Abstract
Glucocorticoids stimulate the intestinal absorption of Na+ and water partly by regulation of the Na+/H+ exchanger 3 (NHE3). Previous studies have shown both genomic and nongenomic regulation of NHE3 by glucocorticoids. Serum and glucocorticoid-inducible kinase 1 (SGK1) has been shown to be part of this cascade, where phosphorylation of NHE3 by SGK1 initiates the translocation of NHE3 to the cell surface. In the present work, we examined a series of changes in SGK1 and NHE3 induced by glucocorticoids using human colonic Caco-2 and opossum kidney cells. We found that dexamethasone rapidly stimulated SGK1 mRNAs, but a significant change in protein abundance was not detected. Instead, there was an increase in SGK1 kinase activity as early as at 2 h. An increase in NHE3 protein abundance was not detected until 12 h of dexamethasone exposure, although the transport activity was significantly stimulated at 4 h. These data demonstrate that the changes of SGK1 precede those of NHE3. Chronic regulation (24 h) of NHE3 was blocked completely by prevention of protein synthesis with cycloheximide or actinomycin D and by the glucocorticoid receptor blocker RU486. The acute effect of dexamethasone was similarly abrogated by RU486, but was insensitive to cycloheximide and actinomycin D. Similarly, the stimulation of SGK1 activity by dexamethasone was blocked by RU486 but not by actinomycin D. Together, these data show that the acute effect of glucocorticoids on NHE3 is mediated by a glucocorticoid receptor dependent mechanism that activates SGK1 in a nongenomic manner.
Keywords: Na+/H+ exchanger 3, serum and glucocorticoid-inducible kinase 1
Glucocorticoids are stress hormones that modulate a number of physiological actions involved in metabolic, inflammatory, and cardiovascular processes (12). Glucocorticoid excess, as in Cushing’s syndrome, is associated with obesity, hypertension, hyperlipidemia, and glucose intolerance (33). Glucocorticoids have been used to treat diarrhea associated with inflammatory bowel diseases and nontropical sprue (45). There is evidence that glucocorticoids exert a direct effect on the intestinal salt and water absorption and play an important role in Na and acid homeostasis in the kidney (4, 35, 49). Studies using animals have shown that glucocorticoids induce Na+/H+ exchanger 3 (NHE3) transcripts, proteins, and activity, and suggested that the major mechanism of glucocorticoids is by genomic regulation of NHE3 (9, 49). However, genomic regulation cannot account for all the studies on NHE3 regulation by glucocorticoids. More importantly, genomic induction of NHE3 does not consistently correlate with the changes in Na+/H+ exchange activity (3, 6, 47). The discrepancy could be due to activation of another NHE isoform. Certainly, glucocorticoids have recently been shown to activate NHE1 in human bronchial epithelial cells (37). However, there is profound evidence for nongenomic regulation of NHE3 by glucocorticoids (6, 39, 47).
NHE3 belongs to a family of mammalian membrane transporters (Slc9) that drive H+ efflux in exchange for external Na+ in an electroneutral manner (27, 50, 51). There are nine known genes within this family, each with distinct physiological functions and cellular distribution. NHE3 plays an important role in NaCl and NaHCO3 absorption in epithelia cells. Intestine and kidney are the major sites for NaCl absorption. In the kidney, NHE3 accounts for a major portion of Na+/H+ exchanger activity, whereas NHE3 generally plays a larger role than other NHEs in NaCl absorption in the intestine (27, 50, 51). Genetic disruption of NHE3 expression in mice results in hypotension, hypovolemia, mild diarrhea, and mild acidosis (34). Animals with intestinal NHE3 restored by transgenic expression show some improvement over the NHE3 knockout animals, with better tolerance to low-salt diet but still remain hypovolemic and hypotensive (43).
