Abstract
Growing evidence suggests that socioeconomic attributes of both childhood and adulthood confer risk for cardiovascular morbidity and mortality. In this study, we examine the association of both parental and individual educational attainment with C-reactive protein (CRP), an inflammatory mediator relevant to cardiovascular pathophysiology, in a midlife community sample. Subjects were 811 men and women (394 men/417 women; 87 % European-American/13 % African American), 30–54 years of age. Plasma concentrations of CRP were determined from blood samples obtained at a single session following an overnight fast. Regression analyses adjusting for age and race showed both parental education and individual education to be associated inversely with CRP in women, but not men. The relationship of parental education with CRP in women persisted on multivariable adjustment for both lifestyle risk factors (smoking, alcohol consumption, sleep, exercise, body mass index) and individual SES. Independent of reported personal educational attainment, mid-life adult women whose parents achieved fewer years of educational attainment exhibit higher levels of circulating CRP than women with higher parental education. This association may help explain the increased risk of atherosclerotic cardiovascular morbidity and mortality conferred by low childhood socioeconomic status.
Keywords: C-Reactive Protein, Parental Education
Introduction
Variation in socioeconomic status (SES) is inversely related to cardiovascular mortality (Salonen, 1982;Seigel et al., 1987; Keil et al., 1992), incident coronary heart disease (Rose and Marmot, 1981; Liu et al., 1982; Diez-Roux et al.,1995), and cardiovascular disease risk factors, including cigarette smoking (Zang and Wynder, 1998), generalized obesity (Sobal and Stunkard, 1989), and physical activity (Evenson et al., 2002). In general, these associations hold for both men and women, though the strength of the relationships may vary between sexes. Among women, stronger inverse associations between SES and incident coronary heart disease (Vogels et al., 1999; Thurston et al., 2005), coronary mortality (Chandola, 1998; Pekkanen et al., 1995), and obesity (Sobal and Stunkard, 1989; Langenberg et al. 2003; Zhang and Wang, 2004) have been documented in some, but not all (Marmot et al, 1997; Frank et al., 2003; Diez-Roux et al., 1995) investigations.
Although most literature has focused primarily on adult SES, growing evidence indicates that lower childhood SES, independently of adult social standing, is associated with increased risk of incident coronary heart disease (CHD) outcomes, including myocardial infarction (Notkola et al., 1985; Kaplan and Salonen, 1990; Gliksman et al., 1995; Wannamathee et al., 1996; Wamala et al., 2001; Davey Smith et al., 2002;Lawlor et al., 2004), and several CHD risk factors (Poulton et al., 2002; Blane et al., 1996; Ebrahim et al., 2004). Here, also, sex differences have been documented, with childhood SES predicting serum triglycerides (Parker et al., 2004), HDL cholesterol (Brunner et al., 1999), and metabolic syndrome (Lehman et al., 2005) in women, but not men.
Inflammation is now recognized as a risk factor for the development and exacerbation of atherosclerotic cardiovascular disease (Ross, 1999). C-reactive protein (CRP) is a frequently cited inflammatory marker shown to predict future cardiovascular morbidity and mortality (Danesh et al., 2000; Danesh et al., 2004; Lagrand et al., 1999). Growing evidence also suggests that adult SES, whether indicated by occupation (Owen et al., 2003), employment status (employed/unemployed; Danesh et al, 1999), education (Panagiotakos et al., 2004; Wu et al., 2002); composites of income and education (Jousilahti et al., 2003), or community-level SES (Petersen et al., 2008) is inversely related to CRP.
Extending the literature on SES associations with inflammation, more recent attention has turned to the role of childhood influences on inflammatory processes in adulthood. In this regard, it is suggested that the early-childhood environment can regulate immune development in ways that influence health in adulthood, even when adult SES is taken into account (e.g., Lynch, Kaplan, & Salonen, 1997). Thus, early life variation in exposure to environmental factors that covary inversely with SES and modulate immune function, such as air pollutants, viral infections, maternal smoking, psychological stress and allergen exposure (Donovan and Finn, 1999) may have far-reaching effects. Childhood SES may also affect adult inflammation through more proximal behavioral mechanisms. For example, lower SES families engage in poorer health practices, such as smoking, physical inactivity, poorer dietary choices (Marmot et al., 1991), that have been associated with higher systemic inflammation (Bruunsgaard, 2005; Frohlich et al., 2003). Lifestyle practices are often established early in life, with lower childhood SES predicting poorer health practices in adulthood (Politt et al., 2005). Thus, in examining possible associations between childhood SES and adult inflammation, it is important to determine whether contemporaneous factors such as adult lifestyle choices or remaining in one’s social class of childhood explain observed effects or whether there is something unique about childhood SES that influences later inflammatory processes.
To date, findings from the few studies examining childhood SES in relation to adult CRP levels have been mixed. One study showed a positive association between father’s occupation and CRP in men 45 to 59 years of age (Mendall et al, 2000), and similar findings obtained for a composite of father’s occupation and education in younger adults (Pollitt et al., 2007). A third investigation failed to find parental education associated with CRP (Gimeno et al., 2008), whereas a fourth (Taylor et al., 2006) found that parental education was indirectly associated with adult CRP levels through a pathway encompassing a history of familial adversity, later psychological functioning, and body mass. The model tested in that study did not adjust for adult subjects’ own educational attainments, however, and did not specifically address potential sex differences in the magnitude of association between early life SES and CRP. The purpose of the present study, then, was to further explore the relationship of parental education (as one frequently studied component of childhood SES) with CRP concentrations, adjusting for participants’ own education, among a diverse community sample of mid-life men and women, and to examine whether these relationships might vary by sex.
Methods
Participants
This investigation was based on data derived from 1047 adults (48% male; 17% African-American) who participated in the University of Pittsburgh Adult Health and Behavior (AHAB) project between 2001 and 2005. The AHAB registry is a compendium of behavioral and biological measurements on mid-life community volunteers who were recruited, via mass-mail solicitation, from Southwestern Pennsylvania (principally Allegheny County). Exclusions from AHAB participation included age <30 or >54 years; a reported history of atherosclerotic cardiovascular disease, chronic kidney or liver disease, cancer treatment in the preceding year, and major neurological disorders, schizophrenia or other psychotic illness. Other exclusions included pregnancy and the use of insulin, glucocorticoid, antiarrhythmic, psychotropic, or prescription weight-loss medications. Data collection occurred over multiple laboratory sessions, and informed consent was obtained in accordance with approved protocol and guidelines of the University of Pittsburgh Institutional Review Board.
Because AHAB exclusions did not include common acute illnesses, such as recent colds or allergies, data of participants having circulating CRP levels >10 mg/L were not included in the current analyses (Pearson et al., 2003). Overall, 185 subjects were excluded due to elevated CRP. In order to examine the effect of both fathers’ and mothers’ education on CRP in later life, analyses were limited to two-parent households, which resulted in the exclusion of 78 additional subjects. This yielded a final sample of 811 European-American (EA) and African American (AA) individuals, a sample which included 394 men (37 AA) and 417 women (67 AA). These subjects did not differ in age or sex from AHAB participants who were excluded from the present analyses due to elevated inflammatory markers or childhood household status.
