Abstract
Formation of a functional vasculature during mammalian development is essential for embryonic survival. In addition, imbalance in blood vessel growth contributes to the pathogenesis of numerous disorders. Most of our understanding of vascular development and blood vessel growth comes from investigating the Vegf signaling pathway as well as the recent observation that molecules involved in axon guidance also regulate vascular patterning. In order to take an unbiased, yet focused, approach to identify novel genes regulating vascular development, we performed a three-step ENU mutagenesis screen in zebrafish. We first screened live embryos visually, evaluating blood flow in the main trunk vessels, which form by vasculogenesis, and the intersomitic vessels, which form by angiogenesis. Embryos that displayed reduced or absent circulation were fixed and stained for endogenous alkaline phosphatase activity to reveal blood vessel morphology. All putative mutants were then crossed into the Tg(flk1:EGFP)s843 transgenic background to facilitate detailed examination of endothelial cells in live and fixed embryos.
We screened 4015 genomes and identified 30 mutations affecting various aspects of vascular development. Specifically, we identified 3 genes (or loci) that regulate the specification and/or differentiation of endothelial cells, 8 genes that regulate vascular tube and lumen formation, 8 genes that regulate vascular patterning, and 11 genes that regulate vascular remodeling, integrity and maintenance. Only 4 of these genes had previously been associated with vascular development in zebrafish illustrating the value of this focused screen. The analysis of the newly defined loci should lead to a greater understanding of vascular development and possibly provide new drug targets to treat the numerous pathologies associated with dysregulated blood vessel growth.
Introduction
The vasculature needs to undergo continuous modification and remodeling to accommodate the diverse needs for growth, regeneration and repair during an organism’s life. This dynamic modulation of the vascular system is achieved by intricate interactions between two distinct mechanisms, vasculogenesis (de novo assembly of vessels) and angiogenesis (modification and expansion of pre-existing vessels) (reviewed by Risau and Flamme, 1995; Risau, 1997).
Failure to regulate vasculogenesis and angiogenesis has been implicated in a wide variety of pathological conditions. Excessive vascular formation is usually associated with cancer, psoriasis, arthritis and blindness, while insufficient vascular formation is involved in a variety of inherited diseases, as well as heart and brain ischemia, neurodegeneration, and osteoporosis (reviewed by Carmeliet and Jain, 2000; Carmeliet, 2003; Carmeliet, 2005). The pathological consequences of dysregulated vascular formation have provided the impetus to understand the underlying principles of vascular system development and function, resulting in the identification of various signaling pathways and their downstream effectors (reviewed by Rossant and Howard, 2002).
The earliest event in this developmental cascade is the specification of endothelial cells, the cells lining all blood vessels. Initially, factors such as Bone Morphogenic Proteins (BMPs) (Gupta et al., 2006; Park et al., 2006) and Wnts (Lindsley et al., 2006; Wang et al., 2006) appear to define the number of potential endothelial progenitors within the nascent mesoderm. Subsequently Sonic Hedgehog (SHH) (Lawson et al., 2002; Vokes et al., 2004), Vascular Endothelial Growth Factor (VEGF) (Carmeliet et al., 1996; Ferrara et al., 1996; Cleaver and Krieg, 1998), and Notch (Krebs et al., 2000; Lawson et al., 2001) signaling pathways, as well as transcription factors including Ets family members (Dube et al., 1999; Pham et al., 2006; Sumanas and Lin, 2006), Scl/Tal1 (Kallianpur et al., 1994; Visvader et al., 1998; Patterson et al., 2005), and Coup-TFII (You et al., 2005) play critical roles in the differentiation of endothelial progenitors into arterial and venous endothelial cells. Endothelial cells then migrate towards the midline to form an aggregate known as the vascular cord, which subsequently lumenizes to form functional blood vessels (Torres-Vazquez et al., 2003; Jin et al., 2005). Extracellular matrix proteins such as Fibronectin, and mediators of cell movements such as Rac and Cdc42 provide critical functions during the migration of endothelial cells and vascular lumen formation (Jiang et al., 1994; Wijelath et al., 2002; Kamei et al., 2006). Nascent vascular networks then recruit vascular smooth muscle cells and pericytes, a process that requires the function of Platelet Derived Growth Factor (PDGF) signaling and EphrinB2-EphB4 interaction (Jain, 2003; Betsholtz et al., 2005).
Despite the progress in identifying some of the key factors required for vascular development, the functions of many signaling pathways and the interactions between them are poorly understood due to technical limitations of commonly used model systems. It is technically challenging to study the mechanisms of vascular development since the intricate architecture and context of vascular networks is difficult to reproduce in vitro and the development of the vascular system is strongly influenced by interactions between vessels and neighboring tissues. In addition, inaccessibility of mammalian embryos during development makes in vivo analyses of vascular formation a difficult task. Furthermore, the indispensable function of the placental vasculature during mammalian development constitutes another obstacle for studying the effect of genetic mutations within the developing embryo.
To understand the fundamental principles of vascular development and identify essential genes in this process, we chose to utilize the zebrafish system, since it offers a unique opportunity to overcome the aforementioned technical difficulties. In addition to its well-documented amenability to forward genetic screens (Driever et al., 1996; Haffter et al., 1996; Amsterdam et al., 1999), the externally fertilized and optically clear embryos enable one to analyze the development of the vasculature in vivo at a cellular level. Recently generated vascular specific transgenic lines, such as Tg(flk1:EGFP)s843 (Jin et al., 2005), facilitate the analyses. Although blood circulation starts at 24hpf, zebrafish embryos can survive up to 7 dpf without a functional vasculature or heart beat (Stainier et al., 1995; Stainier, 2001; Sehnert et al., 2002), allowing one to study defects in vascular formation and patterning in the developing embryo over an extended period of time.
Here we present the results of a three-step forward genetic screen in zebrafish. Initially, we identified mutants with vascular defects by observing their blood circulation and subsequently stained them for endogenous alkaline phosphatase activity, which labels endothelial cells. These analyses were followed by an in vivo analysis using a transgenic endothelial specific GFP-reporter line (Tg(flk1:EGFP)s843).
We identified 30 distinct genetic loci that regulate vascular development. Mutations in 11 of these loci interfere with vasculogenesis by causing changes in the number of endothelial cells or in vascular tube and lumen formation. The other 19 mutations affect angiogenesis by disturbing vessel patterning, remodeling, integrity and/or maintenance.
A majority of the identified mutations cause vascular defects in the context of otherwise unaffected embryos. Many of the vascular defects are similar to known human conditions such as hemangioma, aortic dissection, arterio-venous malformation, cerebral cavernous vascular malformation and cerebral hemorrhage. Given the paucity of genetic models for these human vascular conditions, these zebrafish mutants should help understand the molecular and cellular etiology of these disorders as well as provide novel insights into vascular development.
Materials and Methods
ENU mutagenesis and screening
Mutagenesis was performed by treating zebrafish (Danio rerio) males with the chemical mutagen N-nitroso-N-ethylurea, to induce mutations in premeiotic germ cells. Founder males were subjected to three to five treatments with ENU at weekly intervals and used to generate F2 generations, as previously described (Muto et al., 2005).
F3 embryos were first screened visually for defects in circulation to identify potential vascular mutants. Embryos with defective circulation were stained for endogenous alkaline phosphatase activity to highlight the vasculature. Embryos that showed absent or reduced endogenous alkaline phosphatase activity were further analyzed by in situ hybridization with flk1 to determine the extent of their phenotype, and their parents were subsequently crossed into the Tg(flk1:EGFP)s843 line (Jin et al., 2005) to analyze the phenotype at the cellular level. As a second transgenic line for phenotypic analysis we used Tg(tie2:GFP)s849 (Motoike el al., 2000).
Endogenous alkaline phosphatase activity assay, in situ hybridization, confocal analyses, immunohistochemistry, microangiography and video microscopy
Endogenous alkaline phosphatase activity assay was performed as previously described (Childs et al., 2002; Parker et al., 2004). Briefly, embryos were fixed in a 4% paraformaldehyde solution overnight and washed multiple times with 0.1% Triton in PBS then stained in 10mM Tris-HCl (pH 9). Alkaline phosphatase activity was detected by NBT/BCIP color reaction. In situ hybridizations were performed as previously described (Alexander and Stainier, 1999). Riboprobes for flk1 (Liao et al., 1997), ephrinB2a (Chan et al., 2001; Lawson et al., 2001), flt4 (Thompson et al., 1998), tie2 (Lyon et al., 1998), gata1 (Detrich et al., 1995) and ve-cadherin (Larson et al., 2004) were prepared with Roche Digoxigenin labeling kits. Embryos were mounted in benzylbenzoate:benzyl alcohol (2:1) and documented with a Zeiss Axiocam.
For confocal analyses, embryos were processed as previously described (Trinh and Stainier, 2004). Briefly, embryos were fixed overnight with 2% paraformaldehyde and embedded in 4% NuSieve GTG low melting agarose. Embedded embryos were cut with a VT1000S vibratome (Leica) into 250 μm sections. Sections were processed in PBDT [1% BSA, 1% DMSO, and 0.1% Triton X-100 in PBS (pH 7.3)]. Mouse IgG anti-β-catenin (Sigma) at 1:100 (Horne-Badovinac et al., 2003) was used to detect endothelial cell boundaries, and TOPRO (Molecular Probes) at 1:10000 (Oomman et al., 2004) was used to visualize nuclei. Processed samples were mounted in Vectashield (Vector Laboratories) and the images were acquired using a Zeiss LSM5 Pascal confocal microscope.