SGK1 was originally identified in rat mammary epithelial cells as a serum- and glucocorticoid-regulated gene (41). Recently, SGK1 has been established as an important convergence point in the regulation of multiple transport processes (23). It has been shown that SGK1 stimulates the epithelial Na channel and SCN5A, the K+ channels ROMK1, Kv1.3, and KCNE1/KCNQ1, the cation conductance induced by 4F2/LAT1, and the chloride conductance induced by the cystic fibrosis transmembrane conductance regulator (23). We have previously demonstrated (46, 47) that SGK1 plays an important role in NHE3 regulation by glucocorticoids by interacting with NHE regulatory factor 2 (NHERF2). Our recent study (39) has shown that phosphorylation of NHE3 by SGK1 increases the NHE3 protein abundance at the cell surface. In the present work, we determined the time course of changes in NHE3 and SGK1 to further understand the mechanism and the sequences of multiple alteration elicited by glucocorticoids. The molecular mechanisms involved in the regulation of NHE3 have been studied in several different models (27, 50, 51). We have chosen two cell lines, opossum kidney (OK) and Caco-2 cells, to represent renal and intestinal epithelial cells, respectively. Caco-2 cells are derived from human colon cancer but possess many of the properties of the small intestine as such that these cells represent a useful and well-accepted tool for studying the absorptive and/or secretive processes across the intestinal mucosa (15). Similarly, OK cells derived from the renal proximal tubule of the opossum exhibit multiple features similar to the renal proximal tubules, including the presence of NHE3 at the apical membrane and hormonal response (3, 6, 22). Regulation of NHE3 by dexamethasone occurs in both kidney and intestine, and previous studies (27, 50, 51) have shown significant similarities in the mechanisms underlying renal and intestinal NHE3 regulation.
MATERIALS AND METHODS
Cell culture and treatment
Caco-2 cells were grown in DMEM supplemented with 10% fetal bovine serum, 25 mM NaHCO3, 50 μg/ml streptomycin, 50 U/ml penicillin, and 1% nonessential amino acids (47). OK cells stably transfected with NHERF2 (OK/NHERF2) have been previously described (22, 48). OK/NHERF2 cells were cultured in a 1:1 mixture of DMEM/Ham’s F-12 medium supplement with 10% FBS, 50 U/ml penicillin, 50 U/ml streptomycin, and 300 μg/ml G418. Cells were serum deprived for 24 h before exposure to dexamethasone in ethanol. As a control, the same volume of ethanol was added to the cells. In some experiments, cells were pretreated with actinomycin, cycloheximide, or nocodazole for 15 min before the addition of dexamethasone.
RNA extraction and Northern blot analysis
Total RNA from Caco-2 and OK cells was isolated using TRIzol (Invitrogen). Thirty micrograms of total RNA for each sample were hybridized using ExpressHyb hybridization solution (BD Biosciences) at 68°C with continuous shaking for 1 h. The blot was washed at 50°C with three changes of washing solution. OK NHE3 cDNA probe (OK NHE3 aa 475–832) labeled with [α-32P]dATP was used to detect NHE3 mRNA levels in OK cells. OK NHE3 cDNA was kindly provided by Dr. Orson Moe at the University of Texas Southwestern Medical Center. Full-length human SGK1 (NM 005627) was used for the detection of SGK1 mRNA expression in both OK and Caco-2 cells. cDNA probes for SGK2 (NM 170693) and SGK3 (NM 170709) correspond to nucleotides 1033–1599 and 1015–1634, respectively. There is <50% nucleotide identity among SGK isoforms (20) and these probes do not cross react with other isoforms under the experimental conditions. Human phosphoprotein 36B4 was used as a loading control to normalize expression of NHE3 or SGK (21).
Real-time RT-PCR
Total RNA from Caco-2 cells was used to synthesize cDNA using the First Strand Synthesis kit as recommended by manufacturer (Invitrogen). Human NHE3 was amplified by using the primer pair, N1 (5′-AAG CGC CTG GAG TCC TTC AAG TCG-3′) and N2 (5′-GGG GCT CCT CGG TGT CTG AAA GTT-3′). The human β-actin primer pair are G1 (5′-TAC TCC TGC TTG CTG ATC CAC AT-3′) and G2 (5′-TAT GCC AAC ACA GTG CTG TCT GG-3). cDNA was amplified with iQ SYGR Green Supermix (Bio-Rad). The reaction mix consists of 0.5 μl of cDNA, 25 μl of iQ SYGR Green Supermix, 0.2 μM of target primers in total volume of 50 μl. Amplification was carried out at 10 min at 95°C for polymerase activation, and 35 cycles of 95°C for 15 s (denaturation) and 56°C for 1 min (annealing and extension) on the IQ5 real-time detection system (Bio-Rad). The amount of NHE3 mRNA was normalized to human actin as an internal control.
