Abstract
Many plant pathogens secrete toxins that enhance microbial virulence by killing host cells. Usually, these toxins are produced by particular microbial taxa, such as bacteria or fungi. In contrast, many bacterial, fungal and oomycete species produce necrosis and ethylene-inducing peptide 1 (Nep1)-like proteins (NLPs) that trigger leaf necrosis and immunity-associated responses in various plants. We have determined the crystal structure of an NLP from the phytopathogenic oomycete Pythium aphanidermatum to 1.35Å resolution. The protein fold exhibits structural similarities to cytolytic toxins produced by marine organisms (actinoporins). Computational modeling of the 3-dimensional structure of NLPs from another oomycete, Phytophthora parasitica, and from the phytopathogenic bacterium, Pectobacterium carotovorum, revealed a high extent of fold conservation. Expression of the 2 oomycete NLPs in an nlp-deficient P. carotovorum strain restored bacterial virulence, suggesting that NLPs of prokaryotic and eukaryotic origins are orthologous proteins. NLP mutant protein analyses revealed that identical structural properties were required to cause plasma membrane permeabilization and cytolysis in plant cells, as well as to restore bacterial virulence. In sum, NLPs are conserved virulence factors whose taxonomic distribution is exceptional for microbial phytotoxins, and that contribute to host infection by plasma membrane destruction and cytolysis. We further show that NLP-mediated phytotoxicity and plant defense gene expression share identical fold requirements, suggesting that toxin-mediated interference with host integrity triggers plant immunity-associated responses. Phytotoxin-induced cellular damage-associated activation of plant defenses is reminiscent of microbial toxin-induced inflammasome activation in vertebrates and may thus constitute another conserved element in animal and plant innate immunity.
Keywords: crystal structure, immunity, pathogen, plant immunity, phytotoxin
Millions of years of coevolution of plants and microbial pathogens have shaped both the abilities of microbial pathogens to overcome plant disease resistance and the abilities of plants to cope with microbial invasion (1). Phytopathogens from different taxonomic origins secrete structurally unrelated effectors into the apoplast and cytoplasm of plants to establish infection and to suppress host defenses (2–4). In addition, phytopathogenic micro-organisms produce a wide range of cytolytic toxins that function as key virulence determinants (5, 6). In particular, micro-organisms preferring hemibiotrophic or necrotrophic lifestyles produce host-selective and host-nonselective toxins that contribute to microbial virulence by facilitating the killing of plant tissues (6–8).
Microbial pattern recognition is a prerequisite for the initiation of antimicrobial defenses in all multicellular organisms, including plants. The bipartite plant immune system is based upon recognition of pathogen-associated molecular patterns by pattern-recognition receptors (1, 9) as well as upon the activities of resistance proteins that have evolved to recognize the presence or activities of microbial effectors (2). In addition to the recognition of microbial patterns and effectors, plants also possess capacities to sense host-derived “damage” patterns that originate, for example, from the degradation of the plant cell wall by microbial hydrolytic enzyme activities (10). Paradoxically, some phytopathogenic microbe-derived cytolytic toxins have also been reported to elicit plant defenses, including Fusarium spp.-derived fumonisin B1, Phomopsis amygdali-derived fusicoccin, or the host-selective toxin, victorin, from Cochliobolus victoriae (6, 11). However, for virtually all microbial toxins with plant defense-stimulating potential, it is unknown whether activation of plant defenses results from toxin-induced cellular distress or, independently of toxin action, from recognition of toxins as microbial patterns by plant pattern-recognition receptors.
NLPs constitute a superfamily of proteins that are produced by various phytopathogenic micro-organisms, comprising both prokaryotic and eukaryotic organisms (3, 7, 12, 13). Necrosis and ethylene-inducing peptide 1 (Nep1)-like proteins (NLPs) trigger leaf necrosis that is genetically distinct from immunity-associated programmed cell death (11) and stimulate immunity-associated defenses in all dicotyledonous plants tested, but not in monocotyledonous plants (3, 7). Hence, NLPs were proposed to have dual functions in plant pathogen interactions, acting both as triggers of immune responses and as toxin-like virulence factors (11). The broad taxonomic distribution of NLPs, in particular their occurrence in both prokaryotic and eukaryotic species, is quite unusual for known microbial phytotoxins, the production of which is restricted to a narrow range of microbial species (6, 8).
Major open questions regarding the biological activities of NLPs are as to whether (i) NLPs from various microbial origins are functionally conserved, orthologous proteins; (ii) the necrotic activities of NLP proteins contribute to microbial virulence and infection; and (iii) the necrotic and defense-stimulating activities of NLP proteins are mechanistically linked. To address these questions, NLPPya from the phytopathogenic oomycete Pythium aphanidermatum (14) was selected for tertiary structure studies.
Results
The NLP Fold Is Highly Conserved and Is Distantly Related to Cytolytic Actinoporins.
