Abstract
The mitochondrial lipidome influences ETC (electron transport chain) and cellular bioenergetic efficiency. Brain tumours are largely dependent on glycolysis for energy due to defects in mitochondria and oxidative phosphorylation. In the present study, we used shotgun lipidomics to compare the lipidome in highly purified mitochondria isolated from normal brain, from brain tumour tissue, from cultured tumour cells and from non-tumorigenic astrocytes. The tumours included the CT-2A astrocytoma and an EPEN (ependymoblastoma), both syngeneic with the C57BL/6J (B6) mouse strain. The mitochondrial lipidome in cultured CT-2A and EPEN tumour cells were compared with those in cultured astrocytes and in solid tumours grown in vivo. Major differences were found between normal tissue and tumour tissue and between in vivo and in vitro growth environments for the content or composition of ethanolamine glycerophospholipids, phosphatidylglycerol and cardiolipin. The mitochondrial lipid abnormalities in solid tumours and in cultured cells were associated with reductions in multiple ETC activities, especially Complex I. The in vitro growth environment produced lipid and ETC abnormalities in cultured non-tumorigenic astrocytes that were similar to those associated with tumorigenicity. It appears that the culture environment obscures the boundaries of the Crabtree and the Warburg effects. These results indicate that in vitro growth environments can produce abnormalities in mitochondrial lipids and ETC activities, thus contributing to a dependency on glycolysis for ATP production.
Keywords: cancer, culture, glycolysis, lipid, metabolism, shotgun lipidomics
Abbreviations: Cer, ceramide; CerPCho, sphingomyelin; ChoGpl, choline glycerophospholipids; EPEN, ependymoblastoma; ESI/MS, electrospray ionization/MS; ETC, electron transport chain; EtnGpl, ethanolamine glycerophospholipids; lysoPtdCho, lysophosphatidylcholine; MS/MS, tandem MS; NS, non-synaptic; PakCho, plasmanylcholine; PakEtn, plasmanylethanolamine; PlsCho, plasmenylcholine; PlsEtn, plasmenylethanolamine; PtdCho, phosphatidylcholine; PtdEtn, phosphatidylethanolamine; PtdGro, phosphatidylglycerol; PtdIns, phosphatidylinositol; PtdSer, phosphatidylserine
INTRODUCTION
Brain tumours depend heavily on glycolysis to supply ATP for growth and cellular function (Kirsch et al., 1972; Galarraga et al., 1986; Lichtor and Dohrmann, 1986; Floridi et al., 1989; Oudard et al., 1997; Seyfried and Mukherjee, 2005). An irreversible injury to cellular respiration is proposed to underlie the dependence of tumours on glycolytic energy (Warburg, 1956; Kiebish et al., 2008a). Structural defects in brain tumour mitochondria have been described and involve swelling, morphological deformities and cristolysis (Cervos-Navarro et al., 1981; Oudard et al., 1997; Arismendi-Morillo and Castellano-Ramirez, 2008). These defects suggest an impaired ability of mitochondria to effectively produce ATP via oxidative phosphorylation. Mitochondrial membrane lipids are critical for regulating a variety of functions, including membrane fluidity/stability, proton gradient impermeability, membrane potential, ETC (electron transport chain) activities, supercomplex formation and proton leak (Daum, 1985; McMillin and Dowhan, 2002; Zhang et al., 2002). Also, the unique composition of mitochondrial lipids in both the inner and the outer membranes is required for efficient ATP production (Mileykovskaya et al., 2005). Abnormalities in mitochondrial lipid composition could produce uncoupling and impaired energy production, despite continued CO2 production and O2 consumption (Wu et al., 2007).
EtnGpl (ethanolamine glycerophospholipids) and ChoGpl (choline glycerophospholipids) comprise nearly 80% of the total phospholipids in the mitochondrial lipidome (Daum, 1985; Kiebish et al., 2008b). Other mitochondrial lipids in order of abundance include cardiolipin, PtdIns (phosphatidylinositol), PtdSer (phosphatidylserine), PtdGro (phosphatidylglycerol), CerPCho (sphingomyelin), lysoPtdCho (lysophosphatidylcholine) and Cer (ceramide) (Kiebish et al., 2008c). Each phospholipid contains a unique distribution of fatty acid molecular species in the sn-1 and sn-2 positions. Lipids of tumour mitochondria generally contain fatty acids with shorter chain lengths and less unsaturation than lipids of normal tissue mitochondria (Morton et al., 1976; Reitz et al., 1977; Hartz et al., 1982; Canuto et al., 1989; Peskin and Carter, 2008). These fatty acid changes alter mitochondrial membrane fluidity, which lowers bioenergetic efficiency (Ellis et al., 2005). In addition to abnormalities in fatty acid species composition, neoplastic tissues also contain abnormalities in the content of specific lipids, including cardiolipin, lysoPtdCho, PtdSer and CerPCho, as well as ether glycerophospholipids (Bergelson et al., 1970, 1974; Hostetler et al., 1979). The advent of shotgun lipidomics using ESI/MS (electrospray ionization/MS) now provides a rapid and quantitative method for the comprehensive analysis of membrane lipids from normal and diseased tissues even with a limited amount of sample (Han and Gross, 2005).
Cardiolipin is almost exclusively localized in the inner membrane of mitochondria where it is synthesized from condensation of PtdGro and cytidine diphosphate-diacylglycerol to form immature cardiolipin (Hoch, 1992; Schlame et al., 2000). Mature cardiolipin is then formed through a remodelling process involving deacylation and reacylation reactions using donor fatty acids from EtnGpl, ChoGpl and acyl-CoA (Hauff and Hatch, 2006; Kiebish et al., 2008c). Tetralinoleic cardiolipin is the predominant remodelled cardiolipin in heart, liver and muscle, which is maintained by a selective remodelling process (Schlame et al., 2005). Abnormalities in cardiolipin molecular species composition, as found in Barth syndrome, produce severe impairments in oxidative phosphorylation and energy metabolism (Schlame and Ren, 2006). Thus maintenance of cardiolipin molecular species composition is critical for bioenergetic capacity.
In contrast with the predominant tetralinoleic cardiolipin found in most non-neural tissues, cardiolipin in brain mitochondria is comprised of over 100 fatty acid molecular species (Schlame et al., 2005; Cheng et al., 2008; Kiebish et al., 2008b). Moreover, these molecular species are distributed in seven major groups when arranged according to mass-to-charge ratios (Kiebish et al., 2008b). It is unknown which specific acyltransferase(s), transacylase(s) or phospholipase(s) are involved in the remodelling of brain cardiolipin. Regardless, the cardiolipin remodelling machinery should be similar in all cells derived from neural origin, resulting in the increased molecular species distribution as found for brain cardiolipin. If lipid or fatty acid metabolism is altered in brain tumour mitochondria, then the resulting effects will be evident in cardiolipin remodelling due to utilization of donor fatty acids. Abnormalities in cardiolipin remodelling will also reduce ATP production through oxidative phosphorylation, thus altering bioenergetic capacity.
The present study is the first to analyse the mitochondrial lipidome of experimental brain tumours grown in vivo and in vitro. We have also compared the lipidome of the cultured brain tumour cells with that of non-tumorigenic syngeneic astrocytes. ETC activities were measured in isolated mitochondria to determine whether these brain tumour mitochondria have an impaired ability to produce ATP by oxidative phosphorylation. The results show that abnormalities in the mitochondrial lipidome can arise from tumorigenicity, as well as from the in vitro growth environment.
MATERIALS AND METHODS
Mice and brain tumours
The inbred C57BL/6J (B6) mouse strain was obtained from the Jackson Laboratory. B6 mice were propagated at the Boston College Animal Facility and were housed in plastic cages with filter tops containing Sani-Chip bedding (P.J. Murphy Forest Products). The room was maintained at 22°C on a 12 h/12 h light/dark cycle. Food (Prolab RMH 3000; PMI LabDiet) and water were provided ad libitum. The present study was conducted according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and was approved by the Institutional Animal Care Committee.
