Abstract
Background
Psoriasis is one of the most prevalent chronic inflammatory diseases, affecting approximately 2 million people in Germany.
Methods
Selective literature review taking into account the German S1 and S3 guidelines for the treatment of this condition.
Results and conclusions
Psoriasis is a very troublesome disease with a high economic impact. The disease often persists for life, and the patient has an increased risk of cardiovascular diseases and their complications. One out of five patients develops psoriatic arthritis. The clinical picture of psoriasis is highly variable with regard to lesional characteristics and the severity of disease. To improve the management of psoriasis the guidelines must be followed and all appropriate topical and systemic treatment options must be tried, with clearly defined treatment goals. The spectrum of established systemic treatments for psoriasis has been extended by the biologics. These can be used to achieve a good skin status and a clear-cut improvement in quality of life even in patients who do not—or no longer—respond adequately to conventional therapies.
Keywords: psoriasis, treatment concept, genetics, immune-mediated inflammatory disease, guidelines
Recent scientific advances have led to new concepts of the pathogenesis of psoriasis and of the disease entity itself. Our improved appreciation of the effect that this common chronic dermatosis has on its sufferers’ quality of life also necessitates a reappraisal. Similarities in the inflammatory process and the spectrum of associated diseases, as well as in the response to certain types of treatment, have enabled psoriasis to be classified as one of the "immune-mediated inflammatory diseases" (IMID), a group that also includes rheumatoid arthritis, Crohn’s disease, and other conditions. Psoriasis is now established as a model disease in this group: Therapeutic agents, such as therapeutic antibodies, are initially developed specifically and primarily for the treatment of psoriasis and are tested only afterward for other indications. The goal of this article is to provide an overview of the current understanding of the pathogenesis of psoriasis, the significance of its comorbidities, its treatment, and therapy management.
The learning aims of this article are:
to understand psoriasis as a complex of cutaneous manifestations and comorbidities based on a chronic, immunologically mediated inflammatory reaction,
to be aware of the degree to which patients are harmed by the burden of the disease itself and by their impaired health-related quality of life,
to know the possibilities for treatment according to the current guidelines, with particular attention to very recently introduced medications that interfere with the disease process in targeted fashion.
Epidemiology.
About two million people in Germany suffer from psoriasis.
Epidemiology and genetics
The prevalence of psoriasis in western, industrialized countries is relatively constant at 2% to 3%. For Germany, this implies a total of about two million affected persons nationwide (table 1). Psoriasis is, therefore, one of the more common chronic inflammatory diseases (for comparison, the prevalence of rheumatoid arthritis is about 0.8% to 1%, while that of Crohn’s disease is about 0.5%). According to the Central Research Institute for Ambulatory Health Care in Germany (Zentralinstitut für kassenärztliche Versorgung), psoriasis accounted for about one million visits to a physician in the first quarter of the year 2000; half of these visits were made to a dermatologist, the other half to a family physician.
Table 1. The epidemiology and genetics of psoriasis.
| Epidemiology | |
| Prevalence in Germany | 2–3% |
| Number of patients in Germany | ca. 2 million |
| Male/female ratio | 60%/40% |
| Age at first manifestation | |
| < 10 years | 10% of patients |
| < 15 years | 20% of patients |
| < 40 years | 60% of patients |
| Further characteristics | |
| Type 1 psoriasis | ca. 60% of patients |
| Psoriatic arthritis | ca. 20% of psoriasis patients |
| Important susceptibility loci (linkage) | |
| PSORS1, HLA-Cw6 | Chromosome 6p21 |
| PSORS2 | Chromosome 17q |
| Associated gene polymorphisms | |
| TNF-alpha | |
| IL-12/IL-23p40 | |
| IL-23 receptor |
In about 40% of cases, psoriasis appears in a familial cluster in association with certain HLA traits (HLA-Cw6 and -DR7), and the affected patients are typically in their second or third decade of life at the onset of the disease. This form of the disease is called type 1 psoriasis. In type 2 psoriasis, there is neither familial clustering nor an association with any particular HLA trait, and the peak age of onset is in the fifth and sixth decades (1).
Genetics.
Psoriasis is a multigenetic disease.
Because the first symptoms appear before age 21 in half of all cases, patients tend to suffer from psoriasis for many decades, and life-long treatment is often necessary, particularly when the disease takes a severe, chronic course.
