Abstract
Rational
Eszopiclone and zolpidem are hypnotics that differentially affect sleep and waking states in adult animals. Therefore, it was of interest to compare their effects on the states of sleep and wakefulness in aged animals.
Objectives
Our objective was to determine the responses to eszopiclone and zolpidem vis-à-vis sleep and waking states in aged guinea pigs and to compare them with the effects of these hypnotics in adult animals.
Methods
Aged guinea pigs were prepared to monitor sleep and waking states and to perform a frequency analysis of the EEG. Eszopiclone and zolpidem were administered intraperitoneally (1, 3, and 10 mg/kg).
Results
Eszopiclone produced a more rapid and greater increase in NREM sleep as well as longer duration episodes of NREM sleep compared with zolpidem. There was also a significant increase in the latency to REM sleep with eszopiclone, but not with zolpidem. EEG power during NREM sleep increased in the delta band and decreased in the theta band following eszopiclone administration, whereas zolpidem had no effect on any of the frequency bands analyzed.
Conclusions
In aged as well as adult guinea pigs, eszopiclone is a more effective hypnotic insofar as it produces a shorter latency to NREM sleep, a greater amount of NREM sleep and EEG delta waves. Differences in the effects produced by eszopiclone and zolpidem as a function of the aging process likely reflect the fact that they bind to different subunits of the GABAA receptors, which are differentially reactive to the aging process.
Keywords: Hypnotics, NREM sleep, EEG power, Aging
Introduction
In humans, old age is characterized by a decrease in nonrapid eye movement (NREM) and REM sleep as well as an increase in sleep latency and in the number of awakenings (Bliwise 2005). Sleep-related disorders, such as insomnia, sleep apnea, and periodic limb movements, occur more frequently in elderly individuals than in adults (Ancoli-Israel et al. 1985; Foley et al. 1995). There is also an increase in sleep disorders in the aged that is reflected by the enhanced use of hypnotics (Mendelson 1987). In addition, the aging brain appears to be more sensitive to the sedative effects of classic hypnotics, such as benzodiazepines and cognitive dysfunctions, which are more prevalent in the elderly, may be aggravated by their use (Hemmeter et al. 2000). In the elderly, the long plasma elimination half lives of the benzodiazepines and their metabolites are increased due to a reduced capacity for oxidative hydroxylation (Lader 1993). Because aged individuals are less able to compensate for impairments caused by these hypnotics, the use of benzodiazepines to treat insomnia, especially in the elderly, has been considered problematic (Hemmeter et al. 2000). However, unlike classic hypnotics, nonbenzodiazepine hypnotics, such as zopiclone (a cyclopyrrolone derivative) whose s-isomer is eszopiclone and zolpidem (an imidazopyridine derivative) have fewer adverse effects (Declerck et al. 1992; Hemmeter et al. 2000; Lader 1992; Lancel 1999; Morin 2006; Noble et al. 1998).
Zopiclone and zolpidem act by potentiating the benzodiazepine-GABAA receptor complex by binding to different GABAA receptor subunits. Thus, zopiclone (or eszopiclone) exhibits considerable activity at GABAA receptors containing α1, α2, α3, and α5 subunits (Damgen and Luddens 1999; Davies et al. 2000; Smith et al. 2001). On the other hand, zolpidem displays a very high and selective affinity for α1 subunits with significantly less or no affinity for α2, α3, and α5 subunits (Sanna et al. 2002). Certain of the subunits of GABAA receptors that are activated by the preceding hypnotics display age-related changes in activity (Rissman et al. 2007). Therefore, it was of interest to compare the hypnotic actions of eszopiclone and zolpidem in old age. In addition, data from these studies would establish a foundation for understanding the cellular bases that are responsible for changes in sleep patterns as well as the effectiveness of hypnotics in the elderly, which at present are unknown.
We recently examined the hypnotic effects of eszopiclone and zolpidem in the adult guinea pig (Xi and Chase 2008). We found that eszopiclone, compared with zolpidem, has a pharmacological profile in the adult guinea pig (4–7 months old) that is characterized by a more rapid onset of hypnotic action and an increased amount of time spent in NREM sleep. In addition, eszopiclone produced an increase in EEG delta power, whereas zolpidem had no effect on delta activity. While our previous study and a number of other reports have described the hypnotic effects of eszopiclone (or zopiclone) and zolpidem in adult animals (Chen et al. 2005; Depoortere et al. 1991; Gauthier et al. 1997; Gottesmann et al. 1998; Mailliet et al. 2001; Noguchi et al. 2004; Stutzmann et al. 1992; Xi and Chase 2008), there have been no quantitative studies that have directly compared the effects of eszopiclone and zolpidem on the states of sleep and wakefulness in aged animals or related these effects to those produced in adult animals. Therefore, in the present study, we examined the effects of the intraperitoneal injection of eszopiclone and zolpidem on sleep and waking states, as well as on EEG power, in chronically instrumented, unanesthetized aged guinea pigs. We also compared these data with the effects produced by eszopiclone and zolpidem in adult animals.
Materials and methods
Animals and surgical procedures
Experiments were performed on five Hartley guinea pigs (three females and two males; age 28 ± 2 months, body weight 1.10 ± 0.06 kg) obtained from and determined to be in good health by the VA Greater Los Angeles Healthcare System (VAGLAHS). A variety of systems in the guinea pig, and specifically the central nervous system, exhibit age-related changes in animals that are 24 to 36 months old (Abalo et al. 2005; Gabella 2001; Hiller et al. 1992; McDougall and Schuelert 2007; Nozawa et al. 1997; Zhang et al. 2005). Therefore, the guinea pigs that were used in the present study were classified as “aged”. Animals, which were housed in a temperature (22 ± 1°C) and humidity (50–70%) controlled environment with a 12:12-h light/dark cycle (lights on from 600–1800 hours), had ad libitum access to food and water.
The following procedures were carried out in order to monitor the behavioral states of sleep and wakefulness and to determine the effects of intraperitoneally administered eszopiclone and zolpidem. Briefly, under ketamine/xylazine (45/5 mg/kg, im) anesthesia, using sterile surgical procedures, each guinea pig was implanted with electrodes for recording the cortical electroencephalogram (EEG), the electrooculogram (EOG), and the electromyogram (EMG). To record EEG activity, screw electrodes were implanted bilaterally in the calvarium overlying the cortex at the following coordinators (relative to bregma): 2 mm anterior, 2 mm lateral; 5 mm posterior, and 3 mm lateral. EMG electrodes were inserted bilaterally into the dorsal neck muscles. A Winchester plug, connected to these electrodes, was bonded to the calvarium with acrylic cement. Following surgery, antibiotics were administrated both systemically and topically. After recovery from surgery, each guinea pig was adapted to the recording environment on a daily basis for at least 2 weeks.
