Abstract
The CNS (central nervous system) is unquestionably the central organ that regulates directly or indirectly all physiological systems in the mammalian body. Yet, when considering the defence of the CNS from pathogens, the CNS has often been considered passive and subservient to the pro-inflammatory responses of the immune system. In this view, neuroinflammatory disorders are examples of when the tail (the immune system) wags the dog (the CNS) to the detriment of an individual's function and survival.
Keywords: autoimmunity, irradiation chimaeric mouse, MyD88, neurodegeneration, pathogen conserved molecular pattern (PAMP), Toll-like receptor
Abbreviations: CNS, central nervous system; TLR, Toll-like receptor
In a paper recently published in ASN NEURO, Kielian and colleagues use a Staphylococcus aureus model of brain abscess formation to provide data challenging this commonly stated dogma. Their results reinforce the emerging view of the critical and non-redundant control of pathogen defence by the CNS itself. Specifically, Kielian and colleagues not only reveal the ability of the CNS to both initiate anti-bacterial MyD88-dependent pro-inflammatory responses by CNS-resident cells, they also reveal the surprising ability of these CNS-initiated responses to apparently compensate for pathogen-associated susceptibilities intrinsic to peripheral immune system responses. The identification of immune-system-intrinsic compared with CNS-intrinsic regulation of neuroinflammatory responses is not merely an academic exercise. Rather the degree and type of neuroinflammation that can be initiated by the CNS-intrinsic mechanisms will refocus the sites targeted for effective therapeutic intervention of both pathogen- and non-pathogen-associated CNS neuroinflammatory disorders.
WHY DID WE START ASSUMING THAT THE TAIL WAGGED THE DOG?
In part, this viewpoint is derived from a long series of observations begun nearly 100 years ago (reviewed in Carson et al., 2006). Foreign tissue grafts (allografts) survive for greater periods of time when placed within the brain parenchyma than when they are placed under the skin. The prolonged survival of allografts within the brain correlates with the absence in the CNS of the robust and prominent lymphocytic immune response observed in non-CNS graft sites. These and subsequent observations led to the concept of immunologically privileged sites being defined as tissues with very high thresholds for initiating T-cell responses: the CNS being defined as the stereotypical example. The persistence of viruses and bacteria within the brain, even when these pathogens had been cleared from all other tissue compartments in the body, appeared to confirm this ‘neutral-no battle zone' interpretation of immune privilege. The adaptive advantage for allowing persistence of pathogens within the CNS is argued to be an adaptive balance between limiting disruption of CNS function by the pathogen compared with by the pro-inflammatory responses of immune cells. For example, S. aureus-induced formation of brain abscesses clearly interferes with CNS function. However, sequestration of an infection that cannot be cleared within a brain abscess does prevent the pathogen from inducing further harm and simultaneously facilitates the termination of neurotoxic pro-inflammatory anti-S. aureus-directed immune responses.
Numerous studies are now elucidating the multiple molecular mechanisms that actively maintain immune privilege. Notably, CNS neurons and glia express numerous immunomodulatory molecules such as TGFβ (transforming growth factor β), CD200, CD22, fractalkine, CD47 and TREM2 (triggering receptor expressed on myeloid cells 2), all of which have demonstrated immunosuppressive actions on pro-inflammatory immune responses (Takahashi et al., 2007; reviewed in Carson et al., 2006; Carson and Lo, 2007). Microglia, the resident CNS-specific tissue macrophages are substantially less effective in initiating T-cell responses and have substantially lower bactericidal function than peripheral pro-inflammatory macrophages and neutrophils. (reviewed in Carson et al., 2006; Garg et al., 2009). Taken together, these and other results suggest that the CNS can actively modulate immune system function, but that CNS-intrinsic regulation is biased toward limiting, not initiating, pro-inflammatory responses.