The importance of genetic factors has been demonstrated, particularly for type 1 psoriasis. Linkage studies have identified nine psoriasis susceptibility loci (PSORS) to date, the most important of which is PSORS1 on chromosome 6p21 (e1). Variants in the promoter region of the tumor necrosis factor alpha (TNF-alpha) gene have been found to be associated with psoriasis and psoriatic arthritis (2). Further genetic associations involving cytokines have been discovered recently as well. These are thought to play an important role in the psoriatic inflammatory process, particularly interleukin (IL)-12 and IL-23 (3). The interrelatedness of diseases of the IMID class is also illustrated by the fact that some of the genetic variants mentioned, such as the one involving the IL-23 receptor, are risk genes not just for psoriasis, but also for Crohn’s disease. The genetic factors may promote the appearance of mild cutaneous inflammatory reactions in predisposed individuals that then develop into an immunologically mediated chronic inflammation. A model of this type is consistent with the finding that psoriasis often manifests itself initially, or is worsened at some point in its further course, by precipitating factors of various types. The main ones that have been identified are streptococcal upper respiratory infections, certain medications (beta-blockers, ACE inhibitors, lithium salts, interferon-alpha, hydroxychloroquine/chloroquine), and stress (4, 5).
Precipitating factors.
Typical precipitating factors are infections, stress, and certain medications.
Pathogenesis
The current understanding of the molecular pathogenesis of psoriasis assigns central importance to an interaction between acquired and innate immunity (6). At the onset of the disease, as well as during exacerbations in its later course, special dendritic cells (DC) in the epidermis and dermis are activated; among other effects, these cells produce the messenger substances TNF-alpha and IL-23, which, in turn, promote the development of certain subclasses of T cells (Th1, Th17). These T cells secrete mediators that contribute to the vascular and epidermal changes of psoriasis. The activation of intracellular signal transduction pathways plays an essential role in reinforcing the inflammatory immune reaction (e2). Antimicrobial peptides (AMPs) are an important component of the innate immune system and play a major role in the homeostasis of surface organs such as the skin. Nearly all AMPs that have been identified to date are present in increased amounts in psoriasis lesions (7). The overexpression of antimicrobial peptides is characteristic of psoriasis and may be interpreted as a sign of activation of the innate immune system. In accordance with this hypothesis, bacterial infection of psoriatic lesions is not observed in clinical practice (in contrast to atopic dermatitis).
Clinical types of psoriasis
The typical skin change (primary lesion) of psoriasis is a sharply demarcated erythrosquamous plaque; it appears infiltrated and reddened as a clinical correlate of inflammation, and scaly as a sign of hyperparakeratosis. It is itchy in about two-thirds of patients (8).
Clinical presentation.
The typical skin change of psoriasis is a sharply demarcated erythrosquamous plaque.
Most common type of psoriasis.
The most common clinical type is psoriasis vulgaris (plaque-type psoriasis).
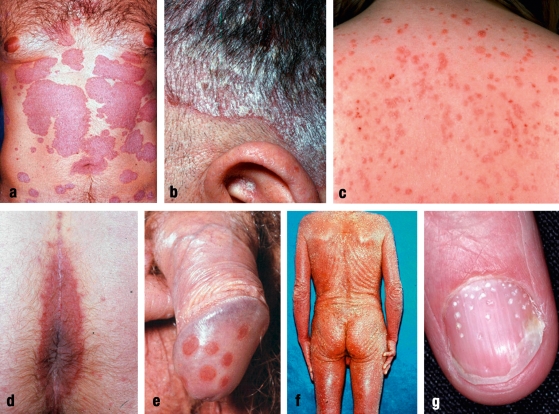
The most common clinical type of psoriasis, affecting some 80% of patients, is psoriasis vulgaris; the North American term for this condition, "plaque-type psoriasis," is now used to an increasing extent in Europe as well (figure 1a). Sites of predilection are the hairy scalp (30% initially and 75% over the course of the disease) (figure 1b), the extensor surfaces of the elbows and knees, and the sacral region, with involvement of the anal fold (this site is often overlooked when the psoriatic involvement is very mild). In children, the face and the genito-anal region are often involved. Stationary plaque psoriasis, which is usually a chronic illness, is distinct from acute eruptive psoriasis, in which there are typically numerous, very small, identical-looking plaques (figure 1c). This clinical picture of so-called psoriasis guttata is often the initial manifestation of psoriasis, e.g., in the aftermath of a streptococcal infection, although not all such cases go on to develop into plaque-type psoriasis. Scaly inflammatory foci may appear in the intertriginous areas (axillae, groin, under the breasts), either in addition to the typical plaques on the extensor surfaces, or as the sole manifestation of psoriasis, known as intertriginous psoriasis. The rare maximal variant of psoriasis vulgaris is called psoriatic erythrodermia and involves the entire skin (figure 1f). The development of pustules in addition to the other signs of psoriasis is the clinical hallmark of pustular psoriasis. Here, there is a further clinical distinction between palmoplantar pustulosis (PPP), which affects only the palms and soles, and other types with generalized pustule formation. PPP is now considered to be a separate entity by itself, because of certain genetic differences. A further special type of localized pustular psoriasis is acrodermatitis continua of Hallopeau, in which the distal phalanges of the fingers and/or toes bear inflammatory pustules, often associated with severe nail abnormalities. In the most severe type of psoriasis, called primary generalized pustular psoriasis, there is acute, generalized pustule formation, together with a usually erythrodermatous psoriasis.
Figure.
