Abstract
Background
Not all drugs prescribed for children and adolescents are certified for use in these age groups. The present study describes the extent of off-label prescriptions in the outpatient sector and identifies deficits in the quality of product information.
Methods
This analysis is based on patient-specific but pseudonymized drug prescription data for approximately 289 000 0- to 16-year-old members of the Gmünder Ersatzkasse, a German statutory health insurance provider, in the year 2002. For each substance prescribed, information regarding the youngest certified age group was derived from the product data.
Results
Of the 1 429 981 prescribed drug packages (726 active substances), 87.4% (66.1% of active substances) were prescribed in accordance with their license and 3.2% (15.7% of active substances) were prescribed off-label. For 9.4% of prescriptions (18.2% of active substances) the licensing status could not be established. For neonates and infants the proportion of licensed prescriptions was below average, at 42.5% and 82.8% of the prescribed packages (20.0% and 38.6% of active substances) respectively. After infancy, prescriptions were predominantly in accordance with licensed use. Deficits were seen in the indication groups "alimentary tract and metabolism," "respiratory system," "dermatologicals," and "sensory organs."
Conclusions
The methodology enables characterization of off-label prescription and identification of fields where further research is needed. With regard to the EU regulation on medicinal products for pediatric use, this could assume increasing importance and contribute to the development of appropriate and safe medicines for children.
Keywords: child health, off-label treatment, drug prescription, drug safety, drug information
In Germany, in the year 2005, approximately 2.1 billion defined daily doses (DDD) of medications were prescribed to roughly 14 million children and adolescents under age 20 and paid for by statutory health insurance (4). Not all of the medications prescribed had been licensed for use in this age group.
Before a medication can be licensed for use in man, its safety and effectiveness must be demonstrated by comprehensive testing. Studies of this type have not been performed in children for all of the medications with which children are treated. The European Parliament and the Council of the European Union stated, as the motivation for their Regulation EC/1901/2006 on pediatric medications, that inappropriate dose recommendations for children elevate the risk of possibly fatal adverse effects, while they may also lead to ineffective treatment if the prescribed dose is too low.
The quality of the information provided to physicians with respect to medication use in children has not yet been studied. Aside from the physician’s own professional knowledge, the information for physicians provided by drug manufacturers is the most important source of information at the physician’s disposal (box). It contains a summary of the modes of use of the medication that have been tested and licensed by the responsible government authority. As it does not state what data were used by this authority to justify the licensed modes of use, the physician cannot determine whether any systematic studies were performed, and, if they were in fact performed, whether they were of adequate quality.
Box. Glossary.
Off-label medication use
The use of a medication outside the specifications of its licensed use with regard to its indications, dosing, patient age group, etc. The subject of the current study is the use of medications in an age group for which they were not licensed.
Unlicensed medication use
The use of a medication that may only be used if licensed by the responsible government authority, but that has not been licensed. Unlicensed use was not the subject of the current study.
Extemporaneous medications
Medications that do not require government licensing, the use of which is not regulated by the German Pharmaceuticals Act (Arzneimittelgesetz, AMG) and thus cannot be the subject of a study on off-label use.
Information for physicians
A summary, for physicians, of the modes of use for which a medication has been licensed (AMG §11a).
Package insert (information for patients)
The same content as the information for physicians, but written so that it can be understood by laypersons and provided to the patient in the package together with the medication (AMG §11).
In this article, we report the first study of the licensing status of medication prescriptions in six pediatric age groups. The Gmünder Ersatzkasse (GEK) is a statutory health insurance provider covering insurees throughout the Federal Republic of Germany; this study concerns the medications prescribed to GEK-insured children and adolescents in Germany in the year 2002. The conformity of each prescription with the licensing status of the medication prescribed, solely with respect to the age of the patient, was determined from the Summary of Product Characteristics (SPC), i.e., the information for physicians for the medication in question. The study was performed in the framework of the research support program of the Children’s Medication Initiative (Initiative Kinderarzneimittel) of the Hexal foundation. This nonprofit initiative was established in 2003 in order to bring about practically relevant contributions to drug safety in children.
