Abstract
BACKGROUND AND OBJECTIVES
Despite the significant burden of delirium among hospitalized adults, there is no approved pharmacologic intervention for delirium. This systematic review evaluates the efficacy and safety of pharmacologic interventions targeting either prevention or management of delirium.
DATA SOURCES
We searched Medline, PubMed, the Cochrane Register of Controlled Trials, and the Cumulative Index to Nursing and Allied Health Literature (CINAHL) information systems from January 1966 to October 2008. We included randomized, controlled trials comparing pharmacologic compounds either to each other or placebo. We excluded non-comparison trials, studies with patients aged < 18 years, a history of an Axis I psychiatric disorder, and patients with alcohol-related delirium.
REVIEW METHODS
Three reviewers independently extracted the data for participants, interventions and outcome measures, and critically appraised each study using the JADAD scale.
RESULTS
We identified 13 studies that met our inclusion criteria and evaluated 15 compounds: second-generation antipsychotics, first-generation antipsychotics, cholinergic enhancers, an antiepileptic agent, an inhaled anesthetic, injectable sedatives, and a benzodiazepine. Four trials evaluated delirium treatment and suggested no differences in efficacy or safety among the evaluated treatment methods (first and second generation antipsychotics). Neither cholinesterase inhibitors nor procholinergic drugs were effective in preventing delirium. Multiple studies, however, suggest either shorter severity and duration, or prevention of delirium with the use of haloperidol, risperidone, gabapentin, or a mixture of sedatives in patients undergoing elective or emergent surgical procedures.
CONCLUSION
The existing limited data indicates no superiority for second-generation antipsychotics over haloperidol in managing delirium. Although preliminary results suggest delirium prevention may be accomplished through various mechanisms, further studies are necessary to prove effectiveness.
INTRODUCTION
Delirium or acute confusional state is a disturbance in consciousness with reduced ability to focus, sustain, or shift attention that occurs over a short period of time and tends to fluctuate over the course of the day.1 It is estimated that among 13 million patients aged 65 and older who were hospitalized in 2002,2 10% to 52% had delirium during their hospitalization.3 Delirium is associated with increased mortality, poorer functional status, limited rehabilitation, increased hospital acquired complications, prolonged length of hospital stay, increased risk of institutionalization, and higher health care expenditures.3
Acute illness is considered the cornerstone of delirium pathogenesis and is usually triggered by multiple etiologies such as infection, hypoxia, hypoperfusion, trauma, and recent surgeries.4–6 The stress of an acute illness leads to various metabolic changes that alter the availability of valuable amino acids from plasma to the brain, in turn modifying the cerebral neurotransmission, and leading to the secretion of cytokines.4,7–9 Therefore, attention to the underlying triggers of the acute illness suffered by patients with delirium and restoring the brain’s imbalanced neurotransmission provide the most logical management approach for delirium, rather than interventions aimed at managing the symptoms of delirium.10–12
Currently, there is no pharmacologic intervention approved by the Federal Drug Agency (FDA) to treat delirium. However, clinicians currently employ various medications to reduce delirium symptoms such as first-generation or second-generation antipsychotics and benzodiazepines.3 The evaluation of the efficacy and safety of these common therapeutics have been limited by poor sample sizes, heterogeneous samples, and variable pharmacologic interventions.3 Recently, two systematic reviews were published to review the efficacy of pharmacological management of delirium13,14. These reviews did not limit inclusion to randomized control trials, excluded delirium prevention, and did not include recent clinical trials that were published in the past two years. We conducted a systematic evidence review of the existing literature to update literature reviews and evaluate the efficacy and safety of the various pharmacological interventions targeting either prevention or treatment of delirium, with a goal of assisting the clinician in appropriate selection of pharmacologic therapy in vulnerable hospitalized patients.