Procedures were conducted in accordance with the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, National Academic Press, Washington DC, 1996) and approved by the Animal Research Committee of the VAGLAHS, and all efforts were made to minimize the number of animals used in this study.
Drug administration
Experimental sessions were conducted between 1100 and 1600 hours. Eszopiclone (Sepracor Canada Ltd; Windsor, Canada) and zolpidem (Sanofi-Aventis USA, Bridgewater, NJ) were separately administered, intraperitoneally, at doses of 1, 3, and 10 mg/kg (0.5 ml). The doses of eszopiclone and zolpidem that were used in the present study are within the range of those typically employed when these substances are administered as hypnotics in experimental animals (Chen et al. 2005; Depoortere et al. 1991; Gauthier et al. 1997; Gottesmann et al. 1998; Mailliet et al. 2001; Noguchi et al. 2002, 2004; Stutzmann et al. 1992; Xi and Chase 2008). Vehicle was injected as a control (50 mM acetate buffer solution, pH 4.5, 0.5 ml). Each animal received injections of doses of 1, 3, and 10 mg/kg of both drugs and vehicle in a randomized protocol, which were administrated during the first 10 min of each experimental session. At least 2 days (>2 days) transpired between each injection session in order to make sure that all drug effects were reversible and did not impact responses produced by subsequent drug injections.
Recording and data analysis
The behavioral states of wakefulness, NREM sleep, and REM sleep were scored on the basis of polygraphic records which included EEG, EOG, and EMG data that were monitored and digitized using a Macintosh computer running AxoGraph X software (AxoGraph Scientific, Sydney, Australia); polygraphic data were scored in 30-s epochs according to previously described criteria (Escudero and Vidal 1996; Ibuka 1984; Tobler and Franken 1993). The following dependent variables were determined for each recording session: (1) percentage of time spent in wakefulness, NREM, and REM sleep; (2) latency to the onset of the first episode of NREM and REM sleep; because injections were carried out during wakefulness, there was no measure of the latency to wakefulness; (3) number of episodes of each behavioral state per hour (frequency); and (4) duration of episodes of each behavioral state. EEG power during each state was determined on the basis of a fast Fourier transform analysis of the EEG. Successive 15-s time segments of EEG signals in 1 Hz bins from 0.5–30 Hz over a period of 2 h were analyzed using AxoGraph X software. The relative power (percent of total) in each of the following frequency bands during each state was determined: delta (0.5–4 Hz); theta (4–9 Hz); sigma (9–14 Hz); and beta (14–30 Hz). Experimental data are expressed as means ± SEM. The statistical significance of the difference between sample means was evaluated using completely randomized one-way analysis of variance (ANOVA). After a one-way ANOVA, post hoc comparisons with the Scheffe method were used to determine the individual levels of significant differences between control (vehicle) and eszopiclone, control and zolpidem, and eszopiclone and zolpidem. A p value of <0.05 was considered to be statistically significant.
Results
In the present study, intraperitoneal injections of 1, 3, and 10 mg/kg of eszopiclone and 1, 3, and 10 mg/kg of zolpidem, as well as control injections of vehicle were performed in order to determine the effects of eszopiclone and zolpidem on sleep and waking states in the aged guinea pig. Control injection of vehicle was ineffective in modifying the states of sleep or wakefulness. No statistically significant changes in the time spent in sleep or wakefulness were observed during the third and fourth hours following the administration of either eszopiclone or zolpidem compared with vehicle control sessions. Thus, the effects of eszopiclone and zolpidem were limited to changes that occurred during the first 2 h following their administration. The following sections describe and compare, quantitatively, the effects of eszopiclone and zolpidem on the states of wakefulness, NREM, and REM sleep, as well as EEG power, during the first 2 h following the administration of these drugs.
Changes in the percentage of time spent in sleep and waking states following the administration of eszopiclone and zolpidem
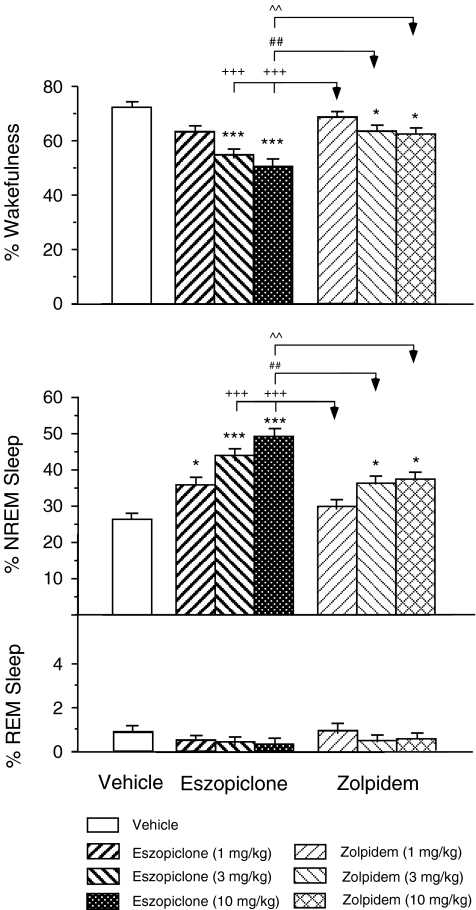
Figure 1 presents an overview of the effects of the administration of eszopiclone and zolpidem on the percentage of time spent in sleep and waking states in the aged guinea pig. The following data are the results of an ANOVA analysis that documents the significant effect on the percentage of time spent in NREM sleep and waking states that occurred a conjunction with the administration of eszopiclone and zolpidem (NREM sleep: df = 6,71, F = 18.35, p < 0.001; wakefulness: df = 6,71, F = 16.33, p < 0.001). Compared to vehicle injections, the administration of eszopiclone at 1, 3, and 10 mg/kg resulted in significant increases in the time spent in NREM sleep by 34.0%, 65.7%, and 85.1% (vehicle 26.8 ± 1.6%, n = 12; 1 mg/kg eszopiclone 35.9 ± 1.7%, n = 12, p = 0.037; 3 mg/kg eszopiclone 44.4 ± 1.7%, n = 12, p < 0.001; 10 mg/kg eszopiclone 49.6 ± 2.1%, n = 8, p < 0.001, post hoc Scheffe method) and decreases in wakefulness by 12.0%, 23.7%, and 30.6% (vehicle 72.2 ± 1.8%, n = 12; 1 mg/kg eszopiclone 63.5 ± 1.7%, n = 12, p = 0.064; 3 mg/kg eszopiclone: 55.1 ± 1.7%, n = 12, p < 0.001; 10 mg/kg eszopiclone 50.1 ± 2.2%, n = 8, p < 0.001, post hoc Scheffe method), respectively.