Immunoblot analysis
Cells were lysed in a lysis buffer composed of 50 mM Tris-Cl, pH 7.4, 150 mM NaCl, 1% Triton X-100, 0.1 mM PMSF, and protease inhibitors (Roche) by sonication, followed by centrifugation at 15,000 g at 4°C for 30 min as described (38). Protein concentration was determined by the TBicinchoninic Acid assay T (Sigma). Sixty micrograms of protein in 1× sample buffer (62.5 mM Tris·HCl, pH 6.8, 1% SDS, 10% glycerol, 0.3 M β-mercaptoethanol) was separated by 10% SDS-PAGE, and Western immunoblot analysis was performed as previously described (38). Monoclonal anti-opossum NHE3 antibody, 3H3, was a gift from Dr. Daniel Biemesderfer at Yale University (6, 38). Polyclonal anti-NHE3 antibody was kindly provided by Dr. Fayez Ghishan at the University of Arizona (10). Polyclonal SGK1 antibody was purchased from Upstate (catalog no. 07-315). In all cases, the blots were stripped and reprobed with an anti-β-actin antibody that was used as a loading control. When the time course was determined, the density of NHE3 or SGK1 protein was first normalized to that of β-actin and then compared with time 0.
In vitro SGK kinase assay
In vitro phosphorylation was performed based on a published procedure with a few modifications (32). Caco-2 cells treated with dexamethasone or ethanol were lysed in lysis buffer supplemented with 100 μM NaF, 5 mM Na pyrophosphate, and 2 mM Na orthovanadate. Lysate was cleared of cell debris by being spun at 15,000 g at 4°C for 10 min. SGK1 was immunoprecipitated by incubating with an anti-SGK1 antibody (Upstate) at 4°C overnight with constant rocking, followed by a 2-h incubation with Protein-A Sepharose beads (Amersham). A protein complex bound to Sepharose beads was spun down and washed three times with lysis buffer. Beads were resuspended in 50-μl SGK kinase buffer composed of 20 mM Tris, 100 μM ATP, 10 mM MgCl2, 1 mM DTT, 10 μCi [γ-32P]ATP, and 1 mM SGK1-specific Crosstide (Upstate) was added to the suspension. After 30 min of reaction at 30°C, the reaction was stopped with stop solution (1 mM ATP, 1% BSA, and 0.6% HCl) and the reaction mix was applied to 2.1 cm p81 Whatman filter paper. After drying at room temperature, the filters were washed four times with 25 mM phosphoric acid, once with acetone, and were then counted in a scintillation counter.
Measurement of Na+-dependent intracellular pH recovery
The Na+-dependent changes in intracellular pH by NHE3 were determined using the ratio-fluorometric, pH-sensitive dye BCECF-AM (Calbiochem) as previously described (22, 39). Briefly, cells were seeded on coverslips, grown to confluence and then serum starved overnight. Cells were washed in Na+ buffer composed of (in mM) 130 NaCl, 20 HEPES, 5 KCl, 1 tetramethylammonium (TMA)-PO4, 2 CaCl2, 1 MgSO4, and 18 glucose, and then dye loaded by incubation for 40 min with 6.5 μM BCECF-AM in the same solution. The coverslips were mounted on a perfusion chamber that was mounted on an inverted microscope, and were then superfused with NH4 buffer composed of (in mM) 40 NH4Cl, 90 NaCl, 20 HEPES, 5 KCl, 1 TMA-PO4, 2 CaCl2, 1 MgSO4, and 18 glucose, and subsequently with a TMA buffer composed of (in mM) 130 TMA-Cl, 20 HEPES, 5 KCl, 1 TMA-PO4, 2 CaCl2, 1 MgSO4, and 18 glucose. Na buffer was then reintroduced to drive Na-dependent pH recovery. Na+/H+ exchange activity in Caco-2 cells was determined in the presence of 30 μM HOE-694 (provided by Drs. Lang and Punter at Aventi Pharma) in Na buffer. Calibration of the fluorescence signal using the K+/H+ ionophore nigericin was performed as described earlier (22, 39).
