Abstract
OBJECTIVE
The reactions of carbohydrate- or lipid-derived intermediates with proteins lead to the formation of Maillard reaction products, which subsequently leads to the formation of advanced glycation/lipoxidation end products (AGE/ALEs). Levels of AGE/ALEs are increased in diseases like diabetes. Unlike AGEs, very little is known about ALE effects in vitro. We hypothesized that ALEs can have proinflammatory effects in monocytes.
RESEARCH DESIGN AND METHODS
In a profiling approach, conditioned media from THP-1 cells either cultured in normal glucose (5.5 mmol/l) or treated with MDA-Lys or MDA alone were hybridized to arrays containing antibodies to 120 known human cytokines/chemokines. Pathway analyses with bioinformatics software were used to identify signalling networks.
RESULTS
Synthetic ALE (malondialdehyde-lysine [MDA-Lys]) (50 μmol/l) could induce oxidant stress and also activate the transcriptional factor nuclear factor-κB (NF-κB) in THP-1 monocytes. MDA-Lys also significantly increased the expression of key candidate proinflammatory genes, interferon-γ–inducible protein-10, β1- and β2-integrins, cyclooxygenase-2 (COX-2), monocyte chemoattractant protein-1 (MCP-1), interleukin-6 and -8, and inducible nitric-oxide synthase, which are also associated with monocyte dysfunction. Several key target proinflammatory proteins were significantly induced by MDA-Lys relative to normal glucose or MDA alone, including MCP-1; tumor necrosis factor ligand superfamily member-14; chemokine CC motif ligand-11 (CCL11); growth-related oncogene-α, -β, and -γ; and chemokine CXC motif ligand-13. Bioinformatics analyses identified a network of chemokine signaling among MDA-Lys–regulated genes. MDA-Lys also increased monocyte binding to vascular smooth muscle and endothelial cells. Furthermore, plasma from diabetic rats showed significantly higher levels of MDA-Lys and CCL11.
CONCLUSIONS
These new results suggest that ALEs can promote monocyte activation and vascular complications via induction of inflammatory pathways and networks.
Maillard reaction products are formed by reactions of proteins with carbohydrate- or lipid-derived intermediates and reactive carbonyls, such as malondialdehyde (MDA), and this subsequently leads to the formation of advanced glycation/lipoxidation end products (AGE/ALEs). AGE/ALE levels increase during aging and in diseases like diabetes (1,2). MDA, a naturally occurring dialdehyde produced in cell membranes by lipid peroxidation, is a strong alkylating agent of primary amino groups in proteins and free amino acids. It occurs mainly in the form of an adduct with Lys, indicating that its predominant reaction in vivo is with Lys residues of proteins (1–3). The biological functions of MDA-Lys [Nε-(2-propenal) lysine] adducts generated with proteins have not been well studied. MDA-Lys epitopes are, however, closely associated with atherogenesis (1,2,4) and recognized as markers of oxidation. Oxidative stress is implicated in the pathogenesis of numerous diseases, including diabetes and atherosclerosis. It promotes the formation of oxidized LDL, which has pleiotrophic effects in cells involved in atherosclerosis (5). Polyunsaturated fatty acid components of LDL are susceptible to peroxidation by reactive oxygen species (ROS), leading to the formation of major end products of toxic and highly reactive aldehydes such as MDA and 4-hydroxy-nonenal (HNE) that can attack intracellular and extracellular proteins (1,2,6). These end products are frequently measured as indicators of lipid peroxidation and oxidative stress in vivo. They form Schiff-base adducts with Lys residues and cross-links with proteins in vitro and in vivo. HNE also reacts with Lys residues, primarily via Michael addition reaction (1–4). These lipid-protein adducts are termed ALEs. Such lipid-aldehyde conjugation of proteins can have diverse effects on cell function, including cross-linking of cell surface receptors, alteration of enzyme activities, and modification of lipoproteins. However, very little is known about their mechanisms of action.
Extensive accumulation of MDA-Lys and carboxymethyl-Lys (CML) was shown in diabetic kidneys (7). Anti–MDA-Lys adduct antibody studies demonstrated extensive presence of MDA-modified proteins in atherosclerotic lesions, primarily in macrophage-derived foam cells (1,6,8,9). ALEs, such as MDA- and HNE-protein adducts, and AGEs, such as CML, accumulate during the development of diabetes and are believed to mediate diabetes complications (1,6,7,10,11).
Certain key factors, including oxidant stress, increased flux through the polyol and hexosamine pathways, protein kinase C (PKC) and mitogen-activated protein kinase (MAPK) activation, and inflammatory gene expression (12–14), have been implicated in diabetes and diabetes-induced accelerated atherosclerosis. Several of these pathways can be triggered by AGEs (11,15,16). In monocytes, these pathways can lead to cellular dysfunction, adhesion to the endothelium, and transmigration into the subendothelial space—all key events in the pathogenesis of atherosclerosis. Several monocyte-macrophage inflammatory cytokines and chemokines have been implicated in these processes.
We and others recently demonstrated that AGEs and ligands of the receptor for AGE, such as S100b, can induce potent inflammatory cytokines and chemokines in human THP-1 monocytes and peripheral blood monocytes and that these genes were also augmented in monocytes from diabetic patients (14,17–21). However, it is not known whether ALEs can elicit similar effects in monocytes or other target cells affected by diabetes. We therefore examined the cellular actions of ALEs by evaluating the effects of MDA-Lys, a prototype ALE, in monocytes. We observed that MDA-Lys induces not only known inflammatory genes but also novel ones, such as eotaxin, and also promotes monocyte activation and dysfunction. We identified nuclear factor-κB (NF-κB) as a key MDA-Lys–activated transcription factor. Furthermore, MDA-Lys seemed to act partly via the receptor for AGEs (RAGE). In addition, we observed increased levels of MDA-Lys and eotaxin in the plasma of diabetic rats, demonstrating in vivo relevance.
RESEARCH DESIGN AND METHODS
The inhibitors used in this study, SB202190, bis-indolylmaleimide (GFX), and N-acetylcysteine (NAC), were purchased from Calbiochem (San Diego, CA); PD98059 was from Cell Signaling (Beverly, MA). Human cytokine antibody arrays and corresponding reagents were from Ray Biotech (Norcross, GA). [32P-γ]ATP (3,000 Ci/mmol) was from New England Nuclear (Boston, MA). RT-PCR and quantitative PCR reagents were from Applied Biosystems (Foster City, CA), and Quantum RNA 18S Internal Standards were from Ambion (Austin, TX). Effectene transfection reagents, plasmid DNA isolation kits, RNeasy, Oligotex kits (for mRNA preparation), and real-time PCR primers for chemokine CC motif ligand (CCL)11, CCL18, and TNFSF14 were from Qiagen (Valencia, CA). ELISA kit for CCL11 was from R&D Systems (Minneapolis, MN). Luciferase assay system was from Promega (Madison, WI). MDA-Lys was quantified with a kit (BIOXYTECH-MDA-586) from OxisResearch (Foster City, CA). Oligonucleotides containing NF-κB, AP-1, and Egr-1 consensus sequences were from Santa Cruz Biotechnology.