DATA SOURCES
We searched OVID Medline, PubMed, Cochrane Central Register of Controlled Trials CENTRAL, and Cumulative Index of Nursing and Allied Health Literature (CINAHL) for clinical trials evaluating the pharmacologic interventions for delirium in hospitalized older patients. The search terms used for this review were: delirium, confusion, agitation, mental status change, inattention, encephalopathy, organic mental disorders, disorientation, pharmacologic treatment, alprazolam, chlordiazepoxide, clonazepam, clorazepate, diazepam, donepezil, estazolam, flunitrazepam, flurazepam, halazepam, ketazolam, lorazepam, midazolam, nitrazepam, oxazepam, quazepam, temazepam, triazolam, gabapentin, chlorpromazine, haloperidol, droperidol, methotrimeprazine, olanzapine, propofol, risperidone, quetiapine, trazodone, ziprasidone, aripiprazole, mesoridazine and thioridazine. The search terms were limited to Human subjects only. We searched articles published from January 1966 through October 2008.
REVIEW METHODS
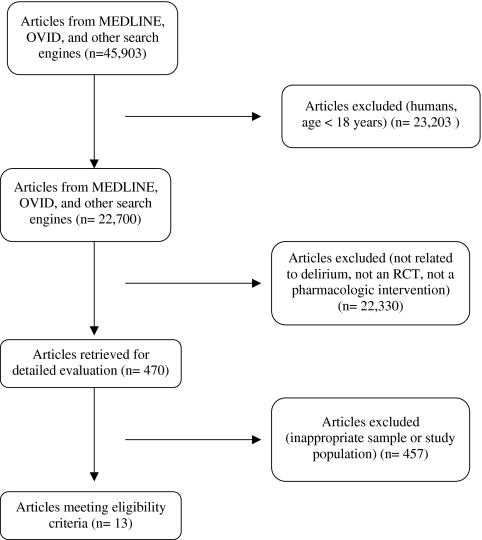
We included any randomized controlled clinical trial that evaluated pharmacologic interventions for the symptomatic control or prevention of delirium using either placebo or active-control comparators. We excluded studies that enrolled patients aged < 18, patients with current or past Axis I psychotic disorders and those that evaluated interventions for alcohol-related delirium (Fig. 1). Titles and abstracts were screened by three reviewers (NC, AA and MB). Copies of full text of the potentially relevant studies and clinical trials were also assessed for inclusion in this review. Bibliographies and references of relevant articles were further screened for other pertinent studies. Any disagreements related to either inclusion or exclusion criteria were resolved by discussions between the reviewers. References of included studies were manually reviewed and articles citing these studies were also considered using appropriate databases and journals. Authors of the included studies were also contacted either through phone or email when it was considered necessary for data extraction.
Figure 1.
Search results.
The methodological quality of each study was independently assessed by three reviewers (NC, AA and MB) using the JADAD scale15. This scale assesses parameters that are critical to the scientific credibility of a clinical trial. This scale allocates a score to each study between 0–5, with higher scores indicating a higher quality in the conduct and/or reporting of the trial15. The studies included in this review were heterogeneous in regard to their interventions, control, clinical setting, and population and therefore were not appropriate to merge into a pooled meta-analytic summary.
RESULTS
Our search strategies identified 45,903 potential studies. We excluded all but thirteen studies as they did not fulfill the inclusion criteria for our systematic evidence review (SER) and used the remaining data to answer our research question (Table 1). Of those meeting our inclusion criteria, three studies prospectively compared haloperidol with other antipsychotics (chlorpromazine16, risperidone17, and olanzapine19) or a benzodiazepine (lorazepam16). One study was included that compared the antipsychotics amisulpride and quetiapine18. No study was identified that compared a pharmacologic intervention with placebo in the treatment of delirium in hospitalized adults. Nine prospective, prevention studies were included with comparisons made between donepezil20,21, haloperidol22, citicoline23, risperidone26, gabapentin27, nitrous oxide28, a combination of sedatives25, and placebo. One other study was identified that compared the use of dexmedetomidine with lorazepam for sedation in critically ill patients24.
Table 1.
