Abstract
Purinergic P2 receptors and gap junctions are two groups of proteins involved in the transmission of ICWs (intercellular calcium waves) between astrocytes. The extent to which ICWs spread among these glial cells depends on the amount of ATP released, which can occur through membrane channels, as well as other pathways. Our previous studies have shown that the pore-forming P2X7R (P2X7 receptor) contributes to the amplification of ICW spread by providing sites of ATP release through Panx1 (Pannexin1) channels. To gain insight into the signal transduction events mediating this response we compared the properties of the P2X7R–Panx1 complex in astrocytes from a mouse strain (C57Bl/6) containing a naturally occurring point mutation (P451L) in the C-terminus of the P2X7R to that of non-mutated receptors (Balb/C mice). Electrophysiological, biochemical, pharmacological and fluorescence imaging techniques revealed that the P451L mutation located in the SH3 domain (a Src tyrosine kinase-binding site) of the C-terminus of the P2X7R attenuates Panx1 currents, ATP release and the distance of ICW spread between astrocytes. Similar results were obtained when using the Src tyrosine inhibitor (PP2) and a membrane-permeant peptide spanning the P451L mutation of the P2X7R of the C57Bl6 astrocytes. These results support the participation of a tyrosine kinase of the Src family in the initial steps mediating the opening of Panx1 channels following P2X7R stimulation and in the transmission of calcium signals among astrocytes.
Keywords: ATP release, gap junction, glia, hemichannel, pannexin, purinergic receptor
Abbreviations: CBX, carbenoxolone; CCD, charge-coupled device; CT, C-terminal; DMEM, Dulbecco’s modified Eagle’s medium; DPBS, Dulbecco’s PBS; FBS, fetal bovine serum; HRP, horseradish peroxidase; ICW, intercellular calcium wave; LDPBS, low-divalent PBS; LY, Lucifer Yellow; MFQ, mefloquine; Panx1, Pannexin1; P2X7R, P2X7 receptor; siRNA, small interfering RNA
INTRODUCTION
Astrocytes in vitro and in vivo express several purinergic P2 receptors of the ionotropic P2X and metabotropic P2Y types (reviewed in Burnstock, 2008). The ionotropic P2X7R (P2X7 receptor) is remarkable in that brief stimulation opens the cation channels, but prolonged stimulation leads to the opening of a pore through which large molecules (up to approx. 800 Da) can pass into or out of the cells (Surprenant et al., 1996; North, 2002). Mutagenic studies have indicated that deletion of the intracellular CT (C-terminal) domain of the P2X7R abrogates membrane permeabilization induced by prolonged agonist stimulation (Surprenant et al., 1996). More recently, a genetic polymorphism was described in mice in which the proline residue at position 451 of the P2X7R CT is replaced by a leucine residue; this single-amino-acid mutation rendered the P2X7R still capable of performing as a cation channel, but largely attenuated pore formation (Adriouch et al., 2002; Le Stunff et al., 2004).
Examination of the region flanking this mutation revealed that it may represent a tyrosine-kinase-binding site, which could suggest a mechanism whereby activation of the pore component of the complex could be selectively modulated. Such modulation could be of considerable importance in long-range signalling in astrocytes where the P2X7R complex provides regenerative signal relay for the transmission of ICWs (intercellular calcium waves) (Anderson and Nedergaard, 2003; Duan et al., 2003; Anderson et al., 2004; Suadicani et al., 2006).
Recent studies have revealed that the gap junction-like protein Panx1 (Pannexin1), a vertebrate homologue of invertebrate gap junction proteins, is part of the pore forming unit of the P2X7R (Pelegrin and Surprenant, 2006; Locovei et al., 2007; Iglesias et al., 2008). As such, non-junctional channels formed by Panx1 provide sites of ATP release from several cell types including erythrocytes, taste bud cells and astrocytes (Locovei et al. 2006a; Huang et al., 2007a; Scemes et al., 2007).
In the present study we used astrocytes obtained from two mouse strains, the C57Bl/6 strain which contains a P451L mutation in the P2X7R CT, and the Balb/C strain which contains non-mutated receptors, to investigate the extent to which activation of Panx1 through the P2X7R depends on the proper amino acid sequence of the P2X7R. Our findings indicate that the sequence spanning the SH3 domain of the P2X7R determines the magnitude of the P2X7R-mediated Panx1 inward currents, ATP release and transmission of calcium waves in astrocytes.
MATERIALS AND METHODS
Astrocyte cultures
Primary cultures of cortical astrocytes derived from neonatal Balb/C and C57Bl/6J mice were prepared as previously described (Scemes and Spray, 1998). Briefly, cortices were separated from whole brains after removal of meninges and trypsinized (0.1% trypsin at 37°C). Cells were collected by centrifugation [355 g for 5 min at room temperature (23°C)], pellets were suspended in DMEM (Dulbecco’s modified Eagle’s medium; CellGro) supplemented with 10% (v/v) FBS (fetal bovine serum; Gibco) and antibiotics (50 units/ml penicillin and 50 μg/ml streptomycin; CellGro), and cells were seeded in 60 mm culture dishes. Astrocyte cultures were maintained for 2–3 weeks in culture (100% humidity; 95% air/5% CO2 at 37°C) at which time approx. 95–98% of the cells in culture were immunopositive for GFAP (glial fibrillary acidic protein). All animal handling and experimental protocols were approved by the Animal Care and Use Committee of the Albert Einstein College of Medicine.
