Abstract
Neurological deficits caused by H-I (hypoxia-ischaemia) to the perinatal brain are often severely debilitating and lead to motor impairment, intellectual disability and seizures. Perinatal brain injury is distinct from adult brain injury in that the developing brain is undergoing the normal process of neuronal elimination by apoptotic cell death and thus the apoptotic machinery is more easily engaged and activated in response to injury. Thus cell death in response to neonatal H-I brain injury is partially due to mitochondrial dysfunction and activation of the apoptosome and caspase 3. An important regulator of the apoptotic response following mitochondrial dysfunction is XIAP (X-linked inhibitor of apoptosis protein). XIAP inhibits apoptosis at the level of caspase 9 and caspase 3 activation, and lack of XIAP in vitro has been shown to lead to increased apoptotic cell death. In the present study we show that mice lacking the gene encoding the XIAP protein have an exacerbated response to neonatal H-I injury as measured by tissue loss at 7 days following the injury. In addition, when the XIAP-deficient mice were studied at 24 h post-H-I we found that the increase in injury correlates with an increased apoptotic response in the XIAP-deficient mice and also with brain imaging changes in T2-weighted magnetic resonance imaging and apparent diffusion coefficient that correspond to the location of apoptotic cell death. These results identify a critical role of XIAP in regulating neuronal apoptosis in vivo and demonstrate the enhanced vulnerability of neurons to injury in the absence of XIAP in the developing brain.
Keywords: apoptosis, caspase, magnetic resonance imaging (MRI), neonatal hypoxia-ischaemia, transgenic mouse, X-linked inhibitor of apoptosis protein (XIAP)
Abbreviations: Apaf-1, apoptotic protease-activating factor 1; DEVD, Asp-Glu-Val-Asp; H-I, hypoxia-ischaemia; KO, knockout; MR, magnetic resonance; MRI, MR imaging; P7 etc., post-natal day 7 etc; TBS, Tris-buffered saline; XIAP, X-linked inhibitor of apoptosis protein
INTRODUCTION
Cerebral H-I (hypoxia-ischaemia) is a major cause of prenatal brain injury (Volpe, 2001a), and leads to significant neurological impairments in many of the survivors (Ferriero, 2004). The manifestation of H-I in the developing brain includes focal ischaemic infarction (Volpe, 2001b) and selective neuronal necrosis and apoptosis (Hagberg, 1992). One of the challenges in the field of neonatal brain injury is prediction of neurological outcome following an hypoxic-ischaemic event. Similar injury patterns can lead to different severities and types of outcome in both humans and in animal models (Vannucci, 1990). Differences in outcome are likely to be caused by a variety of genetic and environmental risk factors; it is well established that females are more resistant to perinatal H-I injury than males (Lauterbach et al., 2001) and, although controversial, an apolipoprotein E genotype may also affect clinical outcome (Kuroda et al., 2007; McMichael et al., 2008). Environmental risk factors probably include energy and antioxidant reserves.
Apoptosis plays an important homoeostatic role in the normal development of the vertebrate nervous system. In this environment of naturally occurring apoptotic signalling, immature neurons are especially vulnerable to insults such as H-I. The apoptotic cascade involves the activation of caspases, a family of proteases that cleave cellular substrates if activated (Cohen, 1997). Caspase activation can be triggered by translocation of cytochrome c from the mitochondria to the cytoplasm (Neame et al., 1998), where cytochrome c induces the formation of the apoptosome, a complex of cytochrome c, Apaf-1 (apoptotic protease-activating factor 1) and pro-caspase 9 (Zou et al., 1999). In this complex, pro-caspase 9 becomes activated and active caspase 9 can go on to activate caspase 3 (Wang et al., 2004), the main contributor to apoptotic cell death following neonatal H-I (Han et al., 2002). Owing to the prominent role of apoptosis in neonatal brain injury it is likely that alterations in the genes and proteins that make up the apoptotic cascade could affect the severity of outcome following perinatal H-I injury.
The activity and ability to activate caspases can be modulated through the activity and expression of a variety of regulatory proteins, including a family of genes encoding IAP (inhibitor of apoptosis) proteins. Among these proteins, XIAP (X-linked IAP) has been found to be a potent inhibitor of caspase 3 activation (Holcik et al., 2001). XIAP blocks the activity of activated caspase 9 in the apoptosome by binding to the neo-N-terminal sequence (ATPF) (Shiozaki et al., 2003), preventing activation of caspase 3 and, in addition, XIAP can directly inhibit activated caspase 3 (Deveraux et al., 1998). In vitro studies have identified endogenous XIAP as a critical post-cytochrome c regulator of caspase-dependent neuronal apoptosis (Potts et al., 2003), and data from studies of mice overexpressing XIAP have shown that increased levels of this protein confer protection against H-I-induced caspase activation in both the adult (Trapp et al., 2003) and neonatal (Wang et al., 2004) brain.
To better understand the anti-apoptotic role of XIAP in vivo, we investigated the effect of neonatal H-I in XIAP-deficient mice. The rationale for using this in vivo model is that neuronal injury following neonatal H-I in rodents has been found to have an apoptotic component which is linked to mitochondrial dysfunction (Cheng et al., 1998; Gibson et al., 2001). We found that at 7 days post-injury the XIAP-KO (knockout) mice have more tissue loss than their wild-type littermates and that this is a reflection of an increased apoptotic response to the H-I insult. Interestingly, the increased tissue loss was specifically observed in the cortex, an area of the brain that is not as affected by neonatal H-I injury in C57Bl6 mice. This suggests that XIAP plays an important role in protecting cells against apoptotic cell death. In addition to the biochemical and histological analysis, we used MRI [MR (magnetic resonance) imaging] to investigate the injury in vivo at 24 h after the insult. We found that the changes observed histologically at both 24 h and 7 days post-injury were strikingly reflected by the MRI at 24 h post-injury. Despite the major advantages of MRI (Allegrini and Sauer, 1992), there are very few publications utilizing MRI in neonatal models (Aden et al., 2002; Wendland et al., 2008). The present study demonstrates the ability of MRI to show areas that correspond to cell death following neonatal H-I brain injury in the post-natal day (P) 7 mouse, and the ability to observe the effects of genetic manipulation on the injured developing brain.
MATERIAL AND METHODS
Animals
Mice deficient in XIAP were obtained from the laboratory of Dr Craig Thompson (University of Pennsylvania, Philadelphia, PA, U.S.A.). They were on a C57Bl6 background. In order to obtain pups of all possible genotypes, breedings were set up with male XIAP−/y crossed with female XIAP+/− mice and male XIAP+/y crossed with female XIAP+/− mice. Mice were housed under a 12 h light/dark cycle and observed daily to establish the date of birth of the pups. Food and water were provided ad libitum and all care was given in compliance with National Institute of Health guidelines on the use of laboratory animals.