Fig. 1.
Effects of eszopiclone and zolpidem on the mean percentage of time spent in wakefulness, NREM sleep, and REM sleep during the first 2-h period following the administration of these drugs at the doses of 1, 3, and 10 mg/kg in the aged guinea pig (n = 5). Each bar represents the mean value; error bars indicate the SEM of each population. *p < 0.05, **p < 0.01, ***p < 0.001 vs. vehicle control group; +++p < 0.001 vs. 1 mg/kg zolpidem group; ##p < 0.01 vs. 3 mg/kg zolpidem group; ^^p < 0.01 vs. 10 mg/kg zolpidem group by post hoc Scheffe method
Following the injection of zolpidem at 1 mg/kg, there were no significant changes in the percentage of time spent in sleep and waking states. However, the administration of zolpidem at 3 and 10 mg/kg significantly increased the percentage of time spent in NREM sleep by 35.4% and 37.7% (3 mg/kg zoipidem 36.4 ± 1.8%, n = 12, p = 0.022; 10 mg/kg zoipidem 36.9 ± 1.8%, n = 10, p = 0.021, post hoc Scheffe method) and decreased the time spent in wakefulness by 12.5% and 13.4% (3 mg/kg zoipidem 63.2 ± 1.8%, n = 12, p = 0.049; 10 mg/kg zoipidem: 62.5 ± 1.9%, n = 10, p = 0.039, post hoc Scheffe method), respectively.
A significant difference in the percentage of NREM sleep and wakefulness was observed on the basis of post hoc analyses following the administration of eszopiclone compared to zolpidem. The administration of eszolpiclone at 3 mg/kg (but not at 1 mg/kg) resulted in an increase in the percentage of time spent in NREM sleep and a decrease in wakefulness that was significantly greater than the time spent in these states after the administration of 1 mg/kg of zolpidem (NREM sleep 3 mg/kg eszopiclone vs. 1 mg/kg zolpidem, p < 0.001; wakefulness 3 mg/kg eszopiclone vs. 1 mg/kg zolpidem, p < 0.001, post hoc Scheffe method). The administration of eszolpiclone at 10 mg/kg also resulted in a significant increase in NREM sleep and a decrease in wakefulness compared to the time spent in these states after the administration of 1, 3, or 10 mg/kg of zolpidem (NREM sleep 10 mg/kg eszopiclone vs. 1 mg/kg zolpidem, p < 0.001, 10 mg/kg eszopiclone vs. 3 mg/kg zolpidem, p = 0.001; 10 mg/kg eszopiclone vs. 10 mg/kg zolpidem, p = 0.004; wakefulness 10 mg/kg eszopiclone vs. 1 mg/kg zolpidem, p < 0.001; 10 mg/kg eszopiclone vs. 3 mg/kg zolpidem, p = 0.002; 10 mg/kg eszopiclone vs. 10 mg/kg zolpidem, p = 0.008, post hoc Scheffe method).
Following the injection of eszopiclone or zolpidem at 1, 3, or 10 mg/kg, there was no significant change in the time spent in REM sleep (df = 6,71, F = 0.86, p = 0.527, ANOVA), although there was a tendency toward a decrease in the REM sleep following the injection of eszopiclone or zolpidem at the higher doses of 3 or 10 mg/kg.
Changes in the latency to NREM and REM sleep following the administration of eszopiclone and zolpidem
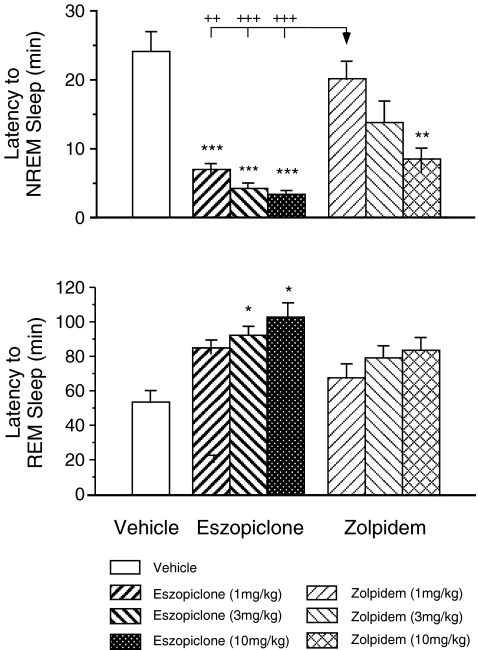
Figure 2 presents the effects of eszopiclone and zolpidem on the mean latency to the onset of the first episode of NREM and REM sleep following the administration of these drugs. Significant drug effects occurred as revealed by ANOVA analysis with respect to the latency to both NREM sleep and REM sleep (NREM sleep: df = 6,71, F = 13.88, p < 0.001; REM sleep: df = 6,27, F = 4.82, p = 0.002).
Fig. 2.
Dosage effects of eszopiclone and zolpidem on the latency to NREM sleep and REM sleep in the aged guinea pig (n = 5). Each bar represents the mean value; error bars indicate the SEM of each population. *p < 0.05, **p < 0.01, ***p < 0.001 vs. vehicle control group; ++p < 0.01, +++p < 0.001 vs. 1 mg/kg zolpidem group by post hoc Scheffe method
Following the injection of eszopiclone at 1, 3, and 10 mg/kg, the mean latency to NREM sleep was significantly shorter compared with vehicle controls, respectively (1 mg/kg eszopiclone vs. vehicle, p < 0.001; 3 mg/kg eszopiclone vs. vehicle, p < 0.001; 10 mg/kg eszopiclone vs. vehicle, p < 0.001, post hoc Scheffe method). Eszopiclone at 3 and 10 mg/kg also significantly increased the mean latency to REM sleep (3 mg/kg eszopiclone vs. vehicle, p = 0.032; 10 mg/kg eszopiclone vs. vehicle, p = 0.034, post hoc Scheffe method).
