Abstract
Humans are continuously exposed to low-level ionizing radiation from natural sources. However, harsher radiation environments persisted during our planet’s early years and mammals survived via an evolutionary gift - a system of radiation-induced natural protective measures (adaptive protection). This system includes antioxidants, DNA repair, apoptosis of severely damaged cells, epigenetically regulated apoptosis (epiapoptosis) pathways that selectively remove precancerous and other aberrant cells, and immunity against cancer. We propose a novel model in which the protective system is regulated at least in part via radiation-stress-stimulated epigenetic reprogramming (epireprogramming) of adaptive-response genes. High-dose radiation can promote epigenetically silencing of adaptive-response genes (episilencing), for example via promoter-associated DNA and/or histone methylation and/or histone deacetylation. Evidence is provided for low linear-energy-transfer (LET) radiation-activated natural protection (ANP) against high-LET alpha-radiation-induced lung cancer in plutonium-239 exposed rats and radon-progeny-exposed humans. Using a revised hormetic relative risk model for cancer induction that accounts for both epigenetic activation (epiactivation) and episilencing of genes, we demonstrate that, on average, >80% of alpha-radiation-induced rat lung cancers were prevented by chronic, low-rate gamma-ray ANP. Interestingly, lifetime exposure to residential radon at the Environmental Protection Agency’s action level of 4 pCi L−1 appears to be associated with on average a > 60% reduction in lung cancer cases, rather than an increase. We have used underlined italics to indicate newly introduced terminology.
Keywords: radiation hormesis, adaptive response, epigenetic reprogramming
INTRODUCTION
An important and essential characteristic of biological organisms is their ability to persist in the face of repeated challenging conditions. Every human alive today owes their existence to the billions of generations of progenitors that survived and reproduced in spite of the previous challenging environmental and other conditions (Lenski et al. 2006). The initial emergence of mammalian life on earth took place in an environment with higher but unknown natural background levels of radiation; even, so mammals, including humans, survived via evolutionarily-derived adaptive responses to the harsher natural radiation environment. The defense repertoire for humans ultimately comprised all levels of the biological hierarchy—from the molecular and biochemical level to the cellular and tissue level to the organ and organ system level (Trosko 1998). Although natural background radiation levels are much lower today, these evolutionarily derived, adaptive-response phenotypes can be invoked by ionizing radiation doses and dose rates somewhat higher than current natural levels (i.e., low doses and dose rates) and are thought to be regulated at least in part epigenetically (Koturbash et al. 2006).
Epigenetic Regulation of Genes
The term epigenetic refers to a heritable change in the pattern of gene activity that is mediated by mechanisms other than alterations in the primary nucleotide sequence of a specified gene (Bird 2002; Russo et al. 1996). Epigenetic regulation (epiregulation) of gene activity is widespread in the genome of eukaryotic cells and can lead to silencing (episilencing) or activation (epiactivation) of gene expression. Epireprogramming is used in this paper to represent both epiactivation and episilencing. We have used underlined italics to indicate newly-introduced terminology. This convention is used throughout the paper.
The control of transcription is regulated by transcription factors, by changes in the methylation status of DNA (the methylation of cytosine residues at 5’ carbon), and by modifications in chromatin (e.g., histone methylation, acetylation, phosphorylation and ubiquitination) (Karpinets and Foy 2005). DNA and histone methylation and histone deacetylation result in a compact chromatin configuration that silences entire regions of DNA. For suppressor gene promoters, the compact chromatin topology restricts the access of the polymerase II complex to regulatory sequence domains, thereby inhibiting tumor suppressor transcription (Karpinets and Foy 2005).
Hypermethylation of gene promoters is a mechanism of long-term gene episilencing. However, episilencing can be reversed by demethylation of DNA.
Demethylation of DNA has been suggested to be involved in DNA repair (Reik 2007) and along with p53 (Camphausen et al. 2003) has been implicated in high-dose radiation abscopal effects (Koturbash et al. 2006). DNA repair is important in adaptive responses of mammalian cells to genotoxic threats, and epigenetic changes have important roles in activating and directing DNA repair pathways; however, mechanisms that govern epireprogramming during DNA repair and replication are not fully understood (Leng et al. 2008).
Methylation of CpG islands in promoter regions of some genes in colon cancer appears to increase with age (Issa et al. 1994). This suggests that life history can impact on the epigenetic profile.
Many genes that are epigenetically reprogrammed via hypermethylation have classic tumor suppressor functions. This includes the VHL gene in renal cancer, the CDKN2A (p16) gene in many types of cancer, and the mismatch-repair gene MLH1 in colorectal and other cancers (Herman and Baylin 2003). We consider any DNA repair or tumor suppressor gene that is activated by low-dose radiation to be an adaptive-response gene. However, new research is needed to clarify any roles that tumor suppressor genes may have in mounting the radiation adaptive responses that suppress cancer occurrence.
Gene episilencing (via promoter hypermethylation) involves the cytosine DNA methyltransferases 1, 3a, and 3b (DNMTs) which act via de novo methylation and recruitment of chromatin remodeling proteins (Damiani et al. 2008). DNMT1 which has both maintenance and de novo methyltransferase activity, is responsible for approximately 90% of the methyltransferase activity in mammalian cells. The DNMT1 protein is rapidly recruited to sites of DNA damage where it participates in de novo methylation and is over-expressed in lung and several other cancers (Damiani et al. 2008). Because significant DNA damage is caused by moderate and high doses of radiation, DNMT1 may participate in inhibiting radiation adaptive-response phenotypes.
High doses and dose rates of radiation are proposed to lead to silencing of adaptive-response genes via mutation induction and/or epireprogramming of promoters (e.g., promoter hypermethylation and/or his-tone deacetylation). This allows for what others have called a two-hit model for inactivation of both gene copies (Knudson 2001). However, dose-related multiple events (e.g., hypermethylation) are implicated for single-gene-copy silencing via methylation. Thus, the “two-hit” terminology appears inappropriate.
Low doses and dose rates of low-LET radiation are proposed to lead to epigenetic changes (up-regulation of adaptive-response genes, e.g., via acetylation) that facilitate managing future threatening genetic hazards. Adaptive-response genes (e.g., tumor suppressor p53) can be stabilized and activated in response to cellular stress (e.g., low dose radiation) through post-translational modifications that include acetylation (Ito et al. 2002). P300/CBP-mediated acetylation of p53 is, however, negatively regulated by MDM2.
Low-Dose-Radiation Stimulated Protective Apoptosis Medicated (PAM) Process
Low doses and dose rates of low-linear-energy-transfer (LET) radiation (e.g., X-rays, gamma rays, and beta radiation) above a stochastic threshold stimulate intracellular and intercellular signaling that leads to activated natural protection (ANP) against cancer and other genomic-instability-associated diseases (Scott 2005; Scott and Di Palma 2006). Pathways that include induced p53-dependent high-fidelity DNA repair along with normal apoptosis (which eliminates seriously damaged cells), activation of an epigenetic protective apoptosis-mediated (PAM) process that selectively removes precancerous and other aberrant cells (Scott and Di Palma 2006), and induced immune functions (Liu 2003, 2007) have been found to be key components to the low-dose radiation ANP. Here we refer to the epigenetic PAM process as epiapoptosis. Others have demonstrated in vitro that reactive oxygen and nitrogen species and specific cytokines (e.g., transforming growth factor β) are involved in this natural protection and that signaling to apoptosis is independent of the p53 gene (Hipp and Bauer 1997; Bauer 2007; Portess et al. 2007).
The PAM process and stimulated immunity (ANP), which are activated by low doses and dose rates of low-LET radiation, appear to be inhibited by moderate and high doses of radiation as well as by high radiation dose rates (Scott and Di Palma 2006; Liu 2007). High-LET alpha radiation does not appear to effectively activate the PAM process (Scott et al. 2007). For exposure to neutrons, the gamma-ray component to the dose appears to activate the PAM process and thereby protect from deleterious neutron-induced stochastic effects (Rithidech and Scott 2008). The level of protection appears to increase as the gamma-ray contribution to the dose increases, which depends on neutron energy (Rithidech and Scott 2008). The PAM process is transient and may not persist for more than a few tens of hours (Scott and Di Palma 2006).
Genetic Polymorphisms in DNA Repair and Other Adaptive-Response Genes and their Influence on Radiation Responses
Radiation-induced repair of DNA double-strand breaks (DSB) is known to require a threshold dose (Rothkamm and Löbrich 2003) that may vary for different individuals, depending on their genetic characteristics (Scott 2005). This is supported by the fact that DNA DSB repair fidelity has been demonstrated to vary for different individuals. A 50% reduction in the mean level of DNA DSB repair capacity was found among lymphocytes from cigarette smokers with a high methylation index, defined as three or more of eight genes methylated in sputum, compared with smokers with no genes methylated. Single nucleotide polymorphisms within the MRE11A, CHEK2, XRCC3, DNA-PKc, and NBN DNA repair genes were highly associated with the methylation index (Leng et al. 2008). These findings provide indirect evidence for genetic/epigenetic links to susceptibility to genomic stresses such as ionizing radiation. Thus, genetic variability provides a plausible basis for variability in response to radiation-induced harm for different individuals.
When harm is evaluated via endpoints such as mutations and neoplastic transformation, stochastic threshold doses (which vary for different individuals) are implicated as previously proposed (Scott 2005). The stochastic thresholds can be protective (e.g., activation of DNA repair) or deleterious (e.g., inhibition of the PAM process).
The radiation dose threshold distributions for activating the different components of adaptive protection (e.g., DNA repair, PAM process, immunity against cancer) likely differ, but this has not been studied. The current risk model for radiation-induced cancer developed at our Institute (Scott and Di Palma 2006) does not allow for different threshold distributions for the different contributors to adaptive protection. Onsets and durations of protective signaling are also not addressed. We plan to address these deficiencies in future research.