Genotyping
XIAP genotyping was performed using primers against the neomycin-resistance cassette to detect the presence of the XIAP-KO allele (forward, 5′-TGCTCCTGCCGAGAAAGTATCCATCATGGC-3′; reverse, 5′-CGCCAAGCTCTTCAGCAATATCACGGGTAG-3′). Presence of the intact XIAP gene was detected using primers for the first exon of the mouse XIAP gene (forward, 5′-TCATTTAACCCCCAGAGAGTTAGCTAGTGC-3′; reverse, 5′-AGAACTCACACCAGATTCACTTCGAACATT-3′). In addition to XIAP genotyping, we also determined the gender in the animals by PCR. This was done using primers against SRY (a Y chromosome-specific gene) (forward, 5′-TTGTCTAGAGAGCATGGAGGGCCATGTCAA-3′; reverse, 5′-CCACTCCTCTGTGACACTTTAGCCCTCCGA-3′).
Neonatal H-I
Neonatal H-I was performed on P7 mouse pups with a body weight between 2.9 and 4.0 g as described previously (West et al., 2006). Briefly, P7 pups were anaesthetized using 5% halothane for induction and 1.5% halothane for maintenance (balance room air). Under anaesthesia, the left carotid artery was permanently ligated with a cauterizer through an incision in the neck. The pups were returned to the dam and allowed to recover for 1–2 h before being placed in a hypoxia chamber through which 8% oxygen (balance nitrogen) flowed for 37 min. Following hypoxia the pups were returned to the dam until they were killed.
Histology and tissue loss determination
At 7 days post-H-I, P14 pups were deeply anaesthetized with pentobarbital (200 mg/kg of body weight) and perfused transcardially with 3 units/ml heparin in PBS, pH 7.4. Following rapid removal, brains were immersion-fixed in 4% (w/v) paraformaldehyde at 4°C for 24 h, and maintained in 30% sucrose until freezing in powdered dry ice followed by sectioning into 50 μm coronal sections with a freezing sliding microtome. Every 6th section (with an approximate distance of 300 μm between slides) from the genu of corpus collosum through to the caudal end of the hippocampus was mounted and stained with Cresyl Violet as described previously (West et al., 2006). Slides were scanned at 1600 dpi using the Silverfast (Lasersoft) plug-in for PhotoShop (Adobe) and saved as TIF files for analysis.
Tissue loss was calculated as the percentage volume loss by comparing volumes of distinct brain regions in the injured (left hemisphere) and non-injured (right hemisphere) hemisphere using the image analysis software SigmaScan Pro 5 (SPSS) (West et al., 2006). Hippocampal volume quantification was performed using the first five sections starting with the most rostral aspect of the hippocampus. Of those, only the first four sections were used to determine the cortical volumes respectively.
Tissue lysis and DEVD (Asp-Glu-Val-Asp) cleavage activity
Pups were sacrificed 24 h following H-I and perfused following the procedure described previously (West et al., 2006). Following brain removal, the right and left hippocampal and cortical regions were dissected on ice and then frozen on dry ice. Samples were stored at −80°C until the time of lysis. The right and left brain samples from all animals were lysed on the same day in 1×lysis buffer (Cell Signaling Technology) with protease inhibitors (Roche) and centrifuged at 17000 g for 15 min at 4°C. Protein concentrations in the lysates were determined using a BCA (bicinchoninic acid) kit (Pierce) and DEVD cleavage activity was measured following a procedure described previously (West et al., 2006). DEVD cleavage activity was normalized to total protein concentration for each of the samples and the values were reported as pmol of AMC (7-amino-4-methylcoumarin) generated per mg of protein per min.
Western blotting
Remaining tissue lysates not used in the DEVDase assay were used for Western blot analysis. To resolve high-molecular-mass proteins, samples were run on 3–8% Tris-acetate NuPage gels. To resolve low-molecular-mass proteins, 4–12% Bis-Tris NuPage gels were used (Invitrogen). Based on the total protein concentration in the lysate, 10 μg of total sample protein was run per lane. Proteins were transferred on to nitocellulose membranes (Bio-Rad) and blocked in TBS-T [Tris-buffered saline (25 mM Tris and 0.15 M NaCl, pH 7.2) containing 0.125% Tween 20] containing 5% (w/v) fat-free milk powder. The membranes were incubated with the following primary antibodies overnight: anti-XIAP (1:1000, R&D Systems), anti-Apaf-1 (1:1000, Alexis Biochemicals), anti-spectrin (1:1000, Chemicon), anti-caspase 9 (1:1000, Sigma) and anti-tubulin (1:4000, Sigma). This was followed by incubation with the appropriate peroxidase-labelled secondary antibodies (horse anti-mouse used at 1:5000 and goat anti-rabbit used at 1:5000). Western blots were developed using either SuperSignal West Pico (Pierce) or ECL Advance (Amersham) and were visualized on an ImageStation (Kodak).
MRI
MR experiments were performed on a Varian UNITY-INOVA 11.74-T MRI system with a 26-cm clear bore diameter horizontal Magnex magnet equipped with 8-cm inner diameter gradient insert assemblies (maximum gradient strength = 120 G/cm). A custom-designed neonatal mouse holder was used together with a 1.5-cm inner diameter birdcage coil (Stark Contrast). A standard spin echo was used for the acquisition of T2-weighted images with the following parameters: TR (repetition time) 4 s, TE (echo time) 80 ms and resolution of 93×93×250 μm.
For MR experiments, P7 animals underwent H-I and were allowed to recover for 24 h post-injury. The P8 pups were then anaesthetized using isoflurane and placed into the animal holder to minimize movement for the duration of the imaging. The animal holder included a temperature and respiration probe. During the imaging the animal was kept under 0.7–1.5% isoflurane anaesthesia (adjusted based on the respiration rate) and heated air was used to control the temperature of the animal.
Immunohistochemistry
At the end of the MRI, animals were sacrificed and the brain tissue was sectioned following the procedure described above for tissue loss determination. A subset of 50 μm free-floating sections (every 6th section) were processed for immunohistochemistry. Endogenous peroxidase activity was blocked with 0.3% H2O2 for 10 min. Non-specific binding was blocked for 30 min with 3% (v/v) goat serum in TBS and 0.25% Triton X-100. Sections were incubated with a rabbit antibody against active caspase 3 (1:1000, Cell Signaling Technologies) at 4°C overnight, followed by incubation with a biotinylated secondary antibody (1:2000, Vector). Visualization was performed using DAB (diaminobenzidine; Sigma) enhanced with 4% nickel chloride and 0.05% H2O2.
