Abstract
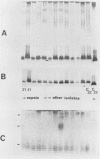
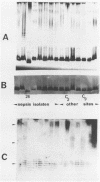
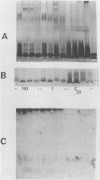
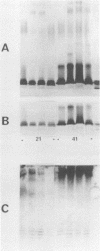
The immunophysical characteristics of 29 Serratia marcescens strains isolated from hospitalized patients in three different cities were studied. Their outer membrane antigens were compared by solid-phase radioimmunoassay inhibition, and their proteinase K-treated, whole-cell lysates were compared by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblot analysis. The strains had a limited number of unique outer membrane lipopolysaccharide (LPS) and capsular polysaccharide (K) antigens. By solid-phase radioimmunoassay inhibition, these strains could be divided into four distinct LPS and five K antigenic groups. By SDS-PAGE, the LPS groups could be further divided into three distinct SDS-PAGE core polysaccharide profiles and five distinct O-side-chain polysaccharide profiles. Immunoblot analysis with rabbit antiserum confirmed the limited heterogeneity of these isolates. Of the strains tested, no PAGE profile was unique to blood or nonblood isolates or to organisms collected from a given hospital. Variability of O and core PAGE profiles was not a function of organism growth cycle. Five representative Serratia strains were tested by SDS-PAGE and immunoblot analysis and in a bactericidal assay with normal human serum. We found that (i) the normal human serum had antibodies to the LPS of each of the strains, (ii) the anti-LPS antibody measured by immunoblot did not correlate with the level of bactericidal activity in the normal human serum, (iii) three of four sepsis isolates were serum sensitive, (iv) two Serratia strains serum sensitive in log-phase growth became serum resistant in late stationary-phase growth and under limiting nutrient conditions, and (v) no LPS PAGE profile distinguished serum-sensitive from serum-resistant strains.
Full text
PDF

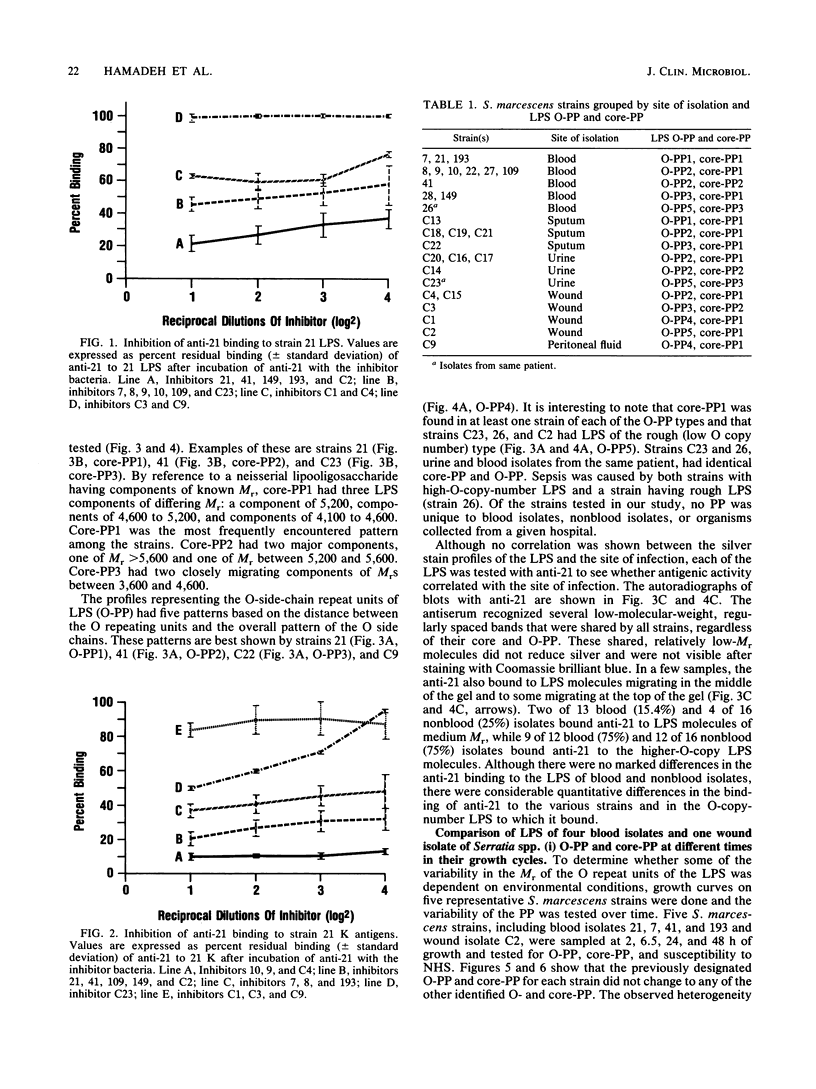
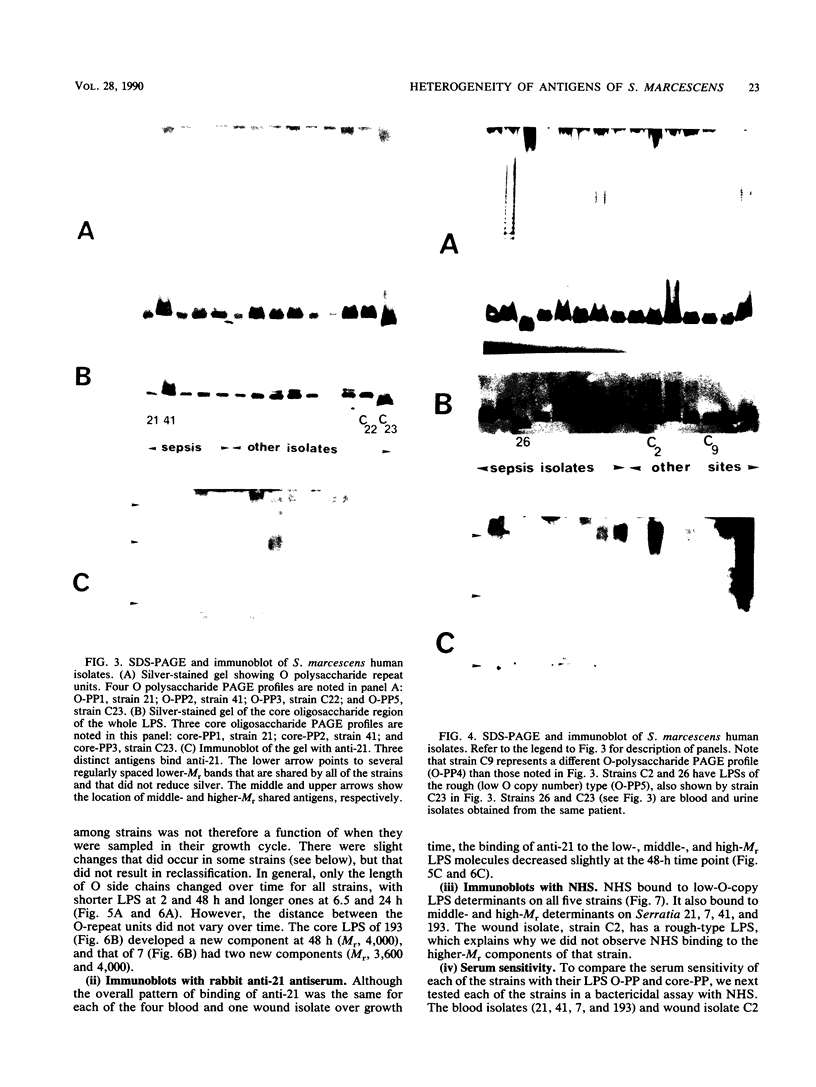
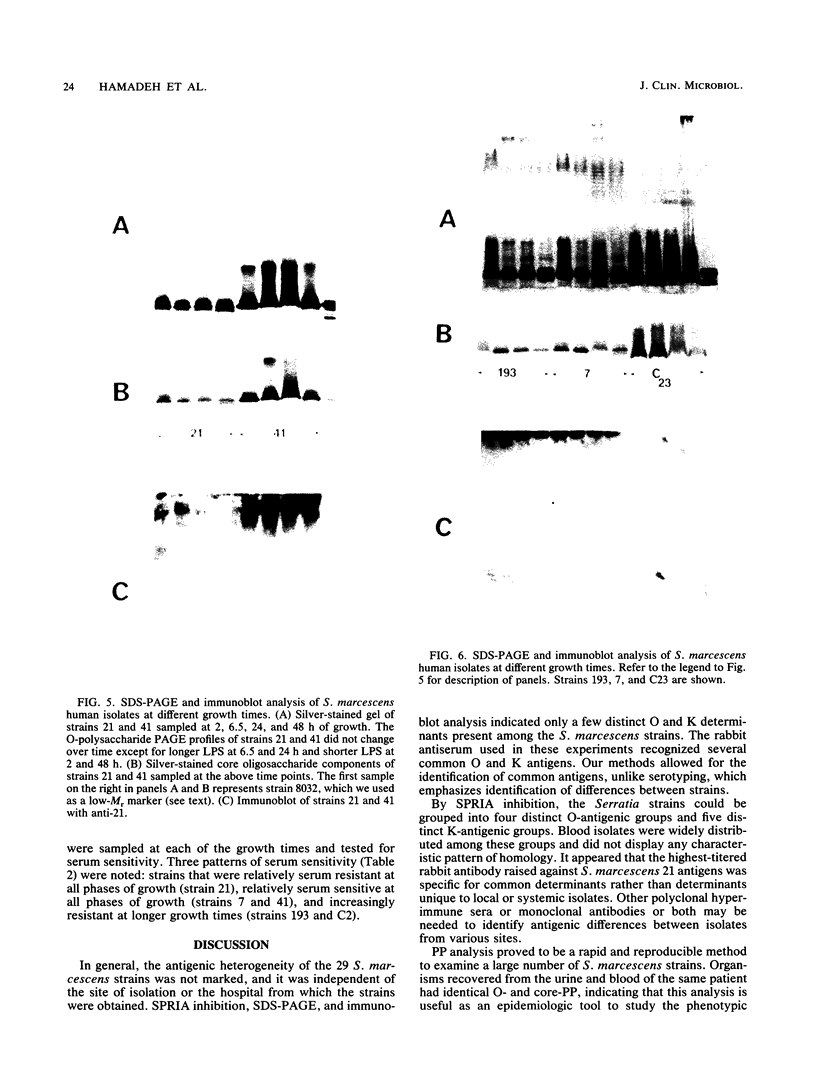


