Abstract
Some people in different parts of Iran use burned mantles as a wound healing medicine. To perform surface area measurement, twenty rats were divided randomly into two groups of 10 animals each. The 1st group received topical burned radioactive lantern mantle powder at 1st-3rd day after making excision wounds. The 2nd group received non-radioactive lantern mantle powder. For histological study, 36 male rats randomly divided into two groups of 18 animals each. Full thickness excision wound (314±31.4 mm2) was made on the dorsal neck in all animals after inducing general anesthesia. For the first 3 days, cases received topical application of the radioactive lantern mantle powder. Finally, to measure the tensile strength, an incision was made on the dorsal neck of the rats. Surface area measurement of the wounds showed a progressive surface reduction in both groups. Histological study showed a significant statistically difference between cases and controls with respect to fibrinoid necrosis and neutrophilic exudate at the days 3 and 14. Considering the existence of granulation tissue, a significant difference was observed between case and control groups at days 3 and 7. Tensile strength study showed no significant difference between the cases and controls until 30 days after excision.
Keywords: Lantern mantle, wound healing, radioactive, Thorium
INTRODUCTION
Despite the scientific basis of medicine, WH Auden, stated “Healing is not a science but the intuitive art of wooing nature” (Roth 1990). Body’s natural process of regenerating dermal and epidermal tissue is usually termed wound healing. After wounding, a set of events takes place to repair the damage. These events can be categorized into 3 separate steps of the inflammatory, proliferative, and remodeling phases. In the inflammatory phase, bacteria and debris are phagocytized and removed and factors are released that lead to the migration and division of cells involved in the proliferative phase (Stadelmann et al. 1998, Iba et al. 2004, Quinn 1998). The proliferative phase is characterized by angiogenesis, collagen deposition, granulation tissue formation, epithelialization, and wound contraction (Midwood et al. 2004). In fact wound healing is a natural restorative response to tissue injury. Healing is the interaction of a complex cascade of cellular events that generates resurfacing, reconstitution, and restoration of the tensile strength of injured skin (Romo and Pearson 2008). To enhance wound healing, a wide variety of methods have been used such as the topical application of herbal medicine (Gupta et al. 2006, Gupta et al. 2005), low level laser therapy (Al-Watban et al. 2007), natural honey (Mphande et al. 2007), ultrasound (Harvey et al. 1975, Dyson and Smalley 1983), ultraviolet light (Nordback et al. 1990), superficial heating (Khan et al. 2004), electrical stimulation (Lawson and Petrofsky 2007, Bayat et al. 2006), pulsed electromagnetic fields (Houghton and Campbell 1999) and fibroblast growth factor (Zheng et al. 1994). In spite of these modalities, the main approach is still the prevention of infection using antibacterial and antiseptic agents (Burleson and Eiseman 1973, Geronemus et al. 1979). Regarding ionizing radiation, it has been shown that irradiation of skin caused slower healing of open wounds (Bernstein et al. 1994).
In different parts of Iran, some educated people use radioactive lantern mantle powder as a therapeutic agent for enhancing wound healing without being aware of its possible dangers. As far as we know, this is the first research which evaluates the stimulatory effects of topical application of radioactive lantern mantel powder on the wound healing. Some lantern mantles which are commonly used for camping contain different levels of thorium compounds (Mohammadi and Mehdizadeh 1983). Thorium is used in mantles to produce incandescence when lantern fuel is burned in the mantle. Although only thorium is initially used in the mantles, the daughters of thorium build up, and when the mantle is used significant quantities of these daughters exist. Some of these daughters are released when the lantern fuel is burned in the mantle. (Luetzelschwab and Googins 1984). Recently, in some developed countries the use of thorium-free mantles has become popular due to the risks associated with the use of a radioactive heavy metal (Poljanc et al. 2007). Thorium oxide is a known human carcinogen. In Australia, in November 1992, the National Health and Medical Research Council (NHMRC) recommended that lantern mantles containing thorium should be withdrawn from sale over time and that in the meantime, packets containing these lantern mantles should carry a warning (ARPANSA 2006).