Procedure
Prior to arrival at the laboratory, participants were asked to fast for 8 hours and avoid exercise for 12 hours, alcohol for 24 hours, and nicotine for 1 hour. All sessions were scheduled in the morning. On arrival to the laboratory, the project nurse completed a medical history and medication use interview, obtained measurements of height and weight for the determination of body mass index (BMI; kg/m2), and drew a 40 cc blood sample in citrate treated tubes. A portion of the blood sample was spun down and plasma collected and stored at −80°C until batched analysis of CRP levels.
Measures
Education
Individual Education
Participant’s were asked to report their years of education and their highest level of academic attainment. Individual education variables were distributed normally. Subjects were well-educated on average, with a mean of 15.6 (SD=2.6) years of schooling. Nonetheless, levels of educational attainment varied appreciably among study participants, with 0.5% lacking a high school diploma, 17.5% having completed high school or technical training only, 23% with some college, 36% with a Bachelors degree, 17.5% with Masters degree, and 5.5% with PhD or doctoral-level professional degree.
Parental Education
Participants reported both their mothers’ [M (SD) = 12.4 (2.5)] and fathers’ [M (SD) = 12.7 (3.3)] years of education completed by the time the participant was age 18 (range: 1 – 24 years). Consistent with other studies (Karlamanga et al., 2005; Taylor et al., 2006), analyses were performed examining the higher of the parents’ education in order to reflect the maximal health-relevant socioeconomic benefits afforded to a particular family. Highest household parental education ranged from 3 – 24 years [M (SD) = 13.5 (2.9)]. Parental education variables were normally distributed. In order to maintain consistent between-family comparisons, analyses were limited to two-parent households to avoid the confounding effect of parental absence and to allow for separate analyses of mothers’ and fathers’ education.
CRP
CRP was measured using the BNII nephelometer from Dade Behring (Deerfield, Illinois) utilizing a particle enhanced immunonephelometric assay. In this procedure, polystyrene particles are coated with monoclonal antibodies to CRP, which, in the presence of antigen agglutinate, cause an increase in the intensity of scattered light. The increase in scattered light is proportional to the amount of CRP in the sample (Ledue et al., 1998). The assay range is 0.175–1100 mg/L. Intra-assay CVs range from 2.3–4.4% and inter-assay CVs range from 2.1– 5.7%. Final CRP values were normalized by reciprocal transformation.
Additional Variables
A number of variables were assessed that might explain associations between SES and inflammation. These variables included age, sex, race, BMI, smoking status, alcohol use, sleep volume, and physical activity. Smoking status was defined by participants’ self-report as being a current cigarette smoker versus all other categories of tobacco use (ex-smoker, non-smoker, other forms of tobacco use). Alcohol use was expressed as drinks/week and estimated from subject’s report of the number of alcoholic drinks consumed over the previous four weeks. Sleep volume was calculated from participant’s reported average hours of sleep during the 7 nights prior to study participation (hours of sleep = (average hours/week night × 5) + (average hours/weekend night × 2)). Physical activity was measured using the Paffenbarger Physical Activity Questionnaire, which estimates kilocalories expended per week (Paffenbarger et al., 1993). The distributions of physical activity scores and number of alcoholic drinks/week were normalized by square root and natural log transformations, respectively.
Statistical Analysis
Associations of education with circulating CRP were evaluated in three linear regression models. In Models IA and IB, demographic covariates (age, sex, and race) were entered on Step 1, individual (i.e., participants’ own) education (IA) or parental education (IB) was entered in Step 2, and an interaction term for sex was entered in Step 3. Models IIA and IIB consisted of sex-specific analyses for circulating CRP, with covariates (age, race) on Step 1, and individual education (IIA) or parental education (IIB) entered on Step 2. In Model III, we examined whether parents’ educational attainment predicted circulating CRP independent of individual education. Additionally, Model IV examined whether parental education was associated with CRP independently of BMI and health practices. Here, demographic characteristics were entered in Step 1, BMI, smoking, exercise, alcohol, and sleep in Step 2, individual education in Step 3, and parental education in Step 4 of the regression equation.
Results
Demographic and health behavior characteristics for the sample are listed in Table 1. Women drank significantly less alcohol than men, smoked less, and had lower BMI, while men engaged in more physical activity. Women had slightly lower mean years of education than men, but highest parental education did not differ by sex. Bivariate correlations describing the associations of subject characteristics with educational variables and with CRP levels are presented in Table 2. For convenience, the signs of correlations involving reciprocally transformed measurements of CRP are reversed (as in all subsequent test statistics), so that positive (and negative) coefficients are interpreted as such. Consistent with prior literature, higher CRP was associated with African-American race, higher BMI, less physical activity, and current smoking (Kasapis and Thompson, 2005; Khera et al., 2005; Mendall et al., 1996). CRP did not covary with age, sex, alcohol use, or sleep duration. Circulating levels of CRP covaried inversely with both parental and individual educational attainment.
Table 1.
Demographic characteristics of sample and for male and female participants separately.
| Characteristic | Total Sample n = 811 M (SD) | Men n = 394 M (SD) | Women n = 417 M (SD) | Test Statistic |
|---|---|---|---|---|
| Age (years) | 44.8 (6.7) | 44.6 (7.0) | 44.9 (6.6) | F1,810 = 0.49 |
| Race (% black) | 13% | 9.5% | 16% | χ2=8.83** |
| BMI (kg/m2) | 27.3 (5.4) | 27.8 (4.7) | 26.4 (5.9) | F1,810 = 23.4** |
| Smoking (% current) | 16% | 18% | 13% | χ2=5.8* |
| Exercise (kilocals) | 2415 (1777) | 2577(1773) | 2287 (1769) | F1,810 = 13.1** |
| Alcohol (drinks/week) | 3.98 (7.5) | 5.91 (9.4) | 2.20 (4.4) | F1, 810 = 59.1** |
| Sleep (hours/week) | 47.9 (7.0) | 47.3 (6.9) | 48.8 (6.9) | F1, 810 = 12.3** |
| CRP | 1.65 (1.9) | 1.49 (1.7) | 1.79 (2.1) | F1, 810 = 0.06 |
| Individual Ed (yrs) | 15.6 (2.6) | 15.9 (2.6) | 15.4 (2.6) | F1, 810 = 4.54* |
| Parental Ed (yrs) | 13.5 (2.9) | 13.5 (2.9) | 13.4 (2.9) | F1, 810 = 0.15 |
P<.01,
P<.05,
P<.10
Race coded 0 = White, 1 = Black. BMI = Body Mass Index; Smoking Status (0=not current, 1=current).
Mean comparisons conducted on the transformed CRP, alcohol, and exercise variables.
Table 2.