Statistics
All values are presented as means±S.E.M. and comparisons between two groups were performed using a Student’s t test for parametric data sets and a Mann–Whitney U test for non-parametric data sets. Statistical significance was set at 0.05. All analysis was performed using GraphPad Prism (GraphPad Software).
RESULTS
Increased tissue loss in the cortex following neonatal H-I in XIAP-KO mice
The volume of tissue loss in the injured hemisphere that occurs following H-I injury to the P7 brain can be assessed 7 days post-injury at P14. Tissue loss 7 days after injury is a useful histological measure of long term outcome of neonatal H-I (Cheng et al., 1998; Almli et al., 2000; Gibson et al., 2001). The damage following neonatal H-I brain injury following unilateral carotid ligation followed by exposure to hypoxic conditions in rats and mice is almostly exclusively restricted to the hemisphere ipsilateral to the ligation and thus the contralateral hemisphere can serve as a control, based on which conclusions about the amount of tissue loss in the same brain can be made (Cheng et al., 1998; Han et al., 2000; Gibson et al., 2001; West et al., 2006). We calculated the extent of tissue loss as the percentage volume loss by comparing the volume of remaining hippocampus and cortex in the left hemisphere to the volume of the same structures in the right hemisphere.
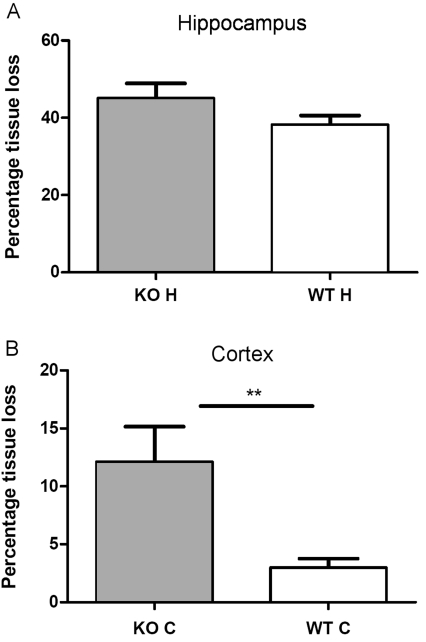
We observed a trend for an increase in tissue loss in the hippocampus of XIAP-KO animals, but this was not significant owing to the high variability of the injury in the hippocampus of the mice (Figure 1A). Interestingly we found that tissue loss in the cortex of XIAP-KO mice [12.1±3.03% (mean±S.E.M.)] was significantly increased by 4-fold as compared with their wild-type littermates (3.00±0.75%; Figure 1B). Previous studies of neonatal H-I in mice have shown that the cortex is largely spared from H-I injury in C57Bl6 mice (Gibson et al., 2001; Han et al., 2001; Hagberg et al., 2004; West et al., 2006; Zhu et al., 2006). We found no significant differences in injury between males and females of the same genotype. Mice heterozygous for the XIAP-KO allele did not have significantly different tissue loss from the wild-type animals, but did have significantly less cortical tissue loss than the XIAP-KO mice (results not shown). However interpretation of data from heterozygous mice is complicated by the fact that the XIAP gene is on the X chromosome which is randomly silenced in females.
Figure 1. Tissue loss following neonatal H-I in XIAP-KO and wild-type mice.
Tissue loss was measured histologically in the hippocampus (A) and the cortex (B) of XIAP-KO and wild-type (WT) littermates at 7 days after the H-I injury. For the hippocampus (A), there is a trend for increased tissue loss in the hippocampus of XIAP-KO mice, but this does not reach significance due to the high variability of the injury (values are mean percentage tissue loss±S.E.M.). However in the cortex (B) there is significantly more tissue loss in the XIAP-KO mice than in the wild-type (WT) littermates (values are mean percentage tissue loss±S.E.M.). **P = 0.009, wild-type compared with KO (as measured by a Student’s t test followed by a Mann–Whitney U post-test). The number of animals for these experiments was 89 (XIAP-KO n = 43, wild-type n = 46).
Caspase 3 activation is significantly increased in XIAP-KO mice compared with wild-type mice
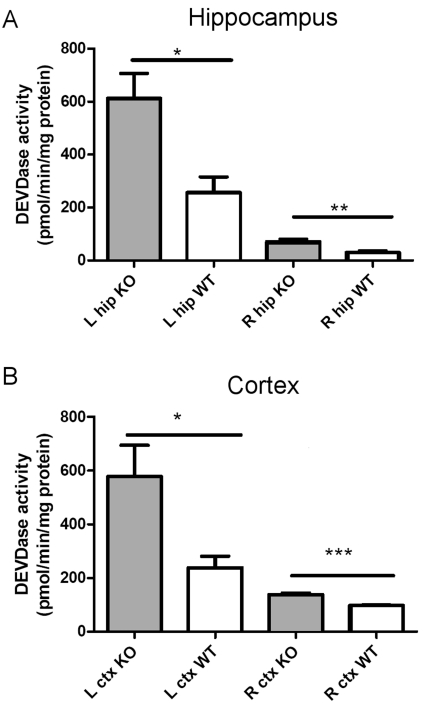
To further investigate the mechanisms behind the patterns of tissue loss observed in XIAP-KO mice, we examined the levels of caspase 3 activation in the hippocampus and cortex at 24 h post-H-I injury. We found that XIAP-KO mice demonstrate more than a 2-fold increase in caspase 3 activity (measured by DEVD cleavage) in both hippocampus and cortex, compared with their wild-type littermates when examined at 24 h post-injury (Figures 2A and 2B). Thus XIAP is important for regulation of the caspase activation in both hippocampus and cortex following neonatal H-I injury. However owing to the severe injury seen in the hippocampus of wild-type mice following this injury at P7, including the very high levels of caspase 3 activity, as well as non-apoptotic injury that occurs in the hippocampus of wild-type mice (Han et al., 2002), increased caspase 3 activation in this brain region did not correspond with an overall increase in tissue loss seen 7 days later.
Figure 2. Increased caspase 3 activation in XIAP-KO mice following neonatal H-I.