All living organisms evolved and exist in a sea of ionizing radiation, much of which is internal. Since the beginning of the discovery of ionizing radiation, there have been two different views on detrimental effects of low doses; the first view states that even low dose radiations pose a danger and there is no threshold dose for the side effects. The other view not only believes in the existence of a threshold dose, but also emphasizes on the beneficial or stimulatory effects of low levels of ionizing radiation based on phenomena such as radiation hormesis and adaptive response (Upton 2001, Prekeges 2003, Feinendegen 2005, Kant et al. 2003).
Due to lack of published reports on the stimulatory effects of topical application of radioactive lantern mantle powder on wound healing, this research was conducted.
MATERIALS & METHODS
Laboratory Animals
In this experimental study 20 Albino N Mary rats which were kept in the Animal laboratory center of Rafsanjan University of Medical Sciences (RUMS) were randomly divided into two groups of 10 (experimental and control). The mean weight of the animals was 200 grams (ranged 190–210 g). All the animals were kept in an identical standard condition. In order to prevent any bias, all the animals were given a code and only after the experiment was done, decoding was performed.
Area Measurement
To perform surface area measurement, twenty rats were divided randomly into two groups of 10 animals each. After inducing general anesthesia, full thickness excision wound was made on the dorsal neck in all animals. The 1st group received topical burned radioactive lantern mantle (Butterfly, China) powder at 1st–3rd day after making excision wounds. The activity of each mantle was about 0.8 kBq. The 2nd group received non-radioactive lantern mantle powder at the same days. Accurate blind surface measurement of the wounds by transparency tracing was used for assessment of the wound healing at 1st, 3rd, 7th, 10th and 15th days after making wounds. The following equation was used for determining the percentage of wound.
In this equation, dayx was the day of wound area measurement (days 3, 5, 7, 10, 15 after wounding) and day0 was the day wounding had done. On the other hand, the percentage of healing was measured as:
Relevant statistical tests (Student-t, and ANOVA) were performed using SPSS (version 15) at p<0.05 as the significant level.
Histological Study
For histological study, 36 male rats randomly divided into two groups of 18 animals each. Full thickness excision wound (314±31.4 mm2) was made on the dorsal neck in all animals after inducing general anesthesia. For the first 3 days, cases received topical application of the radioactive lantern mantle powder while controls received non-radioactive lantern mantle powder. Three, seven and fourteen days after wounding, 6 rats were chosen by random in each group for wound sampling. The four criteria used for histological investigation were 1) fibrinoid necrosis and neutrophilic exudate, 2) granulation tissue, 3) superficial epithelization and 4) collagen fiber synthesis. The minimum and maximum scores for each criterion were 1 (or minus) and 5 (or 4+) respectively. Data analysis was performed by using Mann-Whitney statistical test at p<0.05 as the significant level.
Tensile Strength
Thirty six rats were randomly divided into two groups of case and controls (each consisted of 18 animals). For the first 3 days, cases received topical application of the radioactive lantern mantle powder while controls received non-radioactive lantern mantle powder. To measure the tensile strength, a full thickness incision (20 mm length) was made on the dorsal part of the rats. Samples were obtained at 14th, 21st and 30th days after making incisions. Tensile strength was measured by using a Tensiometer. Student’s t-test and ANOVA were used for data analysis at p<0.05 as the significant level.
RESULTS
Area Measurement
Surface area measurement of the wounds showed a progressive surface reduction in both groups (Table 1). However, for thorium treated group, the rate of recovery was significantly enhanced compared to that of the control group. Although the wound area in the thorium group was not significantly different from that of the control group at the 3rd and 5th days after wounding, a statistically significant difference was observed between the thorium and the control groups at the day7, day10 and day 15. The mean wound surface in thorium and control groups were 150±15.87 and 186±12.68 mm2 at day7 (P<0.001), 93±15.97 and 134±14.19 mm2 at day 10 (P<0.001), 1±0.41 and 9±2.04 mm2 at day15 after wounding, respectively (P<0.01). Healing percentages in control and test groups at different times are presented in Table 2.
TABLE 1.
