Abstract
Aging was associated with increased oxidation of DNA, RNA, and lipids in the cerebellum of male rats. DNA and lipid oxidation was reduced by lifelong (94 weeks) voluntary exercise on a running wheel. A reduction in cerebellar lipid oxidation, but not RNA or DNA oxidation, was observed following 3 months of moderate exercise or dietary supplementation of vitamin E, initiated at 18 months of age. The level of lipid oxidation correlated with measures of forelimb grip strength. The results indicate that lifelong exercise attenuates multiple molecular markers of age-related oxidative damage in the cerebellum. In addition, modest exercise initiated late in life can have a beneficial effect on lipid oxidation and motor function.
Keywords: aging, cerebellum, voluntary exercise, nucleic acid oxidation, lipid peroxidation, rat
1. Introduction
A sedentary life style, as one form of Western culture, has been proposed to be a risk factor that aggravates the aging process, while an active life style is linked to slowing of cognition and motor decline during aging [26,36,45]. The molecular mechanism for beneficial effects of exercise is unknown, though several hypotheses have been proposed. Moderate exercise is associated with enhanced neurogenesis [43], a reduction in markers of aging, including oxidative stress [10], and enhanced trophic influences [23].
Cerebellar Purkinje cells exhibit enhanced DNA damage starting at middle-life [38] and continuing over the course of aging [1,20,25]. Importantly, the extent of degeneration and loss of function is mitigated by lifelong exercise [25]. Interestingly, the cerebellum does not exhibit evidence of neurogenesis [13,24], therefore; we tested the hypothesis that exercise would reduce molecular indicators of oxidative damage in aged animals.
2. Methods
2.1 Animals and treatments
Procedures involving animal subjects have been reviewed and approved by the Institutional Animal Care and Use Committee and were in accordance with guidelines established by the U.S. Public Health Service Policy on Humane Care and Use of Laboratory Animals. Male Fisher-344 rats were originally from an investigation of lifelong exercise on hydrogen peroxide production in the heart [22]. The rats were randomly assigned to one of three groups: sedentary ad libitum fed (Ad Lib, n = 9), sedentary with an 8% caloric restriction (CR, n = 9), or wheel running with 8% food restriction (CR-Run, n = 9). The inclusion of 8% food restriction for animals in the Run group was instituted because rats fed ad libitum tend to abruptly decrease their running activity during aging and a slight food restriction (8-10%) prevents this decline [17,18]. Thus, the CR group was used as an age matched control of food restriction. The diet consisted of rat chow (Harlan Teklad Rodent Diet #8604). For the first set of studies, group assignment was initiated when animals were at 11-weeks of age and the status of each animal was institutionally maintained for 94 weeks. Rats were killed and brains removed at 24 mo of age. Young sedentary rats (Yng, 6-month old, n = 10) were employed to determine age-associated changes in oxidative stress.
In the second study, male Fisher-344 rats (18 mo of age) were randomly assigned to one of four groups: sedentary ad libitum fed (Ad Lib, n = 9), ad libitum fed animals with access to a running wheel (Ad Lib-Run, n = 8), wheel running with 8% food restriction (CR-Run, n = 7) or conditional runners (Con-Run, n = 5), operantly conditioned to run on a wheel (model H10-38R, Coulbourn Instruments, Allentown, PA) for 45 mg food pellets. The criterion for delivery of the pellet was increased such that during that final two months, most animals were running ∼3 meters for each pellet. The brain of each animal was removed, frozen in liquid nitrogen, and stored at −80 °C. Another group of animals, also 18 mo, were provide 3 months of vitamin E supplementation (α-tocopherol 500 IU, Harlan Teklad Madison,WI n = 7) and compared to age-match control animals (n = 7) fed a standard diet (α-tocopherol 50 IU, Harlan Teklad Madison, WI).
2.2 Grip strength
Forelimb grip strength was determined using an automated grip strength meter (Columbus Instruments, Columbus, OH) as previously described [5]. The mean force (grams) was calculated over three trials and was divided by body weight.
2.3 Biochemical Assays
Brains were removed, cerebellar hemispheres dissected and frozen in liquid nitrogen before storage at -80°C. Subsequently, the cerebellar cortex was isolated and lipid peroxidation products were determined by measuring the thiobarbituric acid reactive substance, malondialdehyde (MDA), using a commercially available Kit (ZeptoMetrix Corporation, Buffalo, NY). Briefly, 100 μl of whole homogenate (50 mg/ml protein) was added to a 100 μl SDS solution and 2.44 ml of TBA/buffer and 62.5 μl of 2% (w/v) butylated hydroxytoluene (Sigma, St. Louis, MO). The combined solution was incubated at 95 °C for 60 min followed by an immediate cool in ice for 10 min. After centrifuging the samples at 800 g for 15 min, the supernatant was removed. The sample solution was analyzed using fluorescence spectrophotometry at an excitation wavelength of 530 nm and emission wavelength of 550 nm. Absolute concentrations are generated via comparison with MDA standard curve of fluorescent intensity.