Cell culture and treatments
Human THP-1 monocytic cells were cultured in RPMI 1640 with either 5.5 mmol/l D-glucose (normal glucose) or 50 μmol/l (10 μg/ml) MDA-Lys at 37°C. In some experiments, THP-1 cells were pre-treated with 1 μmol/l SB202190 (p38MAPK inhibitor), 5 μmol/l GFX (PKC inhibitor), 25 μmol/l PD98059 (extracellular signal–regulated kinase [ERK] MAPK pathway inhibitor), or 100 μmol/l NAC (antioxidant). The cells were then incubated in normal glucose or MDA-Lys media for a further 8–12 h. Human vascular smooth muscle cells (HVSMCs) purchased from Clonetics (San Diego, CA) and human umbilical vein endothelial cells (HUVECs) from Cambrex (East Rutherford, NJ) were cultured according to manufacturer’s specifications.
Biological network analysis
The data list of genes unpregulated by MDA-Lys was analyzed and networks generated by Ingenuity Pathways Analysis (Ingenuity Systems; www.ingenuity.com). Details are provided in RESULTS, in the figure legends, and in the online appendix, which is available at http://dx.doi.org/10.2337/db07-1204.
Measurement of secreted CCL11 levels
CCL11 was assayed in conditioned media from THP-1 cells (5×105 cells/ml) cultured in normal glucose, MDA-Lys, or MDA alone for 8 h, using a specific ELISA kit.
MDA-Lys measurement
Rat plasma MDA-Lys levels were measured using MDA-586 assay kit according to manufacturer’s instructions.
Data analyses
Data are expressed as means ± SEM of multiple experiments. Paired Student’s t tests were used to compare two groups and ANOVA with Dunnett’s post tests for multiple comparisons.
Other methods
Other methods are available in the online appendix.
RESULTS
MDA-Lys induces oxidant stress in THP-1 cells
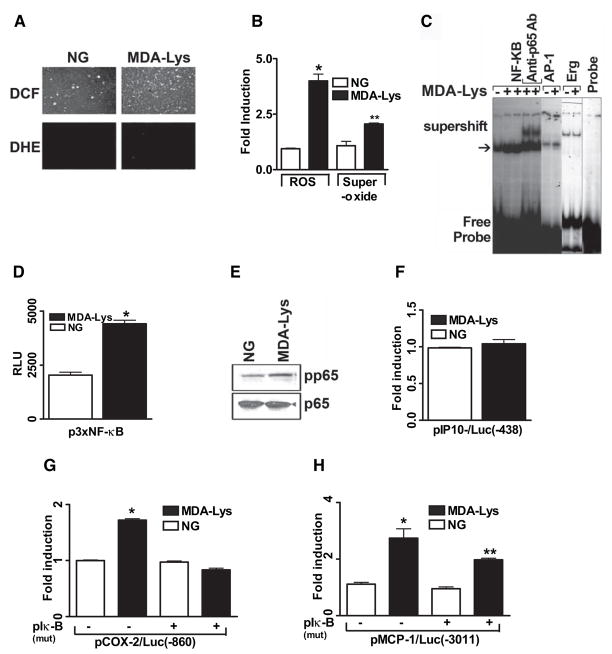
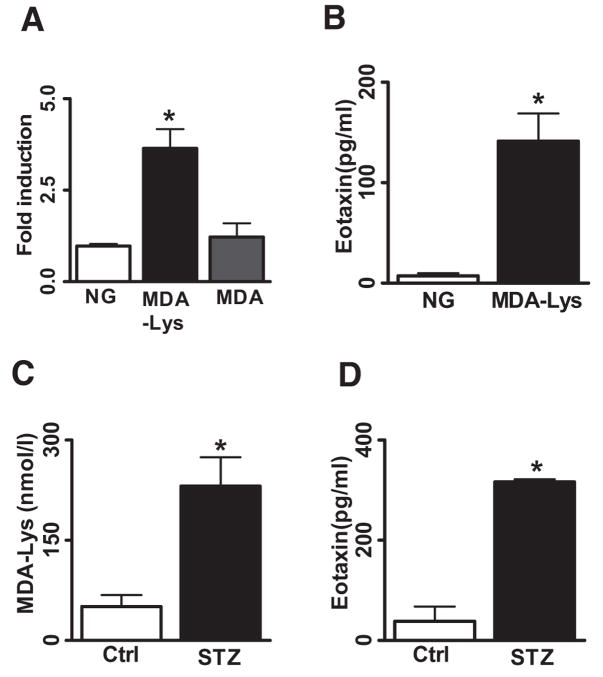
We first examined whether MDA-Lys treatment can induce oxidant stress in monocytes similar to AGEs. We chemically synthesized MDA-Lys needed for these studies and verified its purity by tandem mass spectrometry analysis (online appendix; Supplemental Fig. S1). Purified monomer was used in all experiments. THP-1 monocytes were treated with MDA-Lys for 30 min and stained with cell-permeable fluorescent probes 2′-7′-dichlorofluorescein (DCF) diacetate to detect oxidant stress or dihydroethidine (DHE) to detect superoxide formation, using a fluorescent microscope. MDA-Lys induced a significant increase in oxidant stress (Fig. 1A, top, and B, bar graph) and also superoxide formation (Fig. 1A, bottom, and B, bar graph) compared with untreated control cells grown in normal glucose.
FIG. 1.
MDA-Lys induces oxidant stress and NF-κB activation in THP-1 cells. A: Fluorescence photomicrographs from control THP-1 cells (left panel) and THP-1 cells treated with 50 μmol/l MDA-Lys (right panel) for 30 min exposed to either 1 μmol/l H2DCFDA (DCF, for ROS, top panel) or 1 μmol/l DHE (forsuperoxide, bottom panel) for 15 min. B: Bar graph showing significant fourfold increase (*P < 0.05 vs. normal glucose) in ROS and twofold increase in superoxide formation (**P < 0.01 vs. normal glucose) by MDA-Lys (means ± SE, n = 3). C: EMSAs of nuclear extracts from normal glucose- or MDA-Lys–treated cells incubated with radiolabeled oligonucleotides containing NF-κB, AP-1, or Egr-1 consensus binding sites. Supershifts were performed with nuclear extracts of MDA-Lys treated cell extracts that were pretreated with p65 antibody. D: THP-1 cells were transiently transfected with 500 ng p3xNF-κB reporter plasmid carrying luciferase gene under control of NF-κB consensus elements. After 12 h, cells were treated with or without 50 μmol/l MDA-Lys and cultured for an additional 7 h. Results are from three individual experiments, each performed in triplicate. E: MDA-Lys induces phosphorylation of NF-κB p65. Lysates from THP-1 cells stimulated with MDA-Lys for 1 h were immunoblotted with antibodies to phospho-p65 or total p65 as internal control. F--H: Gene promoter transactivation by MDA-Lys. THP-1 cells were transfected with plasmids containing luciferase gene under the control of respective minimal human promoters of IP-10 (−438/+97) (F), COX-2 (−860/+127) alone or with pCMV-mIκB (G), or MCP-1 (−3,011 to +37) alone or with pCMV-mIκB (H). After overnight recovery, cells were treated with MDA-Lys for 8 h, and luciferase activities determined. Values shown are normalized to 50 μg protein. Means ± SE of three independent experiments (*P < 0.01 vs. normal glucose, **P < 0.05 vs. normal glucose).