Electrophysiology
Astrocytes were plated at low density on coverslips 24–48 h prior to recordings, as previously described (Iglesias et al., 2008; R. Iglesias, G. Dahl, F. Qiu, D.C. Spray and E. Scemes, unpublished data). Briefly, the whole-cell patch–clamp configuration was performed at room temperature on solitary astrocytes bathed in external solution containing 147 mM NaCl, 10 mM Hepes, 13 mM glucose, 2 mM CaCl2, 1 mM MgCl2 and 5 mM KCl (pH 7.4). The pipette solution contained 130 mM CsCl, 10 mM EGTA, 10 mM Hepes and 0.5 mM CaCl2 (pH 7.2). Activation of Panx1 channels by voltage was performed using a ramp protocol with voltages from the holding potential of −60 mV to +100 mV. To analyse the participation of Panx1 channels in agonist-induced P2X7R activation, the P2X7R agonist BzATP (50 μM; Sigma–Aldrich) was superfused for 5–10 s. After the first response to the agonist, test compounds were superfused for 5 min prior to the second addition of the P2X7R agonist and, 5 min after rinsing, agonist was re-applied to test reversibility. Compounds included the gap junction and Panx1 channel blockers MFQ (mefloquine; BioBlocks; used at 100 nM) and CBX (carbenoxolone; Sigma; used at 50 μM), and the Src inhibitor PP2 (Tocris; used at 100 μM). Electrophysiological recordings were obtained using an Axopatch 200B amplifier and pClamp9 software for data acquisition and analysis.
Panx1 siRNA (small interfering RNA)
In some experiments, astrocytes were treated with 50 nM siRNA corresponding to the mouse Panx1 sequence (GGUGCUGAACAUUAAA) using 6 μl of siRNA/1.5 ml of Oligofectamine™ reagent (Invitrogen), as previously described (Locovei et al., 2007; Scemes et al., 2007; Iglesias et al., 2008). After overnight exposure, transfection reagents were removed and cells were incubated for 30 h in DMEM/FBS before use.
TAT-P2X7 peptides
Membrane permeant TAT peptides (SLHDSPPTPGQGGGYGRKKRRQRRR; 78–90% purity) corresponding to the SH3 domain of the Balb/C mouse P2X7R CT were synthesized by GeneScript. For the assays, cells were treated for 15 min with 10 μM of the TAT-P2X7 peptide at 37°C, as previously described (Iglesias et al., 2008).
LY (Lucifer Yellow) microinjections
To evaluate the degree of dye-coupling between astrocytes in confluent cultures derived from C57Bl/6 and Balb/C mice, we used the microinjection assay. Astrocytes were impaled with a microelectrode (25 MΩ when filled with 3 M KCl) filled with the gap-junction-permeant fluorophore LY [5% (w/v) in 150 mM LiCl; Sigma–Aldrich] and the dye was injected by iontophoresis (continuous current of 0.1 μA) for 5 min using an electrometer (Model 3100; A-M Systems). After removal of the microelectrode from the injected cell, images were immediately acquired using a CoolSNAP-HQ2 CCD (charge-coupled device) camera (Photometrics) attached to a Nikon inverted microscope equipped with 10×dry objective (NA 0.3) and FITC filter sets. The number of cell tiers surrounding the injected cell containing LY were counted and the results expressed as the mean±S.E.M.; at least 10 injections were performed in 3–4 different astrocyte cultures.
ATP release
The amount of ATP released in the bathing solution from astrocytes pre-stimulated with BzATP was measured using the luciferin/luciferase assay (Molecular Probes, Invitrogen), as previously described (Striedinger et al., 2007; Striedinger and Scemes, 2008). Confluent cultures of astrocytes plated on 60 mm dishes were washed twice in PBS and then exposed for 3 min at room temperature to PBS containing 300 μM BzATP. After complete removal and washout of the agonist, cells were bathed in PBS for 2 min before collection of samples of BzATP-induced ATP release. Intracellular ATP levels were measured from supernatants following whole-cell lysis in Tris-buffered solution (10 mM Tris and 150 mM NaCl, pH 7.4) containing 1% Triton X-100. Extracellular ATP (released into the bathing solution from stimulated and non-stimulated astrocytes) was measured from aliquots of bathing solution. For each measurement, 50 μl of a buffered solution containing luciferin (50 μM) and luciferase (1.25 μg/ml) was placed in triplicate in a 96-well plate luminometer (Veritas) for background luminescence subtraction. Reactions were started by adding 5 μl of the experimental samples and luminescence values acquired using a 1 s integration time. The amount of ATP in the samples was calculated from standard curves (using ATP from 50 nM to 5000 nM) and normalized for the total amount of protein, using the BCA (bicinchoninic acid) assay (Pierce).
ICWs
Confluent astrocyte cultures plated on glass-bottomed MatTek dishes (MatTek) were loaded for 30 min at 37°C with 5 μM Fluo-3-AM (Molecular Probes, Invitrogen) and then bathed in DPBS (Dulbecco’s PBS, pH 7.4; Cellgro). ICWs were induced by focal mechanical stimulation of single astrocytes and the radius of calcium wave spread from the point of stimulation was measured before and after exposure to LDPBS (low-divalent PBS; containing nominally zero Ca2+ and 43 μM MgCl2, prepared by dissolving 1 mM MgCl2 and 1 mM EDTA in the Ca2+- and Mg+2-free DPBS), as previously described (Suadicani et al., 2006). Images were acquired using a Nikon TE2000 inverted microscope equipped with a CCD camera (Orca-ER; Hamamatsu Photonics) and a 10×Plan Fluor objective (NA 0.3). Changes in Fluo-3 fluorescence intensity emitted at 488 nm excitation (Lambda DG-4; Sutter Instruments) were acquired at 1 Hz using Metafluor software (Molecular Devices).
Western blot analysis
Samples of whole-cell lysates were electrophoresed in SDS/PAGE (4–20%) mini-gels (Bio-Rad) and then transferred to nitrocellulose membranes (Whatman). Immunoblots using primary antibodies were performed after 1 h incubation of membranes with blocking solution [2% (w/v) dry non-fat milk in 1×PBS containing 0.4% Tween 20; Sigma–Aldrich]. After several washes with 1×PBS/Tween 20, membranes were incubated with HRP (horseradish peroxidase)-conjugated secondary antibodies (goat anti-rabbit-HRP, 1:2000; goat anti-mouse-HRP, 1:2000; rabbit anti-chicken-HRP, 1:500; all from Santa Cruz Biotechnology). Detection of bands was performed on X-ray films (Kodak) following incubation with ECL (enhanced chemiluminescence) reagents (Amersham Pharmacia Biotechnology). The rabbit anti-P2X7R antibody (1:500; Alomone), chicken anti-Panx1 antibody (1:2000; a gift from Dr Gerhard Dahl, Department of Physiology and Biophysics, University of Miami, Miami, FL, U.S.A.), and the mouse anti-GAPDH antibody (glyceraldehyde-3-phosphate dehydrogenase, 1:10000; Fitzgerald International) were used in the present study.