Determination of RNA and DNA oxidative damage by measuring 8-oxo-7,8-dihydroguanosine (8-oxoGuo) and 8-oxo-7,8-dihydro-2′-deoxyguanosine (8-oxodGuo), respectively was done using the neutral guanidine isothiocyanate (GTC) method as previously described [16]. Briefly, after homogenization in GTC in the presence of the metal chelator deferoxamine mesylate (DFOM; Sigma), proteins and lipids were removed using organic solvents and centrifugation. After salt/isopropanol precipitation of nucleic acid at 80°C and washing in 70% ethanol, nucleic acids were dissolved in 30 μM DFOM and hydrolyzed using 4 U Nuclease P1 (MP Biomedicals, Irvine, CA) and 5 U alkaline phosphatase (Sigma) in 30 mM sodium acetate, 20 μM ZnCl2, pH 5.3 at 50°C for 60 min. After filtration to remove enzymes, nucleosides were separated using HPLC-EC-UV and analyzed for Guo (RNA) and dGuo (DNA) by UV, and 8-oxoGuo (RNA) and 8-oxodGuo (DNA) electrochemically using a Coulochem III detector from ESA Inc. (Chelmsford, MA) (21). HPLC peaks were quantified against daily made calibration curves of standards from Sigma and Calbiochem (San Diego, CA).
2.4 Immunohistochemistry
One aged and one young animal maintained on an ad lib diet, were anesthetized with 50mg/kg of pentobarbital and perfused with 4% paraformaldehyde-PBS buffer by intra-arterial puncture. The cerebellum was removed, blocked, embedded in Tissue-Tek O.C.T. Compound (Ted Pella, Redding, CA), frozen, and sectioned into 15 μm-thick slices. Immunohistochemical study was performed using the avidin–biotin–peroxidase complex (VECTSTAIN ABC Kit, Vector Laboratories, Burlingame, CA). Briefly, after incubated in 10 mM Tris-HCl, pH 7.5, 1 mM EDTA, 0.4 mM NaCl, and 100 μg/ml RNase for 1 hour at 37°C, the DNA was denatured by soaking the slides in 4 N HCl for 5 minutes followed by neutralizing the sample with 50 mM Tris-base for 5 minutes at room temperature. The sections were then incubated overnight at 4°C with primary mouse monoclonal antibody against 8-hydroxyguanosine (Trevigen Inc., Gaithersburg, MD) at a dilution of 1:300. To exclude non-specific binding sites, sections serving as technical controls were incubated with isotypic IgG: normal mouse IgG (Santa Cruz, Santa Cruz, CA; code sc-2025) instead of the primary antibody. All slices were incubated with the biotinylated secondary antibody (Anti-mouse) with a concentration of 1:100 dilution in PBS for 30 minutes, followed by Strep ABC complex for 30 minutes at room temperature. Diaminobenzamide tetrahydrochloride (DAB substrate kit, Vector Laboratories, Burlingame, CA) was added in to develop the brown color deposit. Photomicrographs were obtained with a Zeiss Axiovert 40 CFL microscope with Axiocam digital CCD camera (Carl Zeiss, Thornwood, NY).
2.5 Statistical analysis
Analyses of variance (ANOVAs) were used to establish main effects of treatment and Fisher's PLSD hoc tests with significance set at p < 0.05 were employed to localize specific differences.
3. Results
3.1 Effects of lifelong exercise
A description of the distances covered in the activity wheel over the course of the lifelong exercise study has previously been reported [22,40]. Briefly, an initial rise in activity was subsequently followed by stable activity over the last 60 weeks of training. For the subgroup of animals examined in the current study, the average distance in meters covered per month was 12827 ± 2670 m and the average distance during the last week prior to sacrifice was 7332 ± 1791 m. While the CR-Run group exhibited a small decrease in overall body weight, this difference was not statistically significant (p = 0.17) (Fig 1a) and no difference was observed for brain weight (data not shown). A significant effect [F(3,32) = 3.68, p < 0.05] of treatment was observed for oxidized DNA due to an increase of DNA oxidation (8-oxodGuo) in the aged Ad Lib group relative to young and aged CR-Run rats (Fig 1b). The age-related increased DNA oxidation could be observed in cerebellar sections immunostained for 8-hydroxyguanosine, a marker DNA oxidative damage (Fig 1 d&e). Similarly a significant effect [F(3,32) = 2.95, p < 0.05] of treatment was observed for oxidized RNA (8-oxoGuo) due to increased oxidation product in the aged Ad Lib group relative to young animals (Fig 1c). Lipid oxidation in the cerebellum, measured as the concentration of MDA exhibited a tendency for a difference across the four conditions [F(3,32) = 2.44, p = 0.08] and post hoc tests indicated that young rats and aged CR-Run rats exhibited a reduction in MDA relative to aged Ad Lib rats (Fig 1f). In addition, there was a tendency for a reduction in lipid peroxidation in CR animals relative to aged Ad Lib rats (p = 0.088).
Figure 1.
