Abstract
Early life adversities are risk factors for later mood and emotional disorders. Repeated separation of infant marmosets from their parents provides a validated primate model of depression vulnerability, producing in vivo biochemical and behavioural effects indicative of persistently altered stress reactivity and mild anhedonia. Here we report the long-term effect (in adolescence) of this intervention on the expression of synaptophysin, GAP-43, VGluT1, VGAT, MAP-2, spinophilin, and 5-HT1A and 5-HT2A receptors, in the anterior cingulate cortex (ACC; supragenual and subgenual areas) and amygdala (lateral, basal and central nuclei). These genes and regions are implicated in the response to stress or in mood disorder. The profile of 5-HT1A receptor binding in ACC was affected by early deprivation, notably in the subgenual region, with a decrease in deep laminae but an increase in superficial laminae. Following early deprivation, spinophilin was reduced in the subgenual ACC. In the amygdala, no significant effects of the manipulation were seen, but expression of several transcripts was sexually dimorphic. There were correlations between expression of some transcripts and in vivo measurements. The results show that early deprivation in a non-human primate has a selective long-term effect on expression of genes in the ACC, particularly the subgenual area. The results differ from those reported in the hippocampus of the same animals, indicating the presence of limbic region-specific long-term molecular responses to early life stress.
Keywords: Subgenual area, serotonin 1A receptor, Depression, Mood disorder, Sexual dimorphism
Introduction
Experimental and epidemiological evidence shows that early life stressors, such as parental separation, are major risk factors for mood and anxiety disorders (DeBellis et al., 1999; Heim and Nemeroff, 2001). The preclinical data come mostly from studies of rodents, but recently some relevant non-human primate models have been developed (Gilmer and McKinney, 2003; Pryce et al., 2005; Sanchez et al., 2001). In particular, in the common marmoset (Callithrix jacchus), a small New World monkey, daily short periods of isolation from the family group during the first month of life (‘early deprivation’; ED) causes endocrine stress responses, and leads to biochemical, cardiovascular, and behavioural effects during juvenility and adolescence, indicative of increased basal activity and reactivity in stress systems, and mild anhedonia (Dettling et al., 2002a,b, 2007; Pryce et al., 2004a,b). These findings indicate that the ED protocol leads to a ‘pro-depressive’ state that persists until at least month 12 of life, which is adolescence in this species. Moreover, ED causes long-term changes in gene expression in the hippocampus, including effects on 5-HT1A receptors and markers of synaptic plasticity (Law et al., 2009) that may reflect the behavioural consequences of ED and which show similarities to alterations reported in the hippocampi of people with mood disorder.
Along with the hippocampus, the anterior cingulate cortex (ACC; Devinsky et al., 1995; Diorio et al., 1993; Paus, 2001; Vogt, 2005) and the amygdala (Davidson, 2002; LeDoux, 2000; Murray, 2007) are brain regions strongly implicated in emotion, mood and the responses to stress. They are also involved centrally in the neuropathology and pathophysiology of a range of psychiatric and neurological conditions, especially mood and anxiety disorders (Drevets et al., 1997; Ebert and Ebmeier, 1996; Harrison, 2002; Mayberg et al., 1997; Nestler et al., 2002). The clinical studies have contributed to the realisation that the ACC is anatomically, functionally, and pathophysiologically, heterogeneous. One key distinction is that between the supragenual and subgenual ACC (Carmichael and Price, 1996; Gittins and Harrison, 2004; Palomero-Gallagher et al., 2008; Vogt et al., 1995). It is the subgenual area that is most implicated in mood disorder (Drevets et al., 1997; Hajek et al., 2008: Koo et al., 2008; Mayberg, 1997; Öngür et al., 1998a) and its treatment (Drevets et al., 2002; Mayberg et al., 2005).
To date, few studies have examined the long-term molecular consequences of early life stress on the ACC or amygdala, and primate data are limited to a single study (Sabatini et al., 2007). This study had two related objectives. First, to discover whether ED in the marmoset has a long-term effect on gene expression in these regions, including a separate analysis of supra- and sub-genual ACC. Second, to identify whether there are correlations between expression of the genes and the biochemical and behavioural effects of ED measured. We focused on three groups of genes, each of which has been implicated in the pathophysiology of mood disorder, and studied previously in the hippocampus of the ED animals (Law et al., 2009): serotonin (5-HT1A and 5-HT2A) receptors, and genes indexing presynaptic (synaptophysin, VGluT1, VGAT, GAP-43) and dendritic (MAP-2, spinophilin) functioning. To the extent that the ED model provides a model of depression vulnerability, the findings also have relevance to the role that early life stress may have in the pathogenesis of ACC or amygdala involvement in mood disorder.
Methods
The animals were bred, and all in vivo studies conducted, at the Laboratory for Behavioural Neurobiology of the Swiss Federal Institute of Technology, Zurich, under experimental permit in accordance with the Swiss Animal Protection Act (1978). The brains were shipped to Oxford for study under licence from the Convention for International Trade in Endangered Species of Wild Fauna and Flora (CITES), administered by the Swiss Federal Office for Veterinary Affairs and the UK Department for Environment, Food and Rural Affairs.
The ED intervention
The ED intervention and its effects in vivo in infancy, juvenility and adolescence have been described fully (Dettling et al., 2002a,b; Law et al., 2009; Pryce et al., 2004a,b). Briefly, the marmoset is characterised by monogamous breeding, dizygotic twins, and high levels of care-giving by both parents. In this study, each set of parents contributed, in random order, one pair of ED twins and one pair of control twins; one set of parents contributed one pair of ED twins only. The total sample was therefore 10 pairs of ED twins and 9 pairs of control twins (Table 1). On post-natal days 2-28, ED infants were separated from their parents for 30-120 minutes each day, using variable durations and timings. ED was carried out consecutively within each twin pair, such that one infant remained with the parents at all times. Controls were briefly handled on the back of the carrying parent each day. After day 28, subjects remained with the family group, and there were no further interventions that differed between ED and control subjects. Subsequent behavioural testing, and the collection of physiological samples, were carried out in the home cage, except that at age 18-20 weeks, animals were studied in six 60-min tests of isolation from the family in a novel physical environment.
Table 1. Details of animals and in vivo data.
| Early deprivation | Controls | Significant in vivo effectsa | |
|---|---|---|---|
| (n=11) | (n=9) | ||
| Sex (male, female) | 7, 4 | 5, 4 | |
| Age (weeks) | 48.4 (range 43-51) | 48.2 (range 47-50) | |
| Terminal CSF cortisol (μg/dl) | 12.2 (1.31) | 10.5 (1.5) | ED x family group interactionc |
| Urinary noradrenaline (ng/mg creatinine)b | 75.9 (6.5) | 56.3 (8.1) | ED main effectd |
| Urinary dopamine (ng/mg creatinine)b | 973 (143) | 690 (86) | ED main effecte |
| Social play in infancy (percent time) | 4.4 (0.4) | 5.6 (1.1) | Trend to ED main effectf |
| Impulsivity in juvenility (from 64 trials) | 13.9 (1.7) (n=9) | 12.3 (3.5) (n=6) | ED x session interactione |
| Reward motivation in adolescence (average rewards) | 8.8 (2.4) (n=8) | 11.9 (1.9) (n=7) | ED main effectd |
Values are mean (SEM). ED = early deprivation.
In vivo studies were carried out with both sets of twins (ED-ED and Control-Control) from each of 5-9 breeding pairs.
Males only. Value averaged from samples taken between weeks 9 and 48.
Laboratory methods
Brains were snap frozen, and stored at -80°C before being coronally sectioned on a cryostat at 10μm thickness and prepared for in situ hybridization (ISH) or receptor autoradiography (Law et al., 2009). Every 20th slide was taken for Nissl staining to identify anatomical landmarks.
