Abstract
The developmental processes underlying gonadal differentiation are conserved across vertebrates, but the triggers initiating these trajectories are extremely variable. The red-eared slider turtle (Trachemys scripta elegans) exhibits temperature-dependent sex determination (TSD), a system where incubation temperature during a temperature-sensitive period of development determines offspring sex. However, gonadal sex is sensitive to both temperature and hormones during this period – particularly estrogen. We present a model for temperature-based differences in aromatase expression as a critical step in ovarian determination. Localized estrogen production facilitates ovarian development while inhibiting male-specific gene expression. At male-producing temperatures aromatase is not upregulated, thereby allowing testis development.
Keywords: temperature-dependent sex determination, Trachemys scripta, estrogen, aromatase, ovary
Introduction
A variety of sex-determining systems exist among vertebrates, ranging from primarily genotypic to primarily environmental [1, 2]. Reptiles as a group are particularly interesting in that examples of the entire range are evident. In this review we will focus on temperature-dependent sex determination (TSD). Many turtles, some lizards, and all crocodilians [3, 4] exhibit this sex-determining mechanism in which gonadal sex1 is determined by the temperature of the embryo. Once thought to be restricted to oviparous species lacking sex chromosomes, recent reports have extended TSD to viviparous and oviparous species with both XX/XY and ZZ/ZW sex-determining systems [6–9].
While the mechanism by which temperature is translated into either testis- or ovarian-determining cues is not known, it has been established that the gonad is sensitive to temperature during a discrete period of development known as the temperature-sensitive period [10–15]. It is significant that sensitivity to steroid hormones generally coincides with the temperature-sensitive period and manipulation of either temperature or the hormonal milieu during this window will redirect the putative sex of the embryo [10, 16, 17]. The best-studied species in this regard is the red-eared slider turtle (Trachemys scripta) and the most extensively studied steroid-induced sex determination involves estrogen.
Estrogen is critical for ovarian development in all vertebrate groups except for placental mammals, although it is clear even in mammals that modifiers of estrogen receptors are involved in some, as yet unknown manner (see below). Exogenous estrogen treatment during development will transiently sex-reverse chickens [18], and permanently sex-reverse reptiles, amphibians, and fish [19–22]. In the slider turtle, administration of exogenous estradiol (E2) to an egg incubating at a male-producing temperature, as well as administration of an aromatase inhibitor (AI), which prevents estrogen production, to an egg incubating at a female-producing temperature, will override the temperature cue and redirect the sexual fate of the embryo [23, 24]. Importantly, warm temperature and estrogen act synergistically – less estrogen is required to sex-reverse embryos from intermediate temperatures than from extreme male-producing temperatures [16]. Because estrogen can override a temperature cue, estrogen production has been proposed to be the proximate mechanism through which temperature exerts its actions [25–27].
Steroid hormones are present in the developing TSD embryo, whether of embryonic or maternal (yolk) origin. Yolk steroid levels are known to vary both seasonally and by clutch, and can affect hatchling sex ratios at intermediate or pivotal (the temperature that produces a 1:1 sex ratio; this will vary according to species) incubation temperatures [28–32]. This variation in steroid hormone levels in the egg may play a role in TSD, although some researchers report no relationship between yolk steroids and offspring sex ratios in the snapping turtle [33] and skink [34]. Recent reviews have also cast doubt on a maternal origin for steroid hormone effects in TSD [35, 36].
Our focus here is on estrogens, but it should be noted that other steroid hormones in the yolk or of embryonic origin may also play a role in sex determination in TSD reptiles. For example, application of exogenous nonaromatizable androgens produce 100% males from a pivotal incubation temperature in the slider turtle [37] but cannot override all-female producing temperatures [38]. Inhibition of reductase, the enzyme that converts testosterone into non-aromatizable dihydrotestosterone (DHT), also sex-reverses hatchlings from a male-biased temperature [23]. In this context, it is particularly interesting to note that combined treatment with DHT and E2 at a pivotal temperature, as well as treatment with high concentrations of testosterone at female-biased temperatures, produces some individuals with ovotestes [23, 37, 39].
In the slider turtle, the interplay between temperature and steroid hormones may trigger ovarian determination through differential activation/repression of sex determining genes during the temperature-sensitive period [40]. Across vertebrate groups, the upstream triggers underlying sex determination are diverse, but the genes involved in sexual differentiation that directly or indirectly respond to those triggers - though not necessarily their expression pattern or developmental timing - are conserved. Homologues for genes first discovered in mammals such as Dmrt1, Sox9, Mis, Sf1, Dax1, WT1, Wnt4, FoxL2, and Rspo1 have been found in fish [41], amphibians [42], birds [43, 44], and reptiles [45–53].
If the same genes and their products are being utilized in gonadal development across vertebrate groups, it may be the timing and manner of gene regulation that allow these core components to respond to distinctive triggers. An interesting example of this phenomenon is in the timing of estrogen sensitivity during ovarian development. In mammals, estrogens play an important role in the development of female secondary sex characteristics rather than ovarian determination itself [54, 55]. Although exogenous estrogen treatments have been shown to sex-reverse male marsupials [56], estrogens have been thought unnecessary for normal ovarian differentiation in placental mammals. It was not until mice were created with both estrogen receptor α (ERα) and estrogen receptor β (ERβ) isoforms knocked out (αβERKO) that estrogen-related sex reversal was observed in a eutherian mammal [57]. In these knockouts, ovaries develop normally, but around puberty the follicles begin to transform into seminiferous tubule-like structures, including the transdifferentiation of mature granulosa cells into Sox9-expressing Sertoli cells [57–59]. Postnatal ovaries of female aromatase knockout (ArKO) mice fed a soy-free diet exhibit the same postnatal sex-reversed phenotype seen in αβERKO ovaries [60, 61].
It should be noted that in the mouse, estrogen is necessary for post-pubertal ovarian maintenance, but not for ovarian determination or development. However, the mouse model is not globally applicable across all mammalian species. Goats and sheep express aromatase during ovarian differentiation [62, 63]; in fact, the goat polled intersex phenotype (now known to be caused by a mutation in FoxL2) probably results from reduced aromatase expression during ovarian differentiation [64]. The slider turtle model system extends the time frame of estrogen sensitivity back further - to the period of ovarian determination.
The remainder of this review will focus on how warm temperatures and estrogen interact with core sex-determining genes early in gonadal development to form an ovary, and in particular on how steroid signaling components respond to temperature and hormones.
Gonadal development in the slider turtle
To understand how temperature and estrogen interact in TSD, and more particularly in ovarian determination, it is necessary to place temperature and estrogen action into the context of the developmental trajectory of the gonad.
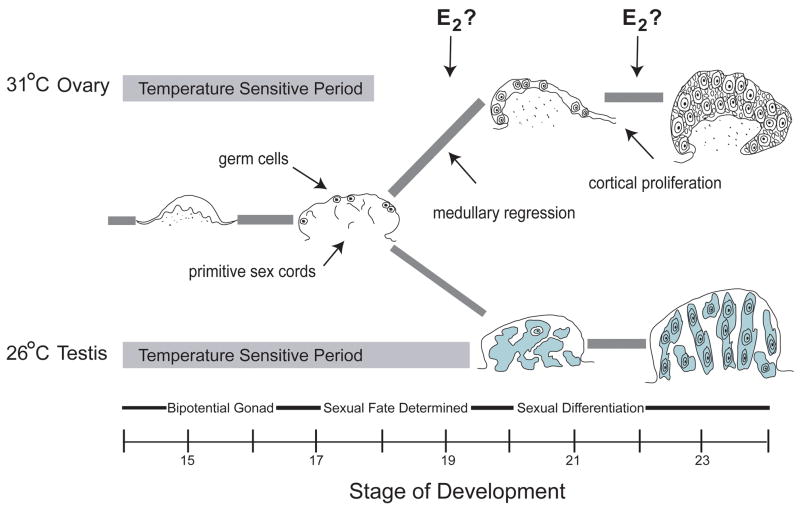
The onset and duration of temperature- and hormone-sensitivity during sex determination have been established in the slider turtle [10, 65] and a schematic of slider turtle gonadal development is represented in Figure 1. The gonad develops as part of the adrenal-kidney-gonad (AKG) complex [66]. The urogenital ridge first forms around stage 13, and hatching occurs at stage 26. Gonadal development encompasses 3 main phases - bipotential (Stages 14–16), sex determination (Stages 16.5/17–19/21), and sexual differentiation (Stages 20/21-hatch). In this species, the temperature-sensitive period encompasses the bipotential and sex determination phases of gonadal development, and occurs in about the middle third of embryonic development [10, 67]. The duration of the temperature-sensitive period varies by temperature. At a female-producing temperature of 31° C, the temperature-sensitive period extends from about Stages 14–18/19, while at a male-producing temperature of 26° C it extends from about Stages 14–20/21.
Figure 1.