Statistical analysis
Statistical significance was assessed by ANOVA. Results are means ± SE.
RESULTS
Dexamethasone stimulates SGK1 activity without altering protein abundance
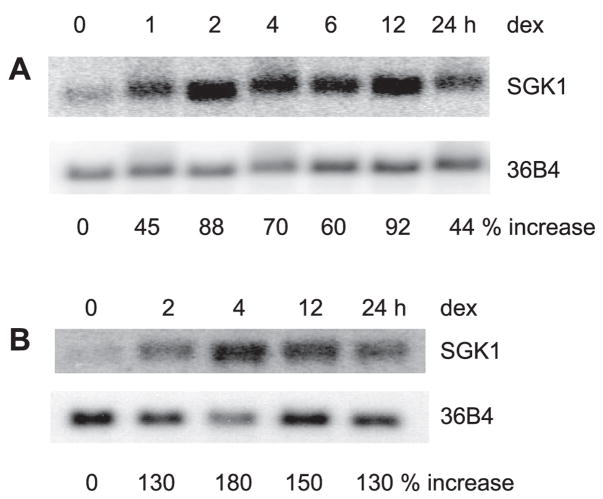
SGK1 plays a pivotal role in regulation of several transport processes, including Na+/H+ exchange by NHE3. A previous study (47) has demonstrated that SGK1 with a defective kinase domain blocked the effect of dexamethasone. To determine the mechanism by which dexamethasone regulates SGK1 and NHE3, we first determined the effect of dexamethasone on SGK1 expression. Caco-2 cells were treated with 1 μM dexamethasone and SGK1 mRNA levels were determined by Northern blot analysis. Figure 1A shows that dexamethasone rapidly induced SGK1 mRNA expression as early as 30 min and maintained the elevated expression for at least 24 h. We have shown previously (47) that the effect of dexamethasone on NHE3 in OK cells is enhanced greatly upon overexpression of NHERF2 in these cells. Hence, we also examined the effect of dexamethasone on SGK1 expression in OK/NHERF2 cells. Similarly, to Caco-2 cells, SGK1 mRNA level in OK/NHERF2 cells was rapidly induced by dexamethasone (Fig. 1B).
Fig. 1.
Dexamethasone (dex) induces serum and glucocorticoid-inducible kinase (SGK1) mRNA. Caco-2 (A) and opossum kidney/Na+/H+ exchanger regulatory factor 2 (OK/NHERF2) (B) cells were serum starved and treated with 1 μM dexamethasone. Total RNA was isolated and the expression of SGK1 mRNA was detected by Northern blot hybridization using human SGK1 cDNA as probe. The amount of SGK1 mRNA was normalized to the expression levels of phosphoprotein 36B4 as a loading control. Values represent changes in the ratio of SGK1 mRNA to 36B4 mRNA relative to that at time 0.
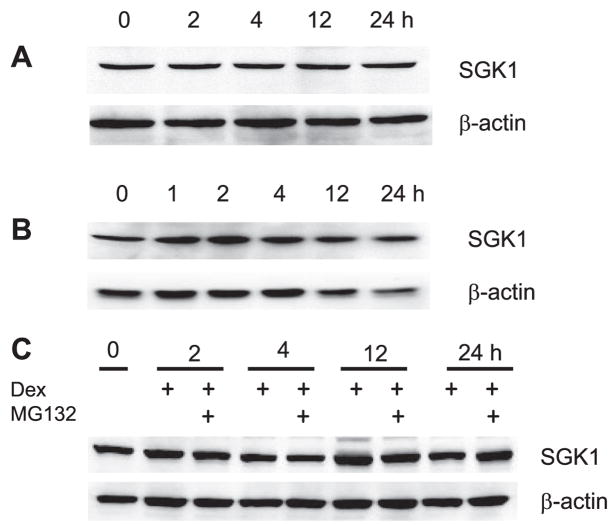
Rapid induction of SGK1 mRNA by glucocorticoids as well as aldosterone has previously been observed (5, 7, 29, 40). However, there is a controversy over the effect of glucocorticoids on SGK1 protein levels and the capacity of glucocorticoids to stimulate SGK1 protein levels widely varies among cell types (1, 24). Unlike the robust effect on mRNA expression, we did not observe a significant change in SGK1 protein expression in Caco-2 or OK/NHERF2 cells (Fig. 2, A and B). Previous studies (8) have shown that SGK1 is subjected to ubiquitination such that SGK1 proteins are rapidly degraded through a proteasome-dependent pathway, which may mask a transient increase in protein abundance. To address this possibility, Caco-2 cells were treated with dexamethasone in the presence of the proteasome inhibitor MG132. Western blots showed that expression levels of SGK1 over 24 h were not altered by the presence of MG132 (Fig. 2C). These data suggest that proteasome-mediated degradation of SGK1 protein is not the reason for the lack of changes in SGK1 protein abundance in Caco-2 and OK cells.