Data material and methods
The insured population and medication prescription data
The evaluation was based on medication prescription data of the GEK from the year 2002, in which the GEK provided insurance coverage to about 1.4 million persons. The percentage of children and adolescents up to age 16 among GEK insurees was 21.1% (table).
Table. The population of insured children.
| Age (years) | Male | Female | Both sexes |
| < 1 | 6111 | 5785 | 11 896 |
| 1-2 | 14 093 | 13 249 | 27 342 |
| 3-6 | 32 318 | 30 925 | 63 243 |
| 7-11 | 44 245 | 42 150 | 86 395 |
| 12-16 | 51 226 | 48 500 | 99 726 |
| All children, age 0-16 years | 147 993 | 140 609 | 288 602 |
| Entire insured population, all ages | 776 423 | 593 638 | 1 370 061 |
| Percentage aged 0-16 years | 19.1% | 23.7% | 21.1% |
The data set included comprehensive information on all medications that were prescribed and charged to the GEK. Only finished formulations were considered. The data were individualized but pseudonymized, so that case-by-case evaluation was possible and the course of individual treatments could be followed, but personal identification of the patient was impossible. Medications were classified by the World Health Organization’s ATC system (Anatomical Therapeutic Chemical Classification System, see http://www.whocc.no/atcddd/), a coding system widely used around the world in research on medication use. The ATC system groups medications hierarchically into active substance and indication groups. Medications that were prescribed, but for which no ATC code was available, were excluded from analysis; these were mainly homeopathic and herbal medications, extemporaneous medications, and imported medications (2, 3).
The following variables were used in the analysis:
the Central Pharmaceutical Number (Pharmazentralnummer, PZN) to identify the medication prescribed;
the number of packages prescribed;
the ATC code of the medication, to identify its active substance and scope of indications;
the age of the patient on the day the medication was prescribed.
The precise designations of the finished formulations prescribed were available in our data set, but were not used, because the evaluation was designed to be carried out solely on the basis of the active substances. Different medications containing the same active substance were evaluated together under their (identical) ATC code.
Age distribution
The prescriptions were divided into six age groups, depending on the age of the patient on the day of the prescription:
Neonates: 0 to 28 days
Infants: 29 days to under 1 year
Toddlers: 1 to 2 years
Preschool children: 3 to 6 years
School-age children: 7 to 11 years
Adolescents: 12 to 16 years.
This classification is finer than the four-level classification used by the European and American drug regulatory agencies, the European Medicines Agency (EMEA), and the United States Food and Drug Administration (FDA). The EMEA/FDA age group "infants and toddlers" was subdivided into "infants" and "toddlers" and the age group "children" was subdivided into "preschool children" and "school-age children." By doing so, the six categories chosen correspond to the ones used in the SPC. They reflect the phases of childhood development more precisely and thus take better account of the varying appropriateness of particular medications at different ages.
Prescriptions for premature neonates were not studied separately. Ambulatory prescriptions are presumably of secondary importance in this patient group.
Determination of licensing status
For each active substance prescribed, it was determined whether a finished formulation with that active substance had been licensed for the treatment of children and adolescents. This was done by means of the information for physicians provided by the expert information service of the "Rote Liste" [Red List] (www.fachinfo.de). The research was performed in June to December 2005. If more than one medicinal product could be identified containing the active substance of the prescribed medication, further evaluation was based on the particular medicinal product that had been licensed, in terms of both dosage and mode of application, for use in the youngest age group. The licensing status of medications for which no information for physicians was available over the Internet was evaluated, as far as possible, by consultation of the 2005 printed versions of the Red and Yellow Lists (first through third quarters of 2005). This study was thus based solely on the drug information supplied by the manufacturers, because only this information is relevant for the identification of off-label use.
