Abstract
Access to effective antimalarial therapy has increased dramatically. As malaria-endemic countries begin to use artemisinin-based combination therapy (ACT) to treat malaria, detecting the emergence and spread of resistance has become more complicated but also more urgent. Clinical efficacy studies may not be sensitive enough to detect the failure of a single component of combination therapy while standardized criteria for in vitro resistance and validated molecular markers are not yet available to many currently deployed drugs. This review discusses the challenges to various methods to monitor antimalarial drug resistance and proposes an integrated approach to the rapid detection and characterization of resistance to ACTs should it arise.
Introduction
Resistance to antimalarial drugs has fueled the ongoing malaria epidemic in sub-Saharan Africa. Until recently, only chloroquine or sulfadoxine-pyrimethamine (SP) were available to treat uncomplicated malaria, despite widespread resistance. The reasons for persistent use of failing antimalarials are complex and include cost, safety and tolerability of alternative medications, ease of administration, and maintenance of adequate supply. Strategies to monitor antimalarial drug resistance were developed when single drugs were used to treat malaria infections. Parasite resistance was identified and characterized only after resistance became widespread.
We are entering a new era in antimalarial therapy. Multilateral support has allowed for the introduction of more effective medication. Currently, malaria-endemic African countries have adopted artemisinin-based combination therapy (ACT) for treating uncomplicated malaria. The artemisinins are highly effective, short-acting drugs derived from Artemisia annua, a plant found in China. Although extract from the plant has been used to treat malaria in China for thousands of years, it was recognized by the international community and became commercially available outside China in the 1980s. Because of their short duration of activity, artemisinins are combined with longer-acting partner drugs in ACTs including artemether-lumefantrine, artesunate-amodiaquine, artesunate-mefloquine, and dihydroartemisinin-piperaquine.
Three strategies have been used to assess the ability of antimalarial drugs to treat malaria infections: clinical in vivo studies, in vitro susceptibility testing, and molecular markers. In discussing these different strategies, it is important to distinguish intrinsic parasite resistance from decreased clinical efficacy. Resistance refers to the ability of a drug to prevent parasite growth in culture, at defined drug concentrations, and in the absence of the human immune response. Changes in efficacy are detected through clinical studies in which parasite intrinsic susceptibility is one of many factors determining outcome. In this review, we discuss the inherent difficulties in interpreting the results of each method and the unique challenges presented by current treatment strategies.
Clinical Studies
In vivo studies are used to assess the clinical efficacy of antimalarial drugs. Patients with malaria illness are enrolled in a study and followed at regular intervals. The World Health Organization (WHO) updates the standard protocol as consensus evolves about the study methodology and end points. According to the current protocol, enrollment occurs on day 0 and follow-up visits take place on days 1, 2, 3, 7, 14, 21, and 28 and any time the patient is ill [1]. Some authorities suggest that longer follow-up is more sensitive in detecting low levels of resistance by capturing episodes of recurrent parasitemia that occur more than 4 weeks after the treatment episode [2].
A successful treatment outcome in a drug efficacy study is adequate clinical and parasitologic response. Failure to have a significant decrease in parasite density or persistent fever in the presence of parasites during the first 3 days of treatment is classified as early treatment failure. Recurrent detectable parasitemia from day 4 until the study’s end is classified as late treatment failure. Treatment failure that occurs with symptomatic infection, usually fever, is often referred to as clinical failure to distinguish it from parasitologic failure that does not take into account the patient’s symptoms.
Recent revisions to the efficacy study procedures have focused on distinguishing recrudescent from new infections in episodes of recurrent parasitemia that occur during the follow-up period. After treatment, the parasites in the blood can fall below the level of detection by microscopy but a small number of resistant parasites may continue to replicate and grow to a detectable level. This is considered a recrudescent infection. In contrast, a malaria infection may be due to a new infectious bite that occurred during the follow-up period. These are differentiated by genotyping of highly polymorphic genes encoding merozoite surface protein (msp) 1 and 2 and the glutamine-rich protein (glurp). Recrudescent infections are considered “true” treatment failures because the parasites survived antimalarial therapy, whereas individuals who experience new infections are treated for their infection and censored from follow-up. The WHO has recently determined that polymerase chain reaction (PCR)—correcting drug efficacy outcomes (using genotyping to distinguish recrudescent from new infections) should be included in reported results [3].
