Abstract
Obesity is a worldwide epidemic that continues to grow at an alarming rate. This condition increases the morbidity and mortality associated with both acute and chronic diseases. Some of the deleterious consequences of obesity have been attributed to its induction of a low-grade chronic inflammatory state that arises from the production and secretion of inflammatory mediators from the expanded pool of activated adipocytes. This review focuses on the mechanisms that underlie the proposed link between obesity and inflammation, and it addresses how obesity-induced inflammation may account for increased morbidity and mortality that is associated with a diverse group of diseases.
Keywords: Adipokines, microcirculation, oxidative stress, immune cells, adhesion molecules, leukocytes, platelets, insulin resistance, endothelial cells
Introduction
Obesity is a serious health problem that increases the morbidity and mortality of a variety of acute and chronic diseases, most notably type 2 diabetes and cardiovascular diseases. In 2005, the World Health Organization estimated that over 400 million adults worldwide are obese (as defined by a body mass index > 30) and the incidence continues to increase at an alarming rate. (1)
The pathophysiological processes that underlie the influence of obesity on the progression and severity of cardiovascular diseases (CVD) remain poorly defined. Metabolic changes related to increased insulin resistance, including oxidative stress, have been implicated in obesity-related pathophysiology (2). However, most attention in recent years has been devoted to the concept that obesity elicits a chronic low-grade systemic inflammatory response that results from a combination of increased insulin resistance and an increased production of inflammatory mediators by the expanding pool of adipocytes. Since other CVD risk factors, such as hypertension and hypercholesterolemia are also known to induce an inflammatory phenotype, albeit via different mechanisms, the combination of obesity with these other risk factors is likely to yield a heightened state of systemic inflammation that enhances the progression and severity of CVD. Given the potential biomedical significance of obesity to human health in general, and CVD in particular, a review of the proposed causes and consequences of the inflammatory state that accompanies obesity is warranted.
An Endocrine Role for Adipocytes in Obesity-Associated Inflammation
The energy storage function of adipose tissue has long been appreciated. However, there is growing recognition that fat is not a passive organ that merely accumulates triglycerides in hypertrophied adipocytes during periods of caloric excess and releases these lipids for use by the organism during periods of caloric restriction. Even the notion that expansion of body fat results exclusively from an increase in the size of adipocytes is now challenged, with newer evidence supporting the view that the increased fat mass in obese individuals also results from hyperplasia or increased number of adipocytes. While the factors regulating adipocyte hypertrophy vs. hyperplasia remain unclear, there is growing evidence suggesting that the processes that allow for fat accumulation also impose significant stress on adipocytes, leading to their activation and consequent production and release of a variety of peptides. These peptides are known to modulate a number of different physiological processes, including appetite, energy expenditure, immunity, and inflammation.
The stress imposed on adipocytes by lipid droplet creation and expansion is manifested prominently in the endoplasmic reticulum (ER) and mitochondria. The ER dysfunction that accompanies adipocyte hypertrophy can result in impaired post-translational modification of proteins, which is manifested as improper folding of newly synthesized proteins, the formation of lipid droplets and impaired cholesterol sensing (3). The accumulation of unfolded proteins in the cytosol of adipocytes can lead to increased release of free fatty acids and inflammatory mediators, which in turn can activate c-Jun-N-terminal kinases (JNK) in muscle, liver, and adipose cells, thereby promoting insulin resistance in these tissues (3).
Mitochondrial dysfunction also appears to add to the stress experienced by hypertrophied adipocytes. Mitochondrial uncoupling caused by the processing of excess fatty acids in adipocytes can result in an enhanced production of reactive oxygen species (ROS) (4). Enhanced ROS production in adipose tissue is evidenced by the accumulation of malondialdehyde and conjugated dienes, which are commonly used as surrogate markers of oxidative stress(5). The pro-oxidative environment resulting from mitochondrial uncoupling is amplified by the enhanced ROS production that is linked to ER stress (6). In response to the stresses imposed on the ER and mitochondria, adipocytes assume both a pro-inflammatory and insulin-resistant phenotype.
Activated adipocytes secrete a variety of biological peptides with cytokine-like properties called adipokines. The adipokines can mediate autocrine, paracrine and endocrine effects, thereby exerting their actions both locally and systemically. Leptin, which is best known for its powerful inhibitory influence on food intake, is produced proportionally to body fat mass. It is known to increase insulin sensitivity (7) and stimulate lipolysis in adipose tissue. Leptin (ob/ob) or leptin-receptor (db/db) deficient mice have been employed as experimental models of obesity. While congenital leptin deficiency associated with obesity has been reported (8), the detection of high leptin levels in obese subjects suggests that leptin resistance (rather than leptin deficiency) is more common in human obesity (9).
