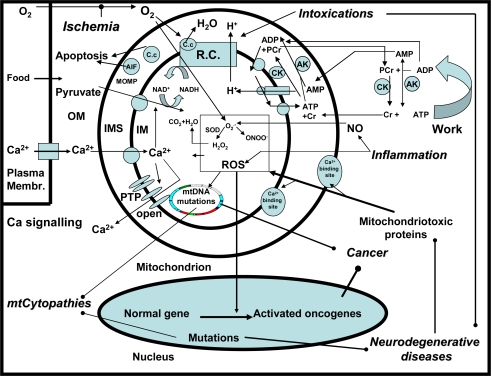
For these reasons and in the light of the recent knowledge, the subsequent discussion provides arguments in favour of idea that the earlier gene-based paradigm of mitochondrial diseases has to be changed into a more general concept which considers that mitochondrial disorders play a central role in majority of pathological processes largely due to their critical function in controlling the cellular energy status, signaling systems and the pathways of cell death. To illustrate this, the Figure 1 depicts the main functions of mitochondria and shows how different clinical entities result in disease-specific impairments of these functions. Most importantly, mitochondria produce ATP for cell work. The mechanism responsible for that process is based on coupling of oxidation of reducing equivalents (e.g. NADH) and electron transport along the respiratory chain to synthesis of ATP by F0F1ATPase, driven by the proton gradient regenerated by the action of the respiratory chain. OXPHOS is coupled to phosphocreatine (PCr) shuttle, which adjusts high phosphorylation potentials for cell work and transports PCr (the ATP equivalent) to the ATP-utilizing enzymes and Cr (the ADP equivalent) back into the intermembrane space (IMS).
Figure 1.
The central role of mitochondria in mitochondrial diseases, neurodegenerative diseases, inflammation, ischemia, intoxication and cancer.
In this system the mitochondrial and extramitochondrial creatine kinases (CK) work in opposite directions, and are functionally coupled to adenine nucleotide translocase (ANT) and ATPases, respectively. Similarly, extramitochondrial adenylate kinase (AK) converts ADP into ATP and AMP and contributes to the ATP regeneration. AMP diffuses as ADP equivalent into the IMS where mitochondrial AK converts AMP and ATP to ADP. In the case of failure or lack of CK-mediated energy transfer pathway, the cytosolic ADP may largely increase in response to increased activity of ATPases, and ADP may diffuse directly into the (IMS) to stimulate OXPHOS. In ischemia the mitochondrial inner membrane (IM) becomes permeable due to the opening of the permeability transition (PT) pore and the outer membrane (OM) becomes leaky, which results in release of the apoptosis inducing factor (AIF) and cytochrome c. These changes induce apoptosis or necrosis, depending on the cellular levels of ATP. In parallel, reactive oxygen species (ROS) are formed due to retarded flow of electrons in the respiratory chain. Normally, O2−. can be eliminated by mitochondrial Mn-dependent superoxide dismutase (MnSOD), a component of the antioxidant defence system. When the ROS formation exceeds the defence capacity, dangerous ROS attack on all biomolecules occurs (oxidative stress), acutely reducing the activity of respiratory chain enzymes, but chronically impairing the mitochondrial and nuclear DNA. In inflammation, increased NO production reinforces the oxidative stress in mitochondria via the reversible inhibition of the respiratory chain and formation of peroxynitrite (ONOO−) from O2−. and NO [30,31–39]. Chronic ROS is assumed to cause gene mutations responsible for cancerogenesis. Hereditary mutations in the mitochondrial genome cause the mitochondrial cytopathies (mt-cytopathies), e.g. due to impairment of respiratory chain complexes. In neurodegenerative diseases, hereditary mutations in non-mitochondrial genes cause the formation of cytotoxic proteins, which give rise to mitochondrial dysfunction. Mitochondriotoxic actions of these pathological proteins are realized by interactions with regulatory Ca2+- binding sites localized at the surface of mitochondria [40]. Ca2+ ions can be accumulated by mitochondria e.g. via the uniporter. Excessive Ca2+ in the matrix can induce the opening of the PT pore (PTP). In case of reversible PT the mitochondria release a fraction of Ca2+ that serves as a signaling messenger, but in conditions of irreversible PT the mitochondria deteriorate and die that leads to serious pathophysiological consequences [41,42]. Finally, the intoxication of mitochondria by medicaments or by specific toxins often impairs the respiratory chain and can cause acute impairments and in some cases symptomatic neurodegenerative diseases.
2. The Cellular Energetic Depression and Mitochondrial Cell Death as Cornerstones of the Diseases
There exist a number of different cell death programs, such as the apoptotic, autophagic, cytoplasmic, and other types [43], initiated and progressing in the course of neurodegenerative, infectious, traumatic, ischemic, and metabolic diseases. For all these diseases and death types, mitochondrial involvement is a common phenomenon, because the pathological processes such as inflammation, hypoxia/ischemia, intoxications, metabolic blockade, and oxidative stress exert deleterious effects on structure and function of mitochondria (Figure 1, Table 2) [43–45]. As an outcome, a decline in cytosolic phosphorylation potential ensues that inhibits the function of Ca2+-ATPases, thereby causing cytosolic Ca2+ overload and suppression of the cell’s ability to work (Figure 2).
Table 2.
Mechanisms leading to mitochondrial cell death.
| Suppression of ATP synthesis | Oxidative stress |
| Ca2+ overload | |
| Decreased substrate supply (O2, fatty acids, glutamate, pyruvate, etc) | |
| Decreased cytosolic adenine nucleotide concentrations | |
| Impaired ADP/ATP transport | |
| Decreased capacity of OXPHOS due to diminished activities of respiratory chain complexes and matrix enzymes resulting from mutations and inhibitions | |
| Impaired biogenesis of mitochondria | |
| Changes leading to or associated with apoptosis | Leaks in mitochondrial outer membrane, loss of cytochrome c |
| Opening of the PT pore | |
| Decreased resistance against Ca2+- and ROS-induced stress |
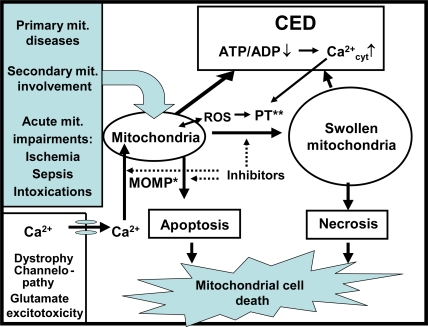
Figure 2.
Mitochondrial cell death. Loss of mitochondrial capacity to synthesize ATP in the processes of OXPHOS leads to cellular energetic depression (CED) characterized by decreased cytosolic phosphorylation potential and increased cytosolic Ca2+ concentration (Ca2+cyt) that leads to reduced ability of cell to do work. The resulting ROS formation and Ca2+ overload further impair the structure and function of mitochondria. In mild stage of CED, when mitochondria can generate some amounts of ATP, mitochondria launch a program of apoptotic cell death by release of cytochrome c. At pronounced CED, when the cytoplasmic ATP levels fall below the levels required for processing the ATP-dependent apoptotic reactions, the cell dies from necrosis. Both, the apoptotic and necrotic death pathways that are mediated by mitochondrial impairments can be classified as of mitochondrial cell death (MCD). The molecular mechanism of mitochondrial outer membrane permeabilization (MOMP) and PT are potential targets for therapeutic interventions preventing mitochondrial cell death.
This condition, for which we propose the term cellular energetic depression (CED), is characterized by a mismatch between ATP production and utilization and enforces mitochondria to accumulate large amounts of Ca2+ that induces the permeability transition (PT) of the mitochondrial inner membrane [46]. This process includes mitochondrial swelling, collapse of the membrane potential (ΔΨ), and splitting of ATP due to reversal of mitochondrial F0F1ATPase. If mitochondria shift into irreversible PT, the cell must die by necrosis. The CED pathway can also start due to intracellular Ca2+ overload caused by degenerative changes of the cell membrane, or channelopathies that lead to increased Ca2+ flux into the cell [47]. Whereas all of these disturbances result in cell’s death, the functional status of mitochondria plays a key role in determining which type of MCD will prevail. If the pathogenic signals induce mitochondrial outer membrane permeabilization (MOMP) [43,44] and CED is relatively mild (i.e. OXPHOS is not yet markedly compromised), cytochrome c and the AIF are released to initiate and execute the apoptotic machinery [43]) (Figure 2). However, along with progression of CED, cellular ATP level may become lower than required for supporting apoptosis with the consequence that cells will enter the necrotic pathway of death. In this review, we provide ample evidence for the involvement of mitochondrial disturbances not only in the regulation of cell death, but also in the progression of major pathological processes, such as inflammation, hypoxia, and carcinogenesis (see Sections 3.2. – 3.4) that all are causal for cell death. Considering the central role of mitochondria in pathogenetic processes, we propose that all forms of cell death, including apoptotic and necrotic cell death, as well as all other death pathways that are linked to mitochondrial impairments, should be envisaged as MCD. MCD contributes to degeneration or atrophy of the affected tissue, and can occur slowly, as in the case of neurodegenerative diseases, or rapidly, as in rhabdomyolysis. Correspondingly, the mitochondria should be regarded as key targets for pharmacological treatment, in order to protect cells from MCD. Indeed, there exist several potent inhibitors of PT, such as cyclosporin A (CsA) [43] and sanglifehrin A [48], that make it possible to attenuate or cure the atrophic processes. It has been shown that CsA can increase the lifespan and decrease the symptoms of degeneration in collagen VI – deficient mice [49]. Also, MOMP, realized via a cascade of molecular reactions should be inhibitable [43,44]. To provide more effective approaches in pharmacological interventions, it may be necessary to discriminate between cell death pathways where mitochondria participate and those that occur without mitochondrial involvement [43]. To our knowledge, the term MCD was first proposed by Kroemer et al., but was only used for the mitochondrial apoptotic pathway including the MOMP [50], a newly discovered phenomenon at that time. We propose to use the term MCD for all modes of death where irreversible mitochondrial impairments, irrespectively of the primary reason and underlying mechanism, are involved.
Studies in the last decades have resulted in understanding that in many types of cells, especially those characterized with high energy turnover, the cellular energy metabolism represents a strictly organized system in which mitochondria and ATPases are linked to each other by specialized energy transfer pathways formed by isoenzymes of creatine kinase (CK) and adenylate kinase (AK) (Figure 1) [51–55]. Therefore, CED (Figure 2) is related not only to injuries of mitochondria, but also to alterations of the energy transfer system. For example, the CK-phosphotransfer network is known to be compromised in conditions of cardiac diseases, e.g. in heart failure [56–58]. The underlying mechanisms include reduced myocardial creatine levels [57], downregulated expression of mitochondrial CK (mi-CK) [57,58], and decoupling between the mi-CK and adenine nucleotide translocase (ANT) [59], these changes suppressing mitochondrial synthesis of phosphocreatine (PCr). On the other hand, reduced expression of extramitochondrial CK isoforms and their inhibition by AMP kinase [58,60,61] decrease the effectiveness of ADP rephosphorylation near ATPases at the expense of PCr [57]. Impaired interaction between mi-CK and ANT might favor opening of the mitochondrial PT pore, thus leading to CED and MCD [62–64]. Fortunately, dysfunction of the CK-phosphotransfer system can be more or less compensated by activation of the AK-mediated system of energy transfer [65,66], because the mitochondrial AK isoform is functionally coupled to ANT and accumulation of ADP near ATP-utilizing enzymes favors using its β-phosphoryls by AK for ATP formation [65]. Also, an inefficient CK system can to some extent overcome by direct ATP/ADP diffusion in order to ensure energy transfer and feedback [67,68].
In the case of neurodegenerative diseases [e.g. Parkinson’s disease (PD), Alzheimer’s disease (AD), Huntington’s disease (HD), and amyotrophic lateral sclerosis (ALS)] mitochondria and the energy transfer systems may be impaired by toxic and disease-specific proteins. Mutated huntingtin binds to the MOM, suppresses the mitochondrial capacity of Ca2+ accumulation [69], and hinders the axonal mobility of mitochondria [70]. In normal brain cells, the function of HK II depends on its interaction with the MOM and the cytoskeletal network [71,72]. On the other hand, mi-CK, localizing in the intermembrane space, is functionally coupled to ANT [73]. Both of these mitochondrial kinases participate in the formation of the PT pore [74,75] and both of them suppress mitochondrial ROS production, due to their stimulative effect on OXPHOS [76,77]. In the case of AD, the CK activity is suppressed, owing to the structural alterations in the brain cell’s cytoskeleton [78]. Decreased CK activity in the spinal cord was also observed in G93A transgenic mice model of familiar ALS [79]. Thus, the neurodegenerative diseases may be associated with impaired functional coupling of mitochondrial kinases to OXPHOS, which could contribute to CED and ROS [80,81].
Recent data demonstrate impairment of CK-mediated energy transfer pathway in sepsis, as the mi-CK activity was found to be reduced and its coupling to OXPHOS impaired in the diaphragm and heart of endotoxin-treated dogs [82].
2.1. Role of cytosolic Ca2+ in regulation of mitochondrial life and death
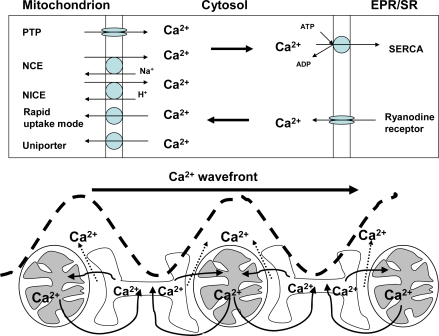
It has been known for a long time that [Ca2+]cyt exerts a regulatory impact on OXPHOS [83,84] and that Ca2+ overload impairs the mitochondrial function [85]. As shown in Figure 3, Ca2+ enters the matrix through the uniporter located in the inner membrane. This process is driven by ΔΨ and can be inhibited by ruthenium red. The KM of the uniporter in brain and muscle mitochondria is about 3 μM Ca2+ [40], which is higher than the cytosolic Ca2+ concentration ([Ca2+]cyt) that usually fluctuates between 50 nM and 1 μM [87]. In the light of these data, substantial Ca2+ uptake into mitochondria can not be expected. However, [Ca2+]cyt is dynamically compartmentalized and spatio-temporally changing, so that its local concentration can reach the values over 40 μM even at normal cellular circumstances [88]. Moreover, polyamines such as spermine, which exist at millimolar concentrations in the cytosol, markedly decrease the KM for Ca2+ uptake by isolated mitochondria [89,90]. Under these conditions, mitochondria can take up large amounts of Ca2+ via uniporter, supported by formation of appatite [91]. On the other hand, Ca2+ can be released from the mitochondrion via the Na+ independent (NICE) and/or the Na+ dependent (NCE) exchange pathways, and via the fast Ca2+ efflux aided by the PT pore. The capacity of the latter exceeds that of NICE and NCE [92] (Figure 3).
Figure 3.
Involvement of mitochondria and endoplasmic/sarcoplasmic reticulum (EPR/SR) in Ca2+ signaling. Upper panel: Mitochondria accumulate Ca2+ via the uniporter and by the rapid uptake mode. Accumulated Ca2+ can be released from mitochondria through reversible Na+ independent (NICE) or Na+ dependent pathways (NCE), but the rates of Ca2+ efflux via these pathways are low in comparison to the fast Ca2+ efflux via the PT pore that can be opened reversibly or irreversibly [46]. Ca2+ accumulation by EPR/SR is realized by SERCA which requires ATP at sufficiently high phosphorylation potentials. To avoid inhibition of SERCA by increasing ADP, it is rephosphorylated by the PCr shuttle (Figure 1). Lower panel: Mitochondria and EPR/SR interact in order to control cytosolic Ca2+ waves and their directed propagation, as modeled in the reconstituted gel system (Table 3 [86]).
The phenomenon of [Ca2+]cyt-induced stimulation of mitochondrial respiration [83,84] has been attributed to accumulation of Ca2+ in the matrix and activation of mitochondrial enzymes, such as pyruvate dehydrogenase (PDH), isocitrate dehydrogenase, and 2-oxoglutarate dehydrogenase [83]. However, due to low sensitivity of dehydrogenases to Ca2+ (S0.5 0.8 – 5 μM [83]), these enzymes likely can not contribute to activation of OXPHOS at lower (nanomolar) ranges of [Ca2+]cyt. Furthermore, increases in Vmax of state 3 respiration due to activation of dehydrogenases are small [83] and computer models assuming the intramitochondrial Ca2+ regulation of OXPHOS can not simulate the measured data [93]. These arguments question the predominant role of intramitochondrial dehydrogenase-mediated mechanisms in regulation of OXPHOS by Ca2+.
Recent data show that aralar 1 [94,95] and citrin, which are the brain and liver isoforms of the Ca2+-dependent aspartate/glutamate carrier, respectively, contain regulatory Ca2+ binding sites localized in the intermembrane space that enable the regulation of both carriers by [Ca2+]cyt (Figure 4). Remarkably, aralar 1 is activated at much lower Ca2+ concentrations (S0.5 = 0.3 μM, [94]) than required for activation of the Ca2+ uniporter. It is a component of the malate/aspartate shuttle that transports the reducing hydrogen into mitochondria [94]. Alternatively, glutamate can enter the mitochondrial matrix by the glutamate/H+ symporter, but the activity of that carrier is low in most organs, except liver and kidney [96–97]. Pardo et al. have demonstrated that elevation of [Ca2+] increases hydrogen transport into brain mitochondria [94], and Palmieri et al. have detected a Ca2+- activation of the glutamate/aspartate carrier, by registrating increased rates of mitochondrial glutamate decarboxylation in human cell line HET-293T in response to enhancement in [Ca2+] [95]. Moreover, other mitochondrial substrate carriers possess the regulatory Ca2+ binding sites to sense [Ca2+]cyt as well. Among them, the Ca2+-regulated ATP-Mg/Pi carrier [102–105] belongs to a subfamily of human Ca2+ binding mitochondrial carriers, named as short Ca2+ binding mitochondrial carriers [105]. Three of them are isoenzymes of the ATP-Mg/Pi carrier, responsible for the net flux of adenine nucleotides into or out of mitochondria. Ca2+ binding motives in the N-terminus of these carriers may serve as sensors of [Ca2+]cyt [103]. Notably, because the mitochondrial Ca2+-uniporter exposes a regulatory Ca2+ binding site into the intermembrane space, it can be activated by extramitochondrial Ca2+ [106,107]. It has been also shown that the PT pore has external binding site for divalent cations, and occupation of that site by Ca2+ and Mg2+ is expected to decrease the PT pore open probability [99]. Finally, the porin pore of the mitochondrial outer membrane, termed as voltage-dependent anion channel (VDAC), is regulated by [Ca2+]cyt [98,108]. VDAC is responsible for the passage of mitochondrial metabolites with a molecular weight < 1,000 Da, but it seems also to participate in formation of the PT pore. Increases in extramitochondrial Ca2+ markedly enhance the permeability of VDAC, probably, through the effect on glutamine residue in position 72 of VDAC, a regulatory Ca2+ binding site of that protein [108].
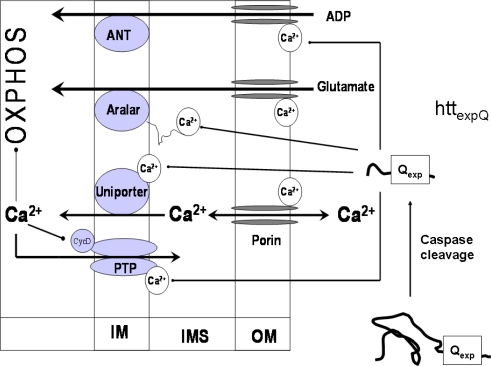
Figure 4.
Mechanisms of regulation of OXPHOS by [Ca2+]cyt that stimulates mitochondrial respiration and ATP synthesis by binding to regulatory sites of several proteins in the mitochondrial outer compartment [40], such as the porin pore [96,98], the PT pore [99], the Ca2+ uniporter, and aralar [94,95]. The Ca2+ binding sites of transporters, PT pore and VDAC may also represent the targets for various pathogenic proteins. As discussed in chapter 3.1.1, huntingtin with an expanded poly Q tract (httexpQ) cleaved by caspases [100,101] can interact with the regulatory Ca2+ binding sites of PT pore and transporters, thereby disturbing the regulation of OXPHOS by [Ca2+]cyt that causes energetic depression, mitochondrial cell death, and tissue atrophy [40].
Our recent data show that the complex I dependent state 3 respiration with glutamate/malate is much lower than complex II dependent respiration with succinate in brain mitochondria if the incubation medium contains very low amounts of Ca2+ [40]. However, the respiration of mitochondria strongly increases in response to elevated [Ca2+] (S0.5 = 0.35 μM). This effect is also observed in the presence of ruthenium red, a blocker of Ca2+ uniporter, which means that activation is exclusively mediated by extramitochondrial Ca2+ [40]. Considering these novel data, the role of interaction of Ca2+ with mitochondrial functions needs to be re-estimated. Firstly, we propose that [Ca2+]cyt exerts significant control over the OXPHOS, independently of entering the mitochondrial matrix (Figure 4). Secondly, it seems reasonable to assume that reversible mitochondrial Ca2+ accumulation, well characterized in many experiments (Figure 3), is not as much required for stimulation of OXPHOS than for fulfilling other tasks. For example, mitochondrial Ca2+ retention may be crucially involved within the redistribution of [Ca2+]cyt, to avoid harmful effects while accumulating in very high concentrations in the close proximity of mitochondria and Ca2+ channels of the cell membrane or EPR/SR [109–111]. In addition, mitochondrial Ca2+ cycling may be vital for governing the cytosolic Ca2+-waves [86,112,113]. To address the latter function of mitochondria within the the intracellular Ca2+ homeostasis, we assessed the spontaneous Ca2+ waves by confocal scanning fluorescence microscopy, using fluo-3 as a Ca2+ sensor in a cell free system (an agarose gel supplemented with isolated SR vesicles, cardiac mitochondria, CK-based ATP regenerating system, and 5 mM ATP) [86]. We found that in such system lacking mitochondria, Ca2+ waves propagated along the gel (Figure 3) with a wavefront velocity of 40 μm/s (Table 3). When the Ca2+-ATPase (SERCA) was inhibited with 10 nM thapsigargin, the velocity of Ca2+ wave propagation decreased by 50% compared to the controls (Table 3).
Table 3.
Influence of mitochondria on the velocity of Ca2+ waves in a SR vesicle agarose gel.
| Excitable medium | Velocity (μm/s) |
|---|---|
| SR vesicles (Control) | 39.2 ± 16.2a (n = 22) |
| SR vesicles + thapsigargin | 19.6 ± 4.4a (n = 8) |
| SR vesicles + RHM | 57.9 ± 12.9b (n = 20) |
| SR vesicles + RHM + antimycin A | 40.9 ± 10.1b (n = 20) |
Spontaneous Ca2+ waves in a SR vesicle agarose gel were assessed by confocal scanning fluorescence microscopy with Fluo-3. SR vesicles were isolated from the m. longissimi dorsi of German landrace pigs. Rat heart mitochondria (RHM) were isolated with standard procedures. SR vesicles were incubated in a solution with the following composition (mM): KCl 100, MgCl2 5, Na2-ATP 4, Phosphocreatine 10, EGTA 0.04, PIPES 20; Fluo-3 0.01; pH = 7.2. The concentration of agarose gel was 0.66% (86). For Ca2+ stimulation we either used a glass tip or a small stripe of paper soaked with Ca2+ solution (200 μM). Under the influence of 10 nM thapsigargin, an inhibitor of the SR Ca2+-ATPase (SERCA), the velocity of Ca2+ waves decreased signifcantly compared to the controls. If mitochondria were added to SR vesicles (together with 10 mM pyruvate plus 2 mM malate as mitochondrial substrates) significantly faster propagating waves were observed. After inhibition of mitochondrial function with 1 μM antimycin A, an inhibitor of complex III of the respiratory chain, this effect was completely abolished. Further details see [86]. Data as mean ± S.D. a, b indicate significant differences between the marked groups (p < 0.01).