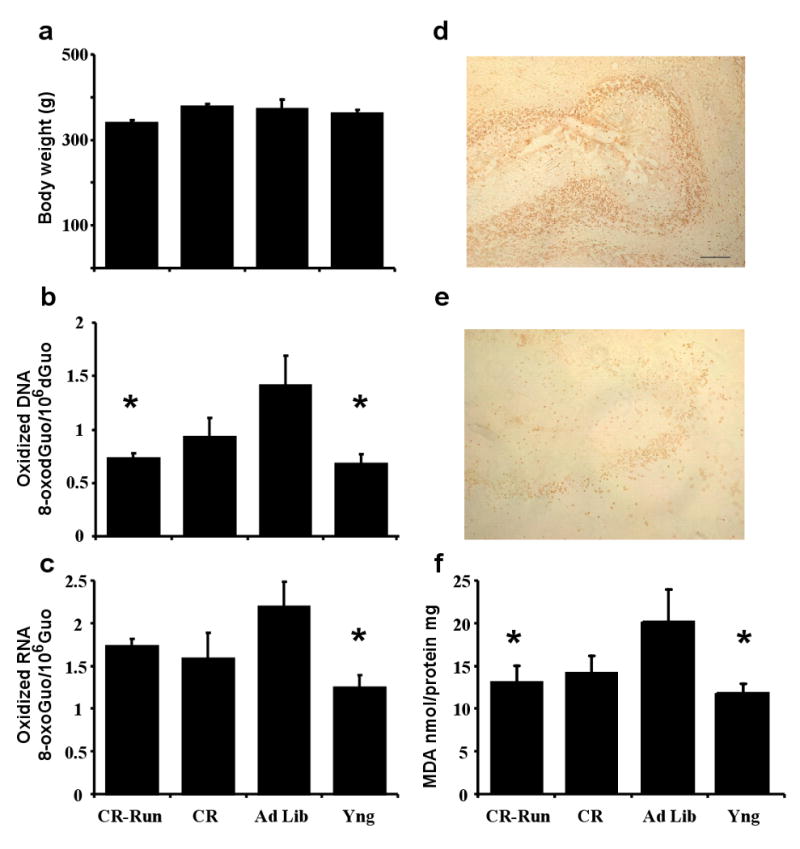
Measures of cerebellar oxidative damage associated with aging and lifelong exercise. a) Body weight at the time of sacrifice. b) DNA oxidation was decreased in animals in the exercise group (CR-Run) and young (Yng) animals relative to aged ad libitum (Ad Lib) fed animals. c) An age-related increase in RNA oxidation was observed for Ad Lib rats. The distribution of 8-hydroxyguanosine–positive cells in the cerebellum of aged (d) young (e) rats. Bar = 200 μm. f) An age-related increase in lipid peroxidation was attenuated by lifelong exercise. For this and subsequent figures, bars represent the mean + SEM. Asterisk indicates significant (<0.05) difference relative to Ad Lib animals.
3.2 Effects of exercise initiated in aged animals
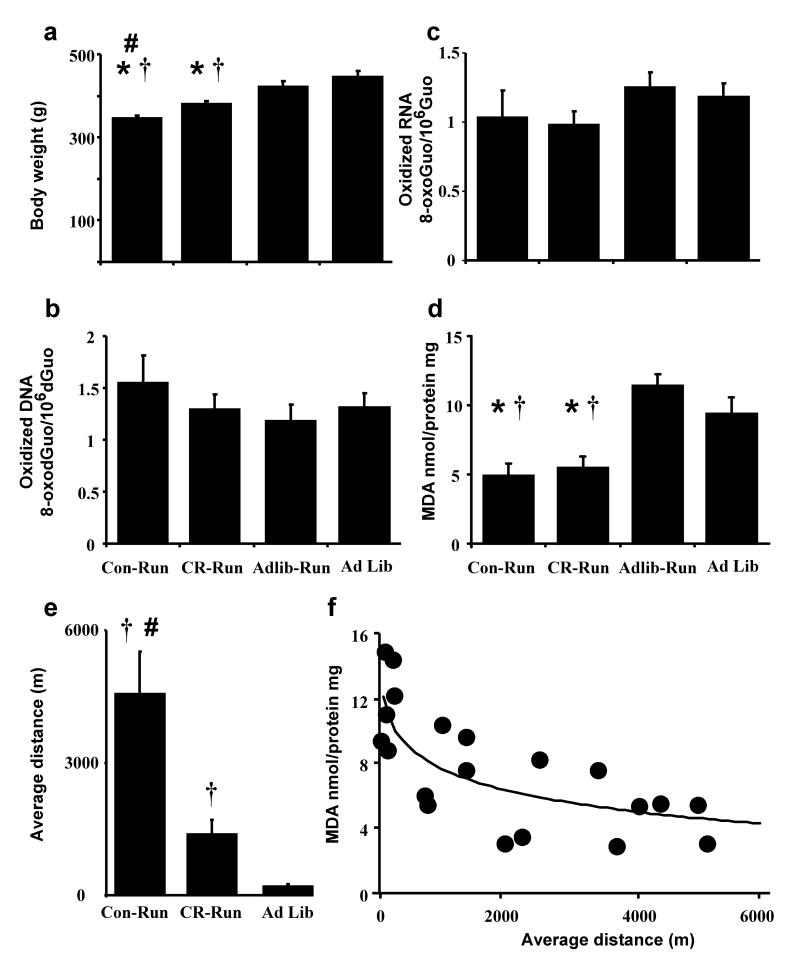
To determine whether the reduction in oxidative damage required exercise across the life-span, a second series of studies were conducted to determine whether exercise initiated in aged animals could influence motor function and reduced cerebellar oxidative damage. For the three groups with access to the running wheel, the treatment conditions differentially influenced the average distance covered over the 3 months [F(2,17) 33.27, p < 0.0001] (Fig 2e) and the average distance covered during the last week [F(2,17) 71.6, p < 0.0001]. During the final week, Con-Run animals averaged 6883 ± 661 m; however, this value likely underestimates the distance covered since animals usually left the wheel and crossed the cage to consume the food pellet as soon as it was delivered. Regardless, the distance was greater than that covered by CR-Run rats (1722 ± 406 m), while Ad Lib-Run animals exhibited relatively little wheel running (345 ± 108 m). Body weight differed across the treatment groups [F(3,25) = 13.78, < 0.0001] due to reduced weight in the CR-Run and Con-Run groups relative to Ad Lib groups (Fig 2a). No treatment effects were observed with regard to oxidation of DNA and