Characteristics of Included Studies
| Study | Country/setting | Comorbid Condition | N | Mean Age (yrs) | Compared Interventions | Mean Dose (mg) | Treatment Length |
|---|---|---|---|---|---|---|---|
| Pharmacologic Management of Delirium | |||||||
| Breitbart16 | USA/Medical Services | AIDS, Medical patients | 30 | 39 | Haloperidol | 1.4 - 2.8 | Up to 6 days |
| Chlorpromazine | 36 - 50 | ||||||
| Lorazepam | 3 - 4.6 | ||||||
| Han17 | Korea/ICU and Medical Services | Medical and surgical patients | 24 | 66 | Haloperidol Risperidone | 1.7 | 7 |
| 1 | |||||||
| Lee18 | South Korea/Medical and Surgical services | Medical and surgical patients | 31 | 62 | Amisulpride Quetiapine | 156 | Up to 7 days |
| 113 | |||||||
| Skrobik19 | Canada/ICU | Medical and surgical patients | 73 | 65 | Haloperidol Olanzapine | 6.5 | 5 days |
| 4.5 | |||||||
| Prevention of Delirium | |||||||
| Liptzin20 | USA/Orthopedic Surgery | Elective knee or hip surgery | 80 | 67 | Donepezil placebo | Not reported | 28 days |
| Sampson21 | UK/Orthopedic Surgery | Elective hip surgery | 33 | 68 | Donepezil placebo | 5 mg daily | 4 days |
| Kalisvaart22 | Netherlands/Orthopedic Surgery | Emergent or elective hip fracture surgery | 430 | 79 | Haloperidol placebo | 0.5 mg three times daily | Minimum 1 day, Maximum 6 days |
| Diaz23 | Chile/Orthopedic Surgery | Elective or emergent hip fracture surgery | 81 | 79 | Citicoline Placebo | 400 mg every 8 hours | 4 days |
| Pandharipande24 | USA/Medical and Surgical Services | Mechanically ventilated adults | 106 | 60 | Dexmedetomidine Lorazepam | 0.74 mcg/hr 3 mg/hr | Up to 120 hours |
| Aizawa25 | Japan/Surgery | Gastric or colon Resection surgery | 42 | 76 | Diazepam, Flunitrazepam, pethidine vs. placebo | 0.1 mg/kg × 1 0.04 mg/kg drip 1 mg/kg drip | 3 days |
| Prakanrattana26 | Thailand/Surgery | Cardiac Surgery | 126 | 61 | Risperidone placebo | 1 mg | 1 day |
| Leung27 | USA/Neurosurgery | Spine surgery | 21 | 60 | Gabapentin placebo | 900 mg/day | 3 days |
| Leung28 | USA/Surgery | Non-cardiac surgery | 228 | 74 | Nitrous oxide + O2 O2 | NR | Procedural administration |
N: Total number of subject in the trial; USA: United States of America; AIDS: Acquired Immunodeficiency Syndrome; ICU: Intensive care unit; NR: Not Reported
Pharmacologic Management of Delirium
Our search strategy identified four studies that evaluated the pharmacologic management of delirium in hospitalized adults16–19. Each study compared either first- or second-generation antipsychotics or benzodiazepines in the management of patients diagnosed with delirium. Studies included in our review evaluated patients with a range of mean ages from 23–75 years with a variety of medical and surgical needs. In general, more males were included in the total study population than females, and most patients were admitted for surgical procedures. The range of study duration was five to seven days. One study performed by Breitbart and colleagues16, included only patients with a history of AIDS who were admitted with an acute medical illness.
Although assessment measures were not consistently reported, the frequency of response was high for all treatment groups (75% with Memorial Delirium Assessment Scale scores less than thirteen17, 80% of patients had a 50% reduction in Delirium Rating Scale-R-9818). Reduction in delirium severity occurred as early as a few hours after initiation of the designated treatment method16 or up to 96 hours after treatment initiation17. Further improvement in delirium symptoms was rarely documented beyond the fourth study day (see Table 2).
Table 2.