Carcinogen-Induced DNA Damage and Related Promoter Episilencing
Carcinogens in cigarette smoke [e.g., benzo(a)pyrene (BAP)] can directly or indirectly cause single and DSB in DNA. Genotoxic but not cytotoxic exposure to a DNA damaging agent was associated with reduced DNA repair capacity and an increased lung cancer risk (Leng et al. 2008). Mounting evidence suggests that a high prevalence of DNA DSBs could be partly responsible for the acquisition of aberrant gene promoter methylation during lung carcinogenesis and influenced by the carcinogen dose. For example, the prevalence of promoter hypermethylation of the cell-cycle-regulating p16 gene was significantly greater (p = 0.03) in adenocarcinomas from workers occupationally exposed to high levels of plutonium-239 (239Pu) alpha radiation (an exposure that efficiently produces DSBs) than in tumors from unexposed smokers after adjusting for smoking (Belinsky 2004; Belinsky et al. 2004). The prevalence of methylation increased as the total radiation dose (and 239Pu organ burden) to the lung increased. In addition, a trend (p = 0.08) was seen for an increase in the number of genes methylated (≥ 2) with increased alpha radiation dose (and 239Pu organ burden). In studies of rodent lung cancer the prevalence of methylation was less for chemicals that mainly produced DNA single-strand breaks than for alpha radiation (Belinsky 2005). These findings support the hypothesis that DSB may play an important role in triggering episilencing of protective genes (e.g., hypermethylation of tumor suppressor gene promoters). Episilencing of protective genes is strongly associated with increased risk for cancer (Belinsky 2004).
The steep rise in the lung cancer incidence in humans above the spontaneous level after moderate and high alpha radiation doses has been explained on the basis of varying individual-specific thresholds for suppression of adaptive protection (e.g., suppression of the immune system) (Scott and Di Palma 2006). The thresholds appear to be related to the DNA-damage-associated episilencing of adaptive-response genes.
Immune System’s Contribution to Radiation Adaptation
It is expected that as low doses and dose rate of low-LET radiation exceed an individual-specific (stochastic) threshold, modulation of the pulmonary immune response will occur in a manner to promote apoptosis and/or immune-mediated clearance of new premalignant cells, leading to a reduced lung cancer risk (Liu 2003, 2007). These changes may manifest as increased numbers or activity of natural killer cells and cytotoxic lymphocytes; increased ability of dendrite cells to recognize and present novel antigens to T cells that in turn induce their proliferation and cytokine secretion (perhaps favoring Th1 differentiation and interferon-gamma production); or enhanced activation of intracellular signaling molecules (leading to up-regulation of nuclear factor κB and downstream gene expression) (Yuan et al. 1992; Wilder and Yuan 1995; Wilder et al. 1996).
There is evidence for enhanced immune system functioning in relation to chronic, low-rate exposure to natural background radiation. Inhabitants of a high natural background area of Iran show a significant increase of CD69 expression on TCD+ stimulated cells and total serum IgE, indicating enhanced immune functioning under conditions of prolonged low-rate, low-dose, low-LET radiation exposure (Ghiassi-Nejad et al. 2004).
Evidence for Novel Adaptive-Response Pathways
Ultra-low radiation doses (below those that stimulate measurable DNA repair, epiapoptosis, and immune system functioning) in excess of natural background radiation (evaluated over the exposure period) are also protective of a subsequent high dose (Day et al. 2007). Thus, additional components to adaptive protection (a hormetic effect) appear to be important and may involve epireprogramming of adaptive-response genes (Karpinets and Foy 2005).
Recently, it was reported that extended, low-rate gamma-ray exposure (1–2 mGy) completely inhibited 239Pu alpha radiation-induced lung cancer, for doses up to approximately 0.6 Gy (600 mGy), in Wistar rats (Scott et al. 2008). The protection lasted for more than 1 year after delivery of the gamma-ray dose, eliminating induced persistent DNA repair, epiapoptosis, and immunity as contributors to the persistent protection. Thus, there appears to be a previously unrecognized, chronic, low-dose, low-LET radiation-induced protective change in normal cells that is persistent and may involve epigenetic memory because gamma-ray doses of 1–2 mGy when delivered at a very low rate produce little damage to DNA and do not increase expression of DNA repair or immune system genes (Rothkamm and Löbrich 2003; Liu 2007). The nature of the persistent protection brought on by exposure to a low dose of low-LET radiation is not resolved but may involve an enhanced DNA repair capacity. This is addressed in the Discussion section of the paper.
In the Wistar rat study discussed, alpha radiation > 10 Gy (10,000 mGy) in conjunction with a small gamma-ray dose efficiently induced lung cancer. The frequency of lung cancer was not as high as that induced by alpha radiation alone. This suggests that protection by low-dose, low-LET radiation was partially suppressed by other changes, possibly involving methylation of adaptive-response genes (Belinsky 2004; Belinsky et al. 2004) as well as other detrimental epigenetic changes (Karpinets and Foy 2005).
In this paper we present results of a more detailed analyses of the Wistar rat lung cancer data, showing that the upper 95% confidence values on the observed zero cancer incidences are relatively large, suggesting that the level of protection may have been < 100%. New modeling results are also presented related to lung cancer suppression in association with residential exposure to radon progeny. The new results are based on data from a recent well-designed epidemiological study (Thompson et al. 2008) and a revised version of an existing dose-response model (Scott and Di Palma 2006) that accounts for radiation adaptive responses. In the revised version, radiation-induced lung cancer is linked to radiation-induced epireprogramming of adaptive-response genes.
THE NATURAL IONIZING RADIATION ENVIRONMENT
Most members of the public believe that we are normally radiation-free entities and any amount of radiation is harmful. This is not correct as radiation is everywhere, including in our bodies (Figure 1). The natural background ionizing radiation encountered on Earth comes from the sun (solar radiation), outer space (cosmic rays), and terrestrial sources (e.g., radionuclides in our environment, homes, and bodies). The main source of internal radiation is ingested potassium-40 (40K) followed by carbon-14 (14C). Table 1 lists estimates of the natural radioactivity in the body of a 70-kg adult human from some of the long-lived radionuclides, based on information available via the web (ISU 2008). For 40K, there are about 380 million disintegrations per day, approximately 90% (342 million) of which involve emission of beta particles. For proponents of the linear-no-threshold (LNT) hypothesis that implicates each beta particle emission as being harmful, there is no scientific basis for such a claim. The following section describes how these millions of beta particle emissions in our bodies each day may in fact be beneficial to our health.
FIGURE 1.
Natural radiation sources: plant, animals, our bodies, soil, rocks, cosmic rays, indoor radon.
TABLE 1.
Natural radioactivity in the body of a typical 70-kg (150-pound) adult human*
| Nuclide | Approximate Total Mass | Disintegrations per Day |
|---|---|---|
| Uranium isotopes | 90 micrograms | 95 thousand |
| Thorium isotopes | 30 micrograms | 9.5 thousand |
| Potassium-40 | 17 miligrams | 380 million |
| Radium isotopes | 31 picograms | 95 thousand |
| Carbon-14 | 22 nanograms | 320 million |
| Tritium | 0.06 picograms | 2 million |
| Polonium isotopes | 0.2 picograms | 3.2 million |
Based on information from Idaho State University’s web site (ISU 2008).
EARTH’S PREVIOUS HIGHER NATURAL BACKGROUND RADIATION LEVELS’ EVOLUTIONARY IMPACT ON MAMMALS
Billions of years ago when mammalian life first evolved on Earth, natural radiation levels were much higher due to the presence of both long-and short-lived radionuclides (Draganić et al. 1990). Since then, all of the short lived isotopes have decayed. Only those initially-present isotopes with very long half lives (e.g., > 100 million years) remain along with the isotopes that formed from the decay of the long-lived isotopes and newly produced isotopes. Evolutionary changes allowed mammals to cope with the higher radiation levels that previously existed via radiation adaptation (Tubiana 2008). The adaptation included acquiring the capability to correct nuclear DNA damage via a number of repair pathways that evolved over time and today can be stimulated by beta particle emissions or secondary electrons (similar to beta particles) produced by gamma rays. These different repair processes are regulated by both genetic and epigenetic pathways.
Epiapoptosis (e.g., PAM process) is now considered to protect mammals from stochastic effects of higher natural background radiation levels, and it was probably important to maintaining mammalian life on Earth through the earlier periods of high natural radiation (Scott 2007). This is supported by the fact that low-LET radiation levels somewhat above current natural background levels stimulate the removal of neoplastically transformed cells (Redpath et al. 2001), likely via epiapoptosis. Evolution also gave mammals an additional, apparently longer lasting type of protection that is stimulated by low doses of low-LET radiation. This protection relates to stimulating the immune system, which protects the body from cancer by eliminating precancerous and cancer cells (Ghiassi-Nejad et al. 2004; Liu 2007).
CURRENT SYSTEM FOR LIMITING HUMAN RADIATION EXPOSURE
The current system for limiting radiation exposure of humans is based on the premise that cancer is the major risk from small radiation doses and cancer risk increases as a linear-no-threshold (LNT) function of radiation dose (NRC 2006). The LNT model claims that any radiation dose, no matter how small, causes some cancers among a very large irradiated population and that doubling the radiation dose doubles the number of cancer cases. Thus, from Table 1 it can be inferred based on the LNT model that the more than 1 million radioactive disintegrations that take place in our bodies each day produce cancer in some people among the world population. Whatever this hypothetical number of people is calculated to be, the number of induced cancer cases would be predicted to double in two days and to further increase with each passing day. However, the LNT model is empirical, is not based on modern science, and there are no data to support it in the context of low-level radiation exposure (Tubiana 2005, 2008; Tubiana et al. 2005).
Because the current system of limiting radiation exposure is tied to the LNT model, demonstrating either a radiation dose threshold for cancer induction or a decrease in cancer risk as radiation exposure increases above natural background exposure levels for a range of doses causes major problems for justifying the continued use of the current LNT-based system.