Trajectory of gonadal developmental in the red-eared slider turtle (Trachemys scripta). The temperature-sensitive period is highlighted in grey. The bipotential gonad contains primitive sex cords in the medullary region, and germ cells in the outer germinal epithelium. At a female-producing incubation temperature, the primitive sex cords degenerate, while the germ and somatic cells in the cortical region proliferate. At a male-producing incubation temperature, the germ cells migrate into the medullary region where they are enclosed within the developing seminiferous tubules. Arrows indicate the two time periods where estrogen may be acting in ovarian development: during medullary regression and cortical proliferation. E2 = 17β-estradiol.
As the gonad develops from the genital ridge, two distinct compartments emerge – an inner medulla and an outer cortical layer. Primitive sex cords are found in the medullary region of the bipotential gonad, regardless of temperature. In the developing ovary, these primitive sex cords will degenerate (~Stages 18–20) while in the testis they will develop into the seminiferous tubules (starting ~Stage 18, and very evident by about stage 20; Figure 1). Interestingly, the cords develop in the testis while sex is still labile, making this process reversible until about Stage 20/21. Germ cells are initially located in the outer germinal epithelium of the gonad. In the ovary, they remain in the cortical region where they will proliferate along with differentiating granulosa cells to form follicles. In the developing testis, germ cells will migrate into the medullary region beginning about Stage 18, where they will be enveloped by the developing seminiferous tubules [10, 68].
Two key events mark ovarian development: regression of the medullary region and proliferation of the cortex (Figure 1). The regression of the medullary sex cords begins at about stage 18/19 in the developing ovary - probably not coincidentally the end of the temperature-sensitive period at female-producing temperatures. The proliferation of the cortical region begins at ~stage 21, and so is an integral part of ovarian differentiation but not sex determination itself. If estrogen action/production is an important part of ovarian development, it could be acting early at the level of determination (medullary regression) and/or late during differentiation (cortical proliferation).
Establishing embryonic steroidogenic capacity during the temperature-sensitive period
Early studies on TSD established steroidogenic capability and activity before, during, and after the temperature-sensitive period in the slider turtle AKG [69], as well as the presence of steroid hormones in both serum and whole embryos [70]. The AKG complexes from embryos incubating at a male-producing temperature are more steroidogenically active following stimulation by pituitary hormones [71]. In addition, whole body and serum measurements indicate that putative males contain more testosterone (T) and E2 during early-to-middle phases of the temperature-sensitive period and more progesterone (P) in the middle of the temperature-sensitive period than embryos at a female-producing temperature [70]. Hydroxysteroid dehydrogenase (HSD) activity is also detected in adrenal (ene-5-3-β-HSD and 3α-HSD) and embryonic kidney (3α- and 17β-HSD) tissue during and after the temperature-sensitive period, but activity does not appear to differ by incubation temperature [72].
Most early work on steroidogenic activity and TSD focused on temperature-specific differences in aromatase, but the evidence for early action of aromatase in female sex determination varied with the model systems being used. Using a tritiated water assay, studies on isolated gonad tissue in the pond turtle (Emys orbicularis) show increased aromatase activity late in the temperature-sensitive period at a female-producing temperature (reviewed in [73]). Shifting pond turtle eggs from a male- to a female-producing temperature also results in increased gonadal aromatase activity [26]. In the diamondback terrapin (Malaclemys terrapin), Jeyasuria and Place [74] used RT-PCR and measured an increase in aromatase expression in the AKG complex late in the temperature-sensitive period. However, in the alligator (Alligator mississippiensis) [75, 76], the painted turtle (Chrysemis picta) [77] and the slider turtle, measures of aromatase activity (via tritiated water assay; [78]) or expression (via RT-PCR; [79]) in AKG tissue show no significant difference in aromatase during the temperature-sensitive period. Both the alligator and slider turtle AKG measures do show a sharp increase later during ovarian differentiation.
While it is possible that the timing of female-specific aromatase upregulation varies across TSD reptiles, more recent work comparing isolated gonad with whole AKG gene expression patterns indicate that expression in adjacent adrenal and kidney tissues may obscure the essential female-specific role for aromatase in the gonad during the temperature-sensitive period [80]. Similarly, in the snapping turtle (Chelydra serpentina), aromatase expression in isolated gonad tissue significantly increases three days after a shift from male- to female-producing temperature [81]. These results point to local production of aromatase within the gonad itself as the key to differential estrogen activity at female-producing temperatures [27, 67].
Most early research focused on steroid hormone production rather than uptake, but E2 uptake sites measured via 3H-E2 in vivo autoradiography localize to saltwater crocodile (Crocodylus porosus) gonads during gonadal differentiation [82]. Conversely, in slider turtle AKG tissue, E2 accumulation occurs in the adrenal and mesonephros tissue rather than the gonad itself [83], although in situ hybridization experiments indicate that ERα expression is localized throughout the slider turtle gonad at both incubation temperatures [84, 85].
Steroid signaling during ovarian development
Our recent research uses an integrated view of multiple steroid signaling system components rather than concentrating on a single gene or element (i.e., estrogen production) [40]. In this review we focus on biological actions of steroid hormones mediated through ligand-specific binding to their respective receptors [86], recognizing that there may also exist in TSD rapid, nongenomic actions for steroid hormones and their receptors [87].
Steroid receptors such as androgen receptor (AR), ERα, and ERβ, as well as aromatase are expressed in adult and embryonic reproductive tissues across vertebrate groups [67, 78, 79, 84, 85, 88–92]. However, prior to our work, ERβ and AR expression had not been examined at all in the slider turtle.
Steroidogenic factor 1 (Sf1) has multiple roles during sexual development across vertebrate groups, including the regulation of aromatase [93–96]. During mammalian development, Sf1 expression is necessary for both the maintenance of the early bipotential gonad (regardless of sex) and for testis differentiation [94, 97], and has recently been implicated as Sry’s coactivator in initiating testis-specific expression of Sox9 [98]. In the mouse, Sf1 is expressed at higher levels in the developing testis than ovary [99], and this pattern is conserved in the slider turtle [51]. In the slider turtle, Sf1 is responsive to sex-reversal via temperature -increasing after shift from a female-producing incubation temperature to a male-producing incubation temperature (FPT→MPT) while decreasing following the converse shift from a male-producing incubation temperature to a female-producing incubation temperature (MPT→FPT). Sf1 is also responsive to sex-reversal via hormonal manipulation - increasing after treatment with aromatase inhibitor (AI) while decreasing after feminizing treatment with exogenous estrogen [100].
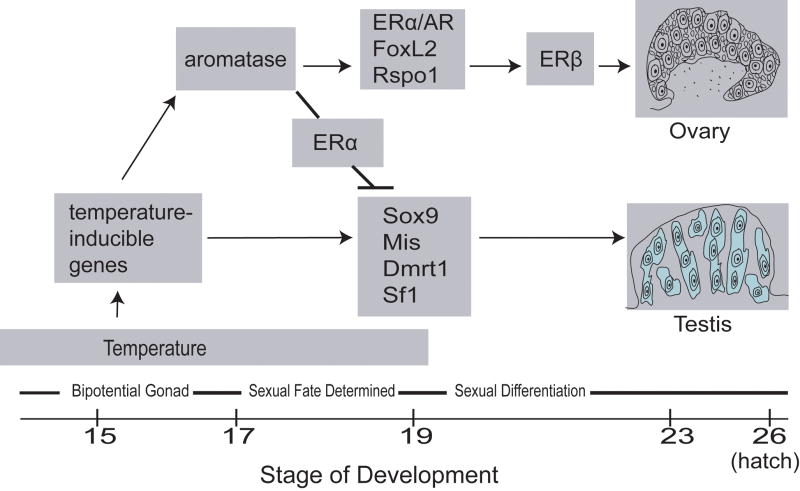
In our integrated approach to steroid signaling during slider turtle sex determination and differentiation, AR, ERα and ERβ expression patterns are critical markers for steroid hormone action. Aromatase expression indicates possible embryonic production of estrogen, and Sf1 is both a marker of testis differentiation and a master steroidogenic regulator. Examining the developmental expression profiles of these five genes serve to illuminate the interplay between temperature and sex steroid hormone action in slider turtle sex determination. As illustrated by our model (Figure 2), we propose that warm temperature either directly or indirectly induces increased gonadal aromatase expression that, coupled with a spike in steroid receptor expression, sets the stage for commitment to ovarian fate. Further, we propose that Sf1, although implicated in the developmental regulation of ovarian aromatase in some vertebrate groups including alligators [48], chickens [101] and amphibians [102], may play a predominantly male-specific role during slider turtle gonadal determination.
Figure 2.
A model for temperature and steroid hormone action during temperature-dependent sex determination in the red-eared slider turtle (Trachemys scripta). Temperature directly or indirectly causes an increase in aromatase expression at female-producing temperatures, which in concert with a spike in expression of ERα and AR and other female-specific genes (i.e., FoxL2, Rspo1), leads to ovarian determination. Increased estrogen levels combined with ERα expression inhibit male-specific gene expression in the developing ovary. ERβ expression increases later in ovarian development, and is associated with ovarian differentiation. In contrast, at male-producing temperatures aromatase expression is not increased, and so estrogen-related inhibition of male development does not occur. Testis determination is associated with increased expression of male-specific genes such as Sox9, Mis, Dmrt1 and Sf1.