MDA-Lys induces NF-κB activation
We next examined whether MDA-Lys can activate oxidant-sensitive transcription factors, such as NF-κB, AP-1, and Egr-1, which are known regulators of proinflammatory genes. Electrophoretic mobility shift assays (EMSAs) were first performed in THP-1 cells after 1 h incubation with MDA-Lys. Figure 1C shows that MDA-Lys can increase DNA binding activities for NF-κB (Fig. 1C, arrow; lanes 2 and 3 vs. control, lane 1) and a slight increase in AP-1 (lane 7 vs. 6) but not Egr-1 (lane 9 vs. 8). To confirm whether the NF-κB complex contains the transcriptionally active p65 subunit, we performed additional EMSAs after pretreatment with an anti-p65 antibody and observed a super shifted band in antibody-treated samples (lanes 4 and 5). To further confirm whether increased DNA binding was associated with transcriptional activity, THP-1 cells were next transiently transfected with plasmids containing heterologous promoters driven by multiple NF-κB or AP-1 response elements upstream of luciferase reporter. MDA-Lys significantly increased NF-κB promoter-driven luciferase activity (Fig. 1D) but not AP-1 (not shown). Furthermore, Western blots showed an increase in phosphorylation of NF-κB p65 by MDA-Lys (Fig. 1E), further supporting increased NF-κB activation.
We next examined whether MDA-Lys can transactivate the promoters of key inflammatory genes known to be regulated by NF-κB and by AGEs in monocytes, namely, monocyte chemoattractant protein-1 (MCP-1), IP-10, and cyclooxygenase-2 (COX-2) (14,18–20). THP-1 cells were transiently transfected with plasmids containing luciferase gene under control of human IP-10 (−438/+97), MCP-1 (−3,011/+52), or COX-2 (−860/+127) promoter fragments. MDA-Lys significantly increased promoter activities of COX-2 (Fig. 1G) and MCP-1(Fig. 1H) but not IP-10 (Fig. 1F). To further confirm the role of NF-κB, we cotransfected cells with an NF-κB super-suppressor IκB-α (mutant) plasmid along with the promoter constructs. Fig. 1G shows that MDA-Lys–induced COX-2 promoter activation was completely blocked by this mutant while MCP-1 promoter activation was partially but significantly blocked (Fig. 1H). These results suggest that MDA-Lys can induce COX-2 and MCP-1 transcription via NF-κB activation, whereas that of IP-10 may be due to mRNA stabilization as noted for IP-10 induction by the RAGE ligand S100b (20) or that the MDA-Lys-response elements for IP-10 are further downstream of the IP-10 promoter fragment used.
MDA-Lys induces the expression of key atherogenic genes associated with monocyte activation
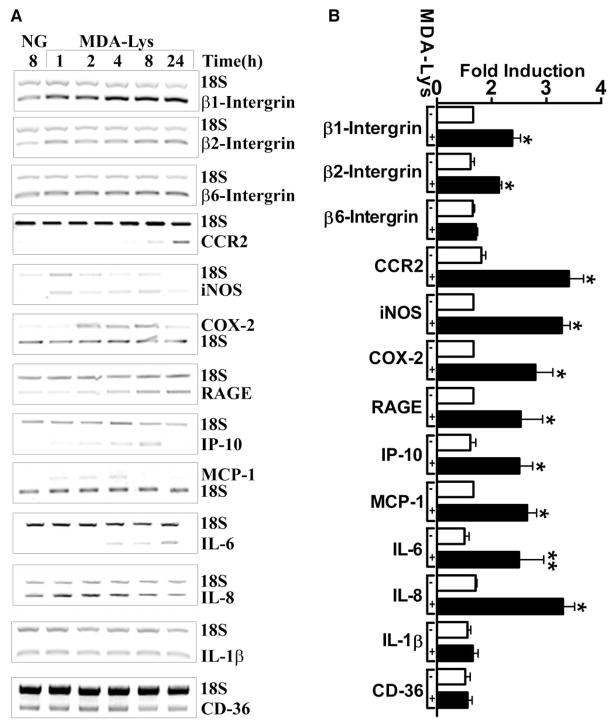
Initial RT-PCR analysis of THP-1 cells treated with increasing concentrations of MDA-Lys (1, 5, 10, and 20 μg/ml) showed that maximal induction of COX-2 mRNA occurred with 10 μg/ml (50 μmol/l) (data not shown); hence, this dose of MDA-Lys was used in subsequent experiments. Evidence shows that AGEs and ligands of RAGE can increase the expression of several inflammatory cytokines and chemokines in monocytes, such as β-integrins, RAGE, interleukin (IL)-1β, COX-2, inducible nitric-oxide synthase (iNOS), COX-1, IP-10, and MCP-1 (14,18–20). In a candidate gene approach, we therefore tested whether MDA-Lys can regulate these genes in THP-1 cells by performing multiplex RT-PCRs using specific primers for the human genes (Supplemental Table S1) paired with 18S rRNA primers as internal controls. Results showed that MDA-Lys increased, in a time-dependent fashion (1–24 h), the expressions of MCP-1, β1- and β2-integrins, RAGE, IP-10, CCR2, iNOS, IL-6, IL-8, and COX-2 but not β6-integrin, IL-1β, or CD-36 (Fig. 2A). Bar graph quantification in Fig. 2B obtained from multiple independent experiments at 4, 8, or 24 h shows that the stimulatory effects on these molecules are significant.
FIG. 2.
Analysis of MDA-Lys-induced candidate genes. Relative RT-PCRs were performed with total RNA isolated from THP-1 cells treated with or without MDA-Lys for 1–24 h, using gene-specific primers (Supplemental Table S1). 18S RNA primers were included in each PCR reaction as internal control. A: Ethidium bromide-stained agarose gels of RT-PCR products. NG indicates control cell grown for 24 h. B: Bar graph showing significant induction of β1- and β2-integrins, CCR2, iNOS, COX-2, RAGE, IP-10, IL-6, IL-8, and MCP-1 mRNAs at 4, 8, or 24 h. Values shown are means ± SE of three independent experiments. *P < 0.001, **P < 0.05 vs. normal glucose.
MDA-Lys induces proinflammatory cytokines and chemokines
The candidate gene approach demonstrated that MDA-Lys and potentially ALEs can induce key known proinflammatory and monocyte activating genes that have also been shown to be induced by high glucose, AGEs, and RAGE ligands in monocytes (14,18–20). However, because ALEs may also induce other ALE-specific inflammatory genes in monocytes, we used commercial human cytokine antibody arrays to profile proteins regulated by MDA-Lys. Results from three independent experiments shown in Fig. 3 and Supplemental Table S2 indicate that the levels of 20 cytokines levels were increased by 1.5- to 5-fold by MDA-Lys relative to control (normal glucose), including CCL11, CCL18, CCL28, TNFSF14, MCP-1, and MCP-2, whereas six cytokines were decreased, including CCL1, CXCL13, CSF-2, and MCP-3. These results show for the first time that multiple inflammatory cytokines and chemokines are regulated by ALEs, such as MDA-Lys in monocytes. Interestingly, many of these genes (Supplemental Table S2; Figs. 2 and 3) are reported to be regulated by NF-κB, suggesting an important regulatory role for NF-κB in the effects of ALEs similar to AGEs. However, ALEs appear to also augment key new genes not reported to be induced by AGEs.
FIG. 3.