RESULTS
P2X7R-mediated Panx1 currents are distinct in astrocytes of two mouse strains
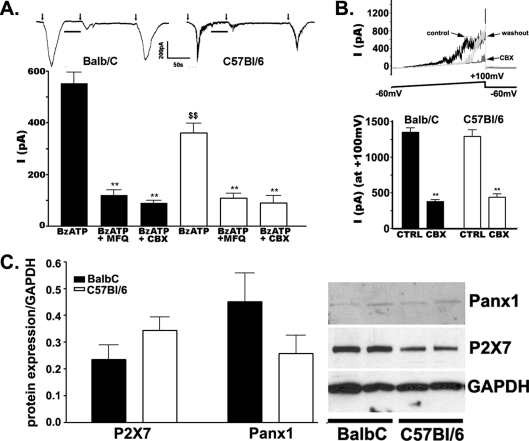
To evaluate whether P2X7R-mediated activation of Panx1 channels in astrocytes involved the domain surrounding the P451L point mutation on the P2X7R, we performed electrophysiological studies on cortical astrocytes derived from C57Bl/6 and Balb/C mice. As shown in Figure 1, 50 μM BzATP induced larger currents in Balb/C astrocytes (461.6±29.3 pA, n = 7 cells) than in C57Bl/6 astrocytes (352.9±19.72 pA, n = 9 cells; P<0.05, measured using an unpaired Student’s t test) (Figure 1A). Bath application of gap junction and Panx1 blockers MFQ (100 nM) or CBX (50 μM), greatly blocked Panx1 currents in both cell types. The remaining MFQ- and CBX-insensitive currents, most likely due to cation flux through the P2X7R channel, were not significantly different in astrocytes of the two mouse strains [Balb/C(MFQ), 120.3±19.6 pA, n = 4 cells; C57Bl/6(MFQ), 108.8±17.7 pA, n = 4 cells; Balb/C(CBX), 89.0±11.4 pA, n = 6 cells; C57Bl/6(CBX), 89.9±28.3 pA, n = 6 cells; P>0.05, measured using an unpaired Student’s t test; Figure 1A].
Figure 1. The proline-rich region of the P2X7R CT mediates activation of Panx1 channels.
(A) Top panel: examples of current traces obtained from Balb/C and C57Bl/6 astrocytes induced by a 5 s application (indicated by arrows) of BzATP (50 μM) are greatly reduced by 60 s pre-incubation (lines) with 100 nM MFQ and 50 μM CBX; after a 5 min washout of blockers, agonist-induced currents were of similar amplitudes as those recorded after the first application. Bottom panel: histograms show the mean values±S.E.M. obtained from 4–6 cells. Note the significantly smaller currents recorded from C57Bl/6 astrocytes compared with those recorded from Balb/C cells following agonist application. Astrocytes were plated on cover slips at a low density and recordings were performed on solitary astrocytes while holding the membrane potential at −60 mV. (B) Top panel: an example of current traces obtained from Balb/C astrocytes recorded during a voltage ramp performed in the absence (control) and presence of CBX (50 μM), and after CBX washout. Bottom panel: histograms showing the mean values±S.E.M. of current amplitudes induced by voltage ramps (−60 to +100 mV) at a −60 mV holding potential recorded from Balb/C and C57Bl/6 astrocytes (n = 4–12 cells) in the absence (CTRL) and presence of 50 μM CBX. Note that current amplitudes at +100 mV were not significantly different in astrocytes derived from the two mouse strains and that CBX significantly reduced voltage-activated outward currents in both cell types. (C) Left-hand panel: histograms showing the mean values±S.E.M. (n = 5 independent experiments) of P2X7R and Panx1 expression levels in the two mouse strains, indicating that in Balb/C and C57Bl/6 astrocytes these two proteins are equally expressed. Right-hand panel: representative blot showing expression of Panx1 and P2X7R in Balb/C and C57Bl/6 astrocytes. **P<0.0001 (compared with the control) was obtained using one-way ANOVA, followed by Newman–Keuls multiple comparison test. $$P<0.05 (measured using a Student’s t test) was obtained when comparing amplitudes of BzATP-induced inward currents in Balb/C and C57Bl/6 astrocytes. GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Evidence that the larger currents evoked by BzATP in Balb/C as compared with C57Bl/6 astrocytes were unrelated to altered activity of Panx1 channels in these strains was obtained using electrophysiological recordings. As shown in Figure 1(B), similar amplitudes of voltage-activated Panx1 currents were recorded in astrocytes from both mouse strains in response to voltage ramps (Balb/C, 472.0±65.4 pA, n = 8 cells; C57Bl/6, 567.2±59.8 pA, n = 12 cells; P>0.05, measured using an unpaired Student’s t test). The gap junction and Panx1 blocker CBX (50 μM) greatly reduced Balb/C and C57Bl/6 voltage-activated outward currents to similar levels [Balb/C(CBX), 383.7±26.6 pA, n = 4 cells; C57Bl/6(CBX), 444.8±47.9 pA, n = 4 cells; P>0.05, measured using an unpaired Student’s t test]. Moreover, since no significant changes in Panx1 and P2X7R protein expression levels were detected by Western blot analysis (Figure 1C), it is likely that current amplitude differences recorded between the two types of astrocytes in response to BzATP (see Figure 1A) are related to the single point mutation of the P2X7R, which is probably an important site for the signal transduction events leading to Panx1 channel opening following receptor activation.
Functional implications of the P2X7R P451L mutation to astrocyte calcium signalling
Our previous studies indicated that the P2X7R and Panx1 contribute to the transmission of calcium signals among astrocytes by providing sites of ATP release (Suadicani et al., 2006; Scemes et al., 2007). In order to evaluate the functional implications of the P451L point mutation of the P2X7R CT for astrocyte intercellular communication, we measured the amount of ATP released and the distance of ICW transmission between astrocytes derived from C57Bl/6 and Balb/C mice.