Caspase 3 activity was measured as the DEVD cleavage in tissue lysates from the injured (left, L) and non-injured (right, R) hippocampus (A) and cortex (B) of XIAP-KO and wild-type (WT) littermates at 24 h following the H-I insult. There is a significant increase in caspase 3 activity in both the hippocampus and the cortex of the XIAP-KO mice on both the injured and the non-injured side of the brain. The number of animals for these experiments was 18 (XIAP KO n = 11, wild-type, n = 7). *P<0.05, **P<0.01, ***P<0.001, for wild-type compared with KO.
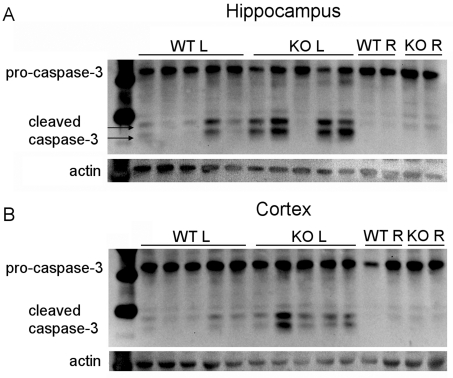
Interestingly, we also observed a significant difference in caspase 3 activation when comparing caspase 3 activity in the hemisphere contralateral to carotid ligation in XIAP-KO compared with wild-type mice (Figures 2A and 2B). This suggests that the inhibitory role of XIAP in the caspase 3 activation cascade plays a role in the hemisphere contralateral to carotid ligation which, at a pathological level, is relatively unaffected. No significant differences in DEVDase activity were observed in XIAP-KO and wild-type non-injured controls (results not shown). To confirm that the higher level of caspase 3 activation observed in the XIAP-KO mice is due to increased proteolytic cleavage of caspase 3 and not just due to changes in activity of the cleaved enzyme, we investigated caspase 3 processing by Western blotting (Figures 3A and 3B). Western blot analysis reveals that XIAP-KO mice have increased levels of both the 17 and 19 kDa forms of cleaved activated caspase 3, suggesting that XIAP is normally reducing the amount of caspase 3 cleavage that occurs in wild-type mice following H-I and thus has an effect upstream of caspase 3 activation. Despite evidence of increased cleaved caspase 3 in injured XIAP-KO brain tissue, we noted no difference in pro-caspase 3 between uninjured XIAP-KO and wild-type brain tissue (results not shown).
Figure 3. Caspase 3 processing in XIAP-KO and wild-type mice.
Western blot analysis for caspase 3 was performed on tissue (hippocampus, A and cortex, B) from the injured hemisphere of XIAP-KO and wild-type (WT) littermates. Pro-caspase 3 is cleaved to form two active forms of caspase 3, the p19 and the p17 form. The presence of these forms in tissue from both XIAP-KO and wild-type mice show that the lack of XIAP does not affect the processing of caspase 3 in either the hippocampus or the cortex. Tissue from the right (non-injured) hippocampus and cortex show that there is a very faint increase in the cleaved form of caspase 3 in the XIAP-KO mice in response to the injury. Western blot analysis for actin is shown as a loading control although this protein, like most cellular proteins, is actually a substrate of caspase 3 and thus cleaved when caspase 3 activity is high.
Spectrin and caspase 9 cleavage in XIAP-KO mice
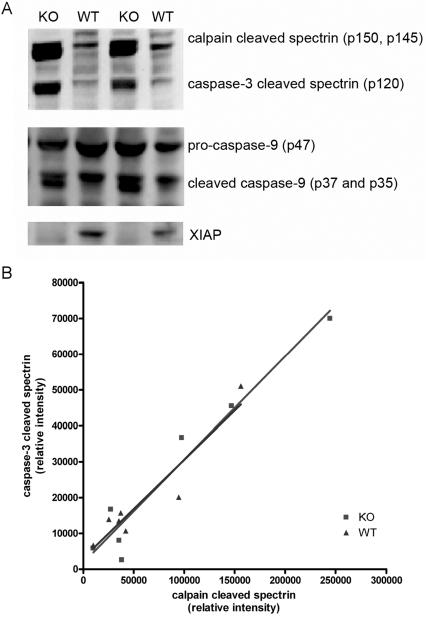
Spectrin acts as an endogenous substrate for caspase 3 activation and is cleaved upon caspase 3 activation post neonatal H-I (Han et al., 2002). In addition to cleavage by caspase 3, spectrin is also cleaved by calpain, a calcium-activated protease which is thought to be a marker for necrotic cell death. Previous studies have found that calpain cleavage of spectrin is independent of caspase activation (Han et al., 2002; West et al., 2006). In the present study we wanted to investigate whether there is a difference in caspase and calpain cleavage of spectrin in the XIAP-KO mice to see if the increased caspase activation in the XIAP-KO mice is independent of calpain cleavage. We found a marked increase in caspase- and calpain-cleaved spectrin in the hippocampus of both XIAP-KO and wild-type animals at 24 h post-H-I (Figure 4A). Interestingly, in the XIAP-KO mice the increased intensity of the calpain-cleaved spectrin band following injury was similar to the increase in intensity of the caspase-cleaved spectrin band suggesting that, in addition to increased apoptosis in the XIAP-KO mice there is also an increase in calpain activation. In addition, there was a strong correlation between the caspase 3-cleaved spectrin and calpain-cleaved spectrin in both XIAP-KO and wild-type mice (Figure 4B). This is possibly due to the fact that when cells are dysfunctional and dying with prominent apoptosis or necrosis, they are not able to maintain normal ion homoeostasis leading to an influx of calcium and activation of calpain.
Figure 4. Spectrin and caspase 9 processing in XIAP-KO and wild-type mice.
Western blot analysis for spectrin, caspase 9 and XIAP was performed on hippocampal tissue lysates from the injured hemisphere of XIAP-KO and wild-type (WT) littermates. Spectrin is cleaved by calpain to yield p150 and p145, and by caspase 3 to yield p120. The intensities of these bands correlate with the activity of the proteases. XIAP-KO mice have increased processing of spectrin by both caspase 3 and spectrin, suggesting that the increased injury in these mice also involves activation of calpain. Processing of caspase 9 is also different between the XIAP-KO and wild-type littermates. The p37 isoform of caspase 9 is caused by autocleavage in the apoptosome, whereas the p35 isoform is caused by caspase 3 cleavage. In wild-type mice only the p37 isoform is present, whereas in the XIAP-KO mice both the isoforms are present. Western blot analysis for XIAP was performed to confirm the genotype of these animals.