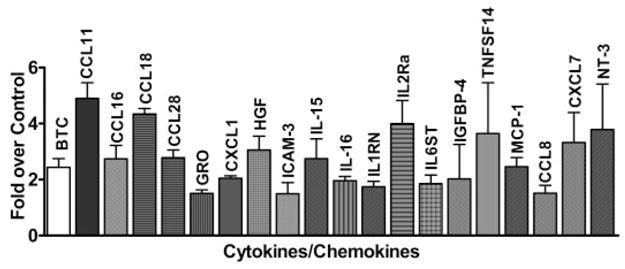
Cytokine antibody array analysis of MDA-Lys–treated THP-1 cells. Conditioned medium from THP-1 monocytes cultured under either normal glucose (5.5 mmol/l) or MDA-Lys (50 μmol/l) conditions for 24 h were hybridized to human cytokine antibody arrays. Relative percentage of spot intensities in the membranes were measured and normalized to normal glucose. Values are average of data from three independent experiments. Cytokines showing significant increases (*P ≤ 0.05) are shown in the bar graph.
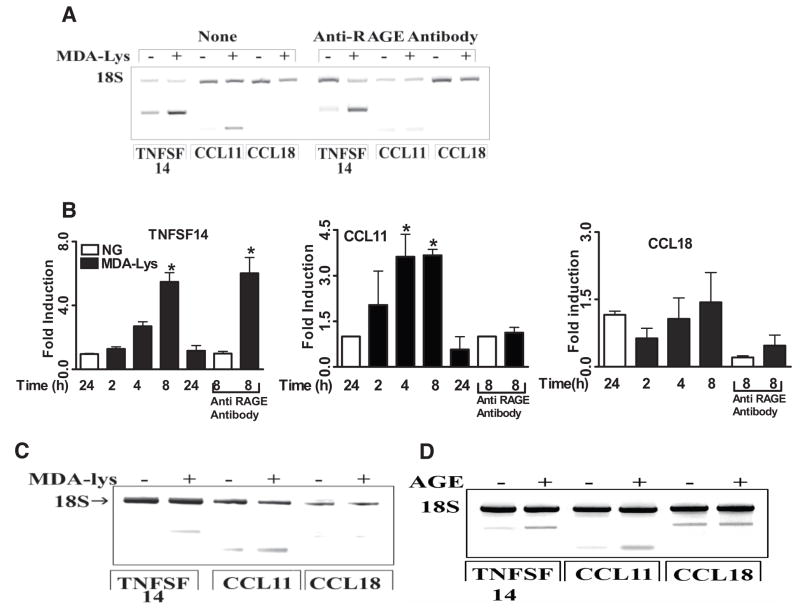
We next examined whether some of these key protein targets induced by MDA-Lys are also modulated at the transcriptional level by analyzing mRNA levels of CCL11, CCL18, and TNFSF14. RT-PCR data in Fig. 4A show that MDA-Lys clearly increased mRNA levels of TNFSF14 and CCL11 but not CCL18 despite increased CCL18 protein levels (Fig. 3; Supplemental Table S2). In addition, we performed real-time quantitative PCR of RNA samples from THP-1 cells treated 1–24 h with MDA-Lys. Bar graphs in Fig. 4B show that MDA-Lys significantly increased CCL11 and TNFSF14 mRNA, but not CCL18 mRNA levels, with peaks at 8 h. These results along with the previous data demonstrate that MDA-Lys can regulate the expression of inflammatory genes in monocytes via both transcriptional and posttranscriptional mechanisms.
FIG. 4.
A: Role of RAGE receptor in MDA-Lys induced CCL11, CCL18, and TNFSF14 in mRNAs. THP-1 cells were pretreated with or without 70 μg/ml anti-RAGE antibody for 1 h followed by 50 μmol/l MDA-Lys for 4 h. mRNA levels analyzed by RT-PCR with 18S internal control. B: Bar graph showing time-course induction of CCL11, CCL18, and TNFSF14 mRNAs by real time quantitative RT-PCR using gene-specific primers and normalized with glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as internal control. C: Effect of MDA-Lys on CCL11, CCL18, and TNFSF14 mRNA expression in peripheral blood primary human monocytes as analyzed by RT-PCR with 18S internal control. D: Agarose gel of RT-PCR products of TNFSF-14, CCL11, and CCL18 mRNA amplification from THP-1 cells treated with or without methylglyoxal modified bovine serum albumin (AGE) for 8 h.
To verify that MDA-Lys can exert similar effects even in primary monocytes, human peripheral blood monocytes were treated for 8 h with MDA-Lys, and mRNA expressions were analyzed by RT-PCR. Fig. 4C shows that CCL11 and TNFSF14 mRNA levels were increased but not CCL18 levels, similar to data obtained in THP-1 cells. We next examined whether these factors are also induced by AGEs. THP-1 cells were treated with or without methyl-glyoxal modified BSA prepared as described previously (19). This AGE-BSA could also induce TNFSF14 and CCL11 mRNAs but not CCL18 (Fig. 4D), similar to the effects of MDA-Lys (Fig. 4C).
To determine whether MDA-Lys can exert some of its cellular effects via the AGE receptor RAGE, THP-1 cells were pretreated with a specific anti-RAGE antibody for 1 h before MDA-Lys. This led to almost complete blockade of MDA-Lys–induced CCL11 mRNA (Fig. 4A, lanes 9 and 10, and B, middle panel) but not TNFSF14 (Fig. 4A, lanes 7 and 8, and B, left panel), suggesting that MDA-Lys can signal not only through RAGE but also via other as yet unidentified receptors. Taken together, these observations demonstrate that ALEs can enhance the expression of key inflammatory chemokines that promote monocyte adhesion, transmigration, and foam cell formation as seen in diabetes and its complications.
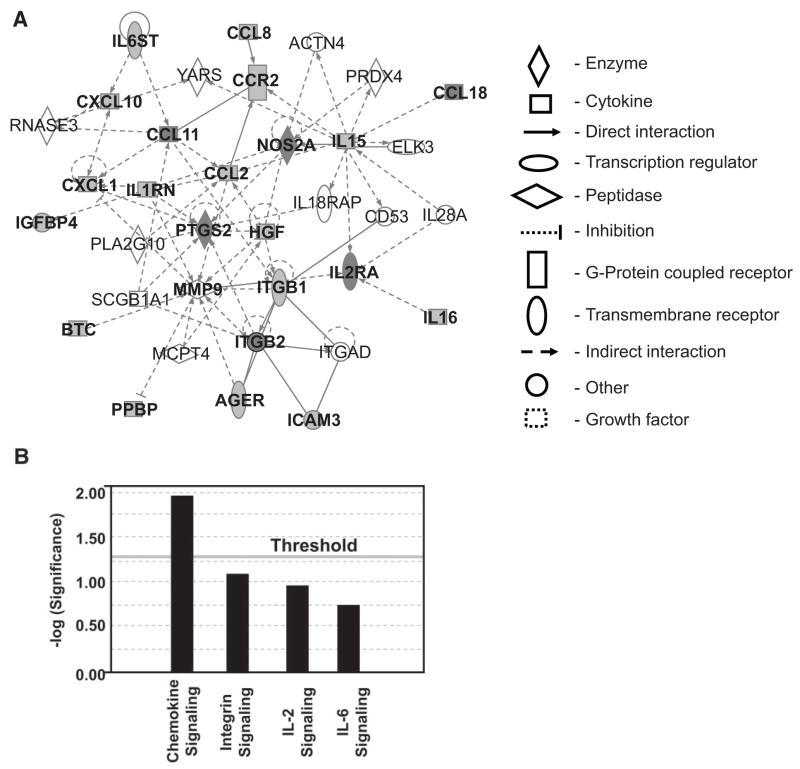
Ingenuity pathway analysis of MDA-Lys–regulated molecules
To identify potential networks of inflammatory signaling that may be triggered by MDA-Lys, we used Ingenuity Pathway Analysis (Ingenuity Systems) to uncover diabetes-related biological networks among MDA-Lys–regulated proteins and mRNAs (Supplemental Table S2; Figs. 2 and 3). Interestingly, this data mining tool uncovered two groups of networks with high scores (Table 1) from the MDA-Lys–induced factors, which include proteins involved in immune response, cellular movement, and cell-to-cell signaling. From these, we chose candidates associated with transcription, gene expression, and cellular activation/deactivation. The resulting network (Fig. 5A) placed our candidate genes in canonical pathways with significant association with chemokine signaling (Fig. 5B) (see figure legends and online appendix for additional details), suggesting that MDA-Lys–regulated molecules belong in large part to families associated with chemokines and their signaling partners. This novel bioinformatics tool has uncovered interactive pathways and functional relevance to further support MDA-Lys as a proinflammatory agent that can promote chemokine signaling and monocyte activation.
