Abstract
Objective
Responses to afferent input during locomotion are organized at the spinal level but modulated by supraspinal centers. The study aim was to examine whether supraspinal influences affect the behavior of complex electromyographic (EMG) responses to single limb perturbations during walking.
Methods
Subjects with motor-complete (MCSCI), motor-incomplete spinal cord injury (MISCI), and non-disabled (ND) subjects participated. Hip or knee joint trajectory was briefly arrested by a robotic device at early or late swing phase. EMG responses from muscles of both legs were analyzed.
Results
Perturbation-induced EMG responses of spinal cord injured and ND individuals were similar in basic structure, with the exception that tibialis anterior onset times were delayed for SCI subjects. Across all groups, perturbations in late swing (i.e., near the swing-to-stance transition) were associated with shorter muscle onset times and higher EMG amplitudes. Knee perturbations were associated with shorter muscle response onset times, while hip perturbations were elicited higher response amplitudes. EMG responses were also evoked in muscles contralateral to the perturbation.
Conclusions
These data indicate that neuronal circuits within the spinal cord deprived of normal supraspinal input respond to swing phase perturbations in a manner that is similar to that of the intact spinal cord.
Significance
The adult human spinal cord is capable of generating complex, phase-appropriate responses much as has been observed in studies of human infants and in spinal animals.
Keywords: walking, spinal reflex, afferent input, modulation
INTRODUCTION
While the motor output associated with human walking is thought to be largely generated by spinal centers, both supraspinal and afferent input contribute considerably to the locomotor output (Dietz, 1992). Afferent input is critical for adapting the locomotor pattern, in a phase-appropriate manner, to unexpected perturbation of the stepping limbs. As such, the neural circuitry underlying locomotor behavior is responsive to a broad range of afferent inputs from muscle and cutaneous afferents. In cat, reliance on afferent input appears to increase following the disruption of supraspinal influence, as occurs following spinal cord damage. For example, hindlimb deafferentation in the otherwise intact cat results in only minor impairment of walking performance on a level surface, with deficits in the performance only in more complex stepping tasks (Bouyer and Rossignol, 2003). Spinalized cats with preserved afferent input show well-preserved stepping coordination (Rossignol, et al. 1996). In contrast, extensive deafferentation of the hindlimb in spinal transected cats, is associated with disordered intralimb (Giuliani and Smith, 1987) and interlimb coordination (Giuliani and Smith, 1987), as is removal of only cutaneous afferent input (Bouyer and Rossignol, 2003). This suggests that afferent input provides information that modulates the timing of locomotor output.
Application of perturbing forces to the moving limb and analysis of compensatory leg muscle electromyography (EMG) provides information about the extent to which external stimuli influence the programmed pattern of muscle activity associated with locomotion. Prior investigations have examined the effect of perturbations applied at various times in the gait cycle in neurologically intact animals, intact adult humans, intact human infants (in whom descending tracts are not yet mature), humans with movement disorders and spinal animals (Zehr and Stein, 1999)). Perturbations applied during swing and/or stance phases of human locomotion have been of various forms, including tactile stimulation (Lam et al., 2003), electrical stimulation (Duysens, 1992; Zehr et al., 1997), unexpected treadmill or support surface accelerations/decelerations (Dietz et al., 1987; Pang and Yang, 2002) or mechanical resistance (Ghori and Luckwill, 1989; Ghori and Luckwill, 1990). The adaptive response pattern observed in the locomotor output was shown to be highly dependent upon the form of the perturbation (Zehr and Stein, 1999).
The purpose of the study was to assess whether supraspinal input influences the behavior of complex responses associated with joint perturbations applied during the swing phase of walking. Two time points for analysis, representing early and late swing phase, were selected. EMG responses in both the perturbed limb (ipsilateral; swing limb) as well as the unperturbed limb (contralateral; stance limb) were studied. We hypothesized that the temporal aspects of the response pattern would be similar across groups, on the basis that the spinal cord would be able to generate similar responses to an external stimulus even in individuals in whom supraspinal influence is absent. Further, we hypothesized that early hip perturbations would be associated with a larger responses as the hip is beginning to move from extension to flexion at this time and afferent signals from hip flexors appear to be critical for control of step timing (Pang and Yang, 2000; Pearson and Rossignol, 1991; Hiebert et al., 1996; Andersson et al., 1978; Grillner and Rossignol, 1978). In addition, we hypothesized that EMG responses in the contralateral limb would not be as large as those of the ipsilateral limb as the most direct effects of the perturbation would likely to be observed in the perturbed limb.
METHODS
Subjects
Subjects were individuals with spinal cord injury (SCI) and non-disabled individuals. The study protocol was approved by the Institutional Review Board at the University of Miami Miller School of Medicine, and each subject provided written informed consent consistent with regulations for protection of human subjects. Subjects were grouped, according to motor function status, into one of three groups: Group 1= ASIA A or B (no voluntary motor function below the level of injury; n = 10). Group 2= ASIA C (sensory and motor function are preserved below the level of the lesion, but at least half of the muscles below the level of the lesion have a grade of less than 3; n = 11). Group 3= non-disabled subjects (n = 10). Subjects in Groups 1 and 2 had chronic SCI as all had sustained a SCI at or above the T10 neurologic level at least one year prior to participation in the study. All subjects with SCI showed signs of spasticity with exaggerated reflexes and muscle hypertonia. Descriptive data for each of the three groups (motor-complete spinal cord injury [MCSCI], motor-incomplete spinal cord injury [MISCI], non-disabled [ND] subjects) are given in Table 1. Subject classification is according to motor function level of International Standards for Neurological Classification of Spinal Cord Injury.(American Spinal Injury Association, 2002) Mean age by group was 37.3, 42.4, 37.0 years in the MCSCI, MISCI, and ND groups respectively. Mean time post injury was 6.1 and 8.0 years in the MCSCI and MISCI groups, respectively.