Leptin receptors are expressed on circulating blood cells (leukocytes and platelets) and vascular endothelial cells. The engagement of leptin with its receptors on these cells appears to result in cell activation. On endothelial cells, the response to leptin includes increased ROS production, which usually results in an increased expression of endothelial cell adhesion molecules and an increased capacity to recruit immune cells. Leptin may also induce this inflammatory phenotype in endothelial cells by stimulating the release of TNF-alpha from circulating monocytes. The proposed pro-inflammatory actions of leptin is further supported by evidence that leptin deficient ob/ob mice are resistant to chronic inflammatory diseases such as inflammatory bowel disease (10) and atherosclerosis (11). In view of the established link between inflammation and thrombosis (12), it is not surprising that leptin also promotes a prothrombotic phenotype in the vasculature. Leptin has been shown to elicit platelet aggregation, enhance platelet adhesion to extracellular matrix proteins, and induce thrombosis in mice. Leptin- and leptin receptor-deficient mice are protected from thrombosis, as are mice treated with a leptin neutralizing antibody, while exogenously administered leptin enhances thrombosis (13).
Adiponectin, which normally exhibits the highest plasma concentration of all adipokines, is known to enhance insulin sensitivity and to act as an anti-inflammatory agent. Since obesity is generally associated with decreased plasma levels of adiponectin, this adipokine has been implicated in the insulin resistance and pro-inflammatory state associated with obesity. The anti-inflammatory effects of adiponectin have been attributed to its ability to enhance nitric oxide (NO) production by endothelial cells and to inhibit cytokine-induced upregulation of endothelial cell adhesion molecules. Adiponectin-deficient mice exhibit an increased expression of endothelial cell adhesion molecules such as E-selectin and VCAM-1, impaired vascular NO production, and increased leukocyte-endothelial cell adhesion, all of which are reversed by administration of recombinant human adiponectin (14). Furthermore, the protective effects of exogenous adiponectin on the leukocyte adhesion responses to adiponectin deficiency is lost when the mice were treated with the endothelial NO synthase inhibitor, L-NAME, supporting a critical role for NO in the anti-inflammatory responses of this adipokine (14). The pro-inflammatory effects of TNF-alpha administration to mice are also blunted by treatment with human recombinant adiponectin (14).
Resistin, an adipokine that induces insulin resistance, is produced by macrophages as well as adipocytes in humans (15). This adipokine exerts a variety of effects on endothelial and immune cells that are consistent with an inflammatory mediator. Resistin is associated with an increased production of pro-inflammatory cytokines and a decreased production of anti-inflammatory cytokines, with the former mediated through NFkB activation. The adipokine acts on endothelial cells to induce oxidative stress, increase the expression of the chemokine MCP-1 and adhesion molecules such as VCAM-1 and ICAM-1, and stimulate the release of endothelin-1. Macrophages also respond to resistin stimulation by producing pro-inflammatory cytokines such as TNF-a, IL-12, and IL-6 (16). In the clinical setting, resistin levels appear to correlate with other inflammatory markers, such as C-reactive protein (CRP), in different pathological conditions (17).
A variety of well-characterized pro-inflammatory cytokines, including TNF-a, IL-6, and CRP, are also produced by adipocytes. The circulating plasma levels of these cytokines are elevated in obese subjects and their levels appear to correlate with the amount of body fat, with weight loss resulting in reduced levels. CRP is largely synthesized by the liver, which enhances its production of the acute phase protein in response to TNF-a and IL-6. Hence, CRP appears to amplify the proinflammatory effects of other adipokines. TNF-a, IL-6, and CRP are known to induce an inflammatory phenotype in vascular endothelial cells that is characterized by increased adhesion molecule expression and ROS production and diminished barrier function.
Macrophages Amplify the Inflammatory Effects of Adipokines
A consequence of the production and local release of cytokines and cytokine-related substances by adipocytes is the recruitment of large numbers of immune cells, including monocytes and T-lymphocytes, into adipose tissue. Support for such a link between immune cell recruitment and adipokine production is provided by the observation that pro-inflammatory cytokine levels and macrophage density in visceral fat depots are much higher than in subcutaneous adipose tissue (18). While the mechanisms underlying the recruitment and activation of macrophages in adipose tissue remain poorly understood, there is emerging evidence that chemokines liberated by adipose tissue (probably adipocytes) are largely responsible for the recruitment, retention and activation of macrophage precursors (monocytes) in fat. Monocyte chemoattractant protein-1 (MCP-1) has been implicated as a major mediator of the monocyte recruitment that occurs in adipose tissue, while macrophage colony stimulating factor (M-CSF) is believed to mediate the conversion of monocytes to macrophages in adipose tissue. A role for MCP-1 and M-CSF is supported by reports of a positive correlation between adiposity and MCP expression in the adipose tissue, the significantly higher expression of MCP in visceral adipose tissue compared to the subcutaneous adipose tissue (19), and the correlation between M-CSF and macrophage infiltration in omental adipose tissue (20). Other candidate adipocyte-derived molecules that have also been implicated in macrophage recruitment/activation in adipose tissue include free fatty acids and lipoprotein lipase(21).