Wound area changes in control and test groups at different times.
| Time of Investigation | Wound Area in Controls [Vehicle Group] (mm2) | Wound Area in Cases (mm2) | P-Value |
|---|---|---|---|
| Day 0 (wounding) | 314 ± 31.40 | 314 ± 31.40 | NA |
| 3 Days after wounding | 258 ± 20.65 | 255 ± 17.20 | NS |
| 5 Days after wounding | 224 ± 11.38 | 218 ± 8.95 | NS |
| 7 Days after wounding | 186 ± 12.68 | 150 ± 15.87 | P < 0.001 |
| 10 Days after wounding | 134 ± 14.19 | 93 ± 15.97 | P < 0.001 |
| 15 Days after wounding | 9 ± 5.76 | 1 ± 1.29 | P < 0.01 |
Mean ± SD NA: Not applicable NS: Non Significant
TABLE 2.
Healing percentages in control and test groups at different times.
| Time of Investigation | Healing Percentage in Controls [Vehicle Group] | Healing Percentage in Cases [Radioactive Group] |
|---|---|---|
| Day 0 (wounding) | 0 | 0 |
| 3 Days after wounding | 17.8 | 18.8 |
| 5 Days after wounding | 28.6 | 30.5 |
| 7 Days after wounding | 40.6 | 52.2 |
| 10 Days after wounding | 57.3 | 70.4 |
| 15 Days after wounding | 97.3 | 99.5 |
Histological Findings
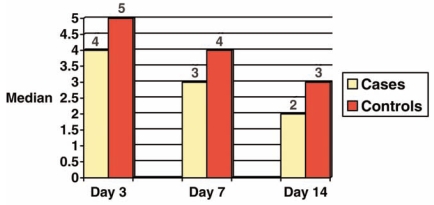
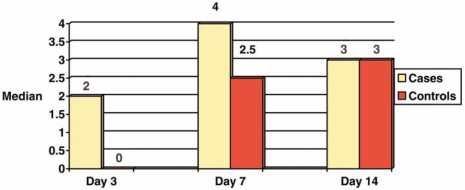
On the other hand, our histological study showed a significant statistically difference between cases and controls with respect to fibrinoid necrosis and neutrophilic exudate at the days 3 and 14 (Figure 1). Considering the existence of granulation tissue, a significant difference was observed between case and control groups at days 3 and 7. No difference was observed in superficial epithelization and collagen fiber synthesis at all days (Figure 2).
FIGURE 1.
Median score of fibrinoid necrosis and neutrophilic exudate at days 3, 7 and 14 in control and case groups.
FIGURE 2.
Median score of granulation tissue at days 3, 7 and 14 in control and case groups.
Tensile Strength
Finally, tensile strength study showed no statistically significant difference between the cases and controls at the days 14 and 21 (Table 3). However, a significant difference was observed at day 30 (P<0.001). Analysis of variance could not detect any significant difference among the means of tensile strengths at days 14, 21 and 30 in cases (Table 4). However, a statistically significant difference was observed among the means of tensile strengths in controls.
TABLE 3.
Tensile strength in case and control groups at days 14, 21 and 30
| Group | No | Tensile Strength (N/mm2) (Mean ±SD) | P-Value (t-test) | |
|---|---|---|---|---|
| Day 14 | Cases | 6 | 2.62±2.29 | NS |
| Controls | 6 | 4.26±2.82 | ||
| Day 21 | Cases | 6 | 2.23±1.07 | NS |
| Controls | 6 | 2.07±0.62 | ||
| Day 30 | Cases | 4 | 4.39±2.58 | P<0.001 |
| Controls | 6 | 11.52±1.99 |
NS: Non Significant
TABLE 4.
Comparison of the means of tensile strengths at days 14, 21 and 30 in case and control groups
| Tensile Strength (N/mm2) (Mean ±SD)
|
||
|---|---|---|
| Cases | Controls | |
| Day 14 | 2.62±2.29 (n=6) | 4.26±2.82 (n=6) |
| Day 21 | 2.23±1.07 (n=6) | 2.07±0.62 (n=6) |
| Day 30 | 4.39±2.58 (n=4) | 11.52±1.99 (n=6) |
| P-Value (ANOVA) | NS | P<0.001 |
NS: Non Significant
DISCUSSION
The results of the current study clearly indicate that topical use of radioactive mantle powder could accelerate the healing process of the wound in rats. As Table 1 indicates, surface area measurement of the wounds at the day 7, day 10 and day 15 showed a statistically significant difference between the case and control groups (P<0.01, P<0.001 and P<0.01, respectively). Based on these data, it could be concluded that as the radioactive thorium is absorbed through the surface area of the wound and when a threshold of radiation is reached, the process of wound healing is accelerated. These results are consistent with the results of numerous studies including the studies of Mortazavi and his colleagues (Ghiasi-nejad et al. 2002, Mortazavi et al. 2003, Mortazavi 2004, Mortazavi et al. 2005a, Mortazavi et al. 2005b, Mortazavi et al. 2006) who showed the appearance of adaptive response in the people living in high background radiation areas above a threshold of dose.