There were no significant changes in the mean latency to NREM sleep following the injection of zolpidem at 1 and 3 mg/kg, although there was a tendency toward a decrease in the latency to NREM sleep at 3 mg/kg. However, zolpidem at 10 mg/kg resulted in a significant decrease in the latency to NREM sleep (10 mg/kg zolpidem vs. vehicle, p = 0.001, post hoc Scheffe method). There was no effect on the latency to REM sleep with any dose of zolpidem.
Following the injection of eszopiclone at 1, 3, and 10 mg/kg, the mean latency to NREM sleep was significantly shorter than that following zolpidem administration at doses of 1 mg/kg, respectively (1 mg/kg eszopiclone vs. 1 mg/kg zolpidem, p = 0.005; 3 mg/kg eszopiclone vs. 1 mg/kg zolpidem, p < 0.001; 10 mg/kg eszopiclone vs. 1 mg/kg zolpidem, p < 0.001, post hoc Scheffe method).
Changes in the duration and frequency of episodes of sleep and wakefulness following the administration of eszopiclone and zolpidem
The effects on the duration of episodes of sleep and waking states were examined after the injection of eszopiclone and zolpidem (Fig. 3). ANOVA revealed significant drug effects vis-à-vis the duration of episodes of NREM sleep (df = 6,71 F = 6.78, p < 0.001). Following the injection of eszopiclone at 3 and 10 mg/kg, the mean duration of the episodes of NREM sleep was significantly longer than the episodes of NREM that occurred with vehicle (3 mg/kg eszopiclone vs. vehicle p = 0.011; 10 mg/kg eszopiclone vs. vehicle p = 0.002, post hoc Scheffe method). Eszopiclone at 3 and 10 mg/kg also resulted in a significant decrease in the mean duration of episodes of wakefulness (3 mg/kg eszopiclone vs. vehicle p = 0.040; 10 mg/kg eszopiclone vs. vehicle p = 0.034, post hoc Scheffe method). No significant effects on the preceding parameters were observed following the injection of eszopiclone at 1 mg/kg.
Fig. 3.
The mean duration of episodes of wakefulness, NREM sleep and REM sleep following the administration of eszopiclone and zolpidem at the doses of 1, 3, and 10 mg/kg in the aged guinea pig (n = 5). *p < 0.05, **p < 0.01 vs. vehicle control group; +p < 0.05 vs. 1 mg/kg zolpidem group; #p < 0.05 vs. 3 mg/kg zolpidem group by post hoc Scheffe method
Following the injection of zolpidem at all doses, the mean duration of episodes of NREM sleep and wakefulness was not significantly different from that following the injection of vehicle. However, there was a tendency for an increase in the duration of episodes of NREM sleep and a decrease in the duration of episodes of wakefulness following the administration of zolpidem, but only at 3 or 10 mg/kg.
Post hoc analyses revealed a significant increase in the mean duration of episodes of NREM sleep following the injection of eszopiclone at 10 mg/kg compared to the duration of NREM sleep episodes following the injection of 1 or 3 mg/kg of zolpidem (10 mg/kg eszopiclone vs. 1 mg/kg zolpidem, p = 0.012; 10 mg/kg eszopiclone vs. 3 mg/kg zolpidem, p = 0.023, post hoc Scheffe method).
Following the administration of eszopiclone and zolpidem at all doses, the frequency of episodes of NREM sleep, REM sleep, and wakefulness was not significantly different from that following the injection of vehicle, although there was a tendency for a reduction in the frequency of episodes of REM sleep following the administration of zolpidem at 3 or 10 mg/kg.
Changes in spectral EEG power following the administration of eszopiclone and zolpidem
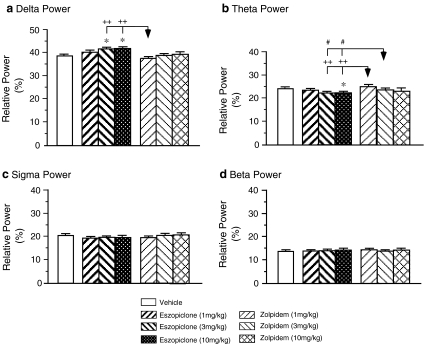
The effects of eszopiclone and zolpidem on EEG power during NREM sleep are shown in Fig. 4, which consists of plots of relative EEG power density during NREM sleep following the injection of eszopiclone, zolpidem, and vehicle. ANOVA revealed significant drug effects on the relative EEG power in the delta and theta bands during NREM sleep which occurred following the injection of eszopiclone and zolpidem (the delta band: df = 6,71 F = 6.73, p < 0.001; the theta band: df = 6,71 F = 7.80, p < 0.001).
Fig. 4.
Effects of eszopiclone and zolpidem on the relative power of EEG of the frontal cortex in the delta, theta, sigma, and beta frequency bands during NREM sleep in the aged guinea pig (n = 5). *p < 0.05 vs. the vehicle control group; +p < 0.05, ++p < 0.01 vs. 1 mg/kg zolpidem group; #p < 0.05 vs. 3 mg/kg zolpidem group by post hoc Scheffe method
Eszopiclone, at a dose of 3 or 10 mg/kg, significantly increased EEG power in the delta band (vehicle 38.6 ± 0.6 %, n = 12; 3 mg/kg eszopiclone 41.8 ± 0.4 %, n = 12, p = 0.026; 10 mg/kg eszopiclone 42.1 ± 0.5 %, n = 8, p = 0.030, post hoc Scheffe method) and reduced EEG power in the theta band (vehicle: 23.8 ± 0.3 %, n = 12; 3 mg/kg eszopiclone 22.2 ± 0.3%, n = 12, p = 0.085; 10 mg/ kg eszopiclone 21.8 ± 0.3%, n = 8, p = 0.037, post hoc Scheffe method). EEG power in the sigma and beta bands were not significantly altered following the administration of eszopiclone at 1, 3, or 10 mg/kg. However, there were no significant changes in EEG power in any of the frequency bands analyzed during NREM sleep following zolpidem administration at any dose.
Comparisons between eszopiclone and zolpidem injections with post hoc analyses revealed that eszopiclone administration at 3 or 10 mg/kg significantly increased the relative power of the EEG in the delta band compared to that after zolpidem administration at 1 mg/kg (3 mg/kg eszopiclone vs. 1 mg/kg zolpidem, p = 0.003; 10 mg/kg eszopiclone vs. 1 mg/kg zolpidem, p = 0.004, post hoc Scheffe method). Eszopiclone administration at 3 or 10 mg/kg also significantly reduced the relative power of the EEG in the theta band compared to that after zolpidem administration at both doses (3 mg/kg eszopiclone vs. 1 mg/kg zolpidem, p = 0.004; 10 mg/kg eszopiclone vs. 1 mg/kg zolpidem, p = 0.002; 3 mg/kg eszopiclone vs. 3 mg/kg zolpidem, p = 0.049; 10 mg/kg eszopiclone vs. 3 mg/kg zolpidem, p = 0.021, post hoc Scheffe method).