There is now abundant evidence that low doses and dose rates of low-LET radiation (gamma rays, X rays, beta radiation) protect, rather than harm. Low doses or low dose rates of low-LET radiation have been demonstrated to do each of the following: (1) protect against spontaneous genomic damage (Feinendegen 2005; Feinendegen et. al 2007); (2) protect against spontaneous and high-radiation-dose-induced mutations (Day et al. 2007); (3) protect against neoplastic transformation (Redpath et al. 2001; Bauer 2007; Portess et al. 2007); (4) protect against high-dose chemical- (Sakai et al. 2003) and alpha-radiation-induced (Sanders 2008; Scott et al. 2008) cancers; (5) enhance immune system defense against cancer (Liu 2003; Cuttler 2007); (6) suppresses metastasis of existing cancer (Liu 2007); (7) extend tumor latency period (Mitchel 2007); (8) protect against diseases other than cancer (Luckey 1991; Sakai 2006), and (9) protect against heritable mutations and fetal malformation (Boreham et al. 2006). A new study (Mancuso et al. 2008) has yield data showing that a total-body X-ray dose of 36 mGy suppressed spontaneous brain cancer in brain-cancer-prone mice, although this feature of the data was apparently not recognized by the researchers. Epigenetic signaling pathways likely are important for the indicated radiation adaptive-response phenotypes. Collectively, the above findings invalidate the LNT model.
LINKING THE HORMETIC RELATIVE RISK MODEL TO EPIREPROGRAMMING OF ADAPTIVE-RESPONSE GENES
This section focuses on lung cancer associated with combined exposure of the lung to low doses and dose rates of low-LET beta/gamma plus high-LET alpha radiation. Mathematical relationships are initially presented for hypothetical groups of people with identical genetic/epigenetic characteristics. We then address heterogeneous populations over which such characteristics are allowed to vary.
Results presented here for beta and gamma radiations also apply to each of these radiations when evaluated separately. With the current version of our stochastic hormetic relative risk (HRR) model, the irradiated subpopulation is separated into two dose, dose-rate, and genetic/epigenetic characteristics dependent parts: (1) those that have maximal ANP and (2) those without ANP. For people with low-LET radiation ANP (a stochastic quantity), the average cancer relative risk is given by
| (2) |
where RRANP is the average relative risk for protected individuals and RRLNT is the relative risk for individuals without ANP and is based on the LNT assumption. Risk is evaluated relative to an unirradiated genetically and epigenetically matched population. The protection factor (profac), whose average over the subpopulation is PROFAC, is allowed to vary for each individual.
The PROFAC (subpopulation average) has values from 0 to 1 and here accounts for prevention of cancer via gamma-ray ANP. PROFAC = 0.25 indicates that cancer would be expected on average to be prevented in 1 of each 4 individuals with radiation ANP for the subpopulation considered. The PROFAC is allowed to vary over a heterogeneous population. The relative risk, RRLNT, applies for alpha-radiation-induced lung cancer and is evaluated based on an LNT function that includes the baseline (spontaneous) cancer incidence B (Scott 2007):
| (3) |
The slope parameter Kα (subpopulation average) is presumed always-positive for people (subpopulation) with similar genetic/epigenetic characteristics, and Dα is the alpha radiation absorbed dose to the target organ. Our use of “spontaneous cancer” is quite general and includes all sporadic and hereditary cancers not associated with the radiation exposure of interest.
Equation 2 is used to evaluate cancer RR for combined exposure to low-LET beta and/or gamma rays and high-LET alpha radiation while Equation 3 applies to exposure to alpha radiation alone and relates to doses below those that cause death from acute effects (Scott 2007). Equation 2 does not apply to high doses and dose rates of low-LET radiation. Also, the PROFAC relates only to the low-LET component of the dose. Setting PROFAC to zero in Equation 2 yields Equation 3.
RRLNT should be set to 1 in Equation 2 for exposure only to low doses and dose rates of low-LET radiation. The average RR is modeled as being independent of the low-LET radiation dose, taking on values < 1 for doses above a very low maximum stochastic threshold for ANP. This solution applies only for low doses and dose rates where epireprogramming of adaptive-response genes is now presumed to enhance one’s capacity to prevent cancer occurrence (adaptive protection/hormesis). Both profac and PROFAC depend on the low-LET radiation dose-rate history and radiation physical characteristics. For very low doses of low-LET radiation, this protection is expected to be progressively lost as the dose decreases and RR is expected to correspondingly increase.
In the original HRR model, DNA repair capacity impacted Kα while the PAM process and stimulated immunity impacted PROFAC (Scott and Di Palma 2006). Here, this characteristic is retained because we can also address deleterious epigenetic changes as follows: changed risk for individuals with adaptive responses suppressed due to dose-related epireprogramming of promoters can be addressed by reassigning risk from Equation 2 to Equation 3. This is done in a stochastic manner as explained in the next section. The expected absolute risk for people with similar genetic/epigenetic characteristics is evaluated by multiplying the RR by the average baseline incidence B for the subpopulation.
All model parameters and variables (including dose) are allowed to vary over a heterogeneous population to account for variation in genetic/epigenetic characteristics and variation in dose. When low doses of low- and high-LET radiation are combined, low-LET radiation-related ANP is assumed to be fully expressed (all protective pathways) except for very low doses (near the natural background radiation level) as previously indicated. The number of individuals with ANP is modeled stochastically as varying with dose to allow for radiation dose-related episilencing of adaptive-response genes promoters.
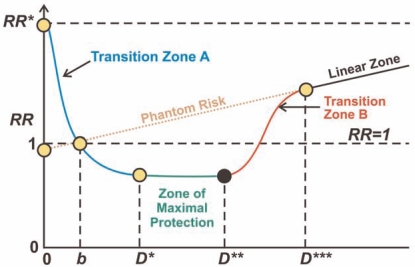
Figure 2 presents a schematic representation of the revised HRR model. The population average RR is plotted vs. the total absorbed dose D for combined exposure to high-LET alpha and low-LET beta/gamma radiation. Dose b is the radiation dose from natural background radiation exposure for the period of interest. The dose indicated by “0” is for a hypothetical environment without any natural background radiation. The dose interval 0 to D* makes up Transition Zone A, where stochastic thresholds (low-LET radiation component) for stimulating (e.g., epiactivating) adaptive-response genes occur. The Zone of Maximal Protection spans the range D* to D** where everyone is presumed to have low-LET radiation ANP against cancer. The dose interval D** to D*** comprises Transition Zone B, where stochastic thresholds for adaptive-response gene promoter silencing (via episilencing and/or induced mutations) occur. For doses just above D*** (Linear Zone) everyone is presumed to have their adaptive response genes silenced. The additional linear increase at higher doses (Linear Zone) relates to additional mutation inductions in adaptive-response genes.
FIGURE 2.
Schematic representation of the cancer relative risk vs. dose relationships according to the HRR model. See the text for explanation of the doses and zones.
IMPLEMENTATION OF THE HRR MODEL
While there is currently little information available on the impact of specific genetic/epigenetic characteristics on cancer risk, employing the HRR model using Bayesian inference methods implemented with Markov chain Monte Carlo (MCMC) allows the distributions (posterior) of model parameters (and functions of the parameters such as RRANP and RRLNT) to be generated. The distributions are expected to at least crudely reflect the impacts of the varying genetic/epigenetic characteristics. The Appendix briefly explains Bayesian inference methods and their implementation using MCMC.
A single very long chain (rather than shorter multiple chains) and uniform prior distributions for model parameters were used for the MCMC results presented in this paper. Chain lengths needed for convergence were judged based on autocorrelations after the first 10,000 iterations. The ratio of the posterior distribution Monte Carlo error to the model parameter standard deviation <0.05 (for each parameter) was judged to be consistent with convergence (Spiegelhalter et al. 2003).
In our analyses of lung cancer dose-response data presented, rather than using point estimates of dose, dose intervals were used that were based on the experimental or epidemiological data fitted. Doses were assumed uniformly distributed over the exposure-group-specific intervals used because detailed information on dose variation over the reported intervals was not available. Results obtained are therefore conditional on the assumed dose distributions.
USING THE HRR MODEL TO DEMONSTRATE GAMMA-RAY PREVENTION OF ALPHA-RADIATION-INDUCED LUNG CANCER IN THE WISTAR RAT MODEL OF LUNG CANCER
Two studies were conducted by Sanders and colleagues that involved inhalation exposure of Wistar rats to alpha-emitting aerosols of 239Pu (Sanders 2008). One study used 239PuO2 alone (Table 2) and a second study (Table 3) used a 169 Yb2O3-239PuO2 aerosol. Only data for alpha radiation doses up to approximately 8000 mGy (8 Gy) are used here. The 169Yb was used as a tracer to track how much 239Pu deposited in the lung (via counting of 169Yb gamma rays). The 169Yb-emitted gamma rays are capable of activating components of the natural protection system already described (i.e., adaptive-response phenotypes). Gamma-ray doses from 169Yb estimated to be 1–2 mGy delivered at very low rates over about 4 months appeared to have completely prevented alpha-radiation-induced lung cancer (Sanders 2008; Scott et al. 2008).
TABLE 2.
Frequency of lung tumors in female Wistar rats after inhalation of 239PuO2 Aerosols (Sanders 2008)
| Number of Rats | Average Alpha Radiation Dose ± Standard Deviation (mGy) | Observed Lung Tumor Incidence | Bayesian Analysis Posterior Distribution Mean Incidence (2.5%, 97.5%)a |
|---|---|---|---|
| 656 | 0 | 0.0015 | 0.0015 (9.8 × 10−4,2.0 × 10−3) |
| 131 | < 100b | 0.015 | 0.0091 (0.0057,0.0138) |
| 51 | 270 ± 120 | 0.078 | 0.05 (0.022,0.083) |
| 26 | 780 ± 170 | 0.346 | 0.135 (0.081,0.21) |
| 38 | 2,550 ± 1,320 | 0.447 | 0.446 (0.273,0.651) |
| 16 | 6,800 ± 1,200 | 0.313 | 0.846 (0.547,1.0) |
Percentiles 2.5 % and 97.5% of the Bayesian analysis posterior distribution for the tumor incidence based on the HRR model. Doses were assumed uniformly distributed over the interval (average − 1.5 standard deviations, average + 1.5 standard deviations).