We also investigated caspase 9 processing by Western blotting. In addition to autocleavage in the apoptosome of pro-caspase 9 at Asp315 (generating the p35 form of caspase 9), leading to the generation of the neo-N-terminal sequence which XIAP can bind to, caspase 9 can also be cleaved by active caspase 3 at Asp330 (generating the p37 form of caspase 9). Interestingly, although the caspase 3 cleavage p37 form of caspase 9 was present in both KO and wild-type animals following H-I injury, the p35 autocleavage-form of caspase 9 was much higher in the XIAP-KO mice than in wild-type mice (Figure 4A). This increase in the p35 form of caspase 9 in the absence of XIAP suggests that, in addition to inhibition of active caspase 9 following autocleavage, XIAP may be able to directly prevent caspase 9 autocleavage in the apoptosome in vivo. In non-injured animals, we found no difference in the levels of cytochrome c, pro-caspase 9, or Apaf-1 in the hippocampus of wild-type and XIAP-KO mice, suggesting that lack of XIAP does not otherwise affect the expression of the components of the apoptosome (results not shown).
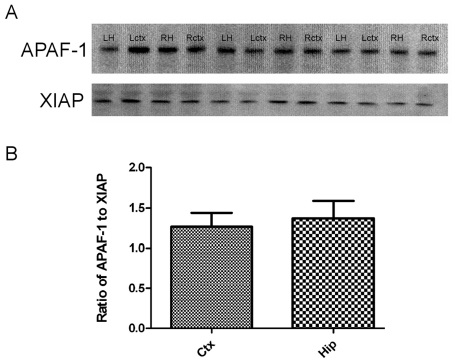
No significant difference in the Apaf-1 expression between the cortex and hippocampus in wild-type animals
The findings of increased apoptosis and tissue loss in the cortex of XIAP-KO mice, a region of the brain usually not markedly affected by neonatal H-I in C57Bl6 mice, suggest that XIAP may play a role in the protection of this region of the brain in wild-type animals. It has been suggested that the ratio of Apaf-1 to XIAP may be what is important in determining whether a XIAP is going to inhibit apoptosis. We used XIAP and Apaf-1 Western blots to investigate whether there is a difference in the ratio between the hippocampus and the cortex in wild-type mice (Figure 5A). Although there is a slight trend towards a lower Apaf-1 to XIAP ratio in the cortex compared with the hippocampus, this was not significant (Figure 5B). In addition to comparing hippocampal and cortical Apaf-1 expression with XIAP expression, we also compared Apaf-1 expression with that of other apoptotic proteins such as cytochrome c, and caspase 3 and 9 (results not shown). We found that there was no difference in expression of these apoptotic proteins between hippocampus and cortex. Thus it is likely that other factors such as expression of other proteins, the vascular system, or other factors are causing the differential vulnerability of the hippocampus and cortex.
Figure 5. Apaf-1 and XIAP expression in the cortex and hippocampus of wild-type mice.
(A) Tissue lysates from both hemispheres (left, L and right, R) of the hippocampus (H) and cortex (ctx) of three P7 wild-type mice were probed with antibodies against Apaf-1 and XIAP to measure the ratio between these two proteins in these two regions of the brain. (B) Bands were quantified as relative intensities, and the ratio of Apaf-1 to XIAP was calculated for both the cortex (Ctx) and the hippocampus (Hip). There was no statistically significant difference between the ratio in the cortex and the hippocampus.
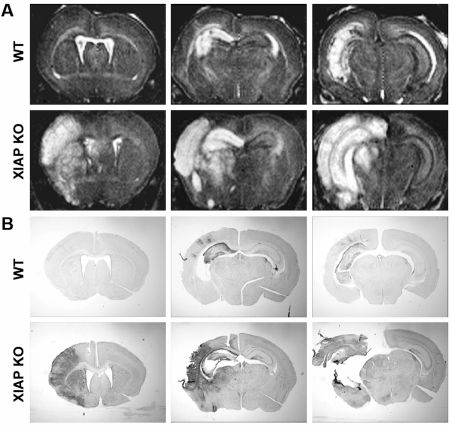
Hippocampal and cortical injury in XIAP-KO mice can be observed at 24 h post-injury by MRI
In addition to measuring caspase 3 activation at 24 h post-injury, we performed MRI on a set of two XIAP-KO mice and two wild-type mice. Immediately following MRI, we sacrificed the animals and sectioned the brains. We found striking differences in the T2-weighted images at 24 h after H-I, especially in the cortex, between the wild-type and XIAP-KO mice that were assessed (Figure 6A). There were areas of increased T2 signal intensity in the hippocampus, corpus callosum, and to a very small extent, the cortex in the wild-type mice ipsilateral to carotid ligation. In XIAP-KO mice, the same areas were affected as in the wild-type mice. However, T2 changes in the cortex, as well as in the striatum and thalamus, were much more extensive. To visualize apoptotic cells, we stained representative sections of the same brains that matched the coronal sections of the brain that we imaged using MRI for active caspase 3. Interestingly, the anatomical regions of MRI hyperintensity corresponded very well with the regions of neuronal caspase 3 activation. Not only did the MRI reflect the increased injury in the cortex of the XIAP-KO mice, it also reflected the anatomical regions where caspase 3 activation was present in general (hippocampus, cortex, striatum and thalamus).
Figure 6. MRI and caspase 3 activation in the brain at 24 h post-injury in XIAP-KO and wild-type mice.
At 24 h after neonatal H-I injury two XIAP-KO mice (second row of images) and two wild-type (WT) mice (top row of images) were imaged using a high-resolution MRI scanner. Following the MRI the mice were killed and the brains were sectioned and stained for active caspase 3 (bottom two rows). High-resolution MRI allows visualization of individual regions in the brain of the P7 mice at a 50 micron in-plane resolution. The T2 image underscores the difference in the pattern of injury observed in the wild-type and XIAP mice. Animals of both genotypes have hyperintensity in the hippocampus, but the cortex of the XIAP-KO mice is much more severely affected than the cortex of the wild-type mice. Immunostaining for active caspase 3 shows a high degree of co-localization with the injury observed by MRI, suggesting that the MRI changes represent increased cell death and apoptosis.