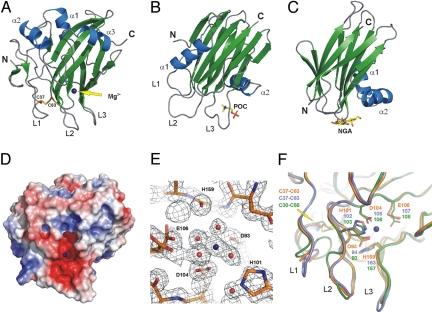
Recombinant NLPPya (lacking the N-terminal signal peptide for secretion) [supporting information (SI) Fig. S1] was produced in Escherichia coli, purified under denaturing conditions and reconstituted into its biologically active form (15). The refolded protein crystallized in orthorhombic crystals whose structure was solved at a resolution of 1.35 Å. The corresponding crystal structure (crystallography statistics given in Table S1) reveals a single-domain molecule with a fold consisting of a central β-sandwich, with 3 strands in the first sheet and a 5-stranded antiparallel second sheet. Three helices encompass the second sheet at the top of the sandwich, giving rise to a flat surface (Fig. 1A, and see Fig. S1). In contrast, an uneven surface is established at the base of the polypeptide mainly by 3 broad loops (L1–L3). Formation of an intramolecular disulfide bridge between 2 conserved cysteine residues that are essential for NLP activities (16) appears to be crucial to anchor loop L1 to the central sheet core (see Fig. 1A and Fig. S1). A cavity exhibiting a strong negative net charge is formed above loops L2/L3 at the surface of the rigid globular fold, with a divalent cation bound therein (Fig. 1D). Residues D93 (β5), D104 (β6), E106 (β6), and H159 (main chain carbonyl group, β10) are directly involved in cation binding, whereas H101 (β6) stabilizes the corresponding water network (see Fig. 1E and Fig. S1).
Fig. 1.
Crystal structure of NLPPya. Secondary structure elements are shown in blue (helices) and green (β-strands) (A–C). The bound magnesium atom is shown as a blue sphere (A, D–F). Residues involved in ion binding (sticks in E and F) or formation of the disulfide bond (sticks in A and F) are labeled. (A–C) Ribbon plot of NLPPya (A), the actinoporin, sticholysin (B), and the lectin, ABL (C). NGA, N-acetylgalactosamine; POC, phosphorylcholine. (D) Electrostatic surface of NLPPya with a color scale that varies from blue to red, representing positive and negative potential, respectively. (E) Electron density for the bound magnesium and its coordinating residues in NLPPya. The 2Fo-Fc density map (contoured at 1 σ) is shown in black. (F) Comparison of NLPPya (orange) with modeled structures of NLPPp (blue) and NLPPcc (green).
Tertiary structure-based comparison of the NLPPya fold [DALI-program (17), http:///www.ebi.ac.uk/dali/] revealed 35 proteins that scored over the default significance level for similarity (DALI Z scores 3.5–2.0) (Tables S2 and S3). Based upon visual inspection of any structural superimposition and in consideration of the physiological context, fungal lectins (Agaricus bisporus, Xerocomus chrysenteron) (18, 19) and actinoporins produced by sea anemones (Actinia equina equinatoxin II, Stichodactyla helianthus sticholysin) (20–22) are most similar to NLPPya (see Fig. 1 A–C). Like NLPPya, these proteins are small, single-domain polypeptides exhibiting a central β-sandwich architecture surrounded by helices. Superimposition of actinoporins and lectins suggests a high degree of structural similarity (score 10.0–10.8), whereas NLPPya represents a more distantly related protein (see Table S3). Actinoporins and lectins are soluble proteins that target specific components of membrane bilayers via a surface-exposed cavity (see Fig. 1 B and C and Fig. S2). Moreover, actinoporins are cytolytic toxins that form transmembrane pores via their flexible N-terminal regions (22). This N-terminal region is absent in lectins (see Fig. 1C), but is found in NLPPya to be required for NLP-induced necrosis and plant defense activation (16). This suggests that NLPs and actinoporins share a cytolytic, membrane-disintegrating mode of action.
The NLPPya fold was used as template to model the 3-dimensional structure of NLPPcc from the phytopathogenic bacterium, Pectobacterium carotovorum (23, 24), and NLPPp from the oomycete Phytophthora parasitica (16). Structurally outstanding regions of these proteins match to a high extent, despite of differences in primary sequences (Fig. 1F and see Fig. S1). In particular, the positions of residues constituting the cation-binding site and the disulfide bridge in NLPPya are conserved (see Fig. 1F). The high degree of tertiary structure conservation among NLPs suggests that these proteins are functionally homologous.
NLPs are Orthologous Virulence-Promoting Cytolytic Phytotoxins.
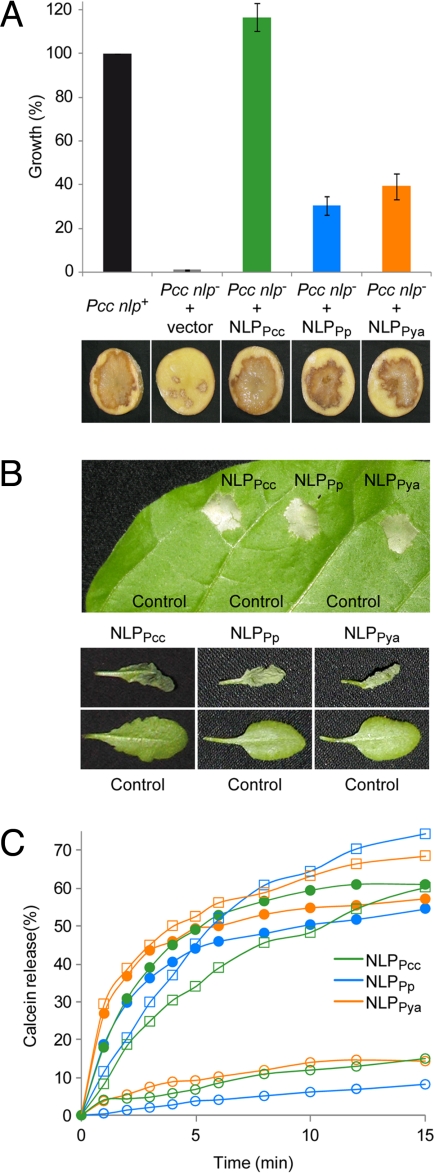
To test the aforementioned hypothesis, we transformed the NLP-deficient Pcc nlp– strain that was recently shown to exhibit reduced aggressiveness on potato (23, 24), with NLPPya or NLPPp-encoding sequences. Both strains showed partial restoration (30–40%) in bacterial virulence and potato tuber maceration relative to a control strain complemented with the endogenous NLPPcc gene (Fig. 2A). Quantitative differences in complementation efficiencies might be a result of NLPPp/NLPPya protein expression levels that were lower than that of NLPPcc (not shown). All together, our data suggest that NLPs are orthologous virulence factors.