CHALLENGING THE ACCEPTED PARADIGM
To understand how Kielian and colleagues (Garg et al., 2009) challenged whether the CNS has an obligate bias towards anti-inflammatory responses, it is important to define how the immune system and CNS detect and initiate cellular responses toward common pathogens. Cells in both the CNS (microglia, astrocytes) and the immune system (neutrophils, macrophages and many others) express receptors such as the TLRs (Toll-like receptors) that recognize PAMPs (pathogen-associated molecular patterns). For example, TLR2 binds the peptioglycan found on the surface of S. aureus. Many (but not all) of the intracellular signalling and pro-inflammatory responses triggered by TLRs are dependent on the presence and function of the intracellular adaptor, MyD88 (also known as myeloid differentiation primary response gene 88). In addition, ablation of MyD88 expression within the immune system not only limits recognition of common pathogens, but also limits the effectiveness and extent of general pro-inflammatory responses to multiple stimuli because MyD88 expression is also required for IL (interleukin)-1 and IL-18 receptor family signalling. Not surprisingly, MyD88-knockout mice begin to lethally succumb to S. aureus infection 24 h following intracerebral infection.
At first glance, the brain abscess model used by Kielian and colleagues (Garg et al., 2009) would appear to confirm the assumed division of labour between the immune system and CNS (pro- compared with anti-inflammatory respectively). Specifically, within hours of intracerebral injection of live S. aureus, robustly activated peripheral immune cells (primarily neutrophils) enter the CNS and come into close apposition with the bacteria. By contrast, microglia preferentially surround the margins of the brain abscess (rather than being in direct contact with S. aureus), consistent with their previously demonstrated functions in limiting both innate and adaptive immune functions. In the commonly accepted paradigm, rapid S. aureus-associated lethality in MyD88-knockout mice is a predictable consequence of a handicapped, immunodeficient peripheral immune system.
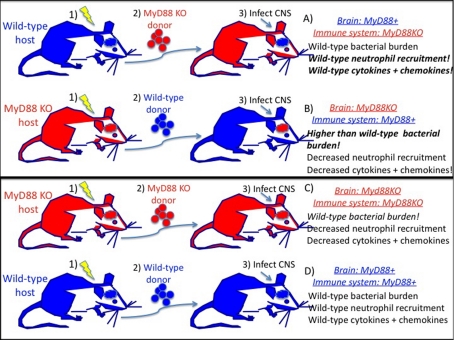
The study by Garg et al. (2009) used irradiation chimaeric mice to selectively remove MyD88 expression from only the peripheral immune system (Figure 1A) or from only the CNS (Figure 1B). The naïve prediction would be that mice in which MyD88 is expressed in the peripheral immune system (Figure 1B) would have immune responses most similar to that observed in wild-type mice (Figure 1D). Similarly, the immune responses in mice expressing MyD88 only in the CNS (Figure 1A) should be most similar to complete MyD88-knockout mice (Figure 1C) if CNS-resident cells play an insignificant pro-inflammatory role in pathogen defence against S. aureus.
Figure 1. MyD88 expression in the CNS, but not the peripheral immune system, leads to acute inflammatory responses similar to wild-type.
The experimental model has three steps. (i) Lethal whole-body irradiation of either wild-type (A and D) or MyD88-knockout (KO) mice (B and C) kills bone marrow and peripheral immune cells, but not CNS microglia or CNS-resident cells. (ii) Supplementation of irradiated mice with donor bone marrow from wild-type (B and D) or MyD88-knockout mice (A and C) leads to the repopulation of the peripheral immune system by the donor bone marrow. This manipulation generates mice with selective expression of MyD88 in either the CNS or the peripheral immune system. (iii) Injection and infection of CNS with live S. aureus. Inflammation is assessed 24 h post-infection. Chimaeras (C) and (D) control for non-specific irradiation effects.
Neither of these results were observed! Rather, expression of MyD88 only in the CNS was sufficient for robust induction of immune cell chemoattractants and recruitment of high numbers of MyD88-knockout neutrophils into the CNS following S. aureus infection. Surprisingly, the levels of pro-inflammatory cytokines produced in the presence of these immunodeficient MyD88-knockout neutrophils and macrophages was equivalent to or higher than that observed in S. aureus-infected wild-type mice.