EEG power during REM sleep was examined following the administration of any doses of eszopiclone and zolpidem. Neither eszopiclone nor zolpidem resulted in any significant change in EEG power in any of the frequency bands analyzed during REM sleep.
Discussion
In the following sections, we discuss the effects of eszopiclone and zolpidem on the states of sleep and wakefulness in the aged guinea pig. These data are also compared with data obtained from our previous study in the adult guinea pig (Xi and Chase 2008) and reports of the effectiveness of these hypnotics in adult animals of other species. The differential binding properties of eszopiclone and zolpidem vis-à-vis specific subunits of the GABAA receptors and the effect of age on the activity of GABAA receptors are suggested to be responsible partially for the changes in the effects of these hypnotics in old age.
Hypnotic effects of eszopiclone in the aged guinea pig
In aged guinea pigs, the administration of eszopiclone resulted in significant changes in NREM sleep at doses of 1, 3, and 10 mg/kg that consisted of an increase in the amount of NREM sleep. This increase was accompanied by a significant decrease in wakefulness. Eszopiclone also significantly reduced the latency to NREM sleep and increased the latency to REM sleep. Furthermore, there was a significant increase in the duration of episodes of NREM sleep and a decrease in the duration of episodes of wakefulness following the administration of eszopiclone at 3 and 10 mg/kg. Finally, no significant effects on the time spent in REM sleep were observed following the administration of eszopiclone, although there was a tendency for eszopiclone to reduce the amount of REM sleep.
Compared with data obtained from our previous study in adult guinea pigs (Xi and Chase 2008), it is interesting to note that the increase in NREM sleep and the reduction in wakefulness following the administration of eszopiclone in the aged guinea pig were smaller than were the changes that occurred when equivalent doses were administrated in adult animals (Table 1). In addition, eszopiclone produced a significant increase in the duration of episodes of NREM sleep at 1 and 3 mg/kg in the adult guinea pig, but there was an increase in the duration of NREM sleep episodes only at a dose of 3 mg/kg in the aged guinea pig. However, eszopiclone (1 and 3 mg/kg) resulted in a significant, and comparable, decrease in the mean latency to NREM sleep in both aged and adult animals.
Table 1.
Hypnotic effects of eszopiclone and zolpidem in adult and aged guinea pigs
| Eszopiclone | Zolpidem | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 mg/kg | 3 mg/kg | 1 mg/kg | 3 mg/kg | ||||||
| Adulta | Aged | Adulta | Aged | Adulta | Aged | Adulta | Aged | ||
| Wakefulness | 25%↓*** | n.c. | 34%↓*** | 24%↓*** | n.c. | n.c. | 13%↓* | 13%↓* | |
| NREM Sleep | 60%↑*** | 34%↑* | 81%↑*** | 66%↑*** | n.c. | n.c. | 32%↑* | 35%↑* | |
| NREM latency | 68%↓*** | 70%↓*** | 85%↓*** | 82%↓*** | n.c. | n.c. | n.c. | n.c. | |
| REM Latency | 82%↑* | n.c. | 117%↑** | 70%↑* | n.c. | n.c. | n.c. | n.c. | |
| Wakefulness Duration | n.c. | n.c. | 35%↓* | 36%↓* | n.c. | n.c. | n.c. | n.c. | |
| NREM Sleep Duration | 35%↑* | n.c. | 65%↑*** | 60%↑* | n.c. | n.c. | n.c. | n.c. | |
| REM Sleep Frequency | n.c. | n.c. | 50%↓* | n.c. | n.c. | n.c. | n.c. | n.c. | |
| Delta Power | 10%↑* | n.c. | 13%↑** | 8%↑* | n.c. | n.c. | n.c. | n.c. | |
| Theta Power | 7%↓** | n.c | 8%↓** | n.c. | n.c. | n.c. | n.c | n.c | |
An increase (↑) or decrease (↓) in the percentage of changes in sleep and waking states compared with control (vehicle) data are presented at 1 and 3 mg/kg for eszopiclone and zolpidem in adult and aged guinea pigs. Data regarding the percentage of time spent in REM sleep, the duration of episodes of REM sleep, the frequency of episodes of NREM sleep and wakefulness, EEG power in sigma and beta bands following the administration of eszopiclone or zolpidem at both doses, which were not significantly different from those of vehicle-injected controls, are not included in this table. *P < 0.05; **P < 0.01; ***P < 0.001. Post hoc comparisons with the Scheffe method were used to determine the levels of significant differences between drugs and control (vehicle)
n.c. no significant change
aXi and Chase (2008)
Our previous study in the adult guinea pig demonstrated that there is a significant increase in EEG power in the delta band (0.5–4.0 Hz) and a decrease in EEG power in the theta band (4.0–9.0 Hz) during NREM sleep following the administration of eszopiclone at either 1 or 3 mg/kg (Xi and Chase 2008). In contrast, the present data revealed that there were no significant changes in EEG power in any of the frequency bands analyzed during NREM sleep following eszopiclone administration at 1 mg/kg in aged animals. However, eszopiclone at 3 mg/kg did produce a significant increase in EEG power in the delta band during NREM sleep in the aged animals. There were no significant changes in either the time spent in REM sleep or EEG power during REM sleep following the injection of eszopiclone at either dose in aged guinea pigs, which is similar to the results in the adult animal (Xi and Chase 2008).
The preceding data indicate that in aged guinea pigs, the effects of eszopiclone on sleep and waking states (an increase in NREM and a decrease in wakefulness) and in the power spectra of the EEG during NREM sleep (an increase in delta power) were significant but not quite as strong as the effects produced in adult animals. Zopiclone (presumably eszopiclone) exhibits considerable binding not only to α1 subunits of the GABAA receptor, but also to GABAA receptors containing α2, α3, and α5 subunits (Damgen and Luddens 1999; Davies et al. 2000; Smith et al. 2001). A recent study has demonstrated that there is a significant decrease in the expression of α3 and α5 subunits of GABAA receptors in aged rats compared with young adult animals (Yu et al. 2006). Therefore, we suggest that a decline in the expression of one or more of these subunits of GABAA receptors in aged animals may provide an explanation for the relatively “weaker” effects of eszopiclone in aged guinea pigs compared to adult guinea pigs.