Since marmoset cDNA sequences were not available, the proposed target region of each transcript was amplified from marmoset cDNA using RT-PCR with primers designed to the human cDNA sequence. RT-PCR products were then sequenced, and oligonucleotide probes designed that were 100% complementary to the marmoset cDNA sequence (available on request). For ISH, the frozen sections were thawed, fixed, acetylated and delipidated (Law et al., 2003). Oligonucleotides were 3′ end-labelled with [35S]dATP (1250Ci / mmol; Perkin Elmer, UK) in a 10:1 molar ratio using terminal deoxynucleotidyl transferase (Promega, UK). The experimental conditions and film exposure times for each probe were optimised in pilot studies; the definitive experiment for each transcript was performed in a single run. Sections were incubated overnight at 40-42°C with hybridization buffer containing 1.0 × 106 cpm of labelled probe, as described (Law et al., 2003). Post-incubation washes were carried out in 1× SSC at 55°C for 3 × 20 min and 1 hour at room temperature. Experimental controls comprised: concurrent hybridization with sense strand probes, hybridization in the presence of 50-fold excess unlabelled probe, and ribonuclease (RNase A 200 μg/ml at 37°C for 20 min) pre-treatment. After air drying, slides were apposed to autoradiographic film (Kodak, Rochester, NY, USA) along with 14C microscales (Amersham Pharmacia Biotech, Sweden) for 3 weeks (GAP-43 and 5-HT1AR), 2 weeks (synaptophysin, spinophilin and VGAT), or 1 week (VGluT1 and MAP2).
Autoradiographic analysis of 5-HT1AR binding site densities was carried out using [3H]WAY100,635 (Burnet et al., 1997). Briefly, sections were thawed and pre-incubated at room temperature in 50mM Tris-HCl buffer (pH7.4) for 30 min. Sections were then incubated in 50mM Tris-HCl containing 3nM [3H]WAY100,635 for 2 h at room temperature. Non-specific binding was determined by incubation of adjacent sections with 10μM 5-HT. Slides were washed with the same buffer for 2 × 4 min at 4°C. Sections were air dried and apposed to Biomax film for 3 weeks. Autoradiography for 5-HT2A receptor binding sites was carried out with 2nM [3H]ketanserin (Eastwood et al., 2001; Pazos et al., 1985); non-specific binding was determined using 10μm mianserin, and film exposure was 6 weeks.
Image and Data Analysis
Densitometric measurements, converted to nCi/g, were taken over the supragenual and subgenual ACC, and over the amygdala (basal, lateral and central nuclei, referred to as BNA, LNA and CNA respectively), with reference to an atlas (Stephan et al., 1980). Within ACC, the average signal across the depth of the grey matter was measured, except for [3H]WAY100,635 binding, for which separate readings were taken over superficial and deep laminae, reflecting its markedly inhomogeneous localisation, with an absence of significant binding in middle layers (Burnet et al., 1997; Hall et al., 1997). Because of limited tissue availability (or low level of expression of some transcripts), not all areas could be measured in every experiment. In each region, signal was measured over the duplicate or triplicate sections and the mean value used for statistical analysis. Readings were converted to nCi/g using co-exposed 14C microscales. All experiments and analyses were conducted blind to treatment group.
Data were examined for normality using the Kolmogorov-Smirnov one-sample test. The effect of ED was measured in each region by ANOVA, with group (ED, control) and sex (male, female) as between-subjects factors. For [3H]WAY100,635 binding in the ACC, repeated measures ANOVA was used with compartment (superficial, deep) as the within subjects factor; the ratio of binding between the two compartments was also measured. Sex was included in all ANOVAs because ED-by-sex interactions were seen in the hippocampus of these animals (Law et al., 2009).
To explore whether the parameters measured here were related to three biochemical and three behavioural indices which were affected by the ED intervention (see Table 1) we used Spearman correlations. As a partial control against multiple testing, we set α=0.02, and followed up each significant correlation by examining whether it persisted after partialling for the effect of ED; we also inspected whether a similar correlation was present (at least as a trend) in control and ED groups considered separately.
Results
Distribution of transcripts and receptor binding sites
Figure 1A shows a representative coronal section of the marmoset brain that includes the subgenual ACC, with the regions wherein supragenual and subgenual measurements were taken. The distribution of VGluT1 (Fig. 1B), VGAT (Fig. 1C), GAP-43 (Fig. 1D), synaptophysin (Fig. 1E), spinophilin (Fig. 1F) mRNAs are shown, as is [3H]WAY100,635 binding (Fig. 1G) and [3H]ketanserin binding (Fig. 1H). Note in Fig. 1G, [3H]WAY100,635 binding showed the anticipated laminar distribution in the supragenual ACC, with a band of prominent signal over the superficial lamina of the grey matter, with a separate, weaker band in the deep laminae. In the subgenual ACC, however, this profile was reversed (Fig. 1G). A representative section through the amygdala is also shown (Fig. 1I).
Figure 1.
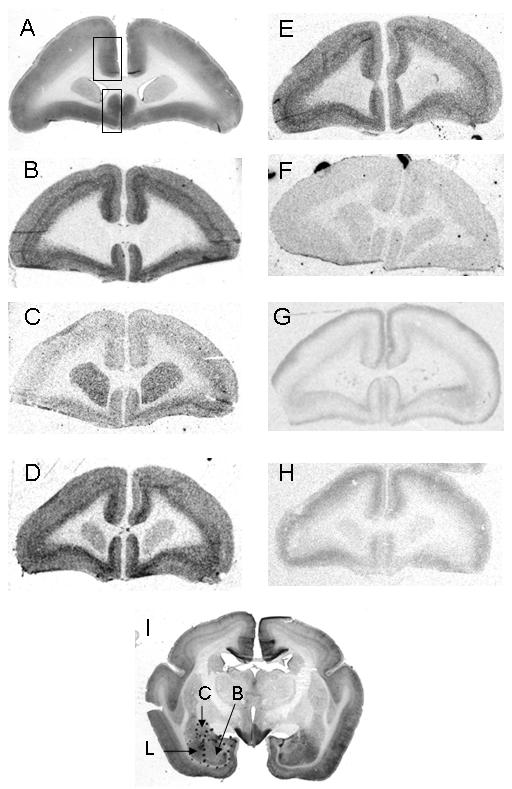
A: Nissl stained coronal section at the level of the ACC (∼A12.5 in the atlas of Stephan et al., 1980). The large rectangle shows where the measurements were taken within the supragenual ACC (cortex cingularis anterior in Stefan et al., 1980; large rectangle) and the subgenual region (cortex subgenualis in Stefan et al., 1980; small rectangle). B: VGluT1 mRNA. C: VGAT mRNA. D: GAP-43 mRNA. E: Synaptophysin mRNA. F: Spinophilin mRNA. G: [3H]WAY100,635 binding. H: [3H]ketanserin binding. I: Nissl stained coronal section through the amygdala, showing the basal (bas), central (cen) and lateral (lat) nuclei (∼A10 in Stephan et al., 1980).
The experimental controls for each probe showed minimal background signal over the ACC and amygdala. In addition to the sequence verification of each probe target, and the fact that each mRNA had the expected distribution based on other primate and rodent studies, this indicates the specificity of signal for each transcript. Similarly, the distribution of radioligand binding to 5-HT1A and 5-HT2A receptors was as anticipated, and showed no detectable labelling after cold displacement.
Effect of ED on expression of pre-synaptic protein genes
VGluT1 mRNA in the ACC did not show a main effect of ED or sex, nor an interaction between them (Table 2). In the BNA, there was a main effect of sex (F1,14=6.74, p=0.021), with VGluT1 mRNA signal greater in males than females (405 ± 13 vs. 338 ± 26 nCi/g; t=-2.59, d.f.16, p=0.022). There was also an ED-by-sex interaction (F1,14=5.42, p=0.035). The latter reflected a trend for BNA VGluT1 mRNA to be increased by ED in females and decreased in males, but neither post hoc test was significant (p=0.35 and p=0.15 respectively). In the CNA, there was a main effect of sex on VGluT1 mRNA (F1,14=5.88, p=0.029), again reflecting higher signal in males than females (487± 22 vs. 402 ± 22 nCi/g; t=-2.61, d.f.16, p=0.019) but no effect of ED nor ED-by-sex interaction.