The primary function of macrophages that reside in adipose tissue remains unclear. It has been proposed that macrophages clear dead (apoptotic and necrotic) cells (2). Hence, adipocytes undergoing necrosis secondary to hypertrophy may lead to macrophage activation (with the accompanying release of inflammatory mediators) and their subsequent clearance from adipose tissue (22). Another potentially important role of adipose tissue macrophages is modulation of adipocyte function. Cross-talk between adipocytes and macrophages is evidenced by the ability of each cell type to enhance the production of protein mediators by the other (23). For example, adipocyte conditioned media can elicit large increases in the production/release of TNFa, IL-6 and NO by macrophages (21), while TNF-a released from macrophages inhibits the production of adiponectin by adipocytes (24). Likely consequences of this cross-talk between macrophages and adipocytes include amplification and perpetuation of the inflammatory phenotype that is induced by the expanding mass of body fat.
Site-specific interactions between adipose tissue and inflamed tissue
While the link between obesity and cardiovascular disease has focused much attention to adipocytes as a remote source of inflammatory mediators, there is also mounting evidence suggesting the possibility of a link between inflammation and adipose tissue that is anatomically associated with certain tissues, such as the colon, lymph nodes, myocardium, and large arteries. Changes in the quantity and quality of adipose tissue within the mesentery and surrounding mesenteric lymph nodes near the inflamed bowel have been described in human inflammatory bowel disease (IBD) (25) and in animal models of experimental IBD (26). This has led to speculation that these activated adipocytes generate mediators that contribute to the inflammatory response that occurs within the adjacent gut wall. A similar relationship has been proposed between epicardial adipose tissue and myocardial tissue in high-risk cardiac patients undergoing coronary artery bypass grafting (27, 28). Increased macrophage accumulation in epicardial adipose tissue may account for the enhanced inflammatory potential described in these reports and this raises the possibility that locally generated adipokines may be a predisposing factor for coronary artery disease (CAD). A detrimental role for epicardial fat in further supported by reports describing the ability of TNF-alpha, leptin and other adipokines to reduce myocardial contractility (28).
While the notion that site-specific accumulation and activation of adipocytes may contribute to chronic inflammatory diseases, such as IBD and CAD, is interesting and potentially important, the available evidence is largely circumstantial and does not yet warrant the development of therapeutic strategies that target such tissue-specific fat depots. A more convincing case however can be made for the involvement of perivascular adipose tissue (PAT) in the regulation of vasomotor function. It is well known that virtually all arteries are surrounded by PAT, which increases during obesity (29). The short distance (<100 microns) between PAT and the adventitial layer of the blood vessel allows adipocyte derived products, including adipokines and ROS, to alter vascular function. While adiponectin is known to relax vascular smooth muscle and produce vasodilation, leptin, resistin, TNF-alpha and other adipokines have been shown to impair endothelium-dependent vasodilation in small and large arteries, probably through the activation of NADPH oxidase and enhanced production of superoxide, which inactivates the endothelium-dependent vasodilator, nitric oxide (29).
A compelling case was recently made for the involvement of PAT in the vasomotor dysfunction associated angiotensin II (AngII)-induced hypertension in mice (30). Chronic AngII infusion leads to hypertension, impaired endothelium-dependent vasodilation and enhanced superoxide formation in large arteries, as well as the infiltration of TNF-alpha-producing T-lymphocytes (mostly CD4+ T-cells) into the PAT surrounding these vessels. The responses were not observed in AngII-treated mice either lacking T-cells or treated with a TNF-alpha antagonist. These observations led the authors to propose that AngII promotes T-cell accumulation and enhanced TNF-alpha release in PAT, which stimulates NADPH oxidase activity and superoxide production in the adjacent vascular smooth muscle layer, leading to impaired vasomotor function and hypertension. It remains unclear whether adipocyte-derived factors underlie the recruitment of T-cells in PAT in this clinically relevant model of hypertension and whether the contribution of PAT to vasomotor dysfunction and hypertension is unique to angiotensin II.
Adipose tissue microcirculation: A microcosm of the systemic inflammatory responses to obesity?