In the presence of mitochondria, the velocity of the wavefront spreading strongly exceeded the velocity measured in the absence of mitochondria, and after addition of antimycin A, an inhibitor of complex III of respiratory chain, a significant retardation of the wave spreading down to the values measured in the absence of mitochondria, was determined (Table 3). These experiments led to the conclusion that mitochondria exert their control of intracellular Ca2+ signaling via two modes of regulation, (i) through providing sufficient ATP for Ca2+-ATPases in the plasmamembrane and EPR/SR and (ii) due to their ability to accumulate and release Ca2+ [86,112,113] (Figure 3). However, in the case of CED, reduced availability of mitochondrial ATP would suppress the activity of Ca2+ ATPases, which in turn would induce a rise in [Ca2+]cyt and affect Ca2+-mediated signaling. This assumption was confirmed by studies on fibroblasts isolated from patients with Leigh disease, which revealed that the Ca2+ uptake by the EPR was significantly reduced due to energy deficiency [114].
3. Involvement of Mitochondria in Pathological Processes and Diseases
3.1. Mitochondria in neurodegenerative diseases
Abnormal protein aggregation and/or inclusion body formation are common factors underlying cellular and molecular mechanisms for neurodegenerative diseases such as AD, PD, HD, ALS, and prion diseases [115–118]. Despite a large clinical and pathophysiological heterogeneity the neurodegenerative diseases have a common ground – the pathophysiologal involvement of mitochondrial impairments [118]. In this review, the energetic aspects of HD and PD are addressed.
3.1.1. Huntington’s disease
HD is a progressive neurodegenerative disorder caused by a CAG repeat expansion in the coding region of the huntingtin (htt) gene resulting in an expanded polyglutamine stretch in the huntingtin (htt) protein (httexpQ) [119,120]. The CAG repeat length of httexpQ correlates inversely with the time point of disease onset [121]. Unmodified htt itself and httexpQ are abundantly expressed in most tissues [120]. To date, numerous proteins have been detected that interact under in vitro conditions with htt [117], but neither the biological function of htt nor the mechanism of cytotoxic action of httexpQ is understood [117].
The symptoms of HD are motor abnormalities including chorea and psychiatric disturbances with gradual dementia, and autopsies have revealed atrophic changes in the striatum [122]. The HD patients also lose body weight despite normal or above-average food intake [123–125], which suggests impairment of energy metabolism. Indeed, alterations in energy metabolism, such as elevated lactate and malonate, decreased activities of the respiratory chain complexes, and abnormal mitochondrial morphology has been found in different brain structures of HD patients [126–132].
Accumulating evidence shows important role of altered mitochondrial Ca2+ signaling in the pathophysiology of HD. Indeed, decreased Ca2+ accumulation capacities of mitochondria isolated from brain of YAC72Q mice [133], liver of htt111Q mice [134], HD patient’s lymphocytes [133], and htt111Q striatal progenitor cells [135] have been reported. Impaired mitochondrial function and Ca2+ dyshomeostasis were also detected in PC12 cells after transfection with httexpQ plasmids [136]. In contrast, increased Ca2+-loading capacities were observed in HD brain mitochondria from several HD mice lines [137,138].
Recently, we presented the first detection of impaired OXPHOS phosphorylation in HD mitochondria from skeletal muscle of R6/2 mice (139). Isolated mitochondria were investigated respirometrically as described previously (140). As shown in Figure 5, exposure of htt150Q mitochondria to elevated Ca2+ concentrations caused a pronounced inhibition of complex I dependent respiration (Figure 5D,F) compared to wild-type (Figure 5C,E).
Figure 5.
Ca2+-induced inhibition of pyruvate-dependent respiration in isolated muscle mitochondria of transgenic R6/2 HD mice. Multi-substrate inhibitor titration of respiration of isolated mitochondria from skeletal muscle of wild-type (WT) (A,C,E) and transgenic mice (htt150Q) (B,D,F) at the age of 14 to 16 weeks. Isolated muscle mitochondria (0.5 mg/mL) were incubated with 10 mM pyruvate and 2 mM malate. Additions: 10 or 20 μM Ca2+ as indicated; ADP, 2 mM ADP; R, 20 μM rotenone; S, 10 mM succinate; CAT, 10 μM CAT. Thin lines indicate the oxygen concentration in the oxygraph (left ordinate) whereas thick lines represent the rate of respiration in nmol O2/min/mg mitochondrial protein (right ordinate). The height of peaks correlates with the rate of respiration. State 3pyr respiration was adjusted by addition of ADP. Rotenone, an inhibitor of complex I, completely inhibited this respiration. Subsequently, succinate addition allowed the measurement of state 3suc respiration. Due to addition of carboxyatractyloside (CAT), the adenine nucleotide translocator (ANT) was inhibited and the state 4 could be measured. Note, that the pyruvate peak (state 3pyr) is absent in the presence of 20 μM Ca2+ in HD mitochondria. Further details see [139].
In general, the succinate, complex II dependent respiration was much less affected, regardless whether htt150Q or WT mitochondria were used. Furthemore, we identified a compromised mitochondrial function in fibroblasts from a HD patient with htt43Q [141]. In situ measurements of mitochondrial respiration revealed a specific decline of OXPHOS in HD150Q striatal cells following NMDA receptor-induced Ca2+ stress [138]. A Ca2+-induced decrease of respiration was also observed in mitochondria isolated from htt111Q striatal cells [135]. Obviously, dysfunction of HD mitochondria and increased extramitochondrial Ca2+ concentration are linked to each other. Therefore, some authors assumed that HD mitochondria are secondarily impaired by elevated Ca2+ [142]. In contrast, we hypothesized that httexpQ directly elicits the mitochondrioxic properties. To prove this hypothesis, we developed an experimental protocol allowing the investigation of OXPHOS under conditions of increasing extramitochondrial Ca2+ concentration in EGTA medium [40]. Experiments were performed using transgenic 21- to 27-months-old HD rat strain with 51 glutamate repeats (htt51Q). In contrast to the htt150Q R6/2 mice, a model of juvenile form of HD [143], this htt51Q rat strain exhibits specifically an adult-related onset of the neurological HD phenotype [144]. We found that the mitochondria from brain of HD rats with htt51Q showed a deficient state 3 respiration, a lower sensitivity to Ca2+ activation and a higher susceptibility to Ca2+-dependent inhibition. Furthermore, htt51Q mitochondria exhibited a diminished membrane potential stability in response to increased Ca2+, and lower capacities and rates of Ca2+ accumulation [40].
Since a mitochondrial localization of htt and httexpQ has been detected [133,136,145], direct interaction of httexpQ with proteins in the mitochondrial outer compartment can be envisaged (Figure 4). The glutamate aspartate carrier aralar [94,95] may be a candidate for one of such httexpQ interacting proteins. This carrier provides a regulatory Ca2+ binding site, which is exposed into the mitochondrial inner membrane space where it is activated by extramitochondrial Ca2+ in the nanomolar range [94]. Therefore, we consider aralar as important target of HD-related effects of httexpQ [40]. Conceivably, extended polyglutamine stretches of httexpQ interact with the regulatory Ca2+ binding site localized in the N-terminal sequence of aralar. As a result, Ca2+-dependent activation of aralar could be affected, leading to insufficient substrate supply for the mitochondrial respiratory chain. We assume that httexpQ could also interact with other Ca2+ binding sites localized at the mitochondrial surface as mentioned above (Figure 4). Besides aralar, other potential target proteins of httexpQ, such as the external Me2+ binding site of the PT pore, deserve attention [40,99]. Indeed, we have provided first evidence for interaction of htt51Q with a regulatory Ca2+ binding site of the PT pore, by revealing that htt51Q effects are opposite on aralar and PT [40]. Due to such interaction, aralar becomes deactivated, followed by a limited mitochondrial substrate supply and thus, a decreased activation of respiration by extramitochondrial Ca2+ concentrations ≤ 2 μM. In parallel, htt51Q sensitizes the PT pore to extramitochondrial Ca2+ levels > 1 μM, leading to its opening and suppression of respiration even in the presence of RR. It is therefore conceivable that htt51Q interacts with the Ca2+ binding site of the PT pore, thereby blocking the protective effect of extramitochondrial Ca2+ against PT (Figure 4). This effect could explain the increased susceptibility of htt51Q mitochondria to PT and their compromised Ca2+ retention capacity [40]. Other potential targets of httexpQ are the isoenzymes of the ATP-Mg/Pi transporters [102,104,105], the assumed Ca2+ binding site of the porin pore [108], and the regulatory Ca2+ binding site of the Ca2+-uniporter [70] that, after interacting with httexp, could cause a decrease in mitochondrial Ca2+ accumulation [40].
While considering the impact of httexpQ protein on mitochondria, it remains to be clarified as to whether this protein can indeed penetrate through the outer membrane of mitochondria in order to reach and interact with the regulatory Ca2+ binding sites of aralar. It is known that proteolytic degradation products of httexpQ protein are more toxic than the intact protein [100]. Furthermore, truncated httexpQ with a size of 3 and 16 kD causes htt-specific protein aggregates along with their cytotoxicity [101]. On the other hand, the dynamic rearrangements of mitochondrial structure can facilitate trapping of cytosolic proteins, including mutated htt. This can occur during fusion/fission-dependent changes, or in a course of formation of contact sites between the mitochondrial outer and inner membranes [146,147]. As these contact sites represent large protein aggregates [146] characterized by rapid redistribution of lipid components [146], they may be able to bind and translocate toxic proteins into the intermembrane space. Based on these arguments, we propose that mutated htt and its truncated polypeptides interact with Ca2+ binding sites in the outer compartment of mitochondria, thereby being responsible for dysregulation of mitochondrial function, CED, MCD, and tissue atrophy in HD [40].
3.1.2. Parkinson’s disease
The involvement of mitochondrial dysfunction in pathogenesis of PD was suggested by the discovery that 1-methyl-4 phenyl-1,2,3,6 tetrahydropyridine (MPTP) causes parkinsonian syndromes by acting through inhibition of complex I of the respiratory chain [148] observed in the substatia nigra [149,150] and platelets of PD patients [151]. In skeletal muscle biopsies of PD patients, the activities of different complexes of the respiratory chain were found to be reduced, in association with increased flux control coefficients of complex I and IV and increased number of point mutations in mtDNA [152].
The etiology of the mitochondrial dysfunction in PD is still unclear. However, this dysfunction can be elicited by MPTP [153], rotenone [154], paraquat [155], endogenous ROS [156], and isoquinolines [157]. So far, mutations or polymorphisms in mtDNA [158,159] and at least in nine nuclear genes were identified as cause or risk factors for PD. The mutated proteins are α-synuclein, the ubiquitin E3 ligase parkin, the antioxidant protein DJ-1, the tensin homologue (PTEN)-induced kinase 1 (PINK1), the leucine-rich-repeat kinase (LRRK2) and the serine protease HTRA2, which are directly or indirectly connected to mitochondrial function [160–170].
α-Synuclein is a major component of the Lewy bodies and its mutations are associated with increased formation of oligomeric and fibrillar aggregates which promote abnormal protein accumulation or degradation with oxidative stress and mitochondrial dysfunction. Overexpression of α-synuclein in transgenic mice impairs mitochondrial function, increases oxidative stress and enhances the MPTP-induced pathology of the substantia nigra [160]. Moreover, overexpression of the A53T mutant α-synuclein gene causes a direct damage of mitochondria [161]. In contrast, an α-synuclein knock-out mice were resistant against MPTP and mitochondrial toxins, e.g., malonate and 3-nitropropionic acid [162].
Mutations in parkin and DJ-1 are associated with autosomal recessive juvenile PD. Parkin-knockout Drosophila [163] and mice [164] strains exhibit impaired mitochondrial function and increased oxidative stress. Leucocytes from patients with parkin mutations showed decreased complex I activities [165]. It is known that parkin can associate with the MOM and thereby prevent mitochondria against swelling and cytochrome c release, but these protective effects are abolished after mutations in parkin protein [166]. The function of DJ-1 protein seems to be the protection of cells against oxidative stress, as it can act as a redox sensor of oxidative stress that causes its translocation into mitochondria. The C106 mutation of DJ-1 prevents this translocation and induces mitochondrial dysfunction [167]. DJ-1 knock-out results in a normal mice phenotype, but sensitizes the animals to toxicity of MPTP, as seen from loss of dopaminergic neurons in response to MPTP [168].
PINK1 is a kinase localized in mitochondria, and it is also considered to be involved in neuroprotection. Overexpression of wild-type PINK1 prevents apoptosis under basal and stauroporine-induced conditions by hindering cytochrome c release, whereas mutated PINK1 antagonizes this effect [169]. PINK1 deficient Drosophila exhibits increased sensitivity to the complex I inhibitor rotenone [170].
It is largely accepted that degeneration of dopaminergic neurons in PD is associated with microglial-mediated inflammation and neurotoxicity (reviewed by Hald and Lotharius [171] and also below). Activation of inflammation is suggested by the finding that PD patients and animal models of PD that were treated with lipopolysaccharide (LPS), MPTP, rotenone or 6-hydroxydopamine exhibited elevated antibody levels against proteins modified by dopamine oxidation products, increased concentrations of cytokines (IL-1, IL-6, IL-10 and TNF-α), and augmented ROS production (171). All these changes were associated with impaired function of complex I of the respiratory chain in dopaminergic neurons. It is likely that modifications of biomolecules by ROS and dopamine-quinones trigger microglia activation that in turn will further promote neurotoxicity [171].
3.2. Mitochondria as mediators and targets of inflammation
Inflammation associates with and complicates many pathological conditions, e.g. cardiac ischemia and reperfusion, cardiac failure, neurodegenerative diseases, diabetes mellitus, and cell necrosis. Increased production of ROS is a hallmark of inflammation [172,173] and recent evidence suggests the mitochondria to be a primary source of ROS generation. This was demonstrated in experiments using mice with targeted disruption of the UCP-2 gene, which exhibited the activation of macrophage phagocytosis and ROS production, in association with increased expression of inducible NO synthase (iNOS), augmented NO production, increased resistance to NO-induced apoptosis, a greater expression of inflammatory cytokines (interferon-γ (IFN-γ) and tumor necrosis factor-α (TNF-α), faster nuclear translocation of nuclear factor-κB (NF-κB), and elevated migration ability compared to wild-type mice in response to bacterial LPS challenge [174,175]. Because TNF-α inhibits mitochondrial oxidation of NADH and FADH2-linked substrates, in association with inhibition of the respiratory chain complexes, it also increases ROS [176–178]. ROS in turn stimulates the expression of proinflammatory cytokines, such as interleukin – 2 (IL-2), TNF-α, and IL-10 [179,180], and activates NF-κB, a common target for TNF-α and IL-1 [181]. While seeking for the mechanisms linking the mitochondrial effects of TNF-α to activation of NF-κB, Itoh et al. [178] found that Dok-4, one of the downstream of tyrosine kinase (Dok) proteins, recruits the cytosolic c-Src protein kinase to be translocated into mitochondria and causes its activation, these changes leading to suppression of complex I and increased mitochondrial ROS production. Mitochondrial ROS and mobilization of Ca2+ trigger the following signalling system comprising of a cascade of kinases (TAK1, MEKK1, NIK, and IκB kinase (IKK)) that eventually activate the NF-κB [182]. Notably, the mitochondria are also the source and target of reactive nitrogen species (RNS), since IL-1, TNF-α and ROS stimulate expression of the iNOS and mitochondrial NOS-l isoforms [30,174,175,183]. NO, while accumulating in relatively low concentrations, reversibly inhibits respiration at the level of cytochrome oxidase (COX) by competing with O2; it also inhibits the activity of the complex II and oxidizes ubiquinol [31, 32]. At higher concentrations, it reacts with superoxide thereby forming a strong oxidant, peroxynitrite (ONOO−). Through S-nitrosylation and/or nitration ONOO− inhibits irreversibly many mitochondrial proteins including the subunits of the complex I and II of the respiratory chain [30] which results in suppression of OXPHOS but increase in ROS production [33–39]. Very recently, a correlation between the electron flux level through the respiratory chain and the type of the mechanism/degree of NO inhibition of respiration, depending on availability in the cell of cytochrome c at the COX site, has been observed in solution and intact lymphocytes [39].
Under conditions of sepsis the endotoxin-induced impairment of mitochondrial function in heart and skeletal muscles manifests as decreased state 3 respiration caused by diminished activities of the complexes I + III as well as II + III [184,185]. Probably, these effects are related to differential effects of TNF-α on isoenzymes of NOS, because TNF-α upregulates iNOS, but downregulates eNOS [176,186–189]. The TNF-α-mediated downregulation of eNOS causes an inhibition of mitochondrial biogenesis, which is positively controlled by eNOS [190]. Suppression of mitochondrial biogenesis in conjunction with the inhibitory effects of NO and ONOO− on mitochondrial respiratory chain should strongly decrease the OXPHOS capacity of the cells. Moreover, in conditions of associated sepsis, CED may worsen, due to inhibition of mitochondrial CK, as demonstrated in the diafragm and heart of endotoxin-treated dogs [82]. In the case that pathogenic bacteria are involved, the mode of action of cytokines on mitochondria is strongly augmented, because bacteria stimulate mitochondrial ROS production via direct effects on mitochondrial membranes. For example, Helicobacter pylori (H. pylori), a major pathogen causing inflammation of gastric mucosa in humans (see also Section 3.4.1), permeabilizes the MOM through translocation of the N-terminal 34 kDa fragment of H. pylori vacA cytotoxin into the mitochondria [191]. This process is associated with depolarization and fragmentation of mitochondrial membranes in association with suppressed ATP synthesis [192,193] and increased production of ROS, NO and ammonia, all of which secondarily exert cyto- and mitochondriotoxic effects [194–196].
Normally, ROS produced by mitochondria are largely detoxified by mitochondrial Mn-dependent superoxide dismutase (MnSOD). However, under conditions of NO excess, this enzyme undergoes nitration that inhibits its own activity [197] and, due to inactivation of NADP+-dependent isocitrate dehydrogenase by ONOO−, less glutathione (GSH) will be regenerated [198]. These cascades facilitate inflammation through establishing the feed-forward circles, as inflammation increases the mitochondrial ROS and RNS and the latter compounds again promote expression of proinflammatory cytokines.
That the cells recruit mitochondria to mediate inflammation seems to be surprising, particularly if one considers that cellular energy metabolism is largely shifted from OXPHOS towards glycolysis under inflammation conditions [172,199]. This could in turn suggest a decreased importance of mitochondria. Nevertheless, the existing evidence indicates that even under conditions of a decreased number of mitochondria, cell fate is maintainly controlled by the remaining ones. On the one hand, mitochondria mediate the prosurvival mechanisms in inflammatory cells. For example, different types of cytokines (e.g. IL-3, IL-5) suppress proapoptotic changes, such as translocation of Bax to the mitochondria, cytochrome c release, activation of caspases, and caspase-independent loss of ΔΨ, as seen in neutrophils and eosinophils [200,201]. NO, a product of inflammatory reactions, also inhibits apoptosis, via suppression of caspases (S-nitration) and PT pore opening, but stimulation of antiapoptotic Bcl2 [200]. In addition, mitochondria sensitize the inflammatory cells to necrotic death, thereby aggravating the inflammatory tissue lesions and complicating the disease phenotype. In this regard, it has been shown that peripheral blood lymphocytes (T cells) of patients with systemic lupus erythematosus displayed persistent mitochondrial hyperpolarization associated with increased ROS production and cellular ATP and GSH depletion, leading to necrotic death in response to IL-10, in contrast to the cells of healthy patients which exhibited transient increase in the ΔΨ that was linked to apoptotic death [201–203]. Along with this evidence, some data suggest that intact mitochondrial function is necessary for supporting the anti-inflammatory properties of the neutrophils, irrespectively of its role in controlling the apoptotic processes [204].
Interestingly, the ways how mitochondria influence the inflammatory processes vary depending on the type of the inflammatory cell and its mode of activation. For example, during differentiation of esosinophils mitochondria loose their capacity to respire and produce ATP, but retain their ability to generate ΔΨ at the expense of glycolytic ATP and to induce apoptosis via cytochrome c release [205]. By these characteristics, eosinophils differ from other inflammatory cells, e.g. neutrophils and macrophages, in which mitochondria contribute to inflammation not only by regulating apoptosis, but also by production of ATP, ROS, and RNS. On the other hand, a remarkable finding is that the Th1 or Th2 cytokines are causal for qualitatively different responses in macrophages. The Th1-derived cytokines (e.g. IFN-γ, TNF-α, LPS, and IL-1) activate the classical signalling pathways predominantly via HIF-1α leading to activation of glycolysis, increased production of NO, ROS, and proinflammatory cytokines (e.g. TNF-α, IL-1, IL-6, and IL-12), which cause marked tissue damage by amplifying the inflammatory reactions [172,199,206]. In contrast, the Th2 cytokines (e.g. IL-4 and IL-13) stimulate oxidative mechanisms via activation of STAT6 (signal transducer and activator of transcription 6) and PGC-1β (PPARγ-coactivator-1β), which induce macrophage programs for fatty acid oxidation and mitochondrial biogenesis. So, it appears that oxidative metabolism is strongly required for establishing the anti-inflammatory phenotype, which helps to limit inflammation and promote reparative processes, such as wound healing and granuloma formation, via secretion of chitinases, chemokines, and collagen [206,207]. In support of this assumption, it has been shown that recovery from Staphylococcous aureus-induced sepsis in mouse liver was associated with activation of mitochondrial biogenesis [208]. This process was clearly controlled by the time-dependent activation of Akt, PKC, and PKA [208]: On day first, activation of PI3K/Akt system, which promoted prosurvival through induction of antiapoptotic reactions, stimulation of mitochondrial biogenesis by phosphorylation of NRF-1, and stabilization of interaction of HK with mitochondria (as shown in Section 3.3), was observed. On day second, activation of PKC-ɛ started, which exerted protection against PTP opening and apoptosis [209,210]. Then, on a third day, activation of PKA was detected which could (i) increase mitochondrial respiratory activity through phosphorylation of complex I subunit [211,212] and (ii) suppress apoptosis by phosphorylation of BAD [213]. As a result, these mechanisms were able to restore oxidative metabolism as an early and important prosurvival factor in liver cells [208].
3.3. Energy metabolism in hypoxia
Tissue hypoxia resulting from oxygen supply-demand mismatch can develop in exercising skeletal muscle, especially in high altitude-hypoxic environment [214] and in conditions of tissue hypoperfusion, as in ischemic myocardium or in the core part of solid tumors [215]. In any of these circumstances, mitochondria represent both sensors and targets of hypoxia. It has been proposed that the electron transport chain reacts to hypoxia as an O2 sensor, by releasing ROS due to retarded flow of electrons along the respiratory chain that reduces the cytochromes and increases a lifetime of the ubisemiquinone radical in complex III [216]. Mitochondrial ROS activate the hypoxia-inducible transcription factor 1α (HIF-1α) [216–223], probably via its stabilization mediated by p38 mitogen-activated protein kinase (MAPK) [221] or by inhibition of HIF prolyl hydroxylases (HPHs) due to oxidation of ferrous iron in the catalytic domain [224]. HIF-1α is a potent inducer of gene transcription [all genes encoding glycolytic enzymes, glucose transporters, vascular endothelial growth factor (VEGF), erythropoietin, and insulin-like growth factor (IGF-2)] [222,223], which enables the cells to survive during the hypoxia period. In parallel, HIF-1α induces pyruvate dehydrogenase kinase 1 (PDK1), which suppresses the mitochondrial O2 consumption by phosphorylating of E1α subunit of PDH [225,226]. Under hypoxic conditions, HIF-1α also regulates cytochrome oxidase subunits by increasing the expression of LON, a mitochondrial protease that degrades COX4-1 and in parallel, activates the expression of COX4-2. This COX4-1 to COX4-2 transition optimizes the efficiency of OXPHOS through increasing the COX activity, respiration rate, and ATP production, while ROS production under conditions of reduced oxygen availability in mitochondria is suppressed [227].