While no reports have compared the effects of eszopiclone or zopiclone on sleep and waking states either in aged guinea pigs or in aged animals of other species, the present results are generally consistent with findings from separate studies in animals and in humans. In adult rats, zopiclone, administered intraperitoneally in doses from 2.5 to 10 mg/kg, was found to increase NREM sleep, decrease wakefulness, decrease the latency to NREM sleep, and increase the latency to REM sleep (Stutzmann et al. 1992); a decrease in the total time spent in REM sleep was also reported (Gutierrez et al. 1997). However, in another study, zopiclone also significantly increased NREM sleep but had no effect on REM sleep in the adult rat (Noguchi et al. 2004). Spectral analyses in adult rabbits revealed that zopiclone, at a dose of 2 mg/kg, significantly increased power in the delta frequency band and decreased power in the theta frequency band (Noguchi et al. 2002). In humans, zopiclone has been shown to have a positive effect on sleep (e.g., a reduction in sleep latency and an increase in total NREM sleep time) with few adverse responses in healthy elderly subjects (Hemmeter et al. 2000). Zopiclone also significantly shortened sleep latency and increased total sleep time in elderly patients with insomnia (Scharf et al. 2005). In young healthy subjects, Zopiclone has been reported to produce a minor increase in EEG delta power, a significant decrease in EEG theta power and an increase in EEG sigma power (Kim et al. 1993). However, other studies have reported that zopiclone decreases the power density in other frequency ranges <10 Hz and enhances the power density in the range of 12–15 Hz in adult healthy subjects (Trachsel et al. 1990; Wright et al. 1986).
There was a positive relationship between the increase in EEG power of the delta frequency band during NREM sleep and the increase in the duration of NREM episodes or the amount of NREM sleep following the administration of eszopiclone. The magnitude of EEG slow wave activity in the delta band is considered to be an index of sleep intensity (Borbely and Achermann 2000; Tobler and Borbely 1986). This is particularly interesting with respect to the use of eszopiclone as a pharmacological tool to improve the quality of sleep in old age, especially when one considers the fact that there is a reduction in delta wave activity in aged humans (Mann and Roschke 2004).
Hypnotic effects of zolpidem in the aged guinea pig
In the aged guinea pig, significant changes in sleep and waking states occurred when zolpidem was administered but only at a dose of 3 or 10 mg/kg. At these doses, it increased the percentage of time spent in NREM sleep and decreased the amount of wakefulness. There were no significant effects on the time spent in REM sleep with either dose of zolpidem.
It is interesting to note that the changes in the percentage of time spent in NREM sleep following the administration of zolpidem at 1 and 3 mg/kg in the aged guinea pig were similar to those that occurred when the same doses were administrated to adult guinea pigs (Xi and Chase 2008; Table 1). In addition, there were no significant changes in EEG power in any of the frequency bands analyzed during NREM sleep following zolpidem administration in the aged guinea pig, which mirrors our findings in the adult guinea pig (Xi and Chase 2008). Therefore, the present data indicate that there are no marked differences in the effects of zolpidem on sleep and waking states (increase in NREM and decrease in wakefulness) and in the power spectra of the EEG during NREM sleep in aged guinea pigs compared with adult guinea pigs.
Zolpidem is known to exert its hypnotic effects by acting primarily on α1 subunits of GABAA receptors (Sanna et al. 2002). A number of anatomical studies have shown that there is no prominent change in the expression of α1 subunits of the GABAA receptor in aged rats compared with adult animals (Gutierrez et al. 1997; Mhatre and Ticku 1992; Yu et al. 2006). Therefore, this may explain the lack of marked differences in the effects of zolpidem in aged and adult guinea pigs. However, additional research is needed to unravel bases of the differences in the effects produced by eszopiclone and zolpidem vis-à-vis changes in the subunits of GABAA receptors that are targeted by these hypnotics in aged animals.
Our results with zolpidem in the aged guinea pig are generally similar to data of previous studies in adult animals of other species. For example, studies in adult rats have shown that zolpidem, administered at doses of 3 or 10 mg/kg, increased NREM sleep and the mean duration of episodes of NREM sleep during the light period and dark period, respectively (Chen et al. 2005; Depoortere et al. 1991; Mailliet et al. 2001). Another study in the adult rat reported that the latency to NREM was reduced following the administration of zolpidem at 2.5, 5, and 7.5 mg/kg, and the latency to REM sleep was only increased at the two higher doses. In addition, the amount of REM sleep decreased at the highest dose (Gottesmann et al. 1998).
In normal elderly human subjects, zolpidem, in doses between 5 and 20 mg, shortens the latency to NREM sleep and increases the total time spent in NREM sleep (Fairweather et al. 1992; Scharf et al. 1991). In elderly patients with insomnia, zolpidem has been reported to shorten sleep latency and increase total sleep time (Ancoli-Israel et al. 1999; Walsh et al. 2008).
Differences between hypnotic effects of eszopiclone and zolpidem in the aged guinea pig
While the administration of both eszopiclone and zolpidem in aged guinea pigs produced hypnotic effects in the present study, our quantitative analyses reveal significant differences in the effects of these two hypnotics. Firstly, the increase in the percentage of time spent in NREM sleep and the decrease in the time spent in wakefulness following the administration of eszopiclone at 3 or 10 mg/kg were significantly greater than the time spent in NREM sleep and wakefulness after zolpidem administration (which was ineffective at a dose of 1 mg/kg). Secondly, eszopiclone at 1 and 3 mg/kg induced a significant decrease in the mean latency to NREM sleep that was significantly shorter than that which followed the administration of zolpidem at 1 mg/kg. Thirdly, eszopiclone at 3 or 10 mg/kg resulted in a significant increase in the mean duration of episodes of NREM sleep, whereas zolpidem did not produce any significant change in the mean duration of episodes of NREM sleep at any doses. Finally, there was a significant increase in EEG power in the delta band and a decrease in EEG power in the theta band during NREM sleep following the administration of eszopiclone at 3 or 10 mg/kg. In contrast, there were no significant changes in EEG power in any of the frequency bands analyzed during NREM sleep following zolpidem administration at any dose. Therefore, the present data suggest that eszopiclone has a greater effect on NREM sleep and the generation of NREM delta activity than does zolpidem in the aged guinea pig, which is similar to the results obtained in the adult guinea pig (Xi and Chase 2008).