The CT-2A and EPEN (ependymoblastoma) brain tumours were originally produced from the implantation of 20-methylcholanthrene into the brains of B6 mice as previously described (Zimmerman and Arnould, 1941; Seyfried et al., 1992). The CT-2A tumour arose in the cerebral cortex and was characterized as a malignant anaplastic astrocytoma, whereas the EPEN tumour arose in the cerebral ventricle and was characterized as an ependymoblastoma (Rubin and Sutton, 1968; Seyfried et al., 1992). Male B6 mice (8–12 weeks of age) were used as tumour recipients. Tumour pieces from donor mice were diced and resuspended in ice-cold PBS at pH 7.4. Mice were anaesthetized with isoflurane (Halocarbon) and 0.1 ml of diced tumour tissue suspended in 0.2 ml of PBS was implanted subcutaneously in the right flank by injection using a 1 cc tuberculin syringe and an 18-gauge needle.
Cell culture
The cultured cell lines were prepared from the CT-2A and EPEN tumours as described previously (Seyfried et al., 1992; El-Abbadi and Seyfried, 1994; Bai and Seyfried, 1997). The C8-D1A type 1 astrocyte cell line was purchased from A.T.C.C. The genetic background of these cells is highly similar to that of the tumour cells since the C8-D1A cells were isolated initially from the cerebella of 8-day-old C57BL/6 mice (Alliot and Pessac, 1984). It is important to mention that no carcinogens or viruses were used to transform the C8-D1A cells. Although the authors described the cells as arising from spontaneous transformation, this is debatable, as the event(s) responsible for the onset of cell growth was similar to that in cultures originating from 1- and 15-day postnatal mouse cerebellum (Alliot and Pessac, 1984). All cultured cells were maintained under identical conditions in DMEM (Dulbecco's modified Eagle's medium; Sigma) supplemented with 10% (v/v) FBS (fetal bovine serum; Atlanta Biologicals), 25 mM glucose and 0.5% penicillin/streptomycin (Sigma) in a humidified atmosphere containing 95% air and 5% CO2 at 37°C. Cells were grown to 90% confluency and removed using a BD Falcon cell scraper (Becton Dickinson).
Mitochondrial isolation
Mitochondria were isolated in a cold room (4°C) and all reagents were kept on ice. NS (non-synaptic) mouse brain mitochondria and brain tumour mitochondria were isolated as previously described using a series of discontinuous Ficoll and sucrose gradients (Kiebish et al., 2008b, 2008c). Briefly, the cerebral cortexes (a pool of six/sample) or a pool of four tumours/sample were homogenized on ice and differentially centrifuged to remove membrane contamination. A crude mitochondrial pellet was layered on a 7.5%/12% Ficoll discontinuous gradient and centrifuged at 73000 g for 36 min in a Sorval SW 28 rotor. An enriched mitochondrial pellet was collected below the 12% Ficoll layer. The enriched mitochondrial pellet was then layered on a 0.8 M/1.0 M/1.3 M/1.6 M discontinuous sucrose gradient and centrifuged at 50000 g for 2 h in a Sorvall SW 28 rotor. Purified NS brain or brain tumour mitochondria were collected at the interface between 1.3 M and 1.6 M sucrose.
Mitochondria were isolated from the cultured CT-2A, EPEN and non-tumorigenic astrocytes cell lines as a single enriched mitochondrial fraction as previously described (Yang et al., 1997; Pon and Schon, 2001). A pellet obtained from cells grown to 90% confluency on six T150 flasks was resuspended in MIB [mitochondrial isolation buffer; 0.32 M sucrose, 10 mM Tris/HCl and 1 mM potassium EDTA (pH 7.4)] and homogenized on ice using a teflon-coated homogenizer attached to a hand-held drill. Samples were homogenized using ten up and down strokes at 500 rev./min. The homogenate was then centrifuged at 800 g for 5 min. The supernatant was collected and centrifuged at 1000 g for 5 min. The pellet was discarded and the supernatant was collected and centrifuged at 14000 g for 10 min. The pellet was collected and resuspended in MIB and recentrifuged at 8600 g for 10 min. The supernatant was discarded and the pellet was resuspended in MIB and layered on a 0.8 M/1.0 M/1.7 M discontinuous sucrose gradient and centrifuged at 80000 g for 2 h in a Sorvall SW 55 Ti rotor. Purified mitochondria were collected at the 1.0 M/1.7 M sucrose interface. The collected band was resuspended in MIB and centrifuged at 19000 g for 15 min. The pellet was resuspended in MIB and centrifuged at 10200 g for 10 min. The pellet was resuspended twice more in MIB and recentrifuged at 8200 g for 10 min. The collected pellet contained purified mitochondria. The protein concentration of isolated mitochondria was determined by the Dc Protein Assay using BSA standards (Bio-Rad).
Materials for MS
Synthetic phospholipids including 14:1-14:1 PtdCho (phosphatidylcholine), 16:1-16:1 PtdEtn (phosphatidylethanolamine), 15:0-15:0 PtdGro (1,2-dipentadecanoyl-sn-glycero-3-phosphoglycerol), 14:0-14:0 PtdSer (1,2-dimyristoyl-sn-glycero-3-phosphoserine), N12:0 CerPCho (N-lauroryl sphingomyelin), T14:0 cardiolipin (1,1′,2,2′-tetramyristoyl cardiolipin), N17:0 Cer (heptadecanoyl ceramide) and 17:0 lysoPtdCho (1-heptadecanoyl-2-hydroxy-sn-glycero-3-phosphocholine) were purchased from Avanti Polar Lipids. It should be noted that the prefix ‘N' denotes the amide-linked acyl chain. All the solvents were obtained from Burdick and Jackson (Honeywell International). All other chemicals were purchased from Sigma–Aldrich.
Sample preparation for mass spectrometric analysis
An aliquot of the mitochondrial preparation was transferred to a disposable culture borosilicate glass tube (16 mm×100 mm). Internal standards were added based on protein concentration and included 16:1-16:1 PtdEtn (100 nmol/mg of protein), 14:1-14:1 PtdCho (45 nmol/mg of protein), T14:0 cardiolipin (3 nmol/mg of protein), 15:0-15:0 PtdGro (7.5 nmol/mg of protein), 14:0-14:0 PtdSer (20 nmol/mg of protein), 17:0 lysoPtdCho (1.5 nmol/mg protein), N12:0 CerPCho (20 nmol/mg of protein) and N17:0 Cer (5 nmol/mg of protein). This allowed the final quantified lipid content to be normalized to the protein content and eliminated potential loss from incomplete recovery. The molecular species of internal standards were selected because they represent <0.1% of the endogenous cellular lipid mass as demonstrated by ESI/MS lipid analysis.
A modified Bligh and Dyer procedure was used to extract lipids from each mitochondrial preparation as previously described (Cheng et al., 2006). Each lipid extract was reconstituted in 500 μl/mg of protein (which was based on the original protein content of the samples as determined from protein measurement) in chloroform/methanol (1:1; v/v). The lipid extracts were flushed with nitrogen, capped and stored at −20°C for ESI/MS analysis. Each lipid solution was diluted approx. 50-fold immediately prior to infusion and lipid analysis.
Instrumentation and MS
A triple-quadrupole mass spectrometer (Thermo Scientific TSQ Quantum Ultra Plus), equipped with an ESI source and Xcalibur system software, was utilized as previously described (Han et al., 2004). The first and third quadrupoles serve as independent mass analysers using a mass resolution setting of peak width 0.7 Th, whereas the second quadrupole serves as a collision cell for MS/MS (tandem MS). The diluted lipid extract was directly infused into the ESI source at a flow rate of 4 μl/min with a syringe pump. Typically, a 2-min period of signal averaging in the profile mode was employed for each mass spectrum. For MS/MS, a collision gas pressure was set at 1.0 mT, but the collision energy varied with the classes of lipids as described previously (Han et al., 2004; Han and Gross, 2005). Typically, a 2- to 5-min period of signal averaging in the profile mode was employed for each MS/MS spectrum. All the mass spectra and MS/MS spectra were automatically acquired by a customized sequence subroutine operated under Xcalibur software. Data processing of two-dimensional MS analyses including ion peak selection, data transferring, peak intensity comparison and quantification was conducted using self-programmed Microsoft Excel macros (Han et al., 2004).
ETC enzyme activities
ETC enzyme analysis for Complex I (NADH-ubiquinone oxidoreductase), Complex II [succinate decylubiquinone DCIP (2,6-dichloroindophenol) oxidoreductase], Complex III (ubiquinol cytochrome c reductase), Complex IV (cytochrome c oxidase), Complex I/III (NADH cytochrome c reductase) and Complex II/III (succinate cytochrome c reductase) was performed as previously described (Ellis et al., 2005; Kiebish et al., 2008b, 2008c).