Due to a shift from OXPHOS to glycolysis a new balance between the cellular energy and redox states is achieved. Therein, ATP is predominantly produced by glycolysis whereas NAD+, necessary for ATP production, is regenerated by LDH. Furthemore, the mitochondrial ROS production is reduced. Neumann et al. [228] have shown that constitutive stabilization of HIF-1α in murine thymocytes leads to overexpression of SERCA2 and diminished intracellular Ca2+ transients in response to T cell receptor stimulation [228]. On the contrary, HIF-1α null cardiac myocytes exhibit suppressed activity of SERCA2 [229]. Thus, HIF-1α not only determines the balance between OXPHOS and glycolysis, but also helps to avoid excess of intracellular Ca2+accumulation, thereby favoring maintenance of cell’s viability.
Energy depletion due to hypoxia or ischemia exerts a direct metabolic regulation through changes in the cellular adenine nucleotides, as an increased cellular AMP/ATP ratio activates the AMP-activated protein kinase (AMPK) [230]. Activation of AMPK is known to stimulate fatty acid uptake and oxidation in muscle cells, through inhibition of acetyl-CoA carboxylase due to its phosphorylation, which in turn suppresses the malonyl-CoA levels, thereby increasing the uptake of long-chain acyl-CoA to mitochondria [230]. The influence of AMPK on oxidative metabolism is also mediated by its stimulatory effects on mitochondrial biogenesis, as it increases the activity of transcription factor NRF1 and expression of co-activator PGC-1α [230,231]. On the other hand, AMPK increases the rate of glycolysis by upregulating the glucose uptake and activities of glycolytic enzymes [230]. In parallel to these effects, AMPK promotes apoptotic cell death, via phosphorylation of IRS-1 that leads to inhibition of phosphatidylinositol 3-kinase/Akt (PKB/Akt) signalling [232], and via promoting translocation of proapoptotic proteins (e.g. Bax) into mitochondria, mediated by activation of p38 MAPK downstream of AMPK [233]. Collectively, these changes, being directed to maximally support the ATP synthesis in conditions of limited availability of oxygen, serve as the cellular adaptive reactions to hypoxia.
3.4. Role of mitochondria in carcinogenesis
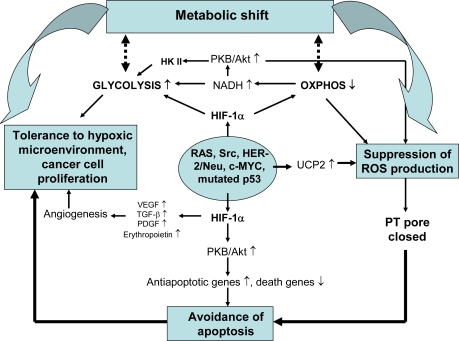
The metabolic shift from OXPHOS to aerobic glycolysis (Warburg effect), tolerance to hypoxic microenvironment, ability to control ROS levels and avoidance of apoptosis are the hallmarks of cancer cells, greatly contributing to their viability, autonomous growth, migration and chemoresistance [234–242] (Figure 6).
Figure 6.
Mechanisms of mitochondrial involvement in cancer development. Activation of oncogenes and HIF-1α, a typical feature of cancer cell, is associated with downregulation of OXPHOS, its coupling to glycolysis via HKII and upregulation of UCP2 which suppress generation of ROS in mitochondria. This change together with altered balance between anti- and pro-apoptotic genes at mitochondrial membranes decreases the susceptibility of cells to apoptotic death. On the other hand, proliferation and survival of cancer cells is promoted by glycolysis and angiogenesis, both activated by HIF-1α.
3.4.1. Mechanisms of metabolic shift in cancer cells
To date, multiple mechanisms of control over the balance between mitochondrial and glycolytic systems have been disclosed. The metabolic shift is primarily driven by specific oncogenes such as RAS, Src, HER-2/Neu, c-MYC, and p53 that activate in response to diverse stresses [243–247]. Activation of c-MYC upregulates the LDH-A isoform [245,247], whereas activation of RAS, Src, and HER-2/Neu triggers induction of glycolytic enzymes through stabilization of HIF-1α. On the other hand, both HIF-1α and c-MYC induce the expression of PDK1, that, by decreasing the activity of pyruvate dehydrogenase (PDH), downregulates the OXPHOS (see above) [248]. Suppression of OXPHOS and activation of glycolysis are feed-forward processes, as they further promote upregulation of HIF-1α through accumulation of pyruvate, other glycolytic intermediates, and oxaloacetate, all of which cause inactivation of the HIF-1α PHDs. As a result, von Hippel-Lindau protein dissociates from HIF-1α, thereby blocking its proteosome-dependent degradation, which increases the levels of active HIF-1α even in aerobic conditions [249–251]. Similarly, in conditions when succinate dehydrogenase is diminished, accumulation of succinate in the cytoplasm hinders the activity of HIF-1α PHDs that stabilizes HIF-1α at high level of activity [252–255]. All these HIF-1α mediated mechanisms strongly promote carcinogenesis in different cell types.
Besides the mechanisms based on activation of HIF-1α, there exist other mechanisms responsible for promoting the metabolic shift. Among those, p53-mediated pathways are of importance [246,256,257]. Normally, activation of p53 leads to stimulation of OXPHOS and mitochondrial respiration, because it stimulates expression of Synthesis of Cytochrome c Oxidase 2 (SCO2) protein that is necessary for the assembly of COX complex [246]. At the same time, p53 suppresses the activity of phosphoglyceromutase and glucose phosphate isomerase and brakes down the PKB/Akt mediated expression of glycolytic enzymes, thus acting as a negative regulator of glycolysis [256,257]. The function of p53 in coordinating OXPHOS and glycolysis is mediated through its interaction with AMPK pathways [258,259]. For example, under glucose deprivation, AMPK causes activation of p53 that results in cell cycle arrest and ensures survival until restoration of glucose supply does occur [258]. At the same time, AMPK can not be activated in the cells lacking p53 [259]. In cancer cells the functions of p53 described are more or less lost due to the mutations in that oncogene. Among many consequences to that change, coordination between OXPHOS and glycolysis [256,257] might be impaired, due to which the OXPHOS would be suppressed, but glycolysis upregulated, similarly to that what would be expected under the influence of HIF-1α (Figure 6).
A balance between glycolysis and OXPHOS can be controlled by the changes in common metabolites as well [245,260]. Because LDH competes with mitochondria for NADH participating in mitochondrial NADH/NAD+ shuttle systems [261], the upregulated glycolysis rapidly consumes NADH for converting pyruvate into lactate, thereby suppressing mitochondrial respiration. It is noteworthy that the NADH-dependent redox state also regulates the activity of PKB/Akt. Accumulation of NADH due to its suppressed consumption by mitochondria causes inactivation of tumor suppressor gene PTEN protein, a negative regulator of PKB/Akt. Hence, PKB/Akt becomes activated and induces expression of the glycolytic enzymes [262]. This redox-dependent mechanism can be amplified by overexpression of PKB/Akt, a characteristic feature of many tumors [262] (Figure 6). Finally, suppression of OXPHOS and upregulation of glycolysis in a course of tumor progression can be stimulated by changes in cytoplasmic adenine nucleotides, as energy stress due to insufficient mitochondrial ATP synthesis activates the AMP-kinase through increased AMP in the cytoplasm, thus stimulating biosynthesis of glycolytic enzymes in cancer cells [242].
3.4.2. Mechanisms of anti-apoptosis in cancer cells
As already was mentioned, mitochondria play a central role in triggering and establishing apoptotic cell death [263]. The importance of mitochondria is explicitly indicated by the following facts. (i) Stimulation of mitochondrial respiration and ROS generation are early events of apoptosis [264 and references therein]. (ii) Activation and oligomerization of proapoptotic Bax and Bak proteins is markedly suppressed after inhibiton of OXPHOS by oligomycin or antimycin A [263]. (iii) Cybrid osteosarcoma cells lacking respiratory chain are unable to undergo apoptosis [265]. Oncogene p53 is likely one of the key factors in the pathways linking cytotoxic stress to mitochondria-dependent apoptosis. p53 activates the transcription of proapoptotic proteins including Bax, Noxa, Puma, and a p53-regulated apoptosis inducing protein-1, but represses the antiapoptotic genes, such as survivin and Bcl-2 [266–271]. A fraction of p53 translocates into mitochondria [272,273], and this process is followed by hyperpolarization-depolarization transient of the inner membrane, increased ROS production, cytochrome c release and caspase activation [274–278]. According to Zhao et al. [272], p53, after having entered the mitochondrion, interacts with MnSOD, thereby decreasing its activity and evoking ROS generation. p53 also binds to BAK and induces its oligomerization, thereby causing permeabilization of the outer mitochondrial membrane and release of cytochrome c, these changes triggering apoptosis [273].
These mitochondria-dependent pro-apoptotic mechanisms appear to be attenuated or even switched off in cancer cells. Obviously, mutations in p53 can be causal for anti-apoptosis. Other reasons for that might be related to specific impairments of mitochondria. In cancer cells, the mitochondria are characterized by defective respiratory chain complexes I and III and decreased β-F1-ATPase [279–291], and the type of mitochondrial impairment appears to determine the clinical phenotype [279,290]. Accordingly, benign oncocytomas are characterized by impaired complex I, but enhanced expression of other respiratory chain complexes and matrix enzymes, together with upregulation of mitochondrial tissue content, the latter changes likely compensating the insufficient complex I. In contrast, malignant renal tumors exhibit downregulation of all respiratory chain complexes and β-F1-ATPase, in correlation with increased tumor aggressiveness and avoidance of apoptosis [279,290]. The second line of discrimination between the cancer cell types goes along their capacity to produce ROS: whereas many types of cancer cells exhibit excess ROS production [239,240,292–297], some cancer forms show very low ROS levels, together with attenuated apoptosis (reviewed by Lu in 2007 [298]). It is known that generation of ROS in mitochondria steeply increases with build-up of transmembrane potential, ΔΨ [299]. In this regard, Santamaria et al. showed recently that oligomycin, an inhibitor of β-F1-ATPase, strongly delayed the stauroporin-induced cell death in liver and hepatoma cell lines; it was concluded that β-F1-ATPase is required to hyperpolarize mitochondria in order to produce ROS for induction of apoptosis [300]. At the same time, it became known that in colon and renal cancers βF1-ATPase is downregulated and the cellular content of mitochondria decreased [279,290]. It was therefore proposed that the cancer cells characterized by reduced activity of β-F1-ATPase and low content of mitochondria are unable to produce mitochondrial ROS in amounts sufficient to induce PTP and apoptosis. This property may represent an adaptive strategy of cancer cells to avoid ROS-mediated cell death that contributes to their increased aggressiveness and chemotherapeutic resistance [279,290].
In fact, the cancer cells possess a variety of other means for suppressing the mitochondrial ROS. In breast cancer cells, estrogen, by binding to its mitochondrial receptors, upregulates mitochondrial MnSOD that in turn slows down mitochondrial ROS production and apoptosis [294]. Colon cancers exhibit increased UCP2 expression [301,302], which, through lowering intracellular ROS levels, confers reduced susceptibility to oxidative damage, apoptosis and drug-resistance [303]. In an attempt to reveal the underlying molecular mechanisms, Derdak et al. overexpressed UCP2 in human colon cancer cells and showed that it was accompanied by reduced ΔΨ and ROS production and increased oxygen consumption, these changes being associated with inactivation of tumor suppressor p53 through its NH2-terminal phosphorylation and induction of the glycolytic phenotype [304]. Notably, realization of the Warburg effect is also linked to promotion of anti-apoptotic and pro-survival mechanisms. Activation of PKB/Akt-dependent signaling through altered redox state and HIF-1α- and IGF-1,2-mediated pathways strongly hinders the apoptotic cell death (Figure 6), as activated PKB/Akt suppresses expression of death genes (Bax, Bak, Smac/Diablo, Fas, Bim, and IGFBP-1), but upregulates antiapoptotic (Bcl-2, Bcl-xL, survivin, XIAP) and proliferation-supporting genes (clAP1, clAP2), probably through activaton of NF-kB and CREB [214,236,305–311]. Because the mutated p53 can not effectively counterbalance this mechanism (see above), the PKB/Akt-mediated effect may take over in cancer cells. Importantly, the anti-apoptotic influence of PKB/Akt can be enhanced through another mechanism – functional coupling between the OXPHOS and glycolysis – which is also controlled by this kinase and observed in several types of transformed cells, e.g. breast and liver cancer cells. These cells overexpress hexokinase (HK) type II [312–315] under stimulation by HIF-1α or c-MYC [207]. HK II effectively binds to the mitochondrial VDAC and this process is activated by protein kinase B or Akt (PKB/Akt) [314,316,317], which blocks the activity of glycogen synthase kinase 3β (GSK3β), an inhibitor of HK binding to VDAC [318]. Interaction of glycolysis with OXPHOS supports cancer growth and protects against apoptotic death by multiple means (Figure 6). Due to forming of the HK II-VDAC complex, ATP synthesized in mitochondria is transported via ANT and porin channels to active sites of HK II and used as a preferable substrate for glucose phosphorylation, whereas ADP, another product of HK reaction is returned into the matrix for ATP synthesis. Thus, coupling of glycolysis to OXPHOS enables to amplify the glycolytic flux by increasing the efficacy of substrate supply and removal of product inhibition [319]. In parallel, HK II binding to VDAC stabilizes the mitochondrial outer membrane, thereby suppressing the release of intermembrane proapoptotic proteins and/or blocking association of exogenous proapoptotic proteins (Bax) with the MOM [317]. It has been proposed that association of HK II with VDAC increases the ATP/ADP turnover that utilizes ΔΨ, thereby suppressing the ΔΨ-dependent ROS production in the respiratory chain [76], which underlies the downregulation of mitochondrial ROS production. As a proof for importance of these mechanisms, inhibition of binding of HK II by 3-bromopyruvate or its detachment from mitochondria could be shown to suppress significantly cellular growth and induced apoptosis via mitochondrial signaling cascades [315,320].
Increased glycolysis advances proliferative growth of cancer cells by several ways other than through improving the availability of ATP. For example, a resultant acidity prepares surrounding tissues for invasion, probably by suppressing immune response [319], protects mitochondria from PT pore opening, and inhibits activation of Bax and Bak, thus favoring antiapoptosis in these cells [263]. High rate of glycolysis activates the pentose phosphate pathway that provides the precursors (G-6-P) for biosynthetic processes [321]. Given that stimulated pentose pathway leads to increased NADPH and high levels of reduced glutathione, it also ends up with lower cellular ROS accumulation, thus supporting survival of the cancer cells. Moreover, the inflammatory mediators (e.g. cytokines, ROS, and NO) suppress apoptosis by causing mutations in Bcl2 and p53 proteins [214] or nitration of caspase 9 [322], whereas HIF-1α supports invasion, migration and tolerance to hypoxia by inducing vascular growth and erythropoietin synthesis [323]. It is also known that in a variety of neoplastic cells expression of peripheral benzodiazepine receptor (PBR), a mitochondrial protein associated with VDAC protein, is strongly upregulated [324]. As the PBR exerts a strong protective effect against ROS damage [325], it supports cancer cell survival, despite increased ROS loading.
In the light of these data, different pharmacological means for stimulating apoptosis in cancer cells are under investigation [326]. For example, it has been found that in many types of cancers an appropriate chemo- and radiotherapy can recover the ability of mitochondria to release cytochrome c and activate apoptosis [327–330].
3.4.3. Development of gastric cancer: possible role of altered mitochondrial function?
The biological model of gastric carcinogenesis can be displayed as an inflammation-atrophy-metaplasia-dysplasia-carcinoma sequence [331] that is based on three different intermingled processes. Firstly, chronic active inflammation caused by H. pylori creates the background for geno- and phenotypic alterations. Secondly, disruption of the balance between apoptosis and cell proliferation results in mucosal atrophy. Thirdly, progressive loss of differentiation favors establishment of intestinal metaplasia characterized by replacement of intestine-type glands for normal glands [332].
To address the role of mitochondria in gastric disease, we have recently characterized the function of OXPHOS in the biopsies of gastric mucosa taken from the patients suffering from chronic active inflammation [333]. We found that compared to non-active gastritis, the active chronic gastritis (confirmed on the basis of the prominent mononuclear infiltration) was associated with decreased complex I dependent ADP-stimulated respiration rate in the corpus mucosa, whereas this parameter was augmented in the antrum mucosa. Increased OXPHOS in the antrum mucosa was unexpected in light of the evidence that H. pylori induces stronger oxidative stress in the antrum than in the corpus [334–336]. However, the inverse changes in OXPHOS in antrum and corpus mucosa could arise from differential effects of H. pylori on the balance between the antiapoptotic and apoptotic pathways in distinct parts of the stomach. Indeed, H. pylori stimulates apoptosis by triggering cytochrome c release [191,337] and translocation of proapoptotic Bax [192,337] or/and the amino-terminal fragment of bacterial cytotoxin VacA into mitochondria [191]. Based on these data and our observation that mitochondria exhibited normal coupling, the suppressed respiration in the gastric corpus mucosa [333] suggests decreased tissue content of mitochondria due to apoptotic loss of mitochondria and cells. However, H. pylori can also impel the cells to slowdown the apoptotic processes, through activation of the cellular inhibitor of apoptosis gene 2 [338] and upregulation of cyclooxygenase-2 (COX-2) [335,339,340]. The products of COX-2 (15d-PGJ2 and PGA1) directly inhibit NF-κB-mediated apoptotic pathways via activating the PPARγ [341,342], whereas PPARγ then accelerates biosynthesis of mitochondria, thus increasing the tissue’s oxidative capacity [343,344]. Given that H. pylori upregulates COX-2 in the antral mucosa to a greater extent than in the corpus mucosa [333], activation of PPARγ is expected to be more pronounced in the antral mucosa, which would explain the increased OXPHOS in this region observed by us.
It is generally accepted that transition from chronic gastric inflammation into atrophic gastritis heralds high risk for gastric adenocarcinoma [331,345,346]. The subcellular mechanisms of that transition may involve the mitochondrial dysfunction, as electron microscopy revealed decreased content of mitochondria and increased fraction of abnormal or damaged mitochondria in mucosa with chronic gastritis [347]. In line with this observation, we found that gastric corpus mucosa of patients with pernicious anemia, which is an end-stage condition of corpus dominant atrophic gastritis, exhibits decreased respiratory capacity compared to non-atrophic mucosa [348]. In addition, mitochondria of the atrophic mucosa showed a deficient respiratory complex I and increased coupling of succinate oxidation to phosphorylation [348]. Thus, our data show that, like it occurs in many other diseases, the gastric mucosal atrophy results in remodeling of the systems of OXPHOS, with specific impairment at the level of complex I of the respiratory chain (see Sections 3.1.1 and 3.1.2), but improved function of more distal complexes that may represent an adaptive response.
As already discussed above, impaired function of the complex I of the respiratory chain can be related to mutations of mtDNA and excess production of mitochondrial ROS that oxidizes the mitochondrial proteins and redox centers. It may also rely on alterations of Ca2+-dependent effects on complex I function, as in the case of pathologically altered mitochondria in brain (see Sections 2.1 and 3.1.1.). However, this possibility remains to be proven in further studies on gastric mucosa. Nevertheless, irrespectively of the reasons for suppressed function of the complex I in mitochondria of corpus mucosa, the major question regards to its pathophysiological role in inflammation, atrophy and carcinogenesis. In this regard, it is interesting that in early stages of carcinogenesis, even before histological evidence of angiogenesis or invasion, the tumor cells in different tissues exhibit overexpression of HIF-1α [349]. In line with these data, analysis of the gastric biopsies of normal mucosa, H. pylori-associated gastritis, intestinal metaplasia, dysplasia, and intestinal and diffuse adenocarcinoma revealed progressively increasing expression of HIF-1α and HIF-2α along with this gastric carcinogenic sequence, and changes in HIF isoforms were accompanied by increased expression of erythropoetin, GLUT-1 and VEGF, all of which represent the targets of HIF [350,351]. Others have found that inhibition of HIF-1α in human gastric cancer TMK-1 cells markedly retarded the tumor growth, angiogenesis, and vessel maturation [352]. Altogether, these data strongly infer that transition from gastric inflammation to cancer is mediated by increased activity of HIF. It is possible that upregulation of HIF isoforms occurs secondarily, in response to altered activities of the upstream PI-3K/Akt/mTOR signaling that promotes growth of tumors [353,354]. This possibility is confirmed by observations that the mammalian target of rapamycin (mTOR), an upstream regulator of HIF, is activated in human gastric cancer, whereas attenuation of PI-3K/Akt/mTOR-mediated signalling by rapamycin effectively blocks HIF-1α in gastric cell lines [355]. Suppressed complex I registered in atrophic gastric mucosa by us [348] may support upregulation of HIF-1α through altered metabolism. (i) ROS, increasingly produced due to impaired complex I, can inhibit the PHDs that should lead to higher activity of HIF-1α (see Section 3.3). (ii) Due to inefficient complex I, utilization of NADH-dependent substrates, like pyruvate, may be hindered, which leads to accumulation of these substrates in the cytoplasm, thereby also stabilizing the PHDs [251] and enabling increases in active HIF-1α. Subsequently, high level of HIF-1α should promote Warburg effect along with suppression of apoptosis, all these changes conferring malignancy to gastric mucosal cells (see Section 3.4, Figure 6). In support of this scenario, the recent data show that human gastric cancer is associated with marked increase in expression of lactate dehydrogenase-5 (LDH-5), in correlation with HIF-1α, VEGF and COX-2. As the overexpression of LDH-5 was found to be more prevalent in advanced tumors exhibiting vessel invasion, and since the patients with lower expression of that enzyme showed higher survival rates compared to those with low levels of expression, expression of LDH-5 was considered to be a useful prognostic marker [356]. Interestingly, we found that mitochondria in atrophic gastric mucosa exhibited improved respiratory control by ADP in the presence of succinate [348]. This suggests that in intact cells, the energetic limitations due to downregulation of OXPHOS and insufficient complex I can be overcome by shifting the preferable substrate for oxidation from pyruvate to succinate. Taken together, it appears that suppressed OXPHOS and deficit in complex I of the respiratory chain may be important factors supporting transition from chronic gastritis to gastric cancer through promoting Warburg effect. Oxygraphic measurement of complex I to complex II activity ratios may therefore serve as a valuable diagnostic tool for detecting the metabolic shift in gastric mucosal cells. Its potential prognostic value requires further experimental and clinical evaluation.
4. Conclusions
R. Luft has classified his discovery of the first patient with a mitochondrial dysfunction as a starting point of a “revolution in mitochondrial medicine” [357]. By now the “revolution” has entered a new phase, with a necessity to consider that dysfunction of mitochondria underlies almost all diseases and pathological processes. Indeed, four large groups of diseases with mitochondrial impairments can be distinguished: 1) primary mitochondrial diseases caused by hereditary or acquired mutations within the mitochondrial genome, 2) secondary mitochondrial diseases which are provoked by mutations in the non-mitochondrial genome leading to mitochondriotoxic effects by mutated proteins or crucially altered regulatory processes, 3) acute mitochondrial insults due to ischemia, inflammation, and intoxications, and 4) changes of mitochondrial regulation and function in cancer cells. In any case, impaired mitochondrial function leads to cellular energetic depression which is characterized by diminished phosphorylation potentials, cytoplasmic and mitochondrial Ca2+ overload, and accumulation of ROS and toxic proteins.