What is the evidence in support of this model?
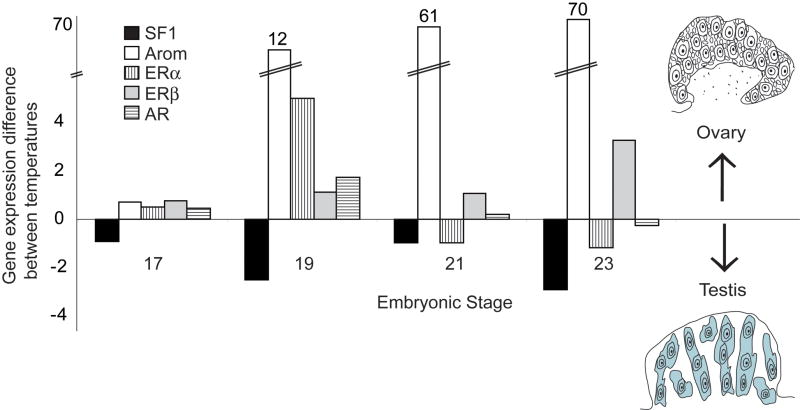
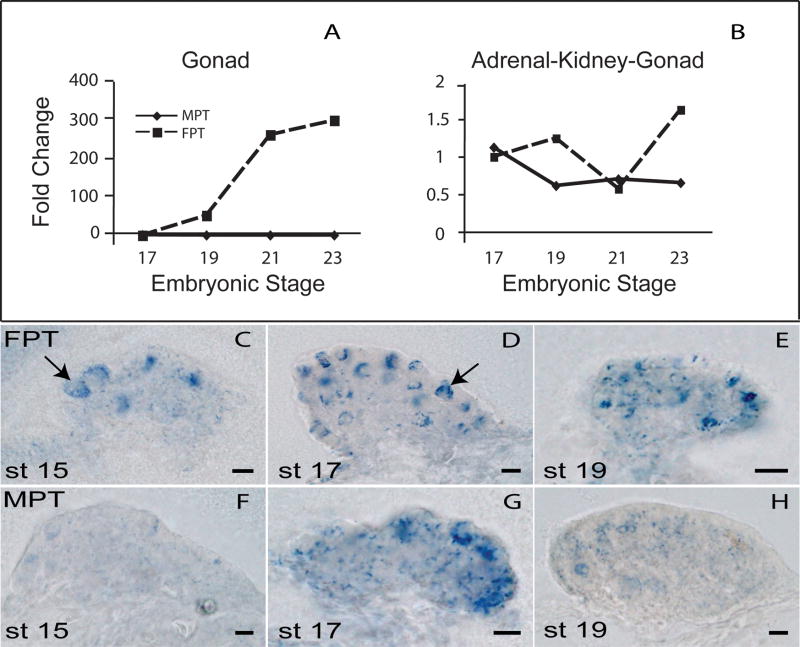
Sf1, aromatase, ERα, ERβ, and AR transcripts are present throughout gonadal development, but are differentially expressed by temperature (Figure 3). Quantitative polymerase chain reaction (qPCR) experiments show that gonadal aromatase is expressed higher at female- than at male-producing temperatures. Using isolated gonad tissue rather than whole AKG tissue as target (Figure 4A,B) reveals that this increase occurs during the temperature-sensitive period itself, while temperature is exerting its action and before ovarian differentiation is committed, and so supports an early role for estrogen production in ovarian determination [67]. Even more striking is the temperature-based difference in aromatase localization patterns early in the temperature-sensitive period (Figure 4C-H). Aromatase is organized into circular patterns (presumably around putative germ cells) at the female-producing temperature by Stage 15 – a time when the gonad is still morphologically bipotential. Both ERα and ERβ are expressed throughout the bipotential gonad at this stage [85], but only at the female-producing temperature are they present in combination with specific and organized estrogen production. These results may reflect early differences in the steroid microenvironment around developing germ cells and in developing follicles, and may be one of the earliest steps toward ovarian determination (Figure 2).
Figure 3.
Expression of components of the steroid signaling network is differential by temperature across development in the red-eared slider turtle (Trachemys scripta). Steroidogenic factor 1 (Sf1), aromatase, estrogen receptors (ERα and ERβ), and androgen receptor (AR) expression levels within the gonad were measured with real-time qPCR, and the normalized expression levels for a male-producing temperature (26° C) were subtracted from female-producing temperature (31° C) values. For each gene, expression was calibrated to female expression levels at Stage 17, and then male expression values were subtracted from the female expression values for each stage. Gene expression levels higher at the female-producing temperature are therefore positive values, while levels higher at male-producing temperature are negative. Aromatase expression is dramatically higher than the other genes at FPT, and the Y-axis is scaled to reflect this (calibrated aromatase value given in parentheses at the top of the bar).
Figure 4.
Aromatase expression during the temperature-sensitive period in the red-eared slider turtle (Trachemys scripta). (A,B) Aromatase expression patterns were compared between isolated gonad and adrenal-kidney-gonad complex (AKG) tissues. Levels were normalized relative to PP1 housekeeping gene and then calibrated across temperatures to the normalized Stage 17 FPT. Therefore, Stage 17 FPT=1, and other values reflect the ratio of aromatase expression level/temp and stage to Stage 17 FPT. (C-H) Aromatase expression patterns were also measured using DIG-labeled whole mount in situ hybridization experiments. Expression patterns at a female-producing temperature (FPT) at Stages 15, 17 and 21 are depicted in panels C,D,E, while expression at a male-producing temperature (MPT) for the same stages is depicted in panels F,G,H. Aromatase is differentially localized into round structures, possibly surrounding germ cells, at the FPT (C,D), and this difference is seen even during the early, bipotential stages of development (C,F). FPT = female-producing temperature (31° C). MPT = male-producing temperature (26° C). Redrawn from [67, 80].
As gonadal development continues, ERα and AR levels are equivalent between male- and female-producing temperatures midway through the temperature-sensitive period, but both exhibit a spike in expression at the close of the temperature-sensitive period at a female-producing temperature [85] (Figure 3). Both then drop and are roughly equivalent, or slightly higher in the developing testis throughout the phase of gonadal differentiation. Importantly, the female-specific spike in expression correlates with the time of ovarian sex determination as well as increased aromatase expression at the female-producing temperature. This difference appears purely quantitative in nature, as none of the steroid receptors exhibited differential localization patterns until after the temperature-sensitive period had closed and gonadal differentiation was complete [85].
The higher AR expression observed during ovarian determination is consistent with growing evidence suggesting it may play a role in ovarian differentiation in species where aromatase expression is key to ovarian development. In the slider turtle, AR expression responds to the inhibition of aromatase activity even before ERα and ERβ patterns change, indicating that AR expression patterns are sensitive to changes in estrogen availability [85]. AR expression levels are also temperature-sensitive in the snapping turtle, showing a significant increase in expression four days after a shift from male- to female-producing temperatures [81]. In the chicken, AR is present in both testes and ovaries during development, but is expressed at higher levels in the left ovary than the testis [90]. Interestingly, in ovo treatment of female chick embryos with the AR antagonist flutamide results in disorganized ovarian cortical development [90]. This disorganization can be rescued by co-application of either testosterone or estradiol with flutamide, indicating a possible role for androgen signaling in sex-specific aromatase action in ovarian development.
ERβ exhibits a unique pattern of expression in the developing slider turtle gonad, particularly when contrasted with ERα (Figure 3). Levels are unchanging at both temperatures throughout the temperature-sensitive period, and it is only later during ovarian differentiation that a strong female-specific increase in ERβ is observed [85] (Figure 3). In mammals, ERβ is predominantly expressed in granulosa cells of the ovary [103, 104], and ERβ expression in the slider turtle ovary coincides with the developmental timing of cortical cell proliferation [10]. If ERβ cell-type expression is conserved between mammals and the slider turtle, then this increase may reflect granulosa cell proliferation, although probably not granulosa cell differentiation. FoxL2, a conserved granulosa cell marker [105–107], is already differentially expressed in the developing cortical compartment and at significantly higher levels at female-producing temperatures by the time of ovarian commitment [50]. The lack of cortex-specific ER expression during ovarian determination indicates granulosa cell differentiation is probably not an estrogen-dependent event in slider turtle ovarian development.
The distinct patterns of expression for ERα and ERβ suggest they may play different roles in slider turtle ovarian development, with ERα mediating the first component of ovarian determination (medullary regression), and ERβ playing a role in estrogen contribution to cortical proliferation later in ovarian development (Figure 2). In addition, these divergent patterns also suggest that unlike ERα, ERβ is not an early target of female-producing temperature in ovarian determination [85].
