Abstract
Background
Gelsolin is a plasma protein that functions to depolymerize actin filaments preventing capillary plug formation following tissue injury. It also functions to mediate the inflammatory response by binding proinflammatory lipids such as lysophosphatidic acid, sphingosine-1-phosphate and phosphoinositides. Clinically, reduced gelsolin concentrations have been associated with increased mortality in critically ill, trauma and burn patients. We have previously shown that following hemorrhagic shock with splanchnic hypoperfusion, mesenteric lymph contains lipid components that cause neutrophil and EC activation and that protein concentrations are severely diluted due to resuscitation. We hypothesized that lipid binding proteins such as gelsolin may be depleted after trauma/hemorrhagic shock leading to increased lipid bioactivity.
Methods
Shock was induced in SD rats by controlled hemorrhage and the mesenteric duct cannulated for lymph collection. Resuscitation was performed by infusing 2x SB volume in NS over 30min, followed by ½ SB volume over 30min, then 2x SB volume in NS over 60min. Pre and post-shock lymph was loaded at equal protein concentrations on 2D-gels, followed by trypsin digestion and identification with mass spectrometry (MS-MS). Proteomics data was confirmed with Western blotting then quantitated by densitometry. ANOVA was used evaluate statistical data.
Results
Gelsolin decreased in mesenteric lymph following hemorrhagic shock.
Conclusion
Gelsolin is found at high levels (comparable to plasma) in mesenteric lymph. Following hemorrhagic shock, gelsolin levels decrease significantly possibly due to consumption by the actin scavenging system. The magnitude of this change in concentration could release lipid bioactivity and predispose the lung and other organs to capillary injury.
Keywords: gelsolin, lymph, proteomics, hemorrhagic shock, actin, mesenteric lymph, post-shock mesenteric lymph, actin scavenging system
Introduction
Post-shock mesenteric lymph (PSML) has been shown to be a conduit via which mediators elaborated by the ischemic gut can provoke distant organ injury after hemorrhagic shock(1). Deitch and colleagues demonstrated that diversion of the mesenteric lymphatics from the thoracic duct before hemorrhagic shock reduced the severity of post-shock acute lung injury(2). Further work from our laboratory has shown that bioactive mediators in PSML can prime neutrophils for augmented superoxide release and can up-regulate ICAM-1 expression in the pulmonary endothelium(3–5). Additionally, prior investigations from our lab have also demonstrated that the overall protein concentration in PSML changes dramatically in that there is a greater than 10-fold decrease in overall protein concentration in the 3rd hour following resuscitation compared to the preshock lymph(6). Consequently, the current investigation of mesenteric lymph has focused primarily on identifying the specific bioactive elements responsible, and in understanding the mechanism by which hemorrhagic shock and resuscitation generates PSML toxicity(7–9). In this study, we focused on the differences between specific proteins in preshock mesenteric lymph and PSML.
Gelsolin is a protein found in both the cytosol and the plasma that has been reported to function primarily as a part of the actin-scavenging system with Gc globulin(10). Cellular disruption or necrosis releases cellular actin, both globular and filamentous fractions, into the circulation which results in capillary plug formation with subsequent tissue ischemia. The plasma actin-scavenging system functions to cleave the filaments, cap the ends preventing repolymerization and assist in the plasma clearance of filamentous actin. Additionally, gelsolin is known for its ability to bind lipids such as lipopolysaccharide, lysophosphatidic acid and phosphotidylinositol (11, 12) and may modulate the inflammatory system. Clinically, plasma gelsolin levels have been shown to decrease following blunt trauma(13). In fact, levels less than 25% of normal (or greater than 2 standard deviations) have been associated with increased incidence of ARDS and increased mortality(14). Experimentally, animals subjected to 40% TBSA burns demonstrated attenuated acute lung injury with the infusion of recombinant gelsolin to normal plasma levels (15). Therefore, we hypothesized that gelsolin is reduced in the mesenteric lymph following hemorrhagic shock and resuscitation and participates in modulating lipid activity.