Microangiography was performed by injection of dextran-AlexaFluor594 (Invitrogen #D22913) into the common cardinal vein as previously described (Isogai et al, 2001). A Toshiba CCD video camera was used to capture the circulatory pattern of the embryos and Pinnacle Studio software was used to process the movies.
Complementation analyses and genetic mapping
Mutations were first categorized according to their phenotypes, and between mutations within the same group heterozygous individuals were crossed for complementation analyses. Mutations were subsequently mapped to linkage groups by bulk segregant analysis with SSLP markers.
Results
Genetic Screen for mutations that cause vascular defects
To identify genes with an essential function in vascular development, we used a forward genetic approach and performed a diploid F3 ENU mutagenesis screen in zebrafish. Specifically, the chemical mutagen ENU was used to induce premeiotic mutations in males which were subsequently bred to generate F2 carrier families as done in previous screens (Driever et al., 1996; Haffter et al., 1996).
F3 embryos were scored for reduced or absent circulation at either 36 or 60 hpf. Those with aberrant circulation pattern were fixed and stained at 36 hpf for endogenous alkaline phosphatase activity to visualize the presence and structure of the vasculature. In order to maximize the specificity of the screen, embryos with vascular defects that are usually associated with hypoxia, such as tortuous subintestinal vessels (SIVs), were not kept for subsequent analyses (data not included). In a subset of potential mutants, in situ hybridization and confocal analyses were performed at various stages to define the time point when the vascular phenotype is first manifest. All recovered mutants were crossed to the Tg(flk1:EGFP)s843 reporter line to analyze their vasculature in detail.
We screened a total of 2392 families, which, taking into account the analyzed number of families and offspring crosses per family (Mullins et al., 1994), can be calculated to correspond to 4015 mutagenized genomes. An extensive series of complementation tests and linkage group assignments indicates that these mutations define 30 different loci (Table 1).
Table 1.
Classification of mutations identified in the screen
| map position | allele | vascular defect | other defects | |
|---|---|---|---|---|
| Group I Mutations affecting the number of endothelial cells | ||||
| groom of cloche (grc) | LG16 | s635 | reduced number of endothelial cells | heart defects |
| mirinay (min) | LG16 | s202 | reduced number of endothelial cells | no blood cells |
| santa (san)* | LG19 | s234 | increased number of endothelial cells | see Stainier et al., 1996 |
| Group II Mutations affecting vasculogenesis | ||||
| Group IIA Mutations affecting the number of vascular lumens | ||||
| ménage a trois (mts) | LG16 | s233 | multiple lumens | endoderm/fin defects |
| solo (sol) | LG14 | s828 | defects in artery/vein segregation | |
| you too (yot)* | LG9 | s406 | defects in vascular cord formation | see Brand et al., 1996 |
| Group IIB Mutations affecting the size of vascular lumens | ||||
| blind alley (bly) | LG2 | s889 | dilated posterior cardinal vein | |
| poongsun (psn) | LG15 | s634 | dilated posterior cardinal vein | blood regurgitation |
| catacomb (ctb) | N/D | s479 | dilated posterior cardinal vein | |
| logelei (log)* | LG9 | s231 | reduced endothelial cells/collapsed lumen | see Schier et al., 1996 |
| valentine (vtn)* | LG20 | s259 | reduced common cardinal vein | see Stainier et al., 1996 |
| Group III Mutations affecting vascular patterning | ||||
| disoriented (did) | LG24 | s240 | missing intersegmental vessels | |
| cacophony (cpn) | LG23 | s236 | missing intersegmental vessels | |
| quo vadis (qad) | LG17 | s840 | defects in brain vasculature | smaller head |
| unfinished (unf) | LG23 | s808 | defects in trunk and brain vasculature | bent body |
| way to go (way) | LG5 | s409 | defects in outflow tract and aortic arches | |
| intersection (int) | LG7 | s413 | defects in dorsal aorta fusion | heart/fin defects, |
| sloppy vein (slv) | N/D | s887 | defects in vein patterning | |
| out of bounds (obd)* | LG8 | s601 | sprouting of intersegmental vessels | see Chen et al., 2001 |
| Group IV Mutations affecting vascular integrity/maintenance | ||||
| adrasteia (adr) | LG 23 | s277 | aortic arch dilation; later: circulation loop | |
| losing grip (lgr) | LG1 | s258 | breakdown of arch vasculature | |
| tomato (tom) | LG21 | s805 | apoptosis of endothelial cells | |
| barolo (bar) | LG8 | s847 | apoptosis of endothelial cells | cardiac edema |
| wadi (wdi) | N/D | s631 | breakdown of dorsal aorta | |
| ruche (ruc) | N/D | s869 | vascular regression | big body |
| stradivari (sra) | N/D | s877 | vascular hemorrhage | |
| reddish (reh) | LG 8 | s587 | vascular hemorrhage | |
| gluten (gln) | N/D | s839 | vascular regression | smaller head |
| nemo (nem) | N/D | s838 | vascular regression | cardiac edema |
| violet beauregarde (vbg)* | LG23 | s407 | defects in artery/vein segregation | see Roman et al. 2002 |
indicates new allele of mutant identified in a previous screen
For all mutants identified in the screen, only one allele is shown.
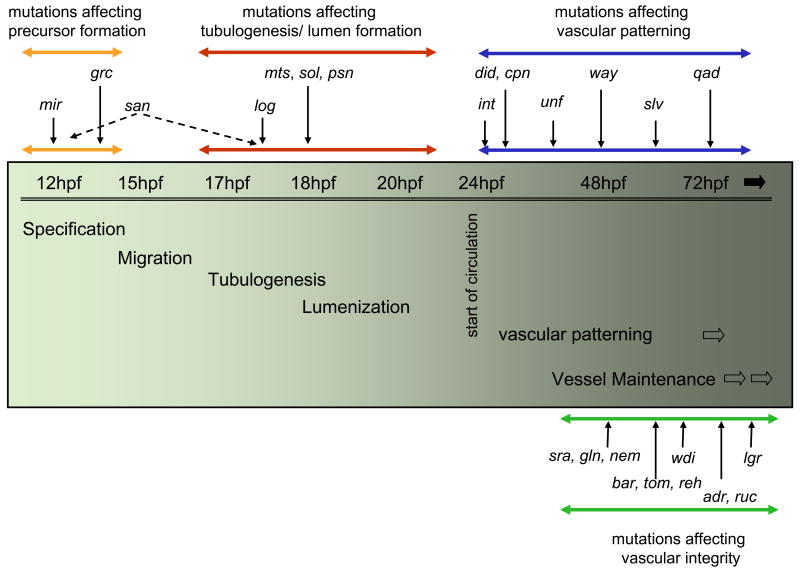
According to the phenotype of the mutants and the onset of their vascular defects, we propose four distinct phases of vascular development: (I) Specification and differentiation of endothelial precursors, (II) migration of these cells and subsequent vascular tube and lumen formation, (III) emergence and patterning of secondary vessels and (IV) maintenance of vessels.
Genes regulating the number of endothelial cells
To identify genes regulating the earlier steps of vascular development such as endothelial specification and/or differentiation, we performed a secondary in situ hybridization screen with embryos that displayed significant changes in the level of endogenous alkaline phosphatase activity, using the vascular specific marker flk1 (Liao et al., 1997; Thompson et al. 1997). Three mutations, mirinays202 (min), groom of cloches635 (grc), and santas234 (san) show discernable changes in flk1 expression (data not included). Further analyses with the Tg(flk1:EGFP)s843 line confirmed that these mutations cause defects in early vascular development, most likely by affecting endothelial precursor formation (Fig. 1C and D; Fig. 2 and data not included). The number of endothelial cells in mins202 and grcs635 mutant embryos is drastically reduced (see Fig. S1), and appears to be comparable to that in the previously identified zebrafish mutant cloche (clo) (Fig. 1B), suggesting that the genes affected by these mutations are critical for endothelial specification and/or differentiation.
Figure 1. Mutations affecting specification of endothelial precursors.
(A–D) Epifluorescent micrographs of 36 hpf wild-type (A), clos5 mutant (B), mins202 mutant (C), and grcs635 mutant (D) embryos visualized by Tg(flk1:EGFP)s843 expression. Morphologically, grcs635 and mins202 mutant embryos show no obvious defects, but the number of endothelial cells appears significantly reduced (C and D), a reduction comparable to that seen in clos5 mutant embryos (B). White arrows point to regions where Tg(flk1:EGFP)s843-positive endothelial cells are conspicuously missing.
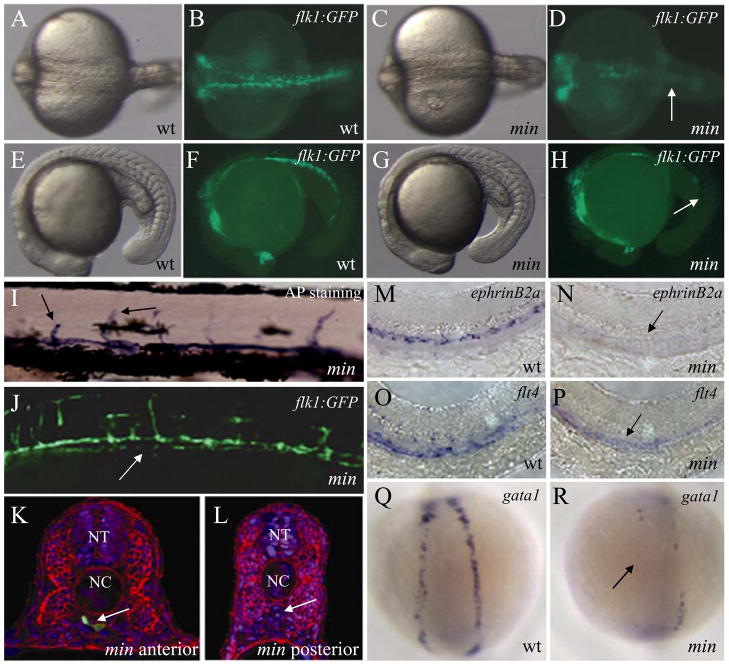
Figure 2. mirinay regulates endothelial and hematopoietic lineages.