Conducting a drug efficacy study is a demanding undertaking. It requires devoted personnel to interact with patients, accurately quantify parasitemia density, and follow up patients who fail to return to the clinic for their scheduled visits. Complete results also require PCR analysis. Health infrastructure, budget, and human resources in malaria-endemic countries cannot support frequent assessments or trials in multiple locales. This is problematic because efficacy changes (usually decreases) over short time periods and may vary at different locations in a single country.
Even with a well-performed clinical study, treatment failures may be due to mechanisms other than intrinsic parasite resistance. In addition to the drug’s effect on the parasites, immunologic and pharmacokinetic factors contribute to the observed outcome. The host immune response plays an important role in removing malaria parasites from the blood. Individuals with pre-existing immunity to malaria may successfully clear parasites that are resistant to the administered drug. Because immunity is acquired with increasing exposure to blood-stage parasites, in malaria-endemic areas immunity is directly related to age. As a result, the age of the study participants is critical for interpreting the efficacy of the antimalarial drug under investigation. Older children and adults who have experienced malaria infections throughout their lives demonstrate better responses in drug efficacy studies than younger children because their immune response is able to clear even resistant parasites [4-6]. Efforts to prevent malaria transmission (eg, using bednets and indoor residual spraying) may mean less exposure to infection and may slow the acquisition of immunity. Less immunity within a population can lead to an observed decrease in drug efficacy at the same level of intrinsic resistance [7]. Other host factors can influence drug efficacy. For example, the efficacy of the antifolate combination sulfadoxine-pyrimethamine varies with blood folate levels [8].
The interaction between HIV and malaria has been difficult to discern and is beyond the scope of this review [8]. However, efficacy assessments conducted in populations with high prevalence of HIV infection can yield misleading results. Drug efficacy studies are conducted in patients who have symptomatic malaria infection. In areas of moderate to high malaria transmission, individuals with HIV infection may have asymptomatic parasitemia while experiencing symptoms of an opportunistic infection that are incorrectly attributed to malaria [10]. Patients may meet the criteria for clinical treatment failure because the underlying cause of the symptoms, such as bacteremia, pneumonia, or viral infection, has not been addressed.
Variability of drug levels may also influence efficacy study outcomes. Inconsistencies in bioavailability and metabolism due to poor-quality drug, interpatient variability in metabolism, and incorrect dosing can lead to differences in the drug level achieved in patients and thus the amount of drug to which the parasites are exposed. Lumefantrine, the non-artemisinin partner drug to artemether in a commonly administered ACT, has best bioavailability when administered with food containing fat and adequate absorption is a key factor in determining the drug efficacy. Lumefantrine level on day 7 is an independent predictor of treatment outcome [11••,12]. Some researchers have proposed including a measurement of the drug level on day 7 as a standard part of the drug efficacy study [13].
Despite the above-mentioned caveats, drug efficacy studies yielded relatively straightforward results for monotherapy for treating malaria. However, in the era of ACTs, the interpretation of outcomes has become more complex. Treatment failure may reflect poor efficacy of the artemisinins, the partner drug, or both. This has important public health implications because of the limited repertoire of antimalarial medications. Without information about each component, if a combination therapy fails, both drugs may be removed from the national treatment policy, leaving less desirable alternative medications.
Clinical resistance to artemisinins is extremely rare. Although not yet fully characterized, evidence exists of decreased efficacy on the Thai-Cambodian border [14]. Initially, investigators in the region noted decreased efficacy of the artesunate-mefloquine combination. To investigate further the contribution of artemisinins resistance to the decreased efficacy, studies are being conducted using a 7-day course of artesunate monotherapy. Unpublished data indicate some decrease in artesunate efficacy [15].
Recurrent parasitemia is known to occur when artemisinins are used as monotherapy for fewer than 5 days. This is not due to resistance but is thought to represent inadequate dosing of the drug, which has a very short half-life, because the recurrent parasites from these patients do not demonstrate decreased in vitro drug susceptibility. High initial parasite burden is strongly associated with recrudescent parasitemia after a 3-day course of artesunate [16]. This association points to the sensitivity of treatment outcomes to the unique pharmacokinetic and pharmacodynamic factors of the artemisinins.