Fig. 2.
NLPs are conserved virulence factors. (A) Complementation of the reduced virulence of the NLP-deficient P. carotovorum strain (Pcc nlp–) by expression of either NLPPcc or the oomycete-derived NLPPp and NLPPya, respectively. The wild-type strain (Pcc nlp+) and Pcc nlp– transformed with the empty vector are shown for comparison. Bacterial growth (Upper) (average +/– SD) as well as soft rot disease development on potato tuber disks (Lower) inoculated with the corresponding strains is indicated. (B) Plant necrosis-promoting activity of NLPs. Leaves from tobacco (Upper) or A. thaliana (Lower) were infiltrated with buffer as control or 0.5 μM NLP. Lesion formation 3 days after infiltration is shown. (C) Membrane permeabilization triggered by NLPPp (blue), NLPPya (orange), or NLPPcc (green). The NLP-induced release of calcein from plasma membrane vesicles obtained either from N. tabacum (closed circles), A. thaliana (open squares), or C. communis (open circles) is shown as the percentage of the maximal release obtained at the end of the assay by addition of the detergent Triton X-100. A representative experiment of 3 yielding similar results is shown.
NLPs trigger necrosis upon infiltration into leaves of dicotyledonous plants (Fig. 2B). We have previously shown that targeting of NLPPp exclusively to the extracellular side of plant plasma membranes results in lesion formation (11). To explore similarities in the molecular mode of action of actinoporins and NLPs we have used a vesicle-leakage assay. Highly enriched plant-plasma membrane vesicles were loaded with a fluorescent dye, calcein, to track membrane damage by measuring dye release (25). All NLPs tested disintegrated vesicles made of tobacco or Arabidopsis plasma membranes within 5 min upon application (Fig. 2C) at similar concentrations (EC50 values determined for tobacco: NLPPya, 0.4 nM; NLPPp, 3.5 nM; NLPPcc, 13.4 nM). In contrast, vesicles made of the monocot Commelina communis proved resistant to NLP treatment (see Fig. 2C), suggesting that NLPs constitute necrosis-promoting proteins that target dicot plasma membrane-specific surface structures and, subsequently, cause membrane disruption.
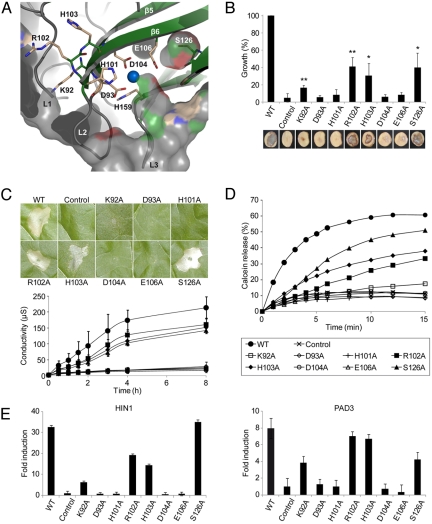
Comparison of NLP primary sequences found in public databases revealed strong conservation of a central hepta-peptide motif “GHRHDWE” (7) (amino acids 100–106 in NLPPya) (see Fig. S1). Remarkably, this region is part of the negatively charged cavity exposed at the protein surface (see Fig. 1 D and E). Three amino acid residues of this motif (H101, D104, E106) and another highly conserved residue (D93) are implicated in coordination of a divalent cation within this cavity (see Figs. 1E, 3A, and Fig. S1), suggesting that this domain is important for the biological activities of NLP proteins. To scrutinize this hypothesis, a total of 8 highly conserved amino acid residues (K92, D93, H101, R102, H103, D104, E106, S126) (see Fig. 3A and Fig. S1) were individually exchanged for alanine in both NLPPcc and NLPPp. The mutant proteins were analyzed for their ability to (i) complement the reduced virulence of the Pcc nlp– strain (NLPPcc mutant derivatives only) (Fig. 3B), (ii) trigger necrosis in tobacco leaves (Fig. 3C and Fig. S3), and (iii) permeabilize tobacco plasma membranes (Fig. 3D and see Fig. S3). We observed a strict qualitative and quantitative correlation of NLP activities. Mutant proteins that were unable to restore P. carotovorum virulence on potato (see Fig. 3B: D93A, H101A, D104A, E106A) failed to cause leaf necrosis (at concentrations up to 5 μM, not shown) (see Fig. 3C: concentration 0.5 μM) and to trigger calcein release from tobacco plasma membrane vesicles (see Fig. 3D). Likewise, mutants that retained the ability to complement the Pcc nlp– mutation (see Fig. 3B: R102A, H103A, S126A) caused necrosis (see Fig. 3C) and disturbed plasma membrane integrity (see Fig. 3D). All mutant proteins were shown to be produced and secreted from Pcc nlp– strains in similar size and amount (Fig. S4). Moreover, circular dichroism analyses of all mutant proteins tested and crystallization of 2 inactive NLPPya mutants (D93A and H101A) (see Fig. S4) revealed no significantly altered tertiary structures within the mutant proteins, suggesting that the observed differences in biological activities of the mutants are the result of subtle changes in the close vicinity of the mutated residues.
