Abstract
The p75NTR (where NTR is neurotrophin receptor) can mediate many distinct cellular functions, including cell survival and apoptosis, axonal growth and cell proliferation, depending on the cellular context. This multifunctional receptor is widely expressed in the CNS (central nervous system) during development, but its expression is restricted in the adult brain. However, p75NTR is induced by a variety of pathophysiological insults, including seizures, lesions and degenerative disease. We have demonstrated previously that p75NTR is induced by seizures in neurons, where it induces apoptosis, and in astrocytes, where it may regulate proliferation. In the present study, we have investigated whether the inflammatory cytokines IL (interleukin)-1β and TNF-α (tumour necrosis factor-α), that are commonly elevated in these pathological conditions, mediate the regulation of p75NTR in neurons and astrocytes. We have further analysed the signal transduction pathways by which these cytokines induce p75NTR expression in the different cell types, specifically investigating the roles of the NF-κB (nuclear factor κB) and p38 MAPK (mitogen-activated protein kinase) pathways. We have demonstrated that both cytokines regulate p75NTR expression; however, the mechanisms governing this regulation are cytokine- and cell-type specific. The distinct mechanisms of cytokine-mediated p75NTR regulation that we demonstrate in the present study may facilitate therapeutic intervention in regulation of this receptor in a cell-selective manner.
Keywords: neurotrophin receptor, nuclear factor κB (NF-κB), p38 mitogen-activated protein kinase (p38 MAPK)
Abbreviations: ERK, extracellular-signal-regulated kinase; IκB, inhibitor of nuclear factor κB; IL-1β, interleukin-1β; IL-1Ra, IL-1 receptor antagonist; JNK, c-Jun N-terminal kinase; MAPK, mitogen-activated protein kinase; MEM, minimal essential medium; NF-κB, nuclear factor κB; NTR, neurotrophin receptor; p-IκB, phosphorylated inhibitor of nuclear factor κB; p-p38, phosphorylated p38 MAPK; RT, reverse transcriptase; TNF-α, tumour necrosis factor-α
INTRODUCTION
The p75NTR (where NTR is neurotrophin receptor) is known to play multiple roles in regulating neuronal survival, death and axonal growth (Greene and Rukenstein, 1981; Rabizadeh et al., 1993; Frade et al., 1996; Maggirwar et al., 1998; Friedman, 2000). p75NTR is more widely expressed during development than in adults (Yan and Johnson, 1988; Friedman, 2000). However, this receptor is re-expressed in several pathological conditions such as traumatic brain injury, seizure, ischaemia, oxidative stress and axonal injury (Kokaia et al., 1998; Roux et al., 1999; Casha et al., 2001; Ramos et al., 2007). The role of p75NTR in these pathological conditions has been proposed to be involved in neurodegeneration. For example, pilocarpine-induced seizures induces p75NTR up-regulation in the hippocampus (Roux et al., 1999) and mediates neuronal apoptosis by activating the caspase 9,6,3 cascade (Troy et al., 2002). These studies suggest that there is a tight correlation between p75NTR expression after injury and neurodegeneration. Although there are many studies demonstrating that p75NTR is up-regulated in pathological conditions, the mechanisms regulating p75NTR expression after injury are not well defined.
IL-1β (interleukin-1β) and TNF-α (tumour necrosis factor-α) are essential pro-inflammatory cytokines released from several cell types, including astrocytes and microglia, after brain injury (Giulian et al., 1986). IL-1β and TNF-α have several physiological functions in neuroinflammation. In particular, IL-1β is known to induce production of other cytokines and growth factors (Benveniste, 1992; Merrill and Benveniste, 1996), change blood flow (Monroy et al., 2001; Maher et al., 2003) and affect neuroendocrine responses and the activity of the HPA (hypothalamic–pituitary–adrenal) axis (Berkenbosch et al., 1987; Sapolsky et al., 1987). In addition, both IL-1β and TNF-α have direct influences on neurodegeneration (Zhao et al., 2001; Thornton et al., 2006), and inhibition of endogenous IL-1β or TNF-α protects against neuronal injury that occurs after cerebral ischaemia (Relton and Rothwell, 1992; Meistrell et al., 1997). In addition to effects on neurodegeneration, IL-1β also affects remyelination by oligodendrocytes after injury (Mason et al., 2001). These cytokines are highly expressed in Alzheimer's disease (Griffin et al., 1989; Fillit et al., 1991; Wang et al., 1997), Parkinson's disease (Mogi et al., 1994, 1996) and spinal cord injury (Wang et al., 1997; Xu et al., 1998), conditions in which p75NTR is induced. IL-1β and TNF-α are up-regulated within 2 h after several types of injury (Minami et al., 1992; Taupin et al., 1993; Buttini et al., 1994; Fan et al., 1996; Eriksson et al., 2000), but p75NTR induction after seizure takes 24 h (Roux et al., 1999). Therefore cytokine induction precedes p75NTR induction after damage, and may play a role in regulation of p75NTR expression. Indeed, results from microarray analysis on astrocytes after IL-1β treatment showed increased p75NTR mRNA (John et al., 2005).
In the present study, we demonstrate that the pro-inflammatory cytokines IL-1β and TNF-α up-regulate p75NTR in hippocampal neurons and astrocytes. However, the underlying signalling mechanisms to induce p75NTR are different with distinct cytokines and cell types, suggesting a diverse mechanism of regulating cell-specific p75NTR expression after injury.
MATERIAL AND METHODS
Materials
IL-1β was generously provided by Dr Ronald Hart (Department of Cell Biology and Neuroscience, Rutgers University, Piscataway, NJ, U.S.A.). TNF-α was purchased from R&D systems. SB203580 and SN-50 were obtained from Alexis Biochemicals. Eagle's MEM (minimal essential medium), Ham's F12 and penicillin/streptomycin were purchased from Invitrogen. All other materials were obtained from Sigma.
Neuronal cultures
Neuronal cultures were prepared as described previously (Farinelli et al., 1998; Friedman, 2000). Rat hippocampi were dissected from embryonic day 18, dissociated, plated on to poly-d-lysine (0.1 mg/ml)-coated dishes, and maintained in serum-free medium. The medium consisted of a 1:1 mixture of Eagle's MEM and Ham's F12 supplemented with glucose (6 mg/ml), putrescine (60 μM), progesterone (20 nM), transferrin (100 μg/ml), selenium (30 nM), penicillin (0.5 units/ml) and streptomycin (0.5 μg/ml). Cultures were maintained in 5% CO2 at 37°C for 5 days before treatment. All animal studies were conducted using the NIH (National Institutes of Health) guidelines for the ethical treatment of animals with approval of the Rutgers Institutional Animal Care and Use Committee.