RESULTS
We used multiple discontinuous gradients to obtain highly purified mitochondria from normal brain and from brain tumour tissue (Kiebish et al., 2008b, 2008c). The length as well as choice of discontinuous gradients employed was designed for the purpose of mitochondrial lipid analysis as well as for assessment of ETC enzyme activities by standard biochemical procedures. We have recently shown that these isolation procedures provide precise information on the content and composition of total mitochondrial lipids when analysed using shotgun lipidomics (Kiebish et al., 2008b). Mitochondria were isolated from the brain tumours grown subcutaneously in order to avoid contamination from normal brain tissue surrounding the tumours. The lipids in tumour mitochondria were compared with those from NS brain mitochondria, which are largely derived from glial cells. Part of the in vivo data in the present study have been presented previously (Kiebish et al., 2008a) and are included here to facilitate comparison with the in vitro data. We also evaluated lipids in mitochondria isolated from the tumour cells and from non-tumorigenic astrocytes cultured under identical conditions. All of the cultured cells and tumours were analysed on the same (B6) genetic background. Our analysis in purified mitochondria eliminates issues regarding differences in mitochondrial content between tumour tissue and normal tissue (Pedersen, 1978; Kiebish et al., 2008b, 2008c). The lipid classes were arranged according to their relative abundance in B6 control brain mitochondria (Table 1).
Table 1. Lipid composition of mitochondria isolated from brain, brain tumour and cells.
Values are expressed as mean nmol/mg of protein±S.D. (n = 3). *P<0.05; **P<0.01 and ***P<0.001, significantly different values from B6 NS or astrocyte mitochondria. N.D., not detected.
| Lipid | In vivo | In vitro | ||||
| BrainC | CT-2A | EPEN | AstrocyteC | CT-2A | EPEN | |
| EtnGpl | 187.4±12.1 | 245.9±13.7** | 368.4±46.4* | 171.4±18.6 | 163.0±6.5 | 211.0±16.7 |
| PtdEtn | 164.9±10.0 | 137.3±6.0* | 259.4±45.7 | 85.9±3.4 | 69.7±3.0** | 98.9±2.3* |
| PlsEtn | 22.5±2.2 | 99.3±7.1** | 147.8±21.4** | 80.5±15.0 | 87.5±9.4 | 106.9±14.4 |
| PakEtn | N.D. | 9.3±0.7** | 12.4±3.0* | 5.0±0.3 | 5.7±0.1* | 5.1±1.1 |
| ChoGpl | 129.9±7.7 | 121.2±3.6 | 160.0±29.5 | 168.5±14.2 | 127.4±13.2* | 194.4±15.7 |
| PtdCho | 119.6±5.3 | 81.4±3.4** | 127.4±25.4 | 124.6±10.5 | 98.1±12.4* | 174.3±13.9** |
| PlsCho | 1.2±0.1 | 19.4±2.1*** | 11.6±4.8 | 22.4±4.0 | 15.8±0.8 | 9.3±0.8* |
| PakCho | 9.1±3.2 | 20.4±2.6** | 17.0±6.2 | 21.5±2.1 | 13.5±0.4* | 10.8±1.1** |
| Cardiolipin | 52.7±4.5 | 26.1±1.0** | 13.5±2.7*** | 28.3±4.3 | 24.6±3.7 | 31.1±4.2 |
| PtdIns | 9.4±0.8 | 9.5±2.6 | 19.4±2.5* | 18.5±2.4 | 18.4±1.6 | 20.5±3.3 |
| PtdGro | 7.1±0.5 | 9.8±0.5** | 16.4±3.6* | 7.7±2.6 | 7.6±1.5 | 4.7±0.3 |
| CerPCho | 5.3±1.2 | 4.6±0.2 | 5.8±1.8 | 9.7±1.4 | 15.9±3.4 | 22.1±1.3** |
| PtdSer | 4.6±1.5 | 9.1±0.6* | 10.4±2.0* | 17.8±0.4 | 28.7±5.7 | 24.1±3.6 |
| LysoPtdCho | 2.7±0.6 | 6.3±0.6** | 2.8±0.4 | 1.5±0.1 | 2.2±0.4 | 2.4±0.6 |
| Cer | 0.7±0.2 | 2.3±0.2*** | 1.7±0.2** | 0.9±0.1 | 1.0±0.5 | 2.0±0.2** |
CB6 NS brain mitochondria or astrocyte (C8-D1A) mitochondria were used as controls.
Comparison of mitochondrial lipids from brain tumours grown in vivo
The most abundant phospholipid in brain mitochondria is EtnGpl, which is comprised of PtdEtn, PlsEtn (plasmenylethanolamine) and PakEtn (plasmanylethanolamine). The total content of EtnGpl was significantly greater in the CT-2A (31%) and EPEN (97%) tumours than in NS mitochondria from normal brain (Table 1). Although PtdEtn was lower in CT-2A than EPEN or normal brain, the significant increase in total EtnGpl was due to increases in PlsEtn and PakEtn content compared with brain mitochondria. PlsEtn content was 4.5- and 6.5-fold higher in CT-2A and EPEN mitochondria respectively, compared with brain mitochondria. PakEtn was expressed in both tumours, but was undetectable in brain mitochondria. Total ChoGpl content was similar in the CT-2A, EPEN and normal brain mitochondria. However, PtdCho content was lower, whereas PlsCho (plasmenylcholine) and PakCho (plasmanylcholine) contents were higher in CT-2A mitochondria than in normal brain mitochondria.
Cardiolipin content was 2- and 4-fold lower in CT-2A and EPEN mitochondria respectively, than in normal brain mitochondria (Table 1). Interestingly, PtdGro (the precursor for cardiolipin) was increased by 38% and 131% in CT-2A and EPEN mitochondria respectively, compared with normal brain mitochondria. PtdIns content was also greater in EPEN mitochondria than in normal brain mitochondria. The content of PtdSer and Cer were higher in tumour mitochondria than in normal brain mitochondria.
Comparison of mitochondrial lipids from brain tumours grown in vitro
Since many studies of cellular metabolism are conducted in dividing cells grown in culture, we examined the lipidome of mitochondria isolated from the brain tumour cells and from non-tumorigenic syngeneic astrocytes grown under identical conditions. The growth rate was similar in the astrocytes and CT-2A cells, but was slower in EPEN cells (Bai and Seyfried, 1997). In contrast with mitochondrial EtnGpl content in the solid tumours grown in vivo, total mitochondrial EtnGpl content was similar in the cultured astrocytes and the tumour cells (Table 1). However, mitochondrial PtdEtn content was lower in CT-2A and higher in EPEN compared with the contents in mitochondria from non-tumorigenic astrocytes. No differences were found in PlsEtn content, whereas PakEtn content was higher in CT-2A compared with astrocyte mitochondria. Total mitochondrial ChoGpl content was lower in CT-2A than in EPEN or astrocytes, primarily due to lower levels of PtdCho and PakCho. The content of CerPCho and Cer was higher in EPEN mitochondria than in astrocyte mitochondria. No differences were detected between non-tumorigenic astrocytes and tumour cells for the content of cardiolipin, PtdIns, PtdGro, PtdSer and lysoPtdCho. Viewed collectively, these findings show that several lipidomic differences observed between normal mitochondria and tumour mitochondria in vivo are not found between non-tumorigenic astrocytes and the tumour cells when grown in vitro.