Table 2. Effects of early deprivation on gene expression in the supragenual and subgenual anterior cingulate cortex.
| Supragenual ACC | Subgenual ACC | |||
|---|---|---|---|---|
| Control | ED | Control | ED | |
| (n=8-9) | (n=10-11) | (n=8-9) | (n=10-11) | |
| Synaptophysin mRNA | 377 (57) | 434 (49) | 466 (71) | 458 (70) |
| VGluT1 mRNA | 687 (48) | 720 (44) | 746 (59) | 755 (53) |
| VGAT mRNA | 93 (5) | 108 (7) | 102 (7) | 123 (7) |
| GAP-43 mRNA | 86 (5) | 88 (4) | 97 (4) | 90 (7) |
| MAP-2 mRNA | 586 (53) | 531 (44) | 643 (95)a | 533 (32) |
| Spinophilin mRNA | 128 (17)b | 112 (5) | 175 (19)b | 118 (9)** |
| 5-HT1AR mRNA | 26 (3) | 31 (3) | 18 (2) | 24 (3) |
| 5-HT2AR mRNA | 67 (3) | 63 (3)c | 58 (2) | 58 (3) |
| [3H]ketanserin binding | 7.8 (0.6)c | 5.8 (0.7)b | 3.1 (0.5)d | 2.6 (0.5)b |
Values are mean (SD) nCi/g.
p=0.017.
N=4.
N=5.
N=7
VGAT mRNA and GAP-43 mRNA showed no effect of ED, sex, nor interaction between them (all P>0.1) in any region measured (Tables 2 and 3).
Table 3. Effects of early deprivation on gene expression in the amygdalaa.
| Basal nucleus | Lateral nucleus | Central nucleus | ||||
|---|---|---|---|---|---|---|
| Control | ED | Control | ED | Control | ED | |
| Synaptophysin mRNAb | 240 (30) | 218 (18) | 325 (38) | 265 (22) | 298 (30) | 275 (29) |
| VGluT1 mRNAb,c | 367 (26) | 391 (16) | 452 (32) | 475 (30) | 427 (28) | 481 (21) |
| GAP-43 mRNA | 78 (3) | 76 (4) | 97 (5) | 88 (6) | 82 (5) | 77 (5) |
| MAP-2 mRNAb | 420 (74) | 455 (80) | 501 (76) | 505 (89) | 409 (76) | 450 (79) |
| 5-HT1AR mRNA | 58 (10) | 52 (4) | ND | ND | ND | ND |
| [3H]WAY100635 binding | 48 (4) | 49 (5) | ND | ND | ND | ND |
| 5-HT2AR mRNA | ND | ND | 56 (3) | 57 (3) | 56 (3) | 54 (8) |
Values are mean (SD) nCi/g. ND - not determined.
VGAT mRNA, spinophilin mRNA, and [3H]ketanserin binding were not determined.
Sex difference (see text)
Sex x ED interaction (see text).
Synaptophysin mRNA did not differ between control and ED groups in ACC (Table 2) or amygdala (Table 3). However, in the LNA, there was an effect of sex (F1,14=5.97, p=0.021), with expression being higher in females than in males (356±42 vs. 251±15 nCi/g; t=2.37, p=0.047). A similar trend was seen in the CNA (F1,14=4.07, p=0.063).
Effect of ED on expression of post-synaptic protein genes
MAP-2 mRNA was not affected by ED, nor did it differ between sexes or show ED-by-sex interactions in either supragenual or subgenual ACC (Table 2); note that the subgenual ACC analyses were underpowered in that due to lack of tissue, only four control animals were available. In the amygdala, there were no effects of ED (Table 3) or ED-by-sex interactions on MAP-2 mRNA (all P>0.25), but there was a main effect of sex (P≤0.05) in each nucleus, with MAP-2 mRNA being higher in males than females: in LNA, 615 (82) vs. 392 (58) nCi/g (t=2.21, p=0.044); in BNA, 552 (78) vs. 323 (45) nCi/g (t=2.55, p=0.027); in CNA, 538 (81) vs. 321 (48) nCi/g (t=2.31, p=0.05).
Spinophilin mRNA in the supragenual ACC did not show main effects of ED or sex, nor an interaction between them. In subgenual ACC there was a main effect of intervention (F1,11=7.98, p=0.017), reflecting a reduction of spinophilin mRNA after ED (Table 2), with no effect of, nor interaction with, sex.
Effect of ED on binding and expression of 5-HT1AR and 5-HT2AR
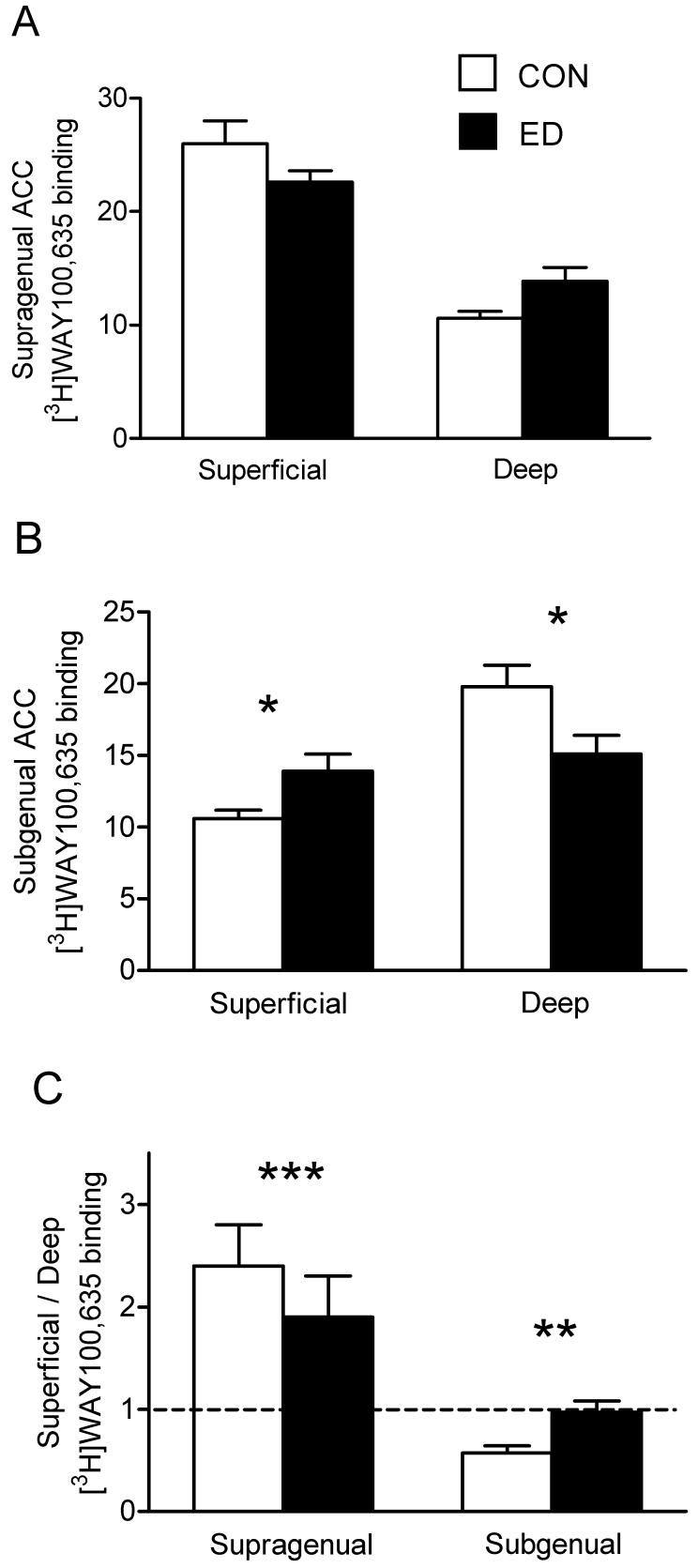
For 5-HT1AR binding, in the supragenual ACC, there was a main effect of laminar compartment (F1,15=169.4, p<0.001), reflecting the higher signal in superficial laminae, and an ED-by-laminar compartment interaction (F1,15=6.76, p=0.020). The effect of ED on [3H]WAY100,635 binding in supragenual ACC was not significant in either compartment considered separately (Fig. 2A), but the superficial : deep ratio of binding was decreased after ED (F1,15=8.67, p=0.010; Fig. 2C, left panel). In the subgenual ACC, there was a main effect of laminar compartment (F1,15=16.2, p=0.001), in this instance arising from the higher level of binding in the deep laminae. There was an ED-by-laminar compartment interaction within subgenual ACC (F1,15=8.15, p=0.012); in superficial laminae, [3H]WAY100,635 binding was increased following ED (Fig. 2B, left panel; F1,15=6.58, p=0.022), whereas in deep laminae, [3H]WAY100,635 binding was reduced after ED (Fig. 2B, right panel; F1,15=5.23, p=0.037). The ratio of binding in subgenual ACC was increased after ED (Fig. 2C, right panel; F1,15=7.03, p=0.018). [3H]WAY100,635 binding was unaffected by ED in the BNA (Table 3). There were no main effects of sex, or ED-by-sex interactions on [3H]WAY100,635 binding in any region.