Bivariate correlations with covariates, CRP, and Parental and Individual Education (n = 811).
| Characteristic | CRP | Parental Ed. | Individual Ed. |
|---|---|---|---|
| r | r | r | |
| Age | −.009 | − .197** | − .060 |
| Gender | .025† | −.018† | −.060+† |
| Race | .099**† | −.118**† | −.188**† |
| BMI (kg/m2) | .458** | −.065* | −.071* |
| Smoking | .070*† | −.076*† | −.172**† |
| Exercise (kilocalories) | −.122** | .084* | .112** |
| Alcohol (drinks/week) | −.032 | − .010 | − .081 |
| Sleep (hours/week) | −.042 | .044 | .050 |
| Individual Ed (yrs) | −.077* | .334** | --- |
| Parental Ed (yrs) | −.099** | --- | --- |
P<.01,
P<.05,
P<.10,
= point biserial correlation.
Race coded 0 = White, 1 = Black. BMI = body mass index; Smoking Status (0=not current, 1=current).
Correlations conducted on transformed CRP, alcohol, and exercise variables, but signs were reversed in the table for ease of interpretation.
In Models IA and IB (not shown in tables), covariates (age, sex, and race) accounted for a significant proportion of the variance in CRP levels (ΔR2 = 0.011, F3, 807 = 2.87, p= 0.036). Both individual education years (β = −0.046, ΔR2 = 0.002, F4, 806 = 2.56, p= 0.037) and highest household parental years of education (β = −0.085, ΔR2 = 0.007, F4, 806 = 3.59, p= 0.007) accounted for significant additional variance in CRP and were both found to interact significantly with sex (individual education: β = −0.47, ΔR2 = 0.005, F4, 805 = 2.89, p= 0.042; parental education: β = −0.174, ΔR2 = 0.009, F4, 805 = 3.54, p= 0.007). Sex-specific analyses were then performed (Model II) to determine whether the variation in CRP accounted for by individual and parental education differed across women and men; these analyses are reported in Table 3. Higher individual education (β = −0.102, ΔR2 = .010, F3,413 = 4.67, p < .04) and parental education (β = −0.153, ΔR2 = .023, F3,413 = 6.49, p < .01) were significant predictors of CRP among women, but no significant effects were seen in men (Table 3). In Table 4, an independent effect of women’s parental education was demonstrated for CRP beyond that of individual education (β = −0.133, ΔR2 = .015, F4,412 = 5.18, p < .01), with individual education no longer accounting for a significant portion of the variance in CRP (β = −0.057, ΔR2 = .010, F3,413 = 4.67, p < .27). Thus, women in our study whose parents had fewer years of education showed higher plasma concentrations of CRP than those with higher parental educational attainment, an effect that was independent of women’s own educational attainment, age, and race. Separate analyses of mothers’ and fathers’ education, controlling for age, race, and individual education, revealed a similar pattern of results. Both mothers’ and fathers’ educational attainment predicted adult CRP levels in women (mother: β = −0.144, ΔR2 = .018, F4,412 = 5.48, p < .01; father: β = −0.116, ΔR2 = .012, F4,412 = 4.82, p < .03), but not in men (data not shown in tables).
Table 3.
Hierarchical linear regressions showing the contributions of age, race, and individual and parental education to the prediction of reciprocally transformed CRP in men and women. Signs have been reversed for ease of interpretation.
| Model IIA | |||||
|---|---|---|---|---|---|
| Males (394) | β | b (SE) | Females (417) | β | b (SE) |
| Step 1: | Step 1: | ||||
| Age | .017 | .001 (.002) | Age | −.027 | −.001 (.002) |
| Race | .033 | .023 (.035) | Race | .135 | .086 (.031) |
|
| |||||
| Step 2: | Step 2: | ||||
| Individual Ed (yrs) | .021 | .002 (.004) | Individual Ed (yrs) | −.102 | −.009 (.004) |
| Model IIB | |||||
|
| |||||
| Step 2: | Step 2: | ||||
| Parental Ed (yrs) | .004 | .000 (.004) | Parental Ed (yrs) | −.153 | −.012 (.004) |
Table 4.
Hierarchical linear regression showing the contributions parental education, adjusted for individual education, to the prediction of reciprocally transformed CRP in women. Signs have been reversed for ease of interpretation.
| Model III | ||
|---|---|---|
| Females (417) | β | b (SE) |
|
Step 1: See Table 3 | ||
| Step 2: | ||
| Individual Ed (yrs) | −.057 | −.005 (.005) |
|
| ||
| Step 3: | ||
| Parental Ed (yrs) | −.133 | −.011 (.004) |
An additional analysis was then performed to determine whether lifestyle factors could account for the association of parental education with CRP in women. Parental education was entered as a predictor after demographic covariates, BMI, health practices (smoking, physical activity, alcohol use, and sleep duration), and individual education (Model IV; Table 5). After controlling for age and race, greater BMI (B = .492, p < .01) predicted higher CRP levels independently of demographic characteristics. Interestingly, individual SES was no longer associated with CRP when entered into the model after demographic covariates, BMI and health behaviors (B=−.053, p > .10). Parental education, on the other hand, remained a significant, independent predictor of CRP (B = −.084, p < .05) with age, race, BMI, smoking status, alcohol use, sleep volume, and physical activity in the model.
Table 5.
Contribution of standard covariates, health behaviors, and individual and parental education to the prediction of reciprocally transformed CRP in women. Signs have been reversed for ease of interpretation.
| Model IV | ||
|---|---|---|
| Females (417) | β | b (SE) |
| Step 1: | ||
| Age | −.047 | −.002 (.002) |
| Race | .018 | .012 (.028) |
| Step 2: | ||
| BMI | .523 | .021 (.002) |
| Alcohol | −.036 | −.007 (.008) |
| Smoking | .041 | .029 (.031) |
| Sleep | .057 | .002 (.001) |
| Exercise | −.012 | −.003 (.012) |
| Step 3: | ||
| Individual Ed (yrs) | −.009 | −.001 (.004) |
| Step 4: | ||
| Parental Ed (yrs) | −.090 | −.007 (.004) |
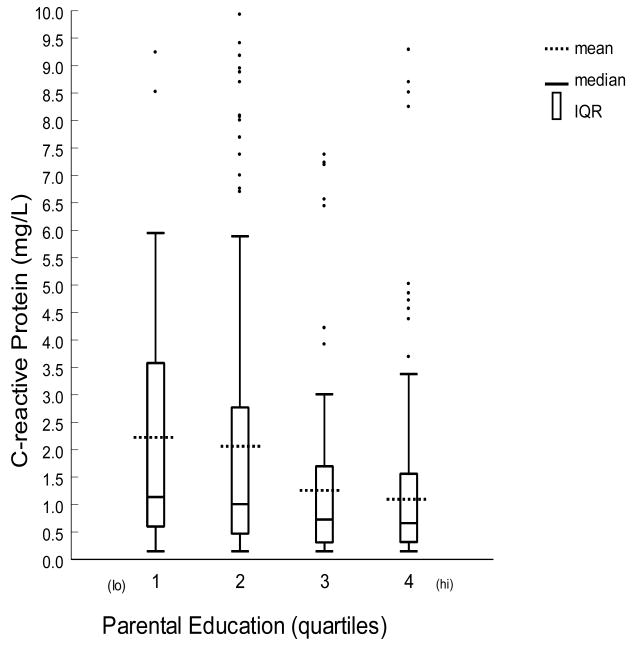
To examine the possibility that the parental education associations reported here might have emerged only in the context of the data transformation required to satisfy parametric assumptions of normality, box-plots of untransformed CRP measurements are reported separately for men and women in Figure 1. The values depicted here are partitioned by quartiles of the distribution of subjects’ parental education (adjusted for correlated variation in age and race). Median values among women comprising the lowest quartile of parental education were greater than those of the highest quartile, and non-parametric statistic (Mann-Whitney U) corroborated end-quartile differences (Z = −2.8, p < 0.006). The Spearman rank-order correlations of parental education with untransformed CRP were likewise significant in women (rho = −0.189, p < 0.001).