Table 1.
Subject demographics.
| Subject Number | Injury Level | Age (years) |
Gender | ASIA Motor Class | Injury duration (years) |
Anti-spastic/anti-spasmodic medication |
|---|---|---|---|---|---|---|
| MCSCI | ||||||
| 101 | C6 | 31 | M | A | 10 | none |
| 102 | T3 | 32 | M | A | 2.3 | none |
| 103 | T7 | 46 | M | A | 2.2 | B |
| 104 | C4 | 47 | F | A | 7 | B |
| 105 | T8 | 29 | M | A | 6.5 | none |
| 106 | C6 | 37 | M | A | 4.5 | none |
| 107 | T11 | 48 | M | A | 10.8 | none |
| 108 | C5 | 26 | F | B | 11.8 | B |
| 109 | T4 | 53 | F | B | 2.5 | G, Tz |
| 110 | C5 | 24 | M | B | 3.1 | B |
| MISCI | ||||||
| 201 | T2 | 51 | M | C | 3.7 | B, G, Tz |
| 202 | C6 | 45 | M | C | 5.7 | G |
| 203 | C7 | 57 | M | C | 18.8 | none |
| 204 | C5 | 41 | F | C | 18.8 | B |
| 205 | T6 | 38 | M | C | 5.8 | none |
| 206 | C7 | 34 | M | C | 12.9 | none |
| 207 | T2 | 30 | M | C | 5 | B |
| 208 | T4 | 32 | M | C | 4.2 | none |
| 209 | C4 | 41 | M | C | 4.6 | B, Tz |
| 210 | C6 | 37 | M | C | 3 | G, Tz |
| 211 | C7 | 60 | M | C | 5.7 | none |
| ND | ||||||
| 301 | 28 | M | ||||
| 302 | 40 | F | ||||
| 303 | 29 | M | ||||
| 304 | 21 | M | ||||
| 305 | 33 | F | ||||
| 306 | 42 | M | ||||
| 307 | 58 | M | ||||
| 308 | 45 | M | ||||
| 309 | 45 | M | ||||
| 310 | 49 | M | ||||
MCSCI=motor-complete SCI, MISCI=motor, ND=non-disabled, C=cervical injury, T=thoracic injury, M=male, F=female, B=baclofen, G=gabapentin Tz=tizanidine
Walking procedures
Subjects were positioned within the driven gait orthosis (DGO; Lokomat®, Hocoma AG, Zurich, Switzerland) according to procedures described elsewhere (Dietz et al., 2004). Briefly, subjects wore a body weight support (BWS) harness and the trunk and pelvis were strapped to the backrest of the DGO. The thigh and shank segments of the DGO exoskeleton were adjusted to align the hip and knee joints with the joint axes of the DGO; thigh and shank cuffs were used to secure the lower extremities within the orthosis. The forefoot was supported by a spring-assisted strap that maintained the ankle in a near-neutral position. The speed of the treadmill and DGO was gradually increased to 2 km/hr and the subjects walked in the device for 2 minutes prior to the onset of testing to allow them to become accustomed to the device. During this 2-minute period abnormal muscle recruitment was monitored in 2 ways: via real-time EMG and via force exerted against the robotic device. If abnormalities in muscle recruitment occurred and did not resolve within 2 minutes, the subject was allowed to rest for 5 minutes before attempting a second trial. If the abnormal muscle recruitment persisted, the subject was excluded from further testing. Cadence was adjusted based on subject height.
Subjects with SCI were tested while walking with 40 – 80% BWS (60 - 20% reduced body weight load on lower extremities). This range of BWS was selected as it was a condition that could be tolerated by all subject groups, subjects who were required greater than 80% BWS to maintain proper walking kinematics (e.g., toe clearance during swing phase, knee extension during stance phase) were excluded from the study. Subjects with SCI walked with the maximum lower extremity load that they were able to tolerate, e.g., without excessive knee flexion in stance phase or toe dragging during swing phase. All ND subjects walked with 40% BWS to confer similar loading conditions. Modifications to the program controlling the DGO step cycle provided perturbations to the stepping limb during the swing phase of the gait cycle which briefly arrested the ongoing movement at the respective joint. The gait cycle duration was 2000ms ± 100ms with differences in cycle duration being due to differences in subject anthropometrics. The swing phase occupied the first 38% of the cycle period (from approximately 0ms to 760ms) and stance phase the latter 62% of the cycle (from approximately 760ms to 2000ms). Perturbations consisted of a 100 ms “hold” during which the trajectory of the thigh (hip) or the shank (knee) was arrested and then released, with the trajectory of the limb recovering to the normal trajectory in an additional 100 ms. With onset of swing phase denoting onset of cycle (0ms; 0% of cycle), these perturbations were programmed to occur either in early (200ms ± 10ms after cycle onset; approximately 10% ± 0.5% of total cycle or 25% of the swing phase) or late swing (700ms ± 10ms; approximately 35% ± 0.5% of total cycle or 90% of the swing phase). This resulted in five possible segment-time perturbation type combinations: late hip (LH), late knee (LK), early hip (EH), early knee (EK), no perturbation (normal unperturbed cycle). The perturbations were administered in a random order with 1 – 2 normal (nonperturbed) steps between perturbed steps. Each of the four modes of perturbations was administered 7 times to the right lower extremity, with a total of ≥ 28 unperturbed cycles interleaved among them. The duration of the swing phase was the same for the perturbed and unperturbed cycles as the DGO returned to the normal limb trajectory approximately 200 ms after the onset of the perturbation. Once the perturbation program was initiated, a single trial was completed in approximately 5 minutes (mean = 320 sec; range 295sec – 345 sec) with time differences being due to variations in the number of interleaved, unperturbed cycles
EMG was recorded with pairs of surface electrodes positioned on the skin overlying target muscles including rectus femoris (RF), biceps femoris (BF), tibialis anterior (TA) and medial gastrocnemius (GM), these muscles were selected as representative, respectively, knee extensor, knee flexor, ankle dorsiflexor, and ankle plantar flexor activity based on the timing of their activity during the swing phase of locomotion (Perry, 1992). EMG was amplified, bandpass filtered (10–1kHz) and sampled at 2kHz then stored for later offline analysis (Datapac, Run Technologies, CA, USA).