The emphasis on reporting clinical outcomes of only recrudescent failures and the removal of study subjects with new infections from the efficacy estimates also may complicate interpretation of efficacy studies [17•]. Combinations that have high efficacy but short duration of action may give the impression of being the best drug choice. However, in areas of high transmission, short-acting combinations do not provide a prolonged period of prophylaxis after treatment, so that another illness episode can occur soon after the initial one. Thus, such combinations may not adequately decrease the malaria burden in areas of high transmission even when the reported efficacy is high.
In Vitro Susceptibility Testing
Given the difficulties of interpreting drug efficacy studies, the objectivity of in vitro susceptibility assessment is appealing. In vitro testing assesses the susceptibility of most microorganisms by growing them in increasing concentrations of the drug. For malaria, parasites from human cases must be collected and cultivated in the laboratory. Once a standardized parasite density has been prepared, infected erythrocytes are incubated in the presence of known concentrations of antimalarial drugs. Parasite growth after 48 to 72 hours is measured in several ways. The “gold standard” method is measurement of tritiated hypoxanthine incorporation as an indicator of parasite growth. Although a robust test, it requires the use of radioactive materials and a scintillation counter. Enzyme-linked immunosorbent assays have been developed and implemented using monoclonal antibodies against Plasmodium lactate dehydrogenase and histidine-rich protein II. The use of fluorescent labeling of DNA with SYBR green also has been established. The result is a concentration of drug at which 50% of the parasite growth is inhibited compared with the control without drug (IC50). The different methods generally produce different IC50 values, although efforts to compare and standardize outcomes have been initiated recently [18-20].
Capacity to collect and preserve parasites is limited to major research centers in malaria-endemic countries. Even under the best conditions, not all parasites are able to be culture-adapted. In areas of high transmission, individuals are typically infected with multiple P. falciparum genotypes. Each genotype may have a different growth rate when cultivated, leading to overgrowth of the strain that is best suited for culture growth but not necessarily reflecting the full spectrum of parasites found in the patient [21]. Fresh parasite isolates can be used to avoid this bias, but field testing is often unavailable at clinical study sites, is less reliable than methods using culture-adapted parasites, and cannot be replicated by other investigators because of the short survival of freshly collected parasites.
Another impediment is the variability of results so that the same strain tested in two laboratories often yields two different IC50 values. One reason for lack of reproducibility is the use of different methodologies. Experts in the field have conceded that achieving uniformity of IC50 values is not likely to occur for most drugs. Rather, the goal is to develop a standardized set of reference strains with established drug susceptibilities. These isolates would be assessed along with experimental ones to serve as a reference for each laboratory [22].
Ideally, a threshold IC50 is established for distinguishing resistant versus susceptible parasites. With malaria drugs, no clear method exists to establish this threshold. It has been difficult to correlate specific IC50 values with clinical outcomes because of variability between human response and in vitro results. Even chloroquine has been controversial in determining an accepted IC50 threshold for resistance [23•]. No data exist to determine the resistance cutoff for the artemisinins or their common partner drugs. Current studies are using clinical phenotypes to define groups. Treatment success versus treatment failure is used when possible, but groups of parasites with signs of decreased susceptibility, such as prolonged parasite clearance time or parasite reduction ratio, are also compared to support the role of impaired artemisinins susceptibility in determining the treatment outcome [14,15].
Another challenge to in vitro susceptibility testing of ACTs is the need to capture the variability of each drug’s pharmacokinetics and pharmacodynamics. During the testing, parasites are exposed to continuous concentrations of a given drug for several days. Although this may accurately capture the blood level experienced by parasites to long-acting drugs, it does not reflect the in vivo exposure of parasites to the artemisinins. The artemisinins have a half-life of 1 to 3 hours and are administered once or twice daily for 3 days when given as part of an ACT for uncomplicated disease. New methods are needed to simulate the physiologic exposure of parasites to drugs with different durations of action.