Using our previously established hemorrhagic shock animal model, we have used quantitative proteomics and immunoblotting to determine relative gelsolin levels in the PSML. The results of our study confirm that the gelsolin concentration is decreased significantly in PSML following shock and resuscitation.
Materials and Methods
Animals
The Institutional Animal Care and Use Committee approved all experiments performed on animals. All products were purchased from Sigma-Aldrich Corp. (St. Louis, MO) unless otherwise specified. Pentobarbital sodium was purchased from Abbott Labs (North Chicago, IL). Polyethylene tubing (PE) was purchased from Intrametic, Fisher Scientific. Heparin was purchased from American Pharmaceutical Partner, Inc (Schaumburg, IL). Precast 8–16% SDS-PAGE gels for western blotting were purchased from Pierce Biotechnology, Inc (Rockford, IL). Nitrocellulose membranes were purchased from Bio-Rad Laboratories (Hercules, CA).
Hemorrhagic shock
Male Sprague-Dawley rats weighing 281mg to 351mg (Colorado State University) were housed in a climate controlled barrier facility with 12hr light/dark cycles with free access to food and water. Each were anesthetized with 50mg/kg Pentobarbital sodium via intraperitoneal injection. The femoral artery and vein were then cannulated with PE 50 tubing and the blood pressure and mean arterial pressure were monitored using a ProPaq invasive monitoring device. A separate skin puncture was created to tunnel the catheters prior to closure of the groin incision. A 3cm midline laparotomy incision was then made just below the xyphoid process. The bowel was eviscerated and rotated to the left. The mesenteric duct and accessory duct (located adjacent to the superior mesenteric artery) were then isolated from the surrounding structures using blunt dissection. The main lymphatic duct was cannulated with PE 100 tubing and secured with 7-0 prolene suture. The accessory duct was then ligated with suture. The catheter was tunneled posteriorly through the skin and the laparotomy incision was closed in a 2-layer fashion. Lymph was collected on ice at 30min intervals and then centrifuged at 5000g for 10min. The supernatant was collected and frozen in liquid nitrogen. After 1hr of lymph collection (preshock lymph), hemorrhagic shock was induced via controlled hemorrhage from the arterial catheter. Blood was withdrawn or returned in order to maintain a MAP of 30mmHg for 40min. Body temperature was monitored rectally and maintained at euthermic levels with a heat lamp. At the completion of the shock period, resuscitation was then initiated with 2x shed blood volume returned in normal saline over 30min, then ½ shed blood volume over 30min, and completed with 2x shed blood volume in normal saline over 1hr via the femoral vein. Lymph was then collected for an additional hour following completion of resuscitation (3rd hour lymph). The animals were then euthanized via pentobarbital overdose. Each catheter was irrigated with heparinized saline prior to insertion. All lymph samples were stored at −80°C until processing.
Immunoblotting
The collected lymph was then analyzed at hourly increments. Protein quantification was performed using the BCA protein analysis method with bovine serum albumin standards to create a regression analysis and estimate overall protein concentration of each hourly sample. Aliquots of each sample were then created to have equal protein concentrations at equal volumes using nanopure water and digestion buffer and boiled at 100°C for 10min. The samples where loaded onto precast SDS-PAGE gels which were run at 100mV for 1hr in tris-hepes running buffer, then transferred to nitrocellulose paper at 100mV for 1hr. The nitrocellulose membrane was probed using anti-Gelsolin antibody (Santa Cruz Biotech; Santa Cruz, CA), anti-F actin antibody (Santa Cruz Biotech, Santa Cruz, Ca) and anti-Pan-actin Antibody (Cell Signaling Technology, Danvers, MA),. Standardization of each protein was performed by comparison to overall protein transferred via ponceau stain and analyzed with densitometry. Purified actin standards obtained from human platelets (Cytoskeleton, Inc; Denver, CO) were included on the gel in known concentrations. A regression analysis was performed on the densitometry of the standards and the actin concentrations in the lymph were then estimated using this equation.