Identification of the bio-positive effects of low doses of ionizing radiation may change the public perception of the occupational, medical and even environmental dangers of radiation. Considering the possible mechanisms of the effects of radioactive powder on the wound healing, we could mention the important role of low dose ionizing radiations in the stimulation of the body’s defense mechanisms. Body’s defense system, especially granulocytes and macrophages, has a considerable role in the healing of the acute wounds. On the other hand, the factors which could cause the weakness of the defense system could also show their final effect as a disturbance in the wound healing. In some cases this disturbance is so strong that it causes the prolonging of the healing and takes it into the persistent phase (Davidson 2001). The reaction of the defense system to the ionizing radiation depends on some determining factors such as radiation dose and the dose rate (Liu and Bai 2000, Liu et al. 2000). On the other hand recently the stimulating effect of ionizing radiation on the defense system has become an important measurement in the evaluation and identification of the bio positive effects of low dose radiation (Liu and Xie 2000). It seems that low levels of radioactive materials, by inducing a kind of stimulation on the defense system, could accelerate the healing process. Results obtained in our study are in contrast with those reported by Bernstein et al. (1994) who showed that irradiation of skin caused slower healing of open wounds. As hormetic effects can only be observed within a narrow range of dose and dose rates, this difference can be clearly explained.
Although still we do not know the entire mechanisms of radiation hormesis, the following theories may explain this process:
DNA repair (Molecular level)
According to this theory, low doses of ionizing radiation induce the production of special proteins, that are involved in DNA repair processes (Ikushima 1996). Studies using two dimensional gel electrophoresis indicated new proteins in cells irradiated with low doses of radiation. Also, it was further shown that cycloheximide, a protein synthesis inhibitor blocks this hormetic effect. The function and importance of these radiation induced proteins is still unknown. Also it was foud that inhibitors of poly ADP-ribose polymerase, an enzyme implicated in DNA strand break rejoining could prevent the induction of adaptive response (for a review see Wolff 1998). More recent In vivo experiments indicated that low doses of ionizing radiation help protect against radiation-induced myeloid leukemia (Mitchel et al., 1999) and spontaneous cancer in mice (Mitchel et al., 2003). These phenomena may be related to adaptations in DNA repair processes.
Free radical detoxification (Molecular level)
In 1987 Feinendengen and his colleagues indicated that low doses of ionizing radiation cause a temporary inhibition in DNA synthesis (the maximum inhibition at 5 hours after irradiation). This temporary inhibition of DNA synthesis would provide a longer time for irradiated cells to recover (Feinendengen et al. 1987). This inhibition also may induce the production of free radical scavengers, so irradiated cells would be more resistant to any further exposures.
Stimulation of immune system (Cellular level)
Despite the fact that high doses of ionizing radiation are immunosupressive, many studies have indicated that low doses radiation may stimulate the function of the immune system. In 1909 Russ first showed that mice treated with low-level radiation were more resistant against bacterial disease (Russ 1909). Later in 1982 Luckey published a large collection of references supporting immunostimulatory effects of low doses of ionizing radiation (Luckey 1982).
Radiation Protection Aspects
Our current radiation protection policy is based on linear extrapolation from the dose-response data of high doses of ionizing radiation. According to the results of many worldwide studies, this hypothetical assumption is not compatible with observed health effects of low levels of radiation. Obviously LNT and current radiation protection regulations exaggerate the risk of low level ionizing radiation (in the range of 1–50 cGy) and cause radiophobia (Yalow 1990).