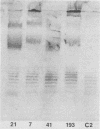
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams G. A., Young R. Constitution of extracellular and capsular polysaccharides of Serratia marcescens. Ann N Y Acad Sci. 1966 Jun 30;133(2):527–545. doi: 10.1111/j.1749-6632.1966.tb52387.x. [DOI] [PubMed] [Google Scholar]
- Bertram M. A., Griffiss J. M., Broud D. D. Response to antigenic determinants of Neisseria meningitidis lipopolysaccharide investigated with a new radioactive antigen-binding assay. J Immunol. 1976 Mar;116(3):842–846. [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Davis J. T., Foltz E., Blakemore W. S. Serratia marcescens. A pathogen of increasing clinical importance. JAMA. 1970 Dec 21;214(12):2190–2192. doi: 10.1001/jama.214.12.2190. [DOI] [PubMed] [Google Scholar]
- Feingold D. S. The serum bactericidal reaction. IV. Phenotypic conversion of Escherichia coli from serum-resistance to serum-sensitivity by diphenylamine. J Infect Dis. 1969 Oct;120(4):437–444. doi: 10.1093/infdis/120.4.437. [DOI] [PubMed] [Google Scholar]
- Glynn A. A., Howard C. J. The sensitivity to complement of strains of Escherichia coli related to their K antigens. Immunology. 1970 Mar;18(3):331–346. [PMC free article] [PubMed] [Google Scholar]
- Hitchcock P. J., Brown T. M. Morphological heterogeneity among Salmonella lipopolysaccharide chemotypes in silver-stained polyacrylamide gels. J Bacteriol. 1983 Apr;154(1):269–277. doi: 10.1128/jb.154.1.269-277.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J. J., Mandrell R. E., Griffiss J. M. Neisseria lactamica and Neisseria meningitidis share lipooligosaccharide epitopes but lack common capsular and class 1, 2, and 3 protein epitopes. Infect Immun. 1989 Feb;57(2):602–608. doi: 10.1128/iai.57.2.602-608.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J. J., Mandrell R. E., Hu Z., Westerink M. A., Poolman J. T., Griffiss J. M. Electromorphic characterization and description of conserved epitopes of the lipooligosaccharides of group A Neisseria meningitidis. Infect Immun. 1988 Oct;56(10):2631–2638. doi: 10.1128/iai.56.10.2631-2638.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Le Minor S., Benazet F., Martin L. Nouveaux facteurs antigéniques O (O23) et H (H26) de Serratia marcescens. Ann Microbiol (Paris) 1983 Nov-Dec;134B(3):447–449. [PubMed] [Google Scholar]