No electronic references were available from which the licensing data for the German market could be automatically downloaded. In order to address the central question of the study without excessive practical difficulties, we decided to limit the study to medications that had been prescribed more often than a defined minimum number of times, and whose active substances could be completely and unambiguously identified through their ATC codes. The evaluation included only active substance groups (level 3 ATC codes) for which at least 25 medication packages were prescribed in 2002; within each active substance group, the evaluation included only ATC categories (active substances, active substance combinations, and active substance subgroups) for which at least 10 medication packages were prescribed. Active substance subgroups and combinations were included if the corresponding active substances were unambiguously identifiable from the ATC code. ATC codes for which no finished pharmaceutical formulation appeared in the 2005 Red List were not included. The word "children" was used to refer to different age groups in the physician information provided for different medications. Nor did the physician information define age groups uniformly. It could not always be unambiguously determined whether a particular drug had been licensed for use in children in a particular age group. For the purpose of classification, the following was done:
The information for physicians, as well as the Red and Yellow Lists, were evaluated by physicians and pharmacists with longstanding experience in the preparation and updating of medication-use information relevant to the process of medication licensing. If no information about an age group was contained in the "Dosage" section, the sections concerning side effects, contraindications, and warnings and precautions were consulted. If the context allowed no determination whether neonates and infants were included, "toddlers" was taken to be the youngest age group for which the medication had been licensed. Each active substance was evaluated as belonging to one of three classes for each age group:
I. Licensed (approved or relatively contraindicated). The active substance received this classification if the information for physicians for at least one finished formulation containing it included dose recommendations for the age group in question. The medication was considered to be appropriate for use in children and adolescents of all ages if dose recommendations were already given for premature babies or neonates, or if dose recommendations were given "im Wachstumsalter" (during the growing years, i.e., in childhood and adolescence).
The classification "licensed" does not necessarily signify that the use of the medication in children was comprehensively and systematically studied. As a result of the historical development of the licensing process, medications approved many years ago are likely to be less well documented by systematic studies than more recently approved ones. Medications were also classified as "licensed" even when their use was explicitly restricted to certain conditions. These relative contraindications were formulated in varying ways, for example:
"only to be used when strongly/urgently indicated"
"(more than usual) caution should be exercised"
"only to be used in exceptional cases, when indicated"
"only to be used after the exclusion of all other medications"
"should only be prescribed by a specialist"
II. Nonlicensed (not approved or absolutely contraindicated). This category contains active substances whose finished formulations had no recommended dosage for the age group in question, or that were not intended for use in children or should not be used in children according to the information supplied by the manufacturer. Medications were considered to be absolutely contraindicated when the information for physicians contained wording such as the following:
"should not/must not be prescribed to children or adolescents"
"is not indicated in / not suitable for use by children or adolescents"
"not to be used by persons under age 18"
"no data are available regarding the use of this medication by children"
III. Licensing status indeterminate (not enough information). For some medications, the information for physicians contains no information at all about their use in children. Their licensing status was considered to be indeterminate.
Statistical evaluation
In the framework of this descriptive study, off-label use in childhood and adolescence was characterized by absolute and relative frequencies among the prescribed medication packages and active substances, which were calculated and graphically represented.
Results
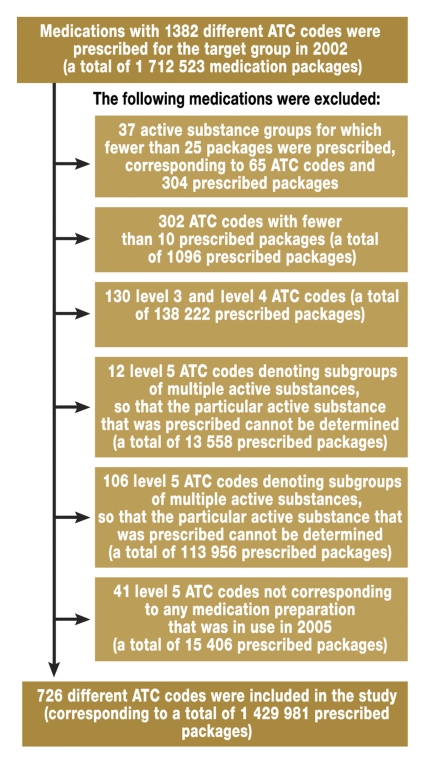
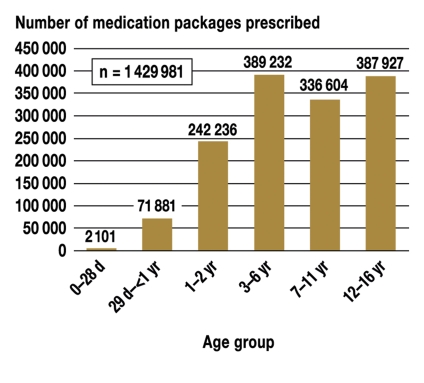
A total of 1 712 523 medication packages were prescribed to GEK insurees aged 0 to 16 years in the year 2002. These prescriptions corresponded to 1382 level 1 ATC codes. The exclusion process presented in further detail in figure 1 resulted in 1 429 981 medication packages that could be included in the analysis, corresponding to 726 different ATC codes. Figure 2 shows the distribution of prescriptions by age group. The spectrum of active substances and quantities prescribed varied widely from one age group to another: 85 active substances were prescribed to neonates, 303 to infants, 463 to toddlers, 572 to preschool children, and 710 to adolescents. There was no age group in which all 726 active substances were prescribed.