Efficacy of Various Pharmacological Interventions on Delirium-related Health Outcomes
| Study | Compared interventions | Delirium Incidence | Delirium severity | Delirium length | Hospital LOS | Adverse events | JADAD Score15 |
|---|---|---|---|---|---|---|---|
| Pharmacologic Management of Delirium | |||||||
| Brietbart16 | Haloperidol vs. Chlorpromazine vs. Lorazepam | NA | Favors haloperidol and chlorpromazine over lorazepam | NA | NA | Favors haloperidol and chlorpromazine over lorazepam | 5 |
| Han17 | Haloperidol vs. Risperidone | NA | ND | ND | NA | ND | 2 |
| Lee18 | Amisulpride vs. Quetiapine | NA | ND | ND | NA | ND | 2 |
| Skrobik19 | Haloperidol vs. Olanzepine | NA | ND | NA | NA | ND | 1 |
| Pharmacologic Prevention of Delirium | |||||||
| Liptzin20 | Donepezil vs. Placebo | ND | ND | ND | ND | ND | 4 |
| Sampson21 | Donepezil vs. Placebo | ND | ND | ND | ND | ND | 5 |
| Kalisvaart22 | Haloperidol vs. Placebo | ND | Favors haloperidol* | Favors haloperidol* | Favors haloperidol* | ND | 5 |
| Diaz23 | Citicoline vs. Placebo | ND | NA | NA | NA | ND | 4 |
| Pandharipande24 | Dexmedetomidine vs. Lorazepam | ND | NA | Favors Dexmede-tomidine | NA | Sinus bradycardia in dexmedetomidine group | 5 |
| Aizawa25 | Diazepam, Flunitrazepam, Pethidine vs. Placebo | Favors treatment* | NA | NA | ND | Morning lethargy in treatment group | 2 |
| Prakanrattana26 | Risperidone vs. Placebo | Favors treatment* | NA | NA | NA | NA | 5 |
| Leung27 | Gabapentin vs. Placebo | Favors treatment* | NA | NA | NA | ND | 5 |
| Leung28 | Nitrous Oxide vs. Usual care | ND | NA | NA | ND | ND | 4 |
LOS = Length of stay; NA = Not available; ND = No difference. *Statistically significant differences found among those experiencing delirium; this difference was not compared with all patients included in the analysis
Although dose-ranging studies have not evaluated higher and lower doses of antipsychotics, results from studies in patients with delirium reveal improvement in delirium symptoms with lower doses of antipsychotics (see Table 1). Most studies included in the review allowed for blinded dose titration when symptoms were not controlled, though the mean doses remained low for all studies. Adverse events were rare across all study participants, with no evidence of serious extrapyramidal symptoms in any treatment group. Only one study group, the lorazepam treatment arm in the study by Breitbart and colleagues16, noted significant differences in sedation and confusion that required intervention, and prematurely ended enrollment in this treatment arm. No study reported changes in electrocardiogram for any treatment arm in studies comparing active interventions with placebo.
The study quality varied significantly amongst studies evaluating delirium treatment. Whereas Breitbart and colleagues16 accounted for appropriate study design measures, JADAD scores in other studies were limited by the lack of description of randomization and blinding techniques used. Studies without randomized patient allocation or appropriately blinded interventions and outcome measurement should be interpreted with much caution.
Prevention of Delirium
Nine studies were included that met our search criteria and evaluated pharmacologic interventions in preventing delirium in hospitalized adults. Patients included in the prevention studies were generally older than those included in the treatment studies (age range in treatment studies: 23–75 years; age range in prevention studies: 45–95), and were admitted to the hospital primarily for surgical procedures (only patients enrolled in one study included patients with an acute medical illness24). Four studies included patients undergoing elective or emergent orthopedic surgeries, one study each enrolled patients undergoing cardiac procedures, spinal surgery, or gastric or colon resection surgery. Finally, one study evaluated older patients admitted to surgical services for any non-cardiac surgery, evaluating any difference in delirium between those who received nitrous oxide with oxygen or oxygen alone during the surgical procedure28. Only two studies stated exclusions of patients with baseline cognitive dysfunction (MMSE)21 or severe dementia (IQCODE)24. Kalisvaart et al.22 recognized four risk factors for delirium (visual impairment, APACHE II scores > 16, MMSE < 24, and dehydration) and included only patients with at least one risk factor for delirium. All other studies included in this review did not identify baseline cognitive performance, and therefore did not stratify study endpoints based on cognitive function.
The study by Kalisvaart and colleagues22 did not show a significant difference in delirium prevention with the use of low-dose haloperidol in a population at high risk of developing delirium, although reductions in delirium severity, duration, and hospital length of stay were noticed. Conversely, a study in cardiac surgery patients randomized to either one dose of risperidone upon awakening after the procedure identified a difference in delirium incidence when compared to those receiving placebo26. The investigators of this study did not report delirium severity, duration, or hospital length of stay (see Table 2).