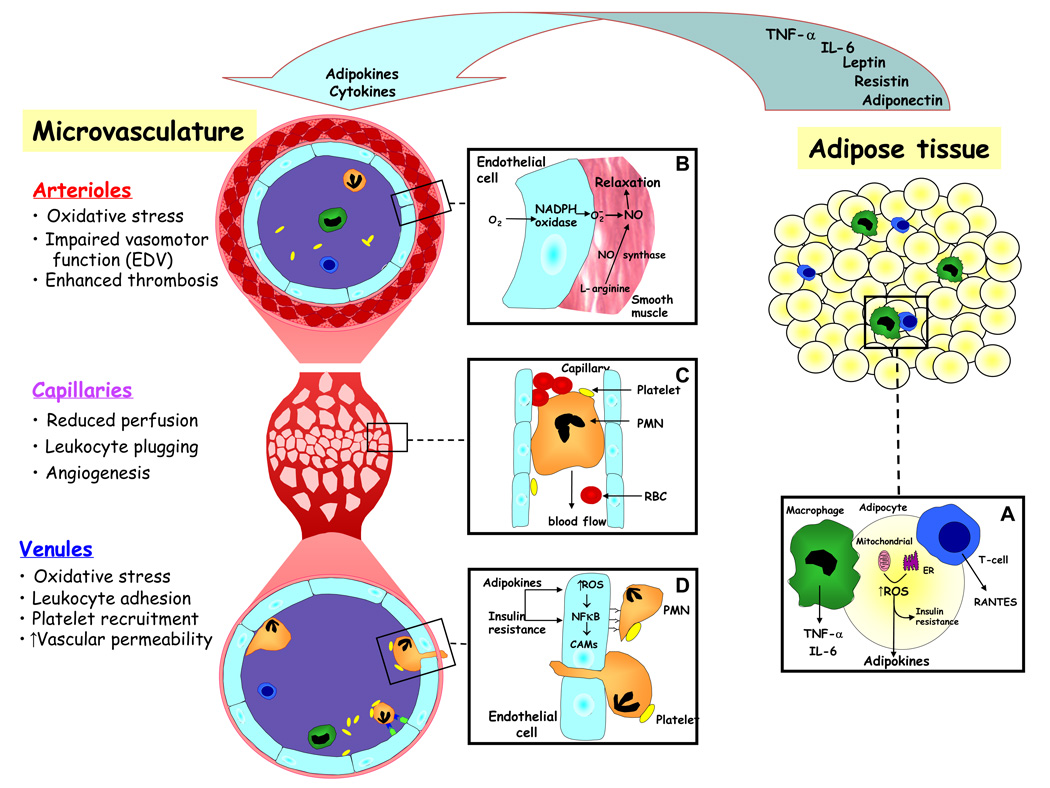
It is generally believed that mediators released from the expanded and activated pool of adipocytes in obese subjects accumulate in plasma to achieve levels that are sufficient to elicit the chronic low-grade systemic inflammatory response that is characteristic of this condition. It is more likely however that the systemic plasma levels achieved by these mediators do not cause overt inflammation in tissues distant from their source (adipose tissue) but instead increase the sensitivity (prime) of cells in these tissues (e.g., endothelial cells) and in circulating blood (e.g., leukocytes, platelets) to subsequent inflammatory stimuli. Within adipose tissue, adipokines would be expected to achieve much higher local concentrations in extracellular fluid, which could result in overt inflammation. Such a scenario is supported by studies that directly examine the microcirculation of visceral adipose tissue in genetically obese (ob/ob) mice and in obese wild type mice placed on a high fat diet (31). These studies reveal that all three segments (arterioles, capillaries, venules) of the microvasculature in visceral adipose tissue respond to obesity in a manner that is consistent with active inflammation (Figure 1), while no such changes are detected in microvessels of subcutaneous fat or skeletal muscle.
Figure 1. Mechanisms linking adipose tissue to inflammation in obesity.
Adipocytes, macrophages and T-lymphocytes residing within obese adipose tissue are activated via mitochondrial and endoplasmic reticulum (ER) dependent mechanisms (Inset A) to produce and secrete a variety of cytokines, chemokines, and adipokines. These substances exert a paracrine action on the local microcirculation and an endocrine action on the vasculature in distant tissues to induce phenotypic changes that are consistent with an inflammatory response. In arterioles (Inset B), adipose tissue-derived mediators induce the production of superoxide, which inactivates the vascular smooth muscle relaxant nitric oxide (NO), leading to vasoconstriction and reduced perfusion. The mediators further impair tissue perfusion by activating leukocytes, which become less deformable and consequently obstruct flow through capillaries (Inset C). The resulting hypoxic state promotes angiogenesis. Venules respond to the adipose tissue-derived mediators by increasing (via ROS mediated, NFkB-dependent transcription) the expression of endothelial cell adhesion molecules (CAMs) that promote the recruitment of leukocytes and platelets (Inset D), which subsequently lead to impaired endothelial barrier function, increased vascular permeability, and interstitial edema.