Figure 1.
Flowchart showing the study inclusion and exclusion criteria for medication prescriptions.
ATC = the Anatomical Therapeutic Chemical Classification System of the WHO
Figure 2.
The number of medication packages prescribed, by age group.
d = days;
yr = years
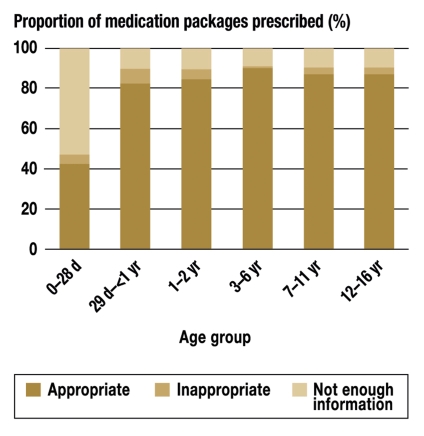
87.4% of the medication packages prescribed were rated as appropriate (licensed) for the age group in question according to the information for physicians, while 3.2% were rated as inappropriate (not licensed). For about one-tenth of the prescribed packages, the licensing status was indeterminate. As shown in figure 3, only 893 of the 2101 medication packages prescribed to neonates were approved; the percentage rose to about 90% in the subsequent age groups. More than half of the packages prescribed to neonates were found to have indeterminate licensing status. In the subsequent age groups, the corresponding figure was no higher than 10%.
Figure 3.
The percentage of medication packages prescribed, by appropriateness and age group.
d = days;
yr = years
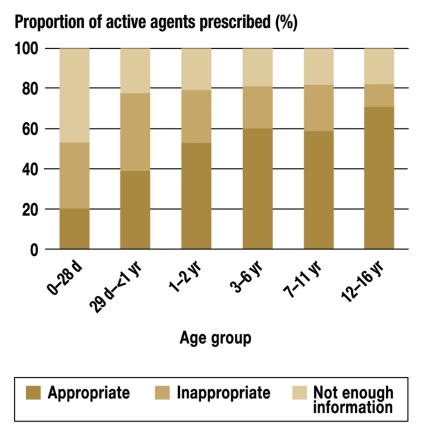
Of the 726 active substances prescribed, 66.1% were approved, 15.7% not approved, and 18.2% of indeterminate licensing status, as judged from the information for physicians. Only one-fifth of the active substances prescribed to neonates were approved, and there was not enough information to determine the licensing status in almost half (figure 4). For infants, roughly 40% of the active substances prescribed were approved, and 40% not approved. In the subsequent age groups, 52% to 70% of the active substances prescribed were approved, 11% to 26% not approved. In all age groups from toddlers onward, one-fifth or more of the active substances prescribed were of indeterminate licensing status.
Figure 4.