RNA (Fig 2b&c). Exercise was associated with a reduction in lipid oxidation [F(3,25) 9.53, p < 0.005] such that MDA levels were reduced in CR-Run and Con-Run groups relative to the Ad Lib groups (Fig 2d). Examination of the correspondence between lipid oxidation and wheel running over the 3 mo period indicated a steep decline in oxidative damage associated with a modest level of exercise (Fig 2f). As such, the negative relationship of MDA levels and the mean distance traveled, per week, during the three months of training was fit by a logarithmic function (R2 = 0.54, p < 0.0005).
Figure 2.
Influence of 3 mo exercise on markers of oxidative damage. a) Body weight at the time of sacrifice. No effect of treatment conditions was observed for (b) DNA and (c) RNA oxidation. d) Reduced lipid oxidation was observed in animals conditioned to run for food (Con-Run) and rats that exercised under mild caloric restriction (CR-Run) relative to Al Lib rats. e) Average weekly distance covered over the 3 mo period for animals with access to a running wheel. f) Relationship between MDA levels and the average weekly distance covered over the 3 mo period. The line is the best fit logarithmic function. Asterisk indicates significant (<0.05) difference relative to Ad Lib animals. † indicates significant (<0.05) difference relative to Ad Lib-Run animals. Pound sign indicates a significant (<0.05) difference between Con-Run and CR-Run groups.
3.3 Effects of vitamin E treatment
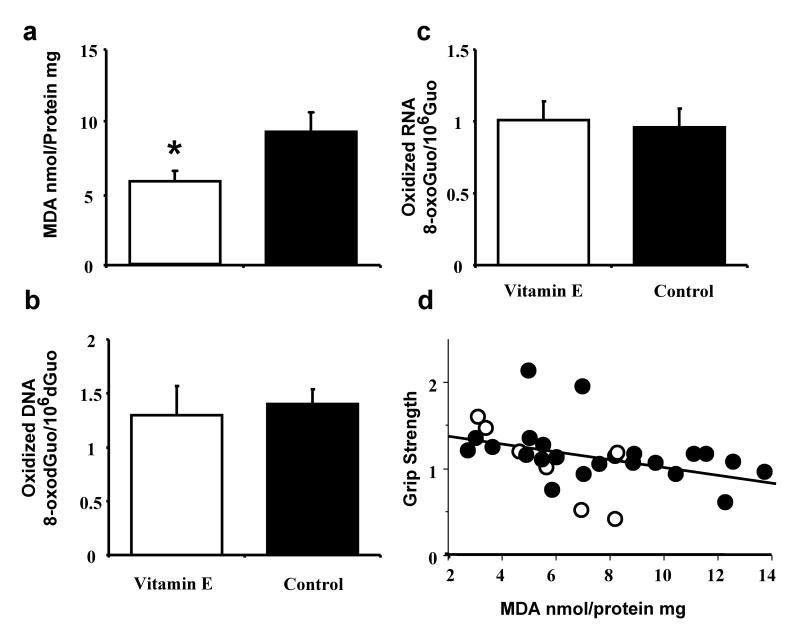
To defend against oxidative stress, cells employ enzymatic mechanisms and antioxidant molecules. Therefore, an additional group of animals (age 18 mo) were provided a relatively short-term (3 months) vitamin E supplementation (α-tocopherol 500 IU, and compared to age-match animals on a standard diet (50 IU). The results indicate that vitamin E supplementation reduced lipid peroxidation [F(1,12) = 5.24, p < 0.05] (Fig 3a), but did not affect oxidization of DNA or RNA (Fig 3 b&c).