Clinical types of psoriasis:
(a) chronic plaque psoriasis with
(b) involvement of the scalp;
(c) eruptive psoriasis (psoriasis guttata);
(d) involvement of the rima ani and
(e) involvement of the genitals;
(f) psoriatic erythrodermia;
(g) nail psoriasis with pitted nail and incipient oil spot.
(Picture credit: Dermatologikum Hamburg)
In some 30% of patients, the psoriatic inflammatory process affects the nail matrix and/or nail bed and causes typical nail changes (figure 1g) known collectively as nail psoriasis. These include whitish spots (leukonychia) and punctate depressions (pitted nails), as well as dystrophy of the nail plate, splinter hemorrhages, and brownish discolorations of the nail bed ("oil spots").
Psoriatic arthritis
In about 20% of patients, an inflammatory disease of the joints called psoriatic arthritis (PsA) arises, usually many years after the initial cutaneous manifestation. Rarely, PsA can also arise before, or even in the absence of, the cutaneous manifestations. It involves the small joints of the fingers and toes, typically the distal interphalangeal joints (polyarthritis; distal interphalangeal [DIP] arthritis), but sometimes also individual large joints (oligoarthritis). About 40% of patients with PsA have involvement of the spine (as well), with spondylarthritis and sacroiliitis. Asymmetrical joint involvement is typical, e.g., arthritis of all the joints of one finger with sparing of, or involvement of only a single joint in, the neighboring fingers. Another typical feature of PsA is inflammation at the sites of attachment of tendons, ligaments, and joint capsules to bone (enthesitis). The most commonly affected site of this type is the attachment of the Achilles tendon. The inflammatory involvement of the tendons and tendon sheaths may lead to inflammatory swelling of affected fingers or toes, which is called dactylitis.
Nail involvement.
The nails are involved in about 30% of patients with psoriasis.
Recent studies have shown that the course of PsA is often more severe than previously assumed. Over the course of the disease, five or more joints are affected in more than 40% of patients; 20% suffer progressive bony changes similar to those of rheumatoid arthritis; and about 5% have an aggressive and destructive variant with rapid joint destruction (arthritis mutilans) (9). Early diagnosis is important, because PsA can now be treated very effectively with TNF-alpha antagonists, which can arrest the progression of the bony changes (evidence level 1–2 [the highest level]) (10). The dermatologist and family physician should look for signs of PsA in any patient with psoriasis. These signs include painful and/or swollen joints with worst symptoms in the morning, enthesitis (e.g., inflammatory swelling of the attachment of the Achilles tendon), and dactylitis with swelling of individual fingers or toes. Once PsA is suspected, the patient should undergo further rheumatological diagnostic evaluation. If cutaneous manifestations and PsA are simultaneously present, the treatment strategy may have to be worked out in an interdisciplinary collaboration. It should be borne in mind that, among the substances that are currently approved in Germany for the treatment of PsA, only MTX and the TNF-alpha antagonists are also effective against the cutaneous manifestations of psoriasis.
The clinical severity of psoriasis
Psoriasis is classified as mild, moderate, or severe. This classification takes account of the severity of the cutaneous manifestations, which are usually rated with the Psoriasis Area and Severity Index (PASI)—an index based on the degree of erythema, infiltration, and scaling and the extent of involvement of the four body areas (head, trunk, arms, and legs) (e3)—or else as a percentage of the total body surface area (BSA).
Psoriasis is classified as mild if the PASI is below 10, and moderate to severe if it is 10 or above; the highest possible PASI value is 72. Values over 40 are only rarely encountered. According to the S3 guidelines, PASI >10 is the criterion for moderate to severe psoriasis. If the BSA is used, BSA >10 is considered to be the criterion for moderate to severe psoriasis (14).
Psoriatic arthritis.
About 20% of patients with psoriasis have psoriatic arthritis.
The classification of psoriasis as mild, moderate, or severe also takes the individual impairment of health-related life quality into account. A useful instrument for assessing this is the Dermatology Life Quality Index (DLQI) (see the downloadable questionnaire at http://www.dermatology.org.uk/index.asp?portal/quality/dlqi.html. The DLQI can take values from 0 to 30. If the DLQI is 0 or 1, the patient’s quality of life is, generally speaking, not impaired by the dermatological disease in any way. Values higher than 10 speak for a very severe impairment of the quality of life (11). All of these parameters are also used to judge the efficacy of treatment in clinical studies and are suitable for therapeutic goal-setting. If the PASI or BSA is greater than 10, and the DLQI is also greater than 10, then the patient is considered to have moderate to severe psoriasis, which generally needs to be treated systemically (12, 13). In assessing the severity of psoriasis, one must also consider further parameters such as the degree of response to earlier treatments, the involvement of visible areas (including the scalp and nails) or of the genital area, and the presence or absence of special symptoms, like itching, that may necessitate systemic treatment in some cases (14).