Comparisons of lipid molecular species in mitochondria from normal and tumour tissue and cells
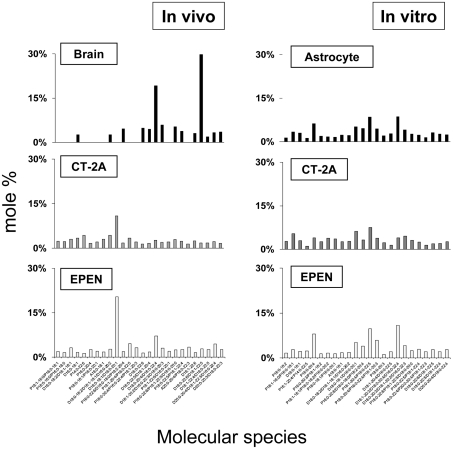
EtnGpl, ChoGpl and cardiolipin comprised approx. 85–90% of the total mitochondrial phospholipid content (Table 1). Differences in the molecular species composition of these phospholipid classes will therefore represent the most significant changes to mitochondrial membrane dynamics. Also, cardiolipin is remodelled using donor fatty acids from EtnGpl and ChoGpl (Hauff and Hatch, 2006; Xu et al., 2006). Consequently, abnormalities in mitochondrial EtnGpl and ChoGpl molecular species will result in alterations of cardiolipin molecular speciation. The molecular species composition for all mitochondrial lipids can be found in Supplementary Tables S1–S9 (at http://www.asnneuro.org/an/001/an001e011.add.htm). EtnGpl from brain NS mitochondria contained predominantly D18:1-22:6 and D18:0-20:4/D16:0-22:4. The first fatty acid species of the pair is presumed to occupy the sn-1 position, whereas the second species of the pair is presumed to occupy the sn-2 position. Besides these major species, approx. 12 other minor molecular species were also detected (Figure 1). In contrast, EtnGpl from the CT-2A and EPEN tumours contained predominantly D18:0-18:1/D16:0-20:1 with lower amounts of D18:0-20:4/D16:0-22:4. The content of molecular species with low mass-to-charge ratios, representing less unsaturated and shorter chain fatty acids, was greater in the CT-2A and the EPEN tumours than in normal brain mitochondria. In contrast, the distribution of EtnGpl molecular species was similar in the cultured astrocytes and in tumour cells, with no molecular species representing a predominant type (Figure 1).
Figure 1. Distribution of EtnGpl molecular species in mitochondria isolated from mouse brain, brain tumours and cultured cells.
Molecular species values are arranged based on the mass-to-charge ratio and are expressed as percentages. The mass content of molecular species can be found in Supplementary Table S1 at http://www.asnneuro.org/an/001/an001e011.add.htm. All values are expressed as the mean of three independent mitochondrial preparations. The in vivo data were obtained from pooling six mouse cerebral cortexes or four brain tumours. The in vitro data were obtained from pooling three independent cell pellets, where each pellet was collected from six T150 culture flasks.
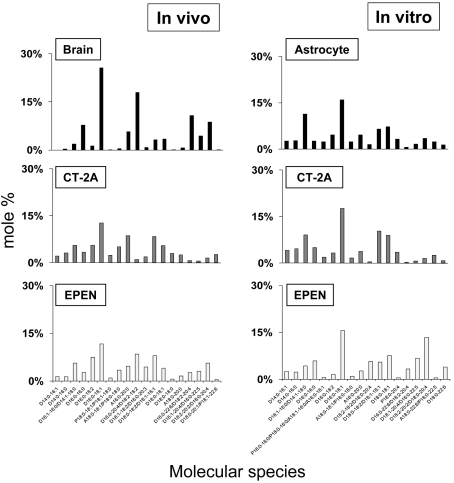
The predominant ChoGpl molecular species in normal brain mitochondria were D16:0-18:1, D18:2-18:2/D16:0-20:4, D16:0-22:6/D18:2-20:4 and D18:2-20:2/D18:0-20:4. These species were also present in the CT-2A and EPEN tumours, but their content was less than that found in normal brain mitochondria (Figure 2). The molecular species of mitochondrial ChoGpl were similar in the astrocytes and tumour cells grown in vitro, with a predominance of D16:0-18:1, D16:1-16:0/D14:1-18:0, D18:0-18:2/D18:1-18:1 and D18:0-18:1 species. However, the D18:2-20:2/D18:0-20:4 species was higher in EPEN cells than in astrocytes (Figure 2).
Figure 2. Distribution of ChoGpl molecular species in mitochondria isolated from mouse brain, brain tumours and cultured cells.
The mass content of molecular species can be found in Supplementary Table S2 at http://www.asnneuro.org/an/001/an001e011.add.htm. Other conditions are as described in the legend to Figure 1.
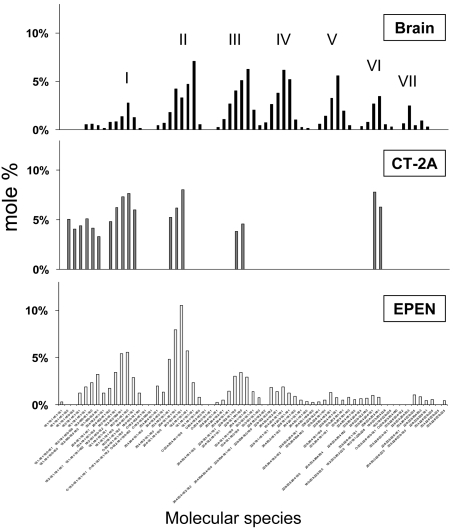
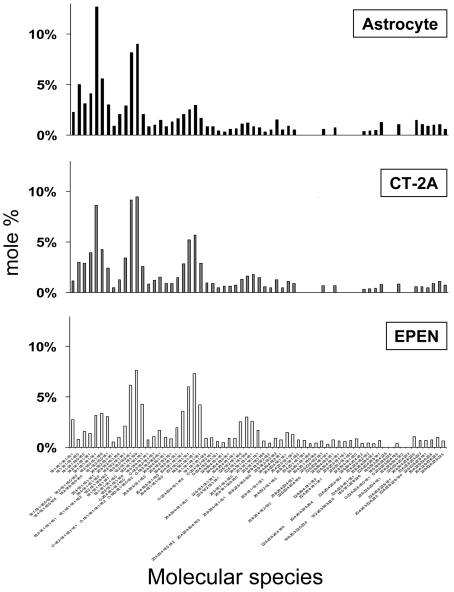
Normal mouse brain cardiolipin contains a unique distribution of molecular species represented by seven major species groups, which are conserved and generated following extensive remodelling (Cheng et al., 2008; Kiebish et al., 2008b). The distribution of cardiolipin molecular species differs markedly between normal brain mitochondria and tumour mitochondria (Figure 3) (Kiebish et al., 2008a). In general, the content of molecular species with low mass-to-charge ratios, representing shorter chain less unsaturated molecular species, was greater in the mitochondria from the CT-2A and EPEN tumours than in mitochondria from normal brain. These low mass-to-charge species probably represent newly synthesized cardiolipin. Also, the number of molecular species groups was noticeably less in the CT-2A tumour than in the EPEN tumour, suggesting that remodelling abnormalities are greater in CT-2A than in EPEN. The total number of cardiolipin molecular species was similar in the EPEN tumour and in normal brain, but their relative distribution in EPEN was markedly abnormal in showing a predominance of shorter chain less unsaturated molecular species as demonstrated by the relative lower mass-to-charge ratios (Figure 3). The distribution of molecular species in cardiolipin is influenced by remodelling from EtnGpl, ChoGpl and acyl CoA fatty acids, as well as by cellular growth rate (Hauff and Hatch, 2006; Xu et al., 2006). The distribution of cardiolipin molecular species in the non-tumorigenic astrocytes was more similar to that of the EPEN and CT-2A tumour cells than to that of normal B6 brain (Figures 3 and 4). Hydroxylated fatty acid molecular species were found in cardiolipin from tumour cells grown in vitro, but were not found in cardiolipin from the solid tumours grown in vivo, suggesting that hydroxylated fatty acids are produced as an artifact of the in vitro environment. No ω−9 fatty acid (mead acid) was found in the mitochondrial lipids of these brain tumours. Although mead acid was previously found in some cancers (Tocher et al., 1995), there are no reports to our knowledge describing mead acid in primary brain tumours.
Figure 3. Distribution of cardiolipin molecular species in mitochondria isolated from mouse brain and brain tumours.
The corresponding mass content of molecular species can be found in Supplementary Table S3 at http://www.asnneuro.org/an/001/an001e011.add.htm. The distribution of cardiolipin molecular species of tumours grown in vivo have been presented previously and are shown here only for comparison with the data for the cultured cells shown in Figure 4. Other conditions are as described in the legend to Figure 1. reproduced from Kiebish et al., 2008a with permission. © 2008, American Society for Biochemistry and Molecular Biology, Inc.
Figure 4. Distribution of cardiolipin molecular species in mitochondria isolated from transformed astrocytes and brain tumour cells grown in vitro.
Conditions are as described in the legend to Figure 1.