Astrocyte cultures
Astrocyte cultures were prepared as described previously (Srinivasan et al., 2004). Rat hippocampi were dissected from embryonic day 21, dissociated, plated on to poly-d-lysine (0.1 mg/ml)-coated flasks, and maintained in Eagle's MEM with Earle's salts, 2 mM l-glutamine, 15% heat-inactivated fetal bovine serum, 6 mg/ml glucose, penicillin (0.5 units/ml), and streptomycin (0.5 μg/ml). After 7–9 days incubation in 5% CO2 at 37°C, cells were shaken at 450 rev./min for 10 min to remove microglia and neurons, followed by a fresh medium change. Cells were shaken at 225 rev./min overnight to eliminate additional non-astrocytic cells. Cells were replenished with fresh medium containing 0.1 mM cytosine arabinoside and maintained for 3 days. Trypsinized and replated cells were kept in 5% CO2 at 37°C for 4 days before treatment (McCarthy and de Vellis, 1980).
Immunoprecipitation And Western Blot Analysis
Cells were lysed in RIPA buffer [50 mM Tris/HCl (pH 7.5), 150 mM NaCl, 5 mM EDTA, 1% Nonidet P40, 0.5% deoxycholic acid and 0.5% SDS] supplemented with a protease inhibitor mixture (Roche Products), 1 mM sodium vanadate and 5 mM sodium fluoride. Proteins were quantified using the Bradford assay (Bio-Rad) and equal amounts of protein were run on a 10% polyacrylamide gel and transferred on to a nitrocellulose membrane. Membranes were blocked in 5% (w/v) non-fat dried skimmed milk in TBST [TBS (Tris-buffered saline; 10 mM Tris and 150 mM NaCI, pH 7.4) containing 0.05% Tween 20] and then probed with antibodies against p75NTR (Upstate Biotechnology), actin (Sigma), p-p38 [phosphorylated-p38 MAPK (mitogen-activated protein kinase)], p-IκB {phosphorylated-IκB [inhibitor of NF-κB (nuclear factor κB)]; Cell Signaling Technology}. p-p38 and p-IκB blots were stripped and reprobed with anti-p38 and anti-IκB (Cell Signaling Technology) antibodies respectively. Bands were visualized by X-ray film exposure using ECL (enhanced chemiluminescence; Pierce). For IκB ubiquitination studies, cells were treated with IL-1β or TNF-α for the indicated periods, then lysed in buffer [50 mM Tris/HCl (pH 7.5), 150 mM NaCl, 1 mM EDTA, 5 mM NaF, 1 mM Na3VO4, 1% Triton X-100 and protease inhibitors], and centrifuged (20817 g for 15 min at 4°C). Supernatants were incubated with anti-IκB antibody overnight at 4°C and then incubated with Protein A–agarose at 4°C for 2 h. Immunoprecipitates were washed three times with lysis buffer and analysed by Western blot for ubiquitin (Santa Cruz Biotechnology). Blots were scanned and quantified using Photoshop. Statistically significant differences were analysed by one-way ANOVA with Tukey's post-hoc analysis.
Biotinylation of cell-surface proteins
Cells were treated with IL-1β or TNF-α for 8 h and washed with pre-chilled PBS once, and with PBS++ (PBS containing 1 mM MgCl2 and 2.5 mM CaCl2) twice. Cell-surface proteins were biotinylated with sulfo-NHS-SS-Biotin (Pierce) at 4°C for 1 h, quenched with glycine, and washed with PBS++ twice. Biotinylated cells were lysed in buffer containing 50 mM Tris, 150 mM NaCl, 1 mM EDTA, 1% Nonidet P40, 0.5% deoxycholate, protease inhibitor mixture, 1 mM sodium vanadate and 5 mM sodium fluoride, and lysates were incubated with streptavidin–agarose (Pierce) overnight at 4°C. After centrifugation (4500 g for 3 min at 4°C), supernatants were saved and pellets were washed with lysis buffer three times. Pellets and supernatants were analysed by Western blot for p75NTR and actin.
Immunostaining
To visualize nuclear translocation of NF-κB, cells were plated on plastic Lab-Tek slide wells and treated with IL-1β or TNF-α with or without pre-incubation with SN-50 for 30 min. Cells were fixed in 4% (w/v) paraformaldehyde, washed with PBS, permeabilized with PBS plus 0.3% Triton X-100, blocked in 5% (v/v) goat serum, and then incubated with anti-p65 antibody (Santa Cruz Biotechnology) overnight at 4°C. Slides were washed three times with PBS, incubated with secondary antibodies coupled to the Alexa Fluor® 555 fluophore (Molecular Probes) for 1 h at room temperature (25°C), then washed with PBS three times. Hoechst 33342 (1 μg/ml; Sigma) was used to visualize the nuclei.
Quantitative real-time RT (reverse transcriptase)–PCR
Primary hippocampal neurons or astrocytes were treated with IL-1β or TNF-α for 2, 4 or 8 h, and mRNA was isolated using TRIzol® reagent (Invitrogen). cDNA was generated using SuperScript™ II RT with random hexamers (Invitrogen), and SYBR-green-based quantitative real-time PCR was performed using primers specific for p75NTR (rat, forward 5′-CTGATGCTGAATGCGAAGAG-3′ and reverse 5′-TCACCATATCCGCCACTGTA-3′) or actin (forward 5′-TCATGAAGTGTGACGTTGACATCCGT-3′ and reverse 5′-CTTAGAAGCATTTGCGGTGCACGATG-3′) with the comparative CT method (ΔΔCT) (ABI).
RESULTS
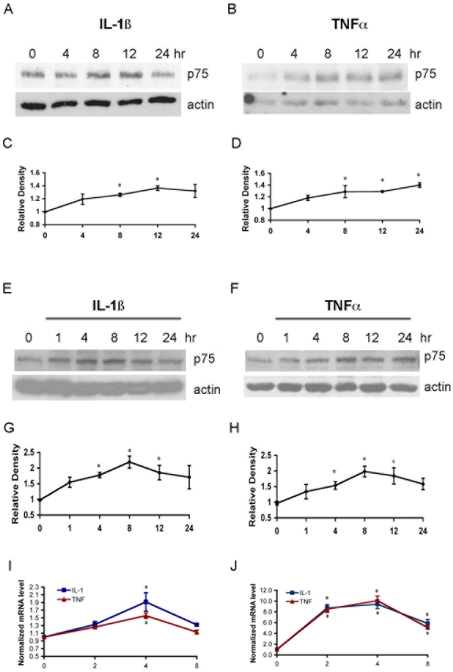
To investigate whether pro-inflammatory cytokines such as IL-1β and TNF-α induce p75NTR in different cell types, cultured hippocampal neurons and astrocytes were treated with IL-1β or TNF-α for 1, 4, 8, 12 or 24 h. Levels of p75NTR protein were evaluated by Western blot analysis, and mRNA was determined by quantitative PCR. The expression of p75NTR was increased by IL-1β and TNF-α in both neurons and astrocytes relative to actin (Figure 1). Elevated p75NTR mRNA expression peaked after 4 h of treatment, and the protein increase was maximal after 8 h of treatment with cytokines.