Proteomics: 2D gel electrophoresis
All procedures were performed following the manufacturer’s protocol and supplies were purchased from Amersham Biosciences/GE Healthcare unless otherwise stated. Fifty μg of protein sample was minimally labeled with either a Cy3 or Cy5 Dye on ice for 30 minutes. Cy3- and Cy5-labeled samples were combined and brought to a final volume of 450μl in rehydration buffer (7M urea, 2M thiourea, 4% CHAPS, 40mM DTT, 0.002% bromophenol blue and 0.5% pharmalytes). The combined sample was passively rehydrated overnight onto a 24cm immobilized pH gradient strip (IPG 3–10 non-linear) and then isoelectric focused using the following parameters: 500V for 100 Vhr, 1,000V for 1,000 Vhr, and 8,000V for 62,500 Vhr on an IPGphor apparatus. IPG strips were equilibrated for 15minutes in a DTT solution, followed by reaction with iodoacetamide solution for 15minutes to reduce and alkylate cysteine residues. Strips were then applied to a precast 8–16% SDS-PAGE gel (Jule Inc., Milford, CT) for electrophoresis at a constant 20W/gel at 25°C in an Ettan DALT 12 electrophoresis unit. Labeled proteins were visualized by scanning the gels at 100μm resolution and appropriate wavelengths for Cy3 and Cy5 fluorescence using the Typhoon 9400 scanner.
Image Analysis
DeCyder software (v. 6.5, Amersham Biosciences, Piscataway, NJ) was used for spot detection and relative quantification of proteins based on the fluorescence images. Peak volumes were measured for each protein spot in the two fluorescent channels (Cy3 and Cy5). Volume ratios were calculated to directly compare abundance of each protein spot in the pre and post-shock pools. Proteins to be excised and identified were selected using the DeCyder Differential In-gel Analysis (DIA) Module.
Spot Picking and Enzymatic Digestion
Protein spot excision and in-gel enzymatic digestion were performed automatically by the Ettan Spot Handling Workstation (Amersham Biosciences, Piscataway, NJ). Spots were excised from the gel with a 2.0mm picking head, and the excised spots transferred to 96-well plates. After the picking was completed, the plates were transferred to the digester where the plugs were washed twice with 100μL of 50mM NH4HCO3/50% methanol for 5min, once with 100μL of 75% acetonitrile for 10min, and once with 100μL of 100% acetonitrile for 10min. The gel plugs were dried at room temperature for 50min, and then trypsin (Promega, San Luis Obispo, CA) was added to each well (10μL, 10μg/μL in 20mM NH4HCO3), and digestion proceeded at room temperature for 16hours.
Protein Identification by Mass Spectrometry
All digests were analyzed using a MALDI-TOF/TOF mass spectrometer (4700, Applied Biosystems, Foster City, CA) and spectra were collected over the range m/z 500–5000. No cleanup of digests was performed. Peptide mass fingerprints were internally calibrated to monoisotopic trypsin autolysis peaks (m/z = 515.33, 842.51, 1045.56, 2211.11). Spectra were processed using the instrument supplied software (Explorer v1.1) to generate centroided peak lists that were submitted to Mascot (v 2.1, Matrix Science, Boston, MA) and Protein Prospector (v 4.21.1, UCSF, CA) for database searching. Spectral processing included defining baseline, noise, and signal-to-noise ratio as well as monoisotopic peak selection. Database searches were conducted using a rodent subset of the non-redundant protein database (NCBI, database version 05/07/2006 with 3625149sequences and 1246451978residues). Key parameters included the following: peptide error tolerance was set at +/− 60ppm, and 0.3 Da for fragment tolerance. Fixed modification of carbamidomethylation of cysteine side chains, and variable modifications of peptide N-terminal Gln to pyroGlu, oxidation of Met, protein N-terminus acetylation and trypsin selected as the enzyme for peptide cleavage with two missed cleavages accepted. Searches were not constrained by pI or molecular weight.
ANOVA was used evaluate statistical data between groups.