CONCLUSION
This study indicated that poorly educated people who have used lantern mantle powder, unaware of its possible dangers, have known the healing power of this radioactive material and in times of need have used it throughout the years. The results of this study should not be considered as a permit for using lantern mantle powders as a wound healing agent. In medicine all the decisions for the use of any chemical or physical therapeutic method is always based on the evaluation of the possible risks and benefits. Considering the known risk of alpha emitting radioactive materials (internal radiation), this study was not concerned with evaluation of possible dangers of such an internal irradiation. In this light further studies are needed to clarify whether low level radioactivity could safely be used as a therapeutic agent for enhancing the process of wound healing.
REFERENCES
- ARPANSA (Australian Radiation Protection and Nuclear Safety Agency) Radioactivity in Lantern Mantles. Fact Sheet. 2006:18. [Google Scholar]
- Al-Watban FA, Zhang XY, Andres BL. Low-level laser therapy enhances wound healing in diabetic rats: a comparison of different lasers. Photomed Laser Surg. 2007;25(2):72–7. doi: 10.1089/pho.2006.1094. [DOI] [PubMed] [Google Scholar]
- Bayat M, Asgari-Moghadam Z, Maroufi M, Rezaie FS, Bayat M, Rakhshan M. Experimental wound healing using microamperage electrical stimulation in rabbits. J Rehabil Res Dev. 2006;43(2):219–26. doi: 10.1682/jrrd.2005.05.0089. [DOI] [PubMed] [Google Scholar]
- Bernstein EF, Harisiadis L, Salomon GD, Harrington F, Mitchell JB, Uitto J, Glatstein E, Russo A. Healing impairment of open wounds by skin irradiation. J Dermatol Surg Oncol. 1994;20(11):757–60. doi: 10.1111/j.1524-4725.1994.tb03200.x. [DOI] [PubMed] [Google Scholar]
- Burleson R, Eiseman B. Effect of skin dressing and topical antibiotics on healing of partial thickness skin wounds in rats. Surg. Gynecol Obstet. 1973;136:958–960. [PubMed] [Google Scholar]
- Davidson JM. Experimental Animal Wound Models. Wounds. 2001;13(1):9–23. [Google Scholar]
- Dyson M, Smalley D. Effets of ultrasound on wound contraction. In: Millner R, Corket U, editors. Ultrasound Interactions in Biology and Medicine. Plenum; New York: 1983. p. 151 . [Google Scholar]
- Feinendegen LE, Muhlensiepen H, Bond VP, Sondhaus CA. Intracellular stimulation of biochemical control mechanisms by low-dose, low-LET irradiation. Health Phys. 1987;52(5):663–669. doi: 10.1097/00004032-198705000-00020. [DOI] [PubMed] [Google Scholar]
- Feinendegen LE. Evidence for beneficial low level radiation effects and radiation hormesis. Br J Radiol. 2005;78(925):3–7. doi: 10.1259/bjr/63353075. [DOI] [PubMed] [Google Scholar]
- Geronemus RG, Mertz PM, Eaglstein WH. Wound healing: The effect of topical antimicrobial agents. Arch. Dermatol. 1979;115:1311–1314. doi: 10.1001/archderm.115.11.1311. [DOI] [PubMed] [Google Scholar]
- Ghiassi-nejad M, Mortazavi SMJ, Cameron JR, Niroomand-rad A, Karam PA. Very High Background Radiation Areas of Ramsar, Iran: Preliminary Biological Studies. Health Physics. 2002;82 (1):87–9. doi: 10.1097/00004032-200201000-00011. [DOI] [PubMed] [Google Scholar]
- Gupta A, Kumar R, Pal K, Banerjee PK, Sawhney RC. A preclinical study of the effects of seabuckthorn (Hippophae rhamnoides L.) leaf extract on cutaneous wound healing in albino rats. Int J Low Extrem Wounds. 2005;4(2):88–92. doi: 10.1177/1534734605277401. [DOI] [PubMed] [Google Scholar]
- Gupta A, Kumar R, Pal K, Singh V, Banerjee PK, Sawhney RC. Influence of sea buckthorn (Hippophae rhamnoides L.) flavone on dermal wound healing in rats. Mol Cell Biochem. 2006;290(1–2):193–8. doi: 10.1007/s11010-006-9187-6. [DOI] [PubMed] [Google Scholar]