Not only the severity of the disease, but also its degree of activity is important. Highly active disease may be suggested by a history of new lesions arising at short intervals, of the expansion of existing foci, and of multiple recurrences after treatment. These factors are particularly important for the choice of therapeutic strategy.
Comorbidities
Patients with psoriasis more commonly suffer from certain accompanying conditions that considerably raise the morbidity and, to some extent, also the mortality of the disease and may shorten patients’ life expectancy (15). The increased rate of cardiovascular diseases such as myocardial infarction and stroke suggests that these are directly or indirectly promoted by the chronic inflammatory process. The direct promotion of cardiovascular diseases, e.g., by endothelial dysfunction caused by permanently elevated levels of mediators such as vascular endothelial growth factor (VEGF), considerably raises the frequency of atherosclerosis even among patients with psoriasis or PsA who have none of the classical risk factors (16, e4). This functional and pathophysiological interrelatedness of conditions is part of the current scientific understanding of comorbidity.
The risk of cardiovascular diseases among patients with psoriasis is also elevated by the increased frequency of metabolic syndrome, which is a clinical complex consisting of arterial hypertension, diabetes, and lipid metabolic disturbance due to obesity (17). The metabolic syndrome is about twice as common in 40- to 60-year-olds with psoriasis as in control subjects (18).
Rating the severity of psoriasis.
The PASI is the most commonly used instrument for rating the severity of psoriasis.
Direct and indirect effects may also underlie the markedly elevated risk of coronary artery sclerosis, myocardial infarction, and stroke among patients with psoriasis, depending on their age and the severity of the disease. Thus, for a 30-year-old patient with severe psoriasis, the risk of myocardial infarction is elevated roughly threefold (19). Patients who must be hospitalized repeatedly at a young age for the treatment of psoriasis have an elevated risk of myocardial infarction or stroke, by a factor of about 2.6 (20).
The therapeutic implication is that particular attention should be paid to comorbidities such as metabolic syndrome and their treatment in patients with psoriasis, and that any coexisting risk factors for cardiovascular diseases should be identified and treated.
Quality of life
Comparative studies and polling of large samples of the members of patient self-help organizations have revealed that patients with psoriasis are emotionally and physically impaired by their disease to an extent comparable to patients with heart disease, cancer, or diabetes (21, 22, e5). A study of patients visiting dermatologists because of psoriasis in Germany in 2005 revealed a high average degree of disease activity and a marked impairment of health-related life quality, with a mean DLQI of 8.6 (23). Patients with psoriasis have not only a higher rate of suicidality, but also a higher rate of depressive disorders and higher degree of alcohol consumption than the general population (24). Like other chronic skin diseases, psoriasis causes a marked stigmatization of the affected persons. Studies have shown that patients with psoriasis also have impaired coping skills.
Guidelines and therapeutic goals for psoriasis
An improved understanding of the marked impairment of the affected patients’ quality of life on the one hand, and the scientific concept of psoriasis as a systemic inflammatory disease associated with major morbidity and increased mortality on the other, have led to a reconsideration of the goals of treatment for psoriasis, especially in view of the fact that patients with this disease are still receiving inadequate care (23).
An improvement of the PASI by at least 75% (PASI 75) and a reduction of the DLQI to 0 or 1 have been suggested as goals for the short- and long-term treatment of psoriasis (14). Whenever the much more modest, minimal goals of a 50% improvement of the PASI and a reduction of the DLQI to below 5 have not been met, the treatment should be changed.
Comorbidities.
Metabolic syndrome and cardiovascular diseases are the most important comorbidities of psoriasis.
In the current S3 therapeutic guidelines, the effectiveness of different types of treatment for psoriasis is discussed on the basis of the available clinical studies (11). The probability that a patient will achieve the PASI 75 therapeutic goal after induction therapy is stated for each of the systemic therapies. These guidelines, as well as various S1 guidelines on individual drugs, also contain information on the practical aspects of treatment, e.g., contraindications, safety issues, and diagnostic tests that should be performed before and after treatment.
Treatment
Topical therapy
Local therapy alone may bring about adequate improvement in mild or moderate cases of psoriasis. Local therapy is also generally used in addition whenever phototherapy or systemic therapy is given.
Quality of life.
The goal of short- and long-term treatment of psoriasis should be a marked, measurable improvement of the quality of life.
Topical corticoids of strength classes II and III remain the medications most commonly used to treat psoriasis. They have a favorable risk/benefit profile when properly used and are also very effective against itching, from which about two-thirds of patients suffer. Topical corticoids should not be used over long periods (i.e., for more than 6 weeks continuously) without interruption in order not to produce typical corticoid side effects such as skin atrophy.