The percentage of active agents prescribed, by appropriateness and age group.
d = days;
yr = years
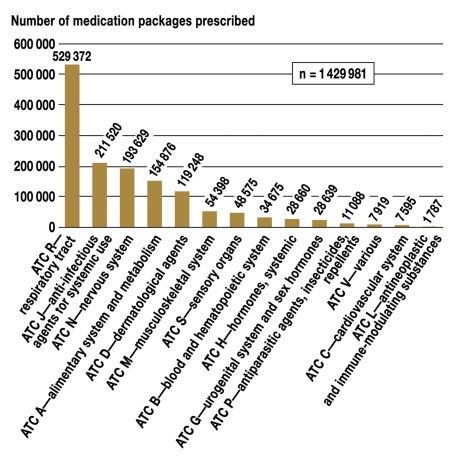
The distribution of medication packages prescribed by major ATC groups is shown in figure 5. 76.2% of all prescribed packages were for four groups of indications/agents: respiratory diseases ("R"), systemic anti-infectious agents ("J"), diseases of the nervous system ("N"), and diseases of the alimentary system and metabolism ("A").
Figure 5.
The number of medication packages prescribed, by indication
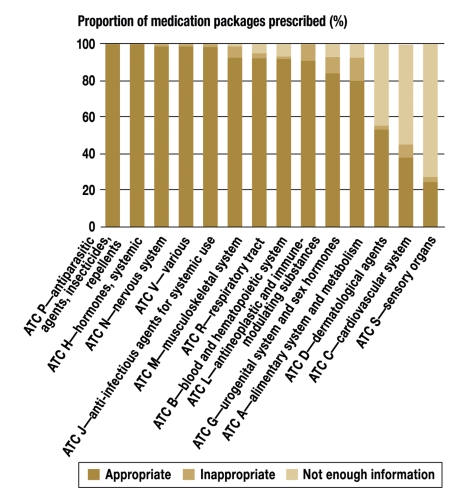
A total of 45 524 medication packages were prescribed whose active substance was contraindicated for the age group in question, according to the SPC. The percentage of contraindicated medication packages in individual indication groups varied from 12.8% (major ATC group A, diseases of the alimentary system and metabolism) and 0.2% (main ATC group V, various) (figure 6).
Figure 6.
The percentage of medication packages prescribed, by appropriateness and indication
Inadequate information about the use of particular medications in children was particularly evident among active substances from the major ATC groups S (sensory organs, 72.3%), C (cardiovascular system, 54.7%), and D (dermatological agents, 44.1%). In the remaining ATC groups, the corresponding percentage varied from 0% to 7%.
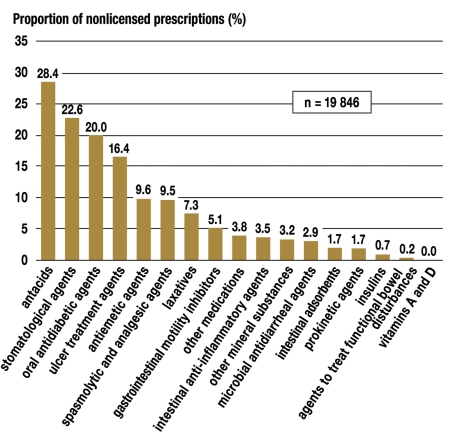
The indication group consisting of diseases of the alimentary system and metabolism had the highest percentage of nonlicensed medication prescriptions according to the information for physicians. Within this indication group, farther broken down by ATC code, nonlicensing was found to be most common among prescriptions for antacids (223 of 785 packages), followed by stomatologic agents (1727 of 75 949 packages), oral antidiabetic agents (10 of 50 packages), and ulcer treatment agents (675 of 4127 packages) (figure 7). All available proton-pump inhibitors in the last-named group were prescribed, even though only a single active agent (omeprazole, A02BC01) was licensed for the treatment of children aged 1 year or older, according to the information for physicians.
Figure 7.
The percentage of prescribed medication packages from ATC group A, "alimentary system and metabolism," in which the prescribed medication was not approved for the patient’s age group
Discussion
This study confirms, as expected, the high rate of use of nonlicensed medications in pediatrics. The finding that the percentage of prescriptions of this type diminishes with increasing age of the child was also expected. This was the first systematic study of the quality of drug information for physicians. For every fifth medication studied, the licensing status was indeterminate because of missing information. Even when the information for physicians and the drug directories contained some information about the use of a particular drug in children, it was not always possible to determine the age group for which it was licensed.
The information for physicians relates licensing data in a particular, specified order. It is supposed to state "the dosing and mode of administration for adults and also for children, if the medication is suitable for use in children."