The central role of mitochondrial dysfunction in cellular pathophysiology is not surprising because (i) the energy state controls directly many of the intra- and intercellular signaling systems, e.g. kinase activities, intracellular Ca2+ homeostasis, and proliferation, (ii) the pathogenetic mechanisms of ischemia/hypoxia, inflammation, cancer, and other pathological processes converge at the level of mitochondrial impairment, and (iii) concentration of cellular ATP determines the type of the cell death. Thus, under conditions of limited but persisting mitochondrial functionality, cytochrome c release through a leaky outer mitochondrial membrane but still effective ATP generation sufficient to support the ATP-dependent apoptotic machinery, mitochondria are able to initiate and execute the program of apoptotic cell death. In contrast, if the mitochondrial function completely vanishes due to irreversible opening of the PT pore, the intracellular ATP levels rapidly decrease to amounts insufficient for maintaining apoptosis and the cell has to switch to necrosis. The latter process initiates negative effects on the, even originally non-diseased, neighboring cells and tissue by inducing inflammation that further worsens the clinical phenotype of the respective disease. Both of cell death modes, apoptosis and necrosis, can be taken as the two sites of the common denominator – a mitochondrial cell death. This type of death governs cell fate in the first three types of diseases listed above. In contrast, in tumor cells (disease group 4 above), the cell death machinery and impaired mitochondrial function can be suppressed and compensated, respectively, (i) by effective anti-apoptotic mechanisms that preserve mitochondrial function at least to some extent and period, and (ii) by upregulation of glycolysis and its functional coupling to OXPHOS, these processes representing the main facets of the Warburg phenotype.
Recognition of the central role of bioenergetic failure in cellular pathophysiology has motivated the scientists to elaborate the therapeutic strategies aimed at salvage of the diseased cells by optimizing their energy balance, with mitochondria as a special target to be protected. Retardation of decay of cellular ATP during unfavorable conditions for cell life, e.g. under oxygen and substrate insufficiency, should be a main goal for such interventions, in order to postpone or avoid the necrotic cell death, but at the same time to support apoptosis and inhibit or even reverse the tumor growth. With these goals set, the inhibitors of PT have been proven to be effective in treatment of various animal models of disease [43,48,49]. Furthermore, recent studies have revealed that antidiabetic agents act via inhibiting the mitochondrial complex I, thereby causing activation of AMP-activated protein kinase followed by downstream metabolic alterations (e.g. stimulation of glycolysis and mitochondrial biogenesis) that improve the cellular energy state in muscle cells and ameliorate the insulin resistance [358,359]. A number of drugs that selectively switch mitochondria from the oxidation of fatty acids to carbohydrate oxidation have been found to be beneficial to protect cardiac muscle cells from ischemia/reperfusion injury [360]. Concerning the metabolic therapy of tumors, the attempts to suppress glycolysis by inhibiting the individual enzymes or via releasing HK from mitochondrial binding sites to reverse the Warburg effect have been rather promising. As a complementary or alternative approach, the drug-induced stimulation of PT pore opening and/or the fine-tuned modulation of pro- and anti-apoptotic signaling cascades is expected to promote apoptotic death of cancer cells [reviewed in 16,361].
Keeping in mind the huge complexity of mitochondria with its more than 1200 different proteins, of which many are unknown in functional terms, and our incomplete knowledge on interaction of mitochondria with other cellular structures and systems, we can optimistically look for novel discoveries and a rapid extension of the frontiers of mitochondrial medicine.
Acknowledgments
This work was supported by the European Huntington network, the DFG (Ge 664/11-2), the German Federal Ministry of Economics and Technology, grant No. IWO72052 (MitoscreenTest), Estonian Ministry of Education and Research (SF0180114As08) and Estonian Science Foundation (grants No. 7117 and 7823).
References
- 1.Warburg O, Geissler AW, Lorenz S. Genesis of tumor metabolism by vitamin B1 deficiency (thiamine deficiency) Z. Naturforsch. B. 1970;25:332–333. [PubMed] [Google Scholar]
- 2.Luft R. Luft's disease revisited. Severe hypermetabolism of nonthyroid origin with a defect in the maintenance of mitochondrial respiratory control. Mt. Sinai J. Med. 1992;59:140–145. [PubMed] [Google Scholar]
- 3.Holt IJ, Harding AE, Morgan-Hughes JA. Deletions of muscle mitochondrial DNA in patients with mitochondrial myopathies. Nature. 1988;331:717–719. doi: 10.1038/331717a0. [DOI] [PubMed] [Google Scholar]
- 4.Wallace DC, Singh G, Lott MT, Hodge JA, Schurr TG, Lezza AM, Elsas LJ, Nikoskelainen EK. Mitochondrial DNA mutation associated with Leber's hereditary optic neuropathy. Science. 1988;242:1427–1430. doi: 10.1126/science.3201231. [DOI] [PubMed] [Google Scholar]
- 5.Triepels RH, Van Den Heuvel LP, Trijbels JM, Smeitink JA. Respiratory chain complex I deficiency. Am. J. Med. Gen. 2001;106:37–45. doi: 10.1002/ajmg.1397. [DOI] [PubMed] [Google Scholar]
- 6.Lestienne P, Ponsot G. Kearns-Sayre syndrome with muscle mitochondrial DNA deletion. Lancet. 1988;8590:885. doi: 10.1016/s0140-6736(88)91632-7. [DOI] [PubMed] [Google Scholar]
- 7.Luft R. The development of mitochondrial medicine. Biochim. Biophys. Acta. 1995;1271:1–6. doi: 10.1016/0925-4439(95)00002-l. [DOI] [PubMed] [Google Scholar]
- 8.Schlame M, Ren M. Barth syndrome, a human disorder of cardiolipin metabolism. FEBS Lett. 2006;80:5450–5455. doi: 10.1016/j.febslet.2006.07.022. [DOI] [PubMed] [Google Scholar]
- 9.Quinzii CM, Dimauro S, Hirano M. Human coenzyme Q(10) deficiency. Neurochem. Res. 2006;32:723–727. doi: 10.1007/s11064-006-9190-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zeviani M, Di Donato S. Mitochondrial disorders. Brain. 2004;127:2153–2172. doi: 10.1093/brain/awh259. [DOI] [PubMed] [Google Scholar]
- 11.Smeitink JA, Zeviani M, Turnbull DM, Jacobs HT. Mitochondrial medicine: a metabolic perspective on the pathology of oxidative phosphorylation disorders. Cell. Metab. 2006;3:9–13. doi: 10.1016/j.cmet.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 12.Morgan-Hughes JA, Hanna MG. Mitochondrial encephalomyopathies: the enigma of genotype versus phenotype. Biochim. Biophys. Acta. 1999;1410:125–145. doi: 10.1016/s0005-2728(98)00162-5. [DOI] [PubMed] [Google Scholar]
- 13.Wiederkehr A, Wollheim CB. Minireview: implication of mitochondria in insulin secretion and action. Endocrinology. 2006;147:2643–2649. doi: 10.1210/en.2006-0057. [DOI] [PubMed] [Google Scholar]
- 14.Halestrap AP. Calcium, mitochondria and reperfusion injury: a pore way to die. Biochem. Soc. Trans. 2006;34:232–237. doi: 10.1042/BST20060232. [DOI] [PubMed] [Google Scholar]
- 15.Chan PH. Mitochondria and neuronal death/survival signaling pathways in cerebral ischemia. Neurochem. Res. 2004;29:1943–1949. doi: 10.1007/s11064-004-6869-x. [DOI] [PubMed] [Google Scholar]
- 16.Galluzzi L, Larochette N, Zamzami N, Kroemer G. Mitochondria as therapeutic targets for cancer chemotherapy. Oncogene. 2006;25:4812–4830. doi: 10.1038/sj.onc.1209598. [DOI] [PubMed] [Google Scholar]
- 17.Fantin VR, Leder P. Mitochondriotoxic compounds for cancer therapy. Oncogene. 2006;25:4787–4797. doi: 10.1038/sj.onc.1209599. [DOI] [PubMed] [Google Scholar]
- 18.Kalman B, Leist TP. A mitochondrial component of neurodegeneration in multiple sclerosis. Neuromolecular Med. 2003;3:147–158. doi: 10.1385/NMM:3:3:147. [DOI] [PubMed] [Google Scholar]
- 19.Perl A, Gergely P, Jr, Banki K. Mitochondrial dysfunction in T cells of patients with systemic lupus erythematosus. Int. Rev. Immunol. 2004;23:293–313. doi: 10.1080/08830180490452576. [DOI] [PubMed] [Google Scholar]
- 20.Liu H, Pope RM. The role of apoptosis in rheumatoid arthritis. Curr. Opin. Pharmacol. 2003;3:317–322. doi: 10.1016/s1471-4892(03)00037-7. [DOI] [PubMed] [Google Scholar]
- 21.Brand MD. The efficiency and plasticity of mitochondrial energy transduction. Biochem. Soc. Trans. 2005;33:897–904. doi: 10.1042/BST0330897. [DOI] [PubMed] [Google Scholar]
- 22.Gellerich FN, Trumbeckaite S, Opalka JR, Chen Y, Neuhoff C, Schlag H, Zierz S. Mitochondrial dysfunction at sepsis: Evidences from bacteraemic baboons and endotoxaemic rabbits. Bioscience Report. 2002;22:99–113. doi: 10.1023/a:1016017224003. [DOI] [PubMed] [Google Scholar]
- 23.Trumbeckaite S, Opalka JR, Neuhof C, Zierz S, Gellerich FN. Different sensitivity of rabbit heart and skeletal muscle to endotoxin-induced impairment of mitochondrial function. Eur. J. Biochem. 2000;268:1422–1429. doi: 10.1046/j.1432-1327.2001.02012.x. [DOI] [PubMed] [Google Scholar]
- 24.Singer M, Brealey D. Mitochondrial dysfunction in sepsis. Biochem. Soc. Symp. 1999;66:149–166. doi: 10.1042/bss0660149. [DOI] [PubMed] [Google Scholar]
- 25.Skulachev VP. Mitochondria in the programmed death phenomena; a principle of biology: “it is better to die than to be wrong”. IUBMB Life. 2000;49:365–373. doi: 10.1080/152165400410209. [DOI] [PubMed] [Google Scholar]
- 26.Wallace DC. A mitochondrial paradigm of metabolic and degenerative diseases, aging, and cancer: a dawn for evolutionary medicine. Annu. Rev. Genet. 2005;39:359–407. doi: 10.1146/annurev.genet.39.110304.095751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gellerich FN, Laterveer FD, Zierz S, Nicolay K. The quantitation of ADP diffusion gradients across the outer membrane of heart mitochondria in the presence of macromolecules. Biochim. Biophys. Acta. 2002;1554:48–56. doi: 10.1016/s0005-2728(02)00212-8. [DOI] [PubMed] [Google Scholar]
- 28.Parone PA, Martinou JC. Mitochondrial fusion and apoptosis: an ongoing trial. Biochim. Biophys. Acta. 2006;1763:522–530. doi: 10.1016/j.bbamcr.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 29.Sickmann A, Reinders J, Wagner Y, Joppich C, Zahedi R, Meyer HE, Schonfisch B, Perschil I, Chacinska A, Guiard B, Rehling P, Pfanner N, Meisinger C. The proteome of Saccharomyces cerevisiae mitochondria. Proc. Natl. Acad. Sci. USA. 2003;100:13207–13212. doi: 10.1073/pnas.2135385100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boveris A, Alvarez S, Navarro A. The role of mitochondrial nitric oxide synthase in inflammation and septic shock. Free Rad. Biol. Med. 2002;33:1186–1193. doi: 10.1016/s0891-5849(02)01009-2. [DOI] [PubMed] [Google Scholar]
- 31.Ramachandran A, Levonen AL, Brookes PS, Ceaser E, Shiva S, Barone MC, Darley-Usmar V. Mitochondria, nitric oxide, and cardiovascular dysfunction. Free Rad. Biol. Med. 2002;33:1469–1474. doi: 10.1016/s0891-5849(02)01142-5. [DOI] [PubMed] [Google Scholar]
- 32.Borutaite V, Moncada S, Brown GC. Nitric oxide from inducible nitric oxide synthse sensitizes the inflamed aorta to hypoxic damage via respiratory inhibition. Shock. 2005;23:319–323. doi: 10.1097/01.shk.0000156672.36439.2d. [DOI] [PubMed] [Google Scholar]
- 33.Frost MT, Wang Q, Moncada S, Singer M. Hyoxia accelerates nitric oxide-dependent inhibition of mitochondrial complex I in activated macrophages. Am. J. Physiol. 2005;288:394–400. doi: 10.1152/ajpregu.00504.2004. [DOI] [PubMed] [Google Scholar]
- 34.Beltran B, Orsi A, Clementi E, Moncada S. Oxidative stress and S-nitrosylation of proteins in cells. British J. Pharmacol. 2000;129:953–960. doi: 10.1038/sj.bjp.0703147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Borutaite V, Budriunaite A, Brown GC. Reversal nitric oxide-, peroxynitrite- and S-nitrosothiol-induced inhibition of mitochondrial respiration or complex I activity by light and thiols. Biochim. Biophys. Acta. 2000;1459:405–412. doi: 10.1016/s0005-2728(00)00178-x. [DOI] [PubMed] [Google Scholar]
- 36.Clementi E, Brown GC, Feelich M, Moncada S. Persistent inhibition of cellular respiration by nitic oxide: crucial role of S-nitrosylation of mitochondrial complex I and protective action of glutathione. Proc. Natl. Acad. Sci. USA. 1998;95:7631–7636. doi: 10.1073/pnas.95.13.7631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moncada S. Nitric oxide and cell respiration: physiology and pathology. Verh. K. Akad. Geneeskd. Belg. 2000;62:171–179. [PubMed] [Google Scholar]
- 38.Takabayashi A, Kawai Y, Iwata S, Kanai M, Denno R, Kawada K, Obama K, Taki Y. Nitric oxide induces a decrease in the mitochondrial membrane potential of peripheral blood lymphocytes, especially in natural killer cells. Antioxid. Redox. Signal. 2000;2:673–680. doi: 10.1089/ars.2000.2.4-673. [DOI] [PubMed] [Google Scholar]
- 39.Masci A, Mastronicola D, Arese M, Piane M, De Amicis A, Blanck T, Chessa L, Sarti P. Control of cell respiration by nitric oxide in Ataxia Telangiectasia lymphoblastoid cells. Biochim. Biophys. Acta. 2008;1777:66–73. doi: 10.1016/j.bbabio.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 40.Gellerich FN, Gizatullina Z, Nguyen HP, Trumbeckaite S, Vielhaber S, Seppet E, Zierz S, Landwehrmeyer B, Ries O, von Hoersten S, Striggow F. Impaired regulation of brain mitochondria by extramitochondrial Ca2+ in transgenic Huntington disease rats. J. Biol. Chem. 2008;283:30715–30724. doi: 10.1074/jbc.M709555200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ichas F, Mazat JP. From calcium signaling to cell death: two conformations for the mitochondrial permeability transition pore. Switching from low- to high-conductance state. Biochim. Biophys. Acta. 1998;1366:33–50. doi: 10.1016/s0005-2728(98)00119-4. [DOI] [PubMed] [Google Scholar]
- 42.Ichas F, Jouaville LS, Sidash SS, Mazat JP, Holmuhamedov EL. Mitochondrial calcium spiking: a transduction mechanism based on calcium-induced permeability transition involved in cell calcium signalling. FEBS Lett. 1994;348:211–215. doi: 10.1016/0014-5793(94)00615-6. [DOI] [PubMed] [Google Scholar]
- 43.Bredesen DE, Rammohan VR, Mehlen P. Cell death in the nervous system. Nature. 2006;443:796–802. doi: 10.1038/nature05293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vieira KL, Kroemer G. Pathophysiology of mitochondrial cell death control. Cell. Mol. Life Sci. 1999;56:971–976. doi: 10.1007/s000180050486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gellerich FN, Trumbeckaite S, Müller T, Chen Y, Deschauer M, Gizatullina Z, Zierz S. Energetic depression caused by mitochondrial dysfunction. Mol Cell Biochem. 2004;256/257:391–405. doi: 10.1023/b:mcbi.0000009885.34498.e6. [DOI] [PubMed] [Google Scholar]
- 46.Bernardi P, Krauskopf A, Basso E, Petronelly V, Blalchy-Dyson E, Di Lisa F, Forte MA. The mitochondrial permeability transition from in vitro artifact to disase target. FEBS J. 2006;273:2077–2099. doi: 10.1111/j.1742-4658.2006.05213.x. [DOI] [PubMed] [Google Scholar]
- 47.Kane GC, Behfar A, Dyer RB, O'Cochlain DF, Liu XK, Hodgson DM, Reyes S, Miki T, Seino S, Terzic A. KCNJ11 gene knockout of the Kir6.2 KATP channel causes maladaptive remodeling and heart failure in hypertension. Hum. Mol. Gen. 2006;15:2285–2297. doi: 10.1093/hmg/ddl154. [DOI] [PubMed] [Google Scholar]
- 48.Clarke SJ, McStay GP, Halestrap AP. Sanglifehrin A acts as a potent inhibitor of the mitochondrial permeability transition and reperfusion injury of the heart by binding to cyclophilin-D at a different site from cyclosporine. J. Biol. Chem. 2002;277:34793–34799. doi: 10.1074/jbc.M202191200. [DOI] [PubMed] [Google Scholar]
- 49.Irwin WA, Bergamin N, Sabatelli P, Reggiani C, Megighian A, Merlini L, Braghetta P, Columbaro M, Volpin D, Bressan GM, Bernardi P, Bonaldo P. Mitochondrial dysfunction and apoptosis in myopathic mice with collagen VI deficiency. Nat. Genet. 2003;35:367–371. doi: 10.1038/ng1270. [DOI] [PubMed] [Google Scholar]
- 50.Green DR, Kroemer G. The pathophysiology of mitochondrial cell death. Science. 2004;305:626–629. doi: 10.1126/science.1099320. [DOI] [PubMed] [Google Scholar]
- 51.Seppet EK, Kaambre T, Sikk P, Tiivel T, Vija H, Tonkonogi M, Sahlin K, Kay L, Appaix F, Braun U, Eimre M, Saks VA. Functional complexes of mitochondria with Ca,MgATPases of myofibrils and sarcoplasmic reticulum in muscle cells. Biochim. Biophys. Acta. 2001;1504:379–395. doi: 10.1016/s0005-2728(00)00269-3. [DOI] [PubMed] [Google Scholar]
- 52.Saks VA, Kaambre T, Sikk P, Eimre M, Orlova E, Paju K, Piirsoo A, Appaix F, Kay L, Regitz-Zagrosek V, Fleck E, Seppet E. Intracellular energetic units in red muscle cells. Biochem. J. 2001;326:643–657. doi: 10.1042/0264-6021:3560643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Saks VA, Kuznetsov AV, Vendelin M, Guerrero K, Kay L, Seppet EK. Functional coupling as a basic mechanism of feedback regulation of cardiac energy metabolism. Mol Cell Biochem. 2004;256/257:185–199. doi: 10.1023/b:mcbi.0000009868.92189.fb. [DOI] [PubMed] [Google Scholar]
- 54.Saks V, Dzeja P, Schlattner U, Vendelin M, Terzic A, Wallimann T. Cardiac system bioenergetics: metabolic basis of the Frank-Starling law. J. Physiol. 2006;571:253–273. doi: 10.1113/jphysiol.2005.101444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Anmann T, Eimre M, Kuznetsov AV, Andrienko T, Kaambre T, Sikk P, Seppet E, Tiivel T, Vendelin M, Seppet E, Saks VA. Calcium-induced contraction of sarcomeres changes the regultaion of mitochondrial respiration in permeabilized cardiac cells. FEBS J. 2005;272:3145–3161. doi: 10.1111/j.1742-4658.2005.04734.x. [DOI] [PubMed] [Google Scholar]
- 56.Neubauer S, Horn M, Pabst T, Godde M, Lubke D, Jilling B, Hahn D, Ertl G. Contributions of 31P-magnetic resonance spectroscopy to the understanding of dilated heart muscle disease. Eur. Heart J. 1995;16:115–118. doi: 10.1093/eurheartj/16.suppl_o.115. [DOI] [PubMed] [Google Scholar]
- 57.Ventura-Claper R, Garnier A, Veksler V. Energy metabolism in heart failure. J. Physiol. 2003;555:1–13. doi: 10.1113/jphysiol.2003.055095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.De Sousa E, Veksler V, Minajeva A, Kaasik A, Mateo P, Mayoux E, Hoerter J, Bigard X, Serrurier B, Ventura-Clapier R. Subcellular creatine kinase alterations. Implications in heart failure. Circ. Res. 1999;85:68–76. doi: 10.1161/01.res.85.1.68. [DOI] [PubMed] [Google Scholar]
- 59.Kaasik A, Minajeva A, De Sousa E, Ventura-Clapier R, Veksler V. Nitric oxide inhibits cardiac energy production via inhibition of mitochondrial creatine kinase. FEBS Lett. 1999;414:75–77. doi: 10.1016/s0014-5793(99)00033-2. [DOI] [PubMed] [Google Scholar]
- 60.Nascimben L, Ingwall JS, Pauletto P, Friedrich J, Gwathmey JK, Saks V, Pessina AC, Allen PD. Creatine kinase system in failing and nonfailing human myocardium. Circulation. 1996;94:1894–1901. doi: 10.1161/01.cir.94.8.1894. [DOI] [PubMed] [Google Scholar]
- 61.Ponticos M, Lu QL, Morgan JE, Hardie DG, Partridge TA, Carling D. Dual regulation of the AMP-activated protein kinase provides a novel mechanism for the control of creatine kinase in skeletal muscle. EMBO J. 1998;17:1688–1699. doi: 10.1093/emboj/17.6.1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dolder M, Walzel B, Speer O, Schlattner U, Wallimann T. Inhibition of the mitochondrial permeability transition by creatine kinase substrates. Requirement for microcompartmentation. J. Biol.Chem. 2003;278:17760–17766. doi: 10.1074/jbc.M208705200. [DOI] [PubMed] [Google Scholar]
- 63.Zoratti M, Szabo I. The mitochondrial permeability transition. Biochim. Biophys. Acta. 1995;1241:139–176. doi: 10.1016/0304-4157(95)00003-a. [DOI] [PubMed] [Google Scholar]
- 64.Gizatullina ZZ, Chen Y, Zierz S, Gellerich FN. Effects of extramitochondrial ADP on permeability transition of mouse liver mitochondria. Biochim. Biophys. Acta. 2005;1706:98–104. doi: 10.1016/j.bbabio.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 65.Gellerich FN. The role of adenylate kinase in dynamic compartmentation of adenine nucleotides in the mitochondrial inter membrane space. FEBS Lett. 1992;297:55–58. doi: 10.1016/0014-5793(92)80326-c. [DOI] [PubMed] [Google Scholar]