In contrast to aromatase, ERα, ERβ and AR, Sf1 is expressed at higher levels at male-producing temperatures than at female-producing temperature during the temperature-sensitive period (Figure 3). Throughout gonadal development, Sf1 abundance and localization patterns do not appear to be correlated with aromatase patterns, although Sf1 is certainly present at both sexes in the early bipotential gonad, and does exhibit low-levels of diffuse signal throughout ovarian commitment and differentiation [51, 67] (Figure 3). While a regulatory relationship between Sf1 and aromatase cannot be excluded, our data do not support such a relationship, particularly in terms of initiating early female-specific aromatase expression. Sf1 expression increases transiently following a shift from a male- to a female-producing temperature in the snapping turtle, however this increase is subsequent to increases in aromatase and FoxL2 [81]. As in the slider turtle, Sf1 does not appear necessary for the initiation of higher aromatase expression in the snapping turtle, but may be a critical regulator for sexually dimorphic steroidogenic activity.
The interaction between aromatase and Sf1 appears complex, and examples of aromatase expression in the absence of Sf1 are found even in mammalian models. For example, aromatase expression is a marker for undifferentiated adipose cells, but these cells do not express Sf1. Instead they express Lrh1, a closely related gene to Sf1 that can also regulate aromatase [108]. In the adult ovary, Sf1 is predominately expressed in (androgenic) theca cells, while aromatase and Lrh1 are predominately expressed in granulosa and luteal cells [109, 110], although it is important to note that ovarian maintenance and function is impaired in granulosa-cell specific Sf-1 knockouts [111],
FoxL2 regulates aromatase in mammals [64, 112], and chickens [105, 113], and so is another strong candidate to regulate aromatase in the slider turtle gonad. In TSD reptiles, FoxL2 is expressed higher at female- than male-producing temperatures [50, 106], and is upregulated along with aromatase following a temperature shift to female-producing temperatures in the snapping turtle [81]. However, additional factors are probably involved in aromatase regulation in the slider turtle gonad because aromatase localization is already differential with temperature early in the bipotential phase of gonadal development, while FoxL2 is not clearly differentially localized until ovarian fate is committed [50, 67].
Temperature-induced versus estrogen-induced sex-reversal
The above material makes clear that incubation temperature, steroid hormones, and genes interact with gonadal sex determination in the slider turtle. The effect of temperature and hormones can be partially uncoupled by examining changes in gene expression patterns following temperature shift or hormonal override of a temperature cue. Manipulating or overriding the temperature cue during this labile period of development means gonadal sex must essentially be re-determined towards the destination sex, and in the case of male-to-female sex-reversal must include both the repression of male development and the activation of female development (Figure 2).
Steroid signaling components may contribute to both of these processes. For example, although the regulatory relationships among slider turtle testis-determining genes are not currently known, two conserved testis-associated genes, Sf1 and Dmrt1, are downregulated in response to exogenous estrogen [100, 114]. In addition, the Sertoli cell markers Sox9 and Mis have ER binding sites (estrogen response element sequences) in their regulatory promoter regions according to genomic screens on mammalian sequences [115, 116].
The effect of temperature on steroid signaling
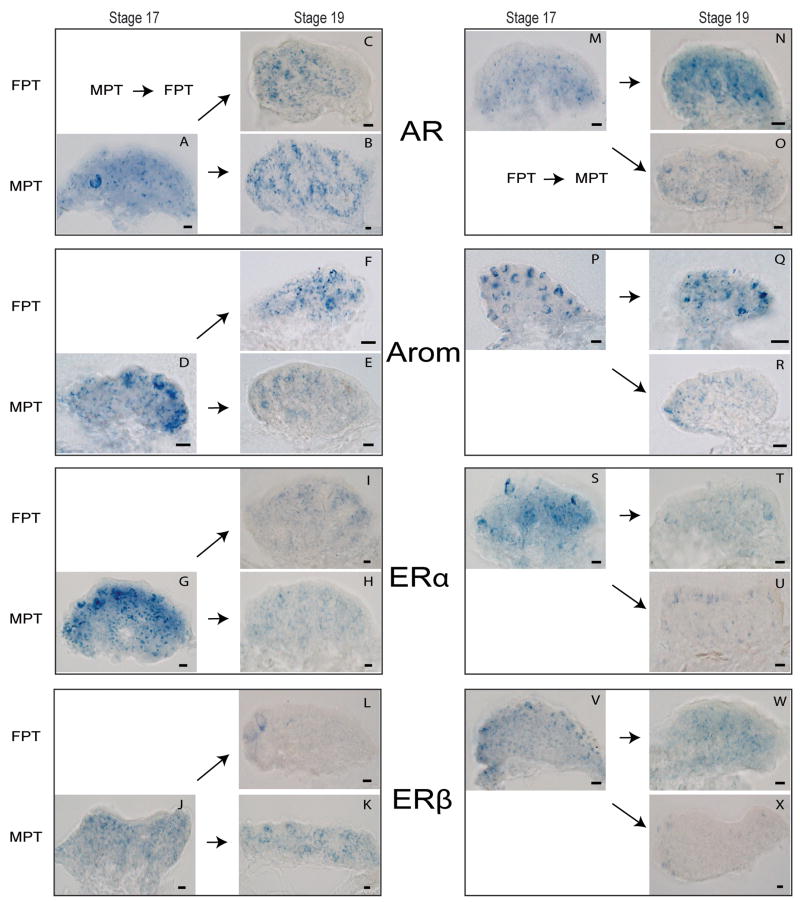
After a male-to-female shift (Figure 5A–K), AR, aromatase, and ERα exhibit organized expression along the degenerating medullary sex cords (Figure 5; C,F,I), while Sf1, presumably reflecting its male-specific role during determination, instead shifts to a more diffuse expression pattern than that seen in unshifted controls (data not shown) [67]. In contrast, ERβ expression declines throughout the medullary region following MPT→FPT shift (Figure 5; L), supporting the idea that ERβ function is primarily later in ovarian differentiation (Figure 2). Aromatase and ERα expression may mediate estrogen action in the medullary compartment following the feminizing temperature shift (Figure 2), however the exact role of AR in ovarian development is still unclear.
Figure 5.
Steriod signaling component expression is altered by temperature shift in the red-eared slider turtle (Trachemys scripta). The left column (Panels A–L) depict gene expression following temperature shifts from MPT → FPT, for AR (A,B,C), aromatase (D,E,F), ERα (G,H,I), ERβ (J,K,L). The right column (Panels M-X) depict gene expression following temperature shift from FPT → MPT, for AR (M,N,O), aromatase (P,Q,R), ERα (S,T,U), ERβ (V,W,X). Temperature shifts occurred at Stage 17, and expression changes were assayed at Stage 19. For each box, horizontal arrows indicate continued incubation at the original temperature, while diagonal arrows indicate a shift to the opposite temperature. Abbreviations as in Figure 4. Redrawn from [67, 85].
The opposing shift from female- to male-producing temperature during the temperature-sensitive period results in testis development (Figure 5M–X). Following a masculinizing shift, aromatase, ERα and ERβ medullary expression is reduced (Figure 5; R, U, X) – possibly relieving estrogen-induced repression of male gene expression. The appearance of male-typical AR expression along the developing seminiferous tubules is delayed in response to the masculinizing shift (Figure 5B,O), indicating that like ERβ in the ovary, AR may play more of a differentiation rather than determination role in testis development [85]. In contrast, Sf1 expression is more rapidly redirected towards a more male-typical medullary expression pattern [67], supporting the idea that in the slider turtle - as it does in the mouse - Sf1 may primarily function in testis development.
The effect of exogenous estrogen on steroid signaling
The estrogen signaling network responds differently to ovarian sex determination via exogenous estrogen than temperature. In contrast to the patterns observed following a feminizing temperature shift, administration of E2 causes both ERα and ERβ to be downregulated in the medullary compartment, but does not alter aromatase expression patterns [67, 85]. The downregulation of ERα was particularly dramatic, and was evident within one developmental stage after E2 application – possibly indicating a feedback relationship between estrogen levels and ERα expression.
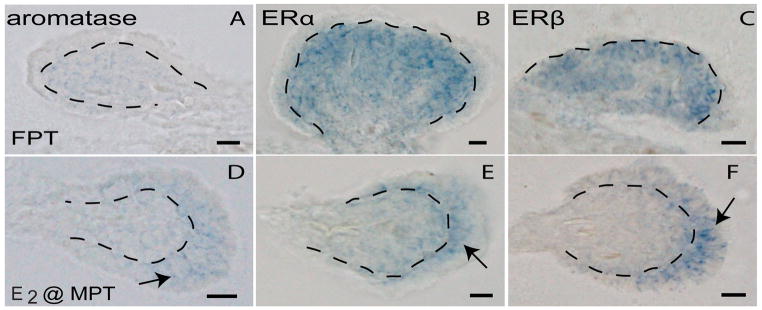
In fully differentiated ovaries formed at female-producing temperatures, aromatase, ERα and ERβ expression is difficult to detect using whole mount in situ hybridization, however, when it is detectable, expression is predominately confined to the medullary region [67, 85] (Figure 6; A,B,C). In contrast, localization for all three genes is shifted towards the cortical compartment in E2-created ovaries incubated at the male-producing temperature (Figure 6; D,E,F). This shift in localization following E2 treatment is not seen with either Sf1 or AR (data not shown) [67, 85]. The altered expression patterns in the estrogen signaling network leads to the intriguing hypothesis that cortical estrogen synthesis and signal transduction may be needed to maintain E2-induced ovarian development at male-producing temperatures, and that under normal conditions of low estrogen concentration at the male-producing temperature, male temperatures may antagonize cortical development.