Data analysis
The EMG data were processed using a customized program (Datapac) that applied following processes, in order, to the EMG activity for each perturbation type: 1) passive demeaning (to eliminate any DC bias), 2) high pass filter (5th order Butterworth, 25 Hz, symmetric; to attenuate any movement artifact), 3) full-wave rectification, 4) signal calibration to mean nonperturbed cycle (resulting in normalization such that 100% represented the average EMG amplitude during nonperturbed cycles in the same channel), 5) subtraction of nonperturbed cycle EMG amplitudes from each of the perturbed cycles (to obtain the difference in the EMG signal associated with that perturbation type). Therefore, in instances in which there was no difference between the perturbed and unperturbed cycles, the EMG amplitude of the subtracted data was zero. For each perturbation type the response to the perturbation was identified as a signal of at least 3 SD greater than background EMG of the subtracted data that fell within a time frame of 30 to 200 ms following the onset of the perturbation, as this represents the time period during which a reflex response to perturbation can be expected. The root mean square (RMS) average of the response to the perturbation (in this 170 ms window) as well as onset and duration of the response were calculated, we refer to this parameter as EMG amplitude henceforth.
Group mean data was calculated for each of the three subject groups for each perturbation type in each of the four muscles in both the perturbed and unperturbed limb. The resulting data set therefore included the following variables: three subject groups (complete SCI, incomplete SCI, non-disabled), four muscle groups (RF, BF, TA, GM), four different perturbation types (LH, LK, EH, EK) and two limbs (perturbed / ipsilateral, unperturbed / contralateral).
Muscle onset times were analyzed using repeated measures analysis of variance (ANOVA) to account for the timing of the perturbation (Early, Late) and joint (Hip, Knee) conditions. The Greenhouse-Geisser correction was applied to account for lack of independence. Each muscle (RF, BF, TA and GM) was assessed individually, and data for the BF muscle was log-transformed in order to meet analysis assumptions. Regression analysis was used to compare whether earlier EMG onset times were associated with the knee/ankle flexor muscle group (BF, TA) versus extensor muscle group (RF, GM). The parameters of the regression model were: subject group, muscle flexor-extensor group, the early-late condition, and the hip-knee condition which were set as dummy variables. Robust standard errors were constructed such that only observations on different persons were considered independent, and the onset data were log-transformed to meet necessary assumptions.
EMG amplitude data were analyzed via two regression models. One included a dummy variable for muscle (RF, BF, TA, GM) and the grouped the muscle data by flexors (BF, TA) and extensors (RF, GM) groups to assess the effect of muscle group. Additional variables in both models were group, the early-late condition and the hip-knee condition. All statistical analysis was conducted used StataSE8.2 (StataCorp LP, College Station, TX, USA).
RESULTS
Ipsilateral EMG response patterns
Exemplary EMG data for one subject for each of the subject groups is illustrated in Figure 2. The leg EMG response ipsilateral to the perturbation (relative to the nonperturbed cycles) was greater in the MCSCI group resulting in a larger amplitude of the subtracted data for this group across all muscles and perturbations tested.
Figure 2.
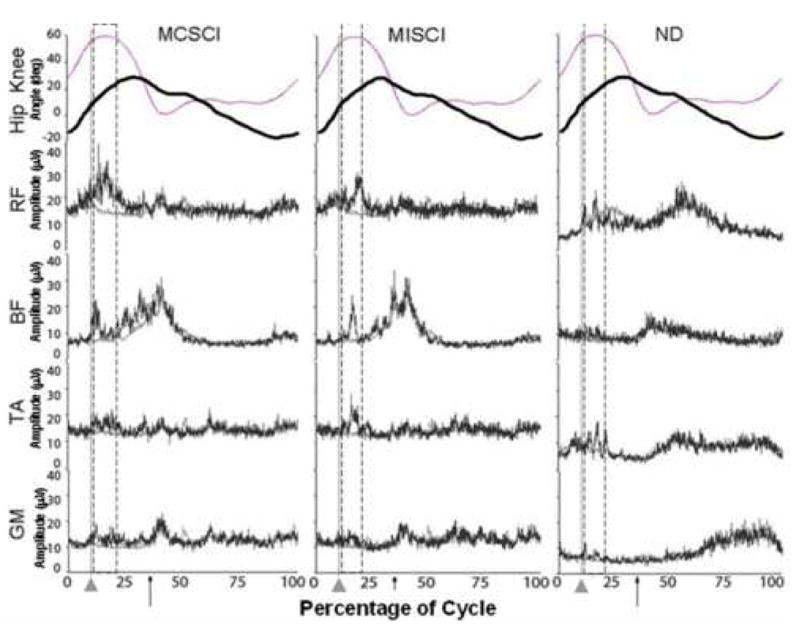
Exemplary individual EMG recordings from ipsilateral leg muscles from each of the subject 3 groups (MCSCI, MISCI, ND) in response to an early hip (EH) perturbation (dark trace) compared to background EMG (light trace). Percent of cycle is given with onset of swing phase representing cycle onset, light vertical line and pointer indicate onset of perturbation, arrow represents onset of stance phase (heel strike). Bracketed region represents period falling 30ms – 200ms after the perturbation onset. The knee (light trace) and hip (dark trace) angles are also noted.