- Mäkelä P. H., Mayer H. Enterobacterial common antigen. Bacteriol Rev. 1976 Sep;40(3):591–632. doi: 10.1128/br.40.3.591-632.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Männel D., Mayer H. Serological and immunological properties of isolated enterobacterial common antigen. Eur J Biochem. 1978 May 16;86(2):371–379. doi: 10.1111/j.1432-1033.1978.tb12319.x. [DOI] [PubMed] [Google Scholar]
- ROWLEY D., WARDLAW A. C. Lysis of gram-negative bacteria by serum. J Gen Microbiol. 1958 Apr;18(2):529–533. doi: 10.1099/00221287-18-2-529. [DOI] [PubMed] [Google Scholar]
- Reynolds B. L., Pruul H. Sensitization of complement-resistant smooth gram-negative bacterial strains. Infect Immun. 1971 Mar;3(3):365–372. doi: 10.1128/iai.3.3.365-372.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simberkoff M. S., Ricupero I., Rahal J. J., Jr Host resistance to Serratia marcescens infection: serum bactericidal activity and phagocytosis by normal blood leukocytes. J Lab Clin Med. 1976 Feb;87(2):206–217. [PubMed] [Google Scholar]
- Taylor P. W. Bactericidal and bacteriolytic activity of serum against gram-negative bacteria. Microbiol Rev. 1983 Mar;47(1):46–83. doi: 10.1128/mr.47.1.46-83.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor P. W., Messner P., Parton R. Effect of the growth environment on cell-envelope components of Escherichia coli in relation to sensitivity to human serum. J Med Microbiol. 1981 Feb;14(1):9–19. doi: 10.1099/00222615-14-1-9. [DOI] [PubMed] [Google Scholar]
- Taylor P. W., Parton R. A protein factor associated with serum resistance in Escherichia coli. J Med Microbiol. 1977 May;10(2):225–232. doi: 10.1099/00222615-10-2-225. [DOI] [PubMed] [Google Scholar]
- Traub W. H. Opsonization requirements of Serratia marcescens. Zentralbl Bakteriol Mikrobiol Hyg A. 1982 Nov;253(2):204–224. [PubMed] [Google Scholar]