DISCUSSION
Previous studies have shown that increasing XIAP expression can protect against H-I-induced injury in both adult (Trapp et al., 2003) and neonatal (Wang et al., 2004) mice. In addition, deletion of the XIAP gene has been shown in vitro to increase neuronal vulnerability to a variety of stressors (Potts PR et al., 2003; Potts MB et al., 2005; Vaughn and Deshmukh, 2007). Based on these studies we hypothesized that mice lacking the endogenous XIAP protein would have an increased apoptotic response to neonatal H-I brain injury and that the increase in apoptosis would lead to increased brain injury. The neonatal rodent H-I model has been found to have an apoptotic component involving the intrinsic apoptotic pathway leading to mitochondrial dysfunction (Cheng et al., 1998). Genetic studies have implicated both pro- and anti-apoptotic members of the Bcl-2 family in cell death following neonatal H-I; overexpression of Bcl-X was found to be protective as was genetic deletion of Bax (Parsadanian et al., 1998; Gibson et al., 2001). Thus the cell death observed in the neonatal H-I model is in part due to activation of the intrinsic apoptotic pathway making this an excellent model for testing the role of XIAP in vivo. For these studies we used XIAP-deficient mice. These mice have been previously reported to have no overt physical or behavioral abnormalities, as well as no histologically determined differences among selected tissues, including brain (Harlin et al., 2001), indicating that the absence of XIAP in the brain does not interfere with postnatal development of the CNS.
By investigating the tissue loss at 7 days post-injury we found that the XIAP-KO mice have significantly more tissue loss in the cortex compared with wild-type littermates. There was a trend for increased hippocampal injury, but this was not statistically significant probably due to the high level of baseline injury to the hippocampus in this model and the variability in injury observed between the animals. The cortex is not normally markedly involved in the injury following neonatal H-I in C57Bl6 mice and thus the cortical injury in the XIAP-KO mice suggests that XIAP plays a role in protecting this region of the brain in wild-type mice (Han et al., 2001; Hagberg et al., 2004; West et al., 2006; Zhu et al., 2006; West et al., 2007).
In addition to measuring tissue loss at 7 days post-injury, we also measured caspase 3 activation in both the hippocampus and cortex at 24 h post-injury. Caspase 3 activation was significantly higher in both the hippocampus and cortex of XIAP-KO mice compared with wild-type littermates. It is likely that the caspase 3 activity seen in wild-type mice in the hippocampus is leading to an almost maximal amount of tissue loss by this mechanism and thus potentiating caspase 3 activity in this brain region probably cannot lead to a greater amount of tissue loss. The increase in caspase 3 activity in XIAP-KO mice is consistent with the proposed role of XIAP as a ‘handbrake’ on apoptosis.
Under normal circumstances, cytochrome c release from the mitochondria leads to formation of the apoptosome complex where pro-caspase 9 cleaves itself to form active caspase 9 (Srinivasula et al., 1998). Active caspase 9 produced in this way can be bound by XIAP, preventing cleavage of pro-caspase 3 and apoptosis (Shiozaki et al., 2003). Deletion of XIAP relieves the inhibition of caspase 9 cleavage of caspase 3 in response to cytochrome c release. In addition, to inhibit caspase 9 cleavage of caspase 3, XIAP can also inhibit caspase 3 directly (Deveraux et al., 1998). By investigating caspase 3 processing, we showed that, in XIAP-KO mice, pro-caspase 3 is cleaved to form the active forms of caspase 3, to a much higher degree than in wild-type mice. Thus the increased caspase 3 activity observed in XIAP-KO mice correlates with increased processing of caspase 3. The fact that deletion of XIAP causes increased caspase 3 activation and processing following neonatal H-I suggests that cytochrome c release, caspase 9 and XIAP all play important roles in the cell death taking place following neonatal H-I brain injury.
We also examined caspase 9 processing in XIAP-KO and wild-type mice by performing Western blot analysis on tissue from the injured hippocampus of mice of both genotypes at 24 h post-injury. Caspase 9 autocleavage takes place on Asp315, generating a p35 and a p12 form of caspase 9, but caspase 9 can also be cleaved by caspase 3 at Asp330, generating a p37 and a p10 form of caspase 9 (Zou et al., 2003). Although caspase 3 cleavage of caspase 9 (in the absence of caspase 9 activation) may not lead to caspase 9 activation (Denault et al., 2007), cleavage of recombinant caspase 9 by caspase 3 in vitro does lead to generation of the p35 isoform of caspase 9 (Zou et al., 2003). We found that the processing of caspase 9 in wild-type mice leads to the production of a major p37 isoform (caspase 3 cleavage) with minimal to no p35 isoform (autocleavage). Interestingly, there was a significant amount of the caspase 9 autocleavage (p35) isoform in the injured hippocampus of XIAP-KO mice, demonstrating that XIAP affects the proteolytic processing of caspase 9. It is likely that this increased autocleavage of caspase 9 is due to feedback of caspase 3 on to the mitochondria. Previous studies have shown that caspase 3 activation can lead to mitochondrial dysfunction and increased cytochrome c release (Chen et al., 2000; Lakhani et al., 2006; Chen et al., 2007). Anecdotally, we did observe increased cytochrome c release in the XIAP-KO mice which would agree with this hypothesis (results not shown).
The difference in tissue loss in the cortex between XIAP-KO and wild-type mice suggests that this brain region is not intrinsically spared from neonatal H-I injury, but rather that in wild-type mice the level of injury in the cortex does not reach the threshold at which cell death is initiated. Thus there may be a difference in the injury threshold between the hippocampus and cortex. It has been shown in vitro that the ratio of Apaf-1 to XIAP can determine whether cells are going to undergo apoptosis or survive (Potts PR et al., 2003; Potts MB et al., 2005). To investigate whether there is a difference in the ratio of Apaf-1 to XIAP between hippocampus and cortex we investigated the levels of these proteins in tissue lysates from wild-type, non-injured P7 animals. We found no significant difference in this ratio between the two brain regions, suggesting that the cause for the difference in vulnerability is not a difference in the ratio of XIAP to Apaf-1. This, however, does not rule out other factors which may influence how effective XIAP is at preventing apoptosis.
To investigate whether the changes in injury severity and patterning observed in the XIAP-KO mice can be observed by in vivo imaging, we performed MRI on a subset of XIAP-KO and wild-type littermates. Using a 12T small animal scanner we were able to get images with less than 100 micron in-plane resolution allowing us to clearly differentiate the different brain structures of the P8 mouse pup. The T2-weighted images clearly show the difference in injury severity between the XIAP-KO and wild-type mice, as well as the difference in vulnerability of the cortex. When brain sections from the imaged animals were stained for active caspase 3, the regions that showed evidence of increased T2 signal on the MRI corresponded to regions of marked caspase 3 activation. To the best of our knowledge, this is one of the the first reports of MRI changes observed after brain injury in a neonatal genetically manipulated mouse, demonstrating the usefulness of this tool in characterizing injury localization and injury severity in animals with genetic mutations. Importantly, MRI should be useful in future studies to demonstrate regions of cell death in the neonatal brain that will allow for non-invasive assessment of injury and the effects of different treatments.