(A–H) Bright-field (A, C, E, and G) and epifluorescent (B, D, F, and H) micrographs of 20 hpf wild-type (A, B, E, and F) and mins202 mutant (C, D, G, and H) embryos, shown in dorsal (A to D) and lateral (E to H) views. Note the reduction of endothelial cells in the posterior region of mins202 mutant embryos (arrows). (I–J) Micrographs of 96 hpf mins202 mutant larvae stained for endogenous alkaline phosphatase activity (I, bright-field) and visualized for Tg(flk1:EGFP)s843 expression (J). Black arrows point to arrested intersegmental vessels (SEs) (I), white arrow points to discontinuous axial vessel (J). (K, L) Transverse sections from anterior (K) and posterior (L) trunk of 36 hpf mins202 mutant embryos, visualized for Tg(flk1:EGFP)s843 expression (green), β-Catenin (red), and TOPRO (blue). Although the vasculature of mins202 mutant embryos eventually recovers, a drastically reduced number of endothelial cells is observed at this stage (white arrow points to the region of axial vessels). (M–R) Defective endothelial cell specification in 24 hpf mins202 mutant embryos (N and P) compared to wild-type embryos (M and O), as assessed by in situ hybridization with the arterial endothelial marker ephrinB2a (M and N), and the venous endothelial marker flt4 (O and P); and defective erythropoiesis in 18 hpf mins202 mutant embryos (R) compared to wild-type (Q), as assesed by examining gata1 expression in dorsal views. Black arrows in N and P point to the reduction in endothelial marker expression in mins202 mutant embryos, and black arrow in R points to the region of the lateral plate mesoderm where erythrocytes form in wild-type embryos.
mins202 mutant embryos show a significant reduction in the number of endothelial precursors during early development, most notably in the posterior portion of the embryos (Fig. 2A to H, S1). This phenotype becomes apparent at approximately 14 hpf. Surprisingly, these embryos form rudimentary axial vessels despite the initial deficit of endothelial cells. However, axial vessels in mins202 mutant embryos appear to be discontinuous and do not form a proper lumen (Fig. 2I to L). Subsequent intersegmental vessel (SE) formation (Fig. 2I and J), and the specification of the arterial and venous endothelial cells also appears to be affected (Fig. 2N and P). In addition, homozygous mins202 mutant embryos have a noticeable decrease in the number of primitive blood cells (Fig. 2R), suggesting that the gene affected by this mutation functions at a level prior to the specification of both lineages, as suggested for clo (Liao et al., 1997).
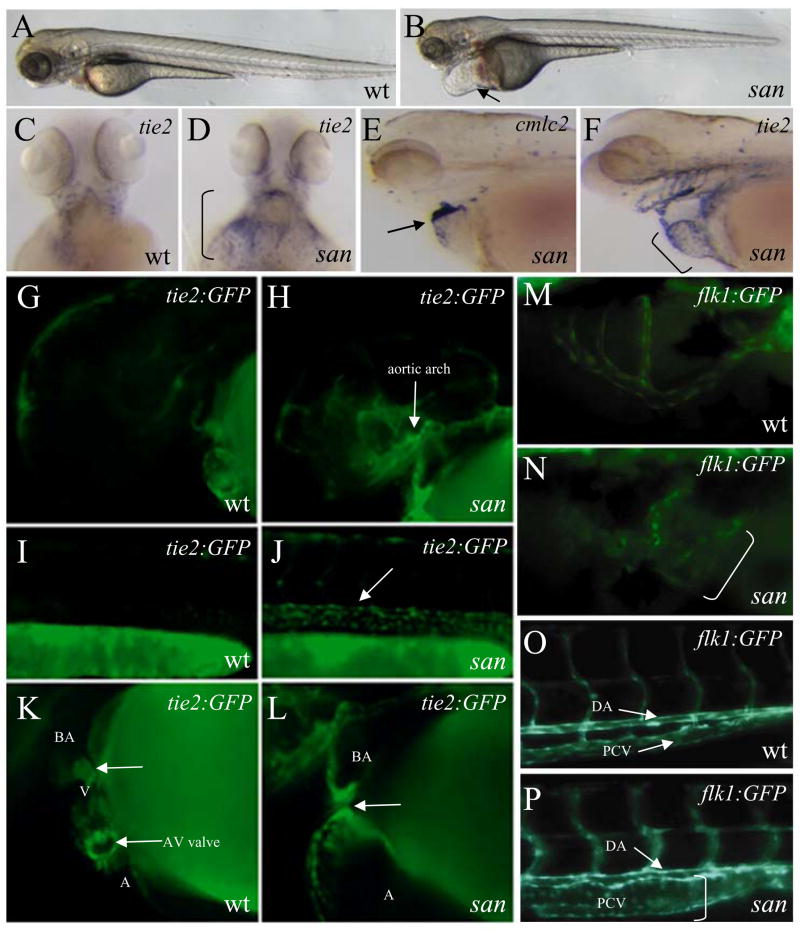
In contrast, sans234 mutant embryos show an elevated expression level of endothelial specific markers (Fig. 3). Complementation analyses showed that the mutation we identified is allelic to a known mutation, sanm775. Recently, positional cloning revealed that this mutation affects the krit1/ccm1 gene (Mably et al., 2006), which has been implicated in cerebral cavernous malformation (Whitehead et al., 2004). However, the early vascular phenotype of san/krit1 mutants has not been reported. In hemizygous Tg(tie2:GFP)s849 embryos, the transgene is hard to detect due to its low expression level (Fig. 3G, I, and K). However, in sans234 mutant embryos, the hemizygous Tg(tie2:GFP)s849 transgene expression is significantly elevated, which results in its easy detection (Fig. 3H, J, and L). Similarly, elevated levels of endogenous tie2 expression were detected in sans234 mutant embryos (Fig. 3D and F). Taken together, these observations suggest that san/krit1 negatively regulates the expression level of endothelial specific genes and/or the number of endothelial cells. At later stages, sans234 mutant embryos show a dilation of major vessels as observed in transgenic mice and human patients (Laberge-le Couteulx et al., 1999, Whitehead et al., 2004), which is most pronounced in the SIV (Fig. 3N) and the posterior cardinal vein (PCV), although the dorsal aorta (DA) appears relatively unaffected (Fig. 3P).
Figure 3. santa negatively regulates vascular specific gene expression and endothelial cell morphology.
(A–F) Bright-field micrographs of 60 hpf wild-type (A) and sans234 mutant (B) larvae. Black arrow points to enlarged heart and pericardial cavity. Endothelial cells visualized by in situ hybridization with tie2 in 60 hpf wild-type (C), and sans234 mutant (D) larvae, shown in ventral view of the heart region. Black bracket in D demarcates the expansion of tie2 expression in this region. Micrographs of cmlc2 (E) and tie2 (F) expression in 60 hpf san mutant larvae. The enlarged heart in sans234 mutant larvae shows an apparent increase of tie2 positive cells (black arrow in E and black bracket in F). (G–L) Epifluorescent micrographs of 36 hpf wild-type (G, I, and K) and san mutant (H, J, and L) Tg(tie2:GFP)s849 embryos showing the aortic arches (G and H), trunk vessels (I and J), and heart (K and L). Note the elevated Tg(tie2:GFP)s849 expression in sans234 mutant embryos (white arrows). (M–P) Epifluorescent micrographs of 60 hpf wild-type (M and O) and san mutant (N and P) Tg(flk1:EGFP)s843 larvae showing the subintestinal vessel (M and N) and posterior trunk (O and P). Vessels in sans234 mutant larvae (white brackets) appear dilated compared to those in wild-type siblings.
Genes regulating vascular tube or lumen structure
A subset of the mutants that display collapsed or dilated vessels were subjected to confocal microscopy analyses to test whether these mutations affect vascular tube and lumen formation (Fig. 4). Through this analysis, we identified seven mutations that specifically affect vascular tube and lumen formation without affecting earlier steps of endothelial cell specification and differentiation (Table 1). These mutations can be subdivided into two groups depending on whether they affect the number (Group IIA) or the diameter (Group IIB) of the vascular lumen.
Figure 4. Mutations affecting vascular tube formation.
(A) Epifluorescent micrograph of 36 hpf Tg(flk1:EGFP)s843 embryo in lateral view (A). White line marks the location of the transverse sections shown in B to F. (B–G) Transverse sections of wild-type (B), sih/cmc2 MO injected (C), sols828 mutant (D), mtss233 mutant (E), logs231 mutant (F), and psns634 mutant (G) Tg(flk1:EGFP)s843 embryos. Lack of circulation does not affect vascular lumen formation (C). Note changes in the number of vascular lumens in sols828 and mtss233 mutant embryos, and in the size and shape of the vascular lumens in logs231 and psns634 mutant embryos.