Figure 1. Distributions of untransformed values of CRP for quartiles of parental education for women (n=417).
Parental Education Years by quartile: 1 = No high school diploma, 2 = H.S. Graduate, 3 = Some college 4 = College graduate or higher
Discussion
The present study shows indices of childhood and adult socioeconomic standing to be associated inversely with a circulating marker of inflammation in a relatively healthy, mid-life community sample. Substantial evidence documents that childhood socioeconomic attributes confer risk for coronary heart disease outcomes (Kaplan and Salonen, 1990; Gliksman et al., 1995; Wannamathee et al.,1996; Wamala et al., 2001; Davey Smith et al., 2002;Lawlor et al., 2004) and risk factors (Blane et al., 1996; Ebrahim et al., 2004; Poulton et al., 2002). Consistent with these findings, our study shows that, when compared with women whose parents were more educated, women with lower parental education have higher levels of circulating CRP, an independent risk factor for cardiovascular events (Danesh et al., 2000; Danesh et al., 2004; Lagrand et al., 1999; Ridker et al., 1997; Thompson et al., 1995). This association was not seen for men in this sample. Additionally, associations between women’s parental education and CRP were independent of age, race, and individual-level SES. Further, participants with lower parental education had higher CRP even after accounting for lifestyle-related risk factors, including BMI, smoking, alcohol consumption, physical activity and sleep. The current results are consistent with evidence that levels of CRP covary inversely with childhood SES (Gimeno et al., 2008; Mendall et al., 2000; Pollitt et al., 2007), though this is the first study to report sex-specific associations. These findings raise the possibility that relationships between childhood SES and increased vulnerability to cardiovascular disease could be mediated, in part, through inflammatory pathways, and that these associations may differ by gender.
The mechanisms by which socioeconomic attributes of childhood affect systemic inflammation are unclear. Childhood socioeconomic conditions are associated with behavioral factors such as smoking, diet, and physical activity (Brunner et al., 1996; Lynch et al., 1997; Winkleby et al., 1992), lifestyle factors which are associated with inflammatory processes (Bruunsgaard, 2005; Frohlich et al., 2003). In our study, however, childhood SES continued to predict CRP levels after controlling for several health practices. Lower parental education may reflect material conditions associated with chronic stress, which may play a role in the etiology of inflammation through endocrine pathways involving the hypothalamic-pituitary adrenal axis or activation of the sympathetic nervous system (Maier and Watkins, 1998; Segerstrom and Miller, 2004). Childhood SES may also contribute to adult health through poor maternal socioeconomic circumstances that may result in dysregulation of a range of metabolic and endocrine systems during fetal growth, as well as material deprivation of the child in early life (Osmond et al., 1993).
Regardless of the mechanisms, our findings were consistent with several studies documenting gender differences in the effects of childhood socioeconomic circumstances on adult health. Although not entirely consistent (Langenberg et al., 2003), substantial evidence demonstrates stronger effects of childhood social class on adult cardiovascular risk factors in women than in men, as seen in relation to smoking (Power et al., 2005), diabetes (Maty et al., 2008), depression (Sadowski et al. 1999), and composite risk profiles (Karlamanga et al., 2005). Our results support other findings that childhood social status influences health outcomes in adulthood, independent of adult socioeconomic indicators, and that these effects may differ by gender. Though this is the first study to report sex-specific associations between parental education and CRP, others have also found levels of CRP to covary inversely with childhood SES (Mendall et al., 2000; Pollitt et al., 2007, Taylor et al., 2006). In any case, the lack of a relationship between childhood SES and CRP in men in our sample warrants further exploration.
An examination of possible mechanisms linking parental education and adult CRP may shed some light on the observed sex difference in our findings. There are numerous ways in which parental education might affect cardiovascular risk factors in later life, and one such mechanism may be the sustained influence of early socioeconomic circumstances on health behaviors. Lower education may predict poor dietary habits and less physical activity, two factors that are related to CRP. It is possible that childhood SES is related to body mass and physical activity differently in males versus females. Kinra et al. (2000) showed that the relationship between socioeconomic “deprivation” and obesity was stronger in girls versus boys, and that this relationship grew stronger with age in girls only. Gender differences in physical activity are well-established, with boys generally being more active than girls (Sallis et al., 2000; Inchley et al., 2005). In their study of Scottish adolescents, Inchley et al. (2005) found that girls from the highest SES groups were less active than boys from the lowest SES groups, suggesting an additive effect of gender and SES that places girls from low SES backgrounds at particular risk of low physical activity. Although our analyses demonstrated that parental education continued to predict CRP in women, but not men, after controlling for both BMI and physical activity, it is possible that further investigation of sex differences in health behaviors related to parental education may advance understanding of this relationship. Another hypothesized link between childhood SES and adult health is that of psychosocial influences, with lower parental education indicating a more “risky” family environment that might lead to adverse alterations in biological systems. Social, behavioral and biological stimuli perceived as “stressful” may play a role in inflammatory processes, as psychosocial stress has been associated with factors related to CRP, including abdominal fat (Lapidus et al., 1989; Wing et al., 1991; Raikkonen et al., 1994) and insulin resistance (Raikkonen et al., 1994; Black, 2003). Perhaps men in our sample were less influenced by risky childhood environmental circumstances than were women, decreasing the association of childhood environment and later inflammatory processes. To our knowledge, however, no data exists which demonstrates sex differences in the psychological effects of lower parental education. Finally, parental education may contribute to adult health through pathways that do not involve behavioral or psychosocial factors. Several studies have suggested that cardiovascular and metabolic risk are related inversely to birth weight (Reynolds et al., 2005), and the association between size at birth and mortality in adult life may be due to socioeconomic factors (Leon et al., 1996). Reduced size at birth may be a marker for poor maternal socioeconomic circumstances that may result in both dysregulation of developing biological systems and inadequate medical or nutritional resources for the child (Osmond et al., 1993). Although research examining sex differences with regard to these associations in humans has been mixed (Phillips et al., 2000; Reynolds et al., 2005), some animal studies have found female rats to be more sensitive than males to activation of the HPA axis (Weinstock et al., 1992), suggesting that relationships between birth size and cardiovascular risk may not be the same in men and women. In terms of the sex differences found in the present study, perhaps these perinatal complications have more serious and long-lasting biological effects on daughters than sons.