Figure 1. IL-1β and TNF-α induce p75NTR in neurons and astrocytes.
(A–D) Cultured hippocampal neurons were treated with either (A) IL-1β (10 ng/ml) or (B) TNF-α (10 ng/ml) for 4, 8, 12 or 24 h, and were then lysed and analysed by Western blot for p75NTR and actin. (C) and (D) Quantification of blots from three experiments as in (A) and (B) respectively. Densitometric values were normalized to actin and are expressed relative to the untreated cells (time 0). (E–H) Cultured hippocampal astrocytes were treated with either (E) IL-1β (10 ng/ml) or (F) TNF-α (10 ng/ml) for 1, 4, 8, 12 or 24 h, and were then lysed and analysed by Western blot for p75NTR and actin. (G) and (H) Quantification of blots from three experiments as in (E) and (F) respectively. Densitometric values were normalized to actin and are expressed relative to the untreated cells (time 0). (I and J) Quantitative real-time PCR analysis of p75NTR mRNA in hippocampal neurons (I) or astrocytes (J) treated with IL-1β or TNF-α expressed relative to untreated control cultures. The significance was determined by ANOVA with Tukey's post-hoc analysis. * indicates values significantly different from control at P<0.05.
Mechanisms of IL-1β regulation of p75NTR
In our previous studies, we have demonstrated that IL-1β activates distinct signalling pathways in neurons and astrocytes. IL-1β activates the classical NF-κB pathway in astrocytes but not in neurons, whereas p38 MAPK [but not JNK (c-Jun N-terminal kinase) or ERK (extracellular-signal-regulated kinase) MAPK] is activated by IL-1β in hippocampal neurons (Srinivasan et al., 2004). Therefore we investigated the underlying signalling mechanisms governing IL-1β regulation of p75NTR in both cell types.
We first confirmed the activation of specific signalling pathways in the different cell types. IL-1β induced robust transient phosphorylation of p38 MAPK in hippocampal neurons (Figure 2A). Activation of NF-κB signalling was monitored by phosphorylation and degradation of IκB, which is necessary for nuclear translocation of NF-κB. In neurons, no IκB phoshorylation was detected (results not shown), consistent with our previous study showing that IL-1β does not activate NF-κB in hippocampal neurons (Srinivasan et al., 2004). However, in astrocytes there was robust increase in p-IκB 5 min after IL-1β treatment, with a subsequent loss of IκB protein (Figure 2B). Interestingly, IL-1β also activated p38 MAPK signalling in astrocytes, p-p38 MAPK increased within 5 min after IL-1β treatment and peaked at 10 min in astrocytes (Figure 2C). Thus IL-1β activates both NF-κB and p38 MAPK signalling in astrocytes, but only p38 MAPK in hippocampal neurons.
Figure 2. IL-1 activates p38 MAPK in both neurons and astrocytes, and NF-κB only in astrocytes.
(A) Western blot analysis of p-p38 MAPK activation by IL-1β in neurons. Hippocampal neurons were cultured for 5 days, and treated with IL-1β for 5, 10, 20, 30 and 60 min and probed for p-p38 MAPK. The blot was stripped and reprobed for total p38 MAPK (bottom panel). (B) Western blot analysis of p-IκB in astrocytes. Cultured astrocytes were treated with IL-1β for 5, 10, 20 and 30 min and probed for p-IκB. The blot was stripped and re-probed for total IκB, which was degraded after phosphorylation (bottom panel). (C) Hippocampal astrocytes were treated with IL-1β for 5, 10, 20, 30 and 60 min and probed for p-p38 MAPK. The blot was stripped and reprobed for total p38 MAPK (bottom panel).
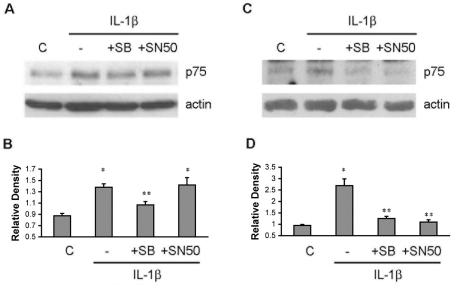
We next examined which signalling pathway was responsible for the IL-1β-mediated p75NTR up-regulation in the different cell types. Neurons or astrocytes were pretreated either with a p38 MAPK inhibitor SB203580 (10 μM) or an NF-κB inhibitor SN-50 (10 μM) for 30 min. IL-1β was then applied to the cells for 8 h, and p75NTR levels were analysed by Western blot. In hippocampal neurons, the p38 MAPK inhibitor prevented the IL-1β-mediated p75NTR up-regulation (Figures 3A and 3B). As expected, the NF-κB inhibitor SN-50 did not prevent the p75NTR expression in neurons. In astrocytes, both the p38 MAPK inhibitor and the NF-κB inhibitor completely prevented the IL-1β induction of p75NTR expression (Figures 3C and 3D), indicating that p75NTR induction in astrocytes requires both the p38 MAPK and NF-κB pathways. Confirmation that the inhibitors blocked the relevant signalling pathways is shown in Supplementary Figure S1 (at http://www.asnneuro.org/an/001/an001e010add.htm).
Figure 3. Signalling pathways required for IL-1β induction of p75NTR in hippocampal neurons and astrocytes.
(A) Hippocampal neurons were pretreated with SB203580 (10 μM) or SN-50 (10 μM) for 30 min and then 10 ng/ml IL-1β was added to the culture for 8 h. Western blot analysis was used to assess p75NTR and actin levels. (B) Quantification of three blots as shown in (A). * indicates values are significantly different from control, P<0.05; ** indicates IL-1β+SB203580 is significantly different from IL-1 alone or IL-1β+SN-50, P<0.05. (C and D) IL-1 induces p75NTR through both the NF-κB and p38 MAPK pathways in astrocytes. Cultured astrocytes were pretreated for 30 min with the p38 MAPK inhibitor SB203580 (10 μM) or the NF-κB inhibitor SN-50 (10 μM) prior to IL-1β treatment for 8 h. Western blot analysis was used to detect the p75NTR and actin levels. (D) Quantification of three blots as shown in (C). * indicates values are significantly different from control, P<0.05; ** indicates IL-1β+SB203580 and IL-1β+SN-50 are significantly different from IL-1 alone, P<0.05. C, control; SB, SB203580.