Often, particularly for externally applied medications that are licensed for both adults and children, no distinction is drawn between adults and children in the dosing recommendations. Furthermore, the German Pharmaceuticals Act does not define the term "children" (Kinder), which is used in the SPC to designate different age groups. Whether the term "children" is meant to include neonates and infants often cannot be determined, or, if so, then only after consultation of the further sections of the physician information sheet—"side effects," "contraindications," or "warnings and precautions."
The designations that were used for pediatric age groups generally did not conform to the model of the EMEA and FDA licensing authorities, and thus the age of the patients that could be treated often could not be determined unambiguously (e.g., "young children under 1 year of age").
Sometimes, too, the suitability of a medication for a particular age group could only be determined by indirect reasoning. It could be concluded, for example, that a medication was to be used by female adolescents, but not by prepubertal girls, from information found in the "indications" section (e.g., "contraception," "painful menstruation") or from the medical context (e.g., "not to be used before the menarche"). A medication for the "supportive treatment of children with difficulty reading and writing" was considered to be intended for school-age children. Indirect reasoning also led to the assumption of nonlicensing for age groups that were not mentioned in the "dosing" section ("children aged 2 years and older / 8 years and older are treated with... ").
The present analysis is based on data routinely obtained by a statutory health insurance provider (the Gmünder Ersatzkasse, GEK) that insures patients throughout the Federal Republic of Germany. It is, therefore, a secondary data analysis. Data from insurance providers are very useful to study the questions posed here, for a number of reasons. The obligatory documentation of the central pharmaceutical number (PZN), a uniform designation used nationwide for finished pharmaceutical formulations, when the medication is dispensed at the pharmacy results in high-quality data that reliably reflect the entire spectrum of prescribed medications and current prescription practices. Observational studies that are designed in other ways can obtain comparably broad and comprehensive data on medication use only with great practical difficulty, or not at all.
With regard to the interval of time that elapsed between data collection and data analysis, it should be noted that the project was originally planned to help the Expert Advisory Board of the Hexal Children’s Medication Initiative in its task of selecting clinical trials to be funded, according to current clinical needs. In view of the fact that support for pediatric clinical research is now a goal across the European Union, it was decided to make this analysis available to the public. The analysis of prescriptions is based on data from the year 2002, but this does not make the conclusions less applicable to the present situation to any significant extent. Aside from a few special indications with relatively low patient numbers, the indication areas in the pediatric sector that are evaluated in this study have changed little in recent years, i.e., there has been little broadening of drug approvals for pediatric use. The information for physicians that can be seen on the initiative’s website, which is known as ZAK (Licensed Medications for Children, Zugelassene Arzneimittel für Kinder, www.zak-kinderarzneimittel.de), confirms the impression that the changes since 2002 have been fairly slight. Thus, the data and their analysis can still be considered current.
There are relatively few limitations on the reimbursement of pediatric medical interventions by the statutory health insurance providers in Germany. Thus, there is no reason to suppose that the medications prescribed and reimbursed for the study population would differ to any significant extent from those that would have been prescribed and reimbursed for a population of the same age structure and disease burden, if it had been covered by another statutory or private health insurance provider.
The analysis showed that child and adolescent outpatients are mainly treated with approved medications, but considerable deficits nevertheless exist in this area for neonates, infants, and toddlers, as well as in the indication groups/organ systems "alimentary system and metabolism," "respiratory tract," and "sensory organs."
The methods used here have enabled us to characterize off-label use in medical prescribing and to identify a need for further research. In the light of the European Union’s Regulation EC/1901/2006 of 12 December 2006 concerning pediatric medications, this type of detailed knowledge regarding off-label use is likely to be very important for the future development of pediatric medications that are appropriate to children’s medical needs. In the EU member states, the new licensing of a medication with a patented active substance requires the submission of data from clinical studies on children, if there is found to be a therapeutic need for the medication in the pediatric age group. From 2009 onward, alterations in the licensing status of already licensed medications will also require such data to be submitted. In compensation for this requirement, the manufacturer of the licensed drug receives an additional six months of patent protection. There are also new incentives to promote the development of pediatric medications with unpatented active substances, e.g., the (voluntary) licensing variant called PUMA (Pediatric Marketing Authorization), which guarantees exclusive use in the European market for ten years. It remains to be seen how quickly these regulatory efforts will improve the medication situation for children.