Molecular Markers
Single nucleotide polymorphisms (SNPs) in the Plasmodium falciparum genome have been linked to drug resistance. The currently accepted molecular markers of drug resistance were identified through assessment of homologues from other organisms [24-26] or through the analysis of progeny of crosses between parasites with resistant and sensitive phenotypes [27-29]. Ecologic and clinical studies confirmed the utility of these markers [30-33]. PCR-based assays to detect SNPs associated with resistance are relatively simple to use in field studies. Preservation of live parasites requires a venipuncture to obtain several milliliters of blood, and the specimen must undergo separation of red blood cells, mixing with a preservative, and deep freezing. In contrast, molecular markers can be assessed by PCR from a drop of blood collected on a piece of filter paper at the same time that the finger is pricked for a malaria smear or rapid diagnostic test.
Like in vitro resistance, the presence of the markers does not necessarily directly predict treatment outcome. It has been difficult to determine the role of molecular markers for the routine monitoring of drug efficacy. Djimde et al. [34] have proposed a genotype failure index (GFI)—the ratio of the prevalence of the resistant genotype to the incidence of clinical treatment failure. The GFI will differ by location based on endemicity and it must be adjusted for age as a correlate of immunity. Once the GFI is established and validated for a given site, routine monitoring of the prevalence of the molecular marker may detect a change in clinical efficacy. Where the prevalence of markers for SP or chloroquine resistance has been less than 50%—when monitoring resistance is most critical because it is not yet widespread—the GFI has been remarkably stable [17•]. When the resistant genotype approaches 100% prevalence, the GFI loses its reliability because host factors become the prevailing determinants of clinical efficacy. Unfortunately, the assessment of GFI using molecular markers for chloroquine and SP resistance is no longer useful because of widespread resistance to these drugs. Nevertheless, the retrospective evaluation of its utility suggests that properly validated molecular markers may have an important role in the future.
Laboratory-adapted strains of malaria that are consistently resistant or susceptible to a specific drug are needed to identify and validate molecular markers to drugs that are now being deployed. As described above, artemisinin resistance has not been characterized in vitro and cases of clinical failure are difficult to identify due to combination therapy, so the search for molecular markers for both artemisinins and the common partner drugs has been challenging. Until now, the search has focused on candidate genes because whole-genome analysis is difficult from field samples. It has been suggested that at least one target of the artemisinins is the SERCA ortholog PfATPase6 [35]. One SNP that is a marker for artemisinins resistance, PfATPase6 S769N, has been proposed based on an ecologic study and supported by differences in IC50 values [36•]. The association has not yet been confirmed. The SNP is not found in China, where the artemisinins have been used the longest, nor has it been identified in Africa [37-39].
One reason for the failure to identify molecular markers to artemisinins and their partner drugs is that they may be lost or changed through culture adaptation. For example, copy number of a multidrug resistance gene has been associated with resistance to lumefantrine, mefloquine, and possibly the artemisinins [11••,40,41]. However, copy number can change with growth in culture so that measurement may change from the fresh clinical isolate to the same isolate after culture adaptation. Although gene copy number can be detected in clinical samples in a relatively straightforward assay [40,42], classic genetic crosses may not yield reliable results. Another possibility is that the mechanism of resistance is an epigenetic phenomenon and requires gene expression analysis.
Choosing the Right Tools
With so many avenues to assess antimalarial drug resistance, and none providing all the necessary information, how should resistance be monitored? The answer depends on the goal of monitoring and assessment. When countries are choosing their first- and second-line treatment regimens, the selection is frequently limited to two or three drugs that are commercially available in sufficient quantity and have an acceptable dosing regimen and tolerability profile. In this scenario, a straightforward drug efficacy study with a minimum of 28 days of follow-up would capture the most relevant information: the rate at which children with disease are cured.
This type of study is not adequate for the clinical trials conducted in the drug development pathway. Although parasitologic and clinical cure rates are critical measures, understanding why a drug fails requires in-depth exploration. For trials associated with drug development, pharmacokinetics and in vitro susceptibility testing must be included to understand how the drug formulation and dosing might be improved to maximize efficacy and minimize adverse effects.