Two lumenized axial vessels, the DA and PCV, can be observed in wild-type embryos (Fig. 4A, B). The formation of the DA and PCV and subsequent lumenization of these vessels does not appear to be affected by lack of circulation, as shown in silent heart/tnnt2 (sih) morpholino-injected embryos (Fig. 4C). Two mutations, solos828 (sol) and ménage a troiss233 (mts), alter the number of lumens in axial vessels (Fig. 4D and E). In sols828 mutant embryos, endothelial cells fail to segregate into the DA and PCV, resulting in a single large vessel in the midline of the embryos (Fig. 4D). In contrast, an extra vascular lumen in the axial vessels is formed in homozygous mtss233 mutant embryos (Fig. 4E). Further in situ analyses suggest that the mtss233 mutation does not cause defects in the differentiation of endothelial cells into arterial or venous endothelial cells (Fig. S2).
Group IIB mutants, which show changes in the diameter of vascular lumen, include new alleles of two previously known mutations, logelei (logs231) (Schier et al., 1996) and valentine (vtns259) (Stainier et al., 1996) and three novel mutations, poongsuns634 (psn), blind alleys889 (bly), and catacombs479 (ctb). Axial vessels initially lumenize, but subsequently collapse in logs231 mutant embryos (Fig. 4F), while the PCV fails to remodel and becomes grossly dilated in homozygous psns634, blys889, and ctbs479 mutant embryos (Fig. 4G and data not included). However, we did not observe any obvious changes in the number or identity of endothelial cells in these mutants (Fig. S2 and data not included), suggesting that the molecular mechanisms that determine the size of vessels can be separated from those that regulate the specification of endothelial cells.
Genes regulating vascular patterning
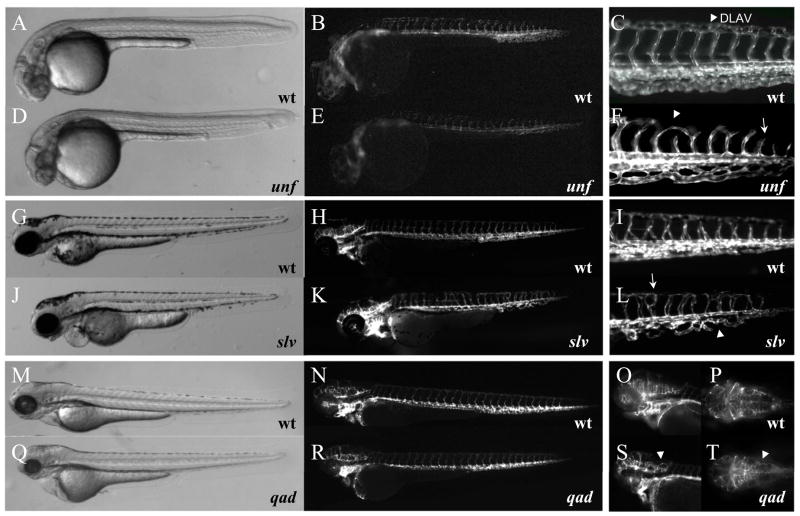
Formation of the complex vascular network requires vascular growth according to a pre-determined pattern. We identified eight mutants with specific defects in vascular patterning, all of which show wild-type like endothelial cell numbers, migration behavior and axial vessel formation. Therefore, these mutations are likely to cause specific patterning defects as results of disturbed angiogenic processes rather than defective vasculogenesis. Four of the identified mutations affect early patterning of the SEs alone or in combination with other patterning events. Complementation analyses showed that we had identified multiple additional alleles of the previously identified out of bounds (obds601) mutation (Chen et al., 2001, Childs et al., 2002), which displays an aberrant guidance of the SEs. In comparison, SEs in disorienteds240 (did), cacophonys236 (cpn), and unfinisheds808 (unf) mutant embryos, show distinct patterning defects: several SEs are missing in did s240 and cpns236 mutant embryos, while partially formed SEs are observed in unfs808 mutant embryos. Although the majority of SEs in unfs808 mutant embryos are properly initiated, they fail to extend and contribute to the dorsal longitudinal anastomotic vessels (DLAVs) (Fig. 5D to F). In severely affected unfs808 mutant embryos, SEs do not form at all (data not included). This failure of angiogenic sprouting also disturbs patterning of the head vasculature in unfs808 mutant embryos. These head vascular defect are characterized by a failure in the sprouting from the primordial midbrain channel (PMBC) and the formation and extension of the hindbrain capillary network (data not included).
Figure 5. Mutations affecting vascular patterning.
(A–T) Bright-field and epifluorescent micrographs of Tg(flk1:EGFP)s843 unfs808 mutant embryos (D, E 30 hpf; F 48 hpf), slvs887 mutant (J–L, 78hpf) and qads840 mutant (Q–R, 78 hpf) larvae and their wild-type (wt) siblings. (A–F) unfs808 mutants have shortened intersegmental vessels (SEs, arrow in F) and fail to form the dorsal longitudinal anastomotic vessel (DLAV, arrowhead in C and F) and appear to have reduced sprouting angiogenesis in the head (E). (G–L) Mis-patterning of the intersegmental vessels (SEs, arrow in L) and failure to remodel the posterior cardinal vein (PCV, arrowhead in L) in slvs887 mutant larvae. (M–S) Loop formation in the hindbrain capillary network in qads840 mutant larvae (P (wt) and T (qads840) are dorsal views focusing on the hindbrain capillary network). Instead of connecting lateral to medial as in wild-type (O, P), in qads840 mutant larvae capillaries connect back to their vessel of origin (arrowheads in S, T). Note that the tail vasculature in qads840 mutants (R) appears indistinguishable from wild-type (N). qads840 mutant embryos and their wild-type siblings were PTU treated to allow better visualization of the head vasculature.
Another angiogenic process, the remodeling of the PCV, appears to be affected in embryos homozygous for the mutation sloppy veins887 (slv) (Fig. 5J–L). Although slvs887 mutants also have minor aberrations in SE development (Fig. 5L), the overall patterning of the trunk and head vasculature appears to be unaffected.
The opposite seems to be the case for quo vadiss840 (qad) mutant embryos. In qads840 mutants (Fig. 5Q to T), misguided patterning of the head vasculature, especially apparent in the hindbrain capillary network, can be observed. However, qads840 mutant embryos have no obvious patterning defects in their trunk and tail vasculature (Fig. 5R). The phenotype becomes apparent relatively late in development: at 48 hpf qads840 mutant embryos are indistinguishable from their siblings, but after 72 hpf mutants can be identified by their looping brain vessels (Fig 5S,T) as well as smaller head and eyes. way to gos409 (way) mutant embryos are characterized by a failure to form the arch vasculature, starting at 48 hpf, and also show no trunk or tail patterning defects (data not included).
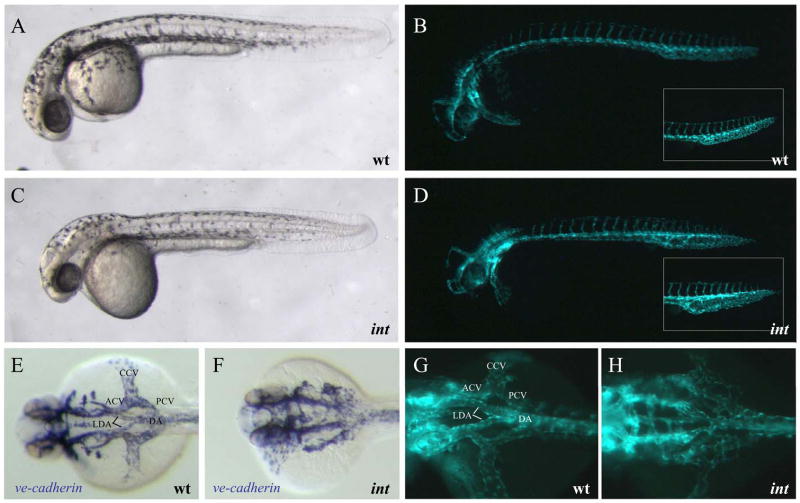
All patterning mutants identified seem to affect angiogenic vascular growth, indicating that many different factors contribute to vessel outgrowth and patterning. However, in intersections413 (int) mutants, specific disruption of patterning leads to a failure in connecting the two lateral dorsal aortae (LDA) into the single DA and a failure in fusing the anterior cardinal veins (ACVs) with their respective PCVs to form the common cardinal veins (CCVs) (Fig. 6). In addition PCV development is affected in ints413 mutants (Fig. 6, inset in D). Since this phenotype can be observed at 24 hpf, it is not clear whether cell migration and vasculogenic processes are also dysregulated in ints413 mutant embryos. Interestingly, ints413 mutant embryos establish a network of endothelial cells (as seen by Tg(flk1:EGFP)s843 expression, Fig. 6H) and express the vascular cell adhesion gene ve-cadherin (Fig. 6F), indicating that assembly, remodeling or arterial versus venous specification rather than cell migration is affected. This observation and the confinement of the phenotype to two sites of arterial-venous remodeling lead us to classify ints413 as a patterning mutant.
Figure 6. intersection regulates vascular patterning.
(A–D) Lateral bright-field and epifluorescent micrographs of 32 hpf embryos. Vascular patterning defects can be observed by visualizing Tg(flk1:EGFP)s843 expression, but not via bright-field microscopy. ints413 mutants fail to connect the lateral dorsal aortae (LDA) to form the dorsal aorta (DA), and the anterior and posterior cardinal veins (ACV and PCV) to the common cardinal vein (CCV). (E–H) Dorsal views of ve-cadherin and Tg(flk1:EGFP)s843 expression. ints413 mutant embryos exhibit a pronounced dilation of the PCV (inset in D).