Key messages.
Medications that have not been licensed for use in children are nevertheless regularly prescribed in ambulatory pediatric practice to an extent that does not conform to the general public’s expectations for medication safety in this vulnerable patient group.
The percentage of off-label prescriptions is higher in younger children.
There seems to be an adequate range of licensed medications to treat the typical childhood diseases.
Fewer licensed medications are available to treat rarer diseases.
The quality of drug information for physicians is deficient with respect to their use in children.
Acknowledgments
The authors thank the Gmünder Ersatzkasse (GEK) and Prof. Dr. Gerd Glaeske of the University of Bremen for the provision of data.
Translated from the original German by Ethan Taub, M.D.
Footnotes
Conflict of interest statement
Professor Mühlbauer maintains cooperative inter-institutional research programs with the Bayer Vital, Janssen Cilag, and Pfizer AG companies. He receives project support from the Stiftung Arzneimittelsicherheit (Drug Safety Foundation) and further support from the German Research Foundation (Deutsche Forschungsgemeinschaft, DFG) and the German Federal Ministry of Education and Research (BMBF). He conducts inter-institutional cooperative projects with the AOK-Bundesverband (a nationwide association of health insurance providers) and the KV Bremen (the Bremen Association of Statutory Health Insurance Physicians).
Dr. Jahnsen is a participant in the GEK-Arzneimittel-Report (GEK Drug Report), a project of the GEK health insurance provider that is supported by third-party funding. She also holds stock in "medicines 4 children GmbH." She receives payments for lectures from the Forum-Insitut für Management GmbH company.
Dr. Pichler served as an assistant for the Hexal Children’s Medication Initiative (Hexal-Initiative Kinderarzneimittel) of the Hexal foundation (Hexal AG) and is an employee of the Hexal AG library.
Dr. Schoettler has been a member of the board of the directors of Hexal AG, and a director of both Sandoz International GmbH and the Hexal Foundation gGmbH.
References
- 1.Bücheler R, Meisner C, Kalchthaler B, et al. „Off-label“ prescribing of drugs in the ambulatory care of children and adolescents. Dtsch Med Wochenschr. 2002;127:2551–2557. doi: 10.1055/s-2002-35819. [DOI] [PubMed] [Google Scholar]
- 2.Glaeske G, Janhsen K. GEK-Arzneimittel-Report 2003. St. Augustin: Asgard; 2003. pp. 68–69. http://media.gek.de/downloads/magazine/ArzneimittelReport03_GEK.pdf. [Google Scholar]
- 3.Glaeske G, Janhsen K. GEK-Arzneimittel-Report 2007. St. Augustin: Asgard; 2007. pp. A1–A8. http://media.gek.de/downloads/magazine/GEK-Arzneimittel-Report-2007.pdf. [Google Scholar]
- 4.Nink K, Schröder H. Arzneiverordnungen nach Alter und Geschlecht. In: Schwabe U, Paffrath D, editors. Arzneiverordnungsreport 2006. Berlin, Heidelberg, New York: Springer; 2007. pp. 966–978. [Google Scholar]
- 5.’t Jong GW, van der Linden PD, Bakker EM, et al. Unlicensed and off-label drug use in a paediatric ward of a general hospital in the Netherlands. Eur J Clin Pharmacol. 2002;58:293–297. doi: 10.1007/s00228-002-0479-9. [DOI] [PubMed] [Google Scholar]
- 6.Turner S, Longworth A, Nunn AJ, Choonara I. Unlicensed and off label drug use in paediatric wards: prospective study. Br Med J. 1998;316:343–345. doi: 10.1136/bmj.316.7128.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Turner S, Nunn AJ, Fielding K, Choonara I. Adverse drug reactions to unlicensed and off-label drugs on paediatric wards: a prospective study. Acta Paediatr. 1999;88:965–968. doi: 10.1080/08035259950168469. [DOI] [PubMed] [Google Scholar]