Another aspect to drug development that has received limited attention is the rational combination of drugs. Drug combinations are studied together with fixed partners, and the opportunity does not exist to study each drug individually or drugs in different combinations. Testing of current drug combinations involves only artemisinins partnered with another drug. Ideally, drugs should be paired based on pharmacokinetic and pharmacodynamic properties that maximize efficacy and deter the emergence and spread of resistance. Several investigators have advocated characterizing the selection window of drug combinations [43,44]. This is the period defined as number of days after drug treatment when resistant parasites survive while susceptible parasites are killed, hence providing a selective advantage for the resistant organisms. Characterizing this period and designing combinations that eliminate or minimize it requires understanding not just the pharmacokinetics of the drugs, but also how the levels encountered at different time periods affect the growth of resistant and susceptible parasites. This is especially critical for designing drugs to be used in hightransmission settings, because individuals continue to be exposed to new infections while antimalarial drug levels are waning after therapy.
International Monitoring for Emergence of Resistance to ACT
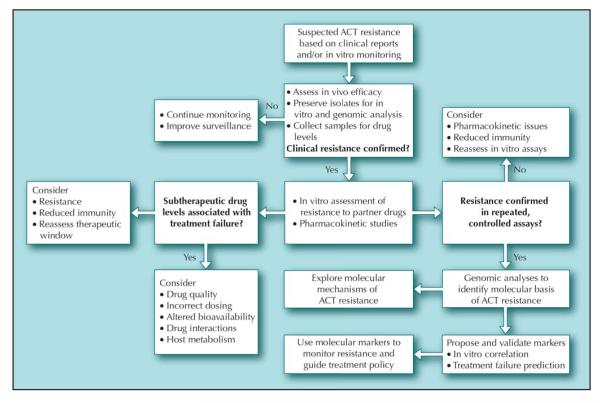
With multilateral support for new, effective therapies to treat malaria, awareness has increased that vigilant surveillance for resistance to these drugs is critical. Efforts must cross national boundaries and incorporate the many facets of drug-resistance monitoring to be sensitive to the first emergence of artemisinin resistance and to monitor for the spread of resistance to partner drugs. We have proposed an integrated system that should be prepared for deployment to detect true cases of resistance to ACT, fashioned after epidemic outbreak investigation (Fig. 1). Areas with suspected ACT resistance should be identified through routine drug efficacy monitoring, especially in regions where ACT use is prevalent. Although episodes of treatment failure would be the clearest indicator of possible intrinsic resistance, more subtle signs of emerging resistance have been used as early warning signals of impending resistance, such as delayed parasite clearance, low parasite reduction ratio, and increased gametocytemia. When routine monitoring leads to concern about possible resistance, intensive efforts must be initiated to collect parasites from selected cases. In this plan, in vitro tests are the key assessment tool to confirm true resistance because ACT may retain clinical efficacy if parasites remain susceptible to one of the two drugs. In vitro testing, preferably using optimized methods, will determine if decreased clinical efficacy is due to intrinsic resistance or other factors such as pharmacokinetic variability, poor drug quality, or interactions with other drugs.
Figure 1.
A proposed algorithm to investigate suspected outbreaks of resistance to artemisinin-based combination therapy (ACT). (From Laufer et al. [17•], with permission.)
Because this level of attention requires unprecedented international coordination, efforts are underway to establish a World Antimalarial Resistance Network (WARN) [45•]. WARN will support four elements of drug-resistance monitoring—clinical, in vitro, molecular, and pharmacokinetic—to ensure the highest possible quality and standardization. Most importantly, it will provide a forum to integrate all the strategies to produce accurate and up-to-date information, protect the useful therapeutic life of current antimalarials, and provide tools to malaria researchers, practitioners, and policy makers.
Conclusions
Monitoring for the emergence and spread of antimalarial drug resistance is more complex than in the past because of the use of combinations of drugs with poorly characterized mechanisms of action and resistance. Such monitoring will require international effort and close collaboration among health policy makers, clinical investigators, and laboratory scientists. A system should be established to quickly investigate, confirm, and characterize resistance to components of combination therapy as soon as it is suspected so that it can be contained and eradicated.