Mechanisms of TNF-α regulation of p75NTR
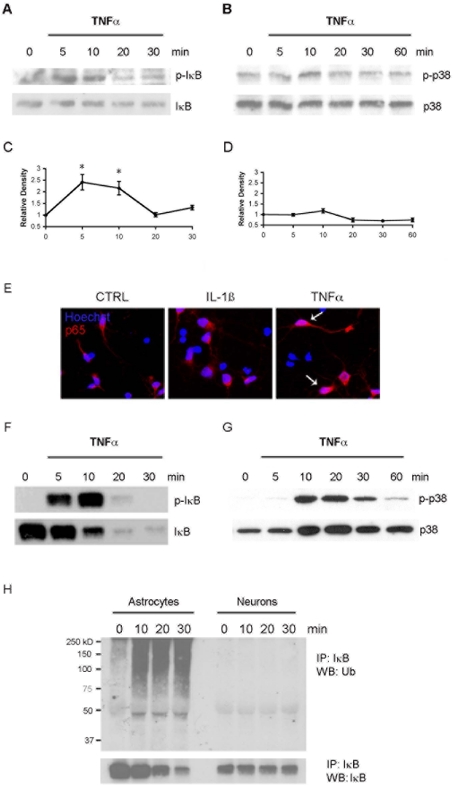
Both IL-1β and TNF-α elicited increased p75NTR expression in hippocampal neurons; however, the signalling mechanisms activated by these cytokines were distinct. In contrast with the effects of IL-1β, TNF-α elicited a modest transient phosphorylation of IκB (Figures 4A and 4C), but failed to induce activation of p38 MAPK (Figures 4B and 4D). Moreover, p65 staining in nuclei was visible with TNF-α-treated neurons, but not with IL-1β-treated neurons (Figure 4E), confirming activation of the NF-κB pathway by TNF-α in neurons.
Figure 4. TNF-α phosphorylates IκB in neurons, and both IκB and p38 MAPK in astrocytes.
(A) Hippocampal neurons were cultured for 5 days and treated with TNF-α for the times indicated. Lysates were probed for p-IκB, stripped and re-probed for total IκB. Note the lack of degradation of IκB. (B) Neuronal lysates were probed for p-p38 MAPK, stripped and re-probed for total p38 MAPK. (C) Quantification of three blots as shown in (A); TNF-α treatment significantly induced p-IκB at 5 and 10 min, *P<0.05. (D) Quantification of three blots as shown in (B); TNF-α treatment did not induce phosphorylation of p38 MAPK in hippocampal neurons. (E) Neurons were treated with IL-1β or TNF-α for 30 min, fixed and immunostained for p65. Nuclei were identified with Hoechst labelling. Note the presence of nuclear p65 in TNF-α-, but not IL-1β-, treated neurons. CTRL, control. (F) Cultured astrocytes were treated with TNF-α for the times indicated. Astrocyte lysates were probed for p-IκB, stripped and re-probed for total IκB. (G) Astrocyte lysates were probed for p-p38 MAPK, stripped and re-probed for total p38 MAPK. (H) Analysis of ubiquitinated IκB in astrocytes and neurons. Cells were treated with TNF-α as indicated. Cell lysates were immunoprecipitated (IP) with anti-IκB and probed for ubiquitin (Ub). Blots were stripped and re-probed for IκB. Note that levels of total IκB decreased over time in the astrocytes, but not in the neurons. The molecular mass in kDa is indicated on the left-hand side of the blot. WB, Western blot.
In astrocytes, TNF-α treatment induced phophorylation of IκB, which peaked at 10 min (Figure 4F) and decreased afterwards. IκB protein was degraded after phosphorylation (Figure 4F). TNF-α also activated the p38 MAPK pathway in astrocytes, inducing robust phosphorylation that peaked at 10–20 min after treatment (Figure 4G). Thus TNF-α, like IL-1β, induced activation of both the NF-κB and p38 MAPK pathways in hippocampal astrocytes.
Interestingly, although TNF-α induced phosphorylation of IκB in both hippocampal neurons and astrocytes, loss of IκB protein was only observed in astrocytes (Figure 4F), not in neurons (Figure 4A). Once IκB is phosphorylated, it goes through polyubiquitination and proteosome-dependent degradation (Karin and Ben-Neriah, 2000). Therefore we investigated whether the ubiquitination of IκB occurred in neurons. Astrocytes and neurons were treated with TNF-α for the indicated times and the cell lysates were immunoprecipitated with anti-IκB antibody, and Western blot analysis was used to detect ubiquitination. There was increased ubiquitinated IκB in the TNF-α-treated astrocytes; however, no ubiquitination was detected in neurons treated with TNF-α (Figure 4H), consistent with the lack of IκB degradation.
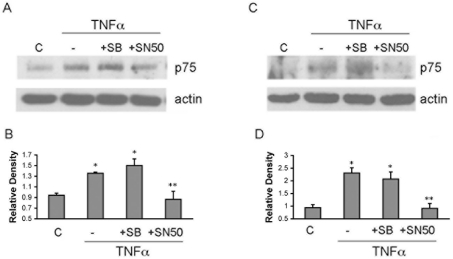
TNF-α induced phosphorylation of IκB and nuclear translocation of NF-κB in hippocampal neurons, suggesting that the increase in p75NTR expression induced by this cytokine might be mediated by NF-κB. Treatment of the neurons with the NF-κB inhibitor SN50, but not the p38 MAPK inhibitor SB203580, prevented the TNF-α-evoked increase in p75NTR (Figures 5A and 5B), demonstrating that TNF-α regulates p75NTR expression via NF-κB signalling, whereas IL-1β regulates p75NTR via p38 MAPK signalling in hippocampal neurons (Figure 3).
Figure 5. TNF-α induces p75NTR via NF-κB in both neurons and astrocytes.
(A) Hippocampal neurons were pretreated with SB203580 (10 μM) or SN-50 (10 μM) for 30 min and then 10 ng/ml TNF-α was provided for 8 h. Western blot analysis was used to assess p75NTR and actin levels. (B) Quantification of three blots as shown in (A). * indicates levels are significantly different from control, P<0.05; ** indicates TNFα+SN-50 is significantly different from TNF-α alone, P<0.05. (C) Hippocampal astrocytes were pretreated with SB203580 (10 μM) or SN-50 (10 μM) for 30 min and then 10 ng/ml TNFα was added to the culture for 8 h. Western blot analysis was used to assess p75NTR and actin. (D) Quantification of three blots as shown in (C). * indicates levels are significantly different from control, P<0.05; ** indicates TNFα+SN-50 is significantly different from TNF-α alone, P<0.05. SB, SB203580. C, control.