Effect of subject group (MCSCI vs. MISCI vs. ND)
Muscle burst onset times for the TA muscle were significantly earlier for the ND subjects (97ms) compared to both the MCSCI subjects (122ms; F=4.26, p=0.04) and the MISCI subjects (126ms; F=6.63, p=0.02). This was the only muscle for which there was a significant difference in onset time among groups. For each subject group, the mean TA EMG onset and offset time is illustrated in Figure 3. Muscle burst onset latencies for all muscles under all conditions for each subject group are given in Table 2.
Figure 3.
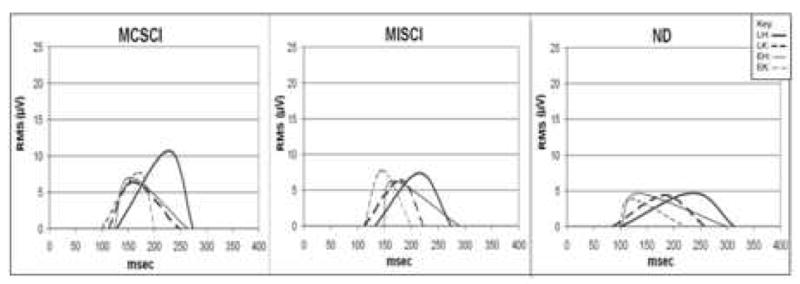
Muscle EMG burst onset times and RMS amplitudes (μV) of the TA muscle for each of four perturbation conditions. The left and right ends of each trace represent the mean burst onset and offset times, respectively, of the EMG burst following the perturbation onset (time = 0). The time of the peak represents the time of the maximum EMG amplitude within the burst period. The amplitude of the peak represents the RMS value of the EMG signal. Only responses in the ipsilateral TA are shown. LH=dark solid line ( — ), LK=dark dashed line (– –), EH=light solid line (—), EK=light dashed line (– –).
Table 2.
Muscle burst onset latencies by subject group
| ONSET LATENCY (msec) | ||||
|---|---|---|---|---|
| Perturbation | Muscle | Group | ||
| MCSCI | MISCI | ND | ||
| EH | RF | 95 | 90 | 103 |
| BF | 154 | 168 | 157 | |
| TA | 124 | 148 | 102* | |
| SOL | 157 | 90 | 120 | |
| EK | RF | 117 | 102 | 128 |
| BF | 92 | 109 | 101 | |
| TA | 100 | 112 | 101* | |
| SOL | 109 | 99 | 87 | |
| LH | RF | 113 | 120 | 133 |
| BF | 88 | 78 | 114 | |
| TA | 154 | 132 | 100* | |
| SOL | 108 | 123 | 121 | |
| LK | RF | 110 | 100 | 110 |
| BF | 104 | 108 | 99 | |
| TA | 113 | 112 | 86* | |
| SOL | 74 | 105 | 107 | |
EH=early hip, EK=early knee, LH=late hip, LK=late knee,
=latency was significantly different in ND subjects compared to SCI groups
Having identified that between-group differences in EMG onset times were restricted to the TA muscle, the data were grouped to assess the behavior of the flexor (TA, BF) versus extensor (GM, RF) muscles. When muscles were grouped on the basis of flexor/extensor group, no significant differences in onset times among the subject groups were observed. The EMG amplitude of the subtracted EMG signal was significantly greater in the MCSCI group compared to the other two groups (see Figs 2, 3, 4). The regression model for RMS values for the four individual muscles indicated that the RMS score for the MCSCI group was higher than for MISCI group (t=−3.26, p=0.003) and for the ND group (t=−4.05, p<0.001). There were no significant differences between the MISCI and ND groups in terms of EMG amplitude. For each subject group, the time of the maximum burst amplitude and burst RMS amplitude for each of the four conditions is illustrated in Figure 3. Note that in each trace, the timing of the peak represents the timing of the peak EMG amplitude (calculated from the raw EMG) while the value of the peak represents the RMS amplitude of the EMG burst.
Figure 4.
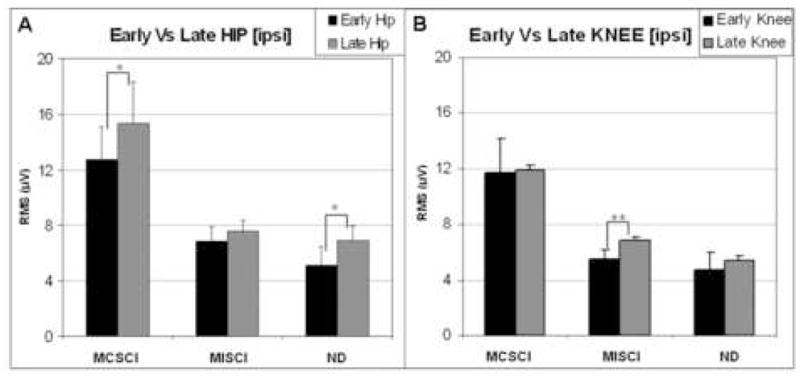
Mean muscle burst amplitude (RMS) associated with ipsilateral EMG responses to early and late perturbations at the hip (A) and knee (B) in each of the three subject groups. Black bars = early responses, gray bars = late responses. Error bars indicate standard error of the mean (SEM). Significant differences in response due to timing of the perturbation are indicated by asterisks (**: p<0.001, *: p<0.1).