Mid-range dose used as an estimate of the average. A subjective upper bound of 20 mGy was used as an upper bound for the standard deviation.
TABLE 3.
Frequency of lung tumors in female Wistar rats following inhalation of 169 Yb2O3-239PuO2 Aerosols (Sanders 2008)
| Number of Rats | Average Alpha Radiation Dose ± Standard Deviation (Gy) | Average Gamma-ray Dose (mGy) | Observed Lung Tumors Incidence | Bayesian Analysis Posterior Distribution Mean Incidence (2.5%, 97.5%)a |
|---|---|---|---|---|
| 1052 | 0 | 0 | 0.00095 | 0.00164 (5.4 × 10−4,0.004) |
| 1389 | 0.056 ± 0.020 | 0.9 | 0 | 0.00146 (6.8 × 10−4, 0.0027) |
| 343 | 0.19 ± 0.09 | 1.8 | 0 | 0.00387 (0.00143, 0.00861) |
| 145 | 0.62 ± 0.16 | 1.3 | 0 | 0.0146 (0.00744,0.0256) |
| 58 | 2.32 ± 0.77 | 2.5 | 0.069 | 0.0627 (0.03, 0.11) |
| 38 | 5.03 ± 0.60 | 5.0 | 0.212 | 0.135 (0.076,0.212) |
| 18 | 7.99 ± 0.67 | 0.8 | 0.278 | 0.21 (0.12,0.33) |
Percentiles 2.5 % and 97.5% of the Bayesian analysis posterior distribution for the tumor incidence based on the HRR model. Doses were assumed uniformly distributed over the interval (average − 1.5 standard deviations, average + 1.5 standard deviations).
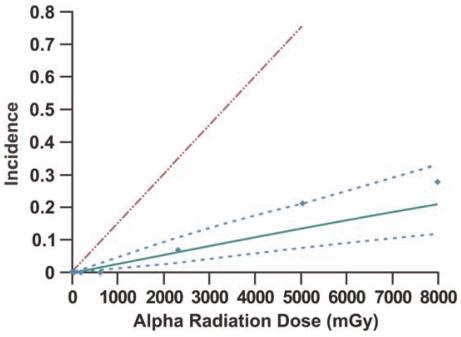
We have fitted the revised HRR model to Sanders’ Wistar rat data using Bayesian inference methods (see Appendix) implemented with MCMC and results are presented in Figures 3 and 4 and Tables 2 and 3. In our analyses we only included alpha radiation doses up to approximately 8,000 mGy (8 Gy) in an attempt to avoid presumed significant life-shortening effects of higher doses. A single MCMC chain was run for 100,000 iterations with the first 80,000 results discarded (this is called burn-in). The ratio of the Monte Carlo error for each parameter relative to the parameter standard deviation was <0.05, consistent with convergence of the indicated long chain. In addition to posterior distribution means, percentiles 2.5% and 97.5% of the posterior distributions were also recorded. Results (posterior distribution mean and percentiles 2.5% and 97.5%) for exposure to only alpha radiation are presented in Table 2. The upper dashed line in both Figures 3 and 4 is based on data for exposure to alpha radiation alone (posterior distribution mean [estimate of population average]). For the alpha-radiation-only line, the posterior distribution mean for Kα (= 1.5 x 10−4 ± 3.2 x 10−5 mGy−1) was used along with a spontaneous cancer incidence of 0.0015. The alpha-radiation-only data are excluded but are presented in Table 2 along with the modeling results (Sanders 2008; Scott et al. 2008). The lower solid curve and upper and lower dashed bands are the Bayesian posterior means and percentiles 2.5% and 97.5% based on the HRR model. Kα was constrained to be fixed at 1.5 x 10−4 mGy−1 (posterior distribution mean for exposure to alpha radiation only) for fitting of the data for combined alpha and gamma irradiation. Thus, results for combined exposure to alpha and gamma rays are conditional on this value for Kα. A high level of gamma-ray ANP is implicated in the results, as PROFAC = 0.83 ± 0.04. This means that on average >80% of sporadic and hereditary lung cancers appear to have been prevented by gamma-ray ANP. The indicated value for PROFAC is very close to the posterior mean of 0.86 ± 0.07 we previously published for gamma-ray ANP against 239Pu alpha radiation-induced lung cancer in Mayak plutonium facility workers (Scott and Di Palma 2006).
FIGURE 3.
Lung cancer incidence in Wistar rats after inhalation exposure to the alpha radiation source 239PuO2 (upper dashed LNT-based line) or 239PuO2 labeled with a 169Yb gamma-emitting tag (data points and associated lower family of curves based on HRR model). The lower family of curves comprise the Bayesian posterior mean (solid curve) and percentiles (dashed curves) 2.5% and 97.5%. The data used are from Sanders (2008).
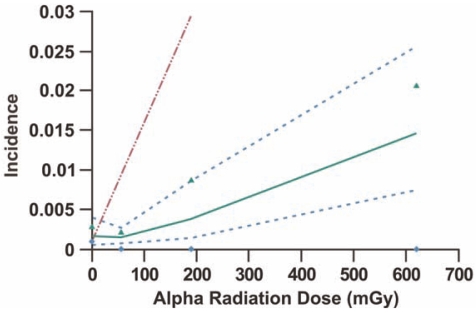
FIGURE 4.
Lower dose-portion of Figure 3 with approximate upper 95% confidence interval (triangles) for an observed incidence of zero (diamonds) added. The upper confidence interval on data points for a zero incidence is based on observing an incidence of zero with a probability of approximately 0.05 (which corresponds to an expected 3 cancer cases). With the assumed Poisson distribution, the probability of 0 events when the expected value is 3 is given by exp(-3) which equals 0.05 (rounded). The data point for the highest dose group is off scale and is not presented so that the shape of the curve at low doses is clearer.
The high level of gamma-ray ANP against alpha radiation induced lung cancer implicated in Figure 3 is thought to relate to the low dose of gamma rays being spread over several months. The low-LET gamma-ray dose to the lung was delivered over about 4 months while the alpha radiation dose accumulated over several years. Thus, the presumably gamma-ray-induced protection appears to have lasted for more than a year after delivery of the protracted gamma-ray dose, ruling out persistent DNA repair, PAM process, and induced immunity as contributing to persistent protection over more than a year. Similar lifetime protection was reported in other in vivo studies (Mitchel 2007). Thus, there appears to be a previously unrecognized chronic, low-dose, low-LET radiation-induced protective epigenetic change (memory effect) in normal cells in vivo (the majority of the irradiated cells in the lung) that persists over long periods because 1–2 mGy gamma-ray doses delivered over a few months would not be expected to sustain DNA repair (Collis et al. 2004) or the PAM process or immune system signaling for more than a year after irradiation. This points to low-dose gamma-ray stimulated epireprogramming of adaptive-response genes as possibly playing a major role in long-term protection against cancer.
We speculate that the indicated protective change in protracted gamma-irradiated Wistar rats may involve a gamma-ray-induced long-lasting epigenetic memory effect in adaptive-response genes so that should future threatening damage occur in nuclear DNA, the damage is quickly recognized (e.g., lower damage response threshold) and adaptive-response genes are more rapidly up-regulated to respond to the damage. The hypothesized novel form of radiation adaptation would help explain why a large mutagenic dose alone that invokes substantial DNA repair and stimulation of immune functions is more mutagenic than when the same dose is preceded by a very small adapting dose of low-LET radiation (which does not appear to invoke DNA repair, the PAM process, or immunity [Day et al. 2007]).
In Sanders’ Wistar rat study (2008) involving combined exposure to alpha and gamma radiations, alpha radiation doses above 10 Gy (10,000 mGy) efficiently induced lung cancer, but these exposures were not as efficient as alpha radiation alone, suggesting that protection against cancer by low-doses of low-LET radiation was reduced by other changes, possibly changes involving differential episilencing of adaptive-response genes (Belinsky 2004; Belinsky et al. 2004; Karpinets and Foy 2005). In this context, epireprogramming of adaptive-response genes would explain the hormetic dose response curve for low-LET or low- plus high-LET radiation induced cancer: low doses produce epiactivation while high doses produce episilencing of adaptive-response gene promoters.
EVIDENCE FOR RESIDENTIAL-RADON-ASSOCIATED ACTIVATION AND SILENCING OF ADAPTIVE-RESPONSE GENES
Radon exposure in the home is considered by regulatory agencies, such as the EPA, to be a health hazard mainly because body organs (especially the lung) are exposed to high-LET alpha radiation from radon progeny. Recently, concern has been raised about radon emanating from granite countertops. Low-LET beta particles and gamma rays are also emitted by radon progeny, although radiation doses are much lower than those for alpha radiation. While there is good evidence that small doses of alpha radiation alone efficiently cause lung cancer (e.g., Figures 3 and 4 ), there is equally good evidence that when small doses of low-LET radiation are also involved, the body’s natural defenses can be stimulated, suppressing lung cancer occurrence (Figures 3 and 4).
More than twenty case-control studies of the lung cancer risk from radon in homes have now been reported for North American, European, and Chinese locations (Thompson et al. 2008). Generally these studies reported increased risk of lung cancer. However, the study designs were deficient in that they had little power for demonstrating odds ratios (OR) < 1, i.e., a radiation adaptive or hormetic response, and were biased for demonstrating OR > 1, usually under the LNT assumption (Scott et al. 2008; Thompson et al. 2008).