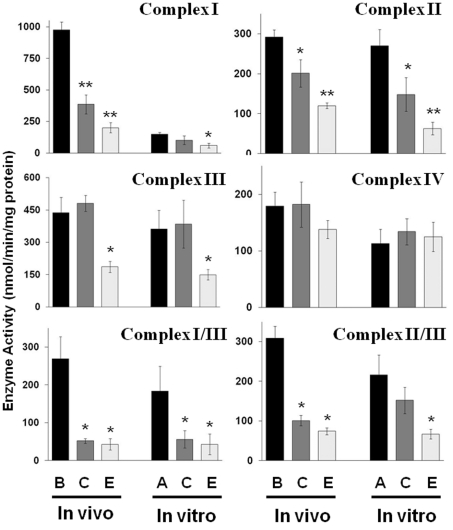
ETC activities of brain, brain tumours and cells grown in culture
ETC activities were measured in mitochondria isolated from brain and brain tumours grown in vivo, as well as from the cells grown in vitro. Both unlinked and linked ETC enzyme activities were examined. The activities of Complex I, II, I/III and II/III were significantly lower in mitochondria from the CT-2A and the EPEN tumours than in mitochondria from normal host brain tissue (Figure 5) (Ellis et al., 2005; Kiebish et al., 2008a). The activity of Complex III was lower only in EPEN, whereas no significant difference was found between the tumours and the normal brain tissue for the activity of Complex IV. The general differences found in the ETC activities in vivo between the tumour tissue and the normal tissue were also found between the non-tumorigenic astrocytes and tumour cells in vitro, with the exception of Complex I activity (Figure 5). Complex I activity was markedly lower in the mitochondria from the cultured astrocytes than in the mitochondria from the brain tissue. Indeed, the Complex I activity of the cultured astrocytes was more similar to the activities of the tumour cells than to those of the brain tissue. These findings indicate that the brain tumour tissue and the cultured tumour cells contain major abnormalities in the activities of most ETC Complex activities. These findings also show that Complex I activity is significantly lower in mitochondria from cultured astrocytes than in mitochondria from normal brain tissue.
Figure 5. ETC enzyme activities in purified mitochondria from mouse brain, brain tumours and cultured tumour cells.
Enzyme activities are expressed as nmol/min per mg of protein as described in the Materials and methods section. B, C, E and A represent enzyme activities from brain, CT-2A, EPEN and astrocytes (non-tumorigenic) respectively. Other conditions are as described in the legend to Figure 1. Asterisks indicate that the activities in the brain tumour samples differ from those of the control samples (either mouse brain or astrocytes) at the *P<0.03 or **P<0.005 levels as determined using the two-tailed Student's t test. The ETC activities of tumours grown in vivo have been presented previously (reproduced from Kiebish et al., 2008a with permission; © 2008, American Society for Biochemistry and Molecular Biology, Inc.), and are presented here only for comparison with the enzyme activities of the cultured cells.
DISCUSSION
Cultured brain tumour cells and non-tumorigenic astrocytes are routinely used for studies of energy metabolism and physiology (Homburg et al., 1961; Rodriguez-Enriquez et al., 2006; Wu et al., 2007; Schieke et al., 2008). Mitochondrial membrane lipids are critical for maintaining numerous enzyme activities involved with energy metabolism (Daum, 1985; Hoch, 1992; Shinzawa-Itoh et al., 2007). Cardiolipin, in particular, regulates ETC activities of Complex I, III, IV and V, as well as regulating supercomplex formation (Fry and Green, 1980, 1981; Chicco and Sparagna, 2007; Bogdanov et al., 2008; Kocherginsky, 2008). Additionally, cardiolipin regulates proton leak and the mitochondrial transport of several bioenergetic molecules to include adenine nucleotide, carnitine, pyruvate and phosphate (Hoch, 1998; Chicco and Sparagna, 2007; Claypool et al., 2008; Kocherginsky, 2008). Abnormalities in cardiolipin content and composition will decrease cellular bioenergetic efficiency (Bogdanov et al., 2008; Kocherginsky, 2008). The extent to which the in vitro growth environment influences the mitochondrial lipidome in non-tumorigenic astrocytes and in brain tumour cells is largely unknown.
In the present study we have found that the mitochondrial lipidome of the CT-2A and EPEN brain tumour cells grown in vitro differed markedly from the mitochondrial lipidome of these same brain tumour cells when they were grown in vivo. In addition, the mitochondrial lipidome of non-tumorigenic astrocytes differed from that of NS brain mitochondria, which are derived largely from glial cells (Kiebish et al., 2008b). Indeed, the mitochondrial lipidome of the non-tumorigenic astrocytes was remarkably similar to that of the brain tumour cells. Since efficiency of oxidative phosphorylation is dependent on the integrity of the mitochondrial lipidome, and especially on the content and composition of cardiolipin, our results indicate that in vitro growth reduces respiratory energy efficiency in these cells.
The effect of passage number, culture conditions and cellular differentiation on fatty acid composition has been extensively studied in transformed and primary cell lines (Murphy and Horrocks, 1993; Murphy et al., 1993, 1997; Cheng et al., 2008). However, no prior studies have examined the influence of growth environment on the mitochondrial lipidome and ETC activities in brain tumour cell lines compared with syngeneic non-tumorigenic astrocytes grown under identical conditions (Springer, 1980; Kunz-Schughart et al., 2001). Respiratory efficiency is dependent to a large extent on the expression of mature cardiolipin, which is synthesized through an extensive remodelling process (Kiebish et al., 2008c). The abundance of immature cardiolipin (short chain saturated/monounsaturated species) indicates that energy through oxidative phosphorylation is compromised in the cultured cells. The markedly reduced ETC activities, especially those associated with Complex I, is consistent with a failure to produce mature cardiolipin. The persistent expression of immature cardiolipin would dissipate the electrochemical concentration gradient, contribute to mitochondrial uncoupling, and reduce energy production through oxidative phosphorylation (Brookes et al., 1998; Hoch, 1998; Bogdanov et al., 2008; Kocherginsky, 2008). In addition, low amounts of long-chain polyunsaturated fatty acids in choline and ethanolamine glycerophospholipids in brain tumour tissue will prevent cardiolipin remodelling to the extent seen in normal non-tumorigenic tissue (Hostetler et al., 1976; Yates et al., 1979; Hartz et al., 1982; Canuto et al., 1989; Campanella, 1992). Accumulation of the cardiolipin precursor, PtdGro, also suggests problems with cardiolipin remodelling and synthesis. An inability to synthesize mature cardiolipin will therefore reduce energy production through oxidative phosphorylation.
Further support for respiratory energy inefficiency in the cultured cells comes from our results that lactic acid production is high in the CT-2A tumour cells and the non-tumorigenic astrocytes when grown under identical in vitro growth conditions (L. Shelton and T.N. Seyfried, unpublished data), indicating that these cells obtain their energy largely from glycolysis. In addition to enhanced glycolysis, an impaired mitochondrial lipidome could also enhance energy production through glutaminolysis and substrate level phosphorylation in the TCA (tricarboxylic acid) cycle itself (DeBerardinis et al., 2007). This energy source, together with glycolysis, could compensate for the energy lost through respiration in order to preserve cell viability (Schwimmer et al., 2005). Viewed collectively, our findings indicate that the in vitro growth environment produces lipidomic and ETC abnormalities in non-tumorigenic astrocytes and in brain tumour cells, which would compromise energy production through oxidative phosphorylation.
Otto Warburg first proposed that cancer originated from irreversible injury to cellular respiration that was accompanied by a gradual dependence on glycolytic energy to compensate for the energy lost from respiration (Warburg, 1956; Kiebish et al., 2008a). A dependence on glycolytic energy in the presence of oxygen is known generally as the ‘Warburg effect' and is the metabolic hallmark of nearly all tumour cells (Warburg, 1956; Kiebish et al., 2008a). Using shotgun lipidomics, we recently proposed that abnormal cardiolipin could underlie the irreversible respiratory injury in brain tumours, thus linking mitochondrial lipid defects to aerobic glycolysis and the Warburg theory of cancer (Kiebish et al., 2008a). However, high levels of glucose and other metabolites in culture media can also increase glycolysis and inhibit oxidative phosphorylation. This effect was first described by Herbert Crabtree in the late 1920s and is referred to as the ‘Crabtree effect' (Crabtree, 1929; Guppy et al., 1993; Frezza and Gottleib, 2008). We do not exclude the possibility that the lipidomic and ETC abnormalities observed in the cultured non-tumorigenic astrocytes and brain tumour cells could arise in part from the Crabtree effect. It is interesting that several lipidomic differences found between the brain tumour mitochondria and the NS mitochondria in the in vivo environment are not seen between the brain tumour cells and non-tumorigenic astrocytes in the in vitro environment. We suggest that rapid cell proliferation in vitro and the Crabtree effect could obscure or mask lipidomic abnormalities between normal and tumour cells due to tumorigenesis. Further studies will be needed to test this hypothesis.