In addition to examining childhood SES, the current study also contributes to an understanding of relationships between individual SES and an inflammatory mediator. Consistent with previous findings (Panagiotakos et al., 2004; Wu et al., 2002), individuals’ educational attainment was associated with levels of CRP, though only in women. We found no significant relationship between individual education and levels of CRP in men after adjusting for demographic characteristics. To our knowledge, no other studies have reported sex-specific associations of education and CRP though our results support previous findings showing stronger relationships of educational level and coronary risk in women (Loucks et al., 2007; Thurston et al., 2005) as compared to men.
These findings should be interpreted in the light of several study limitations. First, the cross-sectional design of this study precludes causal interpretation. Alternative explanations for our results include the possibility that CRP and education are independently related to a third factor such as personality, cognitive ability, or general health. Another limitation is the single assessment of CRP. Although evidence suggests that levels of this inflammatory mediator are relatively stable over extended periods (Rao et al., 1994), multiple assessments over time may provide a more reliable indicator of chronic interindividual variability. Future investigations should include longitudinal assessments, beginning in early life, to evaluate the influence of more proximal socioeconomic characteristics, such as chronic stress and access to health-promoting resources, on inflammatory mediators to better elucidate how education may affect individuals’ health. Additionally, the retrospective nature of reported childhood conditions may involve recall bias, although this is fairly unlikely using educational variables. Although education is a widely used index of SES, and is related to income and occupation, these indices do not fully overlap (Krieger et al., 1997). The use of education as a sole index of both childhood and current SES may have neglected other important dimensions of SES. Also, the range of educational attainment was somewhat limited in comparison with other studies, as the sample as a whole averaged over 15 years of schooling. It is possible that a clearer relationship of individual SES with inflammatory mediators would be seen across a broader spectrum of socioeconomic variation, including a greater representation of individuals from the lowest strata of socioeconomic position.
Nonetheless, our findings provide evidence that both parental and individual education are associated, in a sex-specific manner, with a marker of inflammation thought to play a role in the pathogenesis of cardiovascular and other inflammatory diseases. Furthermore, the relationship of childhood social standing with adulthood CRP is independent of demographic characteristics, measured health practices, and individual educational attainment. Thus, inflammatory processes may mediate independent relationships between childhood SES and vulnerability to cardiovascular disease, particularly in women. Further investigation of this potential pathway is warranted.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Black PH. The inflammatory response is an integral part of the stress response: Implications for atherosclerosis, insulin resistance, type II diabetes and metabolic syndrome X. Brain Behav Immun. 2003;17:350–364. doi: 10.1016/s0889-1591(03)00048-5. [DOI] [PubMed] [Google Scholar]
- Blane D, Hart CL, Davey Smith G, Gillis CR, Hole DJ, Hawthorne VM. Association of cardiovascular disease risk factors with socioeconomic position during childhood and during adulthood. BMJ. 1996;313:1434–1438. doi: 10.1136/bmj.313.7070.1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunner E, Shipley MJ, Blane D, Davey Smith G, Marmot MG. When does cardiovascular risk start? Past and present socioeconomic circumstances and risk factors in adulthood. Journal of Epidemiology and Childhood Health. 1999;5:3757–764. doi: 10.1136/jech.53.12.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruunsgaard H. Physical activity and modulation of systemic low-level inflammation. J Leukoc Biol. 2005;78:819–835. doi: 10.1189/jlb.0505247. [DOI] [PubMed] [Google Scholar]
- Chandola T. Social inequality in coronary heart disease: a comparison of occupational classifications. Soc Sci Med. 1998;47:525–533. doi: 10.1016/s0277-9536(98)00141-5. [DOI] [PubMed] [Google Scholar]
- Chen E, Hanson MD, Paterson LQ, Griffin MJ, Walker HA, Miller GE. Socioeconomic status and inflammatory processes in childhood asthma: The role of psychological stress. Journal of Allergy and Clinical Immunology. 2006;117:1014–1020. doi: 10.1016/j.jaci.2006.01.036. [DOI] [PubMed] [Google Scholar]
- Danesh J, Muir J, Wong Y, Ward M, Gallimore JR, Pepys MB. Risk factors for coronary heart disease and acute-phase proteins. A population-based study Eur Heart J. 1999;20:954–959. doi: 10.1053/euhj.1998.1309. [DOI] [PubMed] [Google Scholar]
- Danesh J, Whincup P, Walker M, Lennon L, Thomson A, Appleby P, Gallimore JR, Pepys MB. Low grade inflammation and coronary heart disease: prospective study and updated meta-analyses. BMJ. 2000;321:199–204. doi: 10.1136/bmj.321.7255.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danesh J, Wheeler JG, Hirschfield GM, Eda S, Eiriksdottir G, Rumley A, Lowe GD, Pepys MB, Gudnason V. C-reactive protein and other circulating markers of inflammation in the prediction of coronary heart disease. N Engl J Med. 2004;350:1387–1397. doi: 10.1056/NEJMoa032804. [DOI] [PubMed] [Google Scholar]
- Davey Smith G, Ben-Shlomo Y, Lynch J. Life course approaches to inequalities in coronary heart disease risk. In: Stansfield SA, Marmot MG, editors. Stress and the Heart. London: BMJ Books; 2002. pp. 20–49. [Google Scholar]
- Diez-Roux AV, Nieto FJ, Tyroler HA, Crum LD, Szklo M. Social inequalities and atherosclerosis. The atherosclerosis risk in communities study. Am J Epidemiol. 1995;141:960–972. doi: 10.1093/oxfordjournals.aje.a117363. [DOI] [PubMed] [Google Scholar]