Two studies evaluated donepezil20,21, and one study evaluated citicoline23 (a precursor of acetylcholine) in the prophylaxis of delirium with results consistently showing no benefit of the cholinergic enhancement in preventing delirium. Donepezil was administered 14 days prior to and following the surgical procedure in one study20, and on the day of surgery and 3 days following the procedure in the other study21. Similarly, citicoline use was started on the day of surgery and continued for three days, with no significant difference identified in delirium outcomes. Studies that did find a significant difference in the incidence of delirium22,24,25–27 started the intervention on the day of surgery, and continued up to three days postoperatively. One study finding a significant reduction in the incidence of delirium26 used only one dose of risperidone one time only following cardiovascular surgery.
Two articles compared non-traditional pharmacologic agents as potential targets in reducing the incidence of delirium in intensive care units: 1) sedative use in ventilated patients in the ICU25, and 2) a comparison of intra-operative agents used for general anesthesia during non-cardiac surgical procedures28 (Table 2). Investigators in the first study evaluated whether sedation with either dexmedetomidine or lorazepam would result in any difference in postoperative cognitive outcomes24. Study results found no difference in delirium incidence, though a difference was identified in the combination of delirium-free and coma-free days alive. Other relevant outcomes favored dexmedetomidine, however lacked statistical significance to support clear superiority. The second study also did not result in a significant difference in delirium outcomes between inhaled anesthesia strategies, though did draw associations between the use of patient-controlled analgesia and benzodiazepine use on post-operative days 1 or 2 with a higher risk of developing delirium28.
Finally, sleep-wake cycles and pain management were evaluated as potential targets in the reduction of delirium following surgical procedures25,27. Both early restoration of sleep cycles with the use of a multi-drug benzodiazepine/opiate combination, and pain management with gabapentin postoperatively reduced the incidence of delirium, though hospital length of stay was not different between the intervention and usual care groups in each study. Both studies were limited by small sample sizes, and the lack of a description of the randomization method further limited the results in the sleep-cycle study25.
DISCUSSION
Pharmacologic management of delirium was evaluated primarily by the use of antipsychotic medications with no identifiable, consistent differences in efficacy and tolerability between first- and second-generation antipsychotics. One study identified a difference in tolerability between antipsychotics and benzodiazepines, suggesting that benzodiazepines be avoided in the delirious population.16 Similarly, pharmacologic treatments for the prevention of incident delirium in hospitalized patients have resulted in controversial outcomes. We found inconsistent results in the development of delirium, with one study suggesting that the duration and severity of delirium symptoms appear to be reduced in patients given haloperidol22. Two trials with donepezil20,21, and one with a cholinergic precursor23, have consistently shown no impact on the development of delirium symptoms when administered to patients undergoing orthopedic surgical procedures.
The studies included in our review enrolled both patients with and without baseline cognitive impairment. Although baseline cognitive function has been recognized as a risk factor for the development of delirium3, no study stratified outcomes based on patient’s baseline cognitive status. Studies also inconsistently reported differences in hospital length of stay. Whereas one study identified a statistically significant difference in hospital length of stay when given haloperidol for the prevention of delirium22, another study identified a difference in hospital length of stay only between those who experienced delirium and those who did not, though this difference was not statistically different26.
Our systematic review identified several similar reports as those recognized in two recently published reviews13,14. Seitz and colleagues used similar search criteria and included 14 studies in their analysis13. Their results evaluated single-agent, non-comparison trials in addition to comparison trials, whereas our search included only comparison studies. The authors identified similar methodological limitations to the available literature as described below, most notably the lack of a placebo comparator. However, the authors recognized that patients exposed to antipsychotics during an acute delirious event did show signs of improvement in nearly all studies. Lacasse and colleagues also identified comparison studies of delirium management in acute settings14, though both reviews excluded studies aimed at delirium prevention. As in our review, these reports have described an improvement in delirium symptoms following the use of antipsychotic medications, with no particular agent proving superior to others that have been studied.