Figure 3.
Comparison of the influence of dietary supplementation with vitamin E for three month period (open bars) relative to a control diet (filled bars). Vitamin E supplementation was associated with a) reduced lipid peroxidation in the absence of an effect on b) DNA oxidation and c) RNA oxidation. d) Relationship between grip strength and MDA levels. Open circles represent animals with dietary supplementation of vitamin E.
A subset of animals exposed to 3 months of exercise (CR-Run: n = 6; Con-Run: n = 4, Ad Lib-Run: n = 4), vitamin E supplementation (n = 7), or ad libitum control conditions (n = 8) were tested for forelimb grip strength. Regression analysis indicated a modest but significant (R2 = 0.16, p < 0.05) relationship, with greater forelimb grip strength associated with lower MDA levels (Fig 3d).
4. Discussion
This series of studies addresses a basic question regarding the mechanism by which lifelong exercise can act to limit the extent of cerebellar damage [1,20,31] and loss of cerebellar motor function [9,19]. These results indicate that aging is coupled to an increase in cerebellar oxidative damage for a broad array of molecules. In confirmation of previous work, aging was associated with an increase in cerebellar markers of lipid [21] and DNA oxidation [4,8,30]. In addition, the currents study provides the first evidence that RNA oxidation increases in the cerebellum with advanced age.
The pattern of oxidation for the CR group was similar to that observed for the CR-Run group (Fig 1) and included a tendency for reduced lipid peroxidation compared to Ad Lib animals. It is unclear whether lifelong CR alone might be protective against oxidative damage. Indeed, it has been suggested that the effects of exercise and CR on the brain are mediated by shared mechanisms [29]. Evidence indicates that CR can reduce oxidative stress in obese individuals, however; it is less clear whether CR has beneficial effects in the general population. A relatively severe restriction of food intake to 60% of normal is associated with reduced DNA oxidative damage [14,41], however, another study found that a dietary restriction to 70% of normal was associated with a large reduction in weight in the absence of a decrease in DNA oxidation [11]. The relatively mild CR employed in the current study may promote a general increase in motor activity, which is facilitated by the availability of a running wheel, such that the level or duration of increased motor activity underlies the extent of protection against oxidative damage.
Lifelong exercise reduced oxidation of DNA and lipids, but did not reduce oxidative damage to RNA. There are several possible explanations for the inability of exercise to reduce oxidation of RNA. First, there are structural considerations that increase the potential for interaction with reactive oxygen species (ROS). In contrast to DNA, RNA is more likely to be single-stranded and less well protected [16]. Furthermore, RNA is widely distributed throughout the cytosol making it more available to different sources of ROS.
The extent and nature of the benefits from exercise are likely to vary with brain region, the type of exercise, and the age at which an exercise program is initiated. Previous studies indicate that several weeks of intense exercise involving 60-260 min of forced swimming, initiated later in life, had little or no influence on overall brain lipid peroxidation [33,35]. In contrast, ∼6 mo of moderate treadmill exercise was associated with a reduction of lipid peroxidation in the brain and superior motor function [32]. The difference may relate to a stress from forced swimming which can decrease brain antioxidants [42] and increase superoxides in the cerebellum [34]. In fact, other studies indicate that milder swimming exercise (30 min/day) has age-sensitive effects that are region specific [7,21]. Similar to the results of the current study, these researchers observed that aging was associated with an increase in cerebellar lipid peroxidation, which was reduced by exercise or vitamin E treatment in aged animals [21]. In the currents study, rats engaged in voluntary wheel running which is likely to represent a relatively low level of exercise relative to forced swimming. Indeed, an examination of the relationship between lipid oxidation and exercises suggests that a relatively modest level of voluntary wheel running (1000 – 2000 m/week) had a large impact on oxidative damage. Measurement of thiobarbituric acid reactive substance is a common method, which provides a global evaluation of lipid oxidation. Future studies should determine whether specific polyunsaturated fatty acids are targets for age-related oxidative damage and exercise mediated protection.
Reduced lipid oxidation in the cerebellum due to exercise or vitamin E treatment was associated with enhanced grip strength. Previous work in humans suggests that vitamin E levels are positively related to grip strength [39]. In addition, variability in grip strength during aging is correlated with other measures of cerebellar function in humans [44] and grip strength is employed as a measure of cerebellar control of motor strength in rodents [12,37]. However, it is important to note that exercise induces a number of anatomical and molecular changes in the cerebellum that could contribute to improved motor function [6]. In addition, exercise can influence age-related changes in motor function by delaying and/or reversing sarcopenia [28]. Thus, oxidative damage measured in the cerebellum represents only one component of an aging motor system.