In summary, the present results demonstrate that in the aged guinea pig, eszopiclone, compared with zolpidem, has a more rapid onset of hypnotic action and results in an increased amount in NREM sleep. In addition, eszopiclone produces an increase in EEG delta power, whereas zolpidem has no effect on delta activity. Compared with previous data obtained in the adult guinea pig, the effects of eszopiclone on sleep and waking states and in the power spectra of the EEG during NREM sleep in the aged guinea pig were “weaker” than those in the adult guinea pig, although the effects of zolpidem were similar in aged and adult guinea pigs (Xi and Chase 2008). We, therefore, suggest that in addition to the potential differences in pharmacokinetics and pharmacodynamics of these two drugs (Drover 2004), the relative binding properties of eszopiclone and zolpidem, and the reduction in the expression of specific subunits of GABAA receptors in aged animals may account for the changes in the relative degree of effectiveness of eszopiclone and zolpidem on the states of sleep and wakefulness as well as the power spectra of the EEG in the aged guinea pig.
Acknowledgements
This work was supported by Sepracor, Inc. There are no other financial conflicts of interest. We thank Mr. Oscar Ramos, Mr. Trent Wenzel, Ms. Ricki-Leigh Malaguti, Ms. Emi Koda, and Mr. Kevin Luke Tsai for their excellent technical assistance. Approval for the use of aged guinea pigs in this study was granted by the Animal Research Committee of Veterans Affairs Greater Los Angeles Healthcare System.
Open Access This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Footnotes
This work was supported by Sepracor, Inc., Marlborough, MA, USA.
References
- Abalo R, Jose Rivera A, Vera G, Isabel Martin M (2005) Ileal myenteric plexus in aged guinea-pigs: loss of structure and calretinin-immunoreactive neurones. Neurogastroenterol Motil 17:123–132 [DOI] [PubMed]
- Ancoli-Israel S, Kripke DF, Mason W, Kaplan OJ (1985) Sleep apnea and periodic movements in an aging sample. J Gerontol 40:419–425 [DOI] [PubMed]
- Ancoli-Israel S, Walsh JK, Mangano RM, Fujimori M (1999) Zaleplon, a novel nonbenzodiazepine hypnotic, effectively treats insomnia in elderly patients without causing rebound effects. Prim Care Companion J Clin Psychiat 1:114–120 [DOI] [PMC free article] [PubMed]
- Bliwise D (2005) Normal aging. In: Kryger M, Roth T, Dement WC (eds) Principles and practice of sleep medicine. Elsevier Saunders, Philadelphia, pp 24–38
- Borbely AA, Achermann P (2000) Sleep homeostasis and models of sleep regulation. Saunders, Philadelphia [DOI] [PubMed]
- Chen HY, Kuo TB, Shaw FZ, Lai CJ, Yang CC (2005) Sleep-related vagotonic effect of zolpidem in rats. Psychopharmacology (Berl) 181:270–279 [DOI] [PubMed]
- Damgen K, Luddens H (1999) Zaleplon displays selectivity to recombinant GABAA receptors different from zolpidem, zopiclone and benzodiazepines. Neurosci Res Commun 25:139–148 [DOI]
- Davies M, Newell JG, Derry JM, Martin IL, Dunn SM (2000) Characterization of the interaction of zopiclone with gamma-aminobutyric acid type A receptors. Mol Pharmacol 58:756–762 [DOI] [PubMed]
- Declerck AC, Ruwe F, O'Hanlon JF, Vermeeren A, Wauquier A (1992) Effects of zolpidem and flunitrazepam on nocturnal sleep of women subjectively complaining of insomnia. Psychopharmacology (Berl) 106:497–501 [DOI] [PubMed]
- Depoortere H, Granger P, Leonardon J, Terzano MG (1991) Evaluation of the cyclic alternating pattern in rats by automatic analysis of sleep amplitude variations: effect of zolpidem. In: Terzano MG, Halasz PL, Declerk AC (eds) Phasic events and dynamic organization of sleep. Raven Press, New York, pp 17–33
- Drover DR (2004) Comparative pharmacokinetics and pharmacodynamics of short-acting hypnosedatives: zaleplon, zolpidem and zopiclone. Clin Pharmacokinet 43:227–238 [DOI] [PubMed]
- Escudero M, Vidal PP (1996) A quantitative study of electroencephalography, eye movements and neck electromyography characterizing the sleep-wake cycle of the guinea-pig. Eur J Neurosci 8:572–580 [DOI] [PubMed]
- Fairweather DB, Kerr JS, Hindmarch I (1992) The effects of acute and repeated doses of zolpidem on subjective sleep, psychomotor performance and cognitive function in elderly volunteers. Eur J Clin Pharmacol 43:597–601 [DOI] [PubMed]
- Foley DJ, Monjan AA, Brown SL, Simonsick EM, Wallace RB, Blazer DG (1995) Sleep complaints among elderly persons: an epidemiologic study of three communities. Sleep 18:425–432 [DOI] [PubMed]
- Gabella G (2001) Development and ageing of intestinal musculature and nerves: the guinea-pig taenia coli. J Neurocytol 30:733–766 [DOI] [PubMed]
- Gauthier P, Arnaud C, Stutzmann JM, Gottesmann C (1997) Influence of zopiclone, a new generation hypnotic, on the intermediate stage and paradoxical sleep in the rat. Psychopharmacology (Berl) 130:139–143 [DOI] [PubMed]
- Gottesmann C, Gandolfo G, Arnaud C, Gauthier P (1998) The intermediate stage and paradoxical sleep in the rat: influence of three generations of hypnotics. Eur J Neurosci 10:409–414 [DOI] [PubMed]
- Gutierrez A, Khan ZU, Miralles CP, Mehta AK, Ruano D, Araujo F, Vitorica J, De Blas AL (1997) GABAA receptor subunit expression changes in the rat cerebellum and cerebral cortex during aging. Brain Res Mol Brain Res 45:59–70 [DOI] [PubMed]
- Hemmeter U, Muller M, Bischof R, Annen B, Holsboer-Trachsler E (2000) Effect of zopiclone and temazepam on sleep EEG parameters, psychomotor and memory functions in healthy elderly volunteers. Psychopharmacology (Berl) 147:384–396 [DOI] [PubMed]