Fig. 3.
Structural requirements for the virulence-promoting cytolytic and plant defense stimulating activity of NLPs are identical. (A) Residues mutated in NLPPcc/NLPPp. Detailed view of the region between the β-sheet core and loops L1–L3. Selected residues that were individually mutated are shown as sticks. Parts of the semitransparent solid surface have been removed for clarity. (B) Complementation of the reduced virulence of the NLP-deficient P. carotovorum strain (Pcc nlp–) by expression of NLPPcc wild-type versus mutant proteins. See Fig. 2A for details. “*” (P-value <0.05) and “**” (P-value <0.005) indicates significant differences (Student's t test) from control values. (C) Plant necrosis-promoting activity of NLPPcc variants visualized by lesion formation (Upper) and electrolyte leakage (Lower) upon infiltration into tobacco leaves. See Fig. 2B for details. Labels are indicated in (D). Data in the lower panel refer to average values from 3 experiments +/– SD. (D) Permeabilization of tobacco plasma membrane vesicles triggered by NLPPcc variants. See Fig. 2C for details. (E) Early defense-related gene expression mediated by NLPPcc variants. Quantitative real-time RT-PCR analysis of HIN1 and PAD3 expression in tobacco leaves harvested 4 h after infiltration of 0.5 μM NLPPcc variants. The gene induction (fold change) by NLP infiltration was compared to the expression level of buffer infiltration (control). The data presented are the average of 3 determinations +/– SD per experiment. The experiment was done 3 times with similar results.
Measurement of necrosis by electrolyte leakage assays (see Fig. 3C) suggested that the cytolytic activity of the active mutants (R102A, H103A, S126A) was reduced. However, this reduction corresponded to partial virulence complementation and membrane disintegration activities of these NLP variants. In this regard, the NLP mutant protein K92A exhibits residual, albeit significantly reduced activity (Fig. 3 B and D) and, consequently, still triggers lesion formation at higher concentrations (5 μM; not shown). All together, our findings support the view that NLPs are toxins whose contribution to microbial virulence is based upon their abilities to permeabilize dicotyledonous plant plasma membranes, thereby causing cytolysis and necrosis.
Residues D93, H101, D104, and E106 are important for the cytolytic activity of NLPs and for the coordination of a Mg2+ ion within the acidic cavity (see Figs. 1E, 3A, and Fig. S3). To investigate the importance of a negative charge therein, we replaced D104 either by a noncharged (D104N), a cationic (D104K), or another anionic residue (D104E). All but one (D104E) abolished the cytolytic activities of the NLP mutant proteins (Fig. S5), suggesting that coordination of a cationic ligand is crucial. The Mg2+-occupancy in NLPPya crystals is not complete (≈60–70%), despite of high Mg2+-concentration in the crystallization buffer (500 mM). This indicates that Mg2+ may not be a physiological ligand. NLPs act at the plant cell surface in a Ca2+-rich environment. Indeed, scavenging extracellular calcium by a membrane-impermeable Ca2+-chelator, BAPTA, abolished the plasma membrane-disintegrating activity of NLPs (see Fig. S5), suggesting that coordinative binding of a Ca2+ ion is crucial for NLP function. Ca2+ may thus, as in the case of the C2 membrane binding domain of several extrinsic membrane proteins (26, 27), mediate docking of NLPs to target membranes, or might alternatively be required for the membrane-permeabilizing activity.
Identical Fold Requirements for NLP-Mediated Virulence and Plant Immunity.
Various microbial phytotoxins have been shown to trigger plant immunity-associated responses (6). In virtually all cases, however, it is unknown whether the toxin mode of action is responsible for the activation of plant defenses. To uncover a causal relationship between the cytolytic and defense-stimulating activities of NLPs, we tested NLP mutant proteins for their abilities to trigger immunity-associated gene expression in intact tobacco plants. Quantitative RT-PCR analysis of HIN1 and PAD3 transcript accumulation (28) revealed that mutant proteins R102A, H103A, and S126A trigger gene expression. In contrast, D93A, H101A, D104A, and E106A mutants are completely inactive, whereas K92A shows residual activity (Fig. 3E). Importantly, the same activity pattern of mutants was observed in virulence complementation, leaf cell cytolysis, and plasma membrane destabilization assays (see Fig. 3 B–D). Altogether, these data suggest that a common fold of a cytolytic toxin is required for both microbial pathogenicity and activation of plant immune responses. Thus, NLP-mediated plant-defense responses are likely the consequence of NLP-mediated cytolysis.