Arterioles, the primary site of resistance to blood flow, exhibit a large reduction in blood flow velocity in obese adipose tissue (31). The reduction in perfusion is sufficient to induce a hypoxic state. The mechanism(s) underlying the flow reduction remain unclear, but it may reflect the ability of leptin, resistin and other adipokines to blunt endothelium-dependent vasodilation (29) or it could result from the plugging of capillaries by adherent leukocytes and platelet aggregates (31). The latter possibility is supported by the observation that capillary perfusion in obese adipose tissue is improved following immunoneutralization of ICAM-1 (31). Capillary proliferation (angiogenesis) is also evident in obese adipose tissue, which may arise from the tissue hypoxia and increased expression of HIF-1 alpha (a pro-angiogenic factor) detected in this inflamed tissue (32).
Venules in obese adipose tissue also exhibit an inflammatory phenotype. Increased expression of the endothelial cell adhesion molecules ICAM-1 and E- and P-selectin is accompanied by an increased recruitment of rolling and firmly adherent leukocytes, and the formation of platelet-leukocyte aggregates (31). The increased expression of these adhesion molecules likely results from elevated local levels of TNF-a, leptin and resistin, reduced levels of adiponectin, and the oxidative stress elicited in obese adipose tissue (33). The increased P-selectin expression detected on platelets in the venous effluent of obese adipose tissue (31) likely accounts for the platelet-leukocyte aggregation. These cell-cell interactions are accompanied by an increased vascular permeability. Since ICAM-1 immunoneutralization blocks the leukocyte-endothelial cell interactions as well as the vascular permeability response, leukocyte adhesion-dependent mechanisms are likely responsible for the altered endothelial barrier function observed in obese adipose tissue (31).
Obesity, inflammation and organ dysfunction
While tissues distant from obese adipose tissue do not exhibit an overt inflammatory response, they are exposed to elevated levels of adipokines that are derived from the expanded and activated pool of adipocytes. The cytokine-like actions of these substances, coupled to their ability to reduce insulin resistance, enhance the responsiveness of distant organs to additional stresses and inflammatory stimuli. This notion is supported by clinical and animal studies of obesity, which reveal exaggerated inflammatory responses that can lead to enhanced tissue injury and organ dysfunction in different organs, including the brain, heart, and intestine. Some examples of clinically relevant conditions that exhibit exaggerated inflammatory and tissue injury responses in the setting of obesity include myocardial ischemia, ischemic stroke, sepsis, and non-alcoholic steatohepatitis (NASH).
Cardiovascular diseases
Large-scale clinical studies indicate that obesity is an independent risk factor for cardiovascular disease (CVD), i.e., significant and progressive increases in mortality are noted as BMI exceeds 30 Kg/m2 (34). The distribution of body fat is an important determinant of the risk posed by obesity, with abdominal fat (which produces larger amounts of adipokines) yielding a higher risk for CVD than subcutaneous fat. There is also evidence indicating that, in addition to increasing the likelihood that a tissue will experience an ischemic episode, obesity may also exacerbate the inflammatory and injury responses to ischemia. For example, a study of diet-induced obesity has revealed that in response to brief repetitive periods of ischemia followed by reperfusion, the heart exhibits an exaggerated inflammatory response and larger infarcts (35). Similarly, it has been reported that hearts of genetically obese (ob/ob) mice do not exhibit the protective ischemic preconditioning response that is evident in their lean counterparts, which renders the hearts of obese mice more vulnerable to larger infarcts following exposure to longer ischemic episodes (36).
Obesity is also considered as a risk factor for ischemic stroke (37) and transient ischemic attacks (TIA) (38). A worse long-term prognosis and higher mortality accompanies the increased risk for stroke in obese patients. Animal studies have revealed larger increases in leukocyte and platelet adhesion in cerebral venules, blood brain barrier permeability, plasma monocyte chemoattractant protein-1 (MCP-1) concentration, and infarct size in obese (ob/ob) mice subjected to focal cerebral ischemia and reperfusion (I/R), when compared to their lean counterparts (39). While leptin administration to the leptin deficient ob/ob mice did not blunt the exaggerated inflammatory and injury responses to I/R, immunoneutralization of MCP-1 significantly reduced infarct volume, implicating a role for this chemokine in the obesity-enhanced injury response to cerebral I/R (39).
Sepsis
Sepsis is the leading cause of mortality in non-coronary intensive care units worldwide (40) While some clinical studies report a positive correlation between mortality in the medical intensive care unit and BMI (41), a recent meta analysis of critically ill patients in both medical and surgical intensive care units shows no such correlation between obesity and increased mortality (42). Notwithstanding the controversy that surrounds the link between obesity and morbidity/mortality in septic patients, animal studies are generally consistent with exaggerated inflammatory and tissue injury responses to sepsis in obese mice, compared to their lean counterparts. Enhanced leukocyte and platelet adhesion and increased endothelial adhesion molecule expression are noted in the cerebral microcirculation of septic lean mice, with much greater responses noted in obese (ob/ob) mice (43). These exaggerated responses are accompanied by larger increases in blood brain barrier (BBB) permeability and more severe behavioral deficits. Leptin deficient ob/ob mice and leptin replete melanocortin-4 receptor knockout mice respond similarly to the sepsis induced by cecal ligation and puncture (CLP)(43). P-selectin immunoneutralization affords protection against the obesity-enhanced inflammatory and BBB permeability induced by CLP, suggesting that the exaggerated tissue injury response is linked to inflammation. A more prominent inflammatory response to sepsis has also been described in the intestinal microcirculation of septic ob/ob mice (43).