Effect of early versus late perturbations
Having identified between-group differences in muscle burst onset times (restricted to the TA muscle), data were collapsed across muscles groups to assess the influence of perturbation time (early swing versus late swing). The analysis indicated that ipsilateral muscle burst onset times differed depending on the timing of the perturbation within the swing phase. Shorter mean burst onset latencies were observed following the late (LH: 115ms, LK: 102ms) compared to the early perturbations (EH: 127ms, EK: 105ms). This difference was significant in three of the four muscles tested: RF (F=5.37, p=0.03), BF (F=7.46, p=0.01) and TA (F=19.65, p=0.002). The GM muscle was an exception, as there was no difference in onset time (F=0.77, p=0.4). Between-times comparisons of EMG amplitude for a given joint perturbation (i.e., EH vs LH, EK vs LK) demonstrated that the amplitude of late perturbations was higher than for early perturbations (Figure 4), this difference was significant (t=−3.00, p=0.01)
Effect of hip versus knee perturbations
Ipsilateral perturbations to the knee were associated with shorter mean muscle burst onset time than were those to the hip (t=−2.83, p=0.01) when data were collapsed across subject groups, muscles and time (early versus late). This difference was statistically significant. This was primarily due to the significant difference in the effect of perturbation to the knee versus the hip in the BF (F=19.11, p=0.002) and TA (F=8.15, p=0.01) muscles, indicating that these muscles had an earlier onset to knee perturbation compared to hip perturbation. Between-joint comparisons of EMG amplitude (data collapsed across muscles and subject groups for a given time in the cycle (i.e., EH vs EK, LH vs LK)) demonstrated that the ipsilateral response to the hip perturbation was usually larger than the response to the knee perturbation applied at the same phase in the cycle (t=−2.66, p=0.01; Figure 4).
Effect of muscle groups
When considered as a group, the extensor muscles responded to the perturbations with an earlier muscle burst onset time than did the flexor muscles (extensors - RF: 110ms, GM: 108; flexors - BF: 114 TA: 115ms; data collapsed across perturbation times and joints). Regression analysis of the onset latency, revealed that perturbations resulted in significantly earlier onset times in extensor compared to flexor muscles (t=−2.21, p=0.035) There was no significant difference in EMG amplitude between the flexor and the extensor muscle groups.
Contralateral EMG response patterns
Effect of subject group (MCSCI vs. MISCI vs. ND)
In the contralateral limb the muscle burst onset time of the GM muscle was significantly different between groups, with the GM onset time being earliest in the MCSCI group and latest in the ND group; these differences were significant (F=6.18, p=0.01). There were no other significant differences between groups with regard to muscle burst onset time. As with the ipsilateral limb, the EMG amplitude of the response in the contralateral limb was greatest in the MCSCI group. It was larger compared to the MISCI (t=−3.12, p=0.003) and the ND group (t=−5.46, p<0.001).
Effect of early versus late perturbations
For the GM muscle there was a significantly earlier muscle burst onset time in response to the late versus the early perturbations (F=4.25, p=0.05). This difference was not significant for the other muscles. While there was also earlier muscle activity in response to the late perturbation on the ipsilateral side, in contrast to earlier GM onset time in the contralateral limb, the earlier ipsilateral activity was observed in all muscles except for the GM. As with the ipsilateral limb, for the individuals with MCSCI the overall EMG amplitude was greater in response to the late compared to the early perturbations (t=−2.73, p=0.01). This effect was not observed in the other groups and there were no other differences due to the timing of perturbation (see Figure 5).
Figure 5.
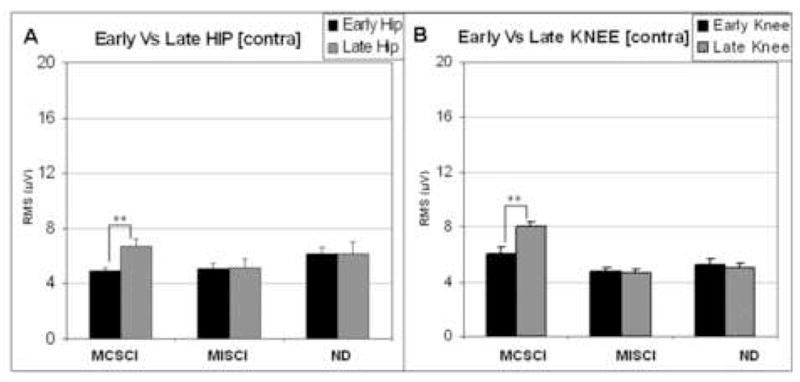
Mean muscle burst amplitude (RMS) in the contralateral limb associated with responses to early and late perturbations at the hip (A) and knee (B) in each of the three subject groups. Black bars = early responses, Gray bars = late responses. Error bars indicate standard error of the mean (SEM). Significant differences in response due to timing of the perturbation are indicated by asterisks (**: p<0.001).
Effect of hip versus knee perturbations
As with the ipsilateral limb, the muscle burst onset was earlier for perturbations to the knee versus those to the hip (t=−5.36, p<0.001). Repeated measures analysis of variance indicated that this difference was due to earlier onset times of BF, RF and TA muscles following perturbations to the knee compared to the hip (F=4.21, p=0.05; F=21.44, p<0.001; F=37.15, p<0.001, respectively). There were no differences in the onset time of the GM muscle or in EMG amplitude of any of the muscles.
Effect of muscle groups
The muscle burst onset time of the proximal muscles (BF, RF) was significantly earlier than for the distal muscles (TA, GM). Relative to RF, BF was earlier (t=2.10, p=0.04), while TA and GM were later (t=4.05, p<0.001; t=2.43, p=0.02, respectively). There were no other statistically significant difference among muscles. Analysis of EMG amplitude with muscles grouped as flexors versus extensors demonstrated that the EMG amplitude associated with the extensor muscles was greater than for flexors (t=2.92, p=0.01). This result is different from the response pattern in the ipsilateral limb in which no difference in EMG amplitude between muscle groups was found.
Ipsilateral versus contralateral EMG response patterns
As might be expected, with all experimental variables considered (subject group, early-late, hip-knee, muscle (or flexor-extensor)) and ipsilateral vs. contralateral leg) the muscle burst onset times occurred earlier in the ispilateral than the contralateral limb (t=15.01, p<0.001). The EMG amplitude was smaller in the contralateral compared to the ipsilateral limb (F=3.40, p=0.002; compare Figures 4 and 5). These differences were observed regardless of whether the muscles were considered individually or grouped as flexor and extensors.