Discussion
We have combined tertiary structure and mutagenesis-based analyses with genetic and biochemical technologies to show that microbial NLPs constitute positive virulence factors accelerating disease and pathogen growth in host plants through disintegration of the plasma membrane and subsequent cytolysis. NLPs are distinguished from other known phytotoxins by their wide distribution across taxa and their broad spectrum of activity against dicotyledonous plants. In particular, the production of orthologous cytolytic toxins in prokaryotic and eukaryotic phytopathogenic microbes is without precedence and suggests that NLPs constitute an evolutionary ancient toxin fold that has been retained, preferably in organisms exhibiting a hemibiotrophic or necrotrophic lifestyle. This view is supported by published reports of NLPs from at least 10 different organisms (including bacterial, oomycete, and genuine fungal species) that have been demonstrated experimentally to possess cytolytic activities against dicotyledonous plant cells (7, 11). Importantly, by using the NLPPp sequence as query in BLAST searches, ≈130 sequences were retrieved from GenBank with E-values higher than that of NLPPcc [P. carotovorum (4e-16)], for which the cytolytic activity was demonstrated. This suggests that numerous, yet untested NLPs harbor a similar cytolytic activity as NLPPp, NLPPya, or NLPPcc. In addition, the high sequence conservation of the heptapeptide motif (GHRHDWE, see Fig. S1) required for all NLP activities further implies a widespread usage of cytolytic NLPs by taxonomically diverse microbial organisms. In the future, it can be expected that phylogeny-based relationship studies will facilitate analyses on the evolutionary origins of NLP. Such analyses may further reveal possible horizontal gene-transfer events that may have resulted in the transfer of prokaryotic sequences into unicellular eukaryotic microbes and that may be the reason for the unprecedented broad occurrence of NLP sequences in phylogenetically different taxa.
Accumulating evidence suggests that NLP gene sequences have also undergone functional diversification. NLP gene families from various Phytophthora species are exceptionally large (> 20 putative NLP-encoding genes) compared to those of other species (7, 11), suggesting that not all NLP proteins may harbor cytolytic activity. Indeed, of 3 different NLPs from P. infestans tested, only one (PiNPP1.1) was found to cause necrosis when expressed in plants (29). Importantly, the 2 noncytolytic NLPs carry mutations in an aspartate residue that corresponds to NLPPya D104, which is essential for the negative charge of the GHRHDWE motif and for the cytolytic activity of NLPs (see Fig. 3 and Fig. S3). Moreover, the genomes of the fungal pathogens Magnaporthe grisea and Mycosphaerella graminicola (30, 31) encode NLP sequences. This is important because the host range of both pathogens is restricted to monocots that are known to be resistant to the cytolytic activities of NLPs (11). In agreement with that finding, the M. graminicola NLP caused cytolysis and plant defense activation in Arabidopsis, but not in wheat (31), suggesting that these proteins might have either lost their physiological importance to the fungus [inactivation of the sole NLP in M. graminicola did not affect its virulence (31)] or might have adopted a yet elusive novel activity. The latter assumption is supported by the fact that the obligate biotroph oomycete, Hyaloperonospora arabidopsidis, produces at least 3 NLP proteins, none of which proved to be cytolytic (A. Cabral, G. van den Ackerveken, personal communication). While the function of these NLPs is currently unclear, it is tempting to speculate that the NLP fold, which is also similar to that of fungal lectins (see Fig. 1C and Fig. S2), has been reshaped during evolution to serve functions in microbial attachment to host plant surfaces.
Our data further imply that toxin-induced interference with cell integrity may culminate in plant immune responses. While the plant defense-stimulating potential of a number of mycotoxins is known for some time (6, 11), our data uniquely demonstrate that the virulence activity of a microbial toxin is also the cause for stimulation of plant defenses. Importantly, microbial toxin-induced innate immunity is also known from animal systems. Cytolytic bacterial (pneumolysin), fungal (nigericin), or marine (maitotoxin) toxins are triggers of the mammalian inflammasome (32, 33). Toxin-mediated activation of mammalian immune responses is based upon the recognition of endogenous, host-derived compounds that are released upon toxin-induced host cell damage and subsequent formation of damage-associated molecular patterns (34–36). Consequently, mammalian inflammasome activation is now considered to be activated not only upon perception of microbial patterns, but also by the action of toxins (32, 33, 37, 38). We suggest that plant cells are also capable of sensing toxin-induced cellular changes. NLP-driven membrane disruption may result in the release of host-derived molecules that serve as endogenous damage-associated molecular patterns. Alternatively, NLP-induced disturbance of the cellular ion homeostasis or membrane potential may signal activation of plant defenses. Remarkably, NLP-induced Ca2+ and H+ influx, as well as K+ efflux, were reported (16, 39) that mimicked synthetic ionophore-induced ion fluxes in plant cells, which themselves were shown to trigger plant defense-associated responses in a nonreceptor-mediated manner (40). Our structure-based analyses suggest that NLPs are cytolytic toxins that trigger plant immunity-associated defenses through interference with plant tissue integrity. Hence, disturbed host integrity as a common signal for the activation of immune defenses adds to the list of conceptual similarities in the organization of innate immunity in the animal and plant lineage.
Materials and Methods
Recombinant NLP Purification and 3-Dimensional Structure Determination of NLPPya.