Similar to the effects in neurons, the p38 MAPK inhibitor did not prevent the TNF-α-mediated p75NTR up-regulation in astrocytes (Figures 5C and 5D), whereas NF-κB inhibition blocked TNF-α-mediated p75NTR expression (Figures 5C and 5D). Confirmation that the inhibitors blocked their respective signalling pathways is shown in Supplementary Figure S1. Thus, in astrocytes, IL-1β requires both the p38 MAPK and NF-κB pathways to regulate p75NTR expression; however, TNF-α regulates p75NTR only through the NF-κB pathway. Moreover, TNF-α signalled via the NF-κB pathway to regulate p75NTR in both hippocampal neurons and astrocytes, whereas IL-1β signalled via the p38 MAPK pathway in both cell types and via NF-κB only in astrocytes.
Cell-surface expression of p75NTR
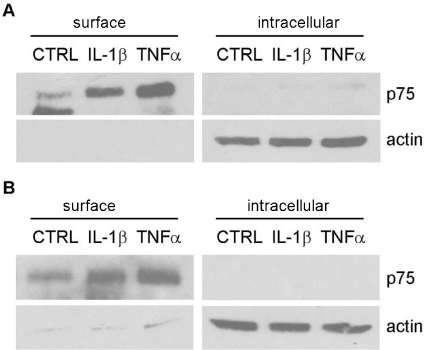
p75NTR is a cell-surface receptor that can bind a variety of different ligands to mediate distinct cellular functions. To assess whether the elevated p75NTR was localized to the cell surface, biotinylation assays were performed on cytokine-treated neurons and astrocytes. Nearly all of the detectable p75NTR was present at the cell surface after 8 h of treatment with IL-1β or TNF-α in neurons (Figure 6A) and little p75NTR was detected in the intracellular fraction. Similar to neurons, IL-1β- and TNF-α-treated astrocytes had elevated p75NTR at the cell surface, but not in cytosolic compartment (Figure 6B), suggesting that p75NTR induced by cytokines is present at the cell surface in both neurons and astrocytes. Specificity of the biotin pulldown is shown in Supplementary Figure S2 (at http://www.asnneuro.org/an/001/an001e010add.htm).
Figure 6. Increased p75NTR expression induced by IL-1β and TNF-α is present on the cell surface in both neurons and astrocytes.
Cultured hippocampal neurons (A) or astrocytes (B) were treated with IL-1β or TNF-α for 8 h, and incubated with biotin for 1 h. Cell lysates were precipitated with streptavidin. Biotinylated cell-surface protein and non-biotinylated intracellular proteins were analysed by Western blotting for p75NTR. Blots were stripped and re-probed for actin, which was only present in the intracellular fraction. CTRL, control.
DISCUSSION
p75NTR is induced in CNS neurons after many types of injury (Kokaia et al., 1998; Roux et al., 1999; Casha et al., 2001; Ramos et al., 2007), and can mediate many different cellular responses, including neuronal apoptosis or survival, and regulation of axonal growth (Rabizadeh et al., 1993; Frade et al., 1996; Maggirwar et al., 1998; Friedman, 2000). However, the signals that regulate p75NTR induction in neurons are not well characterized. Since many different types of pathophysiological conditions induce expression of p75NTR, it is likely that inflammatory events common to these conditions may regulate this receptor. A previous study has demonstrated that hypo-osmotic stress can regulate p75NTR by increasing cellular levels of Sp1 in primary cortical neurons (Ramos et al., 2007). In the present study, we investigated whether the pro-inflammatory cytokines IL-1β and TNF-α, which are highly expressed in the brain following injury (Minami et al., 1992; Taupin et al., 1993; Buttini et al., 1994; Fan et al., 1996; Eriksson et al., 2000), might play a role in p75NTR regulation. Not only are these cytokines highly expressed in the brain under these pathophysiological conditions, but IL-1 is also expressed by microglia during neonatal development (Giulian et al., 1988), a time during which p75NTR is widely expressed throughout the brain. Thus this cytokine may regulate expression of p75NTR developmentally as well as after injury.
In addition to the seizure-induced regulation of p75NTR that we (Troy et al., 2002) and others (Roux et al., 1999), have observed in brain neurons, we recently demonstrated that this receptor is also induced in astrocytes following pilocarpine-induced seizures (Cragnolini et al., 2009). In astrocytes, we have determined that stimulation of p75NTR with NGF negatively regulates cell proliferation (Cragnolini et al., 2009). Thus we investigated the mechanisms that regulate p75NTR induction in both primary hippocampal neurons and astrocytes. We have demonstrated in the present study that the pro-inflammatory cytokines IL-1β and TNF-α induce p75NTR expression in both hippocampal neurons and astrocytes. However, the underlying signalling pathways leading to p75NTR induction was cytokine- and cell-type-specific. Whereas IL-1β induced p75NTR via p38 MAPK in neurons, and via both p38 MAPK and NF-κB in astrocytes, TNF-α induced p75NTR via NF-κB both in neurons and astrocytes. Thus inflammatory cytokines play a key role in regulating p75NTR expression, and the cellular specificity may provide a possible therapeutic target for CNS diseases.
Mechanisms of IL-1β regulation of p75NTR
The release of IL-1β after injury elicits diverse effects including the production of other cytokines and growth factors, thereby promoting inflammatory activity. We have shown in the present study that IL-1β induced p75NTR expression in primary hippocampal neurons and astrocytes. The increased p75NTR expression was transient and returned to baseline by 24 h, which may be due to the actions of IL-1Ra, the naturally occurring IL-1β receptor antagonist (Hannum et al., 1990), providing negative feedback. Many signalling pathways can be activated by IL-1β in different cell types, including the classic NF-κB pathway, as well as the JNK, ERK and p38 MAPK pathways (O'Neill, 2002; Dunne and O'Neill, 2003). We have previously established that hippocampal neurons utilize a different signalling pathway compared with astrocytes (Srinivasan et al., 2004). Although IL-1β activated NF-κB in astrocytes, it failed to do so in hippocampal neurons, activating only p38 MAPK signalling (Srinivasan et al., 2004). Our present study shows that IL-1β activation of p38 MAPK was necessary for p75NTR induction by this cytokine in the hippocampal neurons.
In contrast with neurons, IL-1β activated both the p38 MAPK pathway and the NF-κB pathway in astrocytes. Pharmacological inhibitors were used to determine which of these pathways regulated the induction of p75NTR expression in astrocytes. Interestingly, inhibition of either pathway prevented the increase in p75NTR expression, indicating that both p38 MAPK signalling and the NF-κB pathways are required for p75NTR induction by IL-1β.