Results
Overall lymph production averaged 2.3μL/mg in the preshock period. This increased to an average of 12.1μL/mg in the last hour following resuscitation. Our initial inquiry regarded changes in the specific components of the PSML. Via quantitative 2D-gel electrophoresis using the DIGE technology (Figure 1) and subsequent MS/MS identification, a list of several proteins that had greater than a two-fold change in concentration when standardized for equal protein loading was compiled. Gelsolin was found to decrease 2-fold in mesenteric lymph following hemorrhagic shock and this was confirmed with Western blotting (see figure 2a and 2b, *p<0.01; n=6). Since gelsolin is a known component of the actin scavenging system, we sought to determine the change in actin concentration as well. This was also performed by Western blotting. The filamentous actin concentration in the preshock lymph was approximately 111.1ng/μg/μL of total protein concentration and remained stable in the PSML at 109.5ng/μg/μLof total protein concentration. The pan-actin concentration increased only slightly from an average of 136.7ng/μg/μLof total protein concentration to 148.2ng/μg/μL of total protein concentration (see Figure 3).
Figure 1. 2D gel electrophoresis of PSML.
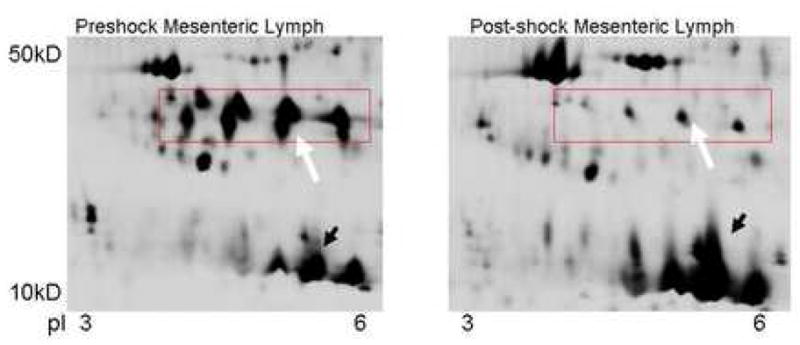
2D gel electrophoresis illustrates differences in several proteins despite equal protein loading. Although, many proteins decreased in the post-shock sample (box with white arrow) some proteins increase in concentration (black arrow) suggesting a selective process. Computerized and manual spot picking were used to identify proteins with significant changes when comparing the pre and post-shock samples which were then analyzed via mass spectrophotometry.
Figure 2.

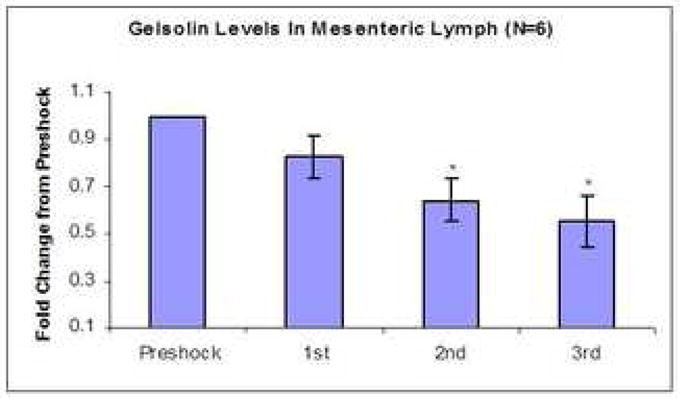
Figure 2a: Western blot of gelsolin in PSML. Each hourly sample was loaded on the SDS-PAGE gel at equal protein concentrations. Analysis by densitometry using a loading control from the ponceau stain shows sequentially decreasing levels in each hour of the post-shock samples.
Figure 2b: Average lymph gelsolin levels. Lymph samples were loaded on SDS-PAGE gels at equal protein concentrations and compared to subsequent hours during and after resuscitation. The overall gelsolin levels decrease sequentially with each subsequent hour of resuscitation and continue to fall after completion of resuscitation during the final hour of the experiment (*p<0.01, n=6).
Figure 3. Western blot of actin in mesenteric lymph.