For functional studies, secretory expression of NLPs was performed either in Pichia pastoris GS115 (secretory expression plasmid pPIC9K, MultiCopy Pichia Expression Kit Instructions, Invitrogen) or in the NLP-deficient P. carotovoum subsp. carotovorum SCC3200 strain (Pcc nlp–) (23). For crystallization of NLPPya and mutants D93A and H101A, recombinant proteins were produced without signal peptides as described in ref. 15. Further experimental details on recombinant protein expression, protein structure elucidation and analysis can be found in SI Materials and Methods.
Plant Material and Growth Conditions.
Nicotiana tabacum cv. Samsun NN, Commelina communis L., and Arabidopsis thaliana Col-0 were grown at 22 °C (15 h light). Solanum tuberosum cv. Atica tubers were obtained from Sa Ka Pflanzenzucht GbR.
Leaf Infiltration and Electrolyte Leakage Assay.
Purified NLPs (0,5 μM, 10 μl) or mock solution were infiltrated abaxialy into leaves of N. tabacum (4–6 weeks old) or A. thaliana (3–4 weeks old). For quantification of necrosis, 1 leaf disc (6-mm diameter) per plant (3 different plants) was removed immediately after infiltration and washed in 1 ml of distilled water. After 30 min, the discs were transferred to 1 ml of fresh water and electrolyte leakage was measured with a conductivity meter (Q cond 2,200). Alternatively, lesion formation was documented 3 days after infiltration of the various NLP proteins.
Pathogenicity Assay.
The NLP-deficient Pcc nlp– strain expressing wild-type NLPPp, NLPPya, NLPPcc, or mutant versions of NLPPcc was grown in liquid culture supplemented with ampicillin until the logarithmic phase was reached. The OD546 was then adjusted to 0.07. Potato tuber slices were inoculated with 15 μl of the bacterial suspension and incubated in water-filled, sealed boxes on a grate 2-cm above the water surface. Rotting symptoms were documented after 3 days. To analyze bacterial growth at that time, 3 potato tuber slices per transgenic strain were pooled and stirred in 25 ml 10 mM MgCl2. Dilutions of this extract were plated on solid LB medium supplemented with ampicillin, chloramphenicol, and cycloheximide to determine colony forming units. Growth of the various transgenic Pcc nlp– strains was calculated relative to the wild-type strain (Pcc nlp+) or the NLPPcc-complemented strain.
Preparation and Permeabilization of Calcein-Loaded Plasma Membrane Vesicles.
Plant plasma membranes were prepared by phase partitioning of microsomal fractions (41) using Dextran and PEG in a concentration of 6.4% (wt/wt) and 3 mM KCl. For preparation of calcein-loaded vesicles, plasma membranes (500 μg protein) were sonified (30 min on ice, 20-sec pulse on, 20-sec pulse off, amplitude 25%) in the presence of calcein (60 mM, pH 7.0 with NaOH). The external calcein was removed by gel filtration using Sephadex G-75 (Sigma) medium column equilibrated in 20 mM Tris pH 8.5, 140 mM NaCl, and 1 mM EDTA. Permeabilization of the vesicles (1 ng protein/μl) induced by 20 nM NLPs (0.04 ng/μl) was assayed at room temperature in 20 mM Mes pH 5.8, 140 mM NaCl by measuring fluorescence (excitation 485 nm, emission 520 nm) in a microplate reader (Sirius HT Injector, MWG). The percentage of calcein release (R) was calculated according to the equation R = (Fmeas–Finit)/(Fmax–Finit)*100, where Fmeas, Finit, and Fmax are the measured, initial, and maximal fluorescence, respectively. Fmax was obtained by the addition of Triton X-100 to 0.5% (vol/vol) final concentration at the end of each measurement. The experiments were repeated 3 times with similar results.
RNA Isolation and RT-PCR.
Tobacco leaves infiltrated with 0.5 μM NLPPcc variants or mock solutions were harvested after 4 h. RNA was isolated using the Tri Reagent method (Sigma). Synthesis of cDNA was performed by means of the RevertAidTM MuLV reverse transcriptase (Fermentas). Quantitative real-time PCR amplification was carried out in the presence of SYBR Green (Bio-Rad) with an iQ5 iCycler (Bio-Rad). Amplification of actin served as internal standard. Data analysis was performed according to the 2-ΔΔCT-method (42). Gene induction (fold change) by NLP was presented as the average of 3 determinations plus or minus standard deviation and compared to the expression level of mock infiltration.
Coordinates.
Crystallographic coordinates have been deposited in the Protein Data Bank under the accession code PDB ID 3GNU.
Supplementary Material
Acknowledgments.
We thank to Mark Gijzen, Georg Felix and Sophien Kamoun for useful comments on the manuscript, the staff of the Swiss Light Source, Villingen, Switzerland, Ursula Neu for technical support, and Ilme Schlichting, Wulf Blankenfeldt, Andrea Scrima, and Nils Schrader for data collection. This work was supported by Grant DFG Se 229/19–3 from the German Federal Ministry of Education and Research (to H.U.S.) and Grant SFB 446 from the German Research Foundation (to C. Oecking and T.N.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The atomic coordinates have been deposited in the Protein Data Bank, www.pdb.org (PDB ID code 3GNU).
This article contains supporting information online at www.pnas.org/cgi/content/full/0902362106/DCSupplemental.