Conversely, despite the disruption of the CNS vasculature and blood–brain barrier following injection of S. aureus into the CNS, immunocompetent MyD88-expressing neutrophils failed to enter the infected MyD88-knockout CNS and no cytokine responses were observed. Furthermore, mice with an immunocompetent MyD88-expressing immune system had higher bacterial burdens than complete MyD88-knockout mice! These results suggest that MyD88 expression in the CNS may play an unusual protective role offsetting the known potential of bacteria to hijack MyD88-dependent pathways in immune cells (Cirl et al., 2008).
One potential caveat to these studies should be considered. Use of irradiation chimaeras recently has come into question because irradiation facilitates inappropriate immune cell entry and colonization of the CNS (reviewed in Ransohoff, 2007). In the present study (Garg et al., 2009), the lower threshold for immune cell entry into the CNS provides a more stringent test of whether there are CNS-intrinsic mechanisms that cannot be replaced by the peripheral immune system.
CONSEQUENCES OF ACTIVE REGULATION
In summary, the majority of recent studies have focused on defining the anti-inflammatory regulatory functions of CNS-resident cells (Takahashi et al., 2007, reviewed in Carson et al., 2006; Carson and Lo, 2007). Garg et al. (2009) provide a clear demonstration that CNS-intrinsic mechanisms exist for induction of pro-inflammatory immune responses that go beyond merely expressing chemokines to recruit immune cells to the site of CNS infection (Garg et al., 2009). Furthermore, these mechanisms cannot be replaced or compensated for by a fully functional peripheral immune system, at least with regard to S. aureus infections of the CNS. These observations are in stark contrast with those generated in models of experimentally induced CNS autoimmunity initiated by injection of strong immune stimulants and adjuvants outside of the CNS (for example, subcutaneous injections of pertussis toxin and complete Freund's adjuvant) (reviewed in Carson et al., 2006). In these types of experimentally induced autoimmune model, CNS-resident cells play no necessary or sufficient role in induction of pro-inflammatory autoimmune responses. These latter types of data have served to focus potential therapeutic manipulation of autoimmune inflammatory responses solely on the peripheral immune system. This focus has yielded a mix of therapeutic approaches with both promising and disappointing results in human clinical trials. The current studies by Garg et al. (2009) suggest that the dysregulation of either CNS-intrinsic anti- or pro-inflammatory regulatory mechanisms could lead to inappropriate initiation of neuroinflammation and could contribute toward the onset of CNS autoimmune or CNS neuroinflammatory disorders.
REFERENCES
- Carson MJ, Doose JM, Melchior B, Schmid CD, Ploix C. CNS immune privilege: hiding in plain sight. Immunol Rev. 2006;213:48–65. doi: 10.1111/j.1600-065X.2006.00441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson MJ, Lo D. Perspective is everything: an irreverent discussion of CNS-immune system interactions as viewed from different scientific traditions. Brain Behav Immun. 2007;21:367–373. doi: 10.1016/j.bbi.2006.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirl C, Wieser A, Yadav M, Duerr S, Schubert S, Fischer H, Stappert D, Wantia N, Rodriguez N, Wagner H, Svanborg C, Miethke T. Subversion of Toll-like receptor signaling by a unique family of bacterial Toll/interleukin-1 receptor domain-containing proteins. Nat Med. 2008;14:399–406. doi: 10.1038/nm1734. [DOI] [PubMed] [Google Scholar]
- Garg S, Nichols JR, Esen N, Liu S, Phulwani NK, Syed MM, Wood WH, Zhang Y, Becker KG, Aldrich A, Kielian T. MyD88 expression by CNS-resident cells is piviotal for eliciting protective immunity in brain abscesses. ASN NEURO. 2009;1(2):art:e00007. doi: 10.1042/AN20090004. doi:10.1042/AN20090004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ransohoff RM. Microgliosis: the questions shape the answers. Nat Neurosci. 2007;10:1507–1509. doi: 10.1038/nn1207-1507. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Prinz M, Stagi M, Chechneva O, Neumann H. TREM2-transduced myeloid precursors mediate nervous tissue debris clearance and facilitate recovery in an animal model of multiple sclerosis. PLoS Med. 2007;4:e124. doi: 10.1371/journal.pmed.0040124. [DOI] [PMC free article] [PubMed] [Google Scholar]