- 66.Pucar D, Janssen E, Dzeja PP, Juranic N, Macura S, Wieringa B, Terzic A. Compromised energetics in the adenylate kinase AK1 gene knockout heart under metabolic stress. J. Biol. Chem. 2000;275:41424–41429. doi: 10.1074/jbc.M007903200. [DOI] [PubMed] [Google Scholar]
- 67.Braun U, Paju K, Eimre M, Seppet E, Orlova E, Kadaja L, Trumbeckaite S, Gellerich FN, Zierz S, Jockusch H, Seppet EK. Lack of dystrophin is associated with altered integration of the mitochondria and ATPases in slow-twitch muscle cells of MDX mice. Biochim. Biophys. Acta. 2001;1505:258–270. doi: 10.1016/s0005-2728(01)00172-4. [DOI] [PubMed] [Google Scholar]
- 68.Kaasik A, Veksler V, Boehm E, Novotova M, Ventura-Clapier R. From energy store to energy flux: a study in creatine kinase-deficient fast skeletal muscle. FASEB J. 2003;17:708–710. doi: 10.1096/fj.02-0684fje. [DOI] [PubMed] [Google Scholar]
- 69.Panov AV, Burke JR, Strittmatter WJ, Greenamyre JT. In vitro effects of polyglutamine tracts on Ca2+-dependent depolarization of rat and human mitochondria: relevance to Huntington's disease. Arch. Biochem. Biophys. 2003;410:1–6. doi: 10.1016/s0003-9861(02)00585-4. [DOI] [PubMed] [Google Scholar]
- 70.Chang TWD, Rintoul LG, Pandipati S, Reynolds IJ. Mutant huntingtin aggregates impair mitochondrial movement and trafficking in cortical neurons. Neurobiol. Dis. 2006;22:388–400. doi: 10.1016/j.nbd.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 71.de Cerqueira Cesar M, Wilson JE. Functional characteristics of hexokinase bound to the type a and type B sites of bovine brain mitochondria. Arch. Biochem. Biophys. 2002;397:106–112. doi: 10.1006/abbi.2001.2639. [DOI] [PubMed] [Google Scholar]
- 72.Wagner G, Kovacs J, Löw P, Orosz F, Ovadi J. Tubulin and microtubule are potential targets for brain hexokinase binding. FEBS Lett. 2001;509:81–84. doi: 10.1016/s0014-5793(01)03136-2. [DOI] [PubMed] [Google Scholar]
- 73.Schlattner U, Forstner M, Eder M, Stachowiak O, Fritz-Wolf K, Wallimann T. Functional aspects of the X-ray structure of mitochondrial creatine kinase: a molecular physiology approach. Mol. Cell. Biochem. 1998;184:125–140. [PubMed] [Google Scholar]
- 74.Beutner G, Rück A, Riede B, Brdiczka D. Complexes between porin, hexokinase, mitochondrial creatine kinase and adenylate translocator display properties of the permeability transition pore. Implication for regulation of permeability transition by the kinases. Biochim. Biophys. Acta. 1998;1368:7–18. doi: 10.1016/s0005-2736(97)00175-2. [DOI] [PubMed] [Google Scholar]
- 75.Speer O, Bäck N, Buerklen T, Brdicka D, Koretsky A, Wallimann T, Eriksson O. Octameric mitochondrial creatine kinase induces and stabilizes contact sites between the inner and outer membrane. Biochem. J. 2005;385:445–450. doi: 10.1042/BJ20040386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.da-Silva WS, Gomez-Puyou A, de Gomez-Puyou MT, Moreno-Sanchez R, De Felice FG, de Meis L, Oliveira MF, Galina A. Mitochondrial bound hexokinase activity as a preventive antioxidant defense: steady-state ADP formation as a regulatory mechanism of membrane potential and reactive oxygen species generation in mitochondria. J. Biol. Chem. 2004;279:39846–39855. doi: 10.1074/jbc.M403835200. [DOI] [PubMed] [Google Scholar]
- 77.Meyer LE, Machado LB, Santiago AP, da-Silva WS, De Felice FG, Holub O, Oliveira MF, Galina A. Mitochondrial creatine kinase activity prevents reactive oxygen species generation: Antioxidant role of mitochondrial kinase-dependent ADP re-cycling activity. J. Biol. Chem. 2006;281:37361–37371. doi: 10.1074/jbc.M604123200. [DOI] [PubMed] [Google Scholar]
- 78.David S, Shoemaker M, Haley BE. Abnormal properties of creatine kinase in Alzheimer's disease brain: correlation of reduced enzyme activity and active site photolabeling with aberrant cytosol-membrane partitioning. Brain Res. Mol. Brain Res. 1998;54:276–287. doi: 10.1016/s0169-328x(97)00343-4. [DOI] [PubMed] [Google Scholar]
- 79.Wendt S, Dedeoglu A, Speer O, Wallimann T, Beal MF, Andreassen O. Reduced creatine kinase activity in transgenic amyotrophic lateral sclerosis mice. Free Rad. Biol. Med. 2002;32:920–926. doi: 10.1016/s0891-5849(02)00784-0. [DOI] [PubMed] [Google Scholar]
- 80.Halliwell B. Oxidative stress and neurodegeneration: where are we now. J Neurochem. 2006;97:1634–1658. doi: 10.1111/j.1471-4159.2006.03907.x. [DOI] [PubMed] [Google Scholar]
- 81.Kwong JQ, Beal MF, Manfredi G. The role of mitochondria in inherited neurodegenerative diseases. J. Neurochem. 2006;97:1659–1675. doi: 10.1111/j.1471-4159.2006.03990.x. [DOI] [PubMed] [Google Scholar]
- 82.Callahan LA, Supinski GS. Diaphragm and cardiac mitochondrial creatine kinases are impaired in sepsis. J. Appl. Physiol. 2007;102:44–53. doi: 10.1152/japplphysiol.01204.2005. [DOI] [PubMed] [Google Scholar]
- 83.Denton RM, McCormack JG. On the role of the calcium transport cycle in heart and other mammalian mitochondria. FEBS Lett. 1980;119:1–8. doi: 10.1016/0014-5793(80)80986-0. [DOI] [PubMed] [Google Scholar]
- 84.McCormack JG, Denton RM. The role of intramitochondrial Ca2+ in the regulation of oxidative phosphorylation in mammalian tissues. TIBS. 1986;11:258–262. doi: 10.1042/bst0210793. [DOI] [PubMed] [Google Scholar]
- 85.Carafoli E. Mitochondrial pathology: An overview. Ann. NY Acad. Sci. 1986;488:1–18. doi: 10.1111/j.1749-6632.1986.tb46544.x. [DOI] [PubMed] [Google Scholar]
- 86.Wussling MHP, Krannich K, Landgraf G, Herrmann-Frank A, Wiedenmann D, Gellerich FN, Podhaisky H. Sarcoplasmatic reticulum vesicles embedded in agarose gel exhibit propagating calcium waves. FEBS Lett. 1999;463:103–109. doi: 10.1016/s0014-5793(99)01595-1. [DOI] [PubMed] [Google Scholar]
- 87.Gunter TE, Pfeiffer DR. Mechanisms by which mitochondria transport calcium. Am. J. Physiol. 1990;258:C755–786. doi: 10.1152/ajpcell.1990.258.5.C755. [DOI] [PubMed] [Google Scholar]
- 88.Montero M, Alonso MT, Carnicero E, Cuchill-Ibanez I, Albillos A, Garcia AG, Garcua-Sancho J, Alvarez J. Chomaffin-cell stimulation triggers fast millimolar mitochondrial Ca2+ transients that modulate secretion. Nat Cell. Biol. 2000;2:57–61. doi: 10.1038/35000001. [DOI] [PubMed] [Google Scholar]
- 89.Nicchitta CV, Williamson JR. Spermine. A regulator of mitochondrial calcium cycling. J. Biol. Chem. 1984;259:12978–12983. [PubMed] [Google Scholar]
- 90.McCormack JG. Effects of spermine on mitochondrial Ca2+ transport and the ranges of extramitochondrial Ca2+ to which the matrix Ca2+-sensitive dehydrogenases respond. Biochem. J. 1989;264:167–174. doi: 10.1042/bj2640167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chalmers S, Nicholls DG. The relationship between free and total calcium concentrations in the matrix of liver and brain mitochondria. J. Biol. Chem. 2003;278:19062–19070. doi: 10.1074/jbc.M212661200. [DOI] [PubMed] [Google Scholar]
- 92.Bernardi P. Mitochondrial transport of cations: channels, exchangers, and permeability transition. Phys. Rev. 1999;79:1127–1155. doi: 10.1152/physrev.1999.79.4.1127. [DOI] [PubMed] [Google Scholar]
- 93.Korzeniewski B. Regulation of oxidative phosphorylation through parallel activation. Biophys. Chem. 2007;129:93–110. doi: 10.1016/j.bpc.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 94.Pardo B, Contreras L, Serrano A, Ramos M, Kobayashi K, Iijima M, Saheki T, Satrustegui J. Essential role of aralar in the transduction of small Ca2+ signals to neuronal mitochondria. J. Biol. Chem. 2006;281:1039–1047. doi: 10.1074/jbc.M507270200. [DOI] [PubMed] [Google Scholar]
- 95.Palmieri L, Pardo B, Lasorsa FM, del Arco A, Kobayashi K, Iijima M, Runswick MJ, Walker JE, Saheki T, Satrustegui J, Palmieri F. Citrin and aralar1 are Ca(2+)-stimulated aspartate/glutamate transporters in mitochondria. EMBO J. 2001;20:5060–5069. doi: 10.1093/emboj/20.18.5060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Molinari F, Raas-Rothschild A, Rio M, Fiermonte G, Encha-Razavi F, Palmieri L, Palmieri F, Ben-Neriah Z, Kadhom N, Vekemans M, Attie-Bitach T, Munnich A, Rustin P, Colleaux L. Impaired mitochondrial glutamate transport in autosomal recessive neonatal myoclonic epilepsy. Am. J. Hum. Genet. 2005;76:334–339. doi: 10.1086/427564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hoek JB, Njogu RM. The role of glutamate transport in the regulation of the pathway of proline oxidation in rat liver mitochondria. J. Biol.Chem. 1980;255:8711–8718. [PubMed] [Google Scholar]
- 98.Gincel D, Zaid H, Shoshan-Barmatz V. Calcium binding and translocation by the voltage-dependent anion channel: a possible regulatory mechanism in mitochondrial function. Biochem. J. 2001;358:147–155. doi: 10.1042/0264-6021:3580147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bernardi P, Veronese P, Petronilli V. Modulation of the mitochondrial cyclosporin A-sensitive permeability transition pore. I. Evidence for two separate Me2+ binding sites with opposing effects on the pore open probability. J. Biol.Chem. 1993;268:1005–1010. [PubMed] [Google Scholar]
- 100.Wellington CL, Ellerby LM, Gutekunst CA, Rogers D, Warby S, Graham RK, Loubser O, van Raamsdonk J, Singaraja R, Yang YZ, Gafni J, Bredesen D, Hersch SM, Leavitt BR, Roy S, Nicholson DW, Hayden MR. Caspase cleavage of mutant huntingtin precedes neurodegeneration in Huntington's disease. J. Neurosci. 2002;22:7862–7872. doi: 10.1523/JNEUROSCI.22-18-07862.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hackam AS, Singaraja R, Wellington CL, Metzler M, McCutcheon K, Zhang T, Kalchman M, Hayden MR. The influence of huntingtin protein size on nuclear localization and cellular toxicity. J. Cell. Biol. 1998;141:1097–105. doi: 10.1083/jcb.141.5.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Nosek MT, Dransfield DT, Aprille JR. Calcium stimulates ATP-Mg/Pi carrier activity in rat liver mitochondria. J. Biol. Chem. 1990;265:8444–8450. [PubMed] [Google Scholar]
- 103.Fiermonte G, De Leonardis F, Todisco S, Palmieri L, Lasorsa FM, Palmieri F. Identification of the mitochondrial ATP-Mg/Pi transporter. Bacterial expression, reconstitution, functional characterization, and tissue distribution. J. Biol. Chem. 2004;279:30722–30730. doi: 10.1074/jbc.M400445200. [DOI] [PubMed] [Google Scholar]
- 104.Hagen T, Lagace CJ, Modica-Napolitano JS, Aprille JR. Permeability transition in rat liver mitochondria is modulated by the ATP-Mg/Pi carrier. Am. J. Physiol. Gastrointest. Liver Physiol. 2003;285:G274–G281. doi: 10.1152/ajpgi.00052.2003. [DOI] [PubMed] [Google Scholar]
- 105.del Arco A, Satrustegui J. Identification of a novel human subfamily of mitochondrial carriers with calcium-binding domains. J. Biol. Chem. 2004;279:24701–24713. doi: 10.1074/jbc.M401417200. [DOI] [PubMed] [Google Scholar]
- 106.Litsky ML, Pfeiffer DR. Regulation of the mitochondrial Ca2+ uniporter by external adenine nucleotides: the uniporter behaves like a gated channel which is regulated by nucleotides and divalent cations. Biochemistry. 1997;36:7071–7080. doi: 10.1021/bi970180y. [DOI] [PubMed] [Google Scholar]
- 107.Igbavboa U, Pfeiffer DR. EGTA inhibits reverse uniport-dependent Ca2+ release from uncoupled mitochondria. Possible regulation of the Ca2+ uniporter by a Ca2+ binding site on the cytoplasmic side of the inner membrane. J. Biol. Chem. 1988;263:1405–1412. [PubMed] [Google Scholar]
- 108.Bathori G, Csordas G, Garcia-Perez C, Davies E, Hajnoczky G. Ca2+-dependent control of the permeability properties of the mitochondrial outer membrane and voltage-dependent anion-selective channel (VDAC) J. Biol. Chem. 2006;281:17347–17358. doi: 10.1074/jbc.M600906200. [DOI] [PubMed] [Google Scholar]
- 109.Rizzuto R, Pozzan T. Microdomains of intracellular Ca2+: molecular determinats and functional consequences. Physiol. Rev. 2006;86:369–408. doi: 10.1152/physrev.00004.2005. [DOI] [PubMed] [Google Scholar]
- 110.Rizzuto R, Simpson AW, Brini M, Pozzan T. Rapid changes of mitochondrial Ca2+ revealed by specifically targeted recombinant aequorin. Nature. 1992;358:325–327. doi: 10.1038/358325a0. [DOI] [PubMed] [Google Scholar]
- 111.Rizzuto R, Brini M, Murgia M, Pozzan T. Microdomains with high Ca2+ close to IP3-sensitive channels that are sensed by neighboring mitochondria. Science. 1993;262:744–747. doi: 10.1126/science.8235595. [DOI] [PubMed] [Google Scholar]
- 112.Ichas F, Jouaville LS, Mazat JP. Mitochondria are excitable organelles capable of generating and conveying electrical and calcium signals. Cell. 1997;89:1145–1153. doi: 10.1016/s0092-8674(00)80301-3. [DOI] [PubMed] [Google Scholar]
- 113.Landgraf G, Gellerich FN, Wussling MHP. Inhibitors of SERCA and mitochondrial Ca-uniporter decrease velocity of calcium waves in rat cardiomyocytes. Mol Cell Biochem. 2004;256/257:379–386. doi: 10.1023/b:mcbi.0000009883.71379.51. [DOI] [PubMed] [Google Scholar]
- 114.Wasniewska M, Karczmarewicz E, Pronicki M, Piekutowska Abramczak K, Zablocki K, Popowska E, Pronicka E, Duszynski J. Abnormal calcium homeostasis in fibroblasts from patients with Leigh disease. Biophys. Biochem. Res. Comm. 2001;283:687–693. doi: 10.1006/bbrc.2001.4834. [DOI] [PubMed] [Google Scholar]
- 115.Rubinsztein DC. The roles of intracellular protein-degradation pathways in neurodegeneration. Nature. 2006;443:780–786. doi: 10.1038/nature05291. [DOI] [PubMed] [Google Scholar]
- 116.Lansbury PT, Lashuel HA. A century-old debate on protein aggregation and neurodegeneration enters the clinic. Nature. 2006;443:774–779. doi: 10.1038/nature05290. [DOI] [PubMed] [Google Scholar]
- 117.Harjes P, Wanker EE. The hunt for huntingtin function: interaction partners tell many different stories. Trends. Biochem. Sci. 2003;28:425–433. doi: 10.1016/S0968-0004(03)00168-3. [DOI] [PubMed] [Google Scholar]
- 118.Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegeneration. Nature. 2006;443:787–795. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- 119.Ambrose CM, Duyao MP, Barnes G, Bates GP, Lin CS, Srinidhi J, Baxendale S, Hummerich H, Lehrach H, Altherr M. Structure and expression of the Huntington's disease gene: evidence against simple inactivation due to an expanded CAG repeat. Somat. Cell. Mol. Genet. 1994;20:27–38. doi: 10.1007/BF02257483. [DOI] [PubMed] [Google Scholar]
- 120.Li SH, Schilling G, Young WS, 3rd, Li XJ, Margolis RL, Stine OC, Wagster MV, Abbott MH, Franz ML, Ranen NG, et al. Huntington's disease gene (IT15) is widely expressed in human and rat tissues. Neuron. 1993;11:985–993. doi: 10.1016/0896-6273(93)90127-d. [DOI] [PubMed] [Google Scholar]
- 121.Langbehn DR, Brinkman RR, Falush D, Paulsen JS, Hayden MR, International Huntington's Disease Collaborative Group A new model for prediction of the age of onset and penetrance for Huntington's disease based on CAG length. Clin. Gen. 2004;65:276–277. doi: 10.1111/j.1399-0004.2004.00241.x. [DOI] [PubMed] [Google Scholar]
- 122.Vonsattel JP, DiFiglia MJ. Huntington disease. J. Neuropathol. Exp. Neurol. 1998;57:369–384. doi: 10.1097/00005072-199805000-00001. [DOI] [PubMed] [Google Scholar]
- 123.Browne SE, Beal MF. The energetics of Huntington’s disease. Neurochem. Res. 2004;29:531–546. doi: 10.1023/b:nere.0000014824.04728.dd. [DOI] [PubMed] [Google Scholar]
- 124.Djousse L, Knowlton, Cupples LA, Marder K, Shoulson I, Myers RH. Weight loss in early stage of Huntington’s disease. Neurologie. 2002;69:1325–1330. doi: 10.1212/01.wnl.0000031791.10922.cf. [DOI] [PubMed] [Google Scholar]
- 125.Sanberg PR, Fibiger HC, Mark RC. Body weight and dietary factors in Huntington’s disease patients compared with matched controls. Med. J. Aust. 1981;1:407–409. doi: 10.5694/j.1326-5377.1981.tb135681.x. [DOI] [PubMed] [Google Scholar]
- 126.Koroshetz WJ, Jenkins BG, Rosen BR, Beal MF. Energy metabolism defects in Huntington’s disease and effects of coenzyme Q10. Ann. Neurol. 1997;41:160–165. doi: 10.1002/ana.410410206. [DOI] [PubMed] [Google Scholar]
- 127.Grunewald T, Beal MF. Bioenergetics in Huntington’s disease. Ann. NY Acad. Sci. 1999;893:203–213. doi: 10.1111/j.1749-6632.1999.tb07827.x. [DOI] [PubMed] [Google Scholar]
- 128.Browne SE, Bowling AC, MacGarvey U, Baik MJ, Berger SC, Muqit MM, Bird ED, Beal MF. Oxidative damage and metabolic dysfunction in Huntington’s disease: selective vulnerability of the basal ganglia. Ann. Neurol. 1997;41:646–653. doi: 10.1002/ana.410410514. [DOI] [PubMed] [Google Scholar]
- 129.Tabrizi SJ, Cleeter MW, Xuereb J, Taanman JW, Cooper JM, Schapira AH. Biochemical abnormalities and excitotoxicity in Huntington’s disease brain. Ann Neurol. 1999;45:25–32. doi: 10.1002/1531-8249(199901)45:1<25::aid-art6>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 130.Goebel HH, Heipertz R, Scholz W, Iqbal K, Tellez-Nagel I. Juvenile Huntington chorea: clinical, ultrastructural, and biochemical studies. Neurology. 1978;28:23–31. doi: 10.1212/wnl.28.1.23. [DOI] [PubMed] [Google Scholar]
- 131.Tellez-Nagel I, Johnson AB, Terry RD. Studies on brain biopsies with Huntington’s chorea. J. Neuropathol. Exp. Neurol. 1974;33:308–332. doi: 10.1097/00005072-197404000-00008. [DOI] [PubMed] [Google Scholar]
- 132.Underwood BR, David Broadhurst D, Dunn WB, Ellis DI, Michell AW, Vacher C, Mosedale DE, Kell DB, Barker RA, Grainger DJ, Rubinsztein DC. Huntington disease patients and transgenic mice have similar pro-catabolic serum metabolite profiles. Brain. 2006;129:877–886. doi: 10.1093/brain/awl027. [DOI] [PubMed] [Google Scholar]
- 133.Panov AV, Gutekunst CA, Leavitt BR, Hayden MR, Burke JR, Strittmatter WJ, Greenamyre JT. Early mitochondrial calcium defects in Huntington's disease are a direct effect of polyglutamines. Nat. Neurosci. 2002;5:731–736. doi: 10.1038/nn884. [DOI] [PubMed] [Google Scholar]
- 134.Choo YS, Johnson GV, MacDonald M, Detloff PJ, Lesort M. Mutant huntingtin directly increases susceptibility of mitochondria to the calcium-induced permeability transition and cytochrome c release. Hum. Mol. Genet. 2004;13:1407–1420. doi: 10.1093/hmg/ddh162. [DOI] [PubMed] [Google Scholar]
- 135.Milakovic T, Quintanilla RA, Johnson GV. Mutant huntingtin expression induces mitochondrial calcium handling defects in clonal striatal cells: functional consequences. J. Biol. Chem. 2006;281:34785–34795. doi: 10.1074/jbc.M603845200. [DOI] [PubMed] [Google Scholar]
- 136.Rockabrand E, Slepko N, Pantalone A, Nukala VN, Kazantsev A, Marsh JL, Sullivan PG, Steffan JS, Sensi SL, Thompson LM. The first 17 amino acids of Huntingtin modulate its sub-cellular localization, aggregation and effects on calcium homeostasis. Hum. Mol. Genet. 2007;16:61–77. doi: 10.1093/hmg/ddl440. [DOI] [PubMed] [Google Scholar]
- 137.Brustovetsky N, LaFrance R, Purl KJ, Brustovetsky T, Keene CD, Low WC, Dubinsky JM. Age-dependent changes in the calcium sensitivity of striatal mitochondria in mouse models of Huntington's Disease. J. Neurochem. 2005;93:1361–1370. doi: 10.1111/j.1471-4159.2005.03036.x. [DOI] [PubMed] [Google Scholar]
- 138.Oliveira JM, Jekabsons MB, Chen S, Lin A, Rego AC, Goncalves J, Ellerby LM, Nicholls DG. Mitochondrial dysfunction in Huntington's disease: the bioenergetics of isolated and in situ mitochondria from transgenic mice. J. Neurochem. 2007;101:241–249. doi: 10.1111/j.1471-4159.2006.04361.x. [DOI] [PubMed] [Google Scholar]
- 139.Gizatullina ZZ, Chen Y, Lindenberg KS, Harjes P, Striggow F, Zierz S, Gellerich FN. Increased calcium sensitivity of respiration and of permeability transition in mitochondria of skeletal muscle in transgenic the R6/2mice with mouse model for Huntington disease. Ann. Neurol. 2006;59:407–411. [Google Scholar]
- 140.Kuznetsov AV, Veksler V, Gellerich FN, Saks V, Margreiter R, Kunz WS. Analysis of mitochondrial function in situ, in permeabilized muscles fibers, tissues and cells. Nature Protoc. 2008;3:965–976. doi: 10.1038/nprot.2008.61. [DOI] [PubMed] [Google Scholar]
- 141.Kosinski CM, Schlangen C, Gellerich FN, Gizatullina Z, Deschauer M, Schiefer J, Young AB, Landwehrmeyer GB, Toyka KV, Sellhaus B, Lindenberg KS. Myopathy as a first symptom of Huntington's disease in a marathon runner. Mov. Disord. 2007;22:637–640. doi: 10.1002/mds.21550. [DOI] [PubMed] [Google Scholar]