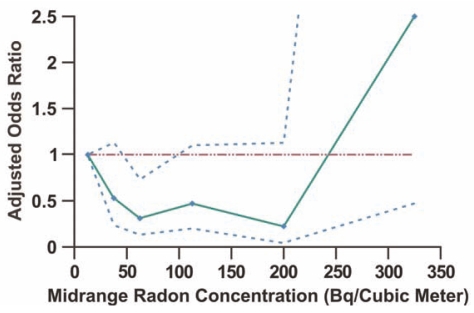
Similar low-LET radiation protection against alpha-radiation-induced lung cancer as presented in Figures 3 and 4 (a modeling result) is now implicated in data from a recent epidemiological study of lung cancer (Figure 5) associated with residential radon progeny exposure (Thompson et al. 2008). The study used a novel design that corrected most of the deficiencies (e.g., lowest exposure [reference] group having a wide rage of exposures is a big problem) associated with other mentioned studies that did not find evidence for OR < 1, due to low power for such a demonstration. The study was performed with 200 cases (58% male, 42% female) and 397 controls matched on age and sex, all from the same health maintenance organization. Emphasis was placed on accurate and extensive year-long dosimetry with etch-track detectors in conjunction with careful questioning about historic patterns of in-house mobility. Conditional logistic regression was used to control for years of residency, smoking, education, income, and years of job exposure to known or potential carcinogens. The researchers were surprised to find the adjusted OR less than 1 (Table 4), which is in good agreement with our HRR model.
FIGURE 5.
Adjusted odds ratio for lung cancer in association with residential radon exposure based on data from Thompson et al. (2008). Odds ratio was evaluated relative to radon concentration in the range 0 to < 25 Bq m−3. Data points are plotted at the midrange of the data in Table 4 with an assigned upper bound of 400 Bq m−3 for the highest exposure level. The dashed curves are based on the 95% confidence values reported by Thompson et al. (2008). Data points were connected by straight lines. The horizontal line marks an adjusted odds ratio equaling 1.
TABLE 4.
Adjusted odds ratio for lung cancer and 95% confidence interval by residential radon concentration category for Worchester County, Massachusetts study of Thompson et al. (2008)
| Radon Concentration in Bq m−3 [pCi L−1]a | Adjusted Odds Ratio (95% Confidence Interval) p value relative to odds ratio = 1 |
|---|---|
| < 25 [< 0.7 (rounded)] | 1.0 (reference group) |
| 25 − < 50 [0.7 − 1.4] | 0.53 (0.24, 1.13), p ≤ 0.1 |
| 50 − < 75 [1.4 − 2.0] | 0.31 (0.13, 0.73), p ≤ 0.05 |
| 75 − 150 [2.0 − 4.1] | 0.47 (0.2, 1.10), p ≤ 0.1 |
| 150 − < 250 [4.1 − 6.8] | 0.22 (0.04, 1.13), p ≤ 0.1 |
| ≥ 250 [> 6.8] | 2.50 (0.47, 13.46) |
Environmental Protection Agency Action Level = 4 pCi L−1.
Under the HRR model, OR and RR have similar shapes and OR would be expected to first decrease to < 1 and then remain below 1 for a range of radiation doses (or radon concentrations) at a roughly fixed level as is illustrated in Figure 2 for RR. At higher doses OR is expected to increase to > 1 as illustrated in Figure 2 for RR. Based on the HRR model, the 4 consecutive data points in Figure 5 with OR < 1 (presumed Zone of Maximal Protection) can be averaged to assess the significance of the adaptive response. Here OR is assumed to be normally distributed for the indicated zone. The average obtained is 0.38 ± 0.14, which is significantly < 1 (p < 10−5). Thus, on average, lung cancer appears to be reduced by 1 – 0.38 = 0.62 (62%) for people residing in homes with radon concentrations between 25 and 250 Bq m−3 (0.676 pCi L−1 and 6.76 pCi L−1) as compared with those residing in homes with lower concentrations. The value of 0.62 can be taken as a lower bound on PROFAC when evaluated relative to the complete absence of any radon in the home. Interestingly, the Environmental Protection Agency’s action level for residential radon of 4 pCi L−1 (USEPA 1993) is in the indicated zone of suppressed lung cancer risk. According to the agency, lifetime exposure of a population of 1000 persons at a level of 4 pCi L−1 could lead to 29 lung cancer cases if these individuals are cigarette smokers (USEPA 1993). The corresponding number for never smokers is 2 lung cancer cases. In contrast, PROFAC > 0.62 indicates that one would expect on average > 62% of spontaneous and smoking related lung cancer cases to be prevented due to the low-level radon exposure in the home (i.e., a protective effect). A similar level of protection against lung cancer occurrence has been reported for radon-exposed Colorado plateau uranium miners (Thomas et al. 1985; USEPA 1993) but not for other miner populations (USEPA 1993). Study design biases can prevent demonstrating cancer suppression after low-level radiation exposure (Scott et al. 2008; Thompson et al. 2008).
Based on the results presented, we can therefore state that for people residing in homes with a radon concentration in the range 25–250 Bq m−3 (0.676 − 6.76 pCi L−1), remediation of their homes to eliminate radon may lead to a substantial increase in their lung caner risk! This finding provides a reason to be cautious about making claims about lung cancers being caused by radon emanating from granite countertops in the home. The radon may actually be protective against cancer, depending on the total level of radon in the home.
DISCUSSION
Lung cancer is the leading cause of cancer mortality in women and men in the United States and now accounts for approximately 30% of all cancer deaths (Belinsky 2004; Jemal et al. 2002). Further, it has been projected that by 2010, annual lung cancer deaths worldwide will climb to about 1.5 million (Parkin et al. 2001). The advent of spiral computed tomography (CT) has led to capabilities of detecting precancerous nodules in the lung as small as 1 – 5 mm (Davis 1991). However, others (Brenner and Hall 2007; Hall and Brenner 2008) have speculated, based on risk extrapolation from atomic bomb survivors in Japan to the clinic in the United States using the LNT model, that many future lung and other cancers are being induced by current usage of CT scans. However, because X-ray doses from single CT scans are in the range for ANP against cancer, Scott et al. (2008) have pointed out that CT scans may actually reduce rather than increase the cancer risk.
We have provided evidence that protracted gamma-ray doses of 1–2 mGy (equivalent to 7 to 13 repeated chest X-rays) significantly prevented lung cancer in 239Pu-exposed (via inhalation) Wistar rats (Sanders 2008). A modest increase in low-LET radiation exposure just above the natural background level is proposed to activate wide-spread, protective epigenetic signaling among normal cells (a mild stress response). Because of the stochastic nature of the interaction of radiation with cells, most cells are not directly hit when the radiation dose is very low (e.g., 0.001 − 0.1 mGy). We speculate that via bystander signaling pathways, large numbers of cells (e.g., normal cells) participate in the adaptive response through mild stress-related epireprogramming. This reprogramming is thought to prime the cells for heightened defense against a future, more threatening insult by a genotoxic agent.
H2AX, the histone guardian of the genome (Fernandez-Capetillo et al. 2004) may play an important role in the low-LET radiation adaptive response. A role for H2AX phosphorylation has been demonstrated in cell cycle checkpoints, DNA repair, regulated gene recombinant events, and tumor suppression. An attractive model that may relate to radiation adaptation was proposed by Fernandez-Capetillo et al. in which chromatin restructuring mediated by H2AX phosphorylation serves to concentrate DNA repair/signaling factors and/or tether DNA ends. If this process is induced by low-dose radiation and if there is a memory for mounting this process in the event of a later enhanced genomic stress (e.g., high dose of carcinogen), more rapid and more efficient DNA repair might be expected to occur. More rapid DNA repair was found for people residing in regions of high natural background low-LET radiation (Masoomi et al. 2006).
For the Wistar rat study previously discussed where 1–2 mGy of gamma rays (early dose) protected from later accumulating alpha radiation doses that built up over more than a year after delivery of the gamma-ray dose, the gamma-ray exposure may have enhanced DNA repair capacity (epigenetic memory effect) for dealing with subsequent damage induced by alpha radiation. The early gamma-ray dose may have provided additional protection via stimulating the PAM process and immune functions.
High radiation doses and chronic exposure to high levels of cigarette smoke are known to stimulate gene promoter hypermethylation in the lung epithelium. Malignant transformation in the epithelium also occurs after years of chronic, sustained genotoxic stress from agents such as tobacco carcinogens (Damiani et al. 2008). Both genetic and epigenetic changes in tumor suppressor and oncogenes are considered to be important in the occurrence of malignant transformation and the development of lung cancer.
Reprogramming of genes via gene promoter hypermethylation now rivals gene mutation in relation to lung cancer occurrence. More than 60 genes have been identified as being episilenced in lung cancer cells (Belinsky et al. 2006). We proposed that among these genes are adaptive-response genes that are epiactivated by low doses of low-LET radiation and episilenced by high doses of low- and high-LET radiation. We speculate that the propensity of gene silencing increases as a dose of a carcinogenic agent increases. Deleterious episilencing of genes (e.g., through methylation) can occur at the very early stages of lung cancer both in histologic precursors to adenocarcinoma and squamous cell carcinoma and in the bronchial epithelium of heavy, long time smokers (Damiani et al. 2008). We hypothesize that episilencing occurs in adaptive-response genes and are planning studies to validate this hypothesis.
Studies already conducted at our Institute have shown that the methylation of the p16 gene occurs in alveolar hyperplasia and basal cell hyperplasia, which are early precursors to adenocarcinoma and squamous cell carcinoma, respectively (Belinsky et al. 2006). For cancer-free smokers, methylation of the p16 gene has also been found in the bronchial epithelium (Belinsky et al. 2006). Such changes are also likely after chronic exposure to high-level radiation as occurred in underground uranium mines and among workers at the Mayak plutonium production facility in Russia during its early years of operation under the former Soviet Union. We speculate that p16 plays an important role in radiation adaptation via cell cycle regulation (e.g., facilitating DNA repair). Whether low doses of low-LET radiation can stimulate demethylation of the p16 gene has not been examined.