Figure 6.
Ovarian differentiation in response to a female-producing temperature versus exogenous estrogen at a male-producing temperature in the red-eared slider turtle (Trachemys scripta). Feminizing temperature and estrogen are not equivalent signals for estrogen signaling components during ovarian differentiation (Stage 23). Aromatase (A,D), ERα (B,E), and ERβ (C,F) are depicted in ovaries created at constant incubation at a female-producing temperature versus ovaries created as a result of estradiol (E2) treatment of eggs at Stage 17. Dotted lines indicate the basement membrane separating the medullary and cortical compartments. Arrows in (D,E,F) highlight the shift in localization towards the cortical region. Abbreviations as in Figure 4. Redrawn from [67, 85].
Conclusions
The slider turtle model system allows unique insight into sex determination and differentiation because of the ability to deconstruct the complex interplay between temperature and steroid hormones. Here we build a model for TSD wherein warm temperatures either directly or indirectly cause increased estrogen production within the gonad that in turn directs ovarian development while inhibiting testis-specific gene expression. As development continues, higher estrogen production within the gonad at female-producing temperatures helps to mediate ovarian-specific processes including regression of the medullary compartment and proliferation of the cortical region.
In contrast, localized estrogen production within the gonad is inhibited at male-producing temperatures. In the absence of aromatase, testis-specific genes such as Dmrt1, Sox9 and Mis are upregulated, resulting in testis determination and development.
Acknowledgments
We thank Raymond Porter, Christina Shoemaker and James Skipper for valuable advice and support, and Creagh Bruener and Thane Wibbels for helpful discussions. This work was supported by NSF grant # IBN 200001269 to DC.
Footnotes
For Special Issue on Environmental Sex Determination in “Seminars in Cell and Developmental Biology”
Sex has many definitions, from primary (gonadal) to gender identity to legal identity [5]. Here, we use the term sex to refer to the type of gonad that forms.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Manolakou P, Lavranos G, Angelopoulou R. Molecular patterns of sex determination in the animal kingdom: a comparative study of the biology of reproduction. Reproductive Biology and Endocrinology. 2006;4:59–81. doi: 10.1186/1477-7827-4-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sarre SD, Georges A, Quinn A. The ends of a continuum: genetic and temperature-dependent sex determination in reptiles. BioEssays. 2004;26:639–45. doi: 10.1002/bies.20050. [DOI] [PubMed] [Google Scholar]
- 3.Bull JJ. Sex determination in reptiles. The Quarterly Review of Biology. 1980;55:3–21. [Google Scholar]
- 4.Janzen FJ, Paukstis GL. Environmental sex determination in reptiles: Ecology, evolution, and experimental design. Quarterly Review of Biology. 1991;66:149–79. doi: 10.1086/417143. [DOI] [PubMed] [Google Scholar]
- 5.Crews D. Functional associations in behavioral endocrinology. In: Reinisch JM, Rosenblum LA, Sanders SA, editors. Masculinity/Feminity: Basic Perspectives. Oxford, England: Oxford University Press; 1987. pp. 83–106. [Google Scholar]
- 6.Quinn AE, Georges A, Sarre SD, Guarino F, Ezaz T, Graves JAM. Temperature sex reversal implies sex gene dosage in a reptile. Science. 2007;316:411. doi: 10.1126/science.1135925. [DOI] [PubMed] [Google Scholar]
- 7.Shine R, Elphick MJ, Donnellan S. Co-occurrence of multiple, supposedly incompatible modes of sex determination in a lizard population. Ecology Letters. 2002;5:486–9. [Google Scholar]
- 8.Robert KA, Thompson MB. Viviparous lizard selects sex of embryos. Nature. 2001;412:698–9. doi: 10.1038/35089135. [DOI] [PubMed] [Google Scholar]
- 9.Radder RS, Quinn AE, Georges A, Sarre SD, Shine R. Genetic evidence for co-occurrence of chromosomal and thermal sex-determining systems in a lizard. Biology Letters. 2008;4:176–8. doi: 10.1098/rsbl.2007.0583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wibbels T, Bull JJ, Crews D. Chronology and morphology of temperature-dependent sex determination. The Journal of Experimental Zoology. 1991;260:371–81. doi: 10.1002/jez.1402600311. [DOI] [PubMed] [Google Scholar]
- 11.Pieau C, Dorizzi M. Determination of temperature sensitive stages for sexual differentiation of the gonads in embryos of the turtle Emys orbicularis. Journal of Morphology. 1981;170:372–82. doi: 10.1002/jmor.1051700308. [DOI] [PubMed] [Google Scholar]
- 12.Yntema CL. Temperature levels and periods of sex determination during incubation of eggs of Chelydra serpentina. Journal of Morphology. 1979;159:17–28. doi: 10.1002/jmor.1051590103. [DOI] [PubMed] [Google Scholar]
- 13.Bull JJ. Temperature-sensitive periods of sex determination in a lizard: Comparisons with turtles and crocodilians. Journal of Experimental Zoology. 1987;241:143–8. doi: 10.1002/jez.1402180315. [DOI] [PubMed] [Google Scholar]
- 14.Bull JJ, Vogt RC. Temperature-sensitive periods of sex determination in emydid turtles. Journal of Experimental Zoology. 1981;218:435–40. doi: 10.1002/jez.1402180315. [DOI] [PubMed] [Google Scholar]
- 15.Ferguson MWJ, Joanen T. Temperature dependent sex determination in Alligator Mississippiensis. Journal of Zoology. 1983;200:143–77. [Google Scholar]
- 16.Wibbels T, Bull JJ, Crews D. Synergism between temperature and estradiol: a common pathway in turtle sex determination? Journal of Experimental Zoology. 1991;260:130–4. doi: 10.1002/jez.1402600117. [DOI] [PubMed] [Google Scholar]
- 17.Merchant-Larios H, Ruiz-Ramirez S, Moreno-Mendoza NA, Marmolejo-Valencia A. Correlation among thermosensitive period, estradiol response, and gonad differentiation in the sea turtle Lepidochelys olivacea. General and Comparative Endocrinology. 1997;107:373–85. doi: 10.1006/gcen.1997.6946. [DOI] [PubMed] [Google Scholar]
- 18.Scheib D. Effects and role of estrogens in avian gonadal differentiation. Differentiation. 1983;23:S87–S92. doi: 10.1007/978-3-642-69150-8_15. [DOI] [PubMed] [Google Scholar]
- 19.Baroiller J-F, Guiguen Y, Fostier A. Endocrine and environmental aspects of sex differentiation in fish. CMLS. 1999;55:910–31. doi: 10.1007/978-3-0348-7781-7_9. [DOI] [PubMed] [Google Scholar]
- 20.Bull JJ, Gutzke WHN, Crews D. Sex reversal by estradiol in three reptilian orders. General and Comparative Endocrinology. 1988;70:425–8. doi: 10.1016/0016-6480(88)90117-7. [DOI] [PubMed] [Google Scholar]
- 21.Freedberg S, Bowden RM, Ewert MA, Sengelaub DR, Nelson CE. Long-term sex reversal by oestradiol in amniotes with heteromorphic sex chromosomes. Biology Letters. 2006;2:378–81. doi: 10.1098/rsbl.2006.0454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wallace H, Badawy GMI, Wallace BMN. Amphibian sex determination and sex reversal. CMLS. 1999;55:901–9. doi: 10.1007/s000180050343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crews D, Bergeron JM. Role of reductase and aromatase in sex determination in the red-eared slider (Trachemys scripta), a turtle with temperature-dependent sex determination. Journal of Endocrinology. 1994;143:279–89. doi: 10.1677/joe.0.1430279. [DOI] [PubMed] [Google Scholar]
- 24.Wibbels T, Gideon P, Bull JJ, Crews D. Estrogen- and temperature-induced medullary cord regression during gonadal differentiation in a turtle. Differentiation. 1993;53:149–54. doi: 10.1111/j.1432-0436.1993.tb00703.x. [DOI] [PubMed] [Google Scholar]
- 25.Crews D, Bergeron JM, Bull JJ, Flores D, Tousignant A, Skipper JK, Wibbels Temperature-dependent sex determination in reptiles: Proximate mechanisms, ultimate outcomes, and practical applications. Developmental Genetics. 1994;15:297–312. doi: 10.1002/dvg.1020150310. [DOI] [PubMed] [Google Scholar]