TABLE 1.
Protein networks identified by pathway analysis
| Proteins in network | Score | Focus protein | Top functions | |
|---|---|---|---|---|
| 1 | ACTN4, RAGE, BTC, CCL2, CCL8, CCL11, CCL18, CCR2, CD53, CXCL1, CXCL10, ELK3, HGF, ICAM3, IGFBP4, IL15, IL16, IL18RAP, IL1RN, IL28A, IL2RA, IL6ST, ITGAD, ITGB1 ITGB2, MCPT4, NOS2A, PLA2G10, PPBP, PRDX4, PTGS2 RNASE3, SCGB1A1, YARS | 56 | 22 | Immune response cellular movement, hematological system development and function |
| 2 | ANG, AXL, BTC, BTN3A3 C10ORF10, CCL8, CHST4, CIB2, CXCL13, DGCR6, FCGR1B, FCGR1C, GSTA4, H60, HRSP12, ICAM3, IFNG, IL6, IL1A, IL1F6, IL1F9, JUN, MAPK14, MYHS, NTF3, RAET1B, SLC28A1, SLC28A2, SPTA1, STAT3, TNF, TNFSF14, TNN, TRAFD1, UBR2 | 14 | 8 | Cell-to-cell signaling and hematological system development and function |
FIG. 5.
Ingenuity pathway analysis identifies key interactive networks among proteins and genes regulated by MDA-Lys in THP-1 cells. A: The network is displayed schematically as nodes (genes/gene products) and edges (solid or dotted lines, depicting biological relationships between the nodes). Filled nodes are identified targets, and open ones are predicted pathway partners. Edges for protein regulations, such as phosphorylation and other modifications, are removed for simplicity. This network was generated by the software based on highest scores obtained from a total of 22 differentially expressed focus proteins noted in our experiments (Supplemental Table 2). In the current study, a score of 14 or higher was used to select significant biological networks regulated by MDA-Lys. A highly significant score of 56 was obtained (Table 1). B: The network score is next displayed as the negative log of the P value, indicating the likelihood of the focus molecules in a network being found together due to random chance. Therefore, −log values of two have at least 99% confidence of not being generated by chance alone as seen for chemokine signaling.
MDA-Lys induces CCL11 protein in THP-1 cells
CCL11, also known as eotaxin, plays a key role in coronary artery disease (22). However, its role in diabetic vascular disease has not been studied. Because our novel observations showed for the first time that MDA-Lys could induce mRNA and protein expression of CCL11 in monocytes, we evaluated it more extensively. The time course of CCL11 mRNA induction showed a peak at 8 h (Fig. 4B) in THP-1 cells, and this was significant compared with equimolar MDA alone or normal glucose (Fig. 6A), suggesting that CCL11 induction was specific to MDA-Lys. Additional ELISAs to quantify CCL11 (Fig. 6B) showed a significant increase (111.1 ± 41.5 pg/ml) in supernates from MDA-Lys–treated cells relative to normal glucose (7.3 ± 2.7 pg/ml).
FIG. 6.
MDA-Lys induces specific and significant induction of CCL11. Total RNA from multiple sets of MDA-Lys, MDA, and normal glucose grown THP-1 cells were used to perform real-time quantitative PCRs using CCL11 specific primers and GAPDH primers. A: Eotaxin levels were calculated by normalizing to internal control GAPDH and results expressed as fold over normal glucose. Values shown are means ± SE of three independent experiments. *P < 0.012. B: Conditioned medium supernatants of THP-1 cells treated with normal glucose or MDA-Lys were assayed for secreted CCL11 (eotaxin) levels by specific ELISA. Results shown are means ± SE from three independent experiments run in triplicate. *P < 0.004. C: Quantification of protein cross-linked MDA in plasma from control and STZ-administered rats (n = 10). *P < 0.003. D: Rat plasma samples assayed for secreted CCL11 levels by specific ELISA. Results shown are mean ± SE from four independent rats run in triplicate. *P < 0.003.
Increased CCL11 levels correlates with diabetic rat plasma MDA
To determine in vivo relevance, we quantified MDA-Lys and CCL11 (eotaxin) levels in plasma from control and streptozotocin (STZ)-injected diabetic rats (32 weeks) (10 control and 10 diabetic) using an ELISA method. MDA-Lys levels were significantly greater in diabetic rat plasma (225.7 ± 43.63 nmol/l) compared with control rat plasma (68.62 ± 14.33 nmol/l) (Fig. 6C). Furthermore, in parallel, we observed a significant increase in eotaxin levels in diabetic rat plasma (231.15 ± 75.04 pg/ml) versus control nondiabetic (50.57 ± 30.12 pg/ml) (Fig. 6D), suggesting that increased CCL11 levels in diabetes may be related to the increased MDA-Lys levels.
MDA-Lys induces adhesion of monocytes to HVSMCs and HUVECs
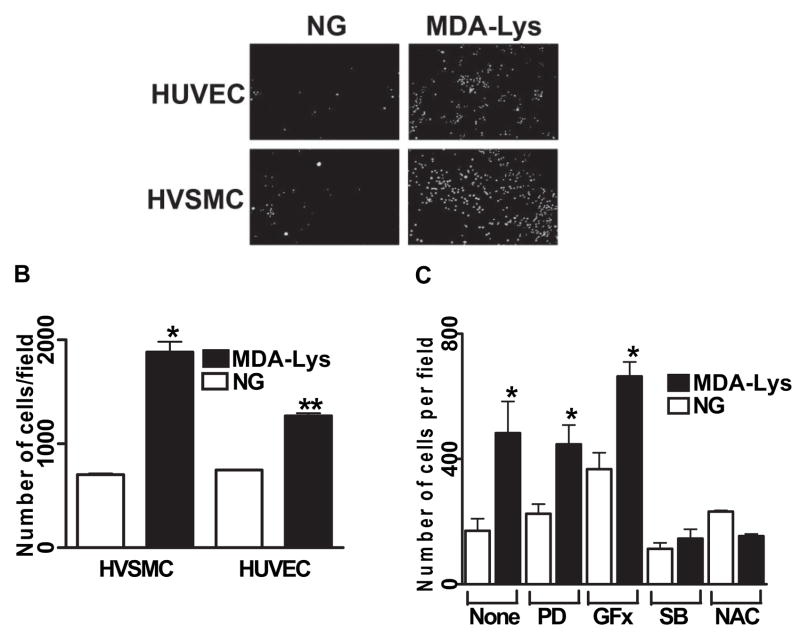
Monocyte adhesion to endothelial cells and vascular smooth muscle cell (VSMCs) are key pathogenic events associated with monocyte activation, subendothelial retention, and atherosclerosis. To evaluate the functional role of MDA-Lys, we next examined whether pretreatment of monocytes with MDA-Lys can enhance their adhesion to HVSMC or HUVECs. THP-1 cells were cultured in normal glucose or with MDA-Lys in serum-depleted medium for 24 h and then allowed to adhere to monolayers of normal HVSMCs and HUVECs as described in RESEARCH DESIGN AND METHODS (online appendix). As seen in Fig. 7A and B, THP-1 cells treated with MDA-Lys adhered two- to threefold more to HVSMCs and HUVECs relative to normal glucose. Thus, elevated ALEs such as MDA-Lys under diabetic conditions can enhance monocyte dysfunction.