The content and molecular composition of EtnGpl and ChoGpl is critical for maintaining mitochondrial membrane fluidity and enzyme activities (Daum, 1985). Fatty acids in the sn-2 position of mitochondrial EtnGpl and ChoGpl can be used as donor acyl chains for cardiolipin remodelling. Consequently, the distribution of cardiolipin molecular species will reflect the acyl chain composition of these glycerophospholipids (Xu et al., 2003). Shorter chain fatty acids with less unsaturation are generally more abundant in phospholipids from tumour cells than from normal cells (Bergelson et al., 1970, 1974; Yates et al., 1979; Canuto et al., 1989). This could arise indirectly from the Warburg effect. Consequently, abnormalities in cardiolipin remodelling can arise in part from disturbances in the content and composition of fatty acids in EtnGpl and ChoGpl. In addition to defects in cardiolipin remodelling using sn-2 fatty acids from EtnGpl and ChoGpl, we do not exclude the possibility that the abnormalities in fatty acid synthesis, resulting from respiratory damage and glycolytic dependence, could also contribute to the expression of abnormal fatty acids in cardiolipin. We also use caution in our interpretation of dynamic metabolic events based on static data. Nevertheless, our findings show multiple lipidomic abnormalities in brain tumour cells and in cultured non-tumorigenic astrocytes that could compromise energy metabolism.
Shotgun lipidomic analysis of EtnGpl and ChoGpl subclasses allows for identification of glycerophospholipids containing vinyl ether linkages (plasmalogens). Plasmalogen glycerophospholipid content is greater in brain tumours than in normal cells (Slagel et al., 1967; Wood and Healy, 1970; Wood et al., 1970; Albert and Anderson, 1977; Yates et al., 1979). Also, cell differentiation state is known to influence plasmalogen content (Murphy et al., 1997). In the present study, we found that the content of PlsEtn and PlsCho was significantly greater in mitochondria from the CT-2A and EPEN tumours grown in vivo than in the NS brain mitochondria. Since plasmalogen lipids possess antioxidant properties, elevated plasmalogen levels in tumour mitochondria could act to buffer free radical production (Zoeller et al., 1988; Paltauf, 1994; Beal et al., 1997; Carew and Huang, 2002). Additionally, elevated plasmalogen levels could compensate for the alterations in other mitochondrial lipids, which could improve membrane structure (Paltauf, 1994; Gorgas et al., 2006). Elevated plasmalogen concentrations, however, were only found in the tumours grown in vivo, but not in the cultured tumour cell lines compared with controls.
In summary, the results of the present study show that the in vitro growth environment can significantly alter the mitochondrial lipidome and ETC activities of brain tumour cells and non-tumorigenic astrocytes. These abnormalities are expected to produce a physiological state different from that which would exist in the in vivo environment. In contrast with the tumour cells, the lipidomic alterations in the astrocytes might not be permanent since these cells are non-tumorigenic. The alterations from the culture environment would reduce energy production through oxidative phosphorylation, thus increasing substrate level phosphorylation to maintain cell viability. In the case of brain tumour cells, growth in the culture environment could obscure lipidomic abnormalities arising from tumorigenesis, which can confound the relationship of altered energy metabolism to tumorigenesis.
Online data
Footnotes
This work was supported by the Boston College Research Expense fund, the National Institutes of Health/National Institute on Aging [grant number AG31675], the National Cancer Institute [grant number CA102135], National Institutes of Health Research Program Projects [grant number L57278]; and the National Institutes of Health [grant number NS055195].
REFERENCES
- Albert DH, Anderson CE. Ether-linked glycerolipids in human brain tumors. Lipids. 1977;12:188–192. doi: 10.1007/BF02533292. [DOI] [PubMed] [Google Scholar]
- Alliot F, Pessac B. Astrocytic cell clones derived from established cultures of 8-day postnatal mouse cerebella. Brain Res. 1984;306:283–291. doi: 10.1016/0006-8993(84)90377-9. [DOI] [PubMed] [Google Scholar]
- Arismendi-Morillo GJ, Castellano-Ramirez AV. Ultrastructural mitochondrial pathology in human astrocytic tumors: potentials implications pro-therapeutics strategies. J Electron Microsc (Tokyo) 2008;57:33–39. doi: 10.1093/jmicro/dfm038. [DOI] [PubMed] [Google Scholar]
- Bai H, Seyfried TN. Influence of ganglioside GM3 and high density lipoprotein on the cohesion of mouse brain tumor cells. J Lipid Res. 1997;38:160–172. [PubMed] [Google Scholar]
- Beal MF, Howell N, Bodis-Wollner I. Mitochondria and Free Radicals in Neurodegerative Diseases. New York: Wiley-Liss. 1997 [Google Scholar]
- Bergelson LD, Dyatlovitskaya EV, Torkhovskaya TI, Sorokina IB, Gorkova NP. Phospholipid composition of membranes in the tumor cell. Biochim Biophys Acta. 1970;210:287–298. doi: 10.1016/0005-2760(70)90173-6. [DOI] [PubMed] [Google Scholar]
- Bergelson LD, Dyatlovitskaya EV, Sorokina IB, Gorkova NP. Phospholipid composition of mitochondria and microsomes from regenerating rat liver and hepatomas of different growth rate. Biochim Biophys Acta. 1974;360:361–365. doi: 10.1016/0005-2760(74)90067-8. [DOI] [PubMed] [Google Scholar]
- Bogdanov M, Mileykovskaya E, Dowhan W. Lipids in the assembly of membrane proteins and organization of protein supercomplexes: implications for lipid-linked disorders. Subcell Biochem. 2008;49:197–239. doi: 10.1007/978-1-4020-8831-5_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brookes PS, Buckingham JA, Tenreiro AM, Hulbert AJ, Brand MD. The proton permeability of the inner membrane of liver mitochondria from ectothermic and endothermic vertebrates and from obese rats: correlations with standard metabolic rate and phospholipid fatty acid composition. Comp Biochem Physiol B Biochem Mol Biol. 1998;119:325–334. doi: 10.1016/s0305-0491(97)00357-x. [DOI] [PubMed] [Google Scholar]
- Campanella R. Membrane lipids modifications in human gliomas of different degree of malignancy. J Neurosurg Sci. 1992;36:11–25. [PubMed] [Google Scholar]
- Canuto RA, Biocca ME, Muzio G, Dianzani MU. Fatty acid composition of phospholipids in mitochondria and microsomes during diethylnitrosamine carcinogenesis in rat liver. Cell Biochem Funct. 1989;7:11–19. doi: 10.1002/cbf.290070104. [DOI] [PubMed] [Google Scholar]
- Carew JS, Huang P. Mitochondrial defects in cancer. Mol Cancer. 2002;1:9. doi: 10.1186/1476-4598-1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervos-Navarro J, Ferszt R, Brackertz M. The ultrastructure of oligodendrogliomas. Neurosurg Rev. 1981;4:17–31. doi: 10.1007/BF01787229. [DOI] [PubMed] [Google Scholar]
- Cheng H, Guan S, Han X. Abundance of triacylglycerols in ganglia and their depletion in diabetic mice: implications for the role of altered triacylglycerols in diabetic neuropathy. J Neurochem. 2006;97:1288–1300. doi: 10.1111/j.1471-4159.2006.03794.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H, Mancuso DJ, Jiang X, Guan S, Yang J, Yang K, Sun G, Gross RW, Han X. Shotgun lipidomics reveals the temporally dependent, highly diversified cardiolipin profile in the mammalian brain: temporally coordinated postnatal diversification of cardiolipin molecular species with neuronal remodeling. Biochemistry. 2008;47:5869–5880. doi: 10.1021/bi7023282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chicco AJ, Sparagna GC. Role of cardiolipin alterations in mitochondrial dysfunction and disease. Am J Physiol Cell Physiol. 2007;292:C33–C44. doi: 10.1152/ajpcell.00243.2006. [DOI] [PubMed] [Google Scholar]