- 26.Desvages G, Pieau C. Aromatase activity in gonads of turtle embryos as a function of the incubation temperature of eggs. J Steroid Biochem Molec Biol. 1992;41:851–3. doi: 10.1016/0960-0760(92)90437-n. [DOI] [PubMed] [Google Scholar]
- 27.Pieau C, Dorizzi M. Oestrogens and temperature-dependent sex determination in reptiles: all is in the gonads. Journal of Endocrinology. 2004;181:367–77. doi: 10.1677/joe.0.1810367. [DOI] [PubMed] [Google Scholar]
- 28.Bowden RM, Ewert MA, Nelson CE. Environmental sex determination in a reptile varies seasonally and with yolk hormones. Proc R Soc Lond B. 200;267:1745–9. doi: 10.1098/rspb.2000.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Janzen FJ, Wilson ME, Tucker JK, Ford SP. Endogenous yolk steroid hormones in turtles with different sex-determining mechanisms. General and Comparative Endocrinology. 1998;111:306–17. doi: 10.1006/gcen.1998.7115. [DOI] [PubMed] [Google Scholar]
- 30.Bowden RM, Ewert MA, Freedberg S, Nelson CE. Maternally derived yolk hormones vary in follicles of the painted turtle, Chrysemys picta. Journal of Experimental Zoology. 2002;293:67–72. doi: 10.1002/jez.10094. [DOI] [PubMed] [Google Scholar]
- 31.Elf PK, Lang JW, Fivizzani AJ. Dynamics of yolk steroid hormones during development in a reptile with temperature-dependent sex determination. General and Comparative Endocrinology. 2002;127:34–9. doi: 10.1016/s0016-6480(02)00018-7. [DOI] [PubMed] [Google Scholar]
- 32.Elf PK. Yolk steroid hormones and sex determination in reptiles with TSD. General and Comparative Endocrinology. 2003;132:349–55. doi: 10.1016/s0016-6480(03)00098-4. [DOI] [PubMed] [Google Scholar]
- 33.St Juliana JR, Bowden RM, Janzen FJ. The impact of behavioral and physiological maternal effects on offspring sex ratio in the common snapping turtle, Chelydra serpentina. Behav Ecol Sociobiol. 2004;56:270–8. [Google Scholar]
- 34.Radder RS, Ali S, Shine R. Offspring sex is not related to maternal allocation of yolk steroids in the lizard Bassiana duperreyi (Scincidae) Physiological and Biochemical Zoology. 2007;80:220–7. doi: 10.1086/510639. [DOI] [PubMed] [Google Scholar]
- 35.Lance VA. Is regulation of aromatase expression in reptiles the key to understanding temperature-dependent sex determination? Journal of Experimental Zoology A. 2008;309A doi: 10.1002/jez.465. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 36.Radder RS. Maternally derived egg yolk steroid hormones and sex determination: Review of a paradox in reptiles. Journal of Biosciences. 2007;32:1213–20. doi: 10.1007/s12038-007-0123-z. [DOI] [PubMed] [Google Scholar]
- 37.Wibbels T, Crews D. Steroid-induced sex determination at incubation temperatures producing mixed sex ratios in a turtle with TSD. General and Comparative Endocrinology. 1995;100:53–60. doi: 10.1006/gcen.1995.1132. [DOI] [PubMed] [Google Scholar]
- 38.Wibbels T, Crews D. Specificity of steroid hormone-induced sex determination in a turtle. Journal of Endocrinology. 1992;133:121–9. doi: 10.1677/joe.0.1330121. [DOI] [PubMed] [Google Scholar]
- 39.Wibbels T, Bull JJ, Crews D. Steroid hormone-induced male sex determination in an amniotic vertebrate. Journal of Experimental Zoology. 1992;262:454–7. doi: 10.1002/jez.1402620413. [DOI] [PubMed] [Google Scholar]
- 40.Crews D, Lou W, Fleming AS, Ogawa S. From gene networks underlying sex determination and gonadal differentiation to the development of neural networks regulating sociosexual behavior. Brain Research. 2006;1126:109–21. doi: 10.1016/j.brainres.2006.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guan G, Kobayashi T, Nagahama Y. Sexually dimorphic expression of two types of DM (Doublesex/Mab-3)-domain genes in a teleost fish, the tilapia (Oreochromis niloticus) Biochemical and Biophysical Research Communications. 200;272:662–6. doi: 10.1006/bbrc.2000.2840. [DOI] [PubMed] [Google Scholar]
- 42.Shibata K, Takase M, Nakamura M. The Dmrt1 expression in sex-reversed gonads of amphibians. General and Comparative Endocrinology. 2002;127:232–41. doi: 10.1016/s0016-6480(02)00039-4. [DOI] [PubMed] [Google Scholar]
- 43.Clinton M. Sex determination and gonadal development: A bird’s eye view. Journal of Experimental Zoology. 1998;281:457–65. doi: 10.1002/(sici)1097-010x(19980801)281:5<457::aid-jez10>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 44.Smith CA, Sinclair AH. Sex determination in the chicken embryo. Journal of Experimental Zoology. 2001;290:691–9. doi: 10.1002/jez.1119. [DOI] [PubMed] [Google Scholar]
- 45.Kettlewell JR, Raymond CS, Zarkower D. Temperature-dependent expression of turtle Dmrt1 prior to sexual differentiation. Genesis. 200;26:174–8. [PubMed] [Google Scholar]
- 46.Maldonado LCT, Piedra AL, Moreno-Mendoza N, Valencia AM, Martinez AM, Merchant-Larios H. Expression profiles of Dax1, Dmrt1, and Sox9 during temperature sex determination in gonads of the sea turtle Lepidochelys olivacea. General and Comparative Endocrinology. 2002;129:20–6. doi: 10.1016/s0016-6480(02)00511-7. [DOI] [PubMed] [Google Scholar]
- 47.Spotila LD, Spotila JR, Hall SE. Sequence and expression analysis of WT1 and Sox9 in the red-eared slider turtle, Trachemys scripta. Journal of Experimental Zoology. 1998;281:417–27. doi: 10.1002/(sici)1097-010x(19980801)281:5<417::aid-jez7>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 48.Western PS, Harry JL, Graves JAM, Sinclair AH. Temperature-dependent sex determination in the American alligator: expression of SF1, WT1, and DAX1 during gonadogenesis. Gene. 200;241:223–32. doi: 10.1016/s0378-1119(99)00466-7. [DOI] [PubMed] [Google Scholar]
- 49.Shoemaker C, Ramsey M, Queen J, Crews D. Expression of Sox9, Mis and Dmrt1 in the gonad of a species with temperature-dependent sex determination. Developmental Dynamics. 2007;236:1055–63. doi: 10.1002/dvdy.21096. [DOI] [PubMed] [Google Scholar]
- 50.Shoemaker C, Queen J, Crews D. Response of candidate sex-determining genes to changes in temperature reveals their involvement in the molecular network underlying temperature-dependent sex determination. Molecular Endocrinology. 2007;21:2750–63. doi: 10.1210/me.2007-0263. [DOI] [PubMed] [Google Scholar]
- 51.Fleming A, Wibbels T, Skipper JK, Crews D. Developmental expression of steroidogenic factor 1 in a turtle with temperature-dependent sex determination. General and Comparative Endocrinology. 1999;116:336–46. doi: 10.1006/gcen.1999.7360. [DOI] [PubMed] [Google Scholar]
- 52.Valenzuela N, LeClere A, Shikano T. Comparative gene expression of steroidogenic factor 1 in Chrysemys picta and Apalone mutica turtles with temperature-dependent and genotypic sex determination. Evolution and Development. 2006;8:424–32. doi: 10.1111/j.1525-142X.2006.00116.x. [DOI] [PubMed] [Google Scholar]
- 53.Smith CA, Shoemaker C, Roeszler KN, Queen J, Crews D, Sinclair AH. Cloning and expression of R-Spondin1 in different vertebrates suggests a conserved role in ovarian development. BMC Developmental Biology. 2008:8–72. doi: 10.1186/1471-213X-8-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Couse JF, Hewitt SC, Korach KS. Receptor null mice reveal contrasting roles for estrogen receptor alpha and beta in reproductive tissues. Journal of Steroid Biochemistry and Molecular Biology. 200;74:287–96. doi: 10.1016/s0960-0760(00)00105-9. [DOI] [PubMed] [Google Scholar]
- 55.Fisher CR, Graves KH, Parlow AF, Simpson ER. Characterization of mice deficient in aromatase (ArKO) because of targeted disruption of the cyp19 gene. PNAS. 1998;95:6965–70. doi: 10.1073/pnas.95.12.6965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Coveney D, Shaw G, Renfree MB. Estrogen-induced gonadal sex reversal in the tammar wallaby. Biology of Reproduction. 2001;65:613–21. doi: 10.1095/biolreprod65.2.613. [DOI] [PubMed] [Google Scholar]
- 57.Couse JF, Hewitt SC, Bunch DO, Sar M, Walker VR, Davis BJ, et al. Postnatal sex reversal of the ovaries in mice lacking estrogen receptors α and β. Science. 1999;286:2328–31. doi: 10.1126/science.286.5448.2328. [DOI] [PubMed] [Google Scholar]