FIG. 7.
A: MDA-Lys treatment increases monocytes adhesion to HVSMC and HUVECs. THP-1 cells were cultured with or without MDA-Lys for 12 h and then labeled with fluorescent dye Calcein-AM for 15 min at 37°C. Labeled THP-1 cells were allowed to adhere to either HUVEC or HVSMC monolayers in 24-well culture dishes. After careful washing, specifically bound monocytes were counted as described previously (19). Results are expressed as number of monocytes bound per high-power field. B: Bar graph shows means ± SE from three to five experiments (*P < 0.005, **P < 0.01 vs. respective controls). C: THP-1 cells were treated for 12 h with the indicated inhibitors or corresponding vehicle and then treated with or without MDA-Lys and then binding to HVSMC examined as above. Results shown are means ± SE (n = 4; *P < 0.01 vs. respective control). PD, 2′-amino-3′methoxyflavone (PD-98059); SB, 4-[4-(4-fluorophenyl)-5-(4-pyridinyl)-1H-imidazol-2-yl]phenol (SB202190).
Identification of signaling pathways involved in MDA-Lys–induced monocyte adhesion
RAGE ligands and high glucose can induce inflammatory genes and monocyte activation via enhanced oxidant stress, activation of NF-κB and AP-1 and key signaling kinases, such as PKC, ERK1/2, and p38 MAPKs (14,20,23–25). Our current data show that MDA-Lys can also activate oxidant stress and NF-κB in monocytes, which may involve kinase activation. We therefore examined the consequences of blocking some of these signaling pathways with specific inhibitors. Figure 7C shows that MDA-Lys–induced monocyte binding to HVSMCs was significantly blocked by the antioxidant 100 μmol/l NAC and by p38MAPK inhibitor (5 μmol/l SB202190) but not by inhibitors of ERK1/2 pathway (PD98059) or PKC (GFX). These results suggest that MDA-Lys–induced inflammatory genes, monocyte activation, and adhesion involve the coordinated activation of multiple pathways, including oxidant stress and p38MAPK.
DISCUSSION
Recent studies suggest that, apart from AGEs, ALEs may also play significant roles in diabetic vasculopathy. However, although an immense amount of work has evaluated in vitro and in vivo functional roles of AGEs, very little is known about the mechanisms by which ALEs may exert their effects despite reports showing increased MDA-Lys in diabetic animal models (7,10,26). The role of MDA-Lys in diabetes-induced atherogenesis is also unclear. Monocytes are orchestrators of the inflammatory response and play key roles in the development of atherosclerosis (27). We recently demonstrated that high glucose or ligation of RAGE with AGEs or S100b in THP-1 monocytes or human blood monocytes leads to increased expression of several inflammatory genes and their receptors (14,18–20,23). Although the cellular effects of ALEs are believed to be similar to AGEs, this has not been tested to date. In the current study, we have explored for the fist time the impact of MDA-Lys on monocyte behavior by studying its effects on inflammatory gene expression and monocyte activation associated with diabetes. Synthetic MDA-Lys was used as a prototype ALE because it is elevated under diabetic conditions. We used 50 μmol/l MDA-Lys because it gave the maximal induction of key inflammatory genes. There are very few reports of MDA-Lys levels in vivo. Miyata et al. (28) have reported ~20 μmol/mol Lys of MDA in normal humans and ~40 μmol/mol Lys in diabetic subjects.
High glucose, AGEs, and RAGE ligation increase intracellular oxidative stress in monocytes (13,29) as manifested by the production of superoxide anion, a potent ROS. ROS function as important second messengers to modulate several downstream signaling molecules like MAPKs and transcription factors, such as NF-κB and AP-1. These in turn can induce key inflammatory genes that promote monocyte activation, migration, and sustained inflammation associated with several diabetes complications, including atherosclerosis. We noted that MDA-Lys could significantly increase oxidant stress, superoxide production, and NF-κB activation and inflammatory gene expression. In a candidate gene profiling approach, MDA-Lys increased the expression of key NF-κB–dependent genes, such as MCP-1, iNOS, RAGE, IP-10, CCR-2, IL-6, IL-8, and COX-2, that are associated with diabetes complications, inflammation, and monocyte activation. Thus, ROS signaling may mediate MDA-Lys–induced NF-κB transactivation during diabetes, vascular dysfunction, and atherosclerosis. Interestingly, IP-10 induction was not transcriptionally regulated, suggesting that MDA-Lys might effect an increase in IP-10 mRNA stability similar to that observed with the RAGE ligand, S100b (20).
In addition, we performed a focused profiling of cytokine, chemokine, and related factors regulated by MDA-Lys in THP-1 cells by using cytokine antibody arrays, with subsequent follow-up validation of key candidates. Interestingly, we noted significant increases in several factors that were primarily secreted, including CCL11, CCL18, TNFSF14, and MCP-1 (Table 1; Fig 3). A review of the literature showed that many of these are regulated by NF-κB (14,19,20,30). Our data also showed increased NF-κB (but not AP-1 or Egr-1) activity, supporting the notion that MDA-Lys–induced NF-κB activation can augment the expression of multiple cytokines implicated in monocyte activation.
On the other hand, although there was a fourfold increase in CCL18 protein levels, there was no change in CCL18 mRNA levels, suggesting that MDA-Lys may exert its effects not only by increasing the transcription of a subset of proinflammatory genes but also by posttranscriptional mechanisms and translational increase in protein synthesis of other genes. We also used a bioinformatics pathway analyses approach to determine whether ALEs can trigger key pathways and networks that modulate cellular function. This grouped the MDA-Lys–induced genes/proteins into two types of high-scoring pathways whose major functions were mainly related to inflammation, cell movement, and cell-to-cell signaling (Table 1), with high significance associated with chemokine family proteins and their partners. These novel query tools clearly demonstrate that MDA-Lys can initiate a chemokine signaling program in monocytes that in turn can promote inflammation, cell migration, adhesion, extravasation, differentiation, and vascular dysfunction.
Although both ALEs and AGEs are formed by Maillard reactions, it is not known whether both molecules at least to some extent share the same receptor. MDA-Lys–induced CCL11 mRNA accumulation was significantly attenuated in THP-1 cells pretreated with anti-RAGE antibody. However, under these conditions, TNFSF14 accumulation was not blocked, suggesting that MDA-Lys–induced TNFSF14 mRNA is RAGE independent and may involve an unidentified ALE receptor. Also, MDA-Lys could directly induce the expression of RAGE but not the scavenger receptor CD-36, which is another AGE receptor (31). Reports show that ALEs, such as MDA and CML, are colocalized with macrophage scavenger receptor-A in atherosclerotic lesions (32). Thus, MDA-Lys may act through multiple receptors, and additional studies are needed to examine these.