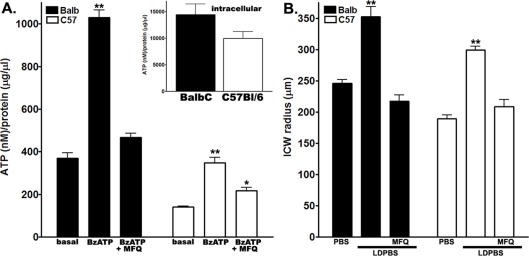
Pre-treatment of astrocytes with 300 μM BzATP for 3 min induced subsequent ATP release (Figure 2A). The amount of ATP present in the bathing solution of stimulated cells was 3-fold higher in Balb/C than in C57Bl/6 astrocytes (Balb/C, 1030.0±35.5 nM; C57Bl/6, 348.0±24.6 nM; n = 12 measurements; P<0.001, measured using an unpaired Student’s t test). Evidence that Panx1 mediates BzATP-induced ATP release was obtained by the finding that MFQ blocked ATP release from astrocytes of both mouse strains (Balb/C, from 1030.0±35.5 nM to 466.7±20.3 nM, n = 9–12 measurements; C57Bl/6, from 348.0±24.6 nM to 217.9±14.4 nM, n = 9–12 measurements; Figure 2A). Because Balb/C and C57Bl/6 cultured astrocytes have similar intracellular ATP levels (Balb/C, 14465.0±1986.0 nM; C57Bl/6, 9967.0±1289.0 nM; n = 12, P>0.05; inset in Figure 2A), and express similar levels of P2X7R and Panx1 proteins (Figure 1C), it is likely that the P451L mutation in the P2X7R of C57Bl/6 interferes with P2X7R–Panx1-mediated ATP release.
Figure 2. Astrocytes from Balb/C and C57Bl/6 mice release different amounts of ATP and display distinct ranges of ICW transmission.
(A) Histograms showing the mean values±S.E.M. of intracellular ATP content (inset) and the amount of ATP released from Balb/C (solid bars) and C57Bl/6 (open bars) astrocytes following BzATP (300 μM) addition in the absence and presence of MFQ (100 nM). Note the similar intracellular ATP levels in both mouse strain astrocytes (inset), and the significantly lower levels of basal and BzATP-induced ATP release in C57Bl/6 compared with Balb/C astrocytes. (B) Histograms showing mean values±S.E.M. of the radius of mechanically induced ICW spread in Balb/C (solid bars) and C57Bl/6 (open bars) astrocyte cultures when bathed in PBS and in LDPBS, in the absence and presence of MFQ (100 nM). Note the shorter distance of ICW spread in C57Bl/6 compared with Balb/C astrocyte cultures and the inhibition of ICW amplification in both types of astrocytes by MFQ. **P<0.0001 (compared with control) was obtained using one-way ANOVA, followed by Newman–Keuls multiple comparison test.
ICW transmission in astrocytes has been shown to be mediated by ATP-sensitive P2Rs and by gap junctions (reviewed in Scemes and Giaume, 2006). For efficient calcium signal transmission between astrocytes, the levels of ATP released play important roles affecting both modes of transmission (extracellular and intercellular). Higher levels of ATP will diffuse longer distances and thus activate cells further away and also will generate higher levels of calcium-mobilizing second messengers that are permeable to gap junction intercellular channels. Thus it would be expected that ICWs would travel shorter distances if the amount of ATP released was reduced. Figure 2(B) shows that, indeed, the radius of ICW spread between C57Bl/6 astrocytes bathed in LDPBS is smaller than that between Balb/C cells (C57Bl/6, 299.0±6.3 μm; Balb/C, 352.5±16.0 μm; P<0.05, measured using a Student’s t test; n = 62–347 radius measurements, from 20–87 fields; Figure 2B). To evaluate for possible differences in gap-junctional coupling among astrocytes from the two mouse strains that might have contributed to the extent of ICW spread, we used the LY microinjection approach. Results from these experiments indicated that the degree of dye-coupling in confluent cultures of Balb/C (4.7±0.2 cell tiers, n = 48 measurements from 12 injections) and C57Bl/6 (5.0±0.2 cell fields, n = 60 measurements from 15 injections) astrocytes was not statistically different (P = 0.30, measured using an unpaired Student’s t test). Taken together these results suggest that decreased P2X7R–Panx1-mediated ATP release from C57Bl/6 astrocytes reduces the distance of spread of ICW compared with that of Balb/C cells.
To determine whether Panx1 participates in the amplification of ICWs, the radius of mechanically induced ICWs was measured in the presence of the Panx1 channel inhibitor MFQ. As shown in Figure 2(B), blockade of Panx1 with 100 nM MFQ prevented amplification of ICWs in both types of astrocytes (Balb/C, from 352.5±16.0 μm to 217.5±9.7 μm, P<0.0001, measured using an unpaired Student’s t test, n = 24–62 radii from 6–16 fields; C57Bl6, from 299.0±6.3 μm to 208.3±11.5 μm, P = 0.0002, measured using an unpaired Student’s t test, n = 24–347 radii from 6–87 fields). This blockade of ICW amplification was similar to that recorded when Panx1 expression was knocked-down using siRNA (Balb/C, from 352.5±16.0 μm to 191.4±7.6 μm, n = 24–80 radii from 6–20 fields; C57Bl/6, from 286.1±16.1 μm to 207.8±8.2 μm, n = 52–80 radii from 13–20 fields; see also Figure 4 in Scemes et al., 2007). These later results therefore support the hypothesis that Panx1 in astrocytes may, under defined conditions, function as a conduit for the release of ATP, as has been shown for other cell types (Huang et al., 2007a; Locovei et al., 2006a).