Fatty liver disease
Obesity is associated with increased risk for non-alcoholic fatty liver disease (NAFLD) and non-alcoholic steatohepatitis (NASH). The prevalence of NAFLD is directly correlated with BMI (44). Oxidative stress and activation of NF kappa B are critical for the initiation of inflammation and tissue injury associated with NASH. The recruitment of inflammatory cells and platelets results in a reduction in the number of perfused liver sinusoids and tissue perfusion, which may amplify the hepatocellular injury mediated by inflammatory cells. Steatosis is also associated with exaggerated inflammatory and injury responses to ischemia and reperfusion, which limits the use of fatty donor livers for transplantation (45).
Conclusions
There is compelling evidence from both clinical and animal studies that supports the view that obesity induces a chronic low-grade inflammatory state. Activation products (adipokines) from an expanded pool of adipocytes likely represent the link between obesity and inflammation. A major target tissue for the proinflammatory actions of locally and systemically released adipokines is the microvasculature, which exhibits impaired function in arterioles, capillaries and venules alike. The obesity-induced microvascular dysfunction contributes to both the initiation and propagation of the inflammatory response. A consequence of the systemic inflammatory state associated with obesity is the priming of tissues for more robust inflammatory and tissue injury responses when challenged with an additional inflammatory stimulus, which may account for the role of obesity as a risk factor for a variety of different diseases.
Acknowledgments
Supported by a grant from the National Heart Lung and Blood Institute (HL26441)
References
- 1.Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser. 2000;894:i–xii. 1–253. [PubMed]
- 2.Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 3.de Ferranti S, Mozaffarian D. The perfect storm: obesity, adipocyte dysfunction, and metabolic consequences. Clin Chem. 2008;54:945–955. doi: 10.1373/clinchem.2007.100156. [DOI] [PubMed] [Google Scholar]
- 4.Wojtczak L, Schonfeld P. Effect of fatty acids on energy coupling processes in mitochondria. Biochim Biophys Acta. 1993;1183:41–57. doi: 10.1016/0005-2728(93)90004-y. [DOI] [PubMed] [Google Scholar]
- 5.Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, Nakayama O, Makishima M, Matsuda M, Shimomura I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest. 2004;114:1752–1761. doi: 10.1172/JCI21625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gregor MG, Hotamisligil GS. Adipocyte stress: The endoplasmic reticulum and metabolic disease. J Lipid Res. 2007 doi: 10.1194/jlr.R700007-JLR200. [DOI] [PubMed] [Google Scholar]
- 7.Ronti T, Lupattelli G, Mannarino E. The endocrine function of adipose tissue: an update. Clin Endocrinol (Oxf) 2006;64:355–365. doi: 10.1111/j.1365-2265.2006.02474.x. [DOI] [PubMed] [Google Scholar]
- 8.Montague CT, Farooqi IS, Whitehead JP, Soos MA, Rau H, Wareham NJ, Sewter CP, Digby JE, Mohammed SN, Hurst JA, et al. Congenital leptin deficiency is associated with severe early-onset obesity in humans. Nature. 1997;387:903–908. doi: 10.1038/43185. [DOI] [PubMed] [Google Scholar]
- 9.Considine RV, Sinha MK, Heiman ML, Kriauciunas A, Stephens TW, Nyce MR, Ohannesian JP, Marco CC, McKee LJ, Bauer TL, et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med. 1996;334:292–295. doi: 10.1056/NEJM199602013340503. [DOI] [PubMed] [Google Scholar]
- 10.Siegmund B, Lehr HA, Fantuzzi G. Leptin: a pivotal mediator of intestinal inflammation in mice. Gastroenterology. 2002;122:2011–2025. doi: 10.1053/gast.2002.33631. [DOI] [PubMed] [Google Scholar]
- 11.Yen TT, Allan JA, Pearson DV, Schinitsky MR. Dissociation of obesity, hypercholesterolemia and diabetes from atherosclerosis in ob/ob mice. Experientia. 1977;33:995–996. doi: 10.1007/BF01945927. [DOI] [PubMed] [Google Scholar]