- Traub W. H. Serotyping of Serratia marcescens: identification of a new O-antigen (O24). Zentralbl Bakteriol Mikrobiol Hyg A. 1985 Jul;259(4):485–488. doi: 10.1016/s0176-6724(85)80080-8. [DOI] [PubMed] [Google Scholar]
- Tsai C. M., Frasch C. E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982 Jan 1;119(1):115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- Tsai C. M. The analysis of lipopolysaccharide (endotoxin) in meningococcal polysaccharide vaccines by silver staining following SDS-polyacrylamide gel electrophoresis. J Biol Stand. 1986 Jan;14(1):25–33. doi: 10.1016/s0092-1157(86)80006-3. [DOI] [PubMed] [Google Scholar]
- Wilfert J. N., Barrett F. F., Kass E. H. Bacteremia due to serratia marcescens. N Engl J Med. 1968 Aug 8;279(6):286–289. doi: 10.1056/NEJM196808082790604. [DOI] [PubMed] [Google Scholar]
- Wilkowske C. J., Washington J. A., 2nd, Martin W. J., Ritts R. E., Jr Serratia marcescens. Biochemical characteristics, antibiotic susceptibility patterns, and clinical significance. JAMA. 1970 Dec 21;214(12):2157–2162. doi: 10.1001/jama.214.12.2157. [DOI] [PubMed] [Google Scholar]
- Wong K. H., Barrera O., Sutton A., May J., Hochstein D. H., Robbins J. D., Robbins J. B., Parkman P. D., Seligmann E. B., Jr Standardization and control of meningococcal vaccines, group A and group C polysaccharides. J Biol Stand. 1977;5(3):197–215. doi: 10.1016/s0092-1157(77)80005-x. [DOI] [PubMed] [Google Scholar]
- Zollinger W. D., Mandrell R. E. Outer-membrane protein and lipopolysaccharide serotyping of Neisseria meningitidis by inhibition of a solid-phase radioimmunoassay. Infect Immun. 1977 Nov;18(2):424–433. doi: 10.1128/iai.18.2.424-433.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zollinger W. D., Mandrell R. E. Type-specific antigens of group A Neisseria meningitidis: lipopolysaccharide and heat-modifiable outer membrane proteins. Infect Immun. 1980 May;28(2):451–458. doi: 10.1128/iai.28.2.451-458.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]