- 58.Dupont S, Dennefeld C, Krust A, Chambon P, Mark M. Expression of Sox9 in granulosa cells lacking the estrogen receptors, ERα and ERβ. Developmental Dynamics. 2003;226:103–6. doi: 10.1002/dvdy.10202. [DOI] [PubMed] [Google Scholar]
- 59.Dupont S, Krust A, Gansmuller A, Dierich A, Chambon P, Mark M. Effect of single and compound knockouts of estrogen receptors alpha (ERα) and beta (ERβ) on mouse reproductive phenotypes. Development. 2000;127:4277–91. doi: 10.1242/dev.127.19.4277. [DOI] [PubMed] [Google Scholar]
- 60.Britt KL, Drummond AE, Dyson M, Wreford NG, Jones MEE, Simpson ER, et al. The ovarian phenotype of the aromatase knockout (ArKO) mouse. Journal of Steroid Biochemistry and Molecular Biology. 2001;79:181–5. doi: 10.1016/s0960-0760(01)00158-3. [DOI] [PubMed] [Google Scholar]
- 61.Britt KL, Kerr J, O’Donnell L, Jones MEE, Drummond AE, Davis SR, et al. Estrogen regulates development of the somatic cell phenotype in the eutherian ovary. FASEB Journal. 2002;16:1389–97. doi: 10.1096/fj.01-0992com. [DOI] [PubMed] [Google Scholar]
- 62.Pailhoux E, Vigier B, Vaiman D, Servel N, Chaffaux S, Cribiu EP, et al. Ontogenesis of female-to-male sex-reversal in XX polled goats. Developmental Dynamics. 2002;224:39–50. doi: 10.1002/dvdy.10083. [DOI] [PubMed] [Google Scholar]
- 63.Quirke LD, Juengel JL, Tisdall DJ, Lun S, Heath DA, McNatty KP. Ontogeny of steroidogenesis in the fetal sheep gonad. Biology of Reproduction. 2001;65:216–28. doi: 10.1095/biolreprod65.1.216. [DOI] [PubMed] [Google Scholar]
- 64.Pannetier M, Fabre S, Batista F, Kocer A, Renault L, Jolivet G, et al. FOXL2 activates P450 aromatase gene transcription: towards a better characterization of the early steps of mammalian ovarian development. Journal of Molecular Endocrinology. 2006;36:399–413. doi: 10.1677/jme.1.01947. [DOI] [PubMed] [Google Scholar]
- 65.Wibbels T, Bull JJ, Crews D. Temperature-dependent sex determination: A mechanistic approach. Journal of Experimental Zoology. 1994;270:71–8. doi: 10.1002/jez.1402600311. [DOI] [PubMed] [Google Scholar]
- 66.Raynaud A, Pieau C. Embryonic development of the genital system. In: Gans C, editor. Biology of the Reptilia. New York: Wiley; 1985. pp. 151–300. [Google Scholar]
- 67.Ramsey M, Shoemaker C, Crews D. Gonadal expression of Sf1 and aromatase during sex determination in the red-eared slider turtle (Trachemys scripta), a reptile with temperature-dependent sex determination. Differentiation. 2007;75:978–91. doi: 10.1111/j.1432-0436.2007.00182.x. [DOI] [PubMed] [Google Scholar]
- 68.Yao HH-C, DiNapoli L, Capel B. Cellular mechanisms of sex determination in the red-eared slider turtle (Trachemys scripta) Mechanisms of Development. 2004;121:1393–401. doi: 10.1016/j.mod.2004.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.White RB, Thomas P. Adrenal-kidney and gonadal steroidogenesis during sexual differentiation of a reptile with temperature-dependent sex determination. General and Comparative Endocrinology. 1992;88:10–19. doi: 10.1016/0016-6480(92)90189-q. [DOI] [PubMed] [Google Scholar]
- 70.White RB, Thomas P. Whole-body and plasma concentrations of steroids in the turtle, Trachemys scripta, before, during, and after the temperature-sensitive period for sex determination. Journal of Experimental Zoology. 1992;264:159–66. [Google Scholar]
- 71.White RB, Thomas P. Stimulation of the in vitro steroidogenesis by pituitary hormones in a turtle (Trachemys scripta) within the temperature-sensitive period for sex determination. Biology of Reproduction. 1992;47:952–9. doi: 10.1095/biolreprod47.6.952. [DOI] [PubMed] [Google Scholar]
- 72.Thomas EO, Licht P, Wibbels T, Crews D. Hydroxysteroid dehydrogenase activity associated with sexual differentiation in embryos of the turtle Trachemys scripta. Biology of Reproduction. 1992;46:140–5. doi: 10.1095/biolreprod46.1.140. [DOI] [PubMed] [Google Scholar]
- 73.Pieau C, Dorizzi M, Richard-Mercier N. Temperature-dependent sex determination and gonadal differentiation in reptiles. CMLS. 1999;55:887–900. doi: 10.1007/s000180050342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jeyasuria P, Place AR. Embryonic brain-gonadal axis in temperature-dependent sex determination of reptiles: A role for P450 aromatase (CYP19) Journal of Experimental Zoology. 1998;281:428–49. [PubMed] [Google Scholar]
- 75.Smith CA, Elf PK, Lang JW, Joss JMP. Aromatase enzyme activity during gonadal sex differentiation in alligator embryos. Differentiation. 1995;58:281–90. [Google Scholar]
- 76.Gabriel WN, Blumberg B, Sutton S, Place AR, Lance VA. Alligator aromatase cDNA sequence and its expression in embryos at male and female incubation temperatures. Journal of Experimental Zoology. 2001;290:439–48. doi: 10.1002/jez.1087. [DOI] [PubMed] [Google Scholar]
- 77.Valenzuela N, Shikano T. Embryological ontogeny of aromatase gene expression in Chrysemys picta and Apalone mutica turtles: comparative patterns within and across temperature-dependent and genotypic sex-determining mechanisms. Dev Genes Evol. 2007;217:55–62. doi: 10.1007/s00427-006-0106-3. [DOI] [PubMed] [Google Scholar]
- 78.Willingham E, Baldwin R, Skipper JK, Crews D. Aromatase activity during embryogenesis in the brain and adrenal-kidney-gonad of the red-eared slider turtle, a species with temperature-dependent sex determination. General and Comparative Endocrinology. 2000;119:202–7. doi: 10.1006/gcen.2000.7505. [DOI] [PubMed] [Google Scholar]
- 79.Murdock C, Wibbels T. Cloning and expression of aromatase in a turtle with temperature-dependent sex determination. General and Comparative Endocrinology. 2003;130:109–19. doi: 10.1016/s0016-6480(02)00573-7. [DOI] [PubMed] [Google Scholar]
- 80.Ramsey M, Crews D. Adrenal-kidney-gonad complex measurements may not predict gonad-specific changes in gene expression patterns during temperature-dependent sex determination in the red-eared slider turtle (Trachemys scripta elegans) Journal of Experimental Zoology A. 2007;307:463–70. doi: 10.1002/jez.399. [DOI] [PubMed] [Google Scholar]
- 81.Rhen T, Metzger K, Schroeder A, Woodward R. Expression of putative sex-determining genes during the thermosensitive period of gonad development in the snapping turtle, Chelydra serpentina. Sexual Development. 2007;1:255–70. doi: 10.1159/000104775. [DOI] [PubMed] [Google Scholar]
- 82.Smith CA, Joss JMP. Uptake of 3H-estradiol by embryonic crocodile gonads during the period of sexual differentiation. Journal of Experimental Zoology. 1994;270:219–24. [Google Scholar]
- 83.Gahr M, Wibbels T, Crews D. Sites of estrogen uptake in embryonic Trachemys scripta, a turtle with temperature-dependent sex determination. Biology of Reproduction. 1992;46:458–63. doi: 10.1095/biolreprod46.3.458. [DOI] [PubMed] [Google Scholar]
- 84.Bergeron JM, Gahr M, Horan K, Wibbels T, Crews D. Cloning and in situ hybridization of estrogen receptor in the developing gonad of the red-eared slider turtle, a species with temperature-dependent sex determination. Develop Growth Differ. 1998;40:243–54. doi: 10.1046/j.1440-169x.1998.00013.x. [DOI] [PubMed] [Google Scholar]
- 85.Ramsey M, Crews D. Steroid signaling system responds differently to temperature and hormone manipulation in the red-eared slider turtle (Trachemys scripta elegans), a reptile with temperature-dependent sex determination. Sexual Development. 2007;1:181–96. doi: 10.1159/000102107. [DOI] [PubMed] [Google Scholar]
- 86.Tsai M-J, O’Malley BW. Molecular mechanisms of action of steroid/thyroid receptor superfamily members. Annual Review of Biochemistry. 1994;63:451–86. doi: 10.1146/annurev.bi.63.070194.002315. [DOI] [PubMed] [Google Scholar]
- 87.Zang D, Trudeau VL. Integration of membrane and nuclear estrogen receptor signaling. Comparative Biochemistry and Physiology Part A. 2006;144:306–35. doi: 10.1016/j.cbpa.2006.01.025. [DOI] [PubMed] [Google Scholar]