Vitamin D3 analogues (cacipotriol, tacalcitol) have come into wide use in recent years. These, too, have a favorable risk/benefit profile, though they are somewhat less effective than the corticoids. Side effects such as local irritation at the beginning of treatment, are rare. A combined preparation consisting of the vitamin D3 analogue calcipotriol together with a corticoid of intermediate strength, which was studied in a controlled trial over a study interval of an entire year, is very effective and is often used as first-line treatment. The medication can be applied once daily, and its safety, tolerability, and effectiveness are high (25, e6). Anthralin is still used with good results, mainly in the inpatient setting, but it is impractical for use by outpatients. This drug, too, causes local irritation ("anthralin dermatitis"). Tars have been shown to have an unfavorable risk-benefit profile and are no longer used in the topical treatment of psoriasis (11). Other skin-care products can be used to good effect alongside the specifically antipsoriatic medications, or during treatment intervals.
Phototherapy
For moderately severe psoriasis or psoriasis that has not responded to topical treatment alone, the application of ultraviolet light is suitable for inducing a remission but not for maintenance therapy, because its prolonged use is associated with a higher risk of skin cancer. The most commonly used type at present is narrow-spectrum UVB therapy (311 nm). UVA irradiation after the administration of a photosensitizer (psoralen) by the oral route, in bathwater, or as a cream (PUVA) is highly effective.
Topical therapy.
Topical corticoids of class II and III strength are the medications most commonly used to treat psoriasis.
A combination of the vitamin D3 analogue calcipotriol with a moderately potent corticoid can be used successfully.
Systemic therapy
Systemic treatment plays a role mainly for patients with moderate to severe psoriasis and for patients with frequent recurrences and highly active disease. Over the last few years, the indications for systemic therapy have expanded: Systemic therapy should always be considered when other treatments have not resulted in a clinical benefit. This criterion includes patients with severe nail psoriasis or with particularly severe involvement of visible areas.
The spectrum of approved medications is relatively broad. In Germany, fumaric acid esters are the most commonly used medications for the systemic treatment of psoriasis. They are both effective and safe for long-term use, although they can cause gastrointestinal symptoms. Methotrexate (MTX) is mainly given to treat pustular psoriasis and psoriatic arthritis. This medication, too, has been in use for many years. When it is properly administered, hepatic damage from cumulative toxicity is rare. A routine liver biopsy after a total cumulative dose of 1.5 g is now no longer recommended. The retinoid acitretin, because of its relatively low efficacy and frequent adverse effects, is now given almost exclusively in combination with UV therapy, but monotherapy with this drug may be effective against pustular psoriasis. Women of child-bearing age should not be treated with acitretin. Ciclosporin is highly effective and thus suitable for induction therapy, though not for long-term maintenance therapy, because of the risk of irreversible renal failure and also because of the elevated risk of skin cancer in patients who have previously undergone phototherapy.
A major expansion of the therapeutic armamentarium against psoriasis has come about with the approval of a number of "biologics" (biological agents) (table 2), which have a good benefit-risk profile overall and are also effective in patients who cannot (or can no longer) be treated adequately with the systemic therapies available till now. These biologics are, by definition, genetically or biotechnologically generated products of living cells. They are cytokines, fusion proteins composed of surface molecules and the constant fragment of immunoglobulins, or antibodies that compensate for the disequilibrium of the inadequately regulated immune system, leading to improvement of the clinical manifestations.
Table 2. The treatment of psoriasis vulgaris (induction therapy).
| Severity of psoriasis | Treatment | Effectiveness*1 | Evidence level*1 | |
| Agent | Administration | |||
| Mild*2 | Dithranol | Local | ++ | 2 |
| Corticoids (class II-IV agents) | Local | ++++ | 1 | |
| Vitamin D3 and its analogues | Local | +++ | 1 | |
| Moderate to severe | UVB | Local | +++ | 2 |
| PUVA | Local | +++ to ++++ | 2 | |
| Acitretin | Oral | + | 3 | |
| Ciclosporine | Oral | ++ to +++ | 1 | |
| Fumarates | Oral | ++ | 2 | |
| Methotrexate | Preferably subcutaneous | ++ | 3 | |
| Efalizumab | Subcutaneous | + | 1 | |
| Etanercept | Subcutaneous | + to ++ | 1 | |
| Infliximab | Intravenous | +++ to ++++ | 1 | |
| Adalimumab | Subcutaneous | +++ to ++++ | (1)*3 | |
*1 Assessment according to S3 guidelines (14); evaluation scale: – (poor), +/–, +, ++, +++, ++++ (good).
*2 Topical therapy is also used for moderate to severe psoriasis in combination with UV or systemic therapy.
*3 Assessment on the basis of published studies; because this agent had not been approved when the S3 guidelines were written, it is not evaluated there.
Infliximab and adalimumab are therapeutic antibodies directed against TNF-alpha that are highly effective against psoriasis vulgaris and psoriatic arthritis.
The effectiveness of etanercept, a fusion protein directed against TNF-alpha, in the treatment of psoriatic arthritis is comparable to that of the antibodies. To achieve a marked improvement of the cutaneous manifestations, high doses (50 mg twice weekly) are usually used in the first 12 weeks.
Systemic therapy.
Systemic therapy is indicated for all moderate and severe types of psoriasis.