In summary, our results demonstrate that endogenous XIAP plays an important role in regulating the extent of apoptotic brain injury in a mouse model of neonatal H-I brain injury. The fact that loss of function of XIAP can lead to vulnerability of the brain to H-I injury suggests that this variation in the level of function of this protein could underlie some of the variability in response to injury observed in the human neonate. Thus differences in XIAP function or levels are potential risk factors for increasing the neuronal susceptibility to brain injury. Furthermore, there is an important need both in pre-term and term human H-I brain injury for better therapies to improve short- and long-term outcome. The relevance of XIAP deficiency might bring new insight in newborns with marked cortical injury in disproportion to the duration of the H-I. It will be important in future studies to determine whether manipulation of XIAP, either genetically or pharmacologically, may be of use in developing better therapeutics to prevent the consequences of neonatal H-I.
FUNDING
This work was supported by the National Institutes of Health [grant numbers NS35902 (to D.M.H. and J.J.N.), NS42197 (to M.D.)].
REFERENCES
- Aden U, Dahlberg V, Fredholm BB, Lai LJ, Chen Z, Bjelke B. MRI evaluation and functional assessment of brain injury after hypoxic ischemia in neonatal mice. Stroke. 2002;33:1405–1410. doi: 10.1161/01.str.0000014608.78503.db. [DOI] [PubMed] [Google Scholar]
- Allegrini PR, Sauer D. Application of magnetic resonance imaging to the measurement of neurodegeneration in rat brain: MRI data correlate strongly with histology and enzymatic analysis. Magn Reson Imaging. 1992;10:773–778. doi: 10.1016/0730-725x(92)90411-r. [DOI] [PubMed] [Google Scholar]
- Almli CR, Levy TJ, Han BH, Shah AR, Gidday JM, Holtzman DM. BDNF protects against spatial memory deficits following neonatal hypoxia-ischemia. Exp Neurol. 2000;166:99–114. doi: 10.1006/exnr.2000.7492. [DOI] [PubMed] [Google Scholar]
- Chen Q, Gong B, Almasan A. Distinct stages of cytochrome c release from mitochondria: evidence for a feedback amplification loop linking caspase activation to mitochondrial dysfunction in genotoxic stress-induced apoptosis. Cell Death Differ. 2000;7:227–233. doi: 10.1038/sj.cdd.4400629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M, Guerrero AD, Huang L, Shabier Z, Pan M, Tan TH, Wang J. Caspase-9-induced mitochondrial disruption through cleavage of anti-apoptotic Bcl-2 family members. J Biol Chem. 2007;282:33888–33895. doi: 10.1074/jbc.M702969200. [DOI] [PubMed] [Google Scholar]
- Cheng Y, Deshmukh M, D’Costa A, Demaro JA, Gidday JM, Shah A, Sun Y, Jacquin MF, Johnson EM, Holtzman DM. Caspase inhibitor affords neuroprotection with delayed administration in a rat model of neonatal hypoxic-ischemic brain injury. J Clin Invest. 1998;101:1992–1999. doi: 10.1172/JCI2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen GM. Caspases: the executioners of apoptosis. Biochem J. 1997;326:1–16. doi: 10.1042/bj3260001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denault JB, Eckelman BP, Shin H, Pop C, Salvesen GS. Caspase 3 attenuates XIAP (X-linked inhibitor of apoptosis protein)-mediated inhibition of caspase 9. Biochem J. 2007;405:11–19. doi: 10.1042/BJ20070288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deveraux QL, Roy N, Stennicke HR, Van Arsdale T, Zhou Q, Srinivasula SM, Alnemri ES, Salvesen GS, Reed JC. IAPs block apoptotic events induced by caspase-8 and cytochrome c by direct inhibition of distinct caspases. EMBO J. 1998;17:2215–2223. doi: 10.1093/emboj/17.8.2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferriero DM. Neonatal brain injury. N Engl J Med. 2004;351:1985–1995. doi: 10.1056/NEJMra041996. [DOI] [PubMed] [Google Scholar]
- Gibson ME, Han BH, Choi J, Knudson CM, Korsmeyer SJ, Parsadanian M, Holtzman DM. BAX contributes to apoptotic-like death following neonatal hypoxia-ischemia: evidence for distinct apoptosis pathways. Mol Med. 2001;7:644–655. [PMC free article] [PubMed] [Google Scholar]
- Hagberg H. Hypoxic-ischemic damage in the neonatal brain: excitatory amino acids. Dev Pharmacol Ther. 1992;18:139–144. [PubMed] [Google Scholar]
- Hagberg H, Wilson MA, Matsushita H, Zhu C, Lange M, Gustavsson M, Poitras MF, Dawson TM, Dawson VL, Northington F, Johnston MV. PARP-1 gene disruption in mice preferentially protects males from perinatal brain injury. J Neurochem. 2004;90:1068–1075. doi: 10.1111/j.1471-4159.2004.02547.x. [DOI] [PubMed] [Google Scholar]
- Han BH, D’Costa A, Back SA, Parsadanian M, Patel S, Shah AR, Gidday JM, Srinivasan A, Deshmukh M, Holtzman DM. BDNF blocks caspase-3 activation in neonatal hypoxia-ischemia. Neurobiol Dis. 2000;7:38–53. doi: 10.1006/nbdi.1999.0275. [DOI] [PubMed] [Google Scholar]
- Han BH, DeMattos RB, Dugan LL, Kim-Han JS, Brendza RP, Fryer JD, Kierson M, Cirrito J, Quick K, Harmony JA, Aronow BJ, Holtzman DM. Clusterin contributes to caspase-3-independent brain injury following neonatal hypoxia-ischemia. Nat Med. 2001;7:338–343. doi: 10.1038/85487. [DOI] [PubMed] [Google Scholar]
- Han BH, Xu D, Choi J, Han Y, Xanthoudakis S, Roy S, Tam J, Vaillancourt J, Colucci J, Siman R, Giroux A, Robertson GS, Zamboni R, Nicholson DW, Holtzman DM. Selective, reversible caspase-3 inhibitor is neuroprotective and reveals distinct pathways of cell death after neonatal hypoxic-ischemic brain injury. J Biol Chem. 2002;277:30128–30136. doi: 10.1074/jbc.M202931200. [DOI] [PubMed] [Google Scholar]