Figure 2.
[3H]WAY100,635 binding to 5-HT1AR in superficial and deep laminae of the anterior cingulate cortex. A: Supragenual ACC. B: Subgenual ACC. C: The ratio of superficial to deep laminar binding, in supragenual and subgenual ACC. *p<0.05, **p<0.02, ***p=0.01.
5-HT1AR mRNA was not significantly affected by ED in either ACC area (Table 2) or in the amygdala (Table 3), nor were there main effects of sex or ED-by-sex interactions.
[3H]ketanserin binding and 5-HT2AR mRNA were unaffected by ED in ACC (Table 2). There was a trend for binding to be lower, but this did not get close to significance, in part because of the smaller number of subjects available for this analysis (Table 2). 5-HT2AR mRNA was also unaltered after ED in the LNA and CNA (Table 3).
Correlations between gene expression and in vivo measures
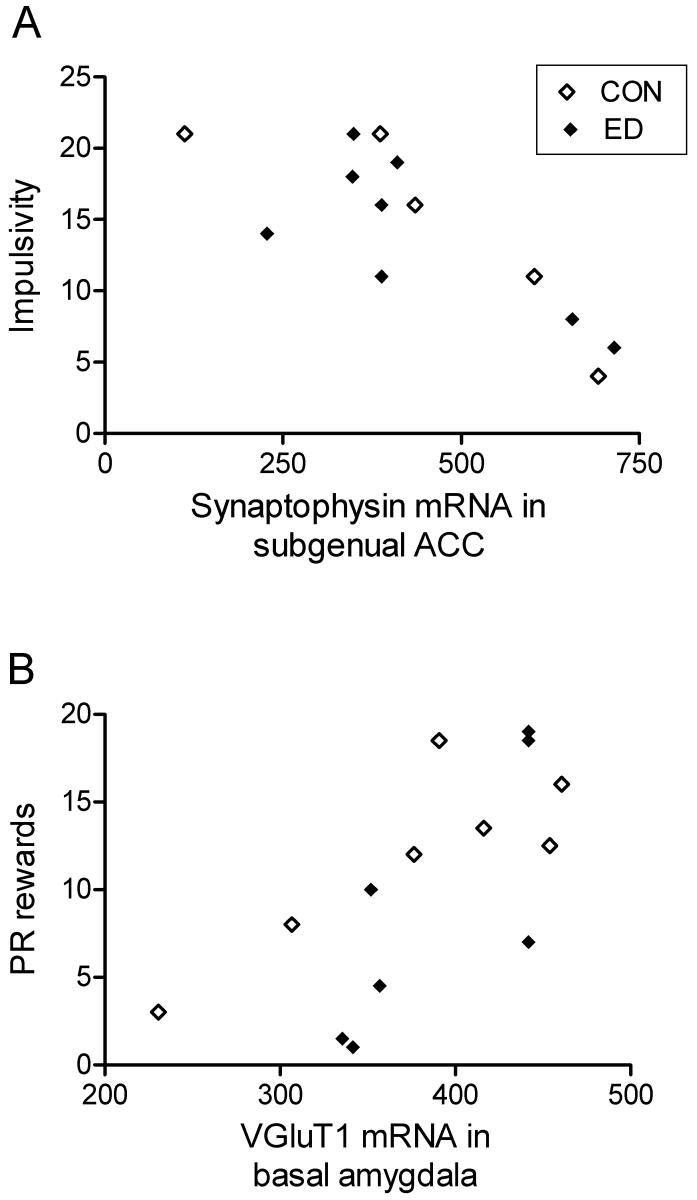
Two correlations between gene expression and behaviour met our criteria for significance (see Materials and Methods). Impulsivity, measured in a palatable-reward reaching task, was increased in ED juveniles compared to controls (Pryce et al., 2004a), and impulsivity scores correlated inversely with synaptophysin mRNA in the subgenual ACC (r=-0.757, n=13, p=0.003; partialling for effect of ED: r=-0.812, d.f.=10, p=0.001), with similar trends in control (r=-0.975, n=5, p=0.005) and ED (r=-0.527, n=8, p=0.18) groups (Fig. 3A). Second, motivation for palatable reward, measured on a progressive ratio (PR) schedule of reinforcement, was reduced in ED adolescents (Pryce et al., 2004b), and scores for rewards obtained correlated with VGluT1 mRNA in BNA (r=0.718, n=14, p=0.004), a relationship seen in control (r=0.750, n=7, p=0.052) and ED (r=0.775, n=7, p=0.041) groups considered separately (Fig. 3B), and which remained significant when partialling for the effect of ED (r=0.742, d.f.11, p=0.004). A similar but weaker relationship was seen for VGluT1 mRNA in subgenual ACC (not shown).
Figure 3.
A: Synaptophysin mRNA in subgenual ACC correlates negatively with impulsivity (n=13, r=-0.757, p=0.003). B: VGluT1 mRNA in the basal nucleus of the amygdala correlates with the number of rewards on the progressive ratio of reinforcement (PR rewards; n=14, r=0.718, p=0.004).
Discussion
The marmoset ED paradigm provides one of the first well-validated primate models of depression vulnerability. It produces not only behavioural and biochemical effects indicative of long-term effects on stress reactivity and mild anhedonia (Dettling et al., 2002a,b, 2007; Pryce et al., 2004a,b), but also leads to several long-term changes in hippocampal gene expression that are indicative of an effect of ED on synaptic plasticity and that are similar to findings reported in subjects with mood disorders (Law et al., 2009; see also Manji et al., 2001; Nestler et al., 2002). Here we report a companion study in two other limbic brain regions implicated in stress responses and emotional regulation, the ACC and the amygdala. The changes were less striking than those in the hippocampus, being limited to a change in the distribution of 5-HT1AR binding, and in expression of MAP-2, in the ACC. We also identified two correlations between gene expression and behavioural measures (impulsivity with synaptophysin mRNA in subgenual ACC, and reward-seeking behaviour with VGluT1 mRNA in BNA), and a sexual dimorphic expression of three transcripts (synaptophysin, VGluT1 and MAP-2) in the amygdala.