Genes regulating vascular integrity
Mutants with defects in vascular integrity initially form and pattern the vascular network as their wild-type siblings, but are characterized by a subsequent partial or complete failure of circulation. Using the Tg(flk1:EGFP)s843 line, we further characterized these mutants and confirmed that these genes regulate the maintenance or integrity of the vasculature.
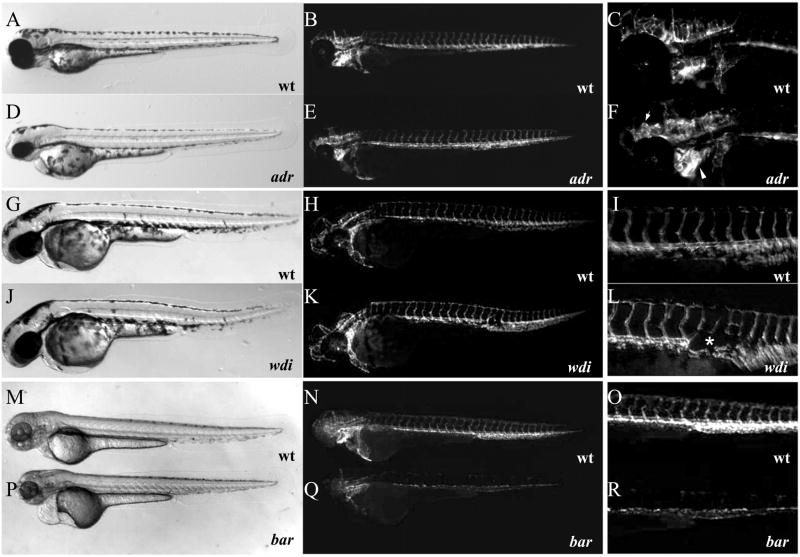
We identified five novel mutations, including a new allele of the the previously identified mutation violet beauregarde (vbgs407) (Roman et al., 2002), that affect vascular maintenance due to the breakdown of specific vessels. Additionally, we identified two mutants stradivaris877 (sra) and reddishs587 (reh) with vascular hemorrhage, and three mutants adrasteias277 (adr), barolos847 (bar) and tomatos805 (tom), which lose circulation gradually. In losing grips258 (lgr) mutant embryos, the breakdown of the vasculature can first be seen after 72 hpf and starts with the stretching or thinning out of the branchial arch vasculature, but the trunk and tail vasculature remain essentially unaffected (data not included).
Similarly, the DA of homozygous wadis631 (wdi) mutant embryos starts to disintegrate at 72 hpf. However, homozygous wdis631 mutant embryos develop a circulation shunt, and are morphologically indistinguishable from their wild-type siblings under the light microscope (Fig. 7G and J, and data not included). Detailed observation with epifluorescent (Fig. 7L) and confocal microscopy (data not included) shows that the DA of wdis631 mutants regresses, and that circulation is rerouted to provide blood to the posterior region of the embryo.
Figure 7. Mutations affecting vascular maintenance and integrity.
(A–R) Bright-field (A, D, G, J, M, and P) and epifluorescent (B, C, E, F, H, I, K, L, N, O, Q, and R) micrographs of Tg(flk1:EGFP)s843 adrs277 mutant larvae (D–F) and wild-type siblings (A–C), wdis631 mutant larvae (J–L) and wild-type siblings (G–I), and bars847 mutant larvae (P–R) and wild-type siblings (M–O) at 72 hpf. Affected areas within epifluorescent micrographs are magnified and shown in panels C, F, I, L, O, and R. Note the massive vascular dilation throughout the head vasculature in adrs277 mutant larvae (F arrowheads), regression of the dorsal aorta in wdis631 mutant larvae (asterix in L; K, L), and apparently disappearing endothelial cells in bars847 mutant larvae (Q, R).
In contrast, in bars847 mutant embryos, the vasculature degenerates much earlier. bar mutant embryos show significant global reduction of Tg(flk1:EGFP)s843 expression in all endothelial cells starting at 48 hpf, and this reduction becomes more pronounced at 72 hpf (Fig. 7Q and R). Furthermore, the bars847 mutation causes severe brain hemorrhage at 72 hpf, due to endothelial cell specific apoptosis (data not included). Surprisingly, these mutant embryos did not show any other major phenotype besides slight cardiac edema, indicating that this phenotype is caused by a disruption of a gene that functions within the endothelium and/or endocardium (Fig. 7P).
Whereas in most mutants of this group the vasculature disintegrates, collapses, or regresses in a mutant-specific way, in adrs277 mutant embryos, we observed the opposite phenotype. A dilation of the head vasculature can first be seen in adrs277 mutant embryos, most notably in the arch vessels, at 72 hpf (Fig. 7E, F). At this time point, circulation is still vigorous throughout the entire embryo. Within the following 48 hpf, circulation becomes progressively reduced, and through the formation of a shunt at 120 hpf blood flow is confined to a minimal loop through the heart (Fig. S3).
Discussion
By using a combination of a fast initial and detailed transgenic line assisted secondary screen, we identified 30 distinct mutations from 4015 mutagenized genomes, that affect processes ranging from early vascular development, such as angioblast specification, to the later maintenance of the vasculature.
In this screen, we identified mutations that cause similar vascular defects to known human conditions, such as hemangioma (in the case of blys889 and psns634), aortic dissection (in case of wdis631), arterio-venous malformations (in the case of ints413 and sols828), vascular hemorrhage (in the case of sras887 and rehs587), and cerebral cavernous vascular malformations (in the case of sans234 and vtns259). With the exception of san and vtn, the aforementioned mutations appear to be novel, a fact that validates our screening strategy and also supports the use of zebrafish for the identification of genes regulating the development and maintenance of the vasculature. In addition, the similarities between the phenotypes of newly identified zebrafish mutants and the symptoms of human vascular conditions indicate that future analyses of these mutants will provide invaluable insights towards understanding the etiology of various human vascular conditions.
Six loci identified in the screen (san, vtn, log, vbg, yot, and obd) had been identified in previous screens (Brand et al., 1996; Driever et al., 1996; Schier et al., 1996; Stainier et al., 1996; Chen et al., 2001, Roman et al., 2002). However only vbg, yot, and obd (Roman et al., 2002; Lawson et al., 2002; Childs et al., 2002) had previously been implicated in vascular development. san, vtn and log had been identified due to other developmental defects (cardiac phenotype in case of san and vtn (Stainier et al., 1996; Mably et al., 2006) and general body shape defects in case of log, and yot (Shier et al., 1996; Brandt et al., 1996)). Multiple alleles were identified for 7 loci. Specifically we identified 2 alleles each of san, sol, log, vtn, adr and vbg as well as 5 alleles of obd. The identification of multiple alleles at certain loci may mean that they are mutagenic hot spots. Alternatively, it might reflect that a certain degree of saturation has been reached in conventional ENU based mutagenesis screens. Nevertheless, a majority of the mutations identified in our screen appear to be novel, suggesting that a modified screening method such as ours can identify new classes of mutants. Thus in order to favor the identification of novel loci, more sophisticated screens, such as a transgene based ones or a screens with a sensitized genetic background should explored.
Use of transgenic fish as a new tool to identify subtle phenotypes
Several in situ based as well as vital staining based ENU mutagenesis screens have been performed to identify novel mutations affecting specific developmental processes (for example, Herzog et al., 2004). However, these secondary staining methods are usually time consuming and labor intensive. To identify mutations displaying subtle defects in vascular development more quickly and effectively, we used a recently generated transgenic line, Tg(flk1:EGFP)s843, to visualize the developing vasculature, which enabled us to identify many mutations by simple observation of embryos under the epifluorescence microscope.
Although many mutants identified in our screen have additional morphological defects, several of them would not have been identified without the Tg(flk1:EGFP) s843 line. As previously shown (Jin et al., 2005), GFP expression in the Tg(flk1:EGFP) s843 line starts within endothelial progenitors at approximately 8 somites (13 hpf), and persists through adulthood. By employing the Tg(flk1:EGFP) s843 line, we were able to identify mutants (e.g. mins202) that show defects in early angioblast specification but recover later during development. A similar transgene-assisted screening approach identified multiple novel mutations affecting endodermal organ development (Ober et al., 2006) and a novel gene affecting vascular patterning (Pham et al., 2007).
Distinct phases of vascular development revealed by distinct phenotypes
The phenotypes of the identified mutants reflect distinct phases of vascular development (Table 1 and Fig. 8): specification of angioblasts, migration and formation of vascular tubes, subsequent vascular patterning, and maintenance of the vasculature. The earliest event in vascular development is the specification of the angioblast, thought to occur as early as shield stage, long before gastrulation is completed. Recent data indicate that the endothelial lineage emerges from at least two distinct sources, the angioblast and the hemangioblast (Vogeli et al., 2006).
Figure 8. Distinct groups of mutants reflect distinct developmental steps of the forming vasculature.
The diagram depicts four distinct stages of vascular development, based on the mutants identified in the screen: Endothelial lineage specification (Orange line), vascular tube and lumen formation (Red line), pattern formation (Blue line), and maintenance (Green line). Mutations affecting each stage are listed accordingly.