- 142.Bezprozvanny I, Hayden MR. Deranged neuronal calcium signaling and Huntington disease Biochem. Biophys. Res. Commun. 2004;322:1310–1317. doi: 10.1016/j.bbrc.2004.08.035. [DOI] [PubMed] [Google Scholar]
- 143.Mangiarini L, Sathasivam K, Seller M, Cozens B, Harper A, Hetherington C, Lawton M, Trottier Y, Lehrach H, Davies SW, Bates GP. Exon 1 of the HD gene with an expanded CAG repeat is sufficient to cause a progressive neurological phenotype in transgenic mice. Cell. 1996;87:493–506. doi: 10.1016/s0092-8674(00)81369-0. [DOI] [PubMed] [Google Scholar]
- 144.von Hörsten S, Schmitt I, Nguyen HP, Holzmann C, Schmidt T, Walther T, Bader M, Pabst R, Kobbe P, Krotova J, Stiller D, Kask A, Vaarmann A, Rathke-Hartlieb S, Schulz JB, Grasshoff U, Bauer I, Vieira-Saecker AM, Paul M, Jones L, Lindenberg KS, Landwehrmeyer B, Bauer A, Li XJ, Riess O. Transgenic rat model of Huntington's disease. Hum. Mol. Genet. 2003;12:617–624. doi: 10.1093/hmg/ddg075. [DOI] [PubMed] [Google Scholar]
- 145.Petrasch-Parwez E, Nguyen HP, Lobbecke-Schumacher M, Habbes HW, Wieczorek S, Riess O, Andres KH, Dermietzel R, Horsten S. Cellular and subcellular localization of Huntingtin [corrected] aggregates in the brain of a rat transgenic for Huntington disease. J. Comp. Neurol. 2007;501:716–730. doi: 10.1002/cne.21272. [DOI] [PubMed] [Google Scholar]
- 146.Grimm S, Brdiczka D. The permeability transition pore in cell death. Apoptosis. 2007;12:841–855. doi: 10.1007/s10495-007-0747-3. [DOI] [PubMed] [Google Scholar]
- 147.Wicker U, Bücheler K, Gellerich FN, Wagner M, Kapischke M, Brdiczka D. Effect of macromolecules on the structure of the mitochondrial inter-membrane space and the regulation of hexokinase. Biochim. Biophys. Acta. 1993;1142:228–239. doi: 10.1016/0005-2728(93)90151-5. [DOI] [PubMed] [Google Scholar]
- 148.Schapira AH, Cooper JM, Dexter D, Jenner P, Clark JB, Marsden CD. Mitochondrial complex I deficiency in Parkinson’s disease. Lancet. 1989;1:1269. doi: 10.1016/s0140-6736(89)92366-0. [DOI] [PubMed] [Google Scholar]
- 149.Schapira AH, Mann VM, Cooper JM, Dexter D, Daniel SE, Jenner P, Clark JB, Marsden CD. Anatomic and diseases specificity of NADH CoQ1 reductase (complex I deficiency) in Parkinson’s disease. J. Neurochem. 1990;55:2142–2145. doi: 10.1111/j.1471-4159.1990.tb05809.x. [DOI] [PubMed] [Google Scholar]
- 150.Krige D, Caroll MT, Cooper JM, Marsden CD, Schapira AH. Platelet mitochondrial function in Parkinson’s disease. The Royal Kings and Queens Parkinson Disease Research Group. Ann. Neurol. 1992;32:782–788. doi: 10.1002/ana.410320612. [DOI] [PubMed] [Google Scholar]
- 151.Parker WD, Jr, Boyson SJ, Parks JK. Abnormalities of the electron transport chain in idiopathic Parkinson’s disease. Ann. Neurol. 1989;26:719–723. doi: 10.1002/ana.410260606. [DOI] [PubMed] [Google Scholar]
- 152.Winkler Stuck K, Kirches E, Mawrin C, Dietzmann K, Lins H, Wallesch CW, Kunz WS, Wiedemann FR. Re-evaluation of the dysfunction of mitochondrial respiratory chain in skeletal muscle of patients with Parkinson’s disease. J. Neural. Transm. 2005;112:499–518. doi: 10.1007/s00702-004-0195-y. [DOI] [PubMed] [Google Scholar]
- 153.Tetrud JW, Langston JW. The effect of deprenyl (selegiline) on the natural history of Parkinson's disease. Science. 1989;245:519–522. doi: 10.1126/science.2502843. [DOI] [PubMed] [Google Scholar]
- 154.Betarbet R, Sherer TB, MacKenzie G, Garcia-Osuna M, Panov AV, Greenamyre JT. Chronic systemic pesticide exposure reproduces features of Parkinson's disease. Nat. Neurosci. 2000;3:1301–1306. doi: 10.1038/81834. [DOI] [PubMed] [Google Scholar]
- 155.McCormack AL, Thiruchelvam M, Manning-Bog AB, Thiffault C, Langston JW, Cory-Slechta DA, Di Monte DA. Environmental risk factors and Parkinson’s disease: selective degeneration of nigral dopaminergic neurons caused by the herbicide paraquat. Neurobiol. Dis. 2002;10:119–127. doi: 10.1006/nbdi.2002.0507. [DOI] [PubMed] [Google Scholar]
- 156.Dawson TM, Dawson VL. Neuroprotective and neurorestorative strategies for Parkinson’s disease. Nat. Neurosci. 2002;5:1058–1061. doi: 10.1038/nn941. [DOI] [PubMed] [Google Scholar]
- 157.Soto-Otero R, Sanmartin-Suarez C, Sanchez-Iglesias S, Hermida-Ameijeiras A, Sanchez-Sellero I, Mendez-Alvarez E. Study on the ability of 1,2,3,4-tetrahydropapaveroline to cause oxidative stress: Mechanisms and potential implications in relation to parkinson’s disease. J. Biochem. Mol. Toxicol. 2006;20:209–220. doi: 10.1002/jbt.20138. [DOI] [PubMed] [Google Scholar]
- 158.Simon DK, Pulst SM, Sutton JP, Browne SE, Beal MF, Johns DR. Familial multisystem degeneration with parkinsonism associated with the 11778 mitochondrial DNA mutation. Neurology. 1999;53:1787–1793. doi: 10.1212/wnl.53.8.1787. [DOI] [PubMed] [Google Scholar]
- 159.Luoma P, Melberg A, Rinne JO, Kaukonen JA, Nupponen NN, Chalmers RM, Oldfors A, Rautakorpi I, Peltonen L, Majamaa K, Somer H, Suomalainen A. Parkinsonism, premature menopause, and mitochondrial DNA polymerase gamma mutations: clinical and molecular genetic study. Lancet. 2004;364:875–882. doi: 10.1016/S0140-6736(04)16983-3. [DOI] [PubMed] [Google Scholar]
- 160.Song DD, Shults CW, Sisk A, Rockenstein E, Masliah E. Enhanced substantia nigra mitochondrial pathology in human alpha-synuclein transgenic mice after treatment with MPTP. Exp. Neurol. 2004;186:158–172. doi: 10.1016/S0014-4886(03)00342-X. [DOI] [PubMed] [Google Scholar]
- 161.Martin LJ, Pan Y, Price AC, Sterling W, Copeland NG, Jenkins NA, Price DL, Lee MK. Parkinson's disease alpha-synuclein transgenic mice develop neuronal mitochondrial degeneration and cell death. J. Neurosci. 2006;26:41–50. doi: 10.1523/JNEUROSCI.4308-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Klivenyi P, Siwek D, Gardian G, Yang L, Starkov A, Cleren C, Ferrante RJ, Kowall NW, Abeliovich A, Beal MF. Mice lacking alpha-synuclein are resistant to mitochondrial toxins. Neurobiol. Dis. 2006;21:541–548. doi: 10.1016/j.nbd.2005.08.018. [DOI] [PubMed] [Google Scholar]
- 163.Pesah Y, Pham T, Burgess H, Middlebrooks B, Verstreken P, Zhou Y, Harding M, Bellen H, Mardon G. Drosophila parkin mutants have decreased mass and cell size and increased sensitivity to oxygen radical stress. Development. 2004;131:2183–2194. doi: 10.1242/dev.01095. [DOI] [PubMed] [Google Scholar]
- 164.Palacino JJ, Sagi D, Goldberg MS, Krauss S, Motz C, Wacker M, Klose J, Shen J. Mitochondrial dysfunction and oxidative damage in parkin-deficient mice. J. Biol. Chem. 2004;279:18614–18622. doi: 10.1074/jbc.M401135200. [DOI] [PubMed] [Google Scholar]
- 165.Muftuoglu M, Elibol B, Dalmizrak O, Ercan A, Kulaksiz G, Ogus H, Dalkara T, Ozer N. Mitochondrial complex I and IV activities in leukocytes from patients with parkin mutations. Mov. Disord. 2004;19:544–548. doi: 10.1002/mds.10695. [DOI] [PubMed] [Google Scholar]
- 166.Darios F, Corti O, Lucking CB, Hampe C, Muriel MP, Abbas N, Gu WJ, Hirsch EC, Rooney T, Ruberg M, Brice A. Parkin prevents mitochondrial swelling and cytochrome c release in mitochondria-dependent cell death. Hum. Mol. Genet. 2003;12:517–526. doi: 10.1093/hmg/ddg044. [DOI] [PubMed] [Google Scholar]
- 167.Canet-Aviles RM, Wilson MA, Miller DW, Ahmad R, McLendon C, Bandyopadhyay S, Baptista MJ, Ringe D, Petsko GA, Cookson MR. The Parkinson's disease protein DJ-1 is neuroprotective due to cysteine-sulfinic acid-driven mitochondrial localization. Proc. Natl. Acad. Sci. USA. 2004;101:9103–9108. doi: 10.1073/pnas.0402959101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Kim RH, Smith PD, Aleyasin H, Hayley S, Mount MP, Pownall S, Wakeham A, You-Ten AJ, Kalia SK, Horne P, Westaway D, Lozano AM, Anisman H, Park DS, Mak TW. Hypersensitivity of DJ-1-deficient mice to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyrindine (MPTP) and oxidative stress. Proc. Natl. Acad. Sci. USA. 2005;102:5215–5220. doi: 10.1073/pnas.0501282102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Petit A, Kawarai T, Paitel E, Sanjo N, Maj M, Scheid M, Chen F, Gu Y, Hasegawa H, Salehi-Rad S, Wang L, Rogaeva E, Fraser P, Robinson B, St George-Hyslop P, Tandon A. Wild-type PINK1 prevents basal and induced neuronal apoptosis, a protective effect abrogated by Parkinson disease-related mutations. J. Biol. Chem. 2005;280:34025–34032. doi: 10.1074/jbc.M505143200. [DOI] [PubMed] [Google Scholar]
- 170.Deng H, Jankovic J, Guo Y, Xie W, Le W. Small interfering RNA targeting the PINK1 induces apoptosis in dopaminergic cells SH-SY5Y. Biochem. Biophys. Res. Commun. 2005;337:1133–1138. doi: 10.1016/j.bbrc.2005.09.178. [DOI] [PubMed] [Google Scholar]
- 171.Hald A, Lotharius J. Oxidative stress and inflammation in Parkinson’s disease: Is there a causal link? Exp. Neuro. 2005;193:179–290. doi: 10.1016/j.expneurol.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 172.Haddad JJ, Harb HL. Cytokines and the regulation of hypoxia-inducible factor (HIF)-1α. Int. Immunopharm. 2005;5:461–483. doi: 10.1016/j.intimp.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 173.Haddad JJ, Land SC. A non-hypoxic, ROS-sensitive pathway mediates TNF-α-dependent regulation of HIF-1α. FEBS Lett. 2001;505:269–274. doi: 10.1016/s0014-5793(01)02833-2. [DOI] [PubMed] [Google Scholar]
- 174.Bai Y, Onuma H, Bai X, Medvedev AV, Misukonis M, Weinberg JB, Cao W, Robidoux J, Floering LM, Daniel KW, Collins S. Persistent nuclear factor- κB activation in Ucp2−/− mice leads to enhanced nitric oxide and inflammatory cytokine production. J. Biol. Chem. 2005;280:19062–19069. doi: 10.1074/jbc.M500566200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Emre Y, Hurtaud C, Nübel T, Criscuolo F, Ricquier D, Cassard-Doulcier AM. Mitochondria contribute to LPS-induced MAPK activation via uncoupling protein UCP2 in macrophages. Biochem. J. 2007;402:271–278. doi: 10.1042/BJ20061430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Schulze-Osthoff K, Bakker AC, Vanhasebroeck B, Beyaert R, Jacob WA, Fiers W. Cytotoxic activity of tumor necrosis factor is mediated by early damage of mitochondrial functions. J. Biol. Chem. 1992;267:5317–5323. [PubMed] [Google Scholar]
- 177.Goossens V, Stange G, Moens K, Pipeleers D, Grooten J. Regulation of tumor necrosis factor-induced, mitochondria- and reactive oxygen species-dependent cell death by the electron flux through the electron transport chain complex I. Antioxid. Redox Signal. 1999;1:285–295. doi: 10.1089/ars.1999.1.3-285. [DOI] [PubMed] [Google Scholar]
- 178.Itoh S, Lemay S, Osawa M, Che W, Duan Y, Tompkins A, Brookes PS, Sheu SS, Abe JI. Mitochondrial Dok-4 recruits Src kinase and regulates NF-kB activation in endothelial cells. J. Biol. Chem. 2005;280:26383–26396. doi: 10.1074/jbc.M410262200. [DOI] [PubMed] [Google Scholar]
- 179.Le Moine O, Louis H, Stordeur P, Collet JM, Goldman M, Deviere J. Role of reactive oxygen intermediates in interleukin 10 release after cold liver ischemia and reperfusion in mice. Gastroenterology. 1997;113:1701–1706. doi: 10.1053/gast.1997.v113.pm9352875. [DOI] [PubMed] [Google Scholar]
- 180.Llorente L, Zou W, Levy Y, Richaud-Patin Y, Wijdenes J, Alcocer-Varele J, Morel-Fourrier B, Brouet JC, Alarcon-Segovia D, Galanaud P, Emilie D. Role of interleukin 10 in the B lymphocyte hyperactivity and antibody production of human systemic lupus erythematosus. J. Exp. Med. 1995;181:839–844. doi: 10.1084/jem.181.3.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Marumo T, Schini-Kerth VB, Fisslthaler B, Busse R. Platelet-derived growth factor-stimulated superoxide anion production modulates activation of transcription factor NF-kappaB and expression of monocyte chemoattractant protein 1 in human aortic smooth muscle cells. Circulation. 1997;96:2361–2367. doi: 10.1161/01.cir.96.7.2361. [DOI] [PubMed] [Google Scholar]
- 182.Mogensen TH, Melchjorsen J, Höllsberg P, Paludan SR. Activation of NF-κB invirus-infected macrophages is dependent on mitochondrial oxidative stress and intracellular calcium: Downstream involvement of the kinases TGF-β-activated kinase 1, mitogen activated kinase/extracellular signal-regulated kinase kinase 1, and IκB kinase. J. Immunol. 2003;170:6224–6233. doi: 10.4049/jimmunol.170.12.6224. [DOI] [PubMed] [Google Scholar]
- 183.Haynes V, Elfering S, Traaseth N, Giulivi C. Mitochondrial nitric-oxide synthase: enzyme expression, characterization, and regulation. J. Bioenerg. Biomembr. 2004;36:341–346. doi: 10.1023/B:JOBB.0000041765.27145.08. [DOI] [PubMed] [Google Scholar]
- 184.Trumbeckaite S, Opalka JR, Neuhof C, Zierz S, Gellerich FN. Different sensitivity of rabbit heart and skeletal muscle to endotoxin-induced impairment of mitochondrial function. Eur. J. Biochem. 2000;268:1422–1429. doi: 10.1046/j.1432-1327.2001.02012.x. [DOI] [PubMed] [Google Scholar]
- 185.Gellerich FN, Trumbeckaite S, Hertel K, Zierz S, Müller-Werdan U, Werdan K, Redl H, Schlag G. Impaired energy metabolism in hearts of septic baboons: diminished activities of complex I and complex II of the mitochondrial respiratory chain. Shock. 1999;11:336–341. [PubMed] [Google Scholar]
- 186.Bojunga J, Dresar-Mayert B, Usadel KH, Kusterer K, Zeuzem S. Antioxidative treatment reverses imbalances of nitric oxide synthase isoform expression and attenuates tissue-cGMP activation in diabetic rats. Biochem Biophys Res Commun. 2004;316:771–780. doi: 10.1016/j.bbrc.2004.02.110. [DOI] [PubMed] [Google Scholar]
- 187.Munoz-Fernandez MA, Fresno M. The role of tumour necrosis factor, interleukin 6, interferon-gamma and inducible nitric oxide synthase in the development and pathology of the nervous system. Prog. Neurobiol. 1998;56:307–340. doi: 10.1016/s0301-0082(98)00045-8. [DOI] [PubMed] [Google Scholar]
- 188.Anderson HD, Rahmutula D, Gardner DG. Tumor necrosis factor-a inhibits endothelial nitric-oxide synthase gene promoter activity in bovine aortic endothelial cells. J. Biol.Chem. 2004;279:963–969. doi: 10.1074/jbc.M309552200. [DOI] [PubMed] [Google Scholar]
- 189.Tatsumi T, Matoba S, Kawahara A, Keira N, Shiraishi J, Akashi K, Kobara M, Tanaka T, Katamura M, Nakagawa C, Ohta B, Shirayama T, Takeda K, Asayama J, Fliss H, Nakagawa M, et al. Cytokine-induced nitric oxide production inhibits mitochondrial energy production and impairs contractile function in rat cardiac myocytes. J Am Coll Cardiol. 2000;35:1338–1346. doi: 10.1016/s0735-1097(00)00526-x. [DOI] [PubMed] [Google Scholar]
- 190.Valerio A, Cardile A, Cozzi V, Bracale R, Tedesco L, Pisconti A, Palomba L, Cantoni O, Clement E, Moncada S, Carruba MO, Nisoli E. TNF-α downregulates eNOS expression and mitochondrial biogenesis in fat and muscle of obese rodents. J. Clin. Invest. 2006;116:2791–2798. doi: 10.1172/JCI28570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Galmiche A, Rassow J, Doye A, Cagnol S, Chambard JC, Contamin S, de Thillot V, Just I, Ricci V, Solcia E, Van Obberghen E, Boquet P. The N-terminal 34 kDa fragment of Helicobacter pylori vacuolating cytotoxin targets mitochondria and induces cytochrome c release. EMBO J. 2000;19:6361–6370. doi: 10.1093/emboj/19.23.6361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Ashktorab H, Frank S, Khaled AR, Durum SK, Kifle B, Smoot DT. Bax translocation and mitochondrial fragmentation induced by Helicobacter pylori. Gut. 2004;53:805–813. doi: 10.1136/gut.2003.024372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Kimura M, Goto S, Wada A, Yahiro K, Niidome T, Hatakeyama T, Aoyagi H, Hirayama T, Kondo T. Vacuolating cytotoxin purified from Helicobacter pylori causes mitochondrial damage in human gastric cells. Microb. Pathog. 1999;26:45–52. doi: 10.1006/mpat.1998.0241. [DOI] [PubMed] [Google Scholar]
- 194.Xia HH, Talley NJ. Apoptosis in gastric epithelium induced by Helicobacter pylori infection: Implications in gastric carcinogenesis. Am. J. Gastroenterol. 2001;96:16–26. doi: 10.1111/j.1572-0241.2001.03447.x. [DOI] [PubMed] [Google Scholar]
- 195.Jung HK, Lee KE, Chu SH, Yi SI. Reactive oxygen species activity, mucosal lipoperoxidation and glutathione in Helicobacter pylori-infected gastric mucosa. J. Gastroenterol. Hepatol. 2001;16:1336–1340. doi: 10.1046/j.1440-1746.2001.02647.x. [DOI] [PubMed] [Google Scholar]
- 196.Kubota Y, Kato K, Dairaku N, Koike T, Iijima K, Imatani A, Sekine H, Ohara S, Matsui H, Shimosegawa T. Contribution of glutamine synthetase to ammonia-induced apoptosis in gastric mucosal cells. Digestion. 2004;69:140–148. doi: 10.1159/000078152. [DOI] [PubMed] [Google Scholar]
- 197.Aulak KS, Koeck T, Crabb JW, Stuehr DJ. Dynamics of protein nitration in cells and mitochondria. Am. J. Physiol. 2004;286:H30–H38. doi: 10.1152/ajpheart.00743.2003. [DOI] [PubMed] [Google Scholar]
- 198.Lee JH, Yang ES, Park JW. Inactivation of NADP+-dependent isocitrate dehydrogenase by peroxynitrite. J. Biol. Chem. 2003;278:52360–52371. doi: 10.1074/jbc.M302332200. [DOI] [PubMed] [Google Scholar]
- 199.Cramer T, Yamanishi Y, Clausen BE, Förster I, Pawlinski R, Mackman N, Haase VH, Jaenisch R, Corr M, Nizet V, Firestein GS, Gerber HP, Ferrara N, Johnson RS. HIF-1α is essential for myeloid cell-mediated inflammation. Cell. 2003;112:645–657. doi: 10.1016/s0092-8674(03)00154-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Dewson G, Cohen GM, Wardlaw AJ. Interleukin-5 inhibits translocation of Bax to the mitochondria, cytochrome c release, and activation of caspoases in human eosinofils. Blood. 2001;98:2239–2247. doi: 10.1182/blood.v98.7.2239. [DOI] [PubMed] [Google Scholar]
- 201.Maianski NA, Mul FPJ, van Buul JD, Roos D, Kuijpers TW. Granulocyte colony-stimulating factor inhibits the mitochondria-dependent activation of caspase-3 in neutrophils. Blood. 2002;99:672–679. doi: 10.1182/blood.v99.2.672. [DOI] [PubMed] [Google Scholar]
- 202.Gergely P, Jr, Niland B, Gonchoroff N, Pullmann R, Jr, Phillips PE, Perl A. Persistent mitochondrial hyperpolarization, increased reactive oxygen intermediate production, and cytoplasmic alkalization characterize altered IL-10 signalling in patients with systemic lupus erythematosus. J. Immunol. 2002;169:1092–1101. doi: 10.4049/jimmunol.169.2.1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Perl A, Gergely P, Jr, Nagy G, Koncz A, Banki K. Mitochondrial hyperpolarization: a checkpoint of T-cell life, death and autoimmunity. Trends Immunol. 2004;25:360–367. doi: 10.1016/j.it.2004.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Fossati G, Moulding DA, Spiller DG, Moots RJ, White MRH, Edwads SW. The mitochondrial network of human neutrophils: Role in chemotaxis, phagocytosis, respiratory burst activation, and commitment of apoptosis. J. Immunol. 2003;170:1964–1972. doi: 10.4049/jimmunol.170.4.1964. [DOI] [PubMed] [Google Scholar]
- 205.Peachman KK, Lyles DS, Bass DA. Mitochondria in eosinophils: Functional role in apoptosis but not respiration. Proc. Natl. Acad. Sci. USA. 2001;98:1717–1722. doi: 10.1073/pnas.98.4.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Vats D, Mukundan L, Odegaard JI, Zhang L, Smith KL, Morel CR, Greaves DR, Murray PJ, Chawla A. Oxidative metabolism and PGC-1β attenuate macrophage-mediated inflammation. Cell Metab. 2006;4:13–24. doi: 10.1016/j.cmet.2006.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Lacy-Hulbert A, Moore KJ. Designer macrophages: oxidative metabolism fuels inflammation repair. Cell Metab. 2006;4:7–8. doi: 10.1016/j.cmet.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 208.Haden D, Suliman HB, Carraway MS, Welti-Wolf KE, Ali AS, Shitara H, Yonekawa H, Piantadosil CA. Mitochondrial biogenesis restores oxidative metabolism during Staphylococcus aureus sepsis. Am. J. Resp. Crit. Car. Med. 2007;176:768–777. doi: 10.1164/rccm.200701-161OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Baines CP, Song CX, Zheng YT, Wang GW, Zhang J, Wang OL, Guo Y, Bolli R, Cardwell EM, Ping P. Protein kinase cepsilon interacts with and inhibits the permeability transition pore in cardiac mitochondria. Circ. Res. 2003;92:873–880. doi: 10.1161/01.RES.0000069215.36389.8D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Baines CP, Zhang J, Wang GW, Zheng YT, Xiu JX, Cardwell EM, Bolli R, Ping P. Mitochondrial PKCe and MAPK form signaling modules in the murine heart: enhanced mitochondrial PKCe-MAPK interactions and differential MAPK activation in PKCe-induced cardioprotection. Circ. Res. 2002;90:390–397. doi: 10.1161/01.res.0000012702.90501.8d. [DOI] [PubMed] [Google Scholar]