- Claypool SM, Oktay Y, Boontheung P, Loo JA, Koehler CM. Cardiolipin defines the interactome of the major ADP/ATP carrier protein of the mitochondrial inner membrane. J Cell Biol. 2008;182:937–950. doi: 10.1083/jcb.200801152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabtree HG. Observations on the carbohydrate metabolism of tumours. Biochem J. 1929;23:536–545. doi: 10.1042/bj0230536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daum G. Lipids of mitochondria. Biochim Biophys Acta. 1985;822:1–42. doi: 10.1016/0304-4157(85)90002-4. [DOI] [PubMed] [Google Scholar]
- DeBerardinis RJ, Mancuso A, Daikhin E, Nissim I, Yudkoff M, Wehrli S, Thompson CB. Beyond aerobic glycolysis: transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. Proc Natl Acad Sci USA. 2007;104:19345–19350. doi: 10.1073/pnas.0709747104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Abbadi M, Seyfried TN. Influence of growth environment on the ganglioside composition of an experimental mouse brain tumor. Mol Chem Neuropathol. 1994;21:273–285. doi: 10.1007/BF02815355. [DOI] [PubMed] [Google Scholar]
- Ellis CE, Murphy EJ, Mitchell DC, Golovko MY, Scaglia F, Barcelo-Coblijn GC, Nussbaum RL. Mitochondrial lipid abnormality and electron transport chain impairment in mice lacking α-synuclein. Mol Cell Biol. 2005;25:10190–10201. doi: 10.1128/MCB.25.22.10190-10201.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floridi A, Paggi MG, Fanciulli M. Modulation of glycolysis in neuroepithelial tumors. J Neurosurg Sci. 1989;33:55–64. [PubMed] [Google Scholar]
- Frezza C, Gottlieb E. Mitochondria in cancer: not just innocent bystanders. Semin Cancer Biol. 2008;19:4–11. doi: 10.1016/j.semcancer.2008.11.008. [DOI] [PubMed] [Google Scholar]
- Fry M, Green DE. Cardiolipin requirement by cytochrome oxidase and the catalytic role of phospholipid. Biochem Biophys Res Commun. 1980;93:1238–1246. doi: 10.1016/0006-291x(80)90622-1. [DOI] [PubMed] [Google Scholar]
- Fry M, Green DE. Cardiolipin requirement for electron transfer in complex I and III of the mitochondrial respiratory chain. J Biol Chem. 1981;256:1874–1880. [PubMed] [Google Scholar]
- Galarraga J, Loreck DJ, Graham JF, DeLaPaz RL, Smith BH, Hallgren D, Cummins CJ. Glucose metabolism in human gliomas: correspondence of in situ and in vitro metabolic rates and altered energy metabolism. Metab Brain Dis. 1986;1:279–291. doi: 10.1007/BF00999357. [DOI] [PubMed] [Google Scholar]
- Gorgas K, Teigler A, Komljenovic D, Just WW. The ether lipid-deficient mouse: tracking down plasmalogen functions. Biochim Biophys Acta. 2006;1763:1511–1526. doi: 10.1016/j.bbamcr.2006.08.038. [DOI] [PubMed] [Google Scholar]
- Guppy M, Greiner E, Brand K. The role of the Crabtree effect and an endogenous fuel in the energy metabolism of resting and proliferating thymocytes. Eur J Biochem. 1993;212:95–99. doi: 10.1111/j.1432-1033.1993.tb17637.x. [DOI] [PubMed] [Google Scholar]
- Han X, Gross RW. Shotgun lipidomics: electrospray ionization mass spectrometric analysis and quantitation of cellular lipidomes directly from crude extracts of biological samples. Mass Spectrom Rev. 2005;24:367–412. doi: 10.1002/mas.20023. [DOI] [PubMed] [Google Scholar]
- Han X, Yang J, Cheng H, Ye H, Gross RW. Toward fingerprinting cellular lipidomes directly from biological samples by two-dimensional electrospray ionization mass spectrometry. Anal Biochem. 2004;330:317–331. doi: 10.1016/j.ab.2004.04.004. [DOI] [PubMed] [Google Scholar]
- Hartz JW, Morton RE, Waite MM, Morris HP. Correlation of fatty acyl composition of mitochondrial and microsomal phospholipid with growth rate of rat hepatomas. Lab Invest. 1982;46:73–78. [PubMed] [Google Scholar]
- Hauff KD, Hatch GM. Cardiolipin metabolism and Barth Syndrome. Prog Lipid Res. 2006;45:91–101. doi: 10.1016/j.plipres.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Hoch FL. Cardiolipins and biomembrane function. Biochim Biophys Acta. 1992;1113:71–133. doi: 10.1016/0304-4157(92)90035-9. [DOI] [PubMed] [Google Scholar]
- Hoch FL. Cardiolipins and mitochondrial proton-selective leakage. J Bioenerg Biomembr. 1998;30:511–532. doi: 10.1023/a:1020576315771. [DOI] [PubMed] [Google Scholar]
- Homburg CJ, Bos CJ, de Bruyn W, Emmelot P. Glycolytic and respiratory properties of malignant and nonmalignant lymphoblasts cultured in vitro. Cancer Res. 1961;21:353–360. [PubMed] [Google Scholar]
- Hostetler KY, Zenner BD, Morris HP. Abnormal membrane phospholipid content in subcellular fractions from the Morris 7777 hepatoma. Biochim Biophys Acta. 1976;441:231–238. doi: 10.1016/0005-2760(76)90166-1. [DOI] [PubMed] [Google Scholar]
- Hostetler KY, Zenner BD, Morris HP. Phospholipid content of mitochondrial and microsomal membranes from Morris hepatomas of varying growth rates. Cancer Res. 1979;39:2978–2983. [PubMed] [Google Scholar]
- Kiebish MA, Han X, Cheng H, Chuang JH, Seyfried TN. Cardiolipin and electron transport chain abnormalities in mouse brain tumor mitochondria: lipidomic evidence supporting the Warburg theory of cancer. J Lipid Res. 2008a;49:2545–2556. doi: 10.1194/jlr.M800319-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiebish MA, Han X, Cheng H, Lunceford A, Clarke CF, Moon H, Chuang JH, Seyfried TN. Lipidomic analysis and electron transport chain activities in C57BL/6J mouse brain mitochondria. J Neurochem. 2008b;106:299–312. doi: 10.1111/j.1471-4159.2008.05383.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiebish MA, Han X, Cheng H, Chuang JH, Seyfried TN. Brain mitochondrial lipid abnormalities in mice susceptible to spontaneous gliomas. Lipids. 2008c;43:951–959. doi: 10.1007/s11745-008-3197-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsch WM, Schulz Q, Van Buskirk J, Nakane P. Anaerobic energy metabolism in brain tumors. Prog Exp Tumor Res. 1972;17:163–191. doi: 10.1159/000393673. [DOI] [PubMed] [Google Scholar]
- Kocherginsky N. Acidic lipids, H+-ATPases, and mechanism of oxidative phosphorylation. Physico-chemical ideas 30 years after P. Mitchell's Nobel Prize award. Prog Biophys Mol Biol. 2008;99:20–41. doi: 10.1016/j.pbiomolbio.2008.10.013. [DOI] [PubMed] [Google Scholar]
- Kunz-Schughart LA, Habbersett RC, Freyer JP. Impact of proliferative activity and tumorigenic conversion on mitochondrial function of fibroblasts in 2D and 3D culture. Cell Biol Int. 2001;25:919–930. doi: 10.1006/cbir.2001.0786. [DOI] [PubMed] [Google Scholar]
- Lichtor T, Dohrmann GJ. Respiratory patterns in human brain tumors. Neurosurgery. 1986;19:896–899. doi: 10.1227/00006123-198612000-00002. [DOI] [PubMed] [Google Scholar]
- McMillin JB, Dowhan W. Cardiolipin and apoptosis. Biochim Biophys Acta. 2002;1585:97–107. doi: 10.1016/s1388-1981(02)00329-3. [DOI] [PubMed] [Google Scholar]
- Mileykovskaya E, Zhang M, Dowhan W. Cardiolipin in energy transducing membranes. Biochemistry (Moscow) 2005;70:154–158. doi: 10.1007/s10541-005-0095-2. [DOI] [PubMed] [Google Scholar]