DISCUSSION
The aim of this study was to investigate the influence of supraspinal drive on complex spinal reflexes evoked by perturbations to a single limb and the autonomy of spinal neuronal circuits to generate appropriate complex responses. This study is the first to compare responses to perturbations applied during the swing phase of locomotion among individuals in whom there are clinically meaningful differences in the amount of supraspinal input available to modulate the spinal circuits. The main finding in this study was the remarkable similarity across subject groups of EMG responses to perturbations applied to a single joint during the swing phase. The results indicate that the spinal cord deprived of normal supraspinal input responds to swing phase perturbations in a manner that is similar to that of any intact supraspinal control.
Autonomy of the spinal cord to generate compensatory response patterns
Across all subject groups, the behavior of the response pattern to perturbation was similar. The only observed temporal difference among subject groups was a delayed TA response in subjects with SCI (both MCSCI and MISCI) compared to ND individuals. The response latencies observed in the TA muscle of the ND subjects were consistent with those reported previously for similar perturbations (Dietz et al., 2004). The delay in TA response observed in subjects with SCI might be due to an impaired or absent input from supraspinal centers which normally function to modulate spinal neuronal responses to afferent input (Berger et al., 1984; Dietz et al., 1989). In subjects with SCI, extensor muscle activity is likely to predominate over that of flexor muscles (Capaday et al., 1999). Therefore the differences in response to perturbation may be assumed to be due in part to underlying differences in central and segmental control of flexor versus extensor muscles.(Iles and Roberts, 1987; Dietz et. al., 1989) This is likely to be particularly true for the TA muscle which is thought to be under a greater cortical control than is the GM (Schubert et al., 1997; Capaday et al., 1999). This observation is also consistent with recent findings that there is reduced synaptic drive to the TA during walking in SCI individuals compared to non-disabled subjects (Hansen et al., 2005).
In all individuals, perturbations were followed by a response pattern which involved muscles of both legs. Stretch reflex activation is unlikely to account for the evoked responses as they consisted of a bilateral, perturbation-induced pattern that occurred at longer latencies and was present in muscles that were not directly affected by the perturbation. These findings indicate that the spinal cord can respond to single joint perturbations in a complex, organized manner, even when it is partially or completely isolated from supraspinal centers. This observation is at variance with prior studies in individuals with hemiplegia due to stroke, in whom the monosynaptic response was exaggerated while compensatory long latency reflex responses were reduced (Berger et al., 1984). This suggests that there may be differences in modulation of spinal neuronal activity in the presence of unilateral supraspinal input (as in the case of stroke) compared to bilaterally impaired or absent supraspinal input (in the case of SCI (Faist et al., 1999). Alternatively, the differences may be attributed to the stronger, single joint displacement induced here compared to the whole leg perturbation applied earlier (Berger et al., 1984).
Larger responses to late swing and to hip perturbations
While we observed few differences attributable to subject group, there were differences in responses based on both timing of the perturbation within the gait cycle and the joint at which the perturbation was directed. Across all subject groups perturbations of the knee or hip applied late in the swing phase (compared to early) were associated with a larger EMG response in both the ipsilateral and contralateral limb as well as a shorter onset latency in the ipsilateral limb. This perturbation occurred just before the swing-to-stance transition. This appears to be a period when the system is particularly sensitive to information related to afferent input such as hip position (Grillner and Rossignol, 1978; Kriellaars et al., 1994). In young infants (Pang and Yang, 2002) a perturbation applied during the stance phase has the greatest influence on the subsequent step cycle when the perturburbation is timed to occur near the stance-to-swing transition. The change from swing phase to stance phase also represents a period of transition. Given that reflex responses to perturbations applied during walking vary in a phase-dependent manner (Dietz, 1992), it seems reasonable that the swing-to-stance transition period is associated with the most robust response to perturbations applied in swing phase.
Across all subject groups, perturbation of the hip was associated with larger amplitude EMG responses than that of the knee. Afferent information related to hip position has been shown to be critical for regulation of step cycle timing (Pang and Yang, 2000; Pearson and Rossignol, 1991; Hiebert et al., 1996; Andersson et al., 1978; Grillner and Rossignol, 1978). Our results are consistent with these works and indicate that hip joint related afferent input is most influential in the generation of a spinal pattern regardless of the degree of available supraspinal influence (Dietz et al., 2002).
Task-dependency of the response pattern
Beyond timing of the perturbation, the mode of the perturbation undoubtedly influences the evoked response (Zehr and Stein, 1999). While several investigations have studied the effects of perturbations applied to the whole limb during the swing phase of walking (Dietz et al., 1986; Lam et al., 2003; Ghori and Luckwill, 1989; Ghori and Luckwill, 1990), the present displacements have been applied at a single joint of a limb that is not freely moving. For this reason comparison with other studies is limited. The longer response latencies observed here may be due to the focused nature of the perturbations. Whole-limb perturbations are likely to activate a larger complement of receptors. The summed effects may evoke a response with earlier onset than that evoked by single-joint perturbations.
In addition, most prior studies have investigated the effects of perturbation on unsupported bipedal locomotion. The DGO utilized in this study greatly reduces the postural requirements normally associated with locomotion. Nevertheless, the pattern of leg muscle activation has been shown to be similar during walking within and without the DGO in ND subjects (Dietz et al., 2004). However, the reduced postural requirements might contribute to differences in the response pattern to perturbations. For example, vestibular influences on spinal responses are likely to be less evident in the stable environment provided by the DGO. Further, as the orthosis controls the timing of the gait cycle, perturbations were not associated with phase resetting which has been observed in studies of freely-moving limbs (Pang and Yang, 2002; Lam et al., 2003).