- Hiller JM, Fan LQ, Simon EJ (1992) Age-related changes in kappa opioid receptors in the guinea-pig brain: a quantitative autoradiographic study. Neuroscience 50:663–673 [DOI] [PubMed]
- Ibuka N (1984) Ontogenesis of circadian sleep-wakefulness rhythms and developmental changes of sleep in the altricial rat and in the precocial guinea pig. Behav Brain Res 11:185–196 [DOI] [PubMed]
- Kim YD, Zhuang HY, Tsutsumi M, Okabe A, Kurachi M, Kamikawa Y (1993) Comparison of the effect of zopiclone and brotizolam on sleep EEG by quantitative evaluation in healthy young women. Sleep 16:655–661 [DOI] [PubMed]
- Lader M (1992) Rebound insomnia and newer hypnotics. Psychopharmacology (Berl) 108:248–255 [DOI] [PubMed]
- Lader M (1993) Pharmacological treatments. In: Albarede JL, Morley JE, Roth T, Vellas BJ (eds) Sleep disorders and insomnia in the elderly, vol 7. facts and research in gerontology. Serdi, Paris, pp 147–154
- Lancel M (1999) Role of GABAA receptors in the regulation of sleep: initial sleep responses to peripherally administered modulators and agonists. Sleep 22:33–42 [DOI] [PubMed]
- Mailliet F, Galloux P, Poisson D (2001) Comparative effects of melatonin, zolpidem and diazepam on sleep, body temperature, blood pressure and heart rate measured by radiotelemetry in Wistar rats. Psychopharmacology (Berl) 156:417–426 [DOI] [PubMed]
- Mann K, Roschke J (2004) Influence of age on the interrelation between EEG frequency bands during NREM and REM sleep. Int J Neurosci 114:559–571 [DOI] [PubMed]
- McDougall JJ, Schuelert N (2007) Age alters the ability of substance P to sensitize joint nociceptors in guinea pigs. J Mol Neurosci 31:289–296 [DOI] [PubMed]
- Mendelson W (1987) Human sleep: research and clinical care. Plenum Press, New York, pp 81–106 323-342
- Mhatre MC, Ticku MK (1992) Aging related alterations in GABAA receptor subunit mRNA levels in Fischer rats. Brain Res Mol Brain Res 14:71–78 [DOI] [PubMed]
- Morin AK (2006) Strategies for treating chronic insomnia. Am J Manag Care 12:S230–S245 [PubMed]
- Noble S, Langtry HD, Lamb HM (1998) Zopiclone. An update of its pharmacology, clinical efficacy and tolerability in the treatment of insomnia. Drugs 55:277–302 [DOI] [PubMed]
- Noguchi H, Kitazumi K, Mori M, Shiba T (2002) Binding and neuropharmacological profile of zaleplon, a novel nonbenzodiazepine sedative/hypnotic. Eur J Pharmacol 434:21–28 [DOI] [PubMed]
- Noguchi H, Kitazumi K, Mori M, Shiba T (2004) Electroencephalographic properties of zaleplon, a non-benzodiazepine sedative/hypnotic, in rats. J Pharmacol Sci 94:246–251 [DOI] [PubMed]
- Nozawa I, Imamura S, Hashimoto K, Shimomura S, Murakami Y (1997) Age-related changes in the compound action potentials of the eighth nerve in guinea pigs. Eur Arch Otorhinolaryngol 254:145–149 [DOI] [PubMed]
- Rissman RA, De Blas AL, Armstrong DM (2007) GABA(A) receptors in aging and Alzheimer's disease. J Neurochem 103:1285–1292 [DOI] [PubMed]
- Sanna E, Busonero F, Talani G, Carta M, Massa F, Peis M, Maciocco E, Biggio G (2002) Comparison of the effects of zaleplon, zolpidem, and triazolam at various GABA(A) receptor subtypes. Eur J Pharmacol 451:103–110 [DOI] [PubMed]
- Scharf MB, Mayleben DW, Kaffeman M, Krall R, Ochs R (1991) Dose response effects of zolpidem in normal geriatric subjects. J Clin Psychiatry 52:77–83 [PubMed]
- Scharf M, Erman M, Rosenberg R, Seiden D, McCall WV, Amato D, Wessel TC (2005) A 2-week efficacy and safety study of eszopiclone in elderly patients with primary insomnia. Sleep 28:720–727 [DOI] [PubMed]
- Smith AJ, Alder L, Silk J, Adkins C, Fletcher AE, Scales T, Kerby J, Marshall G, Wafford KA, McKernan RM, Atack JR (2001) Effect of alpha subunit on allosteric modulation of ion channel function in stably expressed human recombinant gamma-aminobutyric acid(A) receptors determined using (36) Cl ion flux. Mol Pharmacol 59:1108–1118 [DOI] [PubMed]
- Stutzmann JM, Piot O, Reibaud M, Doble A, Blanchard JC (1992) Pharmacological properties and mechanism of action of the cyclopyrrolones. Encephale 18:393–400 [PubMed]
- Tobler I, Borbely AA (1986) Sleep EEG in the rat as a function of prior waking. Electroencephalogr Clin Neurophysiol 64:74–76 [DOI] [PubMed]
- Tobler I, Franken P (1993) Sleep homeostasis in the guinea pig: similar response to sleep deprivation in the light and dark period. Neurosci Lett 164:105–108 [DOI] [PubMed]
- Trachsel L, Dijk DJ, Brunner DP, Klene C, Borbely AA (1990) Effect of zopiclone and midazolam on sleep and EEG spectra in a phase-advanced sleep schedule. Neuropsychopharmacology 3:11–18 [PubMed]
- Walsh JK, Soubrane C, Roth T (2008) Efficacy and safety of zolpidem extended release in elderly primary insomnia patients. Am J Geriatr Psychiatry 16:44–57 [DOI] [PubMed]
- Wright NA, Belyavin A, Borland RG, Nicholson AN (1986) Modulation of delta activity by hypnotics in middle-aged subjects: studies with a benzodiazepine (flurazepam) and a cyclopyrrolone (zopiclone). Sleep 9:348–352 [DOI] [PubMed]
- Xi M, Chase MH (2008) Effects of eszopiclone and zolpidem on sleep and waking states in the adult guinea pig. Sleep 31:1043–1051 [PMC free article] [PubMed]
- Yu ZY, Wang W, Fritschy JM, Witte OW, Redecker C (2006) Changes in neocortical and hippocampal GABAA receptor subunit distribution during brain maturation and aging. Brain Res 1099:73–81 [DOI] [PubMed]
- Zhang JH, Sampogna S, Morales FR, Chase MH (2005) Age-related changes of hypocretin in basal forebrain of guinea pig. Peptides 26:2590–2596 [DOI] [PubMed]