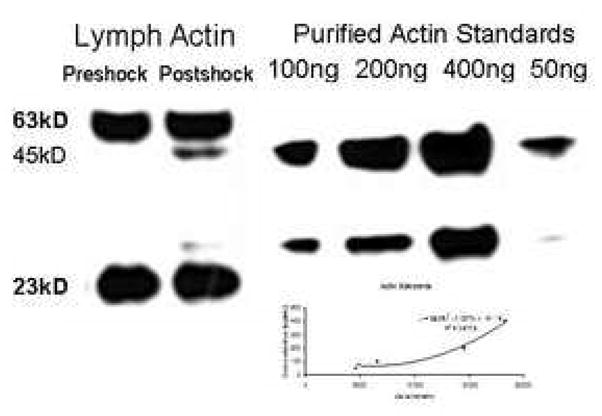
Preshock and PSML were added to precast SDS-PAGE gels at equal protein concentrations. An initial probe was performed to analyze the filamentous portion of actin which appeared as the high and low bands. The membrane was stripped and re-probed for total actin. The high and low bands were again seen, but the globular units also became apparent and 45kD. An increase in actin fragments were visualized in the post-shock sample; however, the overall concentration did not change significantly from preshock values. Actin standards were added to the gel in known concentrations for regression analysis and quantification of actin in the PSML (graph).
Discussion
Mesenteric lymph has been implicated as the mechanistic link by which bioactive mediators elaborated from the ischemic gut during hemorrhagic shock can provoke distant organ injury(1, 2). Collectively, basic bench work with rodent models of hemorrhagic shock from Deitch, et al and our lab have shown PSML to contain factors that can incite acute lung injury through mechanisms of endothelial inflammation and apoptosis as well as neutrophil priming(3–5, 16–20). Current research in this area has emphasized identifying the toxic bioactive mediators in PSML. Initial investigations eliminated the seemingly obvious molecules such as TNF, LPS and proinflammatory cytokines(17, 21). Therefore, given the enormous amount of possible biologic mediators in the lymph, we began our analysis with a global evaluation using a quantitative proteomics approach. This provided an armamentarium of information for further focused studies. Although the results of this current study are observational, we now have an initiating focus to delineate the causative agent(s) and specific components of lymph that contribute to the bioactivity. Previously, we have reported the identification of a neutral phospholipase A2-derived lipid as a potential mediator found in the PSML with proinflammatory activity(8). Conceivably, this lipid mediator may be present in the inactive preshock lymph possibly bound to a carrier protein. The depletion of specific proteins exacerbated by dilution from resuscitation may allow the unbound lipid fraction to increase in effective concentration thereby becoming biologically active. This theory led us to employ proteomics to pursue the identity of lipid-binding proteins that change following hemorrhagic shock and resuscitation.
These results illustrate some of the strengths and disadvantages of proteomic analysis of lymph. Few proteins (albumin, globulins, etc) account for over 80% of the proteinaceous content of the lymph. Therefore, the enormous dynamic range poses a challenge to identifying less abundant proteins. Color differences of fluorescently tagged proteins in preshock vs post-shock lymph, though accurate, often cannot be appreciated with the naked eye. Thus, figure 1 displays a section where the depletion or enrichment of specific proteins can be easily discerned on grayscale, outside the area of the tails of prominent proteins. Where consistent differences can be detected (by quantitative fluorescent contrasts), confident identification of a particular protein remains dependent on being able to generate sufficient fragments (tryptic digests), successful sequencing, and rigorous matching to known proteins within available databases. The identification of gelsolin met all the criteria above including a consistent 2-fold change.
Gelsolin, a known clinical marker(13, 14), was identified to have a significant change in PSML. It is an interesting molecule with several physiologic capabilities that may explain its contribution to lymph bioactivity. Intracellularly, gelsolin (MW 90kD) is regulated by calcium and functions to cleave, then cap actin filaments thereby aiding in chemotaxis and movement of intracellular structures. Plasma gelsolin (MW 93kD) contains an additional 25-amino acid residue at the amino terminus and is believed to be primarily secreted by muscle cells(29). It functions as an actin scavenger by binding actin in the circulation(26), then the complex is cleared by the reticular endothelial system(30). Gelsolin also functions as a lipid carrier with strong binding affinity to several cystolic and plasma lipids such as phosphotidylinositol, lipopolysaccharide, and lysophosphatidic acid(11, 12). The biologic consequences of this particular action are still under investigation.