- Morton R, Cunningham C, Jester R, Waite M, Miller N, Morris HP. Alteration of mitochondrial function and lipid composition in Morris 7777 hepatoma. Cancer Res. 1976;36:3246–3254. [PubMed] [Google Scholar]
- Murphy EJ, Horrocks LA. Effects of differentiation on the phospholipid and phospholipid fatty acid composition of N1E-115 neuroblastoma cells. Biochim Biophys Acta. 1993;1167:131–136. doi: 10.1016/0005-2760(93)90152-y. [DOI] [PubMed] [Google Scholar]
- Murphy EJ, Anderson DK, Horrocks LA. Phospholipid and phospholipid fatty acid composition of mixed murine spinal cord neuronal cultures. J Neurosci Res. 1993;34:472–477. doi: 10.1002/jnr.490340412. [DOI] [PubMed] [Google Scholar]
- Murphy EJ, Rosenberger TA, Horrocks LA. Effects of maturation on the phospholipid and phospholipid fatty acid compositions in primary rat cortical astrocyte cell cultures. Neurochem Res. 1997;22:1205–1213. doi: 10.1023/a:1021924711675. [DOI] [PubMed] [Google Scholar]
- Oudard S, Boitier E, Miccoli L, Rousset S, Dutrillaux B, Poupon MF. Gliomas are driven by glycolysis: putative roles of hexokinase, oxidative phosphorylation and mitochondrial ultrastructure. Anticancer Res. 1997;17:1903–1911. [PubMed] [Google Scholar]
- Paltauf F. Ether lipids in biomembranes. Chem Phys Lipids. 1994;74:101–139. doi: 10.1016/0009-3084(94)90054-x. [DOI] [PubMed] [Google Scholar]
- Pedersen PL. Tumor mitochondria and the bioenergetics of cancer cells. Prog Exp Tumor Res. 1978;22:190–274. doi: 10.1159/000401202. [DOI] [PubMed] [Google Scholar]
- Peskin BS, Carter MJ. Chronic cellular hypoxia as the prime cause of cancer: what is the de-oxygenating role of adulterated and improper ratios of polyunsaturated fatty acids when incorporated into cell membranes? Med Hypotheses. 2008;70:298–304. doi: 10.1016/j.mehy.2007.05.033. [DOI] [PubMed] [Google Scholar]
- Pon LA, Schon EA. Mitochondria. New York: Academic Press. 2001 [Google Scholar]
- Reitz RC, Thompson JA, Morris HP. Mitochondrial and microsomal phospholipids of Morris hepatoma 7777. Cancer Res. 1977;37:561–567. [PubMed] [Google Scholar]
- Rodriguez-Enriquez S, Vital-Gonzalez PA, Flores-Rodriguez FL, Marin-Hernandez A, Ruiz-Azuara L, Moreno-Sanchez R. Control of cellular proliferation by modulation of oxidative phosphorylation in human and rodent fast-growing tumor cells. Toxicol Appl Pharmacol. 2006;215:208–217. doi: 10.1016/j.taap.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Rubin R, Sutton C. The ultrastructure of the mouse ependymoblastoma and its contained virus-like particles. J Neuropathol Exp Neurol. 1968;27:136. [PubMed] [Google Scholar]
- Schieke SM, Ma M, Cao L, McCoy JP, Jr, Liu C, Hensel NF, Barrett AJ, Boehm M, Finkel T. Mitochondrial metabolism modulates differentiation and teratoma formation capacity in mouse embryonic stem cells. J Biol Chem. 2008;283:28506–28512. doi: 10.1074/jbc.M802763200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlame M, Ren M. Barth syndrome, a human disorder of cardiolipin metabolism. FEBS Lett. 2006;580:5450–5455. doi: 10.1016/j.febslet.2006.07.022. [DOI] [PubMed] [Google Scholar]
- Schlame M, Rua D, Greenberg ML. The biosynthesis and functional role of cardiolipin. Prog Lipid Res. 2000;39:257–288. doi: 10.1016/s0163-7827(00)00005-9. [DOI] [PubMed] [Google Scholar]
- Schlame M, Ren M, Xu Y, Greenberg ML, Haller I. Molecular symmetry in mitochondrial cardiolipins. Chem Phys Lipids. 2005;138:38–49. doi: 10.1016/j.chemphyslip.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Schwimmer C, Lefebvre-Legendre L, Rak M, Devin A, Slonimski PP, di Rago JP, Rigoulet M. Increasing mitochondrial substrate-level phosphorylation can rescue respiratory growth of an ATP synthase-deficient yeast. J Biol Chem. 2005;280:30751–30759. doi: 10.1074/jbc.M501831200. [DOI] [PubMed] [Google Scholar]
- Seyfried TN, Mukherjee P. Targeting energy metabolism in brain cancer: review and hypothesis. Nutr Metab (Lond) 2005;2:30. doi: 10.1186/1743-7075-2-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seyfried TN, el-Abbadi M, Roy ML. Ganglioside distribution in murine neural tumors. Mol Chem Neuropathol. 1992;17:147–167. doi: 10.1007/BF03159989. [DOI] [PubMed] [Google Scholar]
- Shinzawa-Itoh K, Aoyama H, Muramoto K, Terada H, Kurauchi T, Tadehara Y, Yamasaki A, Sugimura T, Kurono S, Tsujimoto K, Mizushima T, Yamashita E, Tsukihara T, Yoshikawa S. Structures and physiological roles of 13 integral lipids of bovine heart cytochrome c oxidase. EMBO J. 2007;26:1713–1725. doi: 10.1038/sj.emboj.7601618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slagel DE, Dittmer JC, Wilson CB. Lipid composition of human glial tumour and adjacent brain. J Neurochem. 1967;14:789–798. doi: 10.1111/j.1471-4159.1967.tb10314.x. [DOI] [PubMed] [Google Scholar]
- Springer EL. Comparative study of the cytoplasmic organelles of epithelial cell lines derived from human carcinomas and nonmalignant tissues. Cancer Res. 1980;40:803–817. [PubMed] [Google Scholar]
- Tocher DR, Dick JR, Sargent JR. Occurrence of 22:3n-9 and 22:4n-9 in the lipids of the topminnow (Poeciliopsis lucida) hepatic tumor cell line, PLHC-1. Lipids. 1995;30:555–565. doi: 10.1007/BF02537031. [DOI] [PubMed] [Google Scholar]
- Warburg O. On the origin of cancer cells. Science. 1956;123:309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- Wood R, Healy K. Tumor lipids. Biosynthesis of plasmalogens. J Biol Chem. 1970;245:2640–2648. [PubMed] [Google Scholar]
- Wood R, Walton M, Healy K, Cumming RB. Plasmalogen biosynthesis in Ehrlich ascites cells grown in tissue culture. J Biol Chem. 1970;245:4276–4285. [PubMed] [Google Scholar]
- Wu M, Neilson A, Swift AL, Moran R, Tamagnine J, Parslow D, Armistead S, Lemire K, Orrell J, Teich J, Chomicz S, Ferrick DA. Multiparameter metabolic analysis reveals a close link between attenuated mitochondrial bioenergetic function and enhanced glycolysis dependency in human tumor cells. Am J Physiol Cell Physiol. 2007;292:C125–C136. doi: 10.1152/ajpcell.00247.2006. [DOI] [PubMed] [Google Scholar]
- Xu Y, Kelley RI, Blanck TJ, Schlame M. Remodeling of cardiolipin by phospholipid transacylation. J Biol Chem. 2003;278:51380–51385. doi: 10.1074/jbc.M307382200. [DOI] [PubMed] [Google Scholar]
- Xu Y, Malhotra A, Ren M, Schlame M. The enzymatic function of tafazzin. J Biol Chem. 2006;281:39217–39224. doi: 10.1074/jbc.M606100200. [DOI] [PubMed] [Google Scholar]
- Yang J, Liu X, Bhalla K, Kim CN, Ibrado AM, Cai J, Peng TI, Jones DP, Wang X. Prevention of apoptosis by Bcl-2: release of cytochrome c from mitochondria blocked. Science. 1997;275:1129–1132. doi: 10.1126/science.275.5303.1129. [DOI] [PubMed] [Google Scholar]
- Yates AJ, Thompson DK, Boesel CP, Albrightson C, Hart RW. Lipid composition of human neural tumors. J Lipid Res. 1979;20:428–436. [PubMed] [Google Scholar]
- Zhang M, Mileykovskaya E, Dowhan W. Glueing the respiratory chain together. Cardiolipin is required for supercomplex formation in the inner mitochondrial membrane. J Biol Chem. 2002;277:43553–43556. doi: 10.1074/jbc.C200551200. [DOI] [PubMed] [Google Scholar]
- Zimmerman HM, Arnould H. Experimental brain tumors: I. Tumors produced with methylcholanthrene. Cancer Res. 1941;1:919–938. [Google Scholar]
- Zoeller RA, Morand OH, Raetz CR. A possible role for plasmalogens in protecting animal cells against photosensitized killing. J Biol Chem. 1988;263:11590–11596. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.