Mechanisms of TNF-α regulation of p75NTR
TNF-α is another major pro-inflammatory cytokine that is produced in the brain after injury (Taupin et al., 1993; Fan et al., 1996) and frequently acts synergistically with IL-1β. In contrast with the actions of IL-1β, TNF-α activated the NF-κB pathway in hippocampal neurons, indicated by phosphorylation of IκB and nuclear translocation of NF-κB, demonstrating that these two inflammatory cytokines signal via distinct pathways. Inhibition of NF-κB nuclear translocation with SN-50 prevented TNF-α-mediated p75NTR induction in the hippocampal neurons, confirming that the distinct pathways activated by the two different cytokines both lead to induction of p75NTR.
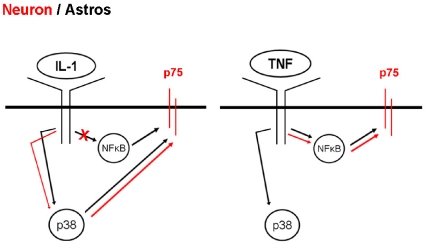
Interestingly, although TNF-α treatment of hippocampal neurons lead to IκB phosphorylation, there was no subsequent ubiquitination and degradation of the protein as normally seen in astrocytes and other cell types (Verstrepen et al., 2008). An alternative pathway for activation of NF-κB has been demonstrated as a MEKK3 (MAPK/ERK kinase kinase 3)-dependent pathway in which IκB is phosphorylated and dissociated from NF-κB, but is not degraded (Yao et al., 2007). Since that is what we have observed in response to TNF-α treatment of neurons, this pathway is likely to be the one involved. In astrocytes, TNF-α activated both the NF-κB and p38 MAPK pathways, similar to the effects of IL-1β. However, blocking NF-κB nuclear translocation with SN-50 prevented TNF-α-mediated induction of p75NTR, whereas the p38 inhibitor had no effect, indicating that the NF-κB pathway mediates p75NTR induction by TNF-α in both neurons and astrocytes. This is in contrast with the regulation of p75NTR by IL-1β in astrocytes, which required signalling via both the NF-κB and p38 MAPK pathways. Thus these two key inflammatory cytokines have a common target in regulation of the p75NTR, but the mechanisms by which they regulate expression of this receptor are distinct both in hippocampal neurons and astrocytes (Figure 7).
Figure 7. Schematic diagram showing the signalling pathways activated by IL-1β and TNF-α in neurons (red) and astrocytes (black) leading to induction of p75NTR.
Since p75NTR is a cell-surface receptor that binds a variety of ligands to mediate different cellular effects, it was important to determine whether the inflammatory cytokines not only increased expression of this receptor in the neurons and astrocytes, but whether the elevated receptor was on the cell surface where it may be activated by ligand. Biotinylation experiments determined that the increased p75NTR expression was nearly completely present on the cell surface, confirming that the receptor would be accessible for ligand binding.
The p75NTR plays an important role in neuronal apoptosis, serving as a receptor for proneurotrophins that are released in the hippocampus following seizures (Volosin et al., 2008). Thus understanding the mechanisms that regulate expression of this receptor may serve as a potential therapeutic target for neuroprotection following seizures or other injuries. There has been contradictory data on the effect of direct administration of IL-1β to the rat brain. Whereas one group has shown that IL-1β and TNF-α administration caused neuronal loss in the substantia nigra (Carvey et al., 2005), most studies have shown that IL-1β alone did not directly affect healthy neurons either in vitro or in vivo (Chao et al., 1995; Lawrence et al., 1998). However, IL-1 is known to exacerbate neuronal degeneration, and the naturally occurring IL-1Ra has been shown to be neuroprotective in models of ischaemia and excitotoxicity (Simi et al., 2007). The neuronal induction of p75NTR by inflammatory cytokines may contribute to neurodegeneration in these pathological conditions.
Our recent studies have demonstrated that p75NTR is induced in hippocampal astrocytes as well as neurons, and serves to attenuate proliferation of these cells (Cragnolini et al, 2009). Since excessive glial proliferation can lead to scarring and inhibition of the potential for regeneration, the induction of p75NTR on astrocytes may be beneficial. Thus it may be therapeutically beneficial to inhibit p75NTR induction in hippocampal neurons to prevent neuronal loss, yet permit p75NTR induction in astrocytes to attenuate glial proliferation. The distinct mechanisms of cytokine-mediated p75NTR regulation that we have identified in the present study may facilitate the cell-type-specific regulation of this receptor and the functional consequences.
Online data
ACKNOWLEDGEMENTS
We would like to thank Richard Farias for technical assistance.
FUNDING
This work was supported by the New Jersey Commission on Brain Injury Research [grant number 08-3211].
REFERENCES
- Benveniste EN. Cytokines: influence on glial cell gene expression and function. Chem Immunol. 1992;52:106–153. [PubMed] [Google Scholar]
- Berkenbosch F, van Oers J, del Rey A, Tilders F, Besedovsky H. Corticotropin-releasing factor-producing neurons in the rat activated by interleukin-1. Science. 1987;238:524–526. doi: 10.1126/science.2443979. [DOI] [PubMed] [Google Scholar]
- Buttini M, Sauter A, Boddeke HW. Induction of interleukin-1β mRNA after focal cerebral ischaemia in the rat. Brain Res Mol Brain Res. 1994;23:126–134. doi: 10.1016/0169-328x(94)90218-6. [DOI] [PubMed] [Google Scholar]
- Carvey PM, Chen EY, Lipton JW, Tong CW, Chang QA, Ling ZD. Intra-parenchymal injection of tumor necrosis factor-α and interleukin 1-β produces dopamine neuron loss in the rat. J Neural Transm. 2005;112:601–612. doi: 10.1007/s00702-004-0222-z. [DOI] [PubMed] [Google Scholar]
- Casha S, Yu WR, Fehlings MG. Oligodendroglial apoptosis occurs along degenerating axons and is associated with FAS and p75 expression following spinal cord injury in the rat. Neuroscience. 2001;103:203–218. doi: 10.1016/s0306-4522(00)00538-8. [DOI] [PubMed] [Google Scholar]