The fact that low doses and dose rates of low-LET radiation can stimulate protective processes that prevent lung cancers associated with alpha irradiation suggests that low-dose-radiation ANP could prevent future cancers in high risk groups (e.g., heavy long-term smokers). The fact that low doses of low-LET radiation stimulate immunity against cancer suggests that such doses could also be used in curing existing cancer (i.e., low-dose cancer therapy). With respect to cancer prevention, factors such as age and genetic characteristics of the irradiated individual would need to be taken into consideration. There is some evidence that the efficiency of radiation ANP against breast cancer increases as age increases above about 50 years of age (Scott et al. 2008). Similar age-related influences may also apply for lung and other cancers. The increased efficiency is thought to possibly relate in part to an increased efficiency of reprogramming of adaptive-response genes as age increases. The increase may relate to the increasing threat posed by rising levels of genomic instability with increasing age. There is now evidence for increased silencing of promoter regions of genes via hypermethylation in the colon with increasing age (Issa et al. 1994; Ahuja et al. 1998; Chan et al. 2002; Herman and Baylin 2003). Regarding low-dose cancer therapy for lung and other cancers, one would have to consider that ANP is age-dependent, transient (for some components), and the different onsets and durations of the protective components (e.g., PAM process, stimulated immunity against cancer) have not been resolved.
The fact that low-rate exposure to gamma rays over an extended period enhances the level of ANP suggests that the protective processes can be repeatedly reactivated. This points to the use of multiple small doses of low-LET radiation in cancer therapy. However, cancer cells resist undergoing apoptosis, implicating a resistance to the PAM process and to killing by components of the immune system. Thus, combined therapy involving multiple low doses of low-LET radiation in combination with multiple low doses of an agent that can sensitize cancer cells to undergo apoptosis might effectively destroy cancer cells.
A recent study by Schwarz et al. (2008) demonstrated that an adapting dose of 30 mGy sensitized cancer cells (a human colorectal cancer cell line) to killing by a subsequent large radiation dose. In contrast, Schwarz et al. (2008) found that normal human cells (fibroblast) were made resistant to cell killing by the subsequent large dose.
Research at our Institute has focused on sensitizing lung cancer cells to undergo apoptosis via novel means such as blockage of nuclear factor-κB and Akt (Wang et al. 2006, 2007, 2008; Chen et al. 2007; Ju et al. 2007). A recent study demonstrated that concurrent blockage of cell survival signaling pathways involving nuclear factor-κB and Akt was quite effective in sensitized lung cancer cells to TNF-induced apoptosis (Wang et al. 2007). This raises the possibility for combination lung cancer therapy that uses low doses of low-LET radiation along with agents that suppress cancer cell survival signaling pathways involving nuclear factor-κB and Akt. An added benefit of low doses of low-LET radiation would be stimulation of immunity against cancer cells and stimulation of the PAM process.
Human exposures to ionizing radiation are managed through an LNT-based system of radiation protection. Effective dose limits govern acceptable levels of radiation exposure. Any amount of radiation adds to the effective dose and therefore supposedly increases cancer risk. However, this system essentially ignores natural radiation protection and in doing so can lead to radiation-phobia associated casualties during radiological emergencies. Based on the material discussed herein and elsewhere (Ketchum 1987; Wolf et al. 1988; Wolff 1989, 1992, 1996; Luckey 1991; Mifune et al. 1992; Hipp and Bauer 1997; Trosko 1998; Redpath et al. 2001; Liu 2003; Sakai et al. 2003; Scott 2004, 2005, 2007, 2008; Jaworowski 2006; Bauer 2007; Day et al. 2007; Feinendegen 2005; Sakai 2006; Scott and Di Palma 2006; Feinendegen et al. 2007; Liu 2007; Mitchel 2007; Portess et al. 2007; ; Strzelczyk et al. 2007; Cohen 2008; Rithidech and Scott 2008; Sanders 2008; Sanders and Scott 2008; Scott et al. 2008; Tubiana 2008), there is a need for a more scientifically valid, updated system of radiation protection that allows for radiation ANP. However, current radiation protection organizations continue to promote the LNT-based system in spite of its problems (Strzelczyk et al. 2007). Thus, it is unlikely that the LNT-based system of radiation protection will be revised any time soon unless pressure to do so comes from better informed members of the world community.
CONCLUSIONS
We humans are not radiation free entities. Each day each of us emit within our bodies millions of beta particles. These emissions are more likely beneficial than harmful, as over time they probably stimulate epiactivation of our adaptive-response genes, making adaptive responses, including DNA repair, more efficient.
The observation that 1–2 mGy of gamma-rays delivered to the lung of the Wistar rat over several months having prevented alpha-radiation-induced lung cancers for absorbed alpha radiation doses up to approximately 600 mGy delivered over more than a year is consistent with the possibility that low doses and dose rates of gamma rays stimulate epireprogramming of adaptive-response genes that persist (memory effect) for an extended period, allowing for rapid up-regulation of adaptive-response genes (e.g., DNA repair genes) at later times when there is a serious threat to the genome.
The rapid increase in lung cancer risk for alpha radiation doses above about 10,000 mGy (10 Gy) combined with 1–2 mGy of gamma rays is consistent with adaptive-response genes (tumor suppressors and other genes) being episilenced via promoter and/or histone methylation and/or histone deacetylation.
Lifetime exposure to residential radon at the Environmental Protection Agency’s action level of 4 pCi L−1 appears to be associated with on average a > 60% reduction in lung cancer cases compared to the cases that would be expected for a radon concentration < 0.7 pCi L−1 (25 Bq m−3).
Low-dose, low-LET-radiation ANP along with other apoptosis-sensitizing agents (targeted to precancerous or cancerous cells) could be exploited in lung cancer prevention and lung cancer therapy; however, considerable research is needed in this area before clinical applications are likely.
ACKNOWLEDGMENTS
This research was supported in part by NIH U01 CA097356, NIH R01 ES008801, U.S. Department of Energy Office for Environmental Safety and Health under cooperative agreement DE-FC03-98EH98028, and by Lovelace Respiratory Research Institute. We are grateful to Vicki Fisher for editorial assistance, to Elaine Marshall for checking radioactivity unit conversions used, and to Dr. Charles Sanders for sharing his data with us. We are also grateful to the journal reviewers for their helpful comments. This paper is being submitted in connection with the first author having participated in the International Dose-Response Society 2008 Conference at the University of Massachusetts, Amherst, MA. However, the contents of the paper go well beyond the research discussed at the conference.
APPENDIX
Estimating HRR Model Parameters Using Bayesian Inference Implemented with MCMC
With the Bayesian approach, model parameters and the biological effects for data of interest (e.g., lung cancer incidence) are considered random variables (Gamerman 1997; Siva, 1998; Miller et al. 2002). If “data” is used to denote the observed biological effects vector and θ is used to denote a model parameters vector, then formal Bayesian inference requires setting up a joint probability distribution P(data,θ) over the quantities in the two vectors (cancer incidences and model parameters). This joint distribution comprises two parts: a prior distribution, P(θ), and a likelihood, P(data|θ). The vertical bar indicates that the data vector is conditional on the parameter vector θ. The joint probability distribution is specified as
| (1) |
Both P(θ) and P(data) are unconditional distributions. When new observations are available, Bayes theorem is used to update the distribution of the parameters P(θ|data) given (i.e., conditional on) the new data. The Bayesian updating relationship can be expressed as (Siva, 1998):
| (2) |
Depending on the complexities of the model being addressed, analytical solutions to Equation 2 may exist. For many exposure scenarios, analytical solutions do not exist for complex stochastic model such as the HRR model. Thus, for this model, the integral is estimated numerically using MCMC (Gilks et al. 1996; Scott and Di Palma 2006).
MCMC methods are a group of sophisticated methods that are often used in complex Bayesian analyses. The most common implementation uses what are called the Metropolis-Hastings algorithm and Gibbs sampling. Markov chains provide a framework under which simulation techniques may be used to explore various distribution properties, generally focused on the posterior distribution. Gibbs sampling is iterative and allows the marginal distribution of model parameters (e.g., slope parameter and profac in the HRR model) to be directly sampled. The Markov chain component of Gibbs sampling provides the framework from which the random samples are generated. However, it is required that the distribution of all parameters, conditional on all other influential parameters and the data fitted, to be specified to allow Monte Carlo sampling.