Antibody array profiling revealed that MDA-Lys can upregulate interesting chemokines CCL11 (eotaxin), TN-FSF14, and CCL18. Similar data were obtained in primary blood monocytes. Eotaxin is a novel chemokine with key emerging functions related to inflammation, atherosclerosis, and associated diseases (22,33). It can stimulate the directional migration, adhesion, accumulation, and recruitment of T-cells during certain types of inflammation (22). Importantly, we noted in vivo relevance because plasma from diabetic rats had significantly elevated levels of not only MDA-Lys but also eotaxin.
TNFSF14, a member of the TNF superfamily, is associated with and released as a soluble ligand upon platelet activation. TNFSF14 can induce inflammatory responses in monocytes and release chemokines. TNFSF14 has been implicated in atherogenesis, plaque destabilization, and other inflammatory disorders involving leukocyte infiltration (34). CCL18 is a chemokine that is induced during inflammation (35).
Evidence shows that HG, AGEs, and S100b can increase monocyte adherence to VSMCs and endothelial cells (14,18,19), key early steps in the pathogenesis of atherosclerosis. Key factors that we noted to be induced by MDA-Lys, such as MCP-1, eotaxin, IL-6, IL-8, β1- and β2-integrins, and COX-2, are associated with monocyte activation, adhesion, and migration. We observed that MDA-Lys treatment of monocytes led to two- to threefold increase in their adherence to HVSMCs and HUVECs. This increased adhesion was significantly blocked by inhibitors of p38 MAPK and oxidant stress. Kumagai et al. (36) showed that another lipid peroxidation–derived electrophile, HNE, can induce COX-2 in macrophages and also activate p38MAPK. However, unlike MDA-Lys, HNE did not activate NF-κB or induce iNOS. Kanayama et al. (37) showed that HNE upregulates CD36 expression, whereas our data show that MDA-Lys had no effect on CD36 mRNA. Thus, MDA-Lys and HNE may act via different signaling mechanisms. Thus, ALEs, such as MDA-Lys, can induce key inflammatory genes in monocytes via oxidant stress, key signaling pathways (p38MAPK), and transcriptional factors (NF-κB) to enhance chemokine expression, monocyte adhesion, retention, and foam cell formation.
Although our results are primarily derived with MDA-Lys, in unpublished results we noted that MDA-modified BSA elicits similar responses, including eotaxin production. Taken together, these results provide, to our knowledge, the first evidence that MDA-Lys may enhance diabetes complications by inducing key pathological genes and, thus, provide new insights into the actions of the excess MDA-Lys and ALEs seen in disease states. Future studies aimed at identifying ALE-specific receptors in target cells may uncover important therapeutic targets for diseases associated with dyslipidemia and enhanced ALEs, such as diabetes and atherosclerosis.
Supplementary Material
Acknowledgments
R.N. has received grants from the National Institutes of Health (National Institute of Diabetes and Digestive and Kidney Diseases and National Heart, Lung, and Blood Institute) and the Juvenile Diabetes Research Foundation. City of Hope has received a General Clinical Research Center Grant MO1RR00043 from National Center for Research Resources.
- AGE
advanced glycation end product
- ALE
advanced lipoxidation end product
- CCL
chemokine CC motif ligand
- CML
carboxymethyl-lysine
- COX-2
cyclooxygenase-2
- DCF
2′-7′-dichlorofluorescein
- DHE
dihydroethidine
- EMSA
electrophoretic mobility shift assay
- ERK
extracellular signal–regulated kinase
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- GFX
bis-indolylmaleimide
- HNE
4-hydroxy-nonenal
- HUVEC
human umbilical vein endothelial cell
- HVSMC
human vascular smooth muscle cell
- IL
interleukin
- iNOS
inducible nitric-oxide synthase
- MAPK
mitogen-activated protein kinase
- MCP-1
monocyte chemoattractant protein-1
- MDA-Lys
malondialdehyde-lysine
- NAC
N-acetylcysteine
- NF-κB
nuclear factor-κB
- PKC
protein kinase C
- RAGE
receptor for advanced glycation end products
- ROS
reactive oxygen species
- STZ
streptozotocin
- VSMC
vascular smooth muscle cell
Footnotes
Additional information for this article can be found in an online appendix at http://dx.doi.org/10.2337/db07-1204.
References
- 1.Thorpe SR, Baynes JW. Maillard reaction products in tissue proteins: new products and new perspectives. Amino Acids. 2003;25:275–281. doi: 10.1007/s00726-003-0017-9. [DOI] [PubMed] [Google Scholar]
- 2.Portero-Otin M, Bellmunt MJ, Requena JR, et al. Protein modification by advanced Maillard adducts can be modulated by dietary polyunsaturated fatty acids. Biochem Soc Trans. 2003;31:1403–1405. doi: 10.1042/bst0311403. [DOI] [PubMed] [Google Scholar]
- 3.Requena JR, Fu MX, Ahmed MU, et al. Quantification of malondialdehyde and 4-hydroxynonenal adducts to lysine residues in native and oxidized human low-density lipoprotein. Biochem J. 1997;322:317–325. doi: 10.1042/bj3220317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang GS, Wang ZP, Wang SC, et al. Intracellular generation of MDA-LYS epitope in foam cells. Life Sci. 1999;65:285–296. doi: 10.1016/s0024-3205(99)00247-7. [DOI] [PubMed] [Google Scholar]
- 5.Parthasarathy S, Steinberg D, Witztum JL. The role of oxidized low-density lipoproteins in the pathogenesis of atherosclerosis. Annu Rev Med. 1992;43:219–225. doi: 10.1146/annurev.me.43.020192.001251. [DOI] [PubMed] [Google Scholar]
- 6.Palinski W, Ord VA, Plump AS, et al. ApoE-deficient mice are a model of lipoprotein oxidation in atherogenesis: demonstration of oxidation-specific epitopes in lesions and high titers of autoantibodies to malondialdehyde-lysine in serum. Arterioscler Thromb. 1994;14:605–616. doi: 10.1161/01.atv.14.4.605. [DOI] [PubMed] [Google Scholar]
- 7.Suzuki D, Miyata T, Saotome N, et al. Immunohistochemical evidence for an increased oxidative stress and carbonyl modification of proteins in diabetic glomerular lesions. J Am Soc Nephrol. 1999;10:822–832. doi: 10.1681/ASN.V104822. [DOI] [PubMed] [Google Scholar]
- 8.Yamada S, Kumazawa S, Ishii T, et al. Immunochemical detection of a lipofuscin-like fluorophore derived from malondialdehyde and lysine. J Lipid Res. 2001;42:1187–1196. [PubMed] [Google Scholar]
- 9.Requena JR, Fu MX, Ahmed MU, et al. Lipoxidation products as biomarkers of oxidative damage to proteins during lipid peroxidation reactions. Nephrol Dial Transplant. 1996;11 (Suppl 5):48–53. doi: 10.1093/ndt/11.supp5.48. [DOI] [PubMed] [Google Scholar]