- Harvey W, Dyson M, Pond JB, Grahame R. The in vitro stimulation of protein synthesis in human fibroblasts by therapeutic levels of ultrasound: proceedings of the second European Congress on Ultrasonics in Medicine. Excerta Medica International Congress Series 363; 1975. p. 10. [Google Scholar]
- Houghton PE, Campbell KE. Choosing an adjunctive therapy for the treatment of chronic wounds. Ostomy Wound Management. 1999;45:43–52. [PubMed] [Google Scholar]
- Iba Y, Shibata A, Kato M, Masukawa T. Possible involvement of mast cells in collagen remodeling in the late phase of cutaneous wound healing in mice. International Immunopharmacology. 2004;4(14):1873–1880. doi: 10.1016/j.intimp.2004.08.009. [DOI] [PubMed] [Google Scholar]
- Ikushima T. Radioadaptive response: Efficient repair of radiation-induced DNA damage in adapted cells. Mutat. Res. 1996;358:193–198. doi: 10.1016/s0027-5107(96)00120-0. [DOI] [PubMed] [Google Scholar]
- Kant K, Chauhan RP, Sharma GS, Chakarvarti SK. Hormesis in humans exposed to low-level ionising radiation. Int J of Low Radiation. 2003;1(1):76–87. [Google Scholar]
- Khan AA, Banwell PE, Bakker MC, Gillespie PG, McGrouther DA, Roberts AH. Topical radiant heating in wound healing: an experimental study in a donor site wound model. Int Wound J. 2004;1(4):233–40. doi: 10.1111/j.1742-4801.2004.00065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson D, Petrofsky JS. A randomized control study on the effect of biphasic electrical stimulation in a warm room on skin blood flow and healing rates in chronic wounds of patients with and without diabetes. Med Sci Monit. 2007;13(6):CR258–CR263. [PubMed] [Google Scholar]
- Liu SZ, Bai O, Chen D, Ye F. Genes and protein molecules involved in the cellular activation induced by low dose radiation. Radia. Res Radiat Proc. 2000;18:175–186. [Google Scholar]
- Liu SZ, Bai O. On mechanistic studies of immune responses following low dose ionizing radiation. In: Yamada T, Mothersill C, Michael BD, Potten SC, editors. International Meeting on Biological Effects of Low Dose Radiation; Cork, Ireland. 25–26 July 1999; Amsterdam: Elsevier Science; 2000. pp. 129–135. [Google Scholar]
- Liu SZ, Xie F. Involvement of the Ca2+-protein kinase C and adenylate cyclase signal pathways in the activation of thymocytes in response to whole-body irradiation with low dose X-rays. Chin Med Sci J. 2000;15:1–7. [PubMed] [Google Scholar]
- Luckey TD. Physiological benefits from low levels of ionizing radiation. Health Phys. 1982;43:771–789. doi: 10.1097/00004032-198212000-00001. [DOI] [PubMed] [Google Scholar]
- Luetzelschwab JW, Googins SW. Radioactivity released from burning gas lantern mantles. Health Phys. 1984;46(4):873–81. doi: 10.1097/00004032-198404000-00013. [DOI] [PubMed] [Google Scholar]
- Midwood KS, Williams LV, Schwarzbauer JE. Tissue repair and the dynamics of the extra-cellular matrix. The International Journal of Biochemistry & Cell Biology. 2004;36(6):1031–1037. doi: 10.1016/j.biocel.2003.12.003. [DOI] [PubMed] [Google Scholar]
- Mitchel REJ, Jackson JS, McCann RA, Boreham DR. The adaptive response modifies latency for radiation induced myeloid leukemia in CBA/H mice. Radiat Res. 1999;152:273–279. [PubMed] [Google Scholar]
- Mitchel REJ, Jakson JS, Morrison DP, Carlisle SM. 2003. Low doses of radiation increase the latency of spontaneous lymphomas and spinal osteosarcomas in cancer prone, radiation sensitive Trp53 heterozygous mice. Radiat Res. 159:320–327. doi: 10.1667/0033-7587(2003)159[0320:ldorit]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Mohammadi H, Mehdizadeh S. Re-identification of 232Th content and relative radioactivity measurements in a number of imported gas mantles. Health Physics. 1983;44 (6):649–653. [PubMed] [Google Scholar]