Three mutants display changes in the number of endothelial cells during early development, possibly affecting early angioblast specification. One of these, mins202 shows defects in both endothelial and hematopoietic lineages as clo does (Stainier et al., 1995), suggesting that min might function in the hemangioblast lineage. Alternatively, min might function in both lineages like scl/tal1 and lmo2 (Liao et al., 1998; Patterson et al., 2006). Another mutation, grcs635, specifically affects the endothelial lineage without affecting the hematopoietic lineage (data not included), suggesting that grc may act downstream of min or clo. We observed the opposite phenotype in a new allele of sans234, which affects krit1 (aka ccm1) (Mably et al., 2006). However, it will be interesting to determine whether san functions to promote the specification of nascent mesoderm into the endothelial lineage or facilitates the proliferation of angioblasts.
After their specification in the lateral plate mesoderm, endothelial cells migrate to the midline to form a vascular cord which then undergoes lumenization (Jin et al., 2005). Previously, Kamei and colleagues (2006) showed that vascular lumen formation in the zebrafish SEs occurs via vesicle fusion similar to what is observed in MDCK cells. Several mutations appear to affect vascular tube and lumen formation without affecting early angioblast lineage specification. Further analyses will need to be performed to assess whether these mutations affect tube and lumen formation of diverse organs such as the pronephros and gut, or specifically vascular tube formation. Interestingly, three mutations, blys889, psns634, and ctbs479 appear to cause dilation specifically in the PCV. Given the late onset of this phenotype and its specificity, it is tempting to speculate that differences between arterial and venous endothelial cells stabilize much later than the initial differentiation detected by molecular markers.
Subsequently, secondary vessels sprout from the lumenized axial vessels through angiogenesis (reviewed by Ema and Rossant, 2003). Increasing lines of evidence suggest that secondary vessels generated by angiogenesis follow a genetically determined pattern. Several key signaling pathways have been identified to regulate the guidance of angiogenic sprouts including Semaphorin-Plexin (Torres-Vasquez et al., 2004), Netrin-DCC (Wilson et al., 2006), and Unc5-DCC (Lu et al., 2004). These findings were instrumental in starting to understand the underlying principles of secondary vessel formation, however, most of these components specifically regulate SE sprouting. Signaling pathways that regulate sprouting of other secondary vessels, such as the brain vasculature or the subintestinal vessel, remain to be identified. We identified four mutations that affect secondary vessels other than SEs. For example, the qads840 mutation specifically affects vascular branching in the brain.
The cellular and molecular mechanisms that govern the maturation and maintenance of developing vessels remain largely unexplored. It is widely accepted that the major vessels become stabilized by recruiting vascular smooth muscle cells, while secondary vessels maintain their flexibility to accommodate constant demands for remodeling. Failure of these events will eventually lead to vascular regression, local hemorrhage, or global vascular cell death. We have identified eleven mutations that affect vessel maturation and maintenance. These newly identified mutants will allow one to analyze this relatively unexplored culmination of vascular development. Mutants such as lgrs258 and wdis631 show a failure of vascular maintenance due to a localized regression of the vasculature. Although detailed analyses need to be performed to further delineate these phenotypes, it is possible that they are due to the a failure to recruit of vascular smooth muscle cells to vascular tubes. In contrast, two mutants, bars847 and toms805, display vascular maintenance defects resulting from endothelial specific apoptosis, which will allow us the investigation this previously unexplored aspect of vascular development.
Ongoing efforts in the molecular isolation of the affected genes and further analyses of the phenotypes reported here will help understand the mechanisms by which the vascular system emerges during development and reveal the underlying genetic networks. These studies may also provide new targets for therapeutic intervention in diseases affected or caused by aberrant vasculogenesis or angiogenesis.
Supplementary Material
(A–D) Transverse sections of 30 hpf Tg(flk1:EGFP)s843 wild-type (A) and mins202 mutant (B) embryos, and 36 hpf wild-type (C) and grcs635 mutant (D) embryos, visualized for Tg(flk1:EGFP)s843 expression (green), β-Catenin (red), and TOPRO (blue). White arrows point to the apparent lack of endothelial cells in mutant embryos (as assessed by Tg(flk1:EGFP)s843 expression). In wild-type embryos, 6 to 7 endothelial cells are present per any given optical section. In comparison, mins202 mutant embryos have less than one endothelial cell per section (n=10), and grcs635 mutant embryos have less than 3 endothelial cells per section (n=14).
(A–H) In situ hybridization with the arterial specific marker ephrinB2a (A to D) and the venous specific marker flt4 (E to H) in 36 hpf logs231 mutant embryos (B and F) and wild-type siblings (A and E), and in 36 hpf mtss233 mutant embryos (D and H) and wild-type siblings (C and G). Black arrows point to the vascular expression of ephrinB2a and flt4. Although these mutants display defective vascular lumen formation, the specification of endothelial cells appears relatively unaffected.
(A–B) Microangiography of wild-type (A) and adrs277 mutant larvae (B) visualizing blood flow at 100 hpf. Note that the blood flow is largely confined to the area adjacent to the heart in adrs277 mutant larvae. (C–D) Circulatory patterns in wild-type (C) and adrs277 mutant (D) larvae at 100 hpf (lateral views, anterior to the right), captured via video microscopy. Although circulation in adrs277 mutant larvae becomes gradually diminished, residual blood flow through the anterior vascular network can still be observed at this time point. Later, the circulation in adrs277 mutant larvae is limited to the loop shown in D.
Acknowledgments
We would like to thank members of the Stainier lab for invaluable discussions. Support for this research came from American Heart Association Post-doctoral fellowships (S. W. J., B. J. and E. A. O.), DFG Post-doctoral Fellowship HE4585/1-1 (W. H.), Human Frontier Science Program Organization Post-doctoral Fellowships (M. M. S., D. B. and H. V.), Canadian Institutes of Health Research Post-doctoral Fellowship (I. C. S.), Cardiovascular Research Institute NIH Training Grant positions (T. S. M., J. F, and L. A. D.), and by grants from the NIH, AHA, and Packard Foundation (D. Y. R. S.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alexander J, Stainier DY. A molecular pathway leading to endoderm formation in zebrafish. Curr Biol. 1999;9:1147–1157. doi: 10.1016/S0960-9822(00)80016-0. [DOI] [PubMed] [Google Scholar]
- Amsterdam A, Burgess S, Golling G, Chen W, Sun Z, Townsend K, Farrington S, Haldi M, Hopkins N. A large-scale insertional mutagenesis screen in zebrafish. Genes Dev. 1999:2713–24. doi: 10.1101/gad.13.20.2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betsholtz C, Lindblom P, Gerhardt H. Role of pericytes in vascular morphogenesis. Exs. 2005:115–25. doi: 10.1007/3-7643-7311-3_8. [DOI] [PubMed] [Google Scholar]
- Brand M, Heisenberg CP, Warga RM, Pelegri F, Karlstrom RO, Beuchle D, Picker A, Jiang YJ, Furutani-Seiki M, van Eeden FJ, et al. Mutations affecting development of the midline and general body shape during zebrafish embryogenesis. Development. 1996;123:129–42. doi: 10.1242/dev.123.1.129. [DOI] [PubMed] [Google Scholar]
- Carmeliet P, Ferreira V, Breier G, Pollefeyt S, Kieckens L, Gertsenstein M, Fahrig M, Vandenhoeck A, Harpal K, Eberhardt C, et al. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature. 1996;380:435–9. doi: 10.1038/380435a0. [DOI] [PubMed] [Google Scholar]
- Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407:249–57. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- Carmeliet P. Angiogenesis in health and disease. Nat Med. 2003;9:653–60. doi: 10.1038/nm0603-653. [DOI] [PubMed] [Google Scholar]
- Carmeliet P. Angiogenesis in life, disease and medicine. Nature. 2005;438:932–6. doi: 10.1038/nature04478. [DOI] [PubMed] [Google Scholar]