Similarly, our review supports the recommendations of delirium management as previously published through the American Psychiatric Association (APA)29 and the Society of Critical Care Medicine (SCCM)30. The APA recommends low-dose haloperidol as a first-line agent in the symptomatic management of delirium episodes, with few comparisons of newer second-generation antipsychotic medications included in their evaluation29. Our review supports the use of haloperidol as the first-line agent in delirium management based on similar results achieved when first-generation and second-generation antipsychotics were compared in head-to-head trials. SCCM guidelines suggest dose titration of haloperidol with the possible need of continuous infusion to control delirium symptoms30; however, our review identified no studies that evaluated the use of continuous infusions of haloperidol, and in fact utilized mean daily doses of no more than 6.5 mg, much lower than the range suggested in the critical care guidelines. Our search results suggest that benzodiazepines should not be considered in delirium in patients without a history of psychiatric illness or alcohol withdrawal due to poor outcomes and limited use in the literature included in this review.
Multiple limitations of this review exist that limit the interpretation of this collected data set. First, several of the available studies lacked or failed to report standard methods of randomization processes and blinding techniques, potentially contaminating their results. Notably, studies that had higher JADAD scores were more likely to identify a difference in delirium incidence, severity, duration, or hospital length of stay. Secondly, the included studies also compared a variety of different pharmacologic interventions in different clinical settings, making universal conclusions regarding delirium management inconclusive. To date, no study has compared the pharmacologic management of delirium symptoms with placebo in hospitalized patients. Similarly, the included studies lacked uniformity in reporting delirium severity, duration and length of hospital stay. The studies included in the review were also limited by the utilization of small sample sizes, thereby exposing the risk of type II errors. No differences in treatment effect could be compared between populations with or without baseline cognitive impairment, those with hypoactive or hyperactive delirium, or those with emergent or elective surgical procedures. With the use of a heterogeneous set of population characteristics, interventions, and evaluation scales in evaluating delirium severity and change over time, it is impossible to pool results to improve the strength of recommendation for clinical practice.
Delirium is commonly encountered in hospitalized older patients especially in medical, surgical and critical care units. A lack of clinical data with strong evidence limits the ability to provide evidence-based recommendations for pharmacologic interventions in this population. In patients at risk for delirium or with identifiable signs of mental status changes, screening with the Confusion Assessment Method (CAM/CAM-ICU) is recommended to confirm a diagnosis of delirium29,30. When appropriate, non-pharmacologic interventions should be performed to minimize the burden of delirium on hospital complications. Haloperidol has been the most widely used and studied agent in hospitalized delirium with suggested benefits in reducing symptom severity and duration of pre-existing delirium episodes. The second-generation antipsychotics may be an alternative in patients who are not candidates for or who do not tolerate first-generation antipsychotics, though the class has shown no benefit over the first-generation antipsychotics on either efficacy or safety parameters in delirium trials. Because of the risks of cardiovascular and musculoskeletal toxicities, the use of antipsychotics in patients with delirium should be continuously evaluated to minimize the potential for toxicity or over-sedation.
The current literature lacks well-designed studies comparing interventions in a controlled environment. Future studies should include appropriate randomization and blinding techniques with adequate sample sizes to ensure accurate and reproducible outcome measurement. Several specific research questions should be addressed, including the most appropriate dose, duration, and time to initiate agents for delirium treatment. Similarly, appropriate prevention interventions should be evaluated with adequately designed studies specifically targeting pain management, sleep cycle restoration, and antipsychotic use as potential targets to reduce delirium episodes.
Acknowledgements
Supported by Grant (K23 AG 26770–01) from the John A. Hartford Foundation, the Atlantic Philanthropies, the Starr Foundation, and the National Institute on Aging.
Conflict(s) of interest None disclosed.
References
- 1.Inouye SK, Dyck CH, Alessi CA, Balkin S, Siegal AP, Horwitz RI. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113:941–8. doi: 10.7326/0003-4819-113-12-941. [DOI] [PubMed] [Google Scholar]
- 2.DeFrances CJ, Hall MJ. 2002 National Hospital Discharge Survey. Advance Data from Vital and Health Statistics, 342. Hyattsville, MD: National Center for Health Statistics; 2004. [PubMed] [Google Scholar]
- 3.Boustani M, Buttar A. Delirium in hospitalized older adults. Primary care geriatrics, a case-based approach. Editors: R Ham, P. Sloane, G. Warshaw. 5th Edition 2007, Chapter 15: 210–218.