The SH3 domain surrounding the P451L mutation of the P2X7R contributes to Panx1 activation and amplification of ICWs
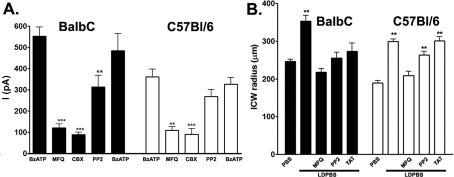
We have recently shown in a macrophage cell line that interaction of a Src tyrosine kinase to the SH3 domain of the P2X7R leads to the activation of Panx1 (Iglesias et al., 2008). To evaluate whether a transduction mechanism similar to that reported in J774 macrophages was also present in astrocytes, we used the Src inhibitor PP2 in electrophysiological studies. As shown in Figure 3(A) our electrophysiological results indicate that PP2 (100 μM) significantly attenuates the BzATP-induced inward current in Balb/C astrocytes by 57% (from 553.0±43.3 pA to 313.0±55.3 pA, n = 4 cells; P<0.05, measured using an unpaired Student’s t test). In contrast with Balb/C astrocytes, PP2 (100 μM) did not significantly decrease inward currents activated by brief application of 50 μM BzATP in C57Bl/6 astrocytes (from 360.4±37.5 pA to 268.1±33.7 pA, n = 4 cells; P>0.05, measured using an unpaired Student’s t test; Figure 3A).
Figure 3. A Src tyrosine kinase mediates Panx1 activation through P2X7R.
(A) Histograms showing the mean values±S.E.M. of current amplitudes induced by BzATP (50 μM) while holding the membrane potential at −60 mV recorded from Balb/C (solid bars) and C57Bl/6 (open bars) astrocytes in the absence and presence of PP2 (10 μM) (n = 4 cells). Note that PP2 significantly reduced current amplitudes in Balb/C, but not in C57Bl/6, astrocytes. (B) Histograms of the mean values±S.E.M. of the ICW radius obtained from Balb/C (solid bars) and C57Bl/6 (open bars) astrocytes when bathed in PBS and LDPBS untreated and treated with MFQ, PP2 and TAT-P2X7 peptide. Note that the exposure to PP2 and the TAT-P2X7 peptide prevented the amplification of ICW seen in LDPBS only in Balb/C astrocytes. **P<0.0001 (compared with control) was obtained using one-way ANOVA, followed by Newman–Keuls multiple comparison test.
Thus these results suggest that the opening of Panx1 channels in Balb/C astrocytes following P2X7R stimulation most likely involves a tyrosine kinase of the Src family. The poor efficacy of PP2 to prevent Panx1 activation in C57Bl/6 astrocytes is probably related to the single point mutation (P451L) in the P2X7R in these cells, which may interfere with interaction with the P2X7R and is necessary for proper Src signalling and Panx1 recruitment into the response.
In agreement with electrophysiological recordings, the amplification of ICWs (from 246.2±5.9 μm to 352.5±16.0 μm, n = 62–118 radii; Figure 3B) seen in Balb/C astrocytes was totally prevented by PP2 (255.2±15.6 μm, n = 21 radii; Figure 3B), whereas this Src inhibitor did not prevent amplification of ICW spread between C57Bl/6 astrocytes [ICW(PBS) = 189.4±6.0 μm, n = 95 radii; ICW(LDPBS) = 299.0±6.3 μm, n = 347 radii; ICW(LDPBS+PP2) = 263.3±10.1 μm, n = 24 radii; Figure 3B].
Further support for the participation of the P2X7R SH3 domain in the transduction signalling events leading to the agonist-induced Panx1 channel opening was obtained by treating astrocytes with a membrane-permeant peptide (TAT-P2X7) spanning the P451L mutation of the P2X7R SH3 domain. As shown in Figure 3(B), the TAT-P2X7 peptide, which we have previously shown to block BzATP-induced Panx1 currents (Iglesias et al., 2008), prevented the amplification of ICWs among astrocytes derived from Balb/C [ICW(LDPBS) = 352.5±16.0 μm, n = 62 radii; ICW(LDPBS+peptide) = 273.3±21.4 μm, n = 12 radii; P<0.05, measured using an unpaired Student’s t test], but not from C57Bl/6 [ICW(LDPBS) = 299.0±6.3 μm, n = 347 radii; ICW(LDPBS+peptide) = 301.0±11.3 μm, n = 20 radii] mice. Our results therefore support the hypothesis that the SH3 domain of the P2X7R is an important site that leads to Panx1 channel opening.
DISCUSSION
Release of ‘gliotransmitters’ from astrocytes allows modulation of neuronal activity, controls blood flow in the brain and contributes to long-range calcium signalling. Diverse mechanisms have been described to be involved in ‘gliotransmitter’ release from astrocytes, including diffusion through regulated secretion and pore-forming membrane channels (Duan and Neary, 2006; Kimelberg et al., 2006; Montana et al., 2006; Oliet and Mothet, 2006; Spray et al., 2006). Among these channels, the pore-forming P2X7R plays a dual role in cell signalling; as a cation channel it contributes to the total calcium influx, promoting the calcium-dependent release of glutamate and GABA from pre-synaptic nerve terminals (Deuchars et al., 2001; Lundy et al., 2002; Sperlagh et al., 2002; Miras-Portugal et al., 2003; Papp et al., 2004), and as a pore-forming channel it plays an important role in glia calcium signalling by providing sites of ATP and glutamate release (Duan et al., 2003; Anderson et al., 2004; Suadicani et al., 2006).
The first demonstration that the P2X7R was associated with a large, non-selective conductance (called the ‘P2Z’ pore) activated by prolonged or repetitive ATP stimulation was provided by Surprenant et al. (1996). At that time, two hypotheses were proposed to explain the permeabilizing nature of the P2X7R: that the changes in permeability were related to conformational alteration of the P2X7R itself provided by its CT, and that the CT interacted with another protein with permeabilization capabilities (Surprenant et al., 1996; Coutinho-Silva and Persechini, 1997; Persechini et al., 1998; Virginio et al., 1999; Smart et al., 2002; Boldt et al., 2003; Faria et al., 2005; Schachter et al., 2008). The second hypothesis gained support, about a decade later, when Panx1 was identified as being the P2X7R pore unit that allows the diffusion of large molecules across the plasma membrane following receptor stimulation (Pelegrin and Surprenant, 2006; Locovei et al., 2007; Iglesias et al., 2008).