References
- 1.Jones JD, Dangl JL. The plant immune system. Nature. 2006;444:323–329. doi: 10.1038/nature05286. [DOI] [PubMed] [Google Scholar]
- 2.Chisholm ST, Coaker G, Day B, Staskawicz BJ. Host-microbe interactions: shaping the evolution of the plant immune response. Cell. 2006;124:803–814. doi: 10.1016/j.cell.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 3.Kamoun S. A catalogue of the effector secretome of plant pathogenic oomycetes. Annu Rev Phytopathol. 2006;44:41–60. doi: 10.1146/annurev.phyto.44.070505.143436. [DOI] [PubMed] [Google Scholar]
- 4.Lindeberg M, Myers CR, Collmer A, Schneider DJ. Roadmap to new virulence determinants in Pseudomonas syringae: insights from comparative genomics and genome organization. Mol Plant Microbe Interact. 2008;21:685–700. doi: 10.1094/MPMI-21-6-0685. [DOI] [PubMed] [Google Scholar]
- 5.Glazebrook J. Contrasting mechanisms of defense against biotrophic and necrotrophic pathogens. Annu Rev Phytopathol. 2005;43:205–227. doi: 10.1146/annurev.phyto.43.040204.135923. [DOI] [PubMed] [Google Scholar]
- 6.van't Slot K, Knogge W. A dual role for microbial pathogen-derived effector proteins in plant disease and resistance. Crit Rev Plant Sci. 2002;21:229–271. [Google Scholar]
- 7.Gijzen M, Nürnberger T. Nep1-like proteins from plant pathogens: Recruitment and diversification of the NPP1 domain across taxa. Phytochemistry. 2006;67:1800–1807. doi: 10.1016/j.phytochem.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 8.Wolpert TJ, Dunkle LD, Ciuffetti LM. Host-selective toxins and avirulence determinants: what's in a name? Annu Rev Phytopathol. 2002;40:251–285. doi: 10.1146/annurev.phyto.40.011402.114210. [DOI] [PubMed] [Google Scholar]
- 9.Zipfel C. Pattern-recognition receptors in plant innate immunity. Curr Opin Immunol. 2008;20:10–16. doi: 10.1016/j.coi.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 10.Vorwerk S, Somerville S, Somerville C. The role of plant cell wall polysaccharide composition in disease resistance. Trends Plants Sci. 2004;9:203–209. doi: 10.1016/j.tplants.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 11.Qutob D, et al. Phytotoxicity and innate immune responses induced by Nep1-like proteins. Plant Cell. 2006;18:3721–3744. doi: 10.1105/tpc.106.044180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bae H, Kim M, Sicher R, Bae H-J, Bailey B. Necrosis- and ethylene-inducing peptide from Fusarium oxysporum induces a complex cascade of transcripts associated with signal transduction and cell death in Arabidopsis. Plant Physiol. 2006;141:1056–1067. doi: 10.1104/pp.106.076869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pemberton CL, Salmond GPC. The Nep1-like proteins—a growing family of microbial elicitors of plant necrosis. Mol Plant Pathol. 2004;5:353–359. doi: 10.1111/j.1364-3703.2004.00235.x. [DOI] [PubMed] [Google Scholar]
- 14.Veit S, Wörle JM, Nürnberger T, Koch W, Seitz HU. A novel protein elicitor (PaNie) from Pythium aphanidermatum induces multiple defense responses in carrot, Arabidopsis, and tobacco. Plant Physiol. 2001;127:832–841. [PMC free article] [PubMed] [Google Scholar]
- 15.Luberacki B, et al. Purification, crystallization and preliminary X-ray diffraction analysis of an oomycete-derived Nep1-like protein. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2008;64:1178–1180. doi: 10.1107/S1744309108037640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fellbrich G, et al. NPP1, a Phytophthora-associated trigger of plant defense in parsley and Arabidopsis. Plant J. 2002;32:375–390. doi: 10.1046/j.1365-313x.2002.01454.x. [DOI] [PubMed] [Google Scholar]
- 17.Holm L, Sander C. The FSSP database: fold classification based on structure-structure alignment of proteins. Nucleic Acids Res. 1996;24:206–209. doi: 10.1093/nar/24.1.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Birck C, et al. A new lectin family with structure similarity to actinoporins revealed by the crystal structure of Xerocomus chrysenteron lectin XCL. J Mol Biol. 2004;344:1409–1420. doi: 10.1016/j.jmb.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 19.Carrizo ME, et al. The antineoplastic lectin of the common edible mushroom (Agaricus bisporus) has two binding sites, each specific for a different configuration at a single epimeric hydroxyl. J Biol Chem. 2005;280:10614–10623. doi: 10.1074/jbc.M411989200. [DOI] [PubMed] [Google Scholar]
- 20.Athanasiadis A, Anderluh G, Macek P, Turk D. Crystal structure of the soluble form of equinatoxin II, a pore-forming toxin from the sea anemone Actinia equina. Structure. 2001;9:341–346. doi: 10.1016/s0969-2126(01)00592-5. [DOI] [PubMed] [Google Scholar]
- 21.Hinds MG, Zhang W, Anderluh G, Hansen PE, Norton RS. Solution structure of the eukaryotic pore-forming cytolysin equinatoxin II: implications for pore formation. J Mol Biol. 2002;315:1219–1229. doi: 10.1006/jmbi.2001.5321. [DOI] [PubMed] [Google Scholar]