- 4.Trzepacz PT. The neuropathogenesis of delirium. A need to focus our research. Psychosomatics. 1994;35:374–91. doi: 10.1016/S0033-3182(94)71759-X. [DOI] [PubMed] [Google Scholar]
- 5.Trzepacz PT. Update on the neuropathogenesis of delirium. Dement Geriatr Cogn Disord. 1999;10(5):330–41. doi: 10.1159/000017164. [DOI] [PubMed] [Google Scholar]
- 6.Trzepacz PT. Is there a final common neural pathway in delirium? Focus on acetylcholine and dopamine. Semin Clin Neuropsychiatry. 2000;5(2):125–31. doi: 10.153/SCNP00500132. [DOI] [PubMed] [Google Scholar]
- 7.van der Mast RC. Delirium: the underlying pathophysiological mechanisms and the need for clinical research. J Psychosomatic Res. 1996;41:109–13. doi: 10.1016/0022-3999(96)00006-2. [DOI] [PubMed] [Google Scholar]
- 8.Flacker JM, Lipsitz LA. Neural mechanisms of delirium: current hypotheses and evolving concepts. Erratum appears in J Gerontol A Biol Sci Med Sci. 1999;54(7):B275. doi: 10.1093/gerona/54.6.b239. [DOI] [PubMed] [Google Scholar]
- 9.van der Mast RC, Fekkes D. Serotonin and amino acids: partners in delirium pathophysiology? Clin Neuropsychiatry. 2000;5(2):125–31. doi: 10.153/SCNP00500125. [DOI] [PubMed] [Google Scholar]
- 10.Inouye SK, Bogardus ST, Jr, Charpentier PA, et al. A multicomponent intervention to prevent delirium in hospitalized older patients. N Engl J Med. 1999;340:669. doi: 10.1056/NEJM199903043400901. [DOI] [PubMed] [Google Scholar]
- 11.Han L, McCusker J, Cole M, et al. Use of medications with anticholinergic effect predicts clinical severity of delirium symptoms in older medical inpatients. Arch Intern Med. 2001;161(8):1099–105. doi: 10.1001/archinte.161.8.1099. [DOI] [PubMed] [Google Scholar]
- 12.Marcantonio ER, Flacker JM, Wright RJ, Resnick NM. Reducing delirium after hip fracture: a randomized trial. J Am Geriatr Soc. 2001;49:516–22. doi: 10.1046/j.1532-5415.2001.49108.x. [DOI] [PubMed] [Google Scholar]
- 13.Seitz DP, Gill SS, Zyl LT. Antipsychotics in the treatment of delirium: a systematic review. J Clin Psychiatry. 2007;68(1):11–21. doi: 10.4088/jcp.v68n0102. [DOI] [PubMed] [Google Scholar]
- 14.Lacasse H, Perreault MM, Williamson DR. Systematic review of antipsychotics for the treatment of hospital-asociated delirium in medically or surgically ill patients. Ann Pharmacother. 2006;40:1966–73. doi: 10.1345/aph.1H241. [DOI] [PubMed] [Google Scholar]
- 15.Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17(1):1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 16.Breitbart W, Marotta R, Platt MM, Weisman H, Derevenco M, Grau C, Corbera K, Raymond S, Lund S, Jacobson P. A double-blind trial of haloperidol, chlorpromazine, and lorazepam in the treatment of delirium in hospitalized AIDS patients. Am J Psychiatr. 1996;153(2):231–7. doi: 10.1176/ajp.153.2.231. [DOI] [PubMed] [Google Scholar]
- 17.Han, Chang-Su, Kim, Yong-Ku A double-blind trial of risperidone and haloperidol for the treatment of delirium. Psychosomatics. 2004;45:297–301. doi: 10.1016/S0033-3182(04)70170-X. [DOI] [PubMed] [Google Scholar]
- 18.Lee KU, Won WY, Lee HK, Kweon YS, Lee CT, Pae CU, Bahk WM. Amisulpride versus quetiapine for the treatment of delirium: a randomized, open prospective study. Int Clin Psychopharmacol. 2005;20(6):311–4. doi: 10.1097/00004850-200511000-00005. [DOI] [PubMed] [Google Scholar]