REFERENCES
- Ahuja N, Li Q, Mohan AL, Baylin SB, Issa JP. Aging and DNA methylation in colorectal mucosa and cancer. Cancer Res. 1998;58:5489–5494. [PubMed] [Google Scholar]
- Bauer G. Low dose radiation and intercellular induction of apoptosis: potential implications for control of oncogenesis. Int J Radiat Biol. 2007;83:873–888. doi: 10.1080/09553000701727523. [DOI] [PubMed] [Google Scholar]
- Belinsky SA. Gene-promoter hypermethylation as a biomarker in lung cancer. Nat Rev Cancer. 2004;4:707–717. doi: 10.1038/nrc1432. [DOI] [PubMed] [Google Scholar]
- Belinsky SA, Klinge DM, Liechty KC, March TH, Kang T, Gilliland FD, Sotnic N, Adamova G, Rusinova G, Telnov V. Plutonium targets the p16 gene for inactivation by promoter hypermethylation in human lung adenocarcinoma. Carcinogenesis. 2004;25:1063–1067. doi: 10.1093/carcin/bgh096. [DOI] [PubMed] [Google Scholar]
- Belinsky SA. Silencing of genes by promoter hypermethylation: key event in rodent and human lung cancer. Carcinogenesis. 2005;26(9):1481–1487. doi: 10.1093/carcin/bgi020. [DOI] [PubMed] [Google Scholar]
- Belinsky SA, Liechty KC, Gentry FD, Wolf HJ, Rogers J, Vu K, Haney J, Kennedy T, Hirsch RF, Miller Y, Franklin WA, Herman JG, Baylin SB, Bunn PA, Byers T. Promoter hypermethylation of multiple genes in sputum precedes lung cancer incidence in a high-risk cohort. Cancer Res. 2006;6:3338–3344. doi: 10.1158/0008-5472.CAN-05-3408. [DOI] [PubMed] [Google Scholar]
- Bird A. DNA methylation patterns and epigenetic memory. Genes Dev. 2002;16:6–21. doi: 10.1101/gad.947102. [DOI] [PubMed] [Google Scholar]
- Boreham D, Dolling J, Somers C, Quinn J, Mitchel REJ. The adaptive response and protection against heritable mutation and fetal malformations. Dose-Response. 2006;4(4):317–326. doi: 10.2203/dose-response.06-104.Boreham. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner DJ, Hall EJ. Computed tomography − an increasing source of radiation exposure. N Engl J Med. 2007;357:2277–2284. doi: 10.1056/NEJMra072149. [DOI] [PubMed] [Google Scholar]
- Camphausen K, Moses MA, Ménard C, Sproull M, Beecken W-D, Folkman J, O’Reilly MS. Radiation abscopal antitumor effect is mediated through p53. Cancer Res. 2003;63:1990–1993. [PubMed] [Google Scholar]
- Chan AO, Broaddus RR, Houlihan PS, Issa JP, Hamilton SR, Rashid A. CpG island methylation in aberrant crypt foci of the colorectum. Am J Pathol. 2002;160:1823–1830. doi: 10.1016/S0002-9440(10)61128-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Wang X, Zhuang J, Zhang L, Lin Y. Induction of death receptor 5 and suppression of survivin contribute to sensitization of TRAIL-induced apoptosis by quercetin in lung cancer cells. Carcinogenesis. 2007;28(10):2114–21. doi: 10.1093/carcin/bgm133. [DOI] [PubMed] [Google Scholar]
- Collis SJ, Schwaninger JM, Ntambi AJ, Keller TW, Nelson WG, Dillehay LE, DeWesse TL. Evasion of early cellular response mechanisms following low level radiation-induced DNA damage. J Biol Chem. 2004;279(48):49624–49632. doi: 10.1074/jbc.M409600200. [DOI] [PubMed] [Google Scholar]
- Cuttler J. Health effects of low level radiation: when will we acknowledge the reality. Dose-Response. 2007;5:292–298. doi: 10.2203/dose-response.07-015.Cuttler. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen BL. The linear no-threshold theory of radiation carcinogenesis should be rejected. J Am Physicians Surg. 2008;13(3):70–76. [Google Scholar]
- Damiani LA, Yingling CM, Leng S, Romo P, Nakamuar J, Belinsky SA. Carcinogen-induced gene promoter hypermethylation is mediated by DNMT1 and causal for transformation of immortalized bronchial epithelial cells. Cancer Res. 2008 doi: 10.1158/0008-5472.CAN-08-1276. (in press) [DOI] [PubMed] [Google Scholar]
- Davis S. CT evaluation for pulmonary metastases in patients with extrathoracic malignancy. Radiology. 1991;180:1–12. doi: 10.1148/radiology.180.1.2052672. [DOI] [PubMed] [Google Scholar]
- Day T, Zeng G, Hooker A, Bhat M, Scott BR, Turner DR, Sykes PJ. Adaptive response for chromosomal inversions in pKZ1 mouse prostate induced by low doses of X radiation delivered after a high dose. Radiat Res. 2007;167:682–692. doi: 10.1667/RR0764.1. [DOI] [PubMed] [Google Scholar]
- Draganić IG, Draganić DD, Adloff JP. Radiation and Radioactivity . CRC Press, Inc; Boca Raton, Florida: 1990. [Google Scholar]
- Feinendegen L. Evidence for beneficial low level radiation effects and radiation hormesis. Br J Radiol. 2005;78:3–7. doi: 10.1259/bjr/63353075. [DOI] [PubMed] [Google Scholar]
- Feinendegen L, Pollycove M, Neumann RD. Whole-body responses to low-level radiation exposure: New concepts in mammalian radiobiology. Experim Hematol. 2007;35:37–46. doi: 10.1016/j.exphem.2007.01.011. [DOI] [PubMed] [Google Scholar]
- Fernandez-Capetillo O, Lee A, Nussenzweig M, Nussenzweig A. H2AX: the histone guardian of the genome. DNA Repair. 2004;3:959–067. doi: 10.1016/j.dnarep.2004.03.024. [DOI] [PubMed] [Google Scholar]
- Gamerman D. Stochastic Simulation for Bayesian Inference. Chapman & Hall; London: 1997. Markov Chain Monte Carlo. [Google Scholar]
- Ghiassi-Nejad M, Zakeri F, Assaei RG, Kariminia A. Long-term immune and cytogenetic effects of high level natural radiation on Ramsar inhabitants in Iran. J Environ Radioact. 2004;74:107–116. doi: 10.1016/j.jenvrad.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Gilks WR, Richardson S, Spiegelhalter DJ, editors. Markov Chain Monte Carlo in Practice. Chapman & Hall; London: 1996. [Google Scholar]
- Hall EJ, Brenner DJ. Cancer risks from diagnostic radiology. Br J Radiol. 2008;81:362–378. doi: 10.1259/bjr/01948454. [DOI] [PubMed] [Google Scholar]
- Herman JG, Baylin SB. Gene silencing in cancer in association with promoter hypermtehylation. N Engl J Med. 2003;341:2042–2054. doi: 10.1056/NEJMra023075. [DOI] [PubMed] [Google Scholar]
- Hipp ML, Bauer G. Intercellular induction of apoptosis in transformed cells does not depend on p53. Oncogene. 1997;17(7):791–797. doi: 10.1038/sj.onc.1201247. [DOI] [PubMed] [Google Scholar]
- Issa JP, Ottaviano YL, Celano P, Hamilton SR, Davidson NE, Baylin SB. Methylation of the estrogen receptor CpG island links ageing and neoplasia in human colon. Nat Genet. 1994;7:536–540. doi: 10.1038/ng0894-536. [DOI] [PubMed] [Google Scholar]
- ISU (Idaho State University) Radioactivity in Nature. 2008. Available at: http://physics.isu.edu/rad-inf/natural.htm.
- Ito A, Kawaguchi Y, Lai CH, Kovacs JJ, Higahimoto Y, Appella E, Yao TP. MDM2-HDAC1-mediated deacetylation of p53 is required for its degradation. EMBO J. 2002;21(22):6236–6245. doi: 10.1093/emboj/cdf616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaworowski Z. Chernobyl: the fear of the unknown. Atomic Insight Guest Column. 2006. Available at http://www.atomicinsights.com/Guests/AGC_05-02-06.html.
- Jemal A, Thomas A, Murray T, Thun M. Cancer statistics. CA Cancer J Clin. 2002;52:23–47. doi: 10.3322/canjclin.52.1.23. [DOI] [PubMed] [Google Scholar]
- Ju W, Wang X, Chen W, Shi H, Belinsky SA, Lin Y. A critical role of luteolin-induced reactive oxygen species in blockage of tumor necrosis factor-activated nuclear factor-kB pathway and sensitization of apoptosis in lung cancer cells. Mol Pharmacol. 2007;71:1381–1388. doi: 10.1124/mol.106.032185. [DOI] [PubMed] [Google Scholar]
- Karpinets TV, Foy BD. Tumorigenesis: the adaptation of mammalian cells to sustained stress environment by epigenetic alterations and succeeding matched mutations. Carcinogenesis. 2005;26(8):1323–1334. doi: 10.1093/carcin/bgi079. [DOI] [PubMed] [Google Scholar]
- Ketchum L. Lessons of Chernobyl: SNM members try to decontaminate world threatened by fallout. J Nucl Med. 1987;28(6):933–942. [PubMed] [Google Scholar]
- Knudson AG. Two genetic hits (more or less) to cancer. Nat Rev Cancer. 2001;1:157–162. doi: 10.1038/35101031. [DOI] [PubMed] [Google Scholar]
- Koturbash I, Rugo RE, Hendricks CA, Loree J, Thibault B, Kutanzi K, Porgribny I, Yanch JC, Endelward BP, Kovalchuk O. Irradiation induced DNA damage and modulates epigenetic effectors in distant bystander tissue in vivo. Oncogene. 2006;25:4267–4275. doi: 10.1038/sj.onc.1209467. [DOI] [PubMed] [Google Scholar]
- Lenski RE, Barrick JE, Ofria C. Balancing robustness and evolvability. PLos Biol. 2006;4(12):2190–2192. doi: 10.1371/journal.pbio.0040428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S. Nonlinear dose-response relationship in the immune system following exposure to ionizing radiation: mechanisms and implications. Nonlin Biol Toxicol Med. 2003;1(1):71–92. doi: 10.1080/15401420390844483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S. Cancer control related to stimulation of immunity by low-dose radiation. Dose-Response. 2007;5:39–47. doi: 10.2203/dose-response.06-108.Liu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leng S, Stidley CA, Willink R, Bernauer A, Do K, Picchi MA, Sheng X, Franco AM, Berg DVD, Gilliand FD, Zima C, Crowell RE, Belinsky SA. Double-strand break damage and associated DNA repair genes predispose smokers to gene methylation. Cancer Res. 2008;68(8):1–8. doi: 10.1158/0008-5472.CAN-07-6344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luckey T. Radiation Hormesis . CRC Press; Baca Raton, Ann Arbor, Boston, London: 1991. [Google Scholar]