Cortical 5-HT1AR distribution is inhomogeneous across the depth of the grey matter. In other primate species, the highest level of mRNA expression (Burnet et al., 1995), immunoreactivity (DeFelipe et al., 2001; Palchaudhuri and Flügge, 2005), and ligand binding (Burnet et al., 1996, 1997; Hall et al., 1997), is in the superficial (supragranular) laminae with a lesser band in the deep (infragranular) laminae. The marmoset corresponds to this profile, although interestingly, in the subgenual ACC, the pattern is reversed, with maximal binding in the deep layers. At a cellular level, cortical 5-HT1ARs are mainly expressed by pyramidal neurons (Burnet et al., 1995; Palchaudhuri and Flügge, 2005; Santana et al., 2004) and most studies agree they are localised to the axon initial segment, with much lower levels elsewhere, e.g. in dendrites (Cruz et al., 2004; DeFelipe et al., 2001; but see Kia et al., 1996). We measured 5-HT1AR expression, and binding, since the serotonin system and these receptors are implicated in stress, depression and anxiety, and they are decreased in the hippocampus after ED (Law et al., 2009) and some other stressors (Flügge, 1995; Preece et al., 2004). Following ED, we found no overall effect on 5-HT1AR binding site density in any region, but in ACC there was a complex shift in binding between the superficial and deep laminae (Figure 2). This pattern was marked in the subgenual ACC (Fig 2B), wherein ED led to a significant increase in binding in superficial layers, and a decrease in deep layers, as well as a significant change in the ratio between them. The opposite redistribution (from superficial to deep) occurred in the supragenual ACC (Fig. 2A). There are precedents for alterations in receptor binding sites of this kind. For example, there is an altered relative laminar distribution of cortical 5-HT1AR binding sites in schizophrenia (Burnet et al., 1996; Simpson et al., 1996), of ACC neurokinin-1 receptors in major depression (Burnet and Harrison, 2000), and of cerebellar 5-HT2A receptors in schizophrenia (Eastwood et al., 2001).
Given the cellular and subcellular localisation of 5-HT1A receptors summarised above, we hypothesize that the shifts in [3H]WAY100,635 binding site distribution after ED represent a differential regulation of these receptors on the axon initial segments of superficial (laminae II-III) versus deep (laminae V-VI) pyramidal neurons. This may result from differences in serotonergic innervation (see DeFelipe et al., 2001), perhaps in turn related to the alterations in serotonergic system and hypothalamo-pituitary axis function produced by ED (Dettling et al., 2002a, 2007; Pryce et al., 2004b) and other early life stressors in primates (Ichise et al., 2006; Rilling et al., 2001). Pyramidal neuron 5-HT1A receptors are hyperpolarizing (Arenada and Andrade, 1991) and play a major role in inhibitory regulation of these cells (Cruz et al., 2004; DeFelipe et al., 2001) and hence the receptor redistribution may impact functionally, by changing ACC output characteristics (Czyrak et al., 2003). The efferent cortical and subcortical connections of the ACC vary between supra- and sub-genual areas and between superficial and deep laminae (Carmichael and Price, 1996; Freedman et al., 2000; Johansen-Berg et al., 2008; Öngür et al., 1998b; Vogt et al., 1995, 2005), and thus any such functional implications will be anatomically complex. For example, the subgenual ACC findings (Fig 2B) suggest, simplistically, that pyramidal neurons projecting to other cortical regions (i.e. those in laminae II/III) are subject to increased inhibition, whereas those projecting to subcortical sites (in laminae V/VI) may be disinhibited. The fact that ACC 5-HT1AR mRNA was unchanged after ED indicates that the alterations in binding site distribution are not secondary to altered gene expression, but likely reflect changes in receptor trafficking.
Spinophilin expression was decreased in the subgenual ACC after ED. Spinophilin is a protein concentrated in dendritic spines (Allen et al., 1997), and its reduced expression after ED implies that there is a change in the function, or the structure (or both) of dendritic spines on neurons within this area (Feng et al., 2000). The fact that there were no alterations in MAP-2 (a dendritic marker; Johnson and Jope, 1992; Pei et al., 1998) or in density of 5-HT2AR binding sites (a receptor localised mainly to dendritic shafts [Jakab and Goldman-Rakic, 1998]), suggests that whatever abnormality is being indexed by reduced spinophilin expression is limited to the spines rather than affecting the dendritic tree as a whole (Eastwood et al., 2007; Law et al., 2004a,b). Since dendritic spines receive most excitatory synapses (whereas inhibitory and monoaminergic synapses more often terminate elsewhere on the neuron), the decrease may indicate a particular decrement of glutamatergic innervation to subgenual neurons. This issue merits further study, including direct assessment of dendritic morphology and spine density using Golgi stains.
In the amygdala, unlike the ACC (and hippocampus), ED had no overall effect on any of the transcripts or binding sites. It is possible that ED does not produce long-term effects on amygdala gene expression. However, more likely, there are alterations which do not include the candidate genes we measured. Notably, a recent microarray study of the amygdala in 3-month old rhesus macaques who had experienced maternal separation in infancy found only 20 out of 8,405 transcripts (0.24%) were differentially expressed, none of which were the genes studied here (Sabatini et al., 2007). Their most striking finding was for GUCY1A3, a soluble nitric oxide receptor, and it would be of interest to measure that transcript in the ED model.
In contrast to the lack of effect of ED in the amygdala, there were several sex differences therein: synaptophysin mRNA was higher in females, whereas VGluT1 and MAP-2 mRNAs were higher in males. The synaptophysin mRNA finding is also seen in the marmoset hippocampus, but MAP-2 and VGluT1 mRNAs are not sexually dimorphic in that region (Law et al., 2009). Sex differences in gene expression have been reported in various brain regions and species (Cahill, 2006; Cosgrove et al., 2007; Rinn and Snyder, 2005), and might contribute to the roles that the amygdala plays in sex-specific social behaviours (Madeira and Lieberman, 1995; Simerly, 2002). To our knowledge there are no prior studies investigating sex differences in these transcripts in the amygdala. In that MAP-2 is a dendritic marker (see above), higher MAP-2 expression may reflect a greater dendritic arborisation in the male amygdala, as is observed in the rat medial amygdala (Cooke et al., 2007; Nishizuka and Arai, 1981). Similarly, elevated VGluT1 mRNA in males may reflect a greater activity or density of glutamate synapses (Wilson et al., 2005) in the male amygdala, which would be compatible with ultrastructural findings in rats (Cooke and Woolley, 2005).
We observed two molecular-behaviour correlations: synaptophysin mRNA (putatively, an index of synaptic density and/or function; see Harrison and Eastwood, 2001) in subgenual ACC correlated inversely with impulsive behaviour, whereas higher VGluT1 mRNA (putatively, a marker of enhanced glutamate neurotransmission; Wilson et al., 2005) in the amygdala correlated with greater motivation for reward. The measure of impulsive behaviour was increased in ED juveniles compared to control juveniles (Pryce et al., 2004a) whereas reward motivation was decreased in ED adolescents compared to control adolescents (Pryce et al., 2004b). The two transcripts involved were unaltered by ED, and the correlations were present in both ED and control groups; as such, they represent molecular correlates of individual variation in the two behaviours. Since synaptophysin and VGluT1 are indexing, albeit indirectly, neural connectivity and/or activity, the correlations may be seen as analogous to those between indices of brain function/activity in human subjects and various behaviours (e.g. Hariri et al., 2006; Smolka et al., 2005).
Many further parameters remain to be determined in this primate model of depression vulnerability. First, stereological assessment of ACC and amygdala volumes and their cellular composition, to establish whether these are affected after ED, as has been reported in mood disorder (Bowley et al., 2002; Chana et al., 2003; Cotter et al., 2001; Hajek et al., 2008; Harrison, 2002; Öngür et al., 1998a; Sheline et al., 1998). Second, investigation of other molecular markers of different cell types and biochemical processes, as a step towards delineating mechanisms that mediate between the ED intervention and the long-term in vivo effects and the alterations in gene expression observed here. Additional experimental studies of this kind have the potential to reveal how early life stress leads to vulnerability to mood and emotional disorders, and in turn may offer the prospect of improved and even preventative therapies.
Acknowledgements
The study was funded by the Wellcome Trust, with additional support from the National Science Foundation, Switzerland (Project grant 3167791.02) and National Center for Competence in Research: Swiss Etiological Study of Adjustment and Mental Health (grant no. 51A240-104890). We thank Phil Burnet, Andrea Dettling, Helen Gordon-Andrews and Mary Walker for their expert contributions.