- 22.Mancheno JM, Martin-Benito J, Martinez-Ripoll M, Gavilanes JG, Hermoso JA. Crystal and electron microscopy structures of sticholysin II actinoporin reveal insights into the mechanism of membrane pore formation. Structure. 2003;11:1319–1328. doi: 10.1016/j.str.2003.09.019. [DOI] [PubMed] [Google Scholar]
- 23.Mattinen L, Tshuikina M, Mae A, Pirhonen M. Identification and characterization of Nip, necrosis-inducing virulence protein of Erwinia carotovora subsp. carotovora. Mol Plant Microbe Interact. 2004;17:1366–1375. doi: 10.1094/MPMI.2004.17.12.1366. [DOI] [PubMed] [Google Scholar]
- 24.Pemberton CL, et al. Novel quorum-sensing-controlled genes in Erwinia carotovora subsp. carotovora: identification of a fungal elicitor homologue in a soft-rotting bacterium. Mol Plant Microbe Interact. 2005;18:343–353. doi: 10.1094/MPMI-18-0343. [DOI] [PubMed] [Google Scholar]
- 25.Kristan K, et al. Pore formation by equinatoxin, a eukaryotic pore-forming toxin, requires a flexible N-terminal region and a stable beta-sandwich. J Biol Chem. 2004;279:46509–46517. doi: 10.1074/jbc.M406193200. [DOI] [PubMed] [Google Scholar]
- 26.Cho W. Membrane targeting by C1 and C2 domains. J Biol Chem. 2001;276:32407–32410. doi: 10.1074/jbc.R100007200. [DOI] [PubMed] [Google Scholar]
- 27.Verdaguer N, Corbalan-Garcia S, Ochoa WF, Fita I, Gomez-Fernandez JC. Ca(2+) bridges the C2 membrane-binding domain of protein kinase Calpha directly to phosphatidylserine. EMBO J. 1999;18:6329–6338. doi: 10.1093/emboj/18.22.6329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee J, Klessig DF, Nürnberger T. A harpin binding site in tobacco plasma membranes mediates activation of the pathogenesis-related gene HIN1 independent of extracellular calcium but dependent on mitogen-activated protein kinase activity. Plant Cell. 2001;13:1079–1093. doi: 10.1105/tpc.13.5.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kanneganti TD, Huitema E, Cakir C, Kamoun S. Synergistic interactions of the plant cell death pathways induced by Phytophthora infestans Nepl-like protein PiNPP1.1 and INF1 elicitin. Mol Plant Microbe Interact. 2006;19:854–863. doi: 10.1094/MPMI-19-0854. [DOI] [PubMed] [Google Scholar]
- 30.Dean RA, et al. The genome sequence of the rice blast fungus Magnaporthe grisea. Nature. 2005;434:980–986. doi: 10.1038/nature03449. [DOI] [PubMed] [Google Scholar]
- 31.Motteram J, et al. Molecular characterization and functional analysis of MgNLP; the sole NPP1-domain containing protein from the fungal wheat leaf pathogen Mycosphaerella graminicola. Mol Plant Microbe Interact. 2009 doi: 10.1094/MPMI-22-7-0790. in press. [DOI] [PubMed] [Google Scholar]
- 32.Mariathasan S, et al. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature. 2006;440:228–232. doi: 10.1038/nature04515. [DOI] [PubMed] [Google Scholar]
- 33.Srivastava A, et al. The apoptotic response to pneumolysin is Toll-like receptor 4 dependent and protects against pneumococcal disease. Infect Immun. 2005;73:6479–6487. doi: 10.1128/IAI.73.10.6479-6487.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Matzinger P. The danger model: a renewed sense of self. Science. 2002;296:301–305. doi: 10.1126/science.1071059. [DOI] [PubMed] [Google Scholar]
- 35.Matzinger P. Friendly and dangerous signals: is the tissue in control? Nat Immunol. 2007;8:11–13. doi: 10.1038/ni0107-11. [DOI] [PubMed] [Google Scholar]
- 36.Ishii KJ, Koyama S, Nakagawa A, Coban C, Akira S. Host innate immune receptors and beyond: making sense of microbial infections. Cell Host & Microbe. 2008;3:352–363. doi: 10.1016/j.chom.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 37.Dostert C, et al. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science. 2008;320:674–677. doi: 10.1126/science.1156995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martinon F, Agostini L, Meylan E, Tschopp J. Identification of bacterial muramyl dipeptide as activator of the NALP3/cryopyrin inflammasome. Curr Biol. 2004;14:1929–1934. doi: 10.1016/j.cub.2004.10.027. [DOI] [PubMed] [Google Scholar]
- 39.Jennings J, et al. Induction of defense responses in tobacco by the protein Nep1 from Fusarium oxysporum. Plant Sci. 2001;161:891–899. [Google Scholar]
- 40.Jabs T, Tschöpe M, Colling C, Hahlbrock K, Scheel D. Elicitor-stimulated ion fluxes and O2– from the oxidative burst are essential components in triggering defense gene activation and phytoalexin synthesis in parsley. Proc Natl Acad Sci USA. 1997;94:4800–4805. doi: 10.1073/pnas.94.9.4800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Larsson C, Sommarin M, Widell S. Isolation of highly purified plant plasma membranes and separation of inside-out and right-side-out vesicles. Methods Enzymol. 1994;228:451–469. [Google Scholar]
- 42.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.