Acknowledgments
Disclosure Dr. Laufer has received research funding from Pfizer.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.World Health Organization . Assessment and Monitoring of Antimalarial Drug Efficacy for the Treatment of Uncomplicated Falciparum Malaria. World Health Organization; Geneva, Switzerland: 2003. [Google Scholar]
- 2.Stepniewska K, Taylor WR, Mayxay M, et al. In vivo assessment of drug efficacy against Plasmodium falciparum malaria: duration of follow-up. Antimicrob Agents Chemother. 2004;48:4271–4280. doi: 10.1128/AAC.48.11.4271-4280.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization . Methods and Techniques for Clinical Trials on Antimalarial Drug Efficacy: Genotyping to Identify Parasite Populations. World Health Organization; Geneva, Switzerland: 2008. [Google Scholar]
- 4.Hoffman SL, Masbar S, Hussein PR, et al. Absence of malaria mortality in villagers with chloroquine-resistant Plasmodium falciparum treated with chloroquine. Trans R Soc Trop Med Hyg. 1984;78:175–178. doi: 10.1016/0035-9203(84)90271-2. [DOI] [PubMed] [Google Scholar]
- 5.Djimde AA, Doumbo OK, Traore O, et al. Clearance of drug-resistant parasites as a model for protective immunity in Plasmodium falciparum malaria. Am J Trop Med Hyg. 2003;69:558–563. [PubMed] [Google Scholar]
- 6.Laufer MK, van Oosterhout JJ, Thesing PC, et al. Malaria treatment efficacy among people living with HIV: the role of host and parasite factors. Am J Trop Med Hyg. 2007;77:627–632. [PubMed] [Google Scholar]
- 7.Greenhouse B, Slater M, Njama-Meya D, et al. Decreasing efficacy of antimalarial combination therapy in Uganda explained by decreasing host immunity rather than increasing drug resistance. J Infect Dis. doi: 10.1086/596741. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dzinjalamala FK, MacHeso A, Kublin JG, et al. Blood folate concentrations and in vivo sulfadoxine-pyrimethamine failure in Malawian children with uncomplicated Plasmodium falciparum malaria. Am J Trop Med Hyg. 2005;72:267–272. [PubMed] [Google Scholar]
- 9.Laufer MK, Plowe CV. The interaction between HIV and malaria in Africa. Curr Infect Dis Rep. 2007;9:47–54. doi: 10.1007/s11908-007-0022-3. [DOI] [PubMed] [Google Scholar]
- 10.Laufer MK, van Oosterhout JJ, Thesing PC, et al. Impact of HIV-associated immunosuppression on malaria infection and disease in Malawi. J Infect Dis. 2006;193:872–878. doi: 10.1086/500245. [DOI] [PubMed] [Google Scholar]
- 11 ••.Price RN, Uhlemann AC, van Vugt M, et al. Molecular and pharmacological determinants of the therapeutic response to artemether-lumefantrine in multidrug-resistant Plasmodium falciparum malaria. Clin Infect Dis. 2006;42:1570–1577. doi: 10.1086/503423.A clinical study demonstrating the complex interplay of factors that contribute to decreased drug efficacy.
- 12.White NJ, van Vugt M, Ezzet F. Clinical pharmacokinetics and pharmacodynamics and pharmacodynamics of artemether-lumefantrine. Clin Pharmacokinet. 1999;37:105–125. doi: 10.2165/00003088-199937020-00002. [DOI] [PubMed] [Google Scholar]
- 13.White NJ, Stepniewska K, Barnes K, et al. Simplified antimalarial therapeutic monitoring: using the day-7 drug level? Trends Parasitol. 2008;24:159–163. doi: 10.1016/j.pt.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 14.World Health Organization . Containment of Malaria Multi-Drug Resistance on the Cambodia-Thailand Border. World Health Organization; Geneva, Switzerland: 2007. Report no. SEA-MAL-246. [Google Scholar]
- 15.Noedl H, Thap LC, Se Y, et al. Artemisinin resistance in Cambodia? [abstract 934]. Presented at the Annual Meeting of the American Society of Tropical Medicine; Philadelphia, PA. November 8, 2007. [Google Scholar]
- 16.Ittarat W, Pickard AL, Rattanasinganchan P, et al. Recrudescence in artesunate-treated patients with falciparum malaria is dependent on parasite burden not on parasite factors. Am J Trop Med Hyg. 2003;68:147–152. [PubMed] [Google Scholar]
- 17 •.Laufer MK, Djimde AA, Plowe CV. Monitoring and deterring drug-resistant malaria in the era of combination therapy. Am J Trop Med Hyg. 2007;77(6 Suppl):160–169.A complete review of the recent controversies surrounding antimalarial drug resistance assessment.