- Chan J, Bayliss PE, Wood JM, Roberts TM. Dissection of angiogenic signaling in zebrafish using a chemical genetic approach. Cancer Cell. 2002;1:257–267. doi: 10.1016/s1535-6108(02)00042-9. [DOI] [PubMed] [Google Scholar]
- Chen JN, van Bebber F, Goldstein AM, Serluca FC, Jackson D, Childs S, Serbedzija GN, Warren KS, Mably JD, Lindahl P, et al. Genetic steps to organ laterality in zebrafish. Comp Funct Genom. 2001;2:60–68. doi: 10.1002/cfg.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childs S, Chen JN, Garrity DM, Fishman MC. Patterning of angiogenesis in the zebrafish embryo. Development. 2002;4:973–82. doi: 10.1242/dev.129.4.973. [DOI] [PubMed] [Google Scholar]
- Cleaver O, Krieg PA. VEGF mediates angioblast migration during development of the dorsal aorta in Xenopus. Development. 1998;125:390514. doi: 10.1242/dev.125.19.3905. [DOI] [PubMed] [Google Scholar]
- Detrich HW, 3rd, Kieran MW, Chan FY, Barone LM, Yee K, Rundstadler JA, Pratt S, Ransom D, Zon LI. Intraembryonic hematopoietic cell migration during vertebrate development. Proc Natl Acad Sci USA. 1995;92:10713–7. doi: 10.1073/pnas.92.23.10713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driever W, Solnica-Krezel L, Schier AF, Neuhauss SC, Malicki J, Stemple DL, Stainier DY, Zwartkruis F, Abdelilah S, Rangini Z, et al. A genetic screen for mutations affecting embryogenesis in zebrafish. Development. 1996;123:37–46. doi: 10.1242/dev.123.1.37. [DOI] [PubMed] [Google Scholar]
- Dube A, Akbarali Y, Sato TN, Libermann TA, Oettgen P. Role of the Ets transcription factors in the regulation of the vascular-specific Tie2 gene. Circ Res. 1999;84:1177–85. doi: 10.1161/01.res.84.10.1177. [DOI] [PubMed] [Google Scholar]
- Ema M, Rossant J. Cell fate decisions in early blood vessel formation. Trends Cardiovasc Med. 2003;13:254–9. doi: 10.1016/s1050-1738(03)00105-1. [DOI] [PubMed] [Google Scholar]
- Ferrara N, Carver-Moore K, Chen H, Dowd M, Lu L, O’Shea KS, Powell-Braxton L, Hillan KJ, Moore MW. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature. 1996;380:439–42. doi: 10.1038/380439a0. [DOI] [PubMed] [Google Scholar]
- Gupta S, Zhu H, Zon LI, Evans T. BMP signaling restricts hemato-vascular development from lateral mesoderm during somitogenesis. Development. 2006;133:2177–87. doi: 10.1242/dev.02386. [DOI] [PubMed] [Google Scholar]
- Haffter P, Granato M, Brand M, Mullins MC, Hammerschmidt M, Kane DA, Odenthal J, van Eeden FJ, Jiang YJ, Heisenberg CP, et al. The identification of genes with unique and essential functions in the development of the zebrafish, Danio rerio. Development. 1996;123:1–36. doi: 10.1242/dev.123.1.1. [DOI] [PubMed] [Google Scholar]
- Herzog W, Sonntag C, Walderich B, Odenthal J, Maischein HM, Hammerschmidt M. Genetic analysis of adenohypophysis formation in zebrafish. Mol Endocrinol. 2004;18:1185–95. doi: 10.1210/me.2003-0376. [DOI] [PubMed] [Google Scholar]
- Horne-Badovinac S, Lin D, Waldron S, Schwarz M, Mbamalu G, Pawson T, Jan Y, Stainier DY, Abdelilah-Seyfried S. Positional cloning of heart and soul reveals multiple roles for PKC lambda in zebrafish organogenesis. Curr Biol. 2001;11:1492–1502. doi: 10.1016/s0960-9822(01)00458-4. [DOI] [PubMed] [Google Scholar]
- Isogai S, Horiguchi M, Weinstein BM. The vascular anatomy of the developing zebrafish: an atlas of embryonic and early larval development. Dev Biol. 2001;230:278–301. doi: 10.1006/dbio.2000.9995. [DOI] [PubMed] [Google Scholar]
- Jain RK. Molecular regulation of vessel maturation. Nat Med. 2003;9:685–93. doi: 10.1038/nm0603-685. [DOI] [PubMed] [Google Scholar]
- Jiang B, Liou GI, Behzadian MA, Caldwell RB. Astrocytes modulate retinal vasculogenesis: effects on fibronectin expression. J Cell Sci. 1994;107:2499–508. doi: 10.1242/jcs.107.9.2499. [DOI] [PubMed] [Google Scholar]
- Jin SW, Beis D, Mitchell T, Chen JN, Stainier DY. Cellular and molecular analyses of vascular tube and lumen formation in zebrafish. Development. 2005;132:5199–209. doi: 10.1242/dev.02087. [DOI] [PubMed] [Google Scholar]
- Kallianpur AR, Jordan JE, Brandt SJ. The SCL/TAL-1 gene is expressed in progenitors of both the hematopoietic and vascular systems during embryogenesis. Blood. 1994;83:1200–8. [PubMed] [Google Scholar]
- Kamei M, Saunders WB, Bayless KJ, Dye L, Davis GE, Weinstein BM. Endothelial tubes assemble from intracellular vacuoles in vivo. Nature. 2006;442:453–6. doi: 10.1038/nature04923. [DOI] [PubMed] [Google Scholar]
- Krebs LT, Xue Y, Norton CR, Shutter JR, Maguire M, Sundberg JP, Gallahan D, Closson V, Kitajewski J, Callahan R, et al. Notch signaling is essential for vascular morphogenesis in mice. Genes Dev. 2000;14:1343–52. [PMC free article] [PubMed] [Google Scholar]
- Laberge-le Couteulx S, Jung HH, Labauge P, Houtteville JP, Lescoat C, Cecillon M, Marechal E, Joutel A, Bach JF, Tournier-Lasserve E. Truncating mutations in CCM1, encoding KRIT1, cause hereditary cavernous angiomas. Nat Genet. 1999;23:189–93. doi: 10.1038/13815. [DOI] [PubMed] [Google Scholar]
- Larson JD, Wadman SA, Chen E, Kerley L, Clark KJ, Eide M, Lippert S, Nasevicious A, Ekker SC, Hackett PB, et al. Expression of VE-cadherin in zebrafish embryos: a new tool to evaluate vascular development. Dev Dyn. 2004;231:204–213. doi: 10.1002/dvdy.20102. [DOI] [PubMed] [Google Scholar]
- Lawson ND, Scheer N, Pham VN, Kim CH, Chitnis AB, Campos-Ortega JA, Weinstein BM. Notch signaling is required for arterial-venous differentiation during embryonic vascular development. Development. 2001;128:3675–83. doi: 10.1242/dev.128.19.3675. [DOI] [PubMed] [Google Scholar]
- Lawson ND, Vogel AM, Weinstein BM. Sonic hedgehog and vascular endothelial growth factor act upstream of the Notch pathway during arterial endothelial differentiation. Dev Cell. 2002;3:127–36. doi: 10.1016/s1534-5807(02)00198-3. [DOI] [PubMed] [Google Scholar]
- Liao EC, Paw BH, Oates AC, Pratt SJ, Postlethwait JH, Zon LI. SCL/Tal-1 transcription factor acts downstream of cloche to specify hematopoietic and vascular progenitors in zebrafish. Genes Dev. 1998;12:621–626. doi: 10.1101/gad.12.5.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao W, Bisgrove BW, Sawyer H, Hug B, Bell B, Peters K, Grunwald DJ, Stainier DY. The zebrafish gene cloche acts upstream of a flk-1 homologue to regulate endothelial cell differentiation. Development. 1997;124:381–389. doi: 10.1242/dev.124.2.381. [DOI] [PubMed] [Google Scholar]
- Lindsley RC, Gill JG, Kyba M, Murphy TL, Murphy KM. Canonical Wnt signaling is required for development of embryonic stem cell-derived mesoderm. Development. 2006;133:3787–96. doi: 10.1242/dev.02551. [DOI] [PubMed] [Google Scholar]
- Lu X, Le Noble F, Yuan L, Jiang Q, De Lafarge B, Sugiyama D, Breant C, Claes F, De Smet F, Thomas JL, et al. The netrin receptor UNC5B mediates guidance events controlling morphogenesis of the vascular system. Nature. 2004;432:179–86. doi: 10.1038/nature03080. [DOI] [PubMed] [Google Scholar]
- Lyons MS, Bell B, Stainier D, Peters KG. Isolation of the zebrafish homologues for the tie-1 and tie-2 endothelium-specific receptor tyrosine kinases. Dev Dyn. 1998;212:133–140. doi: 10.1002/(SICI)1097-0177(199805)212:1<133::AID-AJA12>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Mably JD, Chuang LP, Serluca FC, Mohideen MA, Chen JN, Fishman MC. santa and valentine pattern concentric growth of cardiac myocardium in the zebrafish. Development. 2006;133:3139–46. doi: 10.1242/dev.02469. [DOI] [PubMed] [Google Scholar]
- Motoike T, Loughna S, Perens E, Roman BL, Liao W, Chau TC, Richardson CD, Kawate T, Kuno J, Weinstein BM, et al. Universal GFP reporter for the study of vascular development. Genesis. 2000;28:75–81. doi: 10.1002/1526-968x(200010)28:2<75::aid-gene50>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Mullins MC, Hammerschmidt M, Haffter P, Nusslein-Volhard C. Large-scale mutagenesis in the zebrafish: in search of genes controlling development in a vertebrate. Cur Biol. 1994;1:189–202. doi: 10.1016/s0960-9822(00)00048-8. [DOI] [PubMed] [Google Scholar]
- Muto A, Orger MB, Wehman AM, Smear MC, Kay JN, Page-McCaw PS, Gahtan E, Xiao T, Nevin LM, Gosse NJ, et al. Forward genetic analysis of visual behavior in zebrafish. PLoS Genet. 2005;1:e66. doi: 10.1371/journal.pgen.0010066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ober EA, Verkade H, Field HA, Stainier DY. Mesodermal Wnt2b signalling positively regulates liver specification. Nature. 2006;442:688–9. doi: 10.1038/nature04888. [DOI] [PubMed] [Google Scholar]
- Oomman S, Finckbone V, Dertien J, Attridge J, Henne W, Medina M, Mansouri B, Singh H, Strahlendorf H, Strahlendorf J. Active caspase-3 expression during postnatal development of rat cerebellum is not systematically or consistently associated with apoptosis. J Comp Neurol. 2004;476:154–173. doi: 10.1002/cne.20223. [DOI] [PubMed] [Google Scholar]