- 12.Esmon CT. Crosstalk between inflammation and thrombosis. Maturitas. 2004;47:305–314. doi: 10.1016/j.maturitas.2003.10.015. [DOI] [PubMed] [Google Scholar]
- 13.Bodary PF. Links between adipose tissue and thrombosis in the mouse. Arterioscler Thromb Vasc Biol. 2007;27:2284–2291. doi: 10.1161/ATVBAHA.107.148221. [DOI] [PubMed] [Google Scholar]
- 14.Ouedraogo R, Gong Y, Berzins B, Wu X, Mahadev K, Hough K, Chan L, Goldstein BJ, Scalia R. Adiponectin deficiency increases leukocyte-endothelium interactions via upregulation of endothelial cell adhesion molecules in vivo. J Clin Invest. 2007;117:1718–1726. doi: 10.1172/JCI29623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang YM, Xu ZR, Wu LJ, Huang WD. Study of Resistin gene expression in peripheral blood mononuclear cell and its gene polymorphism in a small range population. J Zhejiang Univ Sci B. 2007;8:132–135. doi: 10.1631/jzus.2007.B0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fu Y, Luo L, Luo N, Garvey WT. Proinflammatory cytokine production and insulin sensitivity regulated by overexpression of resistin in 3T3-L1 adipocytes. Nutr Metab (Lond) 2006;3:28. doi: 10.1186/1743-7075-3-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pang SS, Le YY. Role of resistin in inflammation and inflammation-related diseases. Cell Mol Immunol. 2006;3:29–34. [PubMed] [Google Scholar]
- 18.Moller DE, Kaufman KD. Metabolic syndrome: a clinical and molecular perspective. Annu Rev Med. 2005;56:45–62. doi: 10.1146/annurev.med.56.082103.104751. [DOI] [PubMed] [Google Scholar]
- 19.Ferrante AW., Jr Obesity-induced inflammation: a metabolic dialogue in the language of inflammation. J Intern Med. 2007;262:408–414. doi: 10.1111/j.1365-2796.2007.01852.x. [DOI] [PubMed] [Google Scholar]
- 20.Harman-Boehm I, Bluher M, Redel H, Sion-Vardy N, Ovadia S, Avinoach E, Shai I, Kloting N, Stumvoll M, Bashan N, et al. Macrophage infiltration into omental versus subcutaneous fat across different populations: effect of regional adiposity and the comorbidities of obesity. J Clin Endocrinol Metab. 2007;92:2240–2247. doi: 10.1210/jc.2006-1811. [DOI] [PubMed] [Google Scholar]
- 21.Li L, Renier G. Adipocyte-derived lipoprotein lipase induces macrophage activation and monocyte adhesion: role of fatty acids. Obesity (Silver Spring) 2007;15:2595–2604. doi: 10.1038/oby.2007.311. [DOI] [PubMed] [Google Scholar]
- 22.Cinti S, Mitchell G, Barbatelli G, Murano I, Ceresi E, Faloia E, Wang S, Fortier M, Greenberg AS, Obin MS. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J Lipid Res. 2005;46:2347–2355. doi: 10.1194/jlr.M500294-JLR200. [DOI] [PubMed] [Google Scholar]
- 23.Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fantuzzi G. Adipose tissue, adipokines, and inflammation. J Allergy Clin Immunol. 2005;115:911–919. doi: 10.1016/j.jaci.2005.02.023. quiz 920. [DOI] [PubMed] [Google Scholar]
- 25.Pond CM. Long-term changes in adipose tissue in human disease. Proc Nutr Soc. 2001;60:365–374. doi: 10.1079/pns200198. [DOI] [PubMed] [Google Scholar]
- 26.Karagiannides I, Pothoulakis C. Obesity, innate immunity and gut inflammation. Curr Opin Gastroenterol. 2007;23:661–666. doi: 10.1097/MOG.0b013e3282c8c8d3. [DOI] [PubMed] [Google Scholar]
- 27.Mazurek T, Zhang L, Zalewski A, Mannion JD, Diehl JT, Arafat H, Sarov-Blat L, O'Brien S, Keiper EA, Johnson AG, et al. Human epicardial adipose tissue is a source of inflammatory mediators. Circulation. 2003;108:2460–2466. doi: 10.1161/01.CIR.0000099542.57313.C5. [DOI] [PubMed] [Google Scholar]
- 28.Baker AR, Silva NF, Quinn DW, Harte AL, Pagano D, Bonser RS, Kumar S, McTernan PG. Human epicardial adipose tissue expresses a pathogenic profile of adipocytokines in patients with cardiovascular disease. Cardiovasc Diabetol. 2006;5:1. doi: 10.1186/1475-2840-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guzik TJ, Marvar PJ, Czesnikiewicz-Guzik M, Korbut R. Perivascular adipose tissue as a messenger of the brain-vessel axis: role in vascular inflammation and dysfunction. J Physiol Pharmacol. 2007;58:591–610. [PubMed] [Google Scholar]