Oxidative stress is hypothesized to be a pivotal factor in initiating a cascade of events leading to premature aging in many systems and damage of brain cells [15]. One aspect of this hypothesis is the idea that oxidative damage to genetic material results in the gradual accumulation of mutations and ultimately cell death [2]. Genetic markers of increased oxidative stress and evidence of DNA oxidative damage are apparent in several brain regions by middle-age [3,27]. Importantly, recent work indicates that Purkinje cells exhibit enhanced DNA damage starting at middle-life, while cerebellar granule cells accumulated DNA damage with increasing age [38]. Thus, one possibility is that the relatively early emergence of DNA damage in Purkinje cells contributes to the loss of cells during aging. In this regard, the reduction in DNA oxidation associated with exercise initiated early in life may contribute to neuroprotective effects observed form lifelong treadmill training [25]. However, contrary to lifelong exercise, exercise initiated in older animals did not reduce DNA damage. The inability of exercise, initiated later in life, to reduce DNA damage suggests that once DNA damage and cell loss is established, amelioration is limited. As such, early intervention may be critical.
Acknowledgments
L. Cui and T. Hofer contributed equally to this work. This work was supported by grants to TCF from the National Institute on Aging (AG14979) and National Institute of Mental Health MH (059891); to CL from the National Institute on Aging (AG17994 and AG21042); an American Heart Association Postdoctoral Fellowship to TH (0525346B) and the Evelyn F. McKnight Brain Research Foundation. The authors do not have financial or personal conflicts of interest associated with this work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Andersen BB, Gundersen HJ, Pakkenberg B. Aging of the human cerebellum: a stereological study. J Comp Neurol. 2003;466(3):356–65. doi: 10.1002/cne.10884. [DOI] [PubMed] [Google Scholar]
- 2.Barja G. Free radicals and aging. Trends Neurosci. 2004;27(10):595–600. doi: 10.1016/j.tins.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 3.Blalock EM, Chen KC, Sharrow K, Herman JP, Porter NM, Foster TC, Landfield PW. Gene microarrays in hippocampal aging: statistical profiling identifies novel processes correlated with cognitive impairment. J Neurosci. 2003;23(9):3807–19. doi: 10.1523/JNEUROSCI.23-09-03807.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cardozo-Pelaez F, Song S, Parthasarathy A, Hazzi C, Naidu K, Sanchez-Ramos J. Oxidative DNA damage in the aging mouse brain. Mov Disord. 1999;14(6):972–80. doi: 10.1002/1531-8257(199911)14:6<972::aid-mds1010>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 5.Carter CS, Cesari M, Ambrosius WT, Hu N, Diz D, Oden S, Sonntag WE, Pahor M. Angiotensin-converting enzyme inhibition, body composition, and physical performance in aged rats. J Gerontol A Biol Sci Med Sci. 2004;59(5):416–23. doi: 10.1093/gerona/59.5.b416. [DOI] [PubMed] [Google Scholar]
- 6.Churchill JD, Galvez R, Colcombe S, Swain RA, Kramer AF, Greenough WT. Exercise, experience and the aging brain. Neurobiol Aging. 2002;23(5):941–55. doi: 10.1016/s0197-4580(02)00028-3. [DOI] [PubMed] [Google Scholar]
- 7.Devi SA, Kiran TR. Regional responses in antioxidant system to exercise training and dietary vitamin E in aging rat brain. Neurobiol Aging. 2004;25(4):501–8. doi: 10.1016/S0197-4580(03)00112-X. [DOI] [PubMed] [Google Scholar]
- 8.Dorszewska J, Adamczewska-Goncerzewicz Z. Oxidative damage to DNA, p53 gene expression and p53 protein level in the process of aging in rat brain. Respir Physiol Neurobiol. 2004;139(3):227–36. doi: 10.1016/j.resp.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 9.Forster MJ, Dubey A, Dawson KM, Stutts WA, Lal H, Sohal RS. Age-related losses of cognitive function and motor skills in mice are associated with oxidative protein damage in the brain. Proc Natl Acad Sci U S A. 1996;93(10):4765–9. doi: 10.1073/pnas.93.10.4765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Foster TC. Biological markers of age-related memory deficits: treatment of senescent physiology. CNS Drugs. 2006;20(2):153–66. doi: 10.2165/00023210-200620020-00006. [DOI] [PubMed] [Google Scholar]
- 11.Gedik CM, Grant G, Morrice PC, Wood SG, Collins AR. Effects of age and dietary restriction on oxidative DNA damage, antioxidant protection and DNA repair in rats. European journal of nutrition. 2005;44(5):263–72. doi: 10.1007/s00394-004-0520-0. [DOI] [PubMed] [Google Scholar]