- 18.Kaddouri H, Djimde A, Dama S, et al. Baseline in vitro efficacy of ACT component drugs on Plasmodium falciparum clinical isolates from Mali. Int J Parasitol. 2008;38:791–798. doi: 10.1016/j.ijpara.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 19.Bacon DJ, Latour C, Lucas C, et al. Comparison of a SYBR green I-based assay with a histidine-rich protein II enzyme-linked immunosorbent assay for in vitro antimalarial drug efficacy testing and application to clinical isolates. Antimicrob Agents Chemother. 2007;51:1172–1178. doi: 10.1128/AAC.01313-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rason MA, Randriantsoa T, Andrianantenaina H, et al. Performance and reliability of the SYBR green I based assay for the routine monitoring of susceptibility of Plasmodium falciparum clinical isolates. Trans R Soc Trop Med Hyg. 2008;102:346–351. doi: 10.1016/j.trstmh.2008.01.021. [DOI] [PubMed] [Google Scholar]
- 21.Basco LK. Molecular epidemiology of malaria in Cameroon. XX. Experimental studies on various factors of in vitro drug sensitivity assays using fresh isolates of Plasmodium falciparum. Am J Trop Med Hyg. 2004;70:474–480. [PubMed] [Google Scholar]
- 22.Bacon DJ, Jambou R, Fandeur T, et al. World Antimalarial Resistance Network (WARN) II: in vitro antimalarial drug susceptibility. Malar J. 2007;6:120. doi: 10.1186/1475-2875-6-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23 •.Ekland EH, Fidock DA. In vitro evaluations of antimalarial drugs and their relevance to clinical outcomes. Int J Parasitol. 2008;38:743–747. doi: 10.1016/j.ijpara.2008.03.004.An excellent discussion of the value and shortcomings of in vitro susceptibility testing.
- 24.Bzik DJ, Li WB, Horii T, Inselburg J. Molecular cloning and sequence analysis of the Plasmodium falciparum dihydrofolate reductase-thymidylate synthase gene. Proc Natl Acad Sci U S A. 1987;84:8360–8364. doi: 10.1073/pnas.84.23.8360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Foote SJ, Thompson JK, Cowman AF, Kemp DJ. Amplification of the multidrug resistance gene in some chloroquine-resistant isolates of P. falciparum. Cell. 1989;57:921–930. doi: 10.1016/0092-8674(89)90330-9. [DOI] [PubMed] [Google Scholar]
- 26.Brooks DR, Wang P, Read M, et al. Sequence variation of the hydroxymethyldihydropterin pyrophosphokinase: dihydropteroate synthase gene in lines of the human malaria parasite, Plasmodium falciparum, with differing resistance to sulfadoxine. Eur J Biochem. 1994;224:397–405. doi: 10.1111/j.1432-1033.1994.00397.x. [DOI] [PubMed] [Google Scholar]
- 27.Peterson DS, Walliker D, Wellems TE. Evidence that a point mutation in dihydrofolate reductase-thymidylate synthase confers resistance to pyrimethamine in falciparum malaria. Proc Natl Acad Sci U S A. 1988;85:9114–9118. doi: 10.1073/pnas.85.23.9114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wellems TE, Walker-Jonah A, Panton LJ. Genetic mapping of the chloroquine-resistance locus on Plasmodium falciparum chromosome 7. Proc Natl Acad Sci U S A. 1991;88:3382–3386. doi: 10.1073/pnas.88.8.3382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fidock DA, Nomura T, Talley AK, et al. Mutations in the P. falciparum digestive vacuole transmembrane protein PfCRT and evidence for their role in chloroquine resistance. Mol Cell. 2000;6:861–871. doi: 10.1016/s1097-2765(05)00077-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Plowe CV, Cortese JF, Djimde A, et al. Mutations in Plasmodium falciparum dihydrofolate reductase and dihydropteroate synthase and epidemiologic patterns of pyrimethamine-sulfadoxine use and resistance. J Infect Dis. 1997;176:1590–1596. doi: 10.1086/514159. [DOI] [PubMed] [Google Scholar]