Panx1 forms large conductance (400–500 pS) voltage- and mechano-sensitive channels that can also be activated following P2 receptor stimulation (Bao et al., 2004; Locovei et al., 2006b). Panx1 transcripts are found in astrocytes in vitro and in vivo (Huang et al., 2007b; Cahoy et al., 2008), and the channels formed by this protein possess properties that have been attributed to connexin hemichannels (Figure 1; R. Iglesias, G. Dahl, F. Qiu, D.C. Spray and E. Scemes, unpublished data).
Regarding the mechanisms by which Panx1 is recruited by the P2X7R activation, we recently reported that, in a macrophage cell line, an Src tyrosine kinase, which was found associated to the P2X7R complex, is an important signalling molecule mediating the initial steps leading to activation of Panx1 through the P2X7R (Iglesias et al., 2008). Support for the participation of Src was obtained by showing that both a membrane-permeant peptide spanning the P2X7R SH3 domain and the Src tyrosine kinase inhibitor, PP2, greatly attenuated P2X7R-induced Panx1 currents and membrane permeabilization in this macrophage-like cell (Iglesias et al., 2008).
In the present study we have obtained further evidence pointing to the importance of the SH3 domain located within the P2X7R CT for optimal recruitment of Panx1 into the response following receptor activation. For this, we compared astrocytes derived from two mouse strains in which the P2X7R amino acid sequence differs. In the C57Bl/6 mouse strain, a single amino acid mutation has been shown to impair pore formation (Adriouch et al., 2002; Le Stunff et al., 2004), most likely because the P451L mutation renders a region of the CT of the P2X7R less effective as an SH3-binding domain than in the non-mutated Balb/C strain.
In agreement with these previous studies, our results revealed that Balb/C astrocytes display larger Panx1 currents and release significantly more ATP following P2X7R activation, and display extended transmission of ICWs, than C57Bl/6 astrocytes. Furthermore, our results showing that both a membrane-permeant peptide spanning the P451L point mutation (TAT-P2X7 peptide) and the Src tyrosine kinase inhibitor, PP2, prevented the amplification of ICWs between Balb/C astrocytes, and that PP2 reduced Panx1 currents following P2X7R activation, greatly support the hypothesis that the proline-rich region of the receptor is an important domain for an Src tyrosine kinase-mediated signal transduction event that leads to activation of Panx1 channels.
Our results showing that a spontaneous point mutation (P451L) in the mouse P2X7R has a great impact on receptor function is also in accordance with previous studies performed on rodent and human P2X7R mutants, indicating that polymorphisms in the CT SH3 domain lead to dysfunctional receptors (Adriouch et al., 2002; Le Stunff et al., 2004). Interestingly, from the stand-point of brain function, a single nucleotide polymorphism, resulting in the missense mutation Q460R (12q23–24 region) has been linked to mood disorders such as bipolar and major depressive disorders (Barden et al., 2006; Lucae et al., 2006; McQuillin et al., 2008). It is not yet clear how altered function of the P2X7R would mediate these CNS (central nervous system) disorders. One possible scenario includes the existence of an inflammatory signalling pathway involving the P2X7R–Panx1 complex in astrocytes, as has been described for immune cells. For instance, in macrophages, activation of Panx1 has been shown to play crucial roles in inflammation and to interact with the inflammasome leading to the processing and release of cytokines [IL (interleukin)-1β and IL-18] (Pelegrin and Surprenant, 2006; Kanneganti et al., 2007). The existence of such an inflammatory P2X7R–Panx1 complex in astrocytes may endow these cells with a mechanism to alter production of cytokines, chemokines, and growth and trophic factors, consistent with findings showing changes in circulating inflammatory mediators in blood samples of patients with mood disorders (Song et al., 1994; Maes et al., 1995; Barden et al., 2006). Thus, our present results showing that the P2X7R P451L mutation alters Panx1-mediated ATP release and long-range calcium signalling among astrocytes suggest that interference with such signalling may have a wide impact on brain function.
ACKNOWLEDGEMENTS
We gratefully acknowledge the technical assistance of Ms Melissa Aleksey and Mrs Aisha Cordero.
FUNDING
This work was supported by the National Institutes of Health National Institute of Neurological Disorders and Stroke [grant numbers NS052245 (to E.S.), NS041282 (to D.C.S.)] and the American Heart Association [grant number 0735377N (to S.O.S)].