Finally, other influences such as those from cutaneous input (Bouyer and Rossignol, 2003) and load receptors (Dietz et al, 2002 ; Pang and Yang, 2000) are known to influence the timing of locomotor output. Therefore, we cannot rule out the possibility that cutaneous input to the shank and thigh by the perturbation influenced the motor output. However, prior studies using the DGO have demonstrated that the cutaneous input of the cuffs alone during stepping of SCI subjects while 100% unloaded did not lead to significant leg muscle activation (Dietz et al., 2002). Further, the location and manner of cuff contact is not associated with any known cutaneous reflexes, so we feel it is unlikely that these contributed in a meaningful way to the EMG response pattern. Regarding the influence of load receptors, while small differences in the level of loading are unlikely to have had a significant influence on the perturbed (swing) limb, we cannot discount the possibility that there many have been load-related influences on the contralateral limb.
CONCLUSIONS
The present study demonstrates that the compensatory spinal neuronal response patterns to perturbation applied to a hip or knee joint during the swing phase of gait behave in a similar manner regardless of the amount of supraspinal drive available. The EMG responses cannot be attributed to stretch reflex activities as they consist of longer-latency, complex bilateral responses to the single joint perturbations, many of which are in muscles that are not directly affected by the perturbation. Complete, incomplete SCI and ND individuals show a similar well-organized response pattern with larger responses being associated with perturbations applied late in the swing phase, a finding that is consistent with a time of phase transition. Perturbations to the hip were associated with larger responses than those applied to the knee, providing support for the view that hip-related afferent input is important for the control of walking. Data collected from studies of human infants and spinal animals over the past several decades indicates that the isolated spinal cord is capable of generating complex behaviors. The results of the present study add to the growing body of evidence that the locomotor-related circuitry of the mature human spinal cord performs in much the same manner as has been observed in infant stepping and spinal cat experiments.
Figure 1.
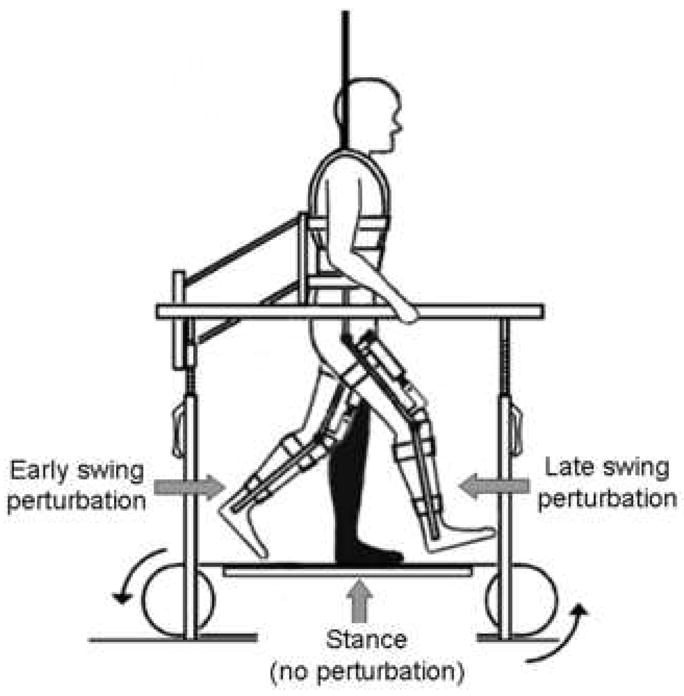
Experimental set up illustrating the position of the swing limb at the time of the early and late perturbations. Subjects walked on a treadmill in the robotic device with body weight support. No perturbations were delivered during stance phase.
Acknowledgments
The authors are grateful to Mohd Khan, Reinhard Schreier, and Andres Hurtado for technical support and assistance and to Dr. Marion McGregor for statistical support. This study was supported in part by a NIH contract NO1-NS-3-351, NIH grant HD41487, a grant from the Schumann Foundation and by The Miami Project to Cure Paralysis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- American Spinal Injury Association International Standards for Neurological Classification of Spinal Cord Injury. Chicago: ASIA; 2002. [DOI] [PubMed] [Google Scholar]
- Andersson O, Grillner S, Lindquist M, Zomlefer M. Peripheral control of the spinal pattern generators for locomotion in cat. Brain Res. 1978;150:625–630. doi: 10.1016/0006-8993(78)90827-2. [DOI] [PubMed] [Google Scholar]
- Berger W, Horstmann G, Dietz V. Tension development and muscle activation in the leg during gait in spastic hemiparesis: independence of muscle hypertonia and exaggerated stretch reflexes. J Neurol Neurosurg Psychiatry. 1984;47:1029–1033. doi: 10.1136/jnnp.47.9.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouyer LJ, Rossignol S. Contribution of cutaneous inputs from the hindpaw to the control of locomotion. II. Spinal cats. J Neurophysiol. 2003;90:3640–3653. doi: 10.1152/jn.00497.2003. [DOI] [PubMed] [Google Scholar]
- Capaday C, Lavoie BA, Barbeau H, Schneider C, Bonnard M. Studies on the corticospinal control of human walking. I. Responses to focal transcranial magnetic stimulation of the motor cortex. J Neurophysiol. 1999;81:129–139. doi: 10.1152/jn.1999.81.1.129. [DOI] [PubMed] [Google Scholar]
- Capaday C, Brigitte AL, Hughes B, Cyril S, Mireille, Bonnard Studies on the Corticospinal Control of Human Walking. I. Reaponses to focal Transcranial Magnetic Stimulation of the Motor Cortex. J Neurophys. 1999;81:129–139. doi: 10.1152/jn.1999.81.1.129. [DOI] [PubMed] [Google Scholar]
- Dietz V, Colombo G, Müller R. Single joint perturbation during gait: neuronal control of movement trajectory. Exp Brain Res. 2004;158:308–316. doi: 10.1007/s00221-004-1904-3. [DOI] [PubMed] [Google Scholar]
- Dietz V, Horstmann GA, Berger W. Interlimb coordination of leg-muscle activation during perturbation of stance in humans. J Neurophys. 1989;62:680–693. doi: 10.1152/jn.1989.62.3.680. [DOI] [PubMed] [Google Scholar]