- Mortazavi SMJ, Cameron JR, Niroomand-rad A. Adaptive response studies may help choose astronauts for long-term space travel. Advances in Space Research. 2003;31 (6):1543–1552. doi: 10.1016/s0273-1177(03)00089-9. [DOI] [PubMed] [Google Scholar]
- Mortazavi SMJ. Adaptive Responses after Exposure to Cosmic and Natural Terrestrial Radiation. Indian Journal of Radiation Research. 2004;1(1):104–112. [Google Scholar]
- Mortazavi SMJ, Monfared A, Ghiassi-Nejad M, Mozdarani H. Radioadaptive Responses Induced in Human Lymphocytes of the Inhabitants of High Level Natural Radiation Areas in Ramsar, Iran. Asian Journal of Experimental Science. 2005a;19:19–31. [Google Scholar]
- Mortazavi SMJ, Shabestani-Monfared A, Ghiassi-Nejad M, Mozdarani H. Radioadaptive responses induced in lymphocytes of the inhabitants in Ramsar, Iran. In: Sugahara T, Morishima M, Sohrabi M, Sasaki Y, Hayata I, Akiba S, editors. High Levels of Natural Radiation and Radon Areas: Radiation Dose and Health Effects. Elsevier; Amsterdam: 2005b. pp. 201–203. [Google Scholar]
- Mortazavi SMJ, Ghiassi-Nejad M, Karam PA, Ikushima T, Niroomand-Rad A, Cameron JR. Cancer incidence in areas with elevated levels of natural radiation. International Journal of Low Radiation. 2006;2(1/2):20–27. [Google Scholar]
- Mphande AN, Killowe C, Phalira S, Jones HW, Harrison WJ. Effects of honey and sugar dressings on wound healing. J Wound Care. 2007;16(7):317–9. doi: 10.12968/jowc.2007.16.7.27053. [DOI] [PubMed] [Google Scholar]
- Nordback I, Kulmala R, Järvinen M. Effect of ultraviolet therapy on rat skin wound healing. J Surg Res. 1990;48(1):68–71. doi: 10.1016/0022-4804(90)90148-u. [DOI] [PubMed] [Google Scholar]
- Poljanc K, Steinhauser G, Sterba JH, Buchtela K, Bichler M. Beyond low-level activity: on a “non-radioactive” gas mantle. Sci Total Environ. 2007;374(1):36–42. doi: 10.1016/j.scitotenv.2006.11.024. [DOI] [PubMed] [Google Scholar]
- Prekeges JL. Radiation hormesis, or, could all that radiation be good for us? J Nucl Med Technol. 2003;31(1):11–7. [PubMed] [Google Scholar]
- Quinn JV. Wound Care. Hamilton: Ont. B.C. Decker, Inc. Electronic book; 1998. Tissue Adhesives. [Google Scholar]
- Romo T, Pearson JM. Wound Healing, Skin. eMedicine. 2008 available at: http://emedicine.medscape.com/article/884594-print.
- Roth S. Psychology: the art of wooing nature . Jason Aronson; Northwale, NJ: 1990. [Google Scholar]
- Russ VK. Consensus of the effect of X rays on bacteria. Hygie. 1909;56:341–344. [Google Scholar]
- Stadelmann WK, Digenis AG, Tobin GR. Physiology and healing dynamics of chronic cutaneous wounds. The American Journal of Surgery. 1998;176(2):26S–38S. doi: 10.1016/s0002-9610(98)00183-4. [DOI] [PubMed] [Google Scholar]
- Upton AC. Radiation hormesis: data and interpretations. Crit Rev Toxicol. 2001;31(4–5):681–95. doi: 10.1080/20014091111956. [DOI] [PubMed] [Google Scholar]
- Wolff S. The adaptive response in radiobiology: evolving insights and implications. Environ. Health Perspect. 1998;106 (Suppl 1):277–283. doi: 10.1289/ehp.98106s1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yalow RS. Concerns with low level ionizing radiation: rational or phobic. J Nucl Med. 1990;31(7):17A–18A. 26A. [PubMed] [Google Scholar]
- Zheng J, Wang S, Guo L. Promotion of wound healing with fibroblast growth factor in combined burn radiation injury. Chinese J Plastic Surg. 1994;10:146–149. [PubMed] [Google Scholar]