- Mancuso M, Pasquali E, Leonardi S, Tanori M, Rebessi S, Di Majo V, Pazzaglia S, Toni MP, Pimpinella M, Covelli V, Saran A. Oncogenic bystander radiation effects in Patched heterozygous mouse cerebellum. PNAS. 2008;105(34):12445–12450. doi: 10.1073/pnas.0804186105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masoomi JR, Mohammadi S, Amino M, Ghiassi-Nejad M. High background radiation areas of Ramsar in Iran: evaluation of DNA damage by alkaline single cell gel electrophoresis (SCGE) J Environ Radioact. 2006;86:176–186. doi: 10.1016/j.jenvrad.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Mifune M, Sobue T, Arimoto H, Komoto Y, Kondo S, Tanooka H. Cancer mortality survey in a spa area (Misasa, Japan) with a high radon background. Jpn J Cancer Res. 1992;83:1–5. doi: 10.1111/j.1349-7006.1992.tb02342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G, Martz HF, Little TT, Guilmette RA. Bayesian internal dosimetry calculations using Markov chain Monte Carlo. Radiat Protect Dosim. 2002;98:191–197. doi: 10.1093/oxfordjournals.rpd.a006709. [DOI] [PubMed] [Google Scholar]
- Mitchel R. Low doses of radiation reduce risk in vivo. Dose-Response. 2007;5:1–10. doi: 10.2203/dose-response.06-109.Mitchel. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NRC (National Research Council) Health Risks from Exposure to Low Levels of Ionizing Radiation Report BEIR-VII, Phase 2. The National Academies Press; 2006. Available at: www.nap.edu. [PubMed] [Google Scholar]
- Parkin DM, Bray FI, Devesa SS. Cancer burden in the year 2000. The global picture. Eur J Cancer. 2001;37:S4–S66. doi: 10.1016/s0959-8049(01)00267-2. [DOI] [PubMed] [Google Scholar]
- Portess D, Bauer G, Hill M, O’Neill P. Low-dose irradiation of nontransformed cells stimulates the selective removal of precancerous cells via intercellular induction of apoptosis. Cancer Res. 2007;67(3):1246–1253. doi: 10.1158/0008-5472.CAN-06-2985. [DOI] [PubMed] [Google Scholar]
- Redpath J, Liang D, Taylor T, James C, Christie E, Elmore E. The shape of the dose-response curve for radiation-induced neoplastic transformation in vitro: evidence for an adaptive response against neoplastic transformation at low doses of low-LET radiation. Radiat Res. 2001;156:700–707. doi: 10.1667/0033-7587(2001)156[0700:tsotdr]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Reik W. Stability and flexibility of epigenetic gene regulation in mammalian development. Nature. 2007;44:425–432. doi: 10.1038/nature05918. [DOI] [PubMed] [Google Scholar]
- Rithidech K, Scott BR. Evidence for radiation hormesis after in vitro exposure of human lymphocytes to low doses of ionizing radiation. Dose-Response. 2008;6:252–271. doi: 10.2203/dose-response.07-024.Rithidech. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothkamm K, Löbrich M. Evidence for a lack of DNA double-strand break repair in human cells exposed to very low x-ray doses. Proc Natl Acad Sci USA. 2003;100(9):5057–5062. doi: 10.1073/pnas.0830918100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo VEA, Martienssen RA, Riggs AD, editors. Epigenetic mechanisms of gene regulation. Cold Spring Harbor Laboratory Press; Plainview, NY: 1996. [Google Scholar]
- Sakai K, Hoshi Y, Nomura T, Oda T, Iwasaki T, Fujita K, Yamada T, Tanooka H. Suppression of carcinogenic process in mice by chronic low dose rate gamma-irradiation. Int J Low Radiat. 2003;1(1):142–146. [Google Scholar]
- Sakai K. Enhancement of bio-protective functions by low dose/dose-rate radiation. Dose-Response. 2006;4(4):327–332. doi: 10.2203/dose-response.06-115.Sakai. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders CL. Inhibition of 239Pu alpha radiation-induced pulmonary carcinogenesis by low dose 169Yb gamma radiation. J Nuclear Soc Thailand. 2008 (in press) [Google Scholar]
- Sanders CL, Scott BR. Smoking and hormesis as confounding factors in radiation pulmonary carcinogenesis. Dose-Response. 2008;5:53–79. doi: 10.2203/dose-response.06-003.Sanders. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz SB, Schaffer PM, Kulka U, Ertl-Wagner B, Hell R, Schaffer M. The effect of radio-adaptive doses on HT29 and GM637 cells. Radiat Oncol. 2008;3(12):1–6. doi: 10.1186/1748-717X-3-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott BR. A biological-based model that links genomic instability, bystander effects, and adaptive response. Mutat Res. 2004;568(1):129–143. doi: 10.1016/j.mrfmmm.2004.06.051. [DOI] [PubMed] [Google Scholar]
- Scott BR. Stochastic thresholds: A novel explanation of nonlinear dose-response relationships. Dose-Response. 2005;3:547–567. doi: 10.2203/dose-response.003.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott B, Di Palma J. Sparsely ionizing diagnostic and natural background radiations are likely preventing cancer and other genomic-instability-associated diseases. Dose-Response. 2006;5:230–255. doi: 10.2203/dose-response.06-002.Scott. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott BR. Natural background radiation-induced apoptosis and the maintenance of mammalian life on earth. In: Vinter LC, editor. New Cell Apoptosis Research. Nova Science Publishers, Inc; Hauppage, NY: 2007. pp. 1–35. [Google Scholar]
- Scott BR, Haque M, Di Palma J. Biological basis for radiation hormesis in mammalian cellular communities. Int J Low Radiat. 2007;4(1):1–16. [Google Scholar]
- Scott BR. Low-dose-radiation-stimulated natural chemical and biological protection against lung cancer. Dose-Response. 2008;6:299–318. doi: 10.2203/dose-response.07-025.Scott. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott BR, Sanders CL, Mitchel REJ, Boreham DR. CT scans may reduce rather than increase the risk of cancer. J Am Physicians and Surg. 2008;13(1):8–11. [Google Scholar]
- Siva DS. Data Analysis, a Bayesian Tutorial . Oxford University Press; New York: 1998. [Google Scholar]
- Spiegelhalter D, Thomas A, Best N, Lunn D, editors. WinBUGS Version 141, Users Manual . MRC Biostatistics Unit; Cambridge, UK: 2003. [Google Scholar]
- Strzelczyk I, Potter W, Zdrojewicz Z. Rad-by-rad (bit-by-bit): Triumph of evidence over activities fostering fear of radiogenic cancer at low doses. Dose-Response. 2007;5:275–283. doi: 10.2203/dose-response.07-021.Strzelczyk. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas DC, McNeill KG, Dougherty C. Estimates of lifetime lung-cancer risks resulting from Rn progeny exposures. Health Phys. 1985;49:825–846. doi: 10.1097/00004032-198511000-00014. [DOI] [PubMed] [Google Scholar]
- Thompson RE, Nelson DF, Popkin JH, Popkin A. Case-control study of lung cancer risk from residential radon exposure in Worchester County, Massachusetts. Health Phys. 2008;94(3):228–241. doi: 10.1097/01.HP.0000288561.53790.5f. [DOI] [PubMed] [Google Scholar]
- Trosko JE. Hierarchical and cybernetic nature of biologic systems and their relevance to homeostatic adaptation to low-level exposure to oxidative stress-inducing agents. Environmental Health Perspect. 1998;106(Suppl 1):331–339. doi: 10.1289/ehp.98106s1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tubiana M. Dose-effect relationship and estimation of the carcinogenic effects of low doses of ionizing radiation: The joint report of the Académie des Sciences (Paris) and of The Académie Nationale de Médicine. Int Radiat Oncol Biol Phys. 2005;63(2):317–319. doi: 10.1016/j.ijrobp.2005.06.013. [DOI] [PubMed] [Google Scholar]
- Tubiana M, Aurengo A, Averbeck D, Bonin A, Le Guen B, Masse R, Monier R, Valleron A-J, de Vathaire F. Dose-effect relationships and estimation of the carcinogenic effects of low doses of ionizing radiation. Académie des Sciences – Académie Nationale de Médicine Report; Paris, France. 2005. [Google Scholar]
- Tubiana M. The 2007 Marie Curie prize: the linear no threshold relationship and advance in our understanding of carcinogenesis. Int J Low Radiat. 2008;5(3) in press. [Google Scholar]
- USEPA (U.S. Environmental Protection Agency) Home Buyers and Sellers Guide to Radon. 1993. Report 402-R-93-003. Available at http://www.radon-levels.com.
- Wang X, Ju W, Renouard J, Aden J, Belinaky A, Lin Y. 17-allylamino-17-demethoxygel-danamycin synergistically potentiates tumor necrosis factor-induced lung cancer cell death by blocking the nuclear factor-κB pathway. Cancer Res. 2006;66:1089–1095. doi: 10.1158/0008-5472.CAN-05-2698. [DOI] [PubMed] [Google Scholar]
- Wang X, Chen W, Lin Y. Sensitization of TNF-induced cytotoxicity in lung cancer cells by concurrent suppression of the NF-κB and Akt pathways. Biochem Biophys Res Commun. 2007;335:807–812. doi: 10.1016/j.bbrc.2007.02.030. [DOI] [PubMed] [Google Scholar]
- Wang X, Chen W, Zeng W, Bai L, Tesfaigzi Y, Belinsky SA, Lin Y. Akt-mediated eminent expression of c-FLIP and Mcl-1 confers acquired resistance to TRAIL-induced cytotoxicity to lung cancer cells. Molecular Cancer Therapeutics. 2008;7:1156–1163. doi: 10.1158/1535-7163.MCT-07-2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilder J, Yuan D. Regulation of IFN-gamma mRNA production in murine natural killer cells. Int Immunol. 1995;7:575–582. doi: 10.1093/intimm/7.4.575. [DOI] [PubMed] [Google Scholar]
- Wilder JA, Koh CY, Yuan D. The Role of NK cells during in vivo antigen-specific antibody responses. J Immunol. 1996;156:146–152. [PubMed] [Google Scholar]
- Wolff S, Afzal V, Wiencke JK, Olivieri G, Michaeli A. Human lymphocytes exposed to low doses of ionizing radiation become refractory to high doses of radiation as well as to chemical mutagens that induced double-strand breaks in DNA. Int J Radiat Biol. 1988;53(1):39–4. doi: 10.1080/09553008814550401. [DOI] [PubMed] [Google Scholar]
- Wolff S. Are radiation-induced effects hormetic? Science. 1989;245:575–621. doi: 10.1126/science.2762808. [DOI] [PubMed] [Google Scholar]
- Wolff S. Failla memorial lecture. Is radiation all bad? The search for adaptation. Radiat Res. 1992;131:117–123. [PubMed] [Google Scholar]
- Wolff S. Aspects of the adaptive response to very low doses of radiation and other agents. Mutat Res. 1996;358:135–142. doi: 10.1016/s0027-5107(96)00114-5. [DOI] [PubMed] [Google Scholar]
- Yuan D, Wilder JA, Dang T, Bennett T, Kumar V. Activation of B lymphocytes by NK cells. Int Immunol. 1992;4:1373–1380. doi: 10.1093/intimm/4.12.1373. [DOI] [PubMed] [Google Scholar]