In our current investigation, gelsolin was found to be present in preshock mesenteric lymph at concentrations that mirror the plasma. Following hemorrhagic shock, gelsolin levels decrease significantly in the lymph. Our results support previous findings of the depletion of gelsolin following significant injury and seen in critically ill patients with increased mortality(13, 22). These clinical investigations have shown that plasma gelsolin concentrations continue to decrease 24hrs to as long as 5days following injury in human subjects(13, 14, 22). This depletion correlates with the fall in other plasma proteins such as albumin; however, the recovery of other proteins is much quicker than that of gelsolin(13, 14) suggesting a specific depletion and lack of replenishment. It may be that the critical level of gelsolin has not yet been reached in our 3rd hour lymph following resuscitation and the actin scavenging system has not been overwhelmed thereby leading to maintained “normal” levels of actin at this particular time point. The eventual consumption may prevent the secondary activity of gelsolin and increase lipid bioactivity possibly predisposing the lung and other organs to endothelial injury.
In vitro experiments have shown decreased inflammatory changes and cellular injury with the addition of gelsolin to samples(28). This has been further supported by in vivo experiments including rats sustaining significant burn injury that have been conducted with the addition of recombinant gelsolin and have shown attenuated lung injury in these subjects(15).
The diverse activity of this particular molecule makes it an interesting culprit in the investigation of mesenteric lymph toxicity. It provides a possible explanation of the precise mechanism of how depletion of gelsolin is associated with a worsened clinical outcome either as a result of tissue injury secondary to impaired actin scavenging or a lack of toxic lipid modulation. More importantly, this provides a potential target to the development of a pharmacotherapy for the treatment or possibly prevention of post-injury multiple organ failure.
In summary, the results of the current study demonstrate the presence and reduction of gelsolin in the PSML. The depletion may be a result of a consumptive process due to actin scavenging to prevent capillary plugging and tissue ischemia. However, this consumptive process may prevent a secondary action of lipid binding and may account for the lipid bioactivity that contributes to the toxicity of PSML and post-injury multiple organ failure.
Acknowledgments
This study was supported in part by the National Institute of Health (grant T32-GM008315 and P50-GM49222)
Footnotes
Presented at the 2nd Annual Academic Surgical Congress February 2007, Phoenix, AZ.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Magnotti LJ, Upperman JS, Xu D, Deitch EA. Gut-derived Mesenteric Lymph but not Portal Blood Increases Endothelial Cell Permeability and Promotes Lung Injury After Hemorrhagic Shock. Ann Surg. 1998;228:518–527. doi: 10.1097/00000658-199810000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Deitch EA, Adams C, Lu Q, Xu DZ. A time course study of the protective effect of mesenteric lymph duct ligation on hemorrhagic shock-induced pulmonary injury and the toxic effects of lymph from shocked rats on endothelial cell monolayer permeability. Surgery. 2001;129 doi: 10.1067/msy.2001.109119. [DOI] [PubMed] [Google Scholar]