- Park C, Lavine K, Mishina Y, Deng CX, Ornitz DM, Choi K. Bone morphogenetic protein receptor 1A signaling is dispensable for hematopoietic development but essential for vessel and atrioventricular endocardial cushion formation. Development. 2006;133:3473–84. doi: 10.1242/dev.02499. [DOI] [PubMed] [Google Scholar]
- Parker LH, Schmidt M, Jin SW, Gray AM, Beis D, Pham T, Frantz G, Palmieri S, Hillan K, Stainier DY, et al. The endothelial-cell-derived secreted factor Egfl7 regulates vascular tube formation. Nature. 2004;428:754–758. doi: 10.1038/nature02416. [DOI] [PubMed] [Google Scholar]
- Patterson LJ, Gering M, Patient R. Scl is required for dorsal aorta as well as blood formation in zebrafish embryos. Blood. 2005;105:3502–11. doi: 10.1182/blood-2004-09-3547. [DOI] [PubMed] [Google Scholar]
- Patterson LJ, Gering M, Eckfeldt CE, Green AR, Verfaillie CM, Ekker SC, Patient R. The transcription factors, Scl and Lmo2, act together during development of the haemangioblast in zebrafish. Blood. 2006 doi: 10.1182/blood-2006-02-003087. [DOI] [PubMed] [Google Scholar]
- Pham VN, Lawson ND, Mugford JW, Dye L, Castranova D, Lo B, Weinstein BM. Combinatorial function of ETS transcription factors in the developing vasculature. Dev Biol. 2007;303:772–8. doi: 10.1016/j.ydbio.2006.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risau W. Mechanisms of angiogenesis. Nature. 1997;386:671–4. doi: 10.1038/386671a0. [DOI] [PubMed] [Google Scholar]
- Risau W, Flamme I. Vasculogenesis. Annu Rev Cell Dev Biol. 1995;11:73–91. doi: 10.1146/annurev.cb.11.110195.000445. [DOI] [PubMed] [Google Scholar]
- Roman BL, Pham VN, Lawson ND, Kulik M, Childs S, Lekven AC, Garrity DM, Moon RT, Fishman MC, Lechleider RJ, et al. Disruption of acvrl1 increases endothelial cell number in zebrafish cranial vessels. Development. 2002;129:3009–19. doi: 10.1242/dev.129.12.3009. [DOI] [PubMed] [Google Scholar]
- Roman BL, Weinstein BM. Building the vertebrate vasculature: research is going swimmingly. Bioessays. 2000;22:882–93. doi: 10.1002/1521-1878(200010)22:10<882::AID-BIES3>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Rossant J, Howard L. Signaling pathways in vascular development. Annu Rev Cell Dev Biol. 2002;18:541–73. doi: 10.1146/annurev.cellbio.18.012502.105825. [DOI] [PubMed] [Google Scholar]
- Schier AF, Neuhauss SC, Harvey M, Malicki J, Solnica-Krezel L, Stainier DY, Zwartkruis F, Abdelilah S, Stemple DL, Rangini Z, et al. Mutations affecting the development of the embryonic zebrafish brain. Development. 1996;123:165–78. doi: 10.1242/dev.123.1.165. [DOI] [PubMed] [Google Scholar]
- Sehnert AJ, Huq A, Weinstein BM, Walker C, Fishman M, Stainier DY. Cardiac troponin T is essential in sarcomere assembly and cardiac contractility. Nat Genet. 2002;31:106–10. doi: 10.1038/ng875. [DOI] [PubMed] [Google Scholar]
- Stainier DY. Zebrafish genetics and vertebrate heart formation. Nat Rev Genet. 2001;2:39–48. doi: 10.1038/35047564. [DOI] [PubMed] [Google Scholar]
- Stainier DY, Fouquet B, Chen JN, Warren KS, Weinstein BM, Meiler SE, Mohideen MA, Neuhauss SC, Solnica-Krezel L, Schier AF, et al. Mutations affecting the formation and function of the cardiovascular system in the zebrafish embryo. Development. 1996;123:285–92. doi: 10.1242/dev.123.1.285. [DOI] [PubMed] [Google Scholar]
- Stainier DY, Weinstein BM, Detrich HW, 3rd, Zon LI, Fishman MC. Cloche, an early acting zebrafish gene, is required by both the endothelial and hematopoietic lineages. Development. 1995;121:3141–50. doi: 10.1242/dev.121.10.3141. [DOI] [PubMed] [Google Scholar]
- Sumanas S, Lin S. Ets1-related protein is a key regulator of vasculogenesis in zebrafish. PLoS Biol. 2006;4:e10. doi: 10.1371/journal.pbio.0040010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson MA, Ransom DG, Pratt SJ, MacLennan H, Kieran MW, Detrich HW, III, Vail B, Huber TL, Paw B, Brownlie AJ, et al. The cloche and spadetail genes differentially affect hematopoiesis and vasculogenesis. Dev Biol. 1998;197:248–269. doi: 10.1006/dbio.1998.8887. [DOI] [PubMed] [Google Scholar]
- Torres-Vazquez J, Kamei M, Weinstein BM. Molecular distinction between arteries and veins. Cell Tissue Res. 2003;314:43–59. doi: 10.1007/s00441-003-0771-8. [DOI] [PubMed] [Google Scholar]
- Torres-Vazquez J, Gitler AD, Fraser SD, Berk JD, Pham VN, Fishman MC, Childs S, Epstein JA, Weinstein BM. Semaphorin-plexin signaling guides patterning of the developing vasculature. Dev Cell. 2004;7:117–23. doi: 10.1016/j.devcel.2004.06.008. [DOI] [PubMed] [Google Scholar]
- Trinh LA, Stainier DY. Fibronectin regulates epithelial organization during myocardial migration in zebrafish. Dev Cell. 2004;6:371–382. doi: 10.1016/s1534-5807(04)00063-2. [DOI] [PubMed] [Google Scholar]
- Visvader JE, Fujiwara Y, Orkin SH. Unsuspected role for the T-cell leukemia protein SCL/tal-1 in vascular development. Genes Dev. 1998;12:473–9. doi: 10.1101/gad.12.4.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogeli KM, Jin SW, Martin GR, Stainier DY. A common progenitor for haematopoietic and endothelial lineages in the zebrafish gastrula. Nature. 2006;443:337–9. doi: 10.1038/nature05045. [DOI] [PubMed] [Google Scholar]
- Vokes SA, Yatskievych TA, Heimark RL, McMahon J, McMahon AP, Antin PB, Krieg PA. Hedgehog signaling is essential for endothelial tube formation during vasculogenesis. Development. 2004;131:4371–80. doi: 10.1242/dev.01304. [DOI] [PubMed] [Google Scholar]
- Wang H, Charles PC, Wu Y, Ren R, Pi X, Moser M, Barshishat-Kupper M, Rubin JS, Perou C, Bautch V, et al. Gene expression profile signatures indicate a role for Wnt signaling in endothelial commitment from embryonic stem cells. Circ Res. 2006;98:1331–9. doi: 10.1161/01.RES.0000220650.26555.1d. [DOI] [PubMed] [Google Scholar]
- Whitehead KJ, Plummer NW, Adams JA, Marchuk DA, Li DY. Ccm1 is required for arterial morphogenesis: implications for the etiology of human cavernous malformations. Development. 2004;131:1437–48. doi: 10.1242/dev.01036. [DOI] [PubMed] [Google Scholar]
- Wijelath ES, Murray J, Rahman S, Patel Y, Ishida A, Strand K, Aziz S, Cardona C, Hammond WP, Savidge GF, et al. Novel vascular endothelial growth factor binding domains of fibronectin enhance vascular endothelial growth factor biological activity. Circ Res. 2002;91:25–31. doi: 10.1161/01.res.0000026420.22406.79. [DOI] [PubMed] [Google Scholar]
- Wilson BD, Ii M, Park KW, Suli A, Sorensen LK, Larrieu-Lahargue F, Urness LD, Suh W, Asai J, Kock GA, et al. Netrins promote developmental and therapeutic angiogenesis. Science. 2006;313:640–4. doi: 10.1126/science.1124704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You LR, Lin FJ, Lee CT, DeMayo FJ, Tsai MJ, Tsai SY. Suppression of Notch signalling by the COUP-TFII transcription factor regulates vein identity. Nature. 2005;435:98–104. doi: 10.1038/nature03511. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A–D) Transverse sections of 30 hpf Tg(flk1:EGFP)s843 wild-type (A) and mins202 mutant (B) embryos, and 36 hpf wild-type (C) and grcs635 mutant (D) embryos, visualized for Tg(flk1:EGFP)s843 expression (green), β-Catenin (red), and TOPRO (blue). White arrows point to the apparent lack of endothelial cells in mutant embryos (as assessed by Tg(flk1:EGFP)s843 expression). In wild-type embryos, 6 to 7 endothelial cells are present per any given optical section. In comparison, mins202 mutant embryos have less than one endothelial cell per section (n=10), and grcs635 mutant embryos have less than 3 endothelial cells per section (n=14).
(A–H) In situ hybridization with the arterial specific marker ephrinB2a (A to D) and the venous specific marker flt4 (E to H) in 36 hpf logs231 mutant embryos (B and F) and wild-type siblings (A and E), and in 36 hpf mtss233 mutant embryos (D and H) and wild-type siblings (C and G). Black arrows point to the vascular expression of ephrinB2a and flt4. Although these mutants display defective vascular lumen formation, the specification of endothelial cells appears relatively unaffected.
(A–B) Microangiography of wild-type (A) and adrs277 mutant larvae (B) visualizing blood flow at 100 hpf. Note that the blood flow is largely confined to the area adjacent to the heart in adrs277 mutant larvae. (C–D) Circulatory patterns in wild-type (C) and adrs277 mutant (D) larvae at 100 hpf (lateral views, anterior to the right), captured via video microscopy. Although circulation in adrs277 mutant larvae becomes gradually diminished, residual blood flow through the anterior vascular network can still be observed at this time point. Later, the circulation in adrs277 mutant larvae is limited to the loop shown in D.