- 19.Skrobik YK, Bergeron N, Dumont M, Gottfried SB. Olanzapine vs Haloperidol: Treating delirium in a critical care setting. Intensive Care Med. 2004;30:444–9. doi: 10.1007/s00134-003-2117-0. [DOI] [PubMed] [Google Scholar]
- 20.Liptzin B, Laki A, Garb JL, Fingeroth R, Krushell R. Donepezil in the prevention and treatment of post surgical delirium. Am J Geriatr Psychiatry. 2005;13:1100–6. doi: 10.1176/appi.ajgp.13.12.1100. [DOI] [PubMed] [Google Scholar]
- 21.Sampson EL, Raven PR, Ndhlovu PN, et al. A randomized, double-blind, placebo-controlled trial of donepezil hydrochloride (Aricept) for reducing the incidence of postoperative delirium after elective total hip replacement. Int J Geriatr Psychiatry. 2007;22:343–9. doi: 10.1002/gps.1679. [DOI] [PubMed] [Google Scholar]
- 22.Kalisvaart KJ, Jonghe JF, Bogaards MJ, Vreeswijk R, Egberts TC, Burger BJ, Eikelenboom P. Haloperidol prophylaxis for elderly hip-surgery patients at risk for delirium: a randomized placebo-controlled study. J Am Ger Soc. 2005;53(10):1658–66. doi: 10.1111/j.1532-5415.2005.53503.x. [DOI] [PubMed] [Google Scholar]
- 23.Diaz V, Rodriguez J, Barrientos P, Serra M, Salina H, et al. Utilizacion de procolinergicos en la prevencion del delirio postoperatorio del adulto mayor sometido a cirugia de fractura de cadera. Ensayo clinico controlado (Use of citicoline in the prevention of delirium in hip fracture surgery in elderly. A randomized controlled trial. Rev Neurol. 2001;33:716–9. [PubMed] [Google Scholar]
- 24.Pandharipande PP, Pun BT, Herr DL, et al. Effect of sedation with dexmedetomidine vs. lorazepam on acute brain dysfunction in mechanically ventilated patients: the MENDS randomized controlled trial. JAMA. 2007;298(22):2644–53. doi: 10.1001/jama.298.22.2644. [DOI] [PubMed] [Google Scholar]
- 25.Aizawa K-I, Kanai T, Saikawa Y, Takabayashi T, Kawano Y, Miyazawa N, Yamamoto T. A novel approach to the prevention of postoperative delirium in the elderly after gastrointestinal surgery. Surg Today. 2002;32:310–4. doi: 10.1007/s005950200044. [DOI] [PubMed] [Google Scholar]
- 26.Prakanrattana U, Prapaitrakool S. Efficacy of risperidone for prevention of postoperative delirium in cardiac surgery. Anaesth Intensive Care. 2007;35(5):714–9. doi: 10.1177/0310057X0703500509. [DOI] [PubMed] [Google Scholar]
- 27.Leung JM, Sands LP, Rico M, Petersen KL, Rowbotham MC, Dahl JB, Ames C, Chou D, Weinstein P. Pilot clinical trial of gabapentin to decrease postoperative delirium in older patients. Neurology. 2006;67(7):1251–3. doi: 10.1212/01.wnl.0000233831.87781.a9. [DOI] [PubMed] [Google Scholar]
- 28.Leung JM, Sands LP, Vaurio LE, Wang Y. Nitrous oxide does not change the incidence of postoperative delirium or cognitive decline in elderly surgical patients. Br J Anaesth. 2006;96:754–760. doi: 10.1093/bja/ael106. [DOI] [PubMed] [Google Scholar]
- 29.American Psychiatric Association Practice guideline for the treatment of patients with delirium. Am J Psychiatry. 1999;156(5 suppl):1–20. [PubMed] [Google Scholar]
- 30.Society of Critical Care Medicine Clinical practice guidelines for the sustained use of sedatives and analgesics in the critically ill adult. Crit Care Med. 2002;30(1):119–41. doi: 10.1097/00003246-200201000-00020. [DOI] [PubMed] [Google Scholar]