REFERENCES
- Adriouch S, Dox C, Welge V, Seman M, Koch-Nolte F, Haag F. Cutting edge: a natural P451L mutation in the cytoplasmic domain impairs the function of the mouse P2X7 receptor. J Immunol. 2002;169:4108–4112. doi: 10.4049/jimmunol.169.8.4108. [DOI] [PubMed] [Google Scholar]
- Anderson CM, Bergher JP, Swanson RA. ATP-induced ATP release from astrocytes. J Neurochem. 2004;88:246–256. doi: 10.1111/j.1471-4159.2004.02204.x. [DOI] [PubMed] [Google Scholar]
- Anderson CM, Nedergaard M. Astrocyte-mediated control of cerebral microcirculation. Trends Neurosci. 2003;26:340–344. doi: 10.1016/S0166-2236(03)00141-3. [DOI] [PubMed] [Google Scholar]
- Bao L, Locovei S, Dahl G. Pannexin membrane channels are mechanosensitive conduits for ATP. FEBS Lett. 2004;572:65–68. doi: 10.1016/j.febslet.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Barden N, Harvey M, Gagné B, Shink E, Tremblay M, Raymond C, Labbé M, Villeneuve A, Rochette D, Bordeleau L, Stadler H, Holsboer F, Müller-Myhsok B. Analysis of single nucleotide polymorphisms in genes in the chromosome 12Q24.31 region points to P2RX7 as a susceptibility gene to bipolar affective disorder. Am J Med Genet B Neuropsychiatr Genet. 2006;141B:374–382. doi: 10.1002/ajmg.b.30303. [DOI] [PubMed] [Google Scholar]
- Boldt W, Klapperstück M, Büttner C, Sadtler S, Schmalzing G, Markwardt F. Glu496Ala polymorphism of human P2X7 receptor does not affect its electrophysiological phenotype. Am J Physiol Cell Physiol. 2003;284:C749–C756. doi: 10.1152/ajpcell.00042.2002. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Purinergic signaling and disorders of the central nervous system. Nat Rev Drug Discov. 2008;7:575–590. doi: 10.1038/nrd2605. [DOI] [PubMed] [Google Scholar]
- Cahoy JD, Emery B, Kaushal A, Foo LC, Zamanian JL, Christopherson KS, Xing Y, Lubischer JL, Krieg PA, Krupenko SA, Thompson WJ, Barres BA. A transcriptome database for astrocytes, neurons, and oligodendrocytes: a new resource for understanding brain development and function. J Neurosci. 2008;28:264–278. doi: 10.1523/JNEUROSCI.4178-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutinho-Silva R, Persechini PM. P2Z purinoceptor-associated pores induced by extracellular ATP in macrophages and J774 cells. Am J Physiol Cell Physiol. 1997;273:C1793–C1800. doi: 10.1152/ajpcell.1997.273.6.C1793. [DOI] [PubMed] [Google Scholar]
- Deuchars SA, Atkinson L, Brooke RE, Musa H, Milligan CJ, Batten TF, Buckley NY, Parson SH, Deuchars J. Neuronal P2X7 receptors are targeted to presynaptic terminals in the central and peripheral nervous systems. J Neurosci. 2001;21:7143–7152. doi: 10.1523/JNEUROSCI.21-18-07143.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan S, Anderson CM, Keung EC, Chen Y, Chen Y, Swanson RA. P2X7 receptor-mediated release of excitatory amino acids from astrocytes. J Neurosci. 2003;23:1320–1328. doi: 10.1523/JNEUROSCI.23-04-01320.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan S, Neary JT. P2X7 receptors: properties and relevance to CNS function. Glia. 2006;54:738–746. doi: 10.1002/glia.20397. [DOI] [PubMed] [Google Scholar]
- Faria RX, Defarias FP, Alves LA. Are second messengers crucial for opening the pore associated with P2X7 receptor? Am J Physiol. 2005;288:C260–C270. doi: 10.1152/ajpcell.00215.2004. [DOI] [PubMed] [Google Scholar]
- Huang YJ, Maruyama Y, Dvoryanchikov G, Pereira E, Chaudhari N, Roper SD. The role of pannexin 1 hemichannels in ATP release and cell–cell communication in mouse taste buds. Proc Natl Acad Sci USA. 2007a;104:6436–6441. doi: 10.1073/pnas.0611280104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Grinspan JB, Abrams CK, Scherer SS. Pannexin1 is expressed by neurons and glia but does not form functional gap junctions. Glia. 2007b;55:46–56. doi: 10.1002/glia.20435. [DOI] [PubMed] [Google Scholar]
- Iglesias R, Locovei S, Roque A, Alberto AP, Dahl G, Spray DC, Scemes E. P2X7 receptor–Pannexin1 complex: pharmacology and signaling. Am J Physiol Cell Physiol. 2008;295:C752–C760. doi: 10.1152/ajpcell.00228.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanneganti TD, Lamkanfi M, Kim YG, Chen G, Park JH, Franchi L, Vandenabeele P, Núñez G. Pannexin-1-mediated recognition of bacterial molecules activates the cryopyrin inflammasome independent of Toll-like receptor signaling. Immunity. 2007;26:383–385. doi: 10.1016/j.immuni.2007.03.008. [DOI] [PubMed] [Google Scholar]
- Kimelberg HK, MacVicar BA, Sontheimer H. Anion channels in astrocytes: biophysics, pharmacology, and function. Glia. 2006;54:747–757. doi: 10.1002/glia.20423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Stunff H, Auger R, Kanellopoulos J, Raymond MN. The Pro-451 to Leu polymorphism within the C-terminal tail of P2X7 receptor impairs cell death but not phospholipase D activation in murine thymocytes. J Biol Chem. 2004;279:16918–16926. doi: 10.1074/jbc.M313064200. [DOI] [PubMed] [Google Scholar]
- Locovei S, Bao L, Dahl G. Pannexin 1 in erythrocytes: function without a gap. Proc Natl Acad Sci USA. 2006a;103:7655–7659. doi: 10.1073/pnas.0601037103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locovei S, Wang J, Dahl G. Activation of pannexin 1 channels by ATP through P2Y receptors and by cytoplasmic calcium. FEBS Lett. 2006b;580:239–244. doi: 10.1016/j.febslet.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Locovei S, Scemes E, Qiu F, Spray DC, Dahl G. Pannexin1 is part of the pore forming unit of the P2X7 receptor death complex. FEBS Lett. 2007;581:483–488. doi: 10.1016/j.febslet.2006.12.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucae S, Salyakina D, Barden N, Harvey M, Gagné B, Labbé M, Binder EB, Uhr M, Paez-Pereda M, Sillaber I, Ising M, Brückl T, Lieb R, Holsboer F, Müller-Myhsok B. P2RX7, a gene coding for a purinergic ligand-gated ion channel, is associated with major depressive disorder. Hum Mol Genet. 2006;15:2438–2445. doi: 10.1093/hmg/ddl166. [DOI] [PubMed] [Google Scholar]