- Chao CC, Hu S, Ehrlich L, Peterson PK. Interleukin-1 and tumor necrosis factor-α synergistically mediate neurotoxicity: involvement of nitric oxide and of N-methyl-D-aspartate receptors. Brain Behav Immun. 1995;9:355–365. doi: 10.1006/brbi.1995.1033. [DOI] [PubMed] [Google Scholar]
- Cragnolini AB, Huang Y, Gokina P, Friedman WJ. Nerve growth factor attenuates proliferation of astrocytes via the p75 neurotrophin receptor. Glia. 2009 doi: 10.1002/glia.20857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunne A, O'Neill LA. The interleukin-1 receptor/Toll-like receptor superfamily: signal transduction during inflammation and host defense. Sci STKE. 2003;2003:re3. doi: 10.1126/stke.2003.171.re3. [DOI] [PubMed] [Google Scholar]
- Eriksson C, Tehranian R, Iverfeldt K, Winblad B, Schultzberg M. Increased expression of mRNA encoding interleukin-1β and caspase-1, and the secreted isoform of interleukin-1 receptor antagonist in the rat brain following systemic kainic acid administration. J Neurosci Res. 2000;60:266–279. doi: 10.1002/(SICI)1097-4547(20000415)60:2<266::AID-JNR16>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Fan L, Young PR, Barone FC, Feuerstein GZ, Smith DH, McIntosh TK. Experimental brain injury induces differential expression of tumor necrosis factor-α mRNA in the CNS. Brain Res Mol Brain Res. 1996;36:287–291. doi: 10.1016/0169-328x(95)00274-v. [DOI] [PubMed] [Google Scholar]
- Farinelli SE, Greene LA, Friedman WJ. Neuroprotective actions of dipyridamole on cultured CNS neurons. J Neurosci. 1998;18:5112–5123. doi: 10.1523/JNEUROSCI.18-14-05112.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillit H, Ding WH, Buee L, Kalman J, Altstiel L, Lawlor B, Wolf-Klein G. Elevated circulating tumor necrosis factor levels in Alzheimer's disease. Neurosci Lett. 1991;129:318–320. doi: 10.1016/0304-3940(91)90490-k. [DOI] [PubMed] [Google Scholar]
- Frade JM, Rodriguez-Tebar A, Barde YA. Induction of cell death by endogenous nerve growth factor through its p75 receptor. Nature. 1996;383:166–168. doi: 10.1038/383166a0. [DOI] [PubMed] [Google Scholar]
- Friedman WJ. Neurotrophins induce death of hippocampal neurons via the p75 receptor. J Neurosci. 2000;20:6340–6346. doi: 10.1523/JNEUROSCI.20-17-06340.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giulian D, Baker TJ, Shih LCN, Lachman LB. Interleukin-1 of the central nervous system is produced by amoeboid microglia. J Exp Med. 1986;164:594–604. doi: 10.1084/jem.164.2.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giulian D, Young DG, Woodward J, Brown DC, Lachman LB. Interleukin-1 is an astroglial growth factor in the developing brain. J Neurosci. 1988;8:709–714. doi: 10.1523/JNEUROSCI.08-02-00709.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene LA, Rukenstein A. Regulation of acetylcholinesterase activity by nerve growth factor. Role of transcription and dissociation from effects on proliferation and neurite outgrowth. J Biol Chem. 1981;256:6363–6367. [PubMed] [Google Scholar]
- Griffin WS, Stanley LC, Ling C, White L, MacLeod V, Perrot LJ, White 3rd CL, Araoz C. Brain interleukin 1 and S-100 immunoreactivity are elevated in Down syndrome and Alzheimer disease. Proc Natl Acad Sci USA. 1989;86:7611–7615. doi: 10.1073/pnas.86.19.7611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannum CH, Wilcox CJ, Arend WP, Joslin FG, Dripps DJ, Heimdal PL, Armes LG, Sommer A, Eisenberg SP, Thompson RC. Interleukin-1 receptor antagonist activity of a human interleukin-1 inhibitor. Nature. 1990;343:336–340. doi: 10.1038/343336a0. [DOI] [PubMed] [Google Scholar]
- John GR, Lee SC, Song X, Rivieccio M, Brosnan CF. IL-1-regulated responses in astrocytes: relevance to injury and recovery. Glia. 2005;49:161–176. doi: 10.1002/glia.20109. [DOI] [PubMed] [Google Scholar]
- Karin M, Ben-Neriah Y. Phosphorylation meets ubiquitination: the control of NF-κB activity. Annu Rev Immunol. 2000;18:621–663. doi: 10.1146/annurev.immunol.18.1.621. [DOI] [PubMed] [Google Scholar]
- Kokaia Z, Andsberg G, Martinez-Serrano A, Lindvall O. Focal cerebral ischemia in rats induces expression of P75 neurotrophin receptor in resistant striatal cholinergic neurons. Neuroscience. 1998;84:1113–1125. doi: 10.1016/s0306-4522(97)00579-4. [DOI] [PubMed] [Google Scholar]
- Lawrence CB, Allan SM, Rothwell NJ. Interleukin-1β and the interleukin-1 receptor antagonist act in the striatum to modify excitotoxic brain damage in the rat. Eur J Neurosci. 1998;10:1188–1195. doi: 10.1046/j.1460-9568.1998.00136.x. [DOI] [PubMed] [Google Scholar]
- Maggirwar SB, Sarmiere PD, Dewhurst S, Freeman RS. Nerve growth factor-dependent activation of NF-κB contributes to survival of sympathetic neurons. J Neurosci. 1998;18:10356–10365. doi: 10.1523/JNEUROSCI.18-24-10356.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher CO, Anderson RE, Martin HS, McClelland RL, Meyer FB. Interleukin-1β and adverse effects on cerebral blood flow during long-term global hypoperfusion. J Neurosurg. 2003;99:907–912. doi: 10.3171/jns.2003.99.5.0907. [DOI] [PubMed] [Google Scholar]
- Mason JL, Suzuki K, Chaplin DD, Matsushima GK. Interleukin-1β promotes repair of the CNS. J Neurosci. 2001;21:7046–7052. doi: 10.1523/JNEUROSCI.21-18-07046.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy KD, de Vellis J. Preparation of separate astroglial and oligodendroglial cell cultures from rat cerebral tissue. J Cell Biol. 1980;85:890–902. doi: 10.1083/jcb.85.3.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meistrell 3rd ME, Botchkina GI, Wang H, Di Santo E, Cockroft KM, Bloom O, Vishnubhakat JM, Ghezzi P, Tracey KJ. Tumor necrosis factor is a brain damaging cytokine in cerebral ischemia. Shock. 1997;8:341–348. [PubMed] [Google Scholar]