Efalizumab, an antibody against the cell-surface adhesion molecule LFA-1 (leukocyte function-associated antigen 1), inhibits the migration of inflammatory cells into the skin and is effective only against psoriasis vulgaris, not against psoriatic arthritis. Successful treatment is achieved after six months in about 40% of patients, but some patients can also benefit from long-term therapy.
All biologics that have been approved for the treatment of psoriasis confer an elevated risk of infection while they are being used. When the TNF-alpha antagonists are used, there is also a risk of reactivating tuberculosis. Detailed information on the evidence-based efficacy of the various local and systemic drugs that are used to treat psoriasis, as well as numerous practical recommendations for their use, can be found in the current "Evidence-based (S3) guidelines for the treatment of psoriasis vulgaris" (11).
Biologics.
All biologics that have been approved for the treatment of psoriasis confer an elevated risk of infection while they are being used.
Further information.
This article has been certified by the North Rhine Academy for Postgraduate and Continuing Medical Education.
Deutsches Ärzteblatt provides certified continuing medical education (CME) in accordance with the requirements of the Chambers of Physicians of the German federal states (Länder). CME points of the Chambers of Physicians can be acquired only through the Internet, not by mail or fax, by the use of the German version of the CME questionnaire within 6 weeks of publication of the article. See the following website: www.aerzteblatt.de/cme.
Participants in the CME program can manage their CME points with their 15-digit "uniform CME number" (einheitliche Fortbildungsnummer, EFN). The EFN must be entered in the appropriate field in the www.aerzteblatt.de website under "meine Daten" ("my data"), or upon registration. The EFN appears on each participant’s CME certificate.
The solutions to the following questions will be published in volume 9/2009. The CME unit "Tonsillectomy in Children" (volume 49/2008) can be accessed until 16 January 2009.
For volume 5/2009 we plan to offer the topic "Lyme Disease—Current State of Knowledge."
Solutions to the CME questionnaire in volume 45/2008:
Bschor T, Adli M: Treatment of depressive disorders:1/e, 2/a, 3/c, 4/c, 5/e, 6/d, 7/d, 8/e, 9/c, 10/b
Please answer the following questions to participate in our certified Continuing Medical Education program. Only one answer is possible per question. Please select the answer that is most appropriate.
Question 1
Which of the following genes is a risk gene for psoriasis?
The interleukin-2 gene
The interferon gene
The interleukin-23 receptor gene
The interleukin-8 gene
The TGF-beta gene
Question 2
How old are most patients on the initial manifestation of type 1 psoriasis?
Under 22 years old
Between 27 and 32 years old
Between 33 and 38 years old
Between 39 and 44 years old
Above 45 years old
Question 3
Which cells are especially important for the generation of a psoriatic lesion?
Dendritic cells
B cells
Fibroblasts
Merkel cells
Melanocytes
Question 4
Which of the following is typical of the localization of psoriatic arthritis?
The spine is spared.
It is limited to the major joints.
It is restricted to synovial inflammation.
It affects the joints asymmetrically.
It affects the joints symmetrically.
Question 5
Psoriasis is today counted among the "immune-mediated inflammatory diseases" along with rheumatoid arthritis and Crohn’s disease. What is the relative prevalence of these three diseases in Germany?
Psoriasis > Crohn’s disease > rheumatoid arthritis
Rheumatoid arthritis > psoriasis > Crohn’s disease
Crohn’s disease > rheumatoid arthritis > psoriasis
Psoriasis > rheumatoid arthritis > Crohn’s disease
Rheumatoid arthritis > Crohn’s disease > psoriasis
Question 6
Which of the following medications is often used in Germany for the systemic treatment of psoriasis vulgaris?
Corticoids
Azathioprine
Sulfasalazine
Cyclophosphamide
Fumaric acid esters
Question 7
Which of the following is a precipitating factor that can bring about the initial manifestation of psoriasis or a worsening in its later course?
The use of beta-blockers
The use of quinolone antibiotics
The use of tramadol
The use of paracetamol (acetaminophen)
The use of citrus extracts
Question 8
Which of the following is an important comorbidity of psoriasis?
Helicobacter pylori infection
Cholecystolithiasis
Metabolic syndrome
Adrenal insufficiency
Cardiomyopathy
Question 9
Which types of psoriasis can be treated with ultraviolet light to induce remission?
Moderately severe psoriasis, as maintenance therapy
Mild psoriasis and psoriasis that responds to topical treatment
Moderate psoriasis and psoriasis that does not respond to topical treatment
Severe psoriasis, as maintenance therapy
Mild psoriasis and psoriasis that does not respond to topical treatment
Question 10
Which of the following medications is/are suitable for the topical treatment of psoriasis?
Benzoyl peroxide
Vitamin E analogues
Vitamin D3 analogues
Antiseptic agents
Antihistamines
Acknowledgments
Translated from the original German by Ethan Taub, M.D.