- 30.Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, Goronzy J, Weyand C, Harrison DG. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J Exp Med. 2007;204:2449–2460. doi: 10.1084/jem.20070657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nishimura S, Manabe I, Nagasaki M, Seo K, Yamashita H, Hosoya Y, Ohsugi M, Tobe K, Kadowaki T, Nagai R, et al. In vivo imaging in mice reveals local cell dynamics and inflammation in obese adipose tissue. J Clin Invest. 2008;118:710–721. doi: 10.1172/JCI33328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nishimura S, Manabe I, Nagasaki M, Hosoya Y, Yamashita H, Fujita H, Ohsugi M, Tobe K, Kadowaki T, Nagai R, et al. Adipogenesis in obesity requires close interplay between differentiating adipocytes, stromal cells, and blood vessels. Diabetes. 2007;56:1517–1526. doi: 10.2337/db06-1749. [DOI] [PubMed] [Google Scholar]
- 33.Singer G, Granger DN. Inflammatory responses underlying the microvascular dysfunction associated with obesity and insulin resistance. Microcirculation. 2007;14:375–387. doi: 10.1080/10739680701283158. [DOI] [PubMed] [Google Scholar]
- 34.Flegal KM, Graubard BI, Williamson DF, Gail MH. Cause-specific excess deaths associated with underweight, overweight, and obesity. JAMA. 2007;298:2028–2037. doi: 10.1001/jama.298.17.2028. [DOI] [PubMed] [Google Scholar]
- 35.Thakker GD, Frangogiannis NG, Zymek PT, Sharma S, Raya JL, Barger PM, Taegtmeyer H, Entman ML, Ballantyne CM. Increased Myocardial Susceptibility to Repetitive Ischemia With High-fat diet-induced Obesity. Obesity (Silver Spring) 2008 doi: 10.1038/oby.2008.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bouhidel O, Pons S, Souktani R, Zini R, Berdeaux A, Ghaleh B. Myocardial ischemic postconditioning against ischemia-reperfusion is impaired in ob/ob mice. Am J Physiol Heart Circ Physiol. 2008;295:H1580–H1586. doi: 10.1152/ajpheart.00379.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hu G, Tuomilehto J, Silventoinen K, Sarti C, Mannisto S, Jousilahti P. Body mass index, waist circumference, and waist-hip ratio on the risk of total and type-specific stroke. Arch Intern Med. 2007;167:1420–1427. doi: 10.1001/archinte.167.13.1420. [DOI] [PubMed] [Google Scholar]
- 38.Winter Y, Rohrmann S, Linseisen J, Lanczik O, Ringleb PA, Hebebrand J, Back T. Contribution of Obesity and Abdominal Fat Mass to Risk of Stroke and Transient Ischemic Attacks. Stroke. 2008 doi: 10.1161/STROKEAHA.108.523001. [DOI] [PubMed] [Google Scholar]
- 39.Terao S, Yilmaz G, Stokes KY, Ishikawa M, Kawase T, Granger DN. Inflammatory and injury responses to ischemic stroke in obese mice. Stroke. 2008;39:943–950. doi: 10.1161/STROKEAHA.107.494542. [DOI] [PubMed] [Google Scholar]
- 40.Sands KE, Bates DW, Lanken PN, Graman PS, Hibberd PL, Kahn KL, Parsonnet J, Panzer R, Orav EJ, Snydman DR, et al. Epidemiology of sepsis syndrome in 8 academic medical centers. JAMA. 1997;278:234–240. [PubMed] [Google Scholar]
- 41.El-Solh A, Sikka P, Bozkanat E, Jaafar W, Davies J. Morbid obesity in the medical ICU. Chest. 2001;120:1989–1997. doi: 10.1378/chest.120.6.1989. [DOI] [PubMed] [Google Scholar]
- 42.Akinnusi ME, Pineda LA, El Solh AA. Effect of obesity on intensive care morbidity and mortality: a meta-analysis. Crit Care Med. 2008;36:151–158. doi: 10.1097/01.CCM.0000297885.60037.6E. [DOI] [PubMed] [Google Scholar]
- 43.Vachharajani V, Russell JM, Scott KL, Conrad S, Stokes KY, Tallam L, Hall J, Granger DN. Obesity exacerbates sepsis-induced inflammation and microvascular dysfunction in mouse brain. Microcirculation. 2005;12:183–194. doi: 10.1080/10739680590904982. [DOI] [PubMed] [Google Scholar]
- 44.Preiss D, Sattar N. Non-alcoholic fatty liver disease: an overview of prevalence, diagnosis, pathogenesis and treatment considerations. Clin Sci (Lond) 2008;115:141–150. doi: 10.1042/CS20070402. [DOI] [PubMed] [Google Scholar]
- 45.Farrell GC, Teoh NC, McCuskey RS. Hepatic microcirculation in fatty liver disease. Anat Rec (Hoboken) 2008;291:684–692. doi: 10.1002/ar.20715. [DOI] [PubMed] [Google Scholar]