- Donovan CE, Finn PW. Immune mechanisms of childhood asthma. Thorax. 1999;54:938–946. doi: 10.1136/thx.54.10.938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebrahim S, Montaner D, Lawlor DA. Clustering of risk factors and social class in childhood and adulthood in British women’s heart and health study: cross sectional analysis. BMJ. 2004;328:861–865. doi: 10.1136/bmj.38034.702836.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evenson KR, Wilcox S, Pettinger M, Brunner R, King AC, McTiernan A. Vigorous Leisure Activity Through Women’s Adult Life – The Women’s Health Initiative Observational Cohort Study. Am J Epidemiol. 2002;156:945–953. doi: 10.1093/aje/kwf132. [DOI] [PubMed] [Google Scholar]
- Frank JW, Cohen R, Yen I, Balfour J, Smith M. Socioeconomic gradients in health status over 29 years of follow-up after midlife: the Alameda County Study. Soc Sci Med. 2003;57:2305–2323. doi: 10.1016/j.socscimed.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Frohlich M, Sund M, Lowel H, Imhof A, Hoffmesiter A, Koenig W. Independent association of various smoking characteristics with markers of inflammation in men. Results from a representative sample of the general population (MONICA Augsburg Survey 1994/1995) Eur Heart J. 2003;24:1365–1372. doi: 10.1016/s0195-668x(03)00260-4. [DOI] [PubMed] [Google Scholar]
- Gimeno D, Ferrie JE, Elovainio M, Pulkki-Raback L, Keltikangas-Jarvinen L, Eklund C, Hurme C, Lehtimäki T, Marniemi J, Viikari JSA, Raitakari OT, Kivimäki M. When do social inequalities in C-reactive protein start? A life course perspective from conception to adulthood in the Cardiovascular Risk in Young Finns Study. Int J Epidemiol. 2008;37:290–298. doi: 10.1093/ije/dym244. [DOI] [PubMed] [Google Scholar]
- Gliksmen MD, Kawachi I, Hunter D, Colditz GA, Manson JE, Stampfer MJ, Speizer FE, Willett WC, Hennekens CH. Childhood socioeconomic status and risk of cardiovascular disease in middle aged US women: a prospective study. Journal of Epidemiology and Childhood Health. 1995;49:10–15. doi: 10.1136/jech.49.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inchley JC, Currie DB, Todd JM, Akhtar PC, Currie CE. Persistent socio-demographic differences in physical activity among Scottish schoolchildren 1990–2002. The European Journal of Public Health. 2005;15:386–388. doi: 10.1093/eurpub/cki084. [DOI] [PubMed] [Google Scholar]
- Jousilahti P, Salomaa V, Rasi V, Vahtera E, Palosuo T. Association of markers of systemic inflammation, C reactive protein, serum amyloid A, and fibrinogen, with socioeconomic status. J Epidemiol Community Health. 2003;57:730–733. doi: 10.1136/jech.57.9.730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan GA, Salonen JT. Socioeconomic conditions in childhood and ischemic heart disease in middle age. BMJ. 1990;301:1121–1123. doi: 10.1136/bmj.301.6761.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlamangla AS, Singer BH, Williams DR. Impact of socioeconomic status on longitudinal accumulation of cardiovascular risk in young adults: the CARDIA Study (USA) Soc Sci Med. 2005;60:999–1015. doi: 10.1016/j.socscimed.2004.06.056. [DOI] [PubMed] [Google Scholar]
- Kasapis C, Thompson PD. The Effects of Physical Activity on Serum C-Reactive Protein and Inflammatory Markers: A Systematic Review. J Am Coll Cardiol. 2005;45:1563–1569. doi: 10.1016/j.jacc.2004.12.077. [DOI] [PubMed] [Google Scholar]
- Keil JE, Sutherland SE, Knapp RG, Tyroler HA. Does Equal Socioeconomic Status in Black and White Men Mean Equal Risk of Mortality? Am J Public Health. 1992;82:1133–1136. doi: 10.2105/ajph.82.8.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khera A, McGuire DK, Murphy SA. Race and gender differences in C-reactive protein levels. J Am Coll Cardiol. 2005;46:464–469. doi: 10.1016/j.jacc.2005.04.051. [DOI] [PubMed] [Google Scholar]
- Kinra S, Nelder RP, Lewendon GJ. Deprivation and childhood obesity: a cross sectional study of 20 973 children in Plymouth, United Kingdom. J Epidemiol Community Health. 2000;54:456–460. doi: 10.1136/jech.54.6.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieger N, Williams D, Moss N. Measuring social class in US public health research: concepts, methodologies, and guidelines. Ann Rev Public Health. 1997;18:341–378. doi: 10.1146/annurev.publhealth.18.1.341. [DOI] [PubMed] [Google Scholar]
- Lagrand WK, Visser CA, Hermens WT, Niessen HWM, Verheught FWA, Wolbink G, Hack E. C-reactive protein as a cardiovascular risk factor: More than an epiphenomenon? Circulation. 1999;100:96–102. doi: 10.1161/01.cir.100.1.96. [DOI] [PubMed] [Google Scholar]
- Langenberg C, Hardy R, Kuh D, Brunner E, Wadsworth M. Central and total obesity in middle aged men and women in relation to lifetime socioeconomic status: evidence from a national birth cohort. J Epidemiol Community Health. 2003;57:816–822. doi: 10.1136/jech.57.10.816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapidus L, Bengtsson C, Hallstrom T, Bjorntorp P. Obesity, adipose tissue distribution and health in women--results from a population study in Gothenburg, Sweden. Appetite. 1989;13:25–35. doi: 10.1016/0195-6663(89)90024-x. [DOI] [PubMed] [Google Scholar]
- Lawlor DA, Ebrahim S, Davey Smith G. Socioeconomic position in childhood and adulthood and insulin resistance: cross sectional survey using data from British women’s heart and health study. BMJ. 2002;325:805–810. doi: 10.1136/bmj.325.7368.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledue TB, Weiner DL, Sipe J, Poulin SE, Collins MF, Rifai N. Analytical evaluation of particle-enhanced immunonephelometric assays for C-reactive protein, serum amyloid A, and mannose binding protein in human serum. Ann Clin Biochem. 1998;35:745–753. doi: 10.1177/000456329803500607. [DOI] [PubMed] [Google Scholar]
- Lehman BJ, Taylor SE, Kiefe CI, Seeman TE. Relation of childhood socioeconomic status and family environment to adult metabolic functioning in the CARDIA Study. Psychosom Med. 2005;67:846–854. doi: 10.1097/01.psy.0000188443.48405.eb. [DOI] [PubMed] [Google Scholar]
- Leon DA, Koupilova I, Lithell HO. Failure to realize growth potential in utero and adult obesity in relation to blood pressure in 50 year old Swedish men. BMJ. 1996;312:401–406. doi: 10.1136/bmj.312.7028.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu K, Cedre LB, Stamler J, Dyer A, Stamler R, Nanas S, Berkson DM, Paul O, Lepper M, Lindberg HA, Marquardt J, Stevens E, Schoenberger JA, Shekelle RB, Collette P, Shekelle S, Garside D. Relationship of education to major risk factors and death from coronary heart disease, cardiovascular diseases and all causes, findings of three Chicago epidemiologic studies. Circulation. 1982;66:1308–1314. doi: 10.1161/01.cir.66.6.1308. [DOI] [PubMed] [Google Scholar]