Fig. 2.
Dexamethasone does not affect SGK1 protein abundance. Lysate was prepared from dexamethasone-treated Caco-2 (A) or OK/NHERF2 (B) cells. The amount of SGK1 protein was determined by Western blot analysis using an anti-SGK1 antibody. Membranes were stripped and probed with an antibody against β-actin as a loading control. Representative Western blots from 4 separate experiments are shown. C: Caco-2 cells were treated with dexamethasone in the absence or presence of MG132. Western blot analysis was performed to determine total SGK1 protein. Representative Western blot from 3 separate experiments is shown.
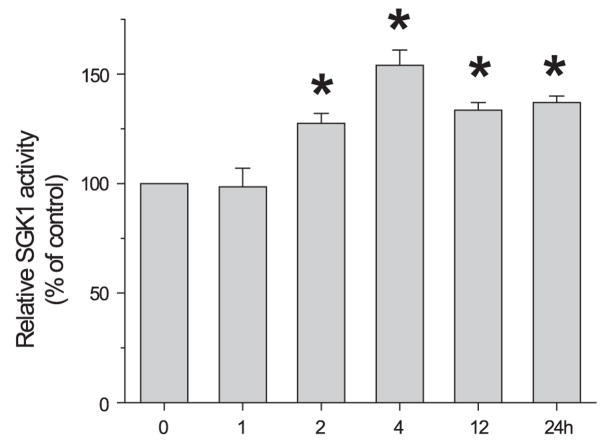
Since no change in SGK1 protein amount was observed, we next tested whether dexamethasone affected the kinase activity of SGK1. SGK1 proteins were immunoprecipitated from the dexamethasone-treated Caco-2 cells and the kinase activity was determined using a synthetic peptide as a substrate (32). Despite the lack of change in protein abundance, there was a significant increase in SGK1 kinase activity at 2 h (Fig. 3). The SGK1 activity further increased at 4 h and sustained its activity for at least 24 h. We could not determine SGK1 activity from OK/NHERF2 cells because the anti-SGK1 antibody from Upstate, Sigma, or Cell Signaling was unable to immunoprecipitate SGK1 from OK/NHERF2 cells despite their ability to recognize a band of 50 kDa on a blot.
Fig. 3.
Dexamethasone stimulates SGK1 kinase activity. Caco-2 cells treated with dexamethasone were lysed and SGK1 was immunoprecipitated. The SGK1-specific substrate crosstide was added to immune complexes in the presence of [γ-32P]ATP, and phosphorylation levels were determined as described in MATERIALS AND METHODS. Results from 4 experiments were represented as means ± SE. *P < 0.05 compared with the kinase activity at time 0.