- 211.Chen Q, Lin RY, Rubin CS. Organelle-specific targeting of protein kinase AII (PKAII): molecular and in situ characterization of murine A kinase anchor proteins that recruit regulatory subunits of PKAII to the cytoplasmic surface of mitochondria. J. Biol. Chem. 1997;272:15247–15257. doi: 10.1074/jbc.272.24.15247. [DOI] [PubMed] [Google Scholar]
- 212.Technikova-Dobrova Z, Sardanelli AM, Speranza F, Scacco S, Signorile A, Lorusso V, Papa S. Cyclic adenosine monophosphate–dependent phosphorylation of mammalian mitochondrial proteins: enzyme and substrate characterization and functional role. Biochemistry. 2001;4:13941–13947. doi: 10.1021/bi011066p. [DOI] [PubMed] [Google Scholar]
- 213.Harada H, Becknell B, Wilm M, Mann M, Huang LJ, Taylor SS, Scott JD, Korsmeyer SJ. Phosphorylation and inactivation of BAD by mitochondria-anchored protein kinase A. Mol. Cell. 1999;3:413–422. doi: 10.1016/s1097-2765(00)80469-4. [DOI] [PubMed] [Google Scholar]
- 214.Hoppeler H, Vogt M, Weibel ER, Fluck M. Response of skeletal muscle to hypoxia. Exp. Physiol. 2003;88:109–119. doi: 10.1113/eph8802513. [DOI] [PubMed] [Google Scholar]
- 215.Höckel M, Vaupel P. Tumor hypoxia: definitions and current clinical, biological, and molecular aspects. J. Natl. Cancer Inst. 2001;93:266–76. doi: 10.1093/jnci/93.4.266. [DOI] [PubMed] [Google Scholar]
- 216.Guzy RD, Schumacker PT. Oxygen sensing by mitochondria at complex III: The paradox of increased reactive oxygen species during hypoxia. Exp. Physiol. 2006;91:807–819. doi: 10.1113/expphysiol.2006.033506. [DOI] [PubMed] [Google Scholar]
- 217.Chandel NS, McClintock DS, Feliciano CE, Wood TM, Melendez JA, Rodriguez AM, Schumacker PT. Reactive oxygen species generated at mitochondrial complex III stabilize hypoxia-inducible factor-1α during hypoxia. J. Biol. Chem. 2000;275:25130–25138. doi: 10.1074/jbc.M001914200. [DOI] [PubMed] [Google Scholar]
- 218.Brunelle JK, Bell EL, Quesada NM, Vercauteren K, Tiranti V, Zeviani M, Scarpulla RC, Chandel NS. Oxygen sensing requires mitochondrial ROS but not oxidative phosphorylation. Cell Metab. 2005;1:409–414. doi: 10.1016/j.cmet.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 219.Mansfield KD, Guzy RD, Pan Y, Young RM, Cash TP, Schumacker PT, Simon MC. Mitochondrial dysfunction resulting from loss of cytochrome c impairs cellular oxygen sensing and hypoxic HIF-α activation. Cell Metab. 2005;1:393–399. doi: 10.1016/j.cmet.2005.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Sanjuán-Pla A, Cervera AM, Apostolova N, Garcia-Bou R, Victor VM, Murphy MP, McCreath KJ. A targeted antioxidant reveals the importance of mitochondrial reactive oxygen species in the hypoxic signaling of HIF-1α. FEBS Lett. 2005;579:2669–2674. doi: 10.1016/j.febslet.2005.03.088. [DOI] [PubMed] [Google Scholar]
- 221.Emerling BM, Platanias LC, Black E, Nebreda AR, Davies RJ, Chandel NS. Mitochondrial reactive oxygen species activation of p38 mitogen-activated protein kinase is required for hypoxia signaling. Mol. Cell. Biol. 2005;25:4853–4862. doi: 10.1128/MCB.25.12.4853-4862.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Semenza GL. HIF-1 and tumor progression: pathophysiology and therapeutics. Trends Mol. Med. 2002;8:S62–S67. doi: 10.1016/s1471-4914(02)02317-1. [DOI] [PubMed] [Google Scholar]
- 223.Iyer NV, Kotch LE, Agani F, Leung SW, Laughner E, Wenger RH, Gassmann M, Gearhart JD, Lawler AM, Yu AY, Semenza GL. Cellular and developmental control of O2 homeostasis by hypoxia-inducible factor 1 alpha. Genes Dev. 1998;12:149–162. doi: 10.1101/gad.12.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Gerald D, Berra E, Frapart YM, Chan DA, Giaccia AJ, Mansuy D, Pouyssegur J, Yaniv M, Mechta-Grigoriou F. JunD reduces tumor angiogenesis by protecting cells from oxidative stress. Cell. 2004;118:781–794. doi: 10.1016/j.cell.2004.08.025. [DOI] [PubMed] [Google Scholar]
- 225.Papandreou I, Cairns RA, Fontana L, Lim AL, Denko NC. HIF-1 mediates adaptation to hypoxia by actively downregulating mitochondrial oxygen consmption. Cell Metab. 2006;3:187–197. doi: 10.1016/j.cmet.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 226.Kim JW, Tchernyshyov I, Semenza GL, Dang CV. HIF-1 mediated expression of pyruvate. Metabolism. 2006;3:177–185. doi: 10.1016/j.cmet.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 227.Fukuda R, Zhang H, Kim JW, Shimoda L, Dang CV, Semenza GL. HIF-1 regulates cytochrome oxidase subunits to optimize efficiency of respiration in hypoxic cells. Cell. 2007;129:111–122. doi: 10.1016/j.cell.2007.01.047. [DOI] [PubMed] [Google Scholar]
- 228.Neumann AK, Yang J, Biju M, Joseph SK, Johnson RS, Haase VH, Freedman BD, Turka LA. Hypoxia inducible factor 1α regulates T cell receptor signal transduction. Proc. Natl. Acad. Sci. USA. 2005;102:17071–17076. doi: 10.1073/pnas.0506070102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Huang Y, Hickey RP, Yeh JL, Liu D, Dadak A, Young LH, Johnson RS, Giordano FJ. Cardiac myocyte-specific HIF-1α deletion alters vascularization, energy availabilty, calcium flux, and contractility in the normoxic heart. FASEB J. 2004;18:1138–1140. doi: 10.1096/fj.04-1510fje. [DOI] [PubMed] [Google Scholar]
- 230.Kahn BB, Alquier T, Carling D, Hardie DG. AMP-activated protein kionase: Ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab. 2005;1:15–25. doi: 10.1016/j.cmet.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 231.Reznick RM, Shulman GI. The role of AMP-activated protein kinase in mitochondrial biogenesis. J. Physiol. 2006;574:33–39. doi: 10.1113/jphysiol.2006.109512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Tzatsos A, Tsichlis PN. Energy depletion inhibits phosphatidylinositol 3-kinase/Akt signaling and induces apoptosis via AMP-activated protein kinase-dependent phosphorylation of IRS-1 at Ser-794. J. Biol. Chem. 2007;282:18069–18082. doi: 10.1074/jbc.M610101200. [DOI] [PubMed] [Google Scholar]
- 233.Capano M, Crompton M. Bax translocates to mitochondria of heart cells during simulated ischaemia: involvement of AMP-activated and p38 mitogen-activated protein kinases. Biochem. J. 2006;395:57–64. doi: 10.1042/BJ20051654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Zörning M, Hueber AO, Baum W, Evan G. Apoptosis regulators and their role in tumorigenesis. Biochim. Biophys. Acta. 2001;1551:F1–F37. doi: 10.1016/s0304-419x(01)00031-2. [DOI] [PubMed] [Google Scholar]
- 235.Reed JC. Mechanisms of apoptosis avoidance in cancer. Curr. Opinion. Oncol. 1999;11:68–75. doi: 10.1097/00001622-199901000-00014. [DOI] [PubMed] [Google Scholar]
- 236.Burgart LJ, Zheng J, Shu Q, Shibata D. Somatic mitochondrial mutation in gastric cancer. Am. J. Pathol. 1995;147:1105–1111. [PMC free article] [PubMed] [Google Scholar]
- 237.Didelot C, Barberi-Heyob M, Bianchi A, Becuwe P, Mirjolet JF, Dauca M, Merlin JL. Constitutive NF-kappaB activity influences basal apoptosis and radiosensitivity of head-and-neck carcinoma cell lines. Int. J. Radiat. Oncol. Biol. Phys. 2001;51:1354–1360. doi: 10.1016/s0360-3016(01)02608-6. [DOI] [PubMed] [Google Scholar]
- 238.Takeda Y, Togashi H, Matsuo T, Shinzawa H, Takeda Y, Takahashi T. Growth inhibition and apoptosis of gastric cancer cell lines by Anemarrhena asphodeloides Bunge. J. Gastroenterol. 2001;36:79–90. doi: 10.1007/s005350170135. [DOI] [PubMed] [Google Scholar]
- 239.Eapen CE, Madesh M, Balasubramanian K, Pulimood A, Mathan M, Ramakrishna BS. Mucosal mitochondrial function and antioxidant defences in patients with gastric carcinoma. Scand. J. Gastroenterol. 1998;33:975–981. doi: 10.1080/003655298750027010. [DOI] [PubMed] [Google Scholar]
- 240.Carretero J, Obrador E, Pellicer JA, Pascual A, Estrela JM. Mitochondrial glutathione depletion by glutamine in growing tumor cells. Free Rad. Biol. Med. 2000;29:913–923. doi: 10.1016/s0891-5849(00)00392-0. [DOI] [PubMed] [Google Scholar]
- 241.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 242.Wu M, Neilson A, Swift AL, Moran R, Tamagnine J, Parslow D, Armistead S, Lemire K, Orrell J, Teich J, Chomicz S, Ferrick DA. Multiparameter metabolic analysis reveals a close link between attenuated mitochondrial bioenergetic function and enhanced glycolysis dependency in human tumor cells. Am. J. Physiol. 2007;292:C125–C136. doi: 10.1152/ajpcell.00247.2006. [DOI] [PubMed] [Google Scholar]
- 243.Dang CV, Semenza GL. Oncogenic alterations of metabolism. Trends. Biochem. 1999;24:68–72. doi: 10.1016/s0968-0004(98)01344-9. [DOI] [PubMed] [Google Scholar]
- 244.Semenza GL, Artemov D, Bedi A, Bhujwalla Z, Chiles K, Feldser D, Laughner E, Ravi R, Simons J, Taghavi P, Zhong H. ‘The metobolism of tumors’: 70 years later. Novartis Found. Symp. 2001;240:251–260. [PubMed] [Google Scholar]
- 245.Fantin VR, St-Pierre J, Leder P. Attenuation of LDH-A expression uncovers a link between glycolysis, mitochondrial physiology, and tumor maintenance. Cancer Cell. 2006;9:426–434. doi: 10.1016/j.ccr.2006.04.023. [DOI] [PubMed] [Google Scholar]
- 246.Matoba S, Kang JG, Patino WD, Wragg A, Boehm M, Gavrilova O, Hurley PJ, Bunz F, Hwang PM. P53 regulates mitochondrial respiration. Science. 2006;312:1650–1653. doi: 10.1126/science.1126863. [DOI] [PubMed] [Google Scholar]
- 247.Shim H, Dolde C, Lewis BC, Wu CS, Dang G, Jungmann RA, Dalla-Favera R, Dang CV. C-Myc transactivation of LDH-A: implications for tumor metabolism and growth. Proc. Natl. Acad. Sci. USA. 1997;94:6658–6663. doi: 10.1073/pnas.94.13.6658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Dang CV. The interplay between MYC and HIF in the Warburg effect. Ernst Schering Found. Symp. Proc. 2007;4:35–53. doi: 10.1007/2789_2008_088. [DOI] [PubMed] [Google Scholar]
- 249.Lu H, Dalgard CL, Mohyeldin A, McFate T, Tait AS, Verma A. Reversible Inactivation of HIF-1 Prolyl Hydroxylases Allows Cell Metabolism to Control Basal HIF-1a. J. Biol. Chem. 2005;280:41928–41939. doi: 10.1074/jbc.M508718200. [DOI] [PubMed] [Google Scholar]
- 250.McFate T, Mohyeldin A, Lu H, Thakar J, Henriques J, Halim ND, Wu H, Schell MJ, Tsang TM, Teahan O, Zhou S, Califano JA, Jeoung NH, Harris RA, Verma A. Pyruvate dehydrogenase complex activity controls metabolic and malignant phenotype in cancer cells. J. Biol.Chem. 2008;283:22700–22708. doi: 10.1074/jbc.M801765200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Dalgard CL, Lu H, Mohyeldin A, Verma A. Endogenous 2-oxoacids differentially regulate expression of oxygen sensors. Biochem. J. 2004;380:419–424. doi: 10.1042/BJ20031647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Baysal BE, Ferrell RE, Willett-Brozick JE, Lawrence EC, Myssiorek D, Bosch A, van der Mey A, Taschner PE, Rubinstein WS, Myers EN, Richard CW, 3rd, Cornelisse CJ, Devilee P, Devlin B, et al. Mutations in SDHD, a mitochondrial complex II gene, in hereditary paraganglioma. Science. 2000;287:848–851. doi: 10.1126/science.287.5454.848. [DOI] [PubMed] [Google Scholar]
- 253.Briere J, Favier J, Benit P, El Ghouzzi V, Lorenzato A, Babier D, Di Renzo MF, Gimenez-Roqueplo AP, Rustin P. Mitochondrial succinate is instrumental for HIF1a nuclear translocation in SDHA-mutant fibroblasts under normoxic conditions. Human Mol Gen. 2005;14:3263–3268. doi: 10.1093/hmg/ddi359. [DOI] [PubMed] [Google Scholar]
- 254.Selak MA, Armour SM, MacKenzie ED, Boulahbel H, Watson DG, Mansfield KD, Pan Y, Simon MC, Thompson CB, Gottlieb E. Succinate links TCA cycle dysfunction to oncogenesis by inhibiting HIF-alpha prolyl hydroxylase. Cancer Cell. 2005;7:77–85. doi: 10.1016/j.ccr.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 255.Gottlieb E, Tomlinson IP. Mitochondrial tumor suppressors: a genetic and biochemical update. Nat. Rev. Cancer. 2005;5:857–866. doi: 10.1038/nrc1737. [DOI] [PubMed] [Google Scholar]
- 256.Levine AJ, Feng Z, Mak TW, You H, Jin S. Coordination and communication between the p53 and IGF-1-AKT-TOR signal transduction pathways. Genes Dev. 2006;20:267–275. doi: 10.1101/gad.1363206. [DOI] [PubMed] [Google Scholar]
- 257.Kondoh H, Lleonart ME, Gil J, Wang J, Degan P, Peters G, Martinez D, Carnero A, Beach D. Glycolytic enzymes can modulate cellular life span. Cancer Res. 2005;65:177–185. [PubMed] [Google Scholar]
- 258.Jones RG, Plas DR, Kubek S, Buzzai M, Mu J, Xu Y, Birnbaum MJ, Thompson CB. AMP-activated protein kinase induces a p53-dependent metabolic checkpoint. Mol Cell. 2005;18:283–293. doi: 10.1016/j.molcel.2005.03.027. [DOI] [PubMed] [Google Scholar]
- 259.Buzzai M, Jones RG, Amaravadi RK, Lum JJ, DeBerardinis RJ, Zhao F, Viollet B, Thompson C. Systemic treatment with the antidiabetic drug metformin selectively impairs p53-deficient tumor cell growth. Cancer Res. 2007;67:6745–6752. doi: 10.1158/0008-5472.CAN-06-4447. [DOI] [PubMed] [Google Scholar]
- 260.Ainscow EK, Zhao C, Rutter GA. Acute overexpression of lactate dehydrogenase-A perturbs b-cell mitochondrial metabolism and insulin secretion. Diabetes. 2000;49:1149–1155. doi: 10.2337/diabetes.49.7.1149. [DOI] [PubMed] [Google Scholar]
- 261.Golshani-Hebroni SG, Bessman SP. Hexokinase binding to mitochondria: a basis for proliferative energy metabolism. J. Bioenerg. Biomembr. 1997;29:331–338. doi: 10.1023/a:1022442629543. [DOI] [PubMed] [Google Scholar]
- 262.Pelicano H, Xu R, Du M, Feng L, Sasaki R, Carew JS, Hu Y, Ramdas L, Hu L, Keating MJ, Zhang W, Plunkett W, Huang P. Mitochondrial respiration defects in cancer cells cause activation of Akt survival pathway through a redox-mediated mechanism. J. Cell. Biol. 2006;175:913–923. doi: 10.1083/jcb.200512100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Tomiyama A, Serizawa S, Tachibana K, Sakurada K, Samejima H, Kuchino Y, Kitanaka C. Critical role for mitochondrial oxidative phosphorylation in the activation of tumor suppressors Bax and Bak. J Natl Cancer Inst. 2006;98:1462–1473. doi: 10.1093/jnci/djj395. [DOI] [PubMed] [Google Scholar]
- 264.Chandra D, Liu JW, Tang DG. Early mitochondrial activation and cytochrome c upregulation during apoptosis. J. Biol. Chem. 2004;277:50842–50854. doi: 10.1074/jbc.M207622200. [DOI] [PubMed] [Google Scholar]
- 265.Kwong JQ, Henning MS, Starkov AA, Manfredi G. The mitochondrial respiratory chain is a modulator of apoptosis. J. Cell Biol. 2007;179:1163–1177. doi: 10.1083/jcb.200704059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Miyashita T, Reed JC. Tumor suppressor p53 is a direct transcriptional activator of the human bax gene. Cell. 1995;80:293–299. doi: 10.1016/0092-8674(95)90412-3. [DOI] [PubMed] [Google Scholar]
- 267.Miyashita T, Harigai M, Hanada M, Reed JC. Identification of a p53-dependent negative response element in the bcl-2 gene. Cancer Res. 1994;54:3131–3135. [PubMed] [Google Scholar]
- 268.Oda E, Ohki R, Murasawa H, Nemoto J, Shibue T, Yamashita T, Tokino T, Taniguchi T, Tanaka N. Noxa, a BH3-only member of the Bcl-2 family and candidate mediator of p53-induced apoptosis. Science. 2000;288:1053–1058. doi: 10.1126/science.288.5468.1053. [DOI] [PubMed] [Google Scholar]
- 269.Oda K, Arakawa H, Tanaka T, Matsuda K, Tanikawa C, Mori T, Nishimori H, Tamai K, Tokino T, Nakamura Y, Taya Y. P53AIP1, a potential mediator of p53-dependent apoptosis, and its regulation by Ser-46-phosphorylated p53. Cell. 2000;102:849–862. doi: 10.1016/s0092-8674(00)00073-8. [DOI] [PubMed] [Google Scholar]
- 270.Nakano K, Vousden KH. PUMA, a novel proapoptotic gene, is induced by p53. Mol. Cell. 2001;7:683–694. doi: 10.1016/s1097-2765(01)00214-3. [DOI] [PubMed] [Google Scholar]
- 271.Hoffman WH, Biade S, Zilfou JT, Chen J, Murphy M. Transcriptional repression of the anti-apoptotic survivin gene by wild type p53. J. Biol. Chem. 2002;277:3247–3257. doi: 10.1074/jbc.M106643200. [DOI] [PubMed] [Google Scholar]
- 272.Zhao Y, Chaiswing L, Velez JM, Batinic-Haberle I, Colburn NH, Oberley TD, St Clair DK. p53 translocation to mitochondria precedes its nuclear translocation and targets mitochondrial oxidative defense protein-manganese superoxide dismutase. Cancer Res. 2005;65:3745–375. doi: 10.1158/0008-5472.CAN-04-3835. [DOI] [PubMed] [Google Scholar]
- 273.Pietsch EC, Perchiniak E, Canutescu AA, Wang G, Dunbrack RL, Murphy ME. Oligomerization of BAK by p53 utilizes conserved residues of the p53 DNA binding domain. J. Biol. Chem. 2008;283:21294–21304. doi: 10.1074/jbc.M710539200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Li PF, Dietz R, von Harsdorf R. P53 regulates mitochondrial membrane potential through reactive oxygen species and induces cytochrome c-independent aoptosis blocked by Bcl-2. EMBO J. 1999;18:6027–6036. doi: 10.1093/emboj/18.21.6027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Marchenko ND, Zaika AI, Moll UM. Death signal induced localization of p53 protein to mitochondria. A potential role in apoptotic signaling. J. Biol. Chem. 2000;275:16202–16212. doi: 10.1074/jbc.275.21.16202. [DOI] [PubMed] [Google Scholar]
- 276.Erster S, Mihara M, Kim RH, Petrenko O, Moll UM. In vivo mitochondrial p53 translocation triggers a rapid first wave of cell death in response to DNA damage that can precede p53 target gene activation. Mol Cell Biol. 2004;15:6728–6741. doi: 10.1128/MCB.24.15.6728-6741.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Schuler M, Bossy-Wetzel E, Goldstein JC, Fitzgerald P, Green DR. P53 induces apoptosis by caspase activation through mitochondrial cytochrome c release. J. Biol. Chem. 2000;275:7337–7342. doi: 10.1074/jbc.275.10.7337. [DOI] [PubMed] [Google Scholar]
- 278.Ding HF, Lin YL, McGill G, Juo P, Zhu H, Blenis J, Yuan J, Fisher DE. Essential role for caspase-8 in transcription-independent apoptosis triggered by p53. J. Biol. Chem. 2000;275:38905–38911. doi: 10.1074/jbc.M004714200. [DOI] [PubMed] [Google Scholar]
- 279.Simonnet H, Demont J, Pfeiffer K, Guenaneche L, Bouvier R, Brandt U, Schagger H, Godinot C. Mitochondrial complex I is deficient in renal oncocytomas. Carcinogenesis. 2003;24:1461–1466. doi: 10.1093/carcin/bgg109. [DOI] [PubMed] [Google Scholar]
- 280.Bonora E, Porcelli AM, Gasparre G, Biondi A, Ghelli A, Carelli V, Baracca A, Tallini G, Martinuzzi A, Lenaz G, Rugolo M, Romeo G. Defective oxidative phosphorylation in thyroid oncocytic carcinoma is associated with pathogenic mitochondrial DNA mutations affecting complexes I and III. Cancer Res. 2006;66:6087–6096. doi: 10.1158/0008-5472.CAN-06-0171. [DOI] [PubMed] [Google Scholar]
- 281.Petros JA, Baumann AK, Ruiz-Pesini E, Amin MB, Sun CQ, Hall J, Lim S, Issa MM, Flanders WD, Hosseini SH, Marshall FF, Wallace DC. mtDNA mutations increase tumorigenicity in prostate cancer. Proc. Natl. Acad. Sci. USA. 2005;102:719–724. doi: 10.1073/pnas.0408894102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Boitier F, Merad-Boudina M, Guguen-Guillouzo C, Defer N, Ceballos-Picot I, Leroux JP, Marsac C. Impairment of the mitochondrial respiratory chain activity in diethylnitrosamine-induced rat hepatomas: possible involvement of oxygen free radicals. Cancer Res. 1995;55:3028–3035. [PubMed] [Google Scholar]
- 283.Ray S, Ray M. Does excessive adenosine 5′-triphosphate formation in cells lead to malignancy? A hypothesis on cancer. Medical Hypotheses. 1997;48:473–476. doi: 10.1016/s0306-9877(97)90113-4. [DOI] [PubMed] [Google Scholar]
- 284.Capuano F, Varone D, D'Eri N, Russo E, Tommasi S, Montemurro S, Prete F, Papa S. Oxidative phosphorylation and F(O)F(1) ATP synthase activity of human hepatocellular carcinoma. Biochem. Mol. Biol. Int. 1996;38:1013–1022. [PubMed] [Google Scholar]
- 285.Green DW, Grover GJ. The IF(1) inhibitor protein of the mitochondrial F(1)F(0)-ATPase. Biochim. Biophys. Acta. 2000;1458:343–355. doi: 10.1016/s0005-2728(00)00085-2. [DOI] [PubMed] [Google Scholar]