- Lundy PM, Hamilton MG, Gong W, Vair C, Sawyer TW, Frew R. Stimulation of Ca2+ influx through ATP receptors on rat brain synaptosomes: identification of functional P2X7 receptor subtypes. Br J Pharmacol. 2002;135:1616–1626. doi: 10.1038/sj.bjp.0704624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes M, Meltzer HY, Bosmans E, Bergmans R, Vandoolaeghe E, Ranjan R, Desnyder R. Increased plasma concentrations of interleukin-6, soluble interleukin-6, soluble interleukin-2 and transferrin receptor in major depression. J Affect Disord. 1995;34:301–309. doi: 10.1016/0165-0327(95)00028-l. [DOI] [PubMed] [Google Scholar]
- McQuillin A, Bass NJ, Choudhury K, Puri V, Kosmin M, Lawrence J, Curtis D, Gurling HM. Case-control studies show that a non-conservative amino-acid change from a glutamine to arginine in the P2RX7 purinergic receptor protein is associated with both bipolar- and unipolar-affective disorders. Mol Psychiatry. 2008 doi: 10.1038/mp.2008.6. doi: 10.1038/mp.2008.6. [DOI] [PubMed] [Google Scholar]
- Miras-Portugal MT, Diaz-Hernandez M, Giraldez L, Hervas C, Gomez-Villafuertes R, Sen RP, Gualix J, Pintor J. P2X7 receptors in brain: presence in synaptic terminals of granule cells. Neurochem Res. 2003;28:1597–1605. doi: 10.1023/a:1025690913206. [DOI] [PubMed] [Google Scholar]
- Montana V, Malarkey EB, Verderio C, Matteoli M, Parpura V. Vesicular transmitter release from astrocytes. Glia. 2006;54:700–715. doi: 10.1002/glia.20367. [DOI] [PubMed] [Google Scholar]
- North RA. Molecular physiology of P2X receptors. Physiol Rev. 2002;82:1013–1067. doi: 10.1152/physrev.00015.2002. [DOI] [PubMed] [Google Scholar]
- Oliet SH, Mothet JP. Molecular determinants of d-serine-mediated gliotransmission: from release to function. Glia. 2006;54:726–737. doi: 10.1002/glia.20356. [DOI] [PubMed] [Google Scholar]
- Papp L, Vizi ES, Sperlagh B. Lack of ATP-evoked GABA and glutamate release in the hippocampus of P2X7 receptor−/− mice. Neuroreport. 2004;15:2387–2391. doi: 10.1097/00001756-200410250-00017. [DOI] [PubMed] [Google Scholar]
- Pelegrin P, Surprenant A. Pannexin-1 mediates large pore formation and interleukin-1β release by the ATP-gated P2X7 receptor. EMBO J. 2006;25:5071–5082. doi: 10.1038/sj.emboj.7601378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persechini PM, Bisaggio RC, Alves-Neto JL, Coutinho-Silva R. Extracellular ATP in the lymphohematopoietic system: P2Z purinoceptors off membrane permeabilization. Braz J Med Biol Res. 1998;31:25–34. doi: 10.1590/s0100-879x1998000100004. [DOI] [PubMed] [Google Scholar]
- Scemes E, Giaume C. Astrocyte calcium waves: what they are and what they do. Glia. 2006;54:716–725. doi: 10.1002/glia.20374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scemes E, Spray DC. Increased intercellular communication in mouse astrocytes exposed to hyposmotic shocks. Glia. 1998;24:74–84. [PMC free article] [PubMed] [Google Scholar]
- Scemes E, Suadicani SO, Dahl G, Spray DC. Connexin and pannexin mediated cell–cell communication. Neuron Glia Biol. 2007;3:199–208. doi: 10.1017/S1740925X08000069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachter J, Motta AP, Souza Zamorano A, da Silva-Souza HA, Guimaraes MZ, Persechini PM. ATP-induced P2X7-associated uptake of large molecules involves distinct mechanisms for cations and anions in macrophages. J Cell Sci. 2008;121:3261–3270. doi: 10.1242/jcs.029991. [DOI] [PubMed] [Google Scholar]
- Smart ML, Panchal RG, Bowser DN, Williams DA, Petrou S. Pore formation is not associated with macroscopic redistribution of P2X7 receptors. Am J Physiol Cell Physiol. 2002;283:C77–C84. doi: 10.1152/ajpcell.00456.2001. [DOI] [PubMed] [Google Scholar]
- Song C, Dinan T, Leonard BE. Changes in immunoglobulin, complement and acute phase protein levels in the depressed patients and normal controls. J Affect Disord. 1994;30:283–288. doi: 10.1016/0165-0327(94)90135-x. [DOI] [PubMed] [Google Scholar]
- Sperlagh B, Kofalvi A, Deuchars J, Atkinson L, Milligan CJ, Buckley NJ, Vizi ES. Involvement of P2X7 receptors in the regulation of neurotransmitter release in the rat hippocampus. J Neurochem. 2002;81:1196–1211. doi: 10.1046/j.1471-4159.2002.00920.x. [DOI] [PubMed] [Google Scholar]
- Spray DC, Ye ZC, Ransom BR. Functional connexin ‘hemichannels’: a critical appraisal. Glia. 2006;54:758–773. doi: 10.1002/glia.20429. [DOI] [PubMed] [Google Scholar]
- Striedinger K, Meda P, Scemes E. Exocytosis of ATP from astrocyte progenitors modulates spontaneous Ca2+ oscillations and cell migration. Glia. 2007;55:652–662. doi: 10.1002/glia.20494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Striedinger K, Scemes E. Interleukin-1β affects calcium signaling and in vitro cell migration of astrocyte progenitors. J Neuroimmunol. 2008;196:116–123. doi: 10.1016/j.jneuroim.2008.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suadicani SO, Brosnan CF, Scemes E. P2X7 receptors mediate ATP release and amplification of astrocytic intercellular Ca2+ signaling. J Neurosci. 2006;26:1378–1385. doi: 10.1523/JNEUROSCI.3902-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surprenant A, Rassendren F, Kawashima E, North RA, Buell G. The cytolytic P2Z receptor for extracellular ATP identified as a P2X receptor (P2X7). Science. 1996;272:735–738. doi: 10.1126/science.272.5262.735. [DOI] [PubMed] [Google Scholar]
- Virginio C, MacKenzie A, North RA, Surprenant A. Kinetics of cell lysis, dye uptake and permeability changes in cells expressing the rat P2X7 receptor. J Physiol. 1999;519:335–346. doi: 10.1111/j.1469-7793.1999.0335m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]