Footnotes
Statement of Interest
In the past three years PJH has received unrestricted educational grants from GlaxoSmithKline (GSK), and honoraria for educational lectures or chairing scientific meetings from Astra Zeneca, Bristol Myers Squibb, GSK, Janssen, Lilly, Merck, Sanofi and Servier, and has been a scientific advisor to Curidium, Janssen, Merck, and Wyeth. None of the other authors declare any interests.
References
- Allen PB, Ouimet CC, Greengard P. Spinophilin, a novel protein phosphatase 1 binding protein localized to dendritic spines. Proceedings of the National Academy of Sciences USA. 1997;94:9956–9961. doi: 10.1073/pnas.94.18.9956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arenade R, Andrade R. 5-Hydroxytryptamine2 and 5-hydroxytryptamine1A receptors mediate opposing responses on membrane excitability in rat association cortex. Neuroscience. 1991;40:399–412. doi: 10.1016/0306-4522(91)90128-b. [DOI] [PubMed] [Google Scholar]
- Bowley MP, Drevets WC, Öngür D, Price JL. Low glial numbers in the amygdala in major depressive disorder. Biological Psychiatry. 2002;52:404–412. doi: 10.1016/s0006-3223(02)01404-x. [DOI] [PubMed] [Google Scholar]
- Burnet PWJ, Harrison PJ. Substance P (NK1) receptors in the cingulate cortex in unipolar and bipolar mood disorder and schizophrenia. Biological Psychiatry. 2000;47:80–83. doi: 10.1016/s0006-3223(99)00264-4. [DOI] [PubMed] [Google Scholar]
- Burnet PWJ, Eastwood SL, Lacey K, Harrison PJ. The distribution of 5-HT1A and 5-HT2A receptor mRNA in human brain. Brain Research. 1995;676:157–168. doi: 10.1016/0006-8993(95)00104-x. [DOI] [PubMed] [Google Scholar]
- Burnet PWJ, Eastwood SL, Harrison PJ. 5-HT1A and 5-HT2A receptor mRNAs and binding site densities are differentially altered in schizophrenia. Neuropsychopharmacology. 1996;15:442–455. doi: 10.1016/S0893-133X(96)00053-X. [DOI] [PubMed] [Google Scholar]
- Burnet PWJ, Eastwood SL, Harrison PJ. [3H]WAY-100635 for 5-HT1A receptor autoradiography in human brain: A comparison with [3H]8-OH-DPAT and demonstration of increased binding in the frontal cortex in schizophrenia. Neurochemistry International. 1997;30:565–574. doi: 10.1016/s0197-0186(96)00124-6. [DOI] [PubMed] [Google Scholar]
- Cahill L. Why sex matters for neuroscience. Nature Reviews Neuroscience. 2006;7:477–484. doi: 10.1038/nrn1909. [DOI] [PubMed] [Google Scholar]
- Carmichael ST, Price JL. Connectional networks within the orbital and medial prefrontal cortex of macaque monkeys. Journal of Comparative Neurology. 1996;371:179–207. doi: 10.1002/(SICI)1096-9861(19960722)371:2<179::AID-CNE1>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Chana G, Landau S, Beasley C, Everall IP, Cotter D. Two-dimensional assessment of cytoarchitecture in the anterior cingulate cortex in major depressive disorder, bipolar disorder, and schizophrenia: Evidence for decreased neuronal somal size and increased neuronal density. Biological Psychiatry. 2003;53:1086–109. doi: 10.1016/s0006-3223(03)00114-8. [DOI] [PubMed] [Google Scholar]
- Cooke BM, Woolley CS. Sexually dimorphic synaptic organization of the medial amygdala. Journal of Neuroscience. 2005;25:10759–10767. doi: 10.1523/JNEUROSCI.2919-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke BM, Stokas MR, Woolley CS. Morphological sex differences and laterality in the prepubertal medial amygdala. Journal of Comparative Neurology. 2007;501:904–915. doi: 10.1002/cne.21281. [DOI] [PubMed] [Google Scholar]
- Cosgrove KP, Mazure CM, Staley JK. Evolving knowledge of sex differences in brain structure, function, and chemistry. Biological Psychiatry. 2007;62:847–855. doi: 10.1016/j.biopsych.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotter D, MacKay D, Landau S, Kerwin R, Everall I. Reduced glial cell density and neuronal size in the anterior cingulate cortex in major depressive disorder. Archives of General Psychiatry. 2001;58:545–553. doi: 10.1001/archpsyc.58.6.545. [DOI] [PubMed] [Google Scholar]
- Cruz DA, Eggan SM, Azmitia EC, Lewis DA. Serotonin1A receptors at the axon initial segment of prefrontal pyramidal neurons in schizophrenia. American Journal of Psychiatry. 2004;161:739–742. doi: 10.1176/appi.ajp.161.4.739. [DOI] [PubMed] [Google Scholar]
- Czyrak A, Czepiel K, Mackowiak M, Chocyk A, Wedzony KW. Serotonin 5-HT1A receptors might control the output of cortical glutamatergic neurons in rat cingulate cortex. Brain Research. 2003;989:42–51. doi: 10.1016/s0006-8993(03)03352-3. [DOI] [PubMed] [Google Scholar]
- Davidson RJ. Anxiety and affective style: Role of prefrontal cortex and amygdala. Biological Psychiatry. 2002;51:68–80. doi: 10.1016/s0006-3223(01)01328-2. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Baum AS, Birmaher B, Keshavan MS, Eccard CH, Boring AM, Jenkins FJ, Ryan ND. Developmental traumatology. Biological Psychiatry. 1999;45:1259–1270. doi: 10.1016/s0006-3223(99)00044-x. [DOI] [PubMed] [Google Scholar]
- DeFelipe J, Arellano JI, Gomez A, Azmitia EC, Munoz A. Pyramidal cell axons show a local specialization for GABA and 5-HT inputs in monkey and human cerebral cortex. Journal of Comparative Neurology. 2001;433:148–155. doi: 10.1002/cne.1132. [DOI] [PubMed] [Google Scholar]
- Dettling AC, Feldon J, Pryce CR. Early deprivation and behavioral and physiological responses to social separation/novelty in the marmoset. Pharmacology, Biochemistry and Behavior. 2002a;73:259–269. doi: 10.1016/s0091-3057(02)00785-2. [DOI] [PubMed] [Google Scholar]
- Dettling AC, Feldon J, Pryce CR. Repeated parental deprivation in the infant common marmoset (Callithrix jacchus, primates) and analysis of its effects on early development. Biological Psychiatry. 2002b;52:1037–1046. doi: 10.1016/s0006-3223(02)01460-9. [DOI] [PubMed] [Google Scholar]
- Dettling AC, Schnell CR, Maier C, Feldon J, Pryce CR. Behavioral and physiological effects of an infant-neglect manipulation in a bi-parental, twinning primate: impact is dependent on familial factors. Psychoneuroendocrinology. 2007;32:331–349. doi: 10.1016/j.psyneuen.2007.01.005. [DOI] [PubMed] [Google Scholar]
- Devinsky O, Morrell MJ, Vogt BA. Contributions of anterior cingulate cortex to behaviour. Brain. 1995;118:279–306. doi: 10.1093/brain/118.1.279. [DOI] [PubMed] [Google Scholar]
- Diorio D, Viau V, Meaney MJ. The role of the medial prefrontal cortex (cingulate gyrus) in the regulation of hypothalamic-pituitary-adrenal responses to stress. Journal of.Neuroscience. 1993;13:3839–3847. doi: 10.1523/JNEUROSCI.13-09-03839.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets WC, Price JL, Simpson JR, Jr., Todd RD, Reich T, Vannier M, Raichle ME. Subgenual prefrontal cortex abnormalities in mood disorders. Nature. 1997;386:824–827. doi: 10.1038/386824a0. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Bogers W, Raichle ME. Functional anatomical correlates of antidepressant drug treatment assessed using PET measures of regional glucose metabolism. European Neuropsychopharmacology. 2002;12:527–544. doi: 10.1016/s0924-977x(02)00102-5. [DOI] [PubMed] [Google Scholar]
- Eastwood SL, Burnet PWJ, Gittins R, Baker K, Harrison PJ. Expression of serotonin 5-HT2A receptors in the human cerebellum and alterations in schizophrenia. Synapse. 2001;42:104–114. doi: 10.1002/syn.1106. [DOI] [PubMed] [Google Scholar]