- Merrill JE, Benveniste EN. Cytokines in inflammatory brain lesions: helpful and harmful. Trends Neurosci. 1996;19:331–338. doi: 10.1016/0166-2236(96)10047-3. [DOI] [PubMed] [Google Scholar]
- Minami M, Kuraishi Y, Yabuuchi K, Yamazaki A, Satoh M. Induction of interleukin-1β mRNA in rat brain after transient forebrain ischemia. J Neurochem. 1992;58:390–392. doi: 10.1111/j.1471-4159.1992.tb09324.x. [DOI] [PubMed] [Google Scholar]
- Mogi M, Harada M, Riederer P, Narabayashi H, Fujita K, Nagatsu T. Tumor necrosis factor-α (TNF-α) increases both in the brain and in the cerebrospinal fluid from parkinsonian patients. Neurosci Lett. 1994;165:208–210. doi: 10.1016/0304-3940(94)90746-3. [DOI] [PubMed] [Google Scholar]
- Mogi M, Harada M, Narabayashi H, Inagaki H, Minami M, Nagatsu T. Interleukin (IL)-1β, IL-2, IL-4, IL-6 and transforming growth factor-α levels are elevated in ventricular cerebrospinal fluid in juvenile parkinsonism and Parkinson's disease. Neurosci Lett. 1996;211:13–16. doi: 10.1016/0304-3940(96)12706-3. [DOI] [PubMed] [Google Scholar]
- Monroy M, Kuluz JW, He D, Dietrich WD, Schleien CL. Role of nitric oxide in the cerebrovascular and thermoregulatory response to interleukin-1β. Am J Physiol Heart Circ Physiol. 2001;280:H1448–H1453. doi: 10.1152/ajpheart.2001.280.4.H1448. [DOI] [PubMed] [Google Scholar]
- O'Neill LA. Signal transduction pathways activated by the IL-1 receptor/toll-like receptor superfamily. Curr Top Microbiol Immunol. 2002;270:47–61. [PubMed] [Google Scholar]
- Rabizadeh S, Oh J, Zhong LT, Yang J, Bitler CM, Butcher LL, Bredesen DE. Induction of apoptosis by the low-affinity NGF receptor. Science. 1993;261:345–348. doi: 10.1126/science.8332899. [DOI] [PubMed] [Google Scholar]
- Ramos A, Ho WC, Forte S, Dickson K, Boutilier J, Favell K, Barker PA. Hypo-osmolar stress induces p75NTR expression by activating Sp1-dependent transcription. J Neurosci. 2007;27:1498–1506. doi: 10.1523/JNEUROSCI.4806-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Relton JK, Rothwell NJ. Interleukin-1 receptor antagonist inhibits ischaemic and excitotoxic neuronal damage in the rat. Brain Res Bull. 1992;29:243–246. doi: 10.1016/0361-9230(92)90033-t. [DOI] [PubMed] [Google Scholar]
- Roux PP, Colicos MA, Barker PA, Kennedy TE. p75 neurotrophin receptor expression is induced in apoptotic neurons after seizure. J Neurosci. 1999;19:6887–6896. doi: 10.1523/JNEUROSCI.19-16-06887.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky R, Rivier C, Yamamoto G, Plotsky P, Vale W. Interleukin-1 stimulates the secretion of hypothalamic corticotropin-releasing factor. Science. 1987;238:522–524. doi: 10.1126/science.2821621. [DOI] [PubMed] [Google Scholar]
- Simi A, Tsakiri N, Wang P, Rothwell NJ. Interleukin-1 and inflammatory neurodegeneration. 2007;35:1122–1126. doi: 10.1042/BST0351122. [DOI] [PubMed] [Google Scholar]
- Srinivasan D, Yen JH, Joseph DJ, Friedman W. Cell type-specific interleukin-1β signaling in the CNS. J Neurosci. 2004;24:6482–6488. doi: 10.1523/JNEUROSCI.5712-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taupin V, Toulmond S, Serrano A, Benavides J, Zavala F. Increase in IL-6, IL-1 and TNF levels in rat brain following traumatic lesion. Influence of pre- and post-traumatic treatment with Ro5 4864, a peripheral-type (p site) benzodiazepine ligand. J Neuroimmunol. 1993;42:177–185. doi: 10.1016/0165-5728(93)90008-m. [DOI] [PubMed] [Google Scholar]
- Thornton P, Pinteaux E, Gibson RM, Allan SM, Rothwell NJ. Interleukin-1-induced neurotoxicity is mediated by glia and requires caspase activation and free radical release. J Neurochem. 2006;98:258–266. doi: 10.1111/j.1471-4159.2006.03872.x. [DOI] [PubMed] [Google Scholar]
- Troy CM, Friedman JE, Friedman WJ. Mechanisms of p75-mediated death of hippocampal neurons. Role of caspases. J Biol Chem. 2002;277:34295–34302. doi: 10.1074/jbc.M205167200. [DOI] [PubMed] [Google Scholar]
- Verstrepen L, Bekaert T, Chau TL, Tavernier J, Chariot A, Beyaert R. TLR-4, IL-1R and TNF-R signaling to NF-κB: variations on a common theme. Cell Mol Life Sci. 2008;65:2964–2978. doi: 10.1007/s00018-008-8064-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volosin M, Trotter C, Cragnolini A, Kenchappa RS, Light M, Hempstead BL, Carter BD, Friedman WJ. Induction of proneurotrophins and activation of p75NTR-mediated apoptosis via neurotrophin receptor-interacting factor in hippocampal neurons after seizures. J Neurosci. 2008;28:9870–9879. doi: 10.1523/JNEUROSCI.2841-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CX, Olschowka JA, Wrathall JR. Increase of interleukin-1β mRNA and protein in the spinal cord following experimental traumatic injury in the rat. Brain Res. 1997;759:190–196. doi: 10.1016/s0006-8993(97)00254-0. [DOI] [PubMed] [Google Scholar]
- Xu IS, Grass S, Xu XJ, Wiesenfeld-Hallin Z. On the role of galanin in mediating spinal flexor reflex excitability in inflammation. Neuroscience. 1998;85:827–835. doi: 10.1016/s0306-4522(97)00676-3. [DOI] [PubMed] [Google Scholar]
- Yan Q, Johnson EM., Jr An immunohistochemical study of the nerve growth factor receptor in developing rats. J Neurosci. 1988;8:3481–3498. doi: 10.1523/JNEUROSCI.08-09-03481.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao J, Kim TW, Qin J, Jiang Z, Qian Y, Xiao H, Lu Y, Qian W, Gulen MF, Sizemore N, DiDonato J, Sato S, Akira S, Su B, Li X. Interleukin-1 (IL-1)-induced TAK1-dependent versus MEKK3-dependent NFκB activation pathways bifurcate at IL-1 receptor-associated kinase modification. J Biol Chem. 2007;282:6075–6089. doi: 10.1074/jbc.M609039200. [DOI] [PubMed] [Google Scholar]
- Zhao X, Bausano B, Pike BR, Newcomb-Fernandez JK, Wang KK, Shohami E, Ringger NC, DeFord SM, Anderson DK, Hayes RL. TNF-α stimulates caspase-3 activation and apoptotic cell death in primary septo-hippocampal cultures. J Neurosci Res. 2001;64:121–131. doi: 10.1002/jnr.1059. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.