- Harlin H, Reffey SB, Duckett CS, Lindsten T, Thompson CB. Characterization of XIAP-deficient mice. Mol Cell Biol. 2001;21:3604–3608. doi: 10.1128/MCB.21.10.3604-3608.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holcik M, Gibson H, Korneluk RG. XIAP: apoptotic brake and promising therapeutic target. Apoptosis. 2001;6:253–261. doi: 10.1023/a:1011379307472. [DOI] [PubMed] [Google Scholar]
- Kuroda MM, Weck ME, Sarwark JF, Hamidullah A, Wainwright MS. Association of apolipoprotein E genotype and cerebral palsy in children. Pediatrics. 2007;119:306–313. doi: 10.1542/peds.2006-1083. [DOI] [PubMed] [Google Scholar]
- Lakhani SA, Masud A, Kuida K, Porter GA, Jr, Booth CJ, Mehal Wz, Inayat I, Flavell RA. Caspases 3 and 7: key mediators of mitochondrial events of apoptosis. Science. 2006;311:847–851. doi: 10.1126/science.1115035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauterbach MD, Raz S, Sander CJ. Neonatal hypoxic risk in preterm birth infants: the influence of sex and severity of respiratory distress on cognitive recovery. Neuropsychology. 2001;15:411–420. [PubMed] [Google Scholar]
- McMichael GL, Gibson CS, Goldwater PN, Haan EA, Priest K, Dekker GA, MacLennan AH. Association between apolipoprotein E genotype and cerebral palsy is not confirmed in a Caucasian population. Hum Genet. 2008;124:411–416. doi: 10.1007/s00439-008-0564-y. [DOI] [PubMed] [Google Scholar]
- Neame SJ, Rubin LL, Philpott KL. Blocking cytochrome c activity within intact neurons inhibits apoptosis. J Cell Biol. 1998;142:1583–1593. doi: 10.1083/jcb.142.6.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsadanian AS, Cheng Y, Keller-Peck CR, Holtzman DM, Snider WD. Bcl-xL is an antiapoptotic regulator for postnatal CNS neurons. J Neurosci. 1998;18:1009–1019. doi: 10.1523/JNEUROSCI.18-03-01009.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potts MB, Vaughn AE, McDonough H, Patterson C, Deshmukh M. Reduced Apaf-1 levels in cardiomyocytes engage strict regulation of apoptosis by endogenous XIAP. J Cell Biol. 2005;171:925–930. doi: 10.1083/jcb.200504082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potts PR, Singh S, Knezek M, Thompson CB, Deshmukh M. Critical function of endogenous XIAP in regulating caspase activation during sympathetic neuronal apoptosis. J Cell Biol. 2003;163:789–799. doi: 10.1083/jcb.200307130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiozaki EN, Chai J, Rigotti DJ, Riedl SJ, Li P, Srinivasula SM, Alnemri ES, Fairman R, Shi Y. Mechanism of XIAP-mediated inhibition of caspase-9. Mol Cell. 2003;11:519–527. doi: 10.1016/s1097-2765(03)00054-6. [DOI] [PubMed] [Google Scholar]
- Srinivasula SM, Ahmad M, Fernandes-Alnemri T, Alnemri ES. Autoactivation of procaspase-9 by Apaf-1-mediated oligomerization. Mol Cell. 1998;1:949–957. doi: 10.1016/s1097-2765(00)80095-7. [DOI] [PubMed] [Google Scholar]
- Trapp T, Korhonen L, Besselmann M, Martinez R, Mercer EA, Lindholm D. Transgenic mice overexpressing XIAP in neurons show better outcome after transient cerebral ischemia. Mol Cell Neurosci. 2003;23:302–313. doi: 10.1016/s1044-7431(03)00013-7. [DOI] [PubMed] [Google Scholar]
- Vannucci RC. Experimental biology of cerebral hypoxia-ischemia: relation to perinatal brain damage. Pediatr Res. 1990;27:317–326. doi: 10.1203/00006450-199004000-00001. [DOI] [PubMed] [Google Scholar]
- Vaughn AE, Deshmukh M. Essential postmitochondrial function of p53 uncovered in DNA damage-induced apoptosis in neurons. Cell Death Differ. 2007;14:973–981. doi: 10.1038/sj.cdd.4402084. [DOI] [PubMed] [Google Scholar]
- Volpe JJ. Perinatal brain injury: from pathogenesis to neuroprotection. Ment Retard Dev Disabil Res Rev. 2001a;7:56–64. doi: 10.1002/1098-2779(200102)7:1<56::AID-MRDD1008>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Volpe JJ. Neurobiology of the Newborn. Philadelphia: W.B. Saunders; 2001b. 4 Edition. [Google Scholar]
- Wang X, Zhu C, Hagberg H, Korhonen L, Sandberg M, Lindholm D, Blomgren K. X-linked inhibitor of apoptosis (XIAP) protein protects against caspase activation and tissue loss after neonatal hypoxia-ischemia. Neurobiol Dis. 2004;16:179–189. doi: 10.1016/j.nbd.2004.01.014. [DOI] [PubMed] [Google Scholar]
- Wendland MF, Faustino J, West T, Manabat C, Holtzman DM, Vexler ZS. Early diffusion-weighted MRI as a predictor of caspase-3 activation after hypoxic-ischemic insult in neonatal rodents. Stroke. 2008;39:1862–1868. doi: 10.1161/STROKEAHA.107.506352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West T, Atzeva M, Holtzman DM. Caspase-3 deficiency during development increases vulnerability to hypoxic-ischemic injury through caspase-3-independent pathways. Neurobiol Dis. 2006;22:523–537. doi: 10.1016/j.nbd.2005.12.017. [DOI] [PubMed] [Google Scholar]
- West T, Atzeva M, Holtzman DM. Pomegranate polyphenols and resveratrol protect the neonatal brain against hypoxic-ischemic injury. Dev Neurosci. 2007;29:363–372. doi: 10.1159/000105477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu C, Xu F, Wang X, Shibata M, Uchiyama Y, Blomgren K, Hagberg H. Different apoptotic mechanisms are activated in male and female brains after neonatal hypoxia-ischaemia. J Neurochem. 2006;96:1016–1027. doi: 10.1111/j.1471-4159.2005.03639.x. [DOI] [PubMed] [Google Scholar]
- Zou H, Li Y, Liu X, Wang X. An APAF-1–cytochrome c multimeric complex is a functional apoptosome that activates procaspase-9. J Biol Chem. 1999;274:11549–11556. doi: 10.1074/jbc.274.17.11549. [DOI] [PubMed] [Google Scholar]
- Zou H, Yang R, Hao J, Wang J, Sun C, Fesik SW, Wu JC, Tomaselli KJ, Armstrong RC. Regulation of the Apaf-1/caspase-9 apoptosome by caspase-3 and XIAP. J Biol Chem. 2003;278:8091–8098. doi: 10.1074/jbc.M204783200. [DOI] [PubMed] [Google Scholar]