Footnotes
Conflict of interest statement
Professor U. Mrowietz has received honoraria for advisory services and lectures, as well as travel expenses, from the following companies and participated in the performance of their clinical studies and cooperative scientific projects: Abbott, Biogen-Idec, Fumapharm, Essex, Schering-Plough, Centocor, Wyeth, Merck-Serono, Leo Pharma, and Galderma.
Professor K. Reich has received honoraria and/or travel expenses for advisory and/or lecturing activities, and/or participated in the performance of clinical studies for the following companies: Abbott, Biogen-Idec, Bristol-Myers Squibb, Centocor, Essex Pharma, Leo Pharma, Schering-Plough, Merck-Serono, and Wyeth.
References
- 1.Henseler T, Christophers E. Psoriasis of early and late onset: characterization of two types of psoriasis vulgaris. J Am Acad Dermatol. 1985;13:450–456. doi: 10.1016/s0190-9622(85)70188-0. [DOI] [PubMed] [Google Scholar]
- 2.Reich K, Hüffmeier U, König IR, et al. TNF polymorphisms in psoriasis: association of psoriatic arthritis with the promoter polymorphism TNF*-875 independent of the PSORS1 risk allele. Arthritis Rheum. 2007;56:2056–2064. doi: 10.1002/art.22590. [DOI] [PubMed] [Google Scholar]
- 3.Cargill M, Schrodi SJ, Chang M, et al. A large-scale genetic association study confirms IL12B and leads to the identification of IL23R as psoriasis-risk genes. Am J Hum Genet. 2007;80:273–290. doi: 10.1086/511051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Diluvio L, Vollmer S, Besgen P, Ellwart JW, Chimenti S, Prinz JC. Identical TCR beta-chain rearrangements in streptococcal angina and skin lesions of patients with psoriasis vulgaris. J Immunol. 2006;176:7104–7111. doi: 10.4049/jimmunol.176.11.7104. [DOI] [PubMed] [Google Scholar]
- 5.Fry L, Baker BS. Triggering psoriasis: the role of infections and medications. Clin Dermatol. 2007;25:606–615. doi: 10.1016/j.clindermatol.2007.08.015. [DOI] [PubMed] [Google Scholar]
- 6.Schröder JM, Reich K, Kabashima K, et al. Who is really in control of skin immunity under physiological circumstances - lymphocytes, dendritic cells or keratinocytes? Exp Dermatol. 2006;15:913–929. doi: 10.1111/j.1600-0625.2006.00506_1.x. [DOI] [PubMed] [Google Scholar]
- 7.Harder J, Schröder JM. Psoriatic scales: a promising source for the isolation of human skin-derived antimicrobial proteins. J Leukoc Biol. 2005;77:476–486. doi: 10.1189/jlb.0704409. [DOI] [PubMed] [Google Scholar]
- 8.Sampogna F, Gisondi P, Melchi CF, et al. Prevalence of symptoms experienced by patients with different clinical types of psoriasis. Br J Dermatol. 2004;151:594–599. doi: 10.1111/j.1365-2133.2004.06093.x. [DOI] [PubMed] [Google Scholar]
- 9.Gladman DD, Antoni C, Mease P, Clegg DO, Nash P. Psoriatic arthritis: epidemiology, clinical features, course, and outcome. Ann Rheum Dis. 2005;64(Suppl 2):ii14–ii17. doi: 10.1136/ard.2004.032482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Soriano ER, McHugh NJ. Therapies for peripheral joint disease in psoriatic arthritis. A systematic review. J Rheumatol. 2006;331:422–430. [PubMed] [Google Scholar]
- 11.Nast A, Kopp IB, Augustin M, et al. Evidence-based (S3) guidelines for the treatment of psoriasis vulgaris. J Dtsch Dermatol Ges. 2007;5(Suppl 3):111–119. doi: 10.1111/j.1610-0387.2007.06172.x. [DOI] [PubMed] [Google Scholar]
- 12.Finlay AY. Current severe psoriasis and the rule of tens. Br J Dermatol. 2005;152:861–867. doi: 10.1111/j.1365-2133.2005.06502.x. [DOI] [PubMed] [Google Scholar]
- 13.Smith CH, Anstey AV, Barker JN, et al. British Association of Dermatologists. British Association of Dermatologists guidelines for use of biological interventions in psoriasis 2005. Br J Dermatol. 2005;153:486–497. doi: 10.1111/j.1365-2133.2005.06893.x. [DOI] [PubMed] [Google Scholar]
- 14.Reich K, Mrowietz U. Treatment goals in psoriasis. J Dtsch Dermatol Ges. 2007;5:566–574. doi: 10.1111/j.1610-0387.2007.06343.x. [DOI] [PubMed] [Google Scholar]