- Loucks EB, Rehkopf DH, Thurston RC, Kawachi I. Socioeconomic disparities in metabolic syndrome differ by gender: evidence from NHANES III. Ann Epidemiol. 2007;17:19–26. doi: 10.1016/j.annepidem.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Lynch JW, Kaplan GA, Salonen JT. Why do poor people behave poorly? Variation in adult health behaviours and psychosocial characteristics by stages of the socioeconomic lifecourse. Soc Sci Med. 1997;44:809–819. doi: 10.1016/s0277-9536(96)00191-8. [DOI] [PubMed] [Google Scholar]
- Maier SF, Watkins LR. Cytokines for psychologists: Implications of bidirectional immune-to-brain communication for understanding behavior, mood, and cognition. Psychology Review. 1998;105:83–107. doi: 10.1037/0033-295x.105.1.83. [DOI] [PubMed] [Google Scholar]
- Marmot M, Ryff CD, Bumpass LL, Shipley M, Marks NF. Social inequalities in health: next questions and converging evidence. Soc Sci Med. 1997;44:901–910. doi: 10.1016/s0277-9536(96)00194-3. [DOI] [PubMed] [Google Scholar]
- Maty SC, Lynch JW, Raghunathan TE, Kaplan GA. Childhood Socioeconomic Position, Gender, Adult Body Mass Index, and Incidence of Type 2 Diabetes Mellitus Over 34 Years in the Alameda County Study. Am J Public Health. 2008;98:1486–1494. doi: 10.2105/AJPH.2007.123653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendall MA, Patel P, Ballam L, Strachan D, Northfield TC. C reactive protein and its relation to cardiovascular risk factors a population based cross sectional study. BMJ. 1996;312:1061–1065. doi: 10.1136/bmj.312.7038.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendall MA, Strachan DP, Butland BK, Ballam L, Morris J, Sweetnam PM, Elwood PC. C-reactive protein relation to total mortality, cardiovascular mortality and cardiovascular risk factors in men. Eur Heart J. 2000;21:1584–1590. doi: 10.1053/euhj.1999.1982. [DOI] [PubMed] [Google Scholar]
- Notkola V, Punsar S, Karvonen MJ, Haapakoski J. Socio-economic conditions in childhood and mortality and morbidity caused by coronary heart disease in adulthood in rural Finland. Soc Sci Med. 1985;21:517–523. doi: 10.1016/0277-9536(85)90035-8. [DOI] [PubMed] [Google Scholar]
- Osmond C, Barker DJP, Winter PD, Fall CHD, Simmonds SJ. Early growth and death from cardiovascular disease in women. BMJ. 1993;307:1519–1524. doi: 10.1136/bmj.307.6918.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen N, Poulton T, Hay FC, Mohamed-Ali V, Steptoe A. Socioeconomic status, CRP, immune factors, and responses to acute mental stress, Brain Behav. Immun. 2003;17:286–295. doi: 10.1016/s0889-1591(03)00058-8. [DOI] [PubMed] [Google Scholar]
- Paffenbarger RS, Blair SN, Lee IM, Hyde RT. Measurement of physical activity to assess health effects in free-living populations. Med Sci Sports Exerc. 1993;25:60–70. doi: 10.1249/00005768-199301000-00010. [DOI] [PubMed] [Google Scholar]
- Pai JK, Pischon T, Ma J, Manson JE, Hankinson SE, Joshipura K, Curhan GC, Rifai N, Cannuscio CC, Stampfer MJ, Rimm EB. Inflammatory markers and the risk of coronary heart disease in men and women. N Engl J Med. 2004;351:2599–2610. doi: 10.1056/NEJMoa040967. [DOI] [PubMed] [Google Scholar]
- Panagiotakos DB, Pitsavos CE, Chrysohoou CA, Skoumas J, Toutouza M, Belegrinos D, Toutouzas PK, Stefanadis C. The association between educational status and risk factors related to cardiovascular disease in healthy individuals the ATTICA study. Ann Epidemiol. 2004;14:188–194. doi: 10.1016/S1047-2797(03)00117-0. [DOI] [PubMed] [Google Scholar]
- Parker L, Lamont DW, Unwin N, Pearce MS, Bennett SMA, Dickinson HO, White M, Mathers JC, Alberti KGMM, Craft AW. A lifecourse study of risk for hyperinsulemia, dyslipidaemia and obesity (the central metabolic syndrome) at age 49–51 years. Diabetic Medicine. 2003;20:406–415. doi: 10.1046/j.1464-5491.2003.00949.x. [DOI] [PubMed] [Google Scholar]
- Pekkanen J, Tuomilehto J, Uutela A, Vartiainen E, Nissinen A. Social class, health behaviour, and mortality among men and women in eastern Finland. BMJ. 1995;311:589–593. doi: 10.1136/bmj.311.7005.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson TA, et al. Markers of Inflammation and Cardiovascular Disease: Application to Clinical and Public Health Practice. A Statement for Healthcare Professionals From the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107:499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- Petersen KL, Marsland AL, Flory J, Votruba-Drzal E, Muldoon MF, Manuck SB. Community Socioeconomic Status is Associated with Circulating Interleukin-6 and C-Reactive Protein. Psychosom Med. 2008;70:646–652. doi: 10.1097/PSY.0b013e31817b8ee4. [DOI] [PubMed] [Google Scholar]
- Phillips DIW, Walker BR, Reynolds RM, Flanaghan DEH, Wood PJ, Osmond C, et al. Low birth weight predicts elevated plasma cortisol concentrations in adults from 3 populations. Hypertension. 2000;35:1301–1306. doi: 10.1161/01.hyp.35.6.1301. [DOI] [PubMed] [Google Scholar]
- Pollitt RA, Kaufman JS, Rose KM, Diez-Roux AV, Zeng D, Heiss G. Early-life and adult socioeconomic status and inflammatory risk markers in adulthood. Eur J Epidemiol. 2007;22:55–66. doi: 10.1007/s10654-006-9082-1. [DOI] [PubMed] [Google Scholar]
- Poulton R, Avshalom C, Milne BJ, Thompson WM, Taylor A, Sears MR, Moffit TE. Association between children’s experience of socioeconomic disadvantage and adult health: a life-course study. Lancet. 2002;360:1640–45. doi: 10.1016/S0140-6736(02)11602-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power C, Manor O, Matthews S. Child to adult socioeconomic conditions and obesity in a national cohort. Int J Obes Relat Metab Disord. 2003;27:1081–1086. doi: 10.1038/sj.ijo.0802323. [DOI] [PubMed] [Google Scholar]
- Power C, Graham H, Due P. The contribution of childhood and adult socioeconomic position to adult obesity and smoking behaviour: an international comparison. Int J Epidemiol. 2005;34:335–344. doi: 10.1093/ije/dyh394. [DOI] [PubMed] [Google Scholar]
- Raikkonen K, Hautanen A, Keltikangas-Jarvinen L. Association of stress and depression with regional fat distribution in healthy middle-aged men. J Behav Med. 1994;17:605–616. doi: 10.1007/BF01857600. [DOI] [PubMed] [Google Scholar]