Activation of NHE3 expression is delayed compared with that of SGK1
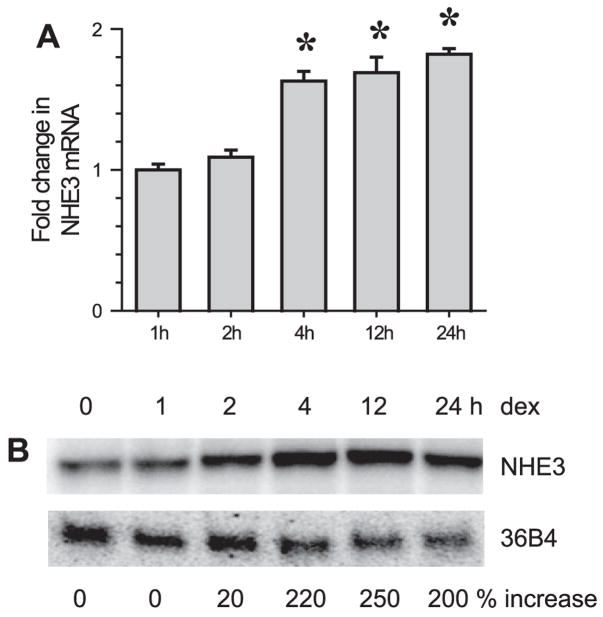
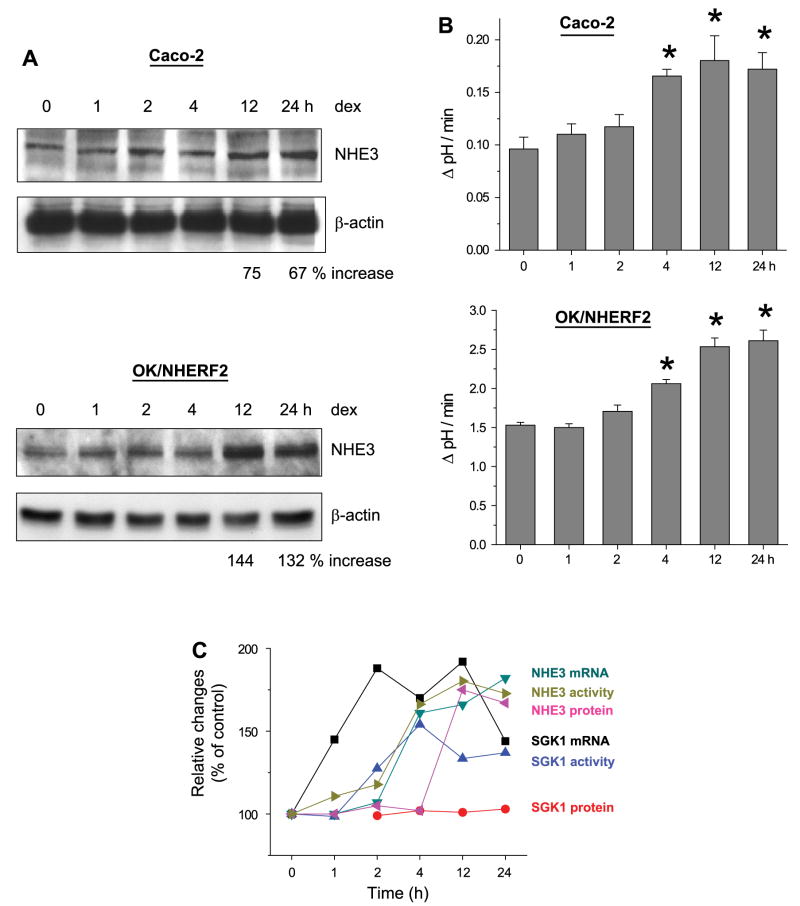
We next determined the effect of dexamethasone on NHE3 expression for two reasons: 1) to compare the time courses of SGK1 and NHE3 expression, and 2) to correlate the increase in NHE3 expression with that of NHE3 activity. We were not able to detect NHE3 mRNA in Caco-2 cells by Northern blot analysis due to the low expression levels and, hence, real-time RT-PCR was used to quantify NHE3 mRNA levels. As shown in Fig. 4A, dexamethasone significantly upregulated NHE3 mRNA at 4 h. NHE3 mRNA levels in OK/NHERF2 cells, determined by Northern blot analysis, were similarly enhanced after the addition of dexamethasone (Fig. 4B). A small increase at 2 h was noted, but the expression increased to near the maximum level between 4 and 12 h of dexamethasone treatment. Despite the increase in mRNA at the early hours, NHE3 protein abundance was not significantly changed at 4 h in both Caco-2 and OK/NHERF2 cells (Fig. 5A). Instead, the total amount of NHE3 protein was not changed until 12 h post treatment. This data shows that there is a delay in the synthesis of NHE3 proteins.