- 15.Mrowietz U, Elder JT, Barker J. The importance of disease associations and concomitant therapy for the long-term management of psoriasis patients. Arch Dermatol Res. 2006;298:309–319. doi: 10.1007/s00403-006-0707-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ludwig RJ, Herzog C, Rostock A, et al. Psoriasis: a possible risk factor for development of coronary artery calcification. Br J Dermatol. 2007;156:271–276. doi: 10.1111/j.1365-2133.2006.07562.x. [DOI] [PubMed] [Google Scholar]
- 17.Sommer DM, Jenisch S, Suchan M, Christophers E, Weichenthal M. Increased prevalence of the metabolic syndrome in patients with moderate to severe psoriasis. Arch Dermatol Res. 2006;298:321–328. doi: 10.1007/s00403-006-0703-z. [DOI] [PubMed] [Google Scholar]
- 18.Gisondi P, Tessari G, Conti A, et al. Prevalence of metabolic syndrome in patients with psoriasis: a hospital-based case-control study. Br J Dermatol. 2007;157:68–73. doi: 10.1111/j.1365-2133.2007.07986.x. [DOI] [PubMed] [Google Scholar]
- 19.Gelfand JM, Neimann AL, Shin DB, Wang X, Margolis DJ, Troxel AB. Risk of myocardial infarction in patients with psoriasis. JAMA. 2006;296:1735–1741. doi: 10.1001/jama.296.14.1735. [DOI] [PubMed] [Google Scholar]
- 20.Mallbris L, Akre O, Granath F, et al. Increased risk for cardiovascular mortality in psoriasis inpatients but not in outpatients. Eur J Epidemiol. 2004;19:225–230. doi: 10.1023/b:ejep.0000020447.59150.f9. [DOI] [PubMed] [Google Scholar]
- 21.Dubertret L, Mrowietz U, Ranki A, et al. European patient perspectives on the impact of psoriasis: the EUROPSO patient membership survey. Br J Dermatol. 2006;155:729–736. doi: 10.1111/j.1365-2133.2006.07405.x. [DOI] [PubMed] [Google Scholar]
- 22.Rapp SR, Feldman SR, Exum ML, Fleischer AB, Jr, Reboussin DM. Psoriasis causes as much disability as other major medical diseases. J Am Acad Dermatol. 1999;41(3 Pt 1):401–407. doi: 10.1016/s0190-9622(99)70112-x. [DOI] [PubMed] [Google Scholar]
- 23.Augustin M, Krüger K, Radtke MA, Schwippl I, Reich K. Disease severity, quality of life and health care in plaque-type psoriasis: a multicenter cross-sectional study in Germany. Dermatology. 2008;216:366–372. doi: 10.1159/000119415. [DOI] [PubMed] [Google Scholar]
- 24.Naldi L, Parazzini F, Brevi A, et al. Family history, smoking habits, alcohol consumption and risk of psoriasis. Br J Dermatol. 1992;127:212–217. doi: 10.1111/j.1365-2133.1992.tb00116.x. [DOI] [PubMed] [Google Scholar]
- 25.Kragballe K, Austad J, Barnes L, et al. Efficacy results of a 52-week, randomised, double-blind, safety study of a calcipotriol/betamethasone dipropionate two-compound product (Daivobet/Dovobet/Taclonex) in the treatment of psoriasis vulgaris. Dermatology. 2006;213:319–326. doi: 10.1159/000096069. [DOI] [PubMed] [Google Scholar]
- e1.Nair RP, Stuart PE, Nistor I, et al. Sequence and haplotype analysis supports HLA-C as the psoriasis susceptibility 1 gene. Am J Hum Genet. 2006;78:827–851. doi: 10.1086/503821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e2.Sano S, Chan KS, Carbajal S, et al. Stat3 links activated keratinocytes and immunocytes required for development of psoriasis in a novel transgenic mouse model. Nat Med. 2005;11:43–49. doi: 10.1038/nm1162. [DOI] [PubMed] [Google Scholar]
- e3.Regionale Psoriasisnetze in Deutschland. www.psonet.de.
- e4.Gonzalez-Juanatey C, Llorca J, Amigo-Diaz E, Dierssen T, Martin J, Gonzalez-Gay MA. High prevalence of subclinical atherosclerosis in psoriatic arthritis patients without clinically evident cardiovascular disease or classic atherosclerosis risk factors. Arthritis Rheum. 2007;57:1074–1080. doi: 10.1002/art.22884. [DOI] [PubMed] [Google Scholar]
- e5.Krueger G, Koo J, Lebwohl M, Menter A, Stern RS, Rolstad T. The impact of psoriasis on quality of life: results of a 1998 National Psoriasis Foundation patient-membership survey. Arch Dermatol. 2001;137:280–284. [PubMed] [Google Scholar]
- e6.Kragballe K, van de Kerkhof PC. Consistency of data in six phase III clinical studies of a two-compound product containing calcipotriol and betamethasone dipropionate ointment for the treatment of psoriasis. J Eur Acad Dermatol Venereol. 2006;20:39–44. doi: 10.1111/j.1468-3083.2005.01343.x. [DOI] [PubMed] [Google Scholar]