- Eastwood SL, Lyon L, George L, Job D, Andrieux A, Harrison PJ. Altered expression of synaptic protein mRNAs in STOP (MAP-6) mutant mice. Journal of Psychopharmacology. 2007;21:635–644. doi: 10.1177/0269881106068825. [DOI] [PubMed] [Google Scholar]
- Ebert D, Ebmeier KP. The role of the cingulate gyrus in depression: From functional anatomy to neurochemistry. Biological.Psychiatry. 1996;39:1044–1050. doi: 10.1016/0006-3223(95)00320-7. [DOI] [PubMed] [Google Scholar]
- Feng J, Yan Z, Ferreira A, Tomizawa K, Liauw JA, Zhuo M, Allen PB, Ouimet CC, Greengard P. Spinophilin regulates the formation and function of dendritic spines. Proceedings of the National Academy of Sciences USA. 2000;97:9287–9292. doi: 10.1073/pnas.97.16.9287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flügge G. Dynamics of central nervous 5-HT1A-receptors under psychosocial stress. Journal of Neuroscience. 1995;15:7132–7140. doi: 10.1523/JNEUROSCI.15-11-07132.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman LJ, Insel TR, Smith Y. Subcortical projections of area 25 (subgenual cortex) of the macaque monkey. Journal of Comparative Neurology. 2000;421:172–188. [PubMed] [Google Scholar]
- Gilmer WS, McKinney WT. Early experience and depressive disorders: human and non-human primate studies. Journal of Affective Disorders. 2003;75:97–113. doi: 10.1016/s0165-0327(03)00046-6. [DOI] [PubMed] [Google Scholar]
- Gittins R, Harrison PJ. A quantitative morphometric study of the human anterior cingulate cortex. Brain Research. 2004;1013:212–222. doi: 10.1016/j.brainres.2004.03.064. [DOI] [PubMed] [Google Scholar]
- Hajek T, Kozeny J, Kopecek M, Alda M, Hoschl C. Reduced subgenual cingulate volumes in mood disorders: a meta-analysis. Journal of Psychiatry and Neuroscience. 2008;33:91–99. [PMC free article] [PubMed] [Google Scholar]
- Hall H, Lundkvist C, Halldin C, Farde L, Pike VW, McCarron JA, Fletcher A, Cliffe IA, Barf T, Wikström H, Sedvall G. Autoradiographic localization of 5-HT1A receptors in the post- mortem human brain using [3H]WAY-100635 and [11C]WAY-100635. Brain Research. 1997;745:96–108. doi: 10.1016/s0006-8993(96)01131-6. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Drabant EM, Weinberger DR. Imaging genetics: Perspectives from studies of genetically driven variation in serotonin function and corticolimbic affective processing. Biological Psychiatry. 2006;59:888–897. doi: 10.1016/j.biopsych.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Harrison PJ. The neuropathology of primary mood disorder. Brain. 2002;125:1428–1449. doi: 10.1093/brain/awf149. [DOI] [PubMed] [Google Scholar]
- Harrison PJ, Eastwood SL. Neuropathological studies of synaptic connectivity in the hippocampal formation in schizophrenia. Hippocampus. 2001;11:508–519. doi: 10.1002/hipo.1067. [DOI] [PubMed] [Google Scholar]
- Heim C, Nemeroff CB. The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies. Biological Psychiatry. 2001;49:1023–1029. doi: 10.1016/s0006-3223(01)01157-x. [DOI] [PubMed] [Google Scholar]
- Ichise M, Vines DC, Gura T, Anderson GM, Suomi SJ, Higley JD, Innis RB. Effects of early life stress on [11C] DASB positron emission tomography imaging of serotonin transporters in adolescent peer- and mother-reared rhesus monkeys. Journal of Neuroscience. 2006;26:4638–4643. doi: 10.1523/JNEUROSCI.5199-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakab RL, Goldman-Rakic PS. 5-Hydroxytryptamine2A serotonin receptors in the primate cerebral cortex: Possible site of action of hallucinogenic and antipsychotic drugs in pyramidal cell apical dendrites. Proceedings of the National Academy of Sciences USA. 1998;95:735–74. doi: 10.1073/pnas.95.2.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen-Berg H, Gutman DA, Behrens TEJ, Matthews PM, Rushworth MFS, Katz E, Lozano AM, Mayberg HS. Anatomical connectivity of the subgenual cingulate region targeted with deep brain stimulation for treatment-resistant depression. Cerebral Cortex. 2008;18:1374–1383. doi: 10.1093/cercor/bhm167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson GVW, Jope RS. The role of microtubule-associated protein-2 (MAP-2) in neuronal growth, plasticity and degeneration. Journal of Neuroscience Research. 1992;33:505–512. doi: 10.1002/jnr.490330402. [DOI] [PubMed] [Google Scholar]
- Kia HK, Miquel MC, Brisorgueil MJ, Daval G, Riad M, El Mestikawy S, Hamon M, Verge D. Immunocytochemical localization of serotonin1A receptors in the rat central nervous system. Journal of Comparative Neurology. 1996;365:289–305. doi: 10.1002/(SICI)1096-9861(19960205)365:2<289::AID-CNE7>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Koo M-S, Levitt JJ, Salisbury DF, Nakamura M, Shenton ME, McCarley RW. A cross-sectional and longitudinal magnetic resonance imaging study of cingulate gyrus gray matter volume abnormalities in first-episode schizophrenia and first-episode affective psychosis. Archives of General Psychiatry. 2008;65:746–760. doi: 10.1001/archpsyc.65.7.746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law AJ, Weickert CS, Webster MJ, Herman MM, Kleinman JE, Harrison PJ. Expression of NMDA receptor NR1, NR2A and NR2B subunit mRNAs during development of the human hippocampal formation. European Journal of Neuroscience. 2003;18:1197–1205. doi: 10.1046/j.1460-9568.2003.02850.x. [DOI] [PubMed] [Google Scholar]
- Law AJ, Weickert CS, Hyde TM, Kleinman JE, Harrison PJ. Reduced spinophilin but not MAP2 expression in the hippocampal formation in schizophrenia and mood disorder: Molecular evidence for a pathology of dendritic spines. American Journal of Psychiatry. 2004a;161:1848–1855. doi: 10.1176/ajp.161.10.1848. [DOI] [PubMed] [Google Scholar]
- Law AJ, Hutchinson LJ, Burnet PWJ, Harrison PJ. Antipsychotics increase microtubule-associated protein 2 (MAP2) mRNA but not spinophilin mRNA in the rat hippocampus and cortex. Journal of Neuroscience Research. 2004b;76:376–382. doi: 10.1002/jnr.20092. [DOI] [PubMed] [Google Scholar]
- Law AJ, Pei Q, Walker M, Gordon-Andrews H, Weickert CS, Feldon J, Pryce CR, Harrison PJ. Early parental deprivation in the marmoset monkey produces long-term changes in hippocampal expression of genes involved in synaptic plasticity and implicated in mood disorder. Neuropsychopharmacology. 2009 Jul 9; doi: 10.1038/npp.2008.106. AOP. 2008; doi:10.1038/npp/2008.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annual Review of Neuroscience. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Madeira MD, Lieberman AR. Sexual dimorphism in the mammalian limbic system. Progress in Neurobiology. 1995;45:275–313. doi: 10.1016/0301-0082(94)00052-j. [DOI] [PubMed] [Google Scholar]
- Manji HK, Drevets WC, Charney DS. The cellular neurobiology of depression. Nature Medicine. 2001;7:541–544. doi: 10.1038/87865. [DOI] [PubMed] [Google Scholar]