Fig. 4.
The effect of dexamethasone on NHE3 mRNA expression. A: Na+/H+ exchanger 3 (NHE3) mRNA expression in dexamethasone-treated Caco-2 cells was determined by real-time RT-PCR. NHE3 expression was normalized to β-actin. Fold changes relative to the NHE3 mRNA level of ethanol treated control Caco-2 cells are shown. Results from 4 experiments were represented as means ± SE. *P < 0.01 compared with control. B: Northern blot analysis was performed on OK/NHERF2 cells using OK NHE3 cDNA as probe to determine NHE3 mRNA levels in response to dexamethasone treatment. Percent increases over the basal level are shown. Representative blot from 3 separate experiments is shown.
Fig. 5.
The effect of dexamethasone on NHE3 protein abundance. A: Caco-2 and OK/NHERF2 cells treated with dexamethasone were lysed and total NHE3 protein expression was determined using anti-NHE3 antibodies. Percent increases relative to the basal level are shown. B: Caco-2 and OK/NHERF2 cells were treated with 1 μM dexamethasone for up to 24 h. NHE3 activity was determined as Na-dependent pH recovery as described in MATERIALS AND METHODS. Results are presented as the means ± SE; n = 8. *P < 0.01 compared with controls. C: summary of the changes in SGK1 and NHE3 in response to dexamethasone is shown.
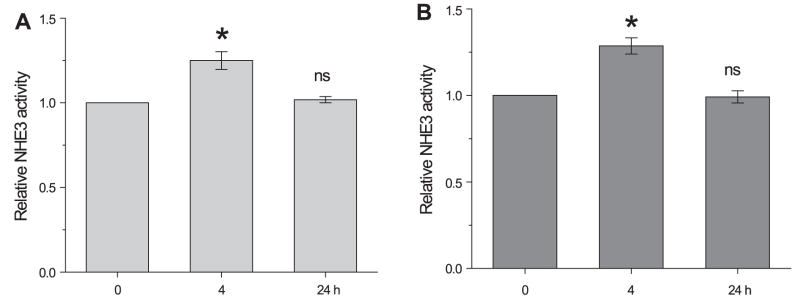
Next, we determined NHE3 activity in response to dexamethasone treatment. Consistent with previous studies (6, 39), NHE3 activity was significantly stimulated by dexamethasone within 4 h in both Caco-2 and OK/NHERF2 cells (Fig. 5B). Together, these data show that the changes in NHE3 protein abundance do not correlate with the increase in NHE3 activity, and that the increase in NHE3 transcription or translation is not the only determinant in the activation of NHE3 activity. A summary of the changes in SGK1 and NHE3 incurred by dexamethasone in Caco-2 cells is shown in Fig. 5C. It shows that the changes in SGK1 activity precede the changes in NHE3 translation and activity. Similar sequences of changes were observed in OK cells.
Actinomycin and cycloheximide block the chronic but not the acute effect
To further examine whether de novo synthesis of NHE3 protein is necessary for the activation of NHE3 activity, the effects of the transcription blocker actinomycin D (10 μM) and the translation blocker cycloheximide (10 μM) were examined. Incubation of OK/NHERF2 cells with actinomycin D or cyclohexmide for 24 h completely blocked dexamethasone-induced activation of NHE3 activity, affirming that the chronic regulation is dependent on the synthesis of NHE3 protein (Fig. 6). On the other hand, these inhibitors did not affect the extent of NHE3 activity stimulated by dexamethasone at 4 h, demonstrating that de novo synthesis of NHE3 is not required for the acute regulation of NHE3.
Fig. 6.
The effects of actinomycin D and cycloheximide on NHE3 activity. OK/NHERF2 cells were treated with dexamethasone for 4 h or 24 h in presence of actinomycin D (A) or cycloheximide (B). Results are presented as the means ± SE; n = 5. *P < 0.01 compared with the control treat cells. ns, not significant.
Glucocorticoid receptor is necessary for the regulation of NHE3