- 88.Andrews JE, Smith CA, Sinclair AH. Sites of estrogen receptor and aromatase expression in the chicken embryo. General and Comparative Endocrinology. 1997;108:182–90. doi: 10.1006/gcen.1997.6978. [DOI] [PubMed] [Google Scholar]
- 89.Couse JF, Lindzey JK, Grandien K, Gustafsson J-A, Korach KS. Tissue distribution and quantitative analysis of estrogen receptor-alpha (ERα) and estrogen receptor-beta (ERβ) messenger ribonucleic acid in the wild-type and ERα-knockout mouse. Endocrinology. 1997;138:4613–21. doi: 10.1210/endo.138.11.5496. [DOI] [PubMed] [Google Scholar]
- 90.Katoh H, Ogino Y, Yamada G. Cloning and expression analysis of androgen receptor gene in chicken embryogenesis. FEBS Letters. 2006;580:1607–15. doi: 10.1016/j.febslet.2006.01.093. [DOI] [PubMed] [Google Scholar]
- 91.Majdic G, Millar MR, Saunders PTK. Immunolocalization of androgen receptor to interstitial cells in fetal rat testes and to mesenchymal and epithelial cells of associated ducts. Journal of Endocrinology. 1995;147:285–93. doi: 10.1677/joe.0.1470285. [DOI] [PubMed] [Google Scholar]
- 92.Smith CA, Andrews JE, Sinclair AH. Gonadal sex differentiation in chicken embryos: Expression of estrogen receptor and aromatase genes. J Steroid Biochem Molec Biol. 1997;60:295–302. doi: 10.1016/s0960-0760(96)00196-3. [DOI] [PubMed] [Google Scholar]
- 93.Morohashi K-I, Omura T. Ad4BP/SF-1, a transcription factor essential for the transcription of steroidogenic cytochrome P450 genes and for the establishment of the reproductive function. FASEB Journal. 1996;10:1569–77. doi: 10.1096/fasebj.10.14.9002548. [DOI] [PubMed] [Google Scholar]
- 94.Parker KL, Rice DA, Lala DS, Ikeda Y, Luo X, Wong M, et al. Steroidogenic factor 1: an essential mediator of endocrine development. Recent progress in hormone research. 2002;57:19–36. doi: 10.1210/rp.57.1.19. [DOI] [PubMed] [Google Scholar]
- 95.Fan W, Yanase T, Morinaga H, Gondo S, Okabe T, Nomura M, et al. Herbicide atrazine activates SF-1 by direct affinity and concomitant co-activators recruitments to induce aromatase expression via promoter II. Biochemical and Biophysical Research Communications. 2007;355:1012–8. doi: 10.1016/j.bbrc.2007.02.062. [DOI] [PubMed] [Google Scholar]
- 96.Fan W, Yanase T, Morinaga H, Gondo S, Okabe T, Nomura M, et al. Atrazine-induced aromatase expression is SF-1 dependent: implications for endocrine disruption in wildlife and reproductive cancers in humans. Environmental Health Perspectives. 2007;115:720–7. doi: 10.1289/ehp.9758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Achermann JC, Ito M, Ito M, Hindmarsh PC, Jameson JL. A mutation in the gene encoding steroidogenic factor-1 causes XY sex reversal and adrenal failure in humans. Nature Genetics. 1999;22:125–6. doi: 10.1038/9629. [DOI] [PubMed] [Google Scholar]
- 98.Sekido R, Lovell-Badge R. Sex determination involves synergistic action of SRY and SF1 on a specific Sox9 enhancer. Nature. 2008;453:930–4. doi: 10.1038/nature06944. [DOI] [PubMed] [Google Scholar]
- 99.Ikeda Y, Shen W-H, Ingraham HA, Parker KL. Developmental expression of mouse steroidogenic factor-1, and essential regulator of the steroid hydroxylases. Molecular Endocrinology. 1994;8:654–62. doi: 10.1210/mend.8.5.8058073. [DOI] [PubMed] [Google Scholar]
- 100.Fleming A, Crews D. Estradiol and incubation temperature modulate regulation of steroidogenic factor 1 in the developing gonad of the red-eared slider turtle. Endocrinology. 2001;142:1403–11. doi: 10.1210/endo.142.4.8043. [DOI] [PubMed] [Google Scholar]
- 101.Smith CA, Smith MJ, Sinclair AH. Expression of chicken steroidogenic factor-1 during gonadal sex differentiation. General and Comparative Endocrinology. 1999;113:187–96. doi: 10.1006/gcen.1998.7214. [DOI] [PubMed] [Google Scholar]
- 102.Mayer LP, Overstreet SL, Dyer CA, Propper CR. Sexually dimorphic expression of steroidogenic factor 1 (SF-1) in developing gonads of the American bullfrog, Rana catesbeiana. General and Comparative Endocrinology. 2002;127:40–7. doi: 10.1016/s0016-6480(02)00019-9. [DOI] [PubMed] [Google Scholar]
- 103.Fitzpatrick SL, Funkhouser JM, Sindoni DM, Stevis PE, Deecher DC, Bapat AR, et al. Expression of estrogen receptor β protein in rodent ovary. Endocrinology. 1999;140:2581–91. doi: 10.1210/endo.140.6.6928. [DOI] [PubMed] [Google Scholar]
- 104.Jefferson WN, Couse JF, Banks EP, Korach KS, Newbold RR. Expression of estrogen receptor β is developmentally regulated in reproductive tissues of male and female mice. Biology of Reproduction. 2000;62:310–7. doi: 10.1095/biolreprod62.2.310. [DOI] [PubMed] [Google Scholar]
- 105.Govoroun M, Pannetier M, Pailhoux E, Cocquet J, Brillard J-P, Couty I, et al. Isolation of chicken homolog of the FOXL2 gene and comparison of its expression patterns with those of aromatase during ovarian development. Developmental Dynamics. 2004;231:859–70. doi: 10.1002/dvdy.20189. [DOI] [PubMed] [Google Scholar]
- 106.Loffler KA, Zarkower D, Koopman P. Etiology of ovarian failure in blepharophimosis ptosis epicanthus inversus syndrome: FOXL2 is a conserved, early-acting gene in vertebrate ovarian development. Endocrinology. 2003;144:3237–43. doi: 10.1210/en.2002-0095. [DOI] [PubMed] [Google Scholar]
- 107.Schmidt D, Ovitt CE, Anlag K, Fehsenfeld S, Gredsted L, Treier A-C, et al. The murine winged-helix transcription factor Foxl2 is required for granulosa cell differentiation and ovary maintenance. Development. 2004;131:933–42. doi: 10.1242/dev.00969. [DOI] [PubMed] [Google Scholar]
- 108.Clyne CD, Speed CJ, Zhou J, Simpson ER. Liver receptor homologue-1 (LRH-1) regulates expression of aromatase in preadipocytes. Journal of Biological Chemistry. 2002;277:20591–7. doi: 10.1074/jbc.M201117200. [DOI] [PubMed] [Google Scholar]
- 109.Fayard E, Auwerx J, Schoonjans K. LRH-1: an orphan nuclear receptor involved in development, metabolism and steroidogenesis. TCB. 2004;14:250–60. doi: 10.1016/j.tcb.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 110.Hinshelwood MM, Repa JJ, Shelton JM, Richardson JA, Mangelsdorf DJ, Mendelson CR. Expression of LRH-1 and SF-1 in the mouse ovary: localization in different cell types correlates with differing function. Molecular and Cellular Endocrinology. 2003;207:39–45. doi: 10.1016/s0303-7207(03)00257-0. [DOI] [PubMed] [Google Scholar]
- 111.Jeyasuria P, Ikeda Y, Jamin SP, Zhoa L, de Rooij DG, Themmen APN, Behringer RR, Parker KL. Cell-specific knockout of steroidogenic factor 1 reveals its essential roles in gonadal function. Molecular Endocrinology. 2004;18:1610–9. doi: 10.1210/me.2003-0404. [DOI] [PubMed] [Google Scholar]
- 112.Batista F, Vaiman D, Dausset J, Fellous M, Veitia RA. Potential targets of FOXL2, a transcription factor involved in craniofacial and follicular development, identified by transcriptomics. PNAS. 2007;104:3330–5. doi: 10.1073/pnas.0611326104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hudson Q, Smith CA, Sinclair AH. Aromatase inhibition reduces expression of FOXL2 in the embryonic chicken ovary. Developmental Dynamics. 2005;233:1052–5. doi: 10.1002/dvdy.20388. [DOI] [PubMed] [Google Scholar]
- 114.Murdock C, Wibbels T. Dmrt1 expression in response to estrogen treatment in a reptile with temperature-dependent sex determination. Journal of Experimental Zoology. 2006;306B:134–9. doi: 10.1002/jez.b.21076. [DOI] [PubMed] [Google Scholar]
- 115.Frasor J, Danes JM, Komm BS, Chang KCN, Lyttle CR, Katzenellenbogen BS. Profiling of estrogen up- and down-regulated gene expression in human breast cancer cells: insights into gene networks and pathways underlying estrogenic control of proliferation and cell phenotype. Endocrinology. 2003;144:4562–74. doi: 10.1210/en.2003-0567. [DOI] [PubMed] [Google Scholar]
- 116.Jin VX, Sun H, Pohar TT, Liyanarachchi S, Palaniswamy SK, Huang TH-M, Davuluri RV. ERTargetDB: an integral information resource of transcription regulation of estrogen receptor target genes. Journal of Molecular Endocrinology. 2005;35:225–30. doi: 10.1677/jme.1.01839. [DOI] [PubMed] [Google Scholar]