- Mayberg H. Limbic-cortical dysregulation: a proposed model of depression. Journal of Neuropsychiatry and Clinical Neuroscience. 1997;9:471–481. doi: 10.1176/jnp.9.3.471. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Brannan SK, Mahurin RK, Jerabek PA, Brickman JS, Tekell JL, Silva JA, McGinnis S, Glass TG, Martin CC, Fox PT. Cingulate function in depression: A potential predictor of treatment response. NeuroReport. 1997;8:1057–1061. doi: 10.1097/00001756-199703030-00048. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, Schwalb JM, Kennedy SH. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45:651–660. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- Murray EA. The amygdala, reward and emotion. Trends in Cognitive Sciences. 2007;11:489–497. doi: 10.1016/j.tics.2007.08.013. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Barrot M, DiLeone RJ, Eisch AJ, Gold SJ, Monteggia LM. Neurobiology of depression. Neuron. 2002;34:13–25. doi: 10.1016/s0896-6273(02)00653-0. [DOI] [PubMed] [Google Scholar]
- Nishizuka M, Arai Y. Sexual dimorphism in synaptic organization in the amygdala and its dependence on neonatal hormone environment. Brain Research. 1981;212:31–38. doi: 10.1016/0006-8993(81)90029-9. [DOI] [PubMed] [Google Scholar]
- Öngür D, Drevets WC, Price JL. Glial reduction in the subgenual prefrontal cortex in mood disorders. Proceedings of the National Academy of Sciences USA. 1998a;95:13290–13295. doi: 10.1073/pnas.95.22.13290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Öngür D, An X, Price JL. Prefrontal cortical projections to the hypothalamus in macaque monkeys. Journal of Comparative Neurology. 1998b;401:480–505. [PubMed] [Google Scholar]
- Palchaudhuri M, Flügge G. 5-HT1A receptor expression in pyramidal neurons of cortical and limbic brain regions. Cell and Tissue Research. 2005;321:159–172. doi: 10.1007/s00441-005-1112-x. [DOI] [PubMed] [Google Scholar]
- Palomero-Gallagher N, Mohlberg H, Zilles K, Vogt B. Cytology and receptor architecture of human anterior cingulate cortex. Journal of Comparative Neurology. 2008;508:906–926. doi: 10.1002/cne.21684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus T. Primate anterior cingulate cortex: where motor control, drive and cognition interface. Nature Reviews Neuroscience. 2001;2:417–424. doi: 10.1038/35077500. [DOI] [PubMed] [Google Scholar]
- Pazos A, Cortes R, Palacios JM. Quantitative autoradiographic mapping of serotonin receptors in the rat brain. II. Serotonin-2 receptors. Brain Research. 1985;346:231–249. doi: 10.1016/0006-8993(85)90857-1. [DOI] [PubMed] [Google Scholar]
- Pei Q, Burnet PJW, Zetterström TSC. Changes in mRNA abundance of microtubule-associated proteins in the rat brain following electroconvulsive shock. NeuroReport. 1998;9:391–394. doi: 10.1097/00001756-199802160-00006. [DOI] [PubMed] [Google Scholar]
- Preece MA, Dalley JW, Theobald DE, Robbins TW, Reynolds GP. Region specific changes in forebrain 5-hydroxytryptamine2A receptors in isolation-reared rats: an in vitro autoradiography study. Neuroscience. 2004;123:725–732. doi: 10.1016/j.neuroscience.2003.10.008. [DOI] [PubMed] [Google Scholar]
- Pryce CR, Dettling AC, Spengler M, Spaete C, Feldon J. Evidence for altered monoamine activity and emotional and cognitive disturbance in marmoset monkeys exposed to early life stress. Annals of the New York Academy of Sciences. 2004a;1032:245–249. doi: 10.1196/annals.1314.030. [DOI] [PubMed] [Google Scholar]
- Pryce CR, Dettling AC, Spengler M, Schnell CR, Feldon J. Deprivation of parenting disrupts development of homeostatic and reward systems in marmoset monkey offspring. Biological Psychiatry. 2004b;56:72–79. doi: 10.1016/j.biopsych.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Pryce CR, Rüedi-Bettschen D, Dettling AC, Weston A, Russig H, Ferger B, Feldon J. Long-term effects of early-life environmental manipulations in rodents and primates: Potential animal models in depression research. Neuroscience and Biobehavioral Reviews. 2005;29:649–674. doi: 10.1016/j.neubiorev.2005.03.011. [DOI] [PubMed] [Google Scholar]
- Rilling JK, Winslow JT, O’Brien D, Gutman DA, Hoffman JM, Kilts CD. Neural correlates of maternal separation in Rhesus monkeys. Biological Psychiatry. 2001;49:146–157. doi: 10.1016/s0006-3223(00)00977-x. [DOI] [PubMed] [Google Scholar]
- Rinn JL, Snyder M. Sexual dimorphism in mammalian gene expression. Trends in Genetics. 2005;21:298–305. doi: 10.1016/j.tig.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Sabatini MJ, Ebert P, Lewis DA, Levitt P, Cameron JL, Mirnics K. Amygdala gene expression correlates of social behavior in monkeys experiencing maternal separation. Journal of Neuroscience. 2007;27:3295–3304. doi: 10.1523/JNEUROSCI.4765-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez MM, Ladd CO, Plotsky PM. Early adverse experience as a developmental risk factor for later psychopathology: Evidence from rodent and primate models. Development and Psychopathology. 2001;13:419–440. doi: 10.1017/s0954579401003029. [DOI] [PubMed] [Google Scholar]
- Santana N, Bortolozzi A, Serrats J, Mengod G, Artigas F. Expression of Serotonin1A and Serotonin2A receptors in pyramidal and GABAergic neurons of the rat prefrontal cortex. Cerebral Cortex. 2004;14:1100–1108. doi: 10.1093/cercor/bhh070. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Gado MH, Price JL. Amygdala core nuclei volumes are decreased in recurrent major depression. NeuroReport. 1998;9:2023–2027. doi: 10.1097/00001756-199806220-00021. [DOI] [PubMed] [Google Scholar]
- Simerly RB. Wired for reproduction: Organization and development of sexually dimorphic circuits in the mammalian forebrain. Annual Review of Neuroscience. 2002;25:507–536. doi: 10.1146/annurev.neuro.25.112701.142745. [DOI] [PubMed] [Google Scholar]
- Simpson MDC, Lubman DI, Slater P, Deakin JFW. Autoradiography with [3H]8-OH-DPAT reveals increases in 5- HT1A receptors in ventral prefrontal cortex in schizophrenia. Biological Psychiatry. 1996;39:919–928. doi: 10.1016/0006-3223(95)00026-7. [DOI] [PubMed] [Google Scholar]
- Smolka MN, Schumann G, Wrase J, Grüsser SM, Flor H, Mann K, Braus DF, Goldman D, Büchel C, Heinz A. Catechol-O-methyltransferase val158met genotype affects processing of emotional stimuli in the amygdala and prefrontal cortex. Journal of Neuroscience. 2005;25:836–840. doi: 10.1523/JNEUROSCI.1792-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan H, Baron G, Schwerdtfeger WK. The Brain of the Common Marmoset (Callithrix Jacchus): A Sterotaxic Atlas. Berlin: Springer-Verlag; 1980. [Google Scholar]
- Vogt BA. Pain and emotion interactions in subregions of the cingulate gyrus. Nature Reviews Neuroscience. 2005;6:533–544. doi: 10.1038/nrn1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt BA, Nimchinsky EA, Vogt LJ, Hof PR. Human cingulate cortex: Surface features, flat maps, and cytoarchitecture. Journal of Comparative Neurology. 1995;359:490–506. doi: 10.1002/cne.903590310. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Vogt L, Farber NB, Bush G. Architecture and neurocytology of monkey cingulate gyrus. Journal of Comparative Neurology. 2005;485:218–239. doi: 10.1002/cne.20512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson NR, Kang J, Hueske EV, Leung T, Varoqui H, Murnick JG, Erickson JD, Liu G. Presynaptic regulation of quantal size by the vesicular glutamate transporter VGLUT1. Journal of Neuroscience. 2005;25:6221–6234. doi: 10.1523/JNEUROSCI.3003-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]