- 10.Furfaro AL, Menini S, Patriarca S, et al. HNE-dependent molecular damage in diabetic nephropathy and its possible prevention by N-acetyl-cysteine and oxerutin. Biofactors. 2005;24:291–298. doi: 10.1002/biof.5520240134. [DOI] [PubMed] [Google Scholar]
- 11.Brownlee M, Cerami A, Vlassara H. Advanced glycosylation end products in tissue and the biochemical basis of diabetic complications. N Engl J Med. 1988;318:1315–1321. doi: 10.1056/NEJM198805193182007. [DOI] [PubMed] [Google Scholar]
- 12.Baynes JW. Role of oxidative stress in development of complications in diabetes. Diabetes. 1991;40:405–412. doi: 10.2337/diab.40.4.405. [DOI] [PubMed] [Google Scholar]
- 13.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 14.Shanmugam N, Reddy MA, Guha M, et al. High glucose-induced expression of proinflammatory cytokine and chemokine genes in monocytic cells. Diabetes. 2003;52:1256–1264. doi: 10.2337/diabetes.52.5.1256. [DOI] [PubMed] [Google Scholar]
- 15.Schmidt AM, Hori O, Brett J, et al. Cellular receptors for advanced glycation end products: implications for induction of oxidant stress and cellular dysfunction in the pathogenesis of vascular lesions. Arterioscler Thromb. 1994;14:1521–1528. doi: 10.1161/01.atv.14.10.1521. [DOI] [PubMed] [Google Scholar]
- 16.Schmidt AM, Yan SD, Wautier JL, et al. Activation of receptor for advanced glycation end products: a mechanism for chronic vascular dysfunction in diabetic vasculopathy and atherosclerosis. Circ Res. 1999;84:489–497. doi: 10.1161/01.res.84.5.489. [DOI] [PubMed] [Google Scholar]
- 17.Morohoshi M, Fujisawa K, Uchimura I, et al. The effect of glucose and advanced glycosylation end products on IL-6 production by human monocytes. Ann N Y Acad Sci. 1995;748:562–570. doi: 10.1111/j.1749-6632.1994.tb17362.x. [DOI] [PubMed] [Google Scholar]
- 18.Figarola JL, Shanmugam N, Natarajan R, et al. Anti-inflammatory effects of the advanced glycation end product inhibitor LR-90 in human monocytes. Diabetes. 2007;56:647–655. doi: 10.2337/db06-0936. [DOI] [PubMed] [Google Scholar]
- 19.Shanmugam N, Kim YS, Lanting L, et al. Regulation of cyclooxygenase-2 expression in monocytes by ligation of the receptor for advanced glycation end products. J Biol Chem. 2003;278:34834–34844. doi: 10.1074/jbc.M302828200. [DOI] [PubMed] [Google Scholar]
- 20.Shanmugam N, Ransohoff RM, Natarajan R. Interferon-gamma-inducible protein (IP)-10 mRNA stabilized by RNA-binding proteins in monocytes treated with S100b. J Biol Chem. 2006;281:31212–31221. doi: 10.1074/jbc.M602445200. [DOI] [PubMed] [Google Scholar]
- 21.Devaraj S, Glaser N, Griffen S, et al. Increased monocytic activity and biomarkers of inflammation in patients with type 1 diabetes. Diabetes. 2006;55:774–779. doi: 10.2337/diabetes.55.03.06.db05-1417. [DOI] [PubMed] [Google Scholar]
- 22.Emanuele E, Falcone C, D’Angelo A, et al. Association of plasma eotaxin levels with the presence and extent of angiographic coronary artery disease. Atherosclerosis. 2006;186:140–145. doi: 10.1016/j.atherosclerosis.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 23.Guha M, Bai W, Nadler JL, et al. Molecular mechanisms of tumor necrosis factor alpha gene expression in monocytic cells via hyperglycemia-induced oxidant stress-dependent and -independent pathways. J Biol Chem. 2000;275:17728–17739. doi: 10.1074/jbc.275.23.17728. [DOI] [PubMed] [Google Scholar]
- 24.Ishii H, Koya D, King GL. Protein kinase C activation and its role in the development of vascular complications in diabetes mellitus. J Mol Med. 1998;76:21–31. doi: 10.1007/s001090050187. [DOI] [PubMed] [Google Scholar]
- 25.Nishikawa T, Edelstein D, Du XL, et al. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature. 2000;404:787–790. doi: 10.1038/35008121. [DOI] [PubMed] [Google Scholar]
- 26.Miyata T, Sugiyama S, Suzuki D, et al. Increased carbonyl modification by lipids and carbohydrates in diabetic nephropathy. Kidney Int. 1999;(Suppl 71):S54–S56. doi: 10.1046/j.1523-1755.1999.07114.x. [DOI] [PubMed] [Google Scholar]
- 27.Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 28.Miyata T, Fu MX, Kurokawa K, van Ypersele de Strihou C, Thorpe SR, Baynes JW. Autooxidation products of both carbohydrates and lipids are increased in uremic plasma: is there oxidative stress in uremia? Kidney Int. 1998;54:1290–1295. doi: 10.1046/j.1523-1755.1998.00093.x. [DOI] [PubMed] [Google Scholar]
- 29.Yan SD, Schmidt AM, Anderson GM, et al. Enhanced cellular oxidant stress by the interaction of advanced glycation end products with their receptors/binding proteins. J Biol Chem. 1994;269:9889–9897. [PubMed] [Google Scholar]
- 30.Feldman M, Durum SK, Hirano T, et al. Cytokine Reference: A Compendium of Cytokines and Other Mediators of Host Defense. 1–2. London: Academic Press; 2001. [Google Scholar]
- 31.Ohgami N, Nagai R, Ikemoto M, et al. CD36, serves as a receptor for advanced glycation endproducts (AGE) J Diabetes Complications. 2002;16:56–59. doi: 10.1016/s1056-8727(01)00208-2. [DOI] [PubMed] [Google Scholar]
- 32.Sakata N, Uesugi N, Takebayashi S, et al. Glycoxidation and lipid peroxidation of low-density lipoprotein can synergistically enhance atherogenesis. Cardiovasc Res. 2001;49:466–475. doi: 10.1016/s0008-6363(00)00262-5. [DOI] [PubMed] [Google Scholar]
- 33.Haley KJ, Lilly CM, Yang JH, et al. Overexpression of eotaxin and the CCR3 receptor in human atherosclerosis: using genomic technology to identify a potential novel pathway of vascular inflammation. Circulation. 2000;102:2185–2189. doi: 10.1161/01.cir.102.18.2185. [DOI] [PubMed] [Google Scholar]
- 34.Otterdal K, Smith C, Oie E, et al. Platelet-derived LIGHT induces inflammatory responses in endothelial cells and monocytes. Blood. 2006;108:928–935. doi: 10.1182/blood-2005-09-010629. [DOI] [PubMed] [Google Scholar]
- 35.Schutyser E, Richmond A, Van Damme J. Involvement of CC chemokine ligand 18 (CCL18) in normal and pathological processes. J Leukoc Biol. 2005;78:14–26. doi: 10.1189/jlb.1204712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kumagai T, Matsukawa N, Kaneko Y, et al. A lipid peroxidation-derived inflammatory mediator: identification of 4-hydroxy-2-nonenal as a potential inducer of cyclooxygenase-2 in macrophages. J Biol Chem. 2004;279:48389–48396. doi: 10.1074/jbc.M409935200. [DOI] [PubMed] [Google Scholar]
- 37.Kanayama M, Yamaguchi S, Shibata T, et al. Identification of a serum component that regulates cyclooxygenase-2 gene expression in cooperation with 4-hydroxy-2-nonenal. J Biol Chem. 2007;282:24166–24174. doi: 10.1074/jbc.M703212200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.