- Dietz V, Muller R, Colombo G. Locomotor activity in spinal man: significance of afferent input from joint and load receptors. Brain. 2002;125:2626–2634. doi: 10.1093/brain/awf273. [DOI] [PubMed] [Google Scholar]
- Dietz V, Quintern J, Boos G, Berger W. Obstruction of the swing phase during gait: phase-dependent bilateral leg muscle coordination. Brain Res. 1986;384:166–169. doi: 10.1016/0006-8993(86)91233-3. [DOI] [PubMed] [Google Scholar]
- Dietz V, Quintern J, Sillem M. Stumbling reactions in man: significance of proprioceptive and pre-programmed mechanisms. J Physiol. 1987;386:149–163. doi: 10.1113/jphysiol.1987.sp016527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz V. Human neuronal control of automatic functional movements: interaction between central programs and afferent input. Physiol Rev. 1992;72:33–69. doi: 10.1152/physrev.1992.72.1.33. [DOI] [PubMed] [Google Scholar]
- Duysens J. Phase-dependent reversal of reflexly induced movements during human gait. Exp Brain Res. 1992;90:404–414. doi: 10.1007/BF00227255. [DOI] [PubMed] [Google Scholar]
- Faist M, Ertel M, Berger W, Dietz V. Impaired modulation of quadriceps tendon jerk reflex during spastic gait: differences between spinal and cerebral lesions. Brain. 1999;122:567–579. doi: 10.1093/brain/122.3.567. [DOI] [PubMed] [Google Scholar]
- Ghori GM, Luckwill RG. Pattern of reflex responses in lower limb muscles to a resistance in walking man. Eur J Appl Physiol Occup Physiol. 1989;58:852–857. doi: 10.1007/BF02332218. [DOI] [PubMed] [Google Scholar]
- Ghori GM, Luckwill RG. Phase-dependent responses in locomotor muscles of walking man. J Biomed Eng. 1990;12:75–78. doi: 10.1016/0141-5425(90)90119-8. [DOI] [PubMed] [Google Scholar]
- Giuliani CA, Smith JL. Stepping behaviors in chronic spinal cats with one hindlimb deafferented. J Neurosci. 1987;7:2537–2546. [PMC free article] [PubMed] [Google Scholar]
- Grillner S, Rossignol S. On the initiation of the swing phase of locomotion in chronic spinal cats. Brain Res. 1978;146:269–277. doi: 10.1016/0006-8993(78)90973-3. [DOI] [PubMed] [Google Scholar]
- Hansen NL, Conway BA, Halliday DM, Hansen S, Pyndt HS, Biering-Sorensen F, Nielsen JB. Reduction of common synaptic drive to ankle dorsiflexor motoneurons during walking in patients with spinal cord lesion. J Neurophysiol. 2005;94:934–942. doi: 10.1152/jn.00082.2005. [DOI] [PubMed] [Google Scholar]
- Hiebert GW, Whelan PJ, Prochazka A, Pearson KG. Contribution of hind limb flexor muscle afferents to the timing of phase transitions in the cat step cycle. J Neurophysiol. 1996;75:1126–1137. doi: 10.1152/jn.1996.75.3.1126. [DOI] [PubMed] [Google Scholar]
- Iles JF, Roberts RC. Inhibition of monosynaptic reflexes in the human lower limb. J Physiol. 1987;385:69–87. doi: 10.1113/jphysiol.1987.sp016484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriellaars DJ, Brownstone RM, Noga BR, Jordan LM. Mechanical entrainment of fictive locomotion in the decerebrate cat. J Neurophysiol. 1994;71:2074–2086. doi: 10.1152/jn.1994.71.6.2074. [DOI] [PubMed] [Google Scholar]
- Lam T, Wolstenholme C, van der LM, Pang MY, Yang JF. Stumbling corrective responses during treadmill-elicited stepping in human infants. J Physiol. 2003;553:319–331. doi: 10.1113/jphysiol.2003.043984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang M, Yang J. Sensory gating for the initiation of the swing phase in different directions of human infant stepping. J Neurosci. 2002;22:5734–5740. doi: 10.1523/JNEUROSCI.22-13-05734.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang M, Yang JF. The initiation of the swing phase in human infant stepping: importance of hip position and leg loading. J Physiol. 2000;528:389–404. doi: 10.1111/j.1469-7793.2000.00389.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson KG, Rossignol S. Fictive motor patterns in chronic spinal cats. J Neurophysiol. 1991;66:1874–1887. doi: 10.1152/jn.1991.66.6.1874. [DOI] [PubMed] [Google Scholar]
- Perry J. Thorofare. NJ: Slack, Inc; 1992. Gait Analysis. [Google Scholar]
- Rossignol S, Chau C, Brustein E, Belanger M, Barbeau H, Drew T. Locomotor capacities after complete and partial lesions of the spinal cord. Acta Neurobiol Exp (Warsz) 1996;56:449–463. doi: 10.55782/ane-1996-1148. [DOI] [PubMed] [Google Scholar]
- Schubert M, Curt A, Jensen K, Dietz V. Corticospinal input in human gait. Modulaton of magnetically evoked motor responses. Exp Brain Res. 1997;115:234–246. doi: 10.1007/pl00005693. [DOI] [PubMed] [Google Scholar]
- Zehr EP, Komiyama T, Stein RB. Cutaneous reflexes during human gait: electromyographic and kinematic responses to electrical stimulation. J Neurophysiol. 1997;77:3311–3325. doi: 10.1152/jn.1997.77.6.3311. [DOI] [PubMed] [Google Scholar]
- Zehr EP, Stein RB. What functions do reflexes serve during human locomotion? Prog Neurobiol. 1999;58:185–205. doi: 10.1016/s0301-0082(98)00081-1. [DOI] [PubMed] [Google Scholar]