- 31.Wang P, Lee CS, Bayoumi R, et al. Resistance to antifolates in Plasmodium falciparum monitored by sequence analysis of dihydropteroate synthetase and dihydrofolate reductase alleles in a large number of field samples of diverse origins. Mol Biochem Parasitol. 1997;89:161–177. doi: 10.1016/s0166-6851(97)00114-x. [DOI] [PubMed] [Google Scholar]
- 32.Djimde A, Doumbo OK, Cortese JF, et al. A molecular marker for chloroquine-resistant falciparum malaria. N Engl J Med. 2001;344:257–263. doi: 10.1056/NEJM200101253440403. [DOI] [PubMed] [Google Scholar]
- 33.Kublin JG, Dzinjalamala FK, Kamwendo DD, et al. Molecular markers for failure of sulfadoxine-pyrimethamine and chlorproguanil-dapsone treatment of Plasmodium falciparum malaria. J Infect Dis. 2002;185:380–388. doi: 10.1086/338566. [DOI] [PubMed] [Google Scholar]
- 34.Djimde A, Doumbo OK, Steketee RW, Plowe CV. Application of a molecular marker for surveillance of chloroquine-resistant falciparum malaria. Lancet. 2001;358:890–891. doi: 10.1016/S0140-6736(01)06040-8. [DOI] [PubMed] [Google Scholar]
- 35.Eckstein-Ludwig U, Webb RJ, Van Goethem ID, et al. Artemisinins target the SERCA of Plasmodium falciparum. Nature. 2003;424:957–961. doi: 10.1038/nature01813. [DOI] [PubMed] [Google Scholar]
- 36 •.Jambou R, Legrand E, Niang M, et al. Resistance of Plasmodium falciparum field isolates to in-vitro artemether and point mutations of the SERCA-type PfATPase6. Lancet. 2005;366:1960–1963. doi: 10.1016/S0140-6736(05)67787-2.The first published study of decreased artemisinin susceptibility in clinical malaria isolates.
- 37.Zhang G, Guan Y, Zheng B, et al. No PfATPase6 S769N mutation found in Plasmodium falciparum isolates from China. Malar J. 2008;7:122. doi: 10.1186/1475-2875-7-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mugittu K, Genton B, Mshinda H, Beck HP. Molecular monitoring of Plasmodium falciparum resistance to artemisinin in Tanzania. Malar J. 2006;5:126. doi: 10.1186/1475-2875-5-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ferreira ID, Lopes D, Martinelli A, et al. In vitro assessment of artesunate, artemether and amodiaquine susceptibility and molecular analysis of putative resistance-associated mutations of Plasmodium falciparum from Sao Tome and Principe. Trop Med Int Health. 2007;12:353–362. doi: 10.1111/j.1365-3156.2006.01789.x. [DOI] [PubMed] [Google Scholar]
- 40.Price RN, Uhlemann AC, Brockman A, et al. Mefloquine resistance in Plasmodium falciparum and increased pfmdr1 gene copy number. Lancet. 2004;364:438–447. doi: 10.1016/S0140-6736(04)16767-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sidhu AB, Uhlemann AC, Valderramos SG, et al. Decreasing pfmdr1 copy number in plasmodium falciparum malaria heightens susceptibility to mefloquine, lumefantrine, halofantrine, quinine, and artemisinin. J Infect Dis. 2006;194:528–535. doi: 10.1086/507115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ferreira ID, Rosario VE, Cravo PV. Real-time quantitative PCR with SYBR Green I detection for estimating copy numbers of nine drug resistance candidate genes in Plasmodium falciparum. Malar J. 2006;5:1. doi: 10.1186/1475-2875-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nyunt MM, Plowe CV. Pharmacologic advances in the global control and treatment of malaria: combination therapy and resistance. Clin Pharmacol Ther. 2007;82:601–605. doi: 10.1038/sj.clpt.6100361. [DOI] [PubMed] [Google Scholar]
- 44.Stepniewska K, White NJ. Pharmacokinetic determinants of the window of selection for antimalarial drug resistance. Antimicrob Agents Chemother. 2008;52:1589–1596. doi: 10.1128/AAC.00903-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45 •.Sibley CH, Barnes KI, Watkins WM, Plowe CV. A network to monitor antimalarial drug resistance: a plan for moving forward. Trends Parasitol. 2008;24:43–48. doi: 10.1016/j.pt.2007.09.008.An introduction to the role of WARN in protecting the useful therapeutic life of antimalarial drugs currently in use.