- 3.Gonzalez RJ, Moore EE, Ciesla DJ, Biffl WL, Johnson JL, Silliman CC. Mesenteric Lymph is responsible for post-hemorrhagic shock systemic neutrophil priming. J Trauma. 2001;51:1069. doi: 10.1097/00005373-200112000-00008. [DOI] [PubMed] [Google Scholar]
- 4.Gonzalez RJ, Moore EE, Ciesla DJ, Nieto JR, Johnson JL, Silliman CC. Post-hemorrhagic shock mesenteric lymph activated human pulmonary microvascular endothelium for in vitro neutrophil-mediated injury: The role of intercellular adhesion molecule-1. J Trauma. 2003;54:219. doi: 10.1097/01.TA.0000047807.12644.95. [DOI] [PubMed] [Google Scholar]
- 5.Sarin EL, Moore EE, Moore JB, et al. Systemic neutrophil priming by lipid mediators in post-shock mesenteric lymph exists across species. J Trauma. 2004;57:950. doi: 10.1097/01.ta.0000149493.95859.6c. [DOI] [PubMed] [Google Scholar]
- 6.Cheng AM, Moore EE, Masuno T, Escobar GA, Sarin EL, Johnson JL, Eckels P, Banerjee A. Normal Mesenteric Lymph Blunts the Pulmonary Inflammatory Response to Endotoxin. J Surg Res. 2006;136:166 – 171. doi: 10.1016/j.jss.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 7.Dayal SD, Hauser CJ, Feketeova E, et al. Shock mesenteric lymph-induced rat polymorphonuclear neutrophil activation and endothelial cell injury is mediated by aqueous factors. J Trauma. 2002;52:1048. doi: 10.1097/00005373-200206000-00005. [DOI] [PubMed] [Google Scholar]
- 8.Gonzalez RJ, Moore EE, Ciesla DJ, Biffl WL, Offner PJ, Silliman CC. Phospholipase A2-derived neutral lipids from posthemorrhagic shock mesenteric lymph prime the neutrophil oxidative burst. Surgery. 2001;130:198 – 203. doi: 10.1067/msy.2001.115824. [DOI] [PubMed] [Google Scholar]
- 9.Gonzalez RJ, Moore EE, Ciesla DJ, Meng X, Biffl WL, Silliman CC. Post-hemorrhagic shock mesenteric lymph lipids prime neutrophils for enhanced cytotoxicity via phospholipase A2. Shock. 2001;16:218. doi: 10.1097/00024382-200116030-00008. [DOI] [PubMed] [Google Scholar]
- 10.Sun HQ, Yamamoto M, Mejillano M, Yin HL. Gelsolin, a Multifunctional Actin Regulatory Protein. J Biol Chem. 1999;274:33179 – 33182. doi: 10.1074/jbc.274.47.33179. [DOI] [PubMed] [Google Scholar]
- 11.Goetzl EJ, Lee H, Azuma T, Stossel TP, Turck CW, Karliner JS. Gelsolin Binding and Cellular Presentation of Lysophosphatidic Acid. J Biol Chem. 2000;275:14573 – 14578. doi: 10.1074/jbc.275.19.14573. [DOI] [PubMed] [Google Scholar]
- 12.Karliner JS, Honbo N, Summers K, Gray MO, Goetzl EJ. The Lysophospholipids Sphingosine-1-Phosphate and Lysophosphatidic Acid Enhance Survival during Hypoxia in Neonatal Rat Cardiac Myocytes. J Mol Cell Card. 2001;33:1713 – 1717. doi: 10.1006/jmcc.2001.1429. [DOI] [PubMed] [Google Scholar]
- 13.Mouzner KC, Moncure M, Smith YR, DiNubile MJ. Relationship of Admission Plasma Gelsolin Levels to Clinical Outcomes in Patients after Major Trauma. Am J Resp Crit Care Med. 1999;160:1673 – 1681. doi: 10.1164/ajrccm.160.5.9807137. [DOI] [PubMed] [Google Scholar]
- 14.Lee P, Drager LR, Stossel TP, Moore FD, Rogers SO. relationship of Plasma Gelsolin Levels to Outcomes in Critically Ill Surgical Patients. Ann Surg. 2006;243:399 – 403. doi: 10.1097/01.sla.0000201798.77133.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rothenbach PA, Dahl B, Schwartz JJ, O’Keefe GE, Yamamoto M, Lee WM, Horton JW, Yin HL, Turnage RH. Recombinant plasma gelsolin infusion attenuates burn-induced pulmonary microvascular dysfunction. J Appl Phys. 2004;96:25 – 31. doi: 10.1152/japplphysiol.01074.2002. [DOI] [PubMed] [Google Scholar]
- 16.Lu Q, Xu D, Davidson MT, Hasko G, Deitch EA. Hemorrhagic shock induces endothelial cell apoptosis, which is mediated by factors contained in mesenteric lymph. Crit Care Med. 2004;32:2464 – 2470. doi: 10.1097/01.ccm.0000147833.51214.03. [DOI] [PubMed] [Google Scholar]