- 286.Bravo C, Minauro-Sanmiguel F, Morales-Rios E, Rodriguez-Zavala JS, Garcia JJ. Overexpression of the inhibitor protein IF(1) in AS-30D hepatoma produces a higher association with mitochondrial F(1)F(0) ATP synthase compared to normal rat liver: functional and cross-linking studies. J. Bioenerg. Biomembr. 2004;36:257–264. doi: 10.1023/b:jobb.0000031977.99479.ea. [DOI] [PubMed] [Google Scholar]
- 287.Cuezva JM, Krajewska M, de Heredia ML, Krajewski S, Santamaria G, Kim H, Zapata JM, Maruzawa H, Chamorro M, Reed JC. The bioenergetic signature of cancer: a marker of tumor progression. Cancer Res. 2002;62:6674–6681. [PubMed] [Google Scholar]
- 288.Cuezva JM, Chen G, Alonso AM, Isidoro A, Misek DE, Hanash SM, Beer DG. The bioenergetic signature of lung adenocarcinomas is a molecular marker of cancer diagnosis and prognosis. Carcinogenesis. 2004;25:1157–1163. doi: 10.1093/carcin/bgh113. [DOI] [PubMed] [Google Scholar]
- 289.Isodoro A, Casado E, Redondo A, Acebo P, Espinosa E, Alonso AM, Cejas P, Hardisson D, Fresno Vara JA, Belda-Iniesta C, Gonzales-Baron M, Cuezva JM. Breast carcinomas fulfill the Warburg hypothesi and provide metabolic markers of cancer prognosis. Carcinogenesis. 2005;26:2095–2104. doi: 10.1093/carcin/bgi188. [DOI] [PubMed] [Google Scholar]
- 290.Simonnet H, Alazard N, Pfeiffer K, Gallou C, Beroud C, Demont J, Bouvie R, Schagger H, Godinot C. Low mitochondrial respiratory chain content correlates with tumor aggressiveness in renal cell carcinoma. Carcinogenesis. 2002;23:759–768. doi: 10.1093/carcin/23.5.759. [DOI] [PubMed] [Google Scholar]
- 291.Kuhnt T, Pelz T, Qu X, Hansgen G, Dunst J, Gellerich FN. Mitochondrial OXPHOS Functions in R1H Rhabdomyosarcoma and Skeletal Muscles of the Rat. Neurochem Res. 2007;32:973–980. doi: 10.1007/s11064-006-9254-0. [DOI] [PubMed] [Google Scholar]
- 292.Spitz DR, Sim JE, Ridnour LA, Galoforo SS, Lee YJ. Glucose deprivation-induced oxidative stress in human tumor cells. A fundamental defect in metabolism? Ann. NY Acad. Sci. 2000;899:349–362. doi: 10.1111/j.1749-6632.2000.tb06199.x. [DOI] [PubMed] [Google Scholar]
- 293.Ahmad IM, Aykin-Burns N, Sim JE, Walsh SA, Higashikubo R, Buettner GR, Venkataraman S, Mackey MA, Flanagan S, Oberley LW, Spitz DR. Mitochondrial O2*- and H2O2 mediate glucose deprivation-induced stress in human cancer cells. J. Biol. Chem. 2005;280:4525–4563. doi: 10.1074/jbc.M411662200. [DOI] [PubMed] [Google Scholar]
- 294.Pedram A, Razandi M, Wallace DC, Levin AR. Functional estrogen receptors in the mitochondria of breast cancer cells. Mol. Biol. Cell. 2006;17:2125–2137. doi: 10.1091/mbc.E05-11-1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Hussain SP, Hofseth LJ, Harris CC. Radical causes of cancer. Nat. Rev. Cancer. 2003;3:276–285. doi: 10.1038/nrc1046. [DOI] [PubMed] [Google Scholar]
- 296.Hervouet E, Simonnet H, Godinot C. Mitochondria and reactive oxygen species in renal cancer. Biochimie. 2007;89:1080–1088. doi: 10.1016/j.biochi.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 297.Schumacker PT. Reactive oxygen species in cancer cells: live by the sword, die by the sword. Cancer Cell. 2006;10:175–176. doi: 10.1016/j.ccr.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 298.Lu F. Reactive oxygen species in cancer, too much or too little? Med. Hypoth. 2007;69:1293–1298. doi: 10.1016/j.mehy.2007.03.017. [DOI] [PubMed] [Google Scholar]
- 299.Korshunov SS, Skulachev VP, Starkov AA. High protonic potential actuates a mechanism of production of reactive oxygen species in mitochondria. FEBS Lett. 1997;416:15–18. doi: 10.1016/s0014-5793(97)01159-9. [DOI] [PubMed] [Google Scholar]
- 300.Santamaría G, Martínez-Diez M, Fabregat I, Cuezva JM. Efficient execution of cell death in non-glycolytic cells requires the generation of ROS controlled by the activity of mitochondrial H+-ATP synthase. Carcinogenesis. 2006;27:925–935. doi: 10.1093/carcin/bgi315. [DOI] [PubMed] [Google Scholar]
- 301.Horimoto M, Resnick MB, Konkin TA, Routhier J, Wands JR, Baffy G. Expression of uncoupling protein-2 in human colon cancer. Clin. Cancer Res. 2004;10:6203–6207. doi: 10.1158/1078-0432.CCR-04-0419. [DOI] [PubMed] [Google Scholar]
- 302.Harper ME, Antoniou A, Villalobos-Menuey E, Russo A, Trauger R, Vendemelio M, George A, Bartholomew R, Carlo D, Shaikh A, Kupperman J, Newell EW, Bespalov IA, Wallace SS, Liu Y, Rogers JR, Gibbs GL, Leahy JL, Camley RE, Melamede R, Newell MK. Characterization of a novel metabolic strategy used by drug-resistant tumor cells. FASEB J. 2002;16:1550–1557. doi: 10.1096/fj.02-0541com. [DOI] [PubMed] [Google Scholar]
- 303.Collins P, Jones C, Choudhury S, Damelin L, Hodgson H. Increased expression of uncoupling protein 2 in HepG2 cells attenuates oxidative damage and apoptosis. Liver Int. 2005;25:880–887. doi: 10.1111/j.1478-3231.2005.01104.x. [DOI] [PubMed] [Google Scholar]
- 304.Derdak Z, Mark NM, Beldi G, Robson SC, Wands JR, Baffy G. The mitochondrial uncoupling protein-2 promotes chemoresistance in cancer cells. Cancer Res. 2008;68:2813–2819. doi: 10.1158/0008-5472.CAN-08-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Chan SL, Yu VC. Proteins of the Bcl-2 family in apoptosis signalling: from mechanistic insights to therapeutic opportunities. Clin. Exp. Pharmacol. Physiol. 2004;31:119–128. doi: 10.1111/j.1440-1681.2004.03975.x. [DOI] [PubMed] [Google Scholar]
- 306.Kirkin V, Joos S, Zörning M. The role of Bcl-2 family members in tumorigenesis. Biochim. Biophys. Acta. 2004;1644:229–249. doi: 10.1016/j.bbamcr.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 307.Sekimura A, Konishi A, Mizuno K, Kobiyashi Y, Sasaki H, Yano M, Fukai I, Fujii Y. Expression of Smac/DIABLO is a novel prognostic marker in lung cancer. Oncol. Rep. 2004;11:797–802. [PubMed] [Google Scholar]
- 308.Mizutani Y, Nakanishi H, Yamamoto K, Li YN, Matsubara H, Mikami K, Okihara K, Kawauchi A, Bonavida B, Miki T. Downregulation of Smac/DIABLO expression in renal cell carcinoma and its prognostic significance. J. Clin. Oncol. 2004;23:448–454. doi: 10.1200/JCO.2005.02.191. [DOI] [PubMed] [Google Scholar]
- 309.Zörning M, Hueber AO, Baum W, Evan G. Apoptosis regulators and their role in tumorigenesis. Biochim. Biophys. Acta. 2001;1551:F1–F37. doi: 10.1016/s0304-419x(01)00031-2. [DOI] [PubMed] [Google Scholar]
- 310.Dohi T, Beltrami E, Wall NR, Plescia J, Altieri DC. Mitochondrial survivin inhibits apoptosis and promotes tumorigenesis. J. Clin. Invest. 2004;114:1117–1127. doi: 10.1172/JCI22222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Hajra KM, Liu JR. Apoptosome dysfunction in human cancer. Apoptosis. 2004;9:691–704. doi: 10.1023/B:APPT.0000045786.98031.1d. [DOI] [PubMed] [Google Scholar]
- 312.Nakashima RA, Paggi MG, Scott LJ, Pedersen PL. Purification and characterization of a bindable form of mitochondrial bound hexokinase from the high glycolytic AS-30D rat hepatoma cell line. Cancer Res. 1988;48:913–919. [PubMed] [Google Scholar]
- 313.Mathupala SP, Rempel A, Pedersen PL. Glucose catabolism in cancer cells. Isolation, sequence, and activity of the promoter for type II hexokinase. J. Biol. Chem. 1995;270:16918–16925. doi: 10.1074/jbc.270.28.16918. [DOI] [PubMed] [Google Scholar]
- 314.Mathupala SP, Ko YH, Pedersen PL. Hexokinase II: cancer’s double edged sword acting as both facilitator and gatekeeper of malignancy when bound to mitochondria. Oncogene. 2006;25:4777–4786. doi: 10.1038/sj.onc.1209603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Gwak GY, Yoon JH, Kim KM, Lee HS, Chung JW, Gores GJ. Hypoxia stimulates proliferation of human hepatoma cells through the induction of hexokinase II expression. J. Hepatol. 2005;42:358–364. doi: 10.1016/j.jhep.2004.11.020. [DOI] [PubMed] [Google Scholar]
- 316.Nicholson KM, Anderson NG. The protein kinase B/Akt signalling pathway in human malignancy. Cell Sign. 2002;14:381–395. doi: 10.1016/s0898-6568(01)00271-6. [DOI] [PubMed] [Google Scholar]
- 317.Majewski N, Nogueira V, Robey RB, Hay N. Akt inhibits apoptosis downstream of BID cleavage via a glucose-dependent mechanism involving mitochondrial hexokinases. Mol. Cell. Biol. 2004;24:730–740. doi: 10.1128/MCB.24.2.730-740.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Pastorino J, Hoek JB, Shulga N. Activation of gkycogen synthase kinase 3b disrupts the binding of hexokinase II to mitochondria by phosphorylating voltage-dependent anion channel and potentiates chemotherapy-induced cytotoxicity. Cancer Res. 2005;65:10545–10554. doi: 10.1158/0008-5472.CAN-05-1925. [DOI] [PubMed] [Google Scholar]
- 319.Mathupala SP, Ko YH, Pedersen PL. Hexokinase-2 bound to mitochondria: cancer's stygian link to the “Warburg Effect” and a pivotal target for effective therapy. Semin Cancer Biol. 2009;19:17–24. doi: 10.1016/j.semcancer.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Chiara F, Castellaro D, Marin O, Petronilli V, Brusilow WS, Juhaszova M, Sollott SJ, Forte M, Bernardi P, Rasola A. Hexokinase II detachment from mitochondria triggers apoptosis through the permeability transition pore independent of voltage-dependent anion channels. PLoS ONE. 2008;3:e1852, 1–11. doi: 10.1371/journal.pone.0001852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Gogvadze V, Orrenius S, Zhivotovsky B. Mitochondria in cancer cells: what is so special about them? Trends Cell Biol. 2008;18:165–173. doi: 10.1016/j.tcb.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 322.Török NJ, Higuchi H, Bronk S, Goers GJ. Nitric oxide inhibits apoptosis downstream of cytochrome c release by nitrosylating caspase 9. Cancer Res. 2001;62:1648–1653. [PubMed] [Google Scholar]
- 323.Lester RD, Jo M, Campana WM, Gonias SL. Erythropoietin promotes MCF-7 breast cancer cell migration by an ERK/mitogen activated protein kinase-dependent pathway and is primarily responsible for the increase in migration observed in hypoxia. J. Biol. Chem. 2005;280:39273–39277. doi: 10.1074/jbc.M509446200. [DOI] [PubMed] [Google Scholar]
- 324.Galiègue S, Casellas P, Kramar A, Tinel N, Simony-Lafontaine J. Immunohistochemical assessment of the peripheral benzodiazepine receptor in breat cancer and its relationship with survival. Clin. Cancer Res. 2004;10:2058–2064. doi: 10.1158/1078-0432.ccr-03-0988. [DOI] [PubMed] [Google Scholar]
- 325.Carayon P, Portier M, Dussossoy D, Bord A, Petiprêtre G, Canat X, Le Fur G, Casellas P. Involvement of peripheral benzodiazepine receptors in the protection of hematopoietic cells against oxygen radical damage. Blood. 1996;87:3170–3178. [PubMed] [Google Scholar]
- 326.Denicourt C, Dowdy S. Targeting apoptotic pathways in cancer cells. Science. 2004;305:1411–1413. doi: 10.1126/science.1102974. [DOI] [PubMed] [Google Scholar]
- 327.Anderson KM, Harris JE. Is induction of type 2 programmed death in cancer cells from solid tumors directly related to mitochondrial mass? Med. Hypoth. 2001;57:87–90. doi: 10.1054/mehy.2001.1330. [DOI] [PubMed] [Google Scholar]
- 328.Grad JM, Cepero E, Boise LH. Mitochondria as targets for established and novel anti-cancer agents. Drug Resist. Updat. 2001;4:85–91. doi: 10.1054/drup.2001.0192. [DOI] [PubMed] [Google Scholar]
- 329.Zhang L, Yu J, Park BH, Kinzler KW, Vogelstein B. Role of BAX in the apoptotic response to anticancer agents. Science. 2000;290:989–992. doi: 10.1126/science.290.5493.989. [DOI] [PubMed] [Google Scholar]
- 330.Zimmermann KC, Waterhouse NJ, Goldstein JC, Schuler M, Green DR. Aspirin induces apoptosis through release of cytochrome c from mitochondria. Neoplasia (NY) 2000;2:505–513. doi: 10.1038/sj.neo.7900120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Correa P. Human gastric carcinogenesis: a multistep and multifactorial process--First American Cancer Society Award Lecture on Cancer Epidemiology and Prevention. Cancer Res. 1992;52:6735–6740. [PubMed] [Google Scholar]
- 332.Correa P. The biological model of gastric carcinogenesis. IARC Sci Pub. 2004:301–310. [PubMed] [Google Scholar]
- 333.Gruno M, Peet N, Seppet E, Kadaja L, Paju K, Eimre M, Orlova E, Peetsalu M, Tein A, Soplepmann J, Schlattner U, Peetsalu A, Seppet EK. Oxidative phosphorylation and its coupling to mitochondrial creatine and adenylate kinases in human gastric mucosa. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006;291:R936–946. doi: 10.1152/ajpregu.00162.2006. [DOI] [PubMed] [Google Scholar]
- 334.Bayerdorffer E, Lehn N, Hatz R, Mannes GA, Oertel H, Sauerbruch T, Stolte M. Difference in expression of Helicobacter pylori gastritis in antrum and body. Gastroenterology. 1992;102:1575–1582. doi: 10.1016/0016-5085(92)91716-h. [DOI] [PubMed] [Google Scholar]
- 335.Fu S, Ramanujam KS, Wong A, Fantry GT, Drachenberg CB, James SP, Meltzer SJ, Wilson KT. Increased expression and cellular localization of inducible nitric oxide synthase and cyclooxygenase 2 in Helicobacter pylori gastritis. Gastroenterology. 1999;116:1319–1329. doi: 10.1016/s0016-5085(99)70496-8. [DOI] [PubMed] [Google Scholar]
- 336.Ozawa K, Kato S, Sekine H, Koike T, Minoura T, Iinuma K, Nagura H. Gastric epithelial cell turnover and mucosal protection in Japanese children with Helicobacter pylori infection. J. Gastroenterol. 2005;40:236–246. doi: 10.1007/s00535-004-1530-7. [DOI] [PubMed] [Google Scholar]
- 337.Maeda S, Yoshida H, Mitsuno Y, Hirata Y, Ogura K, Shiratori Y, Omata M. Analysis of apoptotic and antiapoptotic signalling pathways induced by Helicobacter pylori. Gut. 2002;50:771–778. doi: 10.1136/gut.50.6.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Yanai A, Hirata Y, Mitsuno Y, Maeda S, Shibata W, Akanuma M, Yoshida H, Kawabe T, Omata M. Helicobacter pylori induces antiapoptosis through buclear factor-kappaB activation. J. Infect. Dis. 2003;188:1741–1751. doi: 10.1086/379629. [DOI] [PubMed] [Google Scholar]
- 339.McCarthy CJ, Crofford LJ, Greenson J, Scheiman JM. Cyclooxygenase-2 expression in gastric antral mucosa before and after eradication of Helicobacter pylori infection. Am. J. Gastroenterol. 1999;94:1218–1223. doi: 10.1111/j.1572-0241.1999.01070.x. [DOI] [PubMed] [Google Scholar]
- 340.Sung JJ, Leung WK, Go MY, To KF, Cheng AS, Ng EK, Chan FK. Cyclooxygenase-2 expression in Helicobacter pylori-associated premalignant and malignant gastric lesions. Am. J. Pathol. 2000;157:729–735. doi: 10.1016/S0002-9440(10)64586-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Gupta RA, Polk DB, Krishna U, Israel DA, Yan F, DuBois RN, Peek RM., Jr Activation of peroxisome proliferator-activated receptor gamma suppresses nuclear factor kappa B-mediated apoptosis induced by Helicobacter pylori in gastric epithelial cells. J. Biol. Chem. 2001;276:31059–31066. doi: 10.1074/jbc.M104141200. [DOI] [PubMed] [Google Scholar]
- 342.Peek RM, Jr, Blaser MJ. Helicobacter pylori and gastrointestinal tract adenocarcinomas. Nat. Rev. Cancer. 2002;2:28–37. doi: 10.1038/nrc703. [DOI] [PubMed] [Google Scholar]
- 343.Lehman JJ, Barger PM, Kovacs A, Saffitz JE, Medeiros DM, Kelly DP. Peroxisome proliferator-activated receptor gamma coactivator-1 promotes cardiac mitochondrial biogenesis. J. Clin. Invest. 2000;106:847–856. doi: 10.1172/JCI10268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Czubryt MP, McAnally J, Fishman GI, Olson EN. Regulation of peroxisome proliferator-activated receptor gamma coactivator 1 alpha (PGC-1 alpha) and mitochondrial function by MEF2 and HDAC5. Proc. Natl. Acad. Sci. USA. 2003;100:1711–1716. doi: 10.1073/pnas.0337639100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Valle J, Kekki M, Sipponen P, Ihamaki T, Siurala M. Long-term course and consequences of Helicobacter pylori gastritis. Results of a 32-year follow-up study. Scand. J. Gastroenterol. 1996;31:546–550. doi: 10.3109/00365529609009126. [DOI] [PubMed] [Google Scholar]
- 346.Sipponen P, Kekki M, Haapakoski J, Ihamaki T, Siurala M. Gastric cancer risk in chronic atrophic gastritis: statistical calculations of cross-sectional data. Int. J. Cancer. 1985;35:173–177. doi: 10.1002/ijc.2910350206. [DOI] [PubMed] [Google Scholar]
- 347.Yin GY, Zhang WN, He XF, Chen Y, Shen XJ. Study on the classification of chronic gastritis at molecular biological level. World J. Gastroenterol. 2003;9:836–842. doi: 10.3748/wjg.v9.i4.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Gruno M, Peet N, Tein A, Salupere R, Sirotkina M, Valle J, Peetsalu A, Seppet EK. Atrophic gastritis: deficient complex I of the respiratory chain in the mitochondria of corpus mucosal cells. J. Gastroenterol. 2008;43:780–788. doi: 10.1007/s00535-008-2231-4. [DOI] [PubMed] [Google Scholar]
- 349.Zhong H, De Marzo AM, Laughner E, Lim M, Hilton DA, Zagzag D, Buechler P, Isaacs WB, Semenza GL, Simons JW. Overexpression of hypoxia-inducible factor 1alpha in common human cancers and their metastases. Cancer Res. 1999;59:5830–5835. [PubMed] [Google Scholar]
- 350.Griffits EA, Pritchard SA, Valentine HR, Whitchelo N, Bishop PW, Ebert MP, Proce PM, Welch IM, West CM. Hypoxia-inducible factor-1alpha expression in the gastric carcinogenesis sequence and its prognostic role in gastric and gastro-oesophageal adenocarcinomas. Br. J. Cancer. 2007;96:95–103. doi: 10.1038/sj.bjc.6603524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Griffits EA, Pritchard SA, McGrath SM, Valentine HR, Price PM, Welch IM, West CM. Hypoxia-associated markers in gastric carcinogenesis and HIF-2appha in gastric and gastro-oesophageal cancer prognosis. Br. J. Cancer. 2008;98:965–973. doi: 10.1038/sj.bjc.6604210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Stoeltzing O, McCarty MF, Wey JS, Fan F, Liu W, Belcheva A, Bucana CD, Semenza CL, Ellis LM. Role of hypoxia-inducible factor 1a in gastric cancer cell growth, angiogenesis, and vessel maturation. J. Nat.Cancer Inst. 2004;96:946–956. doi: 10.1093/jnci/djh168. [DOI] [PubMed] [Google Scholar]
- 353.Treins C, Gioergetti-Peraldi S, Murdaca J, Semenza GL, Van Obberghen E. Insulin stimulates hypoxia inducible factor 1 through a phosphatidylinositol 3-kinase/target of rapamycin-dependent signaling pathway. J. Biol. Chem. 2002;277:27975–27981. doi: 10.1074/jbc.M204152200. [DOI] [PubMed] [Google Scholar]
- 354.Mayerhofer M, Valent P, Sperr WR, Griffin JD, Sillaber C. BCR/ABL induces expression of vascular endothelial growth factor and its transcription activator, hypoxia inducible factor-1a, through a pathway involiving phosphoinositide 3-kinase and the mammalian target of rapamycin. Blood. 2002;100:3767–3775. doi: 10.1182/blood-2002-01-0109. [DOI] [PubMed] [Google Scholar]
- 355.Lang SA, Gaumann A, Koehl GE, Seidel U, Bataille F, Klein D, Ellis LM, Bolder U, Hofstaedter F, Schlitt HJ, Geissler EK, Stoeltzing O. Mammalian target of rapamycin is activated in human gastric cancer and serves as a target for therapy in an experimental model. Int. J. Cancer. 2007;120:1803–1810. doi: 10.1002/ijc.22442. [DOI] [PubMed] [Google Scholar]
- 356.Kolev Y, Uetake H, Takagi Y, Sugihara K. Lactate dehydrogenase-5 (LDH-5) expression in human gastric cancer: association with hypoxia-inducible factor (HIF-1alpha) pathway, angiogenic factors production and poor prognosis. Ann. Surg. Oncol. 2008;15:2336–2344. doi: 10.1245/s10434-008-9955-5. [DOI] [PubMed] [Google Scholar]
- 357.Larsson NG, Luft R. Revolution in mitochondrial medicine. FEBS Lett. 1999;455:199–202. doi: 10.1016/s0014-5793(99)00854-6. [DOI] [PubMed] [Google Scholar]
- 358.Zou MH, Kirkpatrick SS, Davie BJ, Nelson JS, Wiles WG, IV, Schlattner U, Neumann D, Brownlee M, Freeman MB, Gioldman MH. Activation of the AMP-activated protein kinase by the antidiabetic drug metformin in vivo. J. Biol. Chem. 2004;279:43940–43951. doi: 10.1074/jbc.M404421200. [DOI] [PubMed] [Google Scholar]
- 359.Brunmair B, Staniek K, Gras F, Scharf N, Althaym A, Clara R, Roden M, Gnaiger E, Nohl H, Waldhäusl W, Fürnsinn C. Thiazolidinediones, like metformin, inhibit respiratory complex I. Diabetes. 2004;53:1052–1059. doi: 10.2337/diabetes.53.4.1052. [DOI] [PubMed] [Google Scholar]
- 360.Wang W, Lopaschuk GD. Metabolic therapy for the treatment of ischemic heart disease: reality and expectations. Exp. Rev. Cardiovasc. Ther. 2007;5:1123–1134. doi: 10.1586/14779072.5.6.1123. [DOI] [PubMed] [Google Scholar]
- 361.Kroemer G. Mitochondria in cancer. Oncogene. 2006;25:4630–4632. doi: 10.1038/sj.onc.1209589. [DOI] [PubMed] [Google Scholar]