- 12.Grady RM, Wozniak DF, Ohlemiller KK, Sanes JR. Cerebellar synaptic defects and abnormal motor behavior in mice lacking alpha- and beta-dystrobrevin. J Neurosci. 2006;26(11):2841–51. doi: 10.1523/JNEUROSCI.4823-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grimaldi P, Rossi F. Lack of neurogenesis in the adult rat cerebellum after Purkinje cell degeneration and growth factor infusion. Eur J Neurosci. 2006;23(10):2657–68. doi: 10.1111/j.1460-9568.2006.04803.x. [DOI] [PubMed] [Google Scholar]
- 14.Hamilton ML, Van Remmen H, Drake JA, Yang H, Guo ZM, Kewitt K, Walter CA, Richardson A. Does oxidative damage to DNA increase with age? Proc Natl Acad Sci U S A. 2001;98(18):10469–74. doi: 10.1073/pnas.171202698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956;11(3):298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- 16.Hofer T, Seo AY, Prudencio M, Leeuwenburgh C. A method to determine RNA and DNA oxidation simultaneously by HPLC-ECD: greater RNA than DNA oxidation in rat liver after doxorubicin administration. Biol Chem. 2006;387(1):103–11. doi: 10.1515/BC.2006.014. [DOI] [PubMed] [Google Scholar]
- 17.Holloszy JO, Schechtman KB. Interaction between exercise and food restriction: effects on longevity of male rats. J Appl Physiol. 1991;70(4):1529–35. doi: 10.1152/jappl.1991.70.4.1529. [DOI] [PubMed] [Google Scholar]
- 18.Holloszy JO, Smith EK, Vining M, Adams S. Effect of voluntary exercise on longevity of rats. J Appl Physiol. 1985;59(3):826–31. doi: 10.1152/jappl.1985.59.3.826. [DOI] [PubMed] [Google Scholar]
- 19.Hu D, Serrano F, Oury TD, Klann E. Aging-dependent alterations in synaptic plasticity and memory in mice that overexpress extracellular superoxide dismutase. J Neurosci. 2006;26(15):3933–41. doi: 10.1523/JNEUROSCI.5566-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang CM, Miyamoto H, Huang RH. The mouse cerebellum from 1 to 34 months: Parallel fibers. Neurobiol Aging. 2005 doi: 10.1016/j.neurobiolaging.2005.09.025. [DOI] [PubMed] [Google Scholar]
- 21.Jolitha AB, Subramanyam MV, Asha Devi S. Modification by vitamin E and exercise of oxidative stress in regions of aging rat brain: studies on superoxide dismutase isoenzymes and protein oxidation status. Exp Gerontol. 2006;41(8):753–63. doi: 10.1016/j.exger.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 22.Judge S, Jang YM, Smith A, Selman C, Phillips T, Speakman JR, Hagen T, Leeuwenburgh C. Exercise by lifelong voluntary wheel running reduces subsarcolemmal and interfibrillar mitochondrial hydrogen peroxide production in the heart. Am J Physiol Regul Integr Comp Physiol. 2005;289(6):R1564–72. doi: 10.1152/ajpregu.00396.2005. [DOI] [PubMed] [Google Scholar]
- 23.Klintsova AY, Dickson E, Yoshida R, Greenough WT. Altered expression of BDNF and its high-affinity receptor TrkB in response to complex motor learning and moderate exercise. Brain Res. 2004;1028(1):92–104. doi: 10.1016/j.brainres.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 24.Kuhn HG, Winkler J, Kempermann G, Thal LJ, Gage FH. Epidermal growth factor and fibroblast growth factor-2 have different effects on neural progenitors in the adult rat brain. J Neurosci. 1997;17(15):5820–9. doi: 10.1523/JNEUROSCI.17-15-05820.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Larsen JO, Skalicky M, Viidik A. Does long-term physical exercise counteract age-related Purkinje cell loss? A stereological study of rat cerebellum. J Comp Neurol. 2000;428(2):213–22. [PubMed] [Google Scholar]
- 26.Laurin D, Verreault R, Lindsay J, MacPherson K, Rockwood K. Physical activity and risk of cognitive impairment and dementia in elderly persons. Arch Neurol. 2001;58(3):498–504. doi: 10.1001/archneur.58.3.498. [DOI] [PubMed] [Google Scholar]
- 27.Lu T, Pan Y, Kao SY, Li C, Kohane I, Chan J, Yankner BA. Gene regulation and DNA damage in the ageing human brain. Nature. 2004;429(6994):883–91. doi: 10.1038/nature02661. [DOI] [PubMed] [Google Scholar]
- 28.Marzetti E, Leeuwenburgh C. Skeletal muscle apoptosis, sarcopenia and frailty at old age. Exp Gerontol. 2006;41(12):1234–8. doi: 10.1016/j.exger.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 29.Mattson MP, Wan R. Beneficial effects of intermittent fasting and caloric restriction on the cardiovascular and cerebrovascular systems. The Journal of nutritional biochemistry. 2005;16(3):129–37. doi: 10.1016/j.jnutbio.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 30.Mecocci P, MacGarvey U, Kaufman AE, Koontz D, Shoffner JM, Wallace DC, Beal MF. Oxidative damage to mitochondrial DNA shows marked age-dependent increases in human brain. Ann Neurol. 1993;34(4):609–16. doi: 10.1002/ana.410340416. [DOI] [PubMed] [Google Scholar]