- 17.Davidson MT, Deitch EA, Lu Q, et al. A study of the biologic activity of trauma-hemorrhagic shock mesenteric lymph over time and the relative role of cytokines. Shock. 2004;136:32. doi: 10.1016/j.surg.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 18.Davidson MT, Deitch EA, Lu Q, et al. Trauma-hemorrhagic shock mesenteric lymph induces endothelial apoptosis that involves both caspase-dependent and caspase-independent mechanisms. Ann Surg. 2004;240:123. doi: 10.1097/01.sla.0000129341.94219.cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Caruso JM, Feketeova E, Dayal SD, Hauser CJ, Deitch EA. Factors in intestinal lymph after shock increase neutrophil adhesion molecule expression and pulmonary leukosequestration. J Trauma. 2003;55:727. doi: 10.1097/01.TA.0000037410.85492.77. [DOI] [PubMed] [Google Scholar]
- 20.Upperman JS, Deitch EA, Guo W, Lu Q, Xu D. Post-hemorrhagic shock mesenteric lymph is cytotoxic to endothelial cells and activates neutrophils. Shock. 1998;10:407. doi: 10.1097/00024382-199812000-00005. [DOI] [PubMed] [Google Scholar]
- 21.Benoit R, Rowe S, Watkins SC, Boyle P, Garrett M, Alber S, Wiener J, Rowe MI, Ford HR. Pure endotoxin does not pass across the intestinal epithelium in vitro. Shock. 1998;10:43 – 48. doi: 10.1097/00024382-199807000-00008. [DOI] [PubMed] [Google Scholar]
- 22.Suhler E, Lin W, Yin HL, Lee WM. Decreased plasma gelsolin concentrations in acute liver failure, myocardial infarction, septic shock, and myonecrosis. Crit Care Med. 1997;25:594 – 598. doi: 10.1097/00003246-199704000-00007. [DOI] [PubMed] [Google Scholar]
- 23.Deitch EA. Multiple Organ Failure Pathophysiology and Potential Future Therapy. Ann Surg. 1992;216:117 – 134. doi: 10.1097/00000658-199208000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Masuno T, Moore EE, Cheng AM, Sarin EL, Banerjee A. Bioactivity of postshock mesenteric lymph depends on the depth and duration of hemorrhagic shock. Shock. 2006;26:285 – 289. doi: 10.1097/01.shk.0000223132.72135.52. [DOI] [PubMed] [Google Scholar]
- 25.Tou J, Gill JS. Lysophosphatidic acid increases phosphatidic acid formation, phospholipase D activity and degranulation by human neutrophils. Cell Sig. 2005;17:77 – 82. doi: 10.1016/j.cellsig.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 26.Way M, Pope B, Weeds AG. Evidence for Functional Homology in the F-actin Binding Domains of Gelsolin and alpha-Actinin: Implications for the Requirements of Severing and Capping. J Cell Biol. 1992;119:835 – 842. doi: 10.1083/jcb.119.4.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zallen G, Moore EE, Johnson JL, Tamura DY, Ciesla DJ, Silliman CC. Posthemorrhagic Shock Mesenteric Lymph Primes Circulating Neutrophils and Provokes Lung Injury. J Surg Res. 1999;83:83 –88. doi: 10.1006/jsre.1999.5569. [DOI] [PubMed] [Google Scholar]
- 28.Bucki R, Georges PC, Espinassous Q, Funaki M, Pastore JJ, Chaby R, Janmey PA. Inactivation of Endotoxin by Human Plasma Gelsolin. Biochem. 2005;44:9590. doi: 10.1021/bi0503504. [DOI] [PubMed] [Google Scholar]
- 29.Kwistkowski DJ, Mehl R, Izumo S, Nadal-Ginard B, Yin HL. Muscle is the major source of plasma gelsolin. J Biol Chem. 1988;263:8239. [PubMed] [Google Scholar]
- 30.Lind SE, Smith DB, Janmey PA, Stossel TP. Role of Plasma Gelsolin and the Vitamin D-binding Protein in Clearing Actin from the Circulation. J Clin Invest. 1986;78:736. doi: 10.1172/JCI112634. [DOI] [PMC free article] [PubMed] [Google Scholar]