- Rao KM, Pieper CS, Currie MS, Cohen HJ. Variability of plasma IL-6 and crosslinked fibrin dimers over time in community dwelling elderly subjects. Am J Clin Pathol. 1994;102:802–805. doi: 10.1093/ajcp/102.6.802. [DOI] [PubMed] [Google Scholar]
- Reynolds RM, Walker BR, Syddall HE, Andrew R, Wood PJ, Phillips DI. Is there a gender difference in the associations of birthweight and adult hypothalamic–pituitary–adrenal axis activity? Eur J Endocrin. 2005;152:249–253. doi: 10.1530/eje.1.01846. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Cushman M, Stampfer MJ, Tracy RP, Hennekens CH. Inflammation, aspirin, and risk of cardiovascular disease in apparently healthy men. N Engl J Med. 1997;336:973–979. doi: 10.1056/NEJM199704033361401. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Buring JE, Shih J, Matias M, Hennekens CH. Prospective study of CRP and risk of future cardiovascular events among apparently healthy women. Circulation. 1998;98:731–733. doi: 10.1161/01.cir.98.8.731. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Hennekens CH, Buring JE, Rifai N. CRP and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342:836–843. doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]
- Rose G, Marmot MG. Social Class and Coronary Heart Disease. BMJ. 1981;45:13–19. doi: 10.1136/hrt.45.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross R. Mechanisms of disease: Atherosclerosis – an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- Sadowski H, Ugarte B, Kolvin I, Kaplan C, Barnes J. Early life family disadvantages and major depression in adulthood. The British Journal of Psychiatry. 1999;174:112–120. doi: 10.1192/bjp.174.2.112. [DOI] [PubMed] [Google Scholar]
- Sallis JF, Prochaska JJ, Taylor WC. A review of correlates of physical activity of children and adolescents. Med Sci Sports Exerc. 2000;32:963–975. doi: 10.1097/00005768-200005000-00014. [DOI] [PubMed] [Google Scholar]
- Salonen JT. Socioeconomic Status and Risk of Cancer, Cerebral Stroke, and Death Due to Coronary Heart Disease and Any Disease: a Longitudinal Study in Eastern Finland. Journal of Epidemiology and Childhood Health. 1982;36:294–297. doi: 10.1136/jech.36.4.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segerstrom SC, Miller GE. Psychological stress and the human immune system: A meta-analytic study of 30 years of inquiry. Psychol Bull. 2004;130:601–630. doi: 10.1037/0033-2909.130.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shemesh T, Rowley KG, Jenkins A, Brimblecombe J, Best JD, O’Dea K. Differential association of C-reactive protein with adiposity in men and women in an Aboriginal community in northeast Arnhem Land of Australia. International Journal of Obesity. 2007;31:103–108. doi: 10.1038/sj.ijo.0803350. [DOI] [PubMed] [Google Scholar]
- Siegel D, Kuller L, Lazarus NB, Black D, Feigal D, Hughes G, Schoenberger JA, Hulley SB. Predictors of Cardiovascular Events and Mortality in the Systolic Hypertension in the Elderly Program Pilot Project. Am J Epidemiol. 1987;126:385–399. doi: 10.1093/oxfordjournals.aje.a114670. [DOI] [PubMed] [Google Scholar]
- Sobal A, Stunkard AJ. Socioeconomic Status and Obesity: A Review of the Literature. Psychol Bull. 1989;105:260–275. doi: 10.1037/0033-2909.105.2.260. [DOI] [PubMed] [Google Scholar]
- Taylor SE, Lehman BJ, Kiefe CI, Seeman TE. Relationship of early life stress and psychological functioning to adult C-reactive protein in the Coronary Artery Risk Development in Young Adults Study. Biol Psychiatry. 2006;60:819–824. doi: 10.1016/j.biopsych.2006.03.016. [DOI] [PubMed] [Google Scholar]
- Thompson SG, Kienast J, Pyke SDM, Haverkate F, van de Loo JCW. Hemostatic factors and the risk of myocardial infarction or sudden death in patients with angina pectoris. N Engl J Med. 1995;332:635–641. doi: 10.1056/NEJM199503093321003. [DOI] [PubMed] [Google Scholar]
- Thurston RC, Kubzansky LD, Kawachi I, Berkman LF. Is the association between socioeconomic position and coronary heart disease stronger in women than in men? Am J Epidemiol. 2005;162:57–65. doi: 10.1093/aje/kwi159. [DOI] [PubMed] [Google Scholar]
- Vogels EA, Lagro-Janssen AL, van Weel C. Sex differences in cardiovascular disease: are women with low socioeconomic status at high risk? British Journal of General Practice. 1999;49:963–966. [PMC free article] [PubMed] [Google Scholar]
- Wamala SP, Lynch J, Kaplan GA. Women’s exposure to early and later life socioeconomic disadvantage and coronary heart disease risk: the Stockholm Female Coronary Risk Study. Int J Epidemiol. 2001;30:275–284. doi: 10.1093/ije/30.2.275. [DOI] [PubMed] [Google Scholar]
- Wannamathee GS, Whincup PH, Shaper G, Walker M. Influence of father’s social class on cardiovascular disease in middle-aged men. Lancet. 1996;348:1259–1263. doi: 10.1016/S0140-6736(96)02465-8. [DOI] [PubMed] [Google Scholar]
- Weinstock M, Matlina E, Maor GI, Rosen H, McEwen BS. Prenatal stress selectively alters the reactivity of the hypothalamic-pituitary adrenal system in the female rat. Brain Res. 1992;595:195–200. doi: 10.1016/0006-8993(92)91049-k. [DOI] [PubMed] [Google Scholar]
- Wing RR, Matthews KA, Kuller LH, Meilahn EL, Plantinga P. Waist to hip ratio in middle-aged women. Associations with behavioral and psychosocial factors and with changes in cardiovascular risk factors. Arterioscler Thromb. 1991;11:1250–1257. doi: 10.1161/01.atv.11.5.1250. [DOI] [PubMed] [Google Scholar]
- Winkleby MA, Jatulis DE, Frank E, Fortmann SP. Socioeconomic Status and Health: How Education, Income, and Occupation Contribute to Risk Factors for Cardiovascular Disease. Am J Public Health. 1992;82:816–820. doi: 10.2105/ajph.82.6.816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T, Dorn JP, Donahue RP, Sempos CT, Trevisan M. Associations of serum C-reactive protein with fasting insulin, glucose, and glycosylated hemoglobin the Third National Health and Nutrition Examination Survey, 1988–1994. Am J Epidemiol. 2002;155:65–71. doi: 10.1093/aje/155.1.65. [DOI] [PubMed] [Google Scholar]
- Zang EA, Wynder EL. Smoking Trends in the United States between 1969 and 1995 Based on Patients Hospitalized with Non-Smoking Related Diseases. Prev Med. 1998;27:854–861. doi: 10.1006/pmed.1998.0369. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Wang Y. Trends in the Association between Obesity and Socioeconomic Status in U.S. Adults: 1971 to 2000. Obesity Research. 2004;12:1622–1632. doi: 10.1038/oby.2004.202. [DOI] [PubMed] [Google Scholar]