- 31.Monteiro RA, Henrique RM, Rocha E, Marini-Abreu MM, Oliveira MH, Silva MW. Age-related changes in the volume of somata and organelles of cerebellar granule cells. Neurobiol Aging. 1998;19(4):325–32. doi: 10.1016/s0197-4580(98)00064-5. [DOI] [PubMed] [Google Scholar]
- 32.Navarro A, Gomez C, Lopez-Cepero JM, Boveris A. Beneficial effects of moderate exercise on mice aging: survival, behavior, oxidative stress, and mitochondrial electron transfer. Am J Physiol Regul Integr Comp Physiol. 2004;286(3):R505–11. doi: 10.1152/ajpregu.00208.2003. [DOI] [PubMed] [Google Scholar]
- 33.Ogonovszky H, Berkes I, Kumagai S, Kaneko T, Tahara S, Goto S, Radak Z. The effects of moderate-, strenuous- and over-training on oxidative stress markers, DNA repair, and memory, in rat brain. Neurochem Int. 2005;46(8):635–40. doi: 10.1016/j.neuint.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 34.Pedreanez A, Arcaya JL, Carrizo E, Mosquera J. Forced swimming test increases superoxide anion positive cells and angiotensin II positive cells in the cerebrum and cerebellum of the rat. Brain Res Bull. 2006;71(13):18–22. doi: 10.1016/j.brainresbull.2006.07.018. [DOI] [PubMed] [Google Scholar]
- 35.Radak Z, Kaneko T, Tahara S, Nakamoto H, Pucsok J, Sasvari M, Nyakas C, Goto S. Regular exercise improves cognitive function and decreases oxidative damage in rat brain. Neurochem Int. 2001;38(1):17–23. doi: 10.1016/s0197-0186(00)00063-2. [DOI] [PubMed] [Google Scholar]
- 36.Richards M, Hardy R, Wadsworth ME. Does active leisure protect cognition? Evidence from a national birth cohort. Soc Sci Med. 2003;56(4):785–92. doi: 10.1016/s0277-9536(02)00075-8. [DOI] [PubMed] [Google Scholar]
- 37.Rogers DC, Fisher EM, Brown SD, Peters J, Hunter AJ, Martin JE. Behavioral and functional analysis of mouse phenotype: SHIRPA, a proposed protocol for comprehensive phenotype assessment. Mamm Genome. 1997;8(10):711–3. doi: 10.1007/s003359900551. [DOI] [PubMed] [Google Scholar]
- 38.Rutten BP, Schmitz C, Gerlach OH, Oyen HM, de Mesquita EB, Steinbusch HW, Korr H. The aging brain: accumulation of DNA damage or neuron loss? Neurobiol Aging. 2007;28(1):91–8. doi: 10.1016/j.neurobiolaging.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 39.Semba RD, Blaum C, Guralnik JM, Moncrief DT, Ricks MO, Fried LP. Carotenoid and vitamin E status are associated with indicators of sarcopenia among older women living in the community. Aging Clin Exp Res. 2003;15(6):482–7. doi: 10.1007/BF03327377. [DOI] [PubMed] [Google Scholar]
- 40.Seo AY, Hofer T, Sung B, Judge S, Chung HY, Leeuwenburgh C. Hepatic oxidative stress during aging: effects of 8% long-term calorie restriction and lifelong exercise. Antioxid Redox Signal. 2006;8(34):529–38. doi: 10.1089/ars.2006.8.529. [DOI] [PubMed] [Google Scholar]
- 41.Sohal RS, Agarwal S, Candas M, Forster MJ, Lal H. Effect of age and caloric restriction on DNA oxidative damage in different tissues of C57BL/6 mice. Mechanisms of ageing and development. 1994;76(23):215–24. doi: 10.1016/0047-6374(94)91595-4. [DOI] [PubMed] [Google Scholar]
- 42.Tsakiris T, Angelogianni P, Tesseromatis C, Tsakiris S, Tsopanakis C. Alterations in antioxidant status, protein concentration, acetylcholinesterase, Na+, K+-ATPase, and Mg2+-ATPase activities in rat brain after forced swimming. Int J Sports Med. 2006;27(1):19–24. doi: 10.1055/s-2005-837506. [DOI] [PubMed] [Google Scholar]
- 43.van Praag H, Shubert T, Zhao C, Gage FH. Exercise enhances learning and hippocampal neurogenesis in aged mice. J Neurosci. 2005;25(38):8680–5. doi: 10.1523/JNEUROSCI.1731-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Washburn RA, Smith KW, Jette AM, Janney CA. The Physical Activity Scale for the Elderly (PASE): development and evaluation. J Clin Epidemiol. 1993;46(2):153–62. doi: 10.1016/0895-4356(93)90053-4. [DOI] [PubMed] [Google Scholar]
- 45.Yaffe K, Barnes D, Nevitt M, Lui LY, Covinsky K. A prospective study of physical activity and cognitive decline in elderly women: women who walk. Arch Intern Med. 2001;161(14):1703–8. doi: 10.1001/archinte.161.14.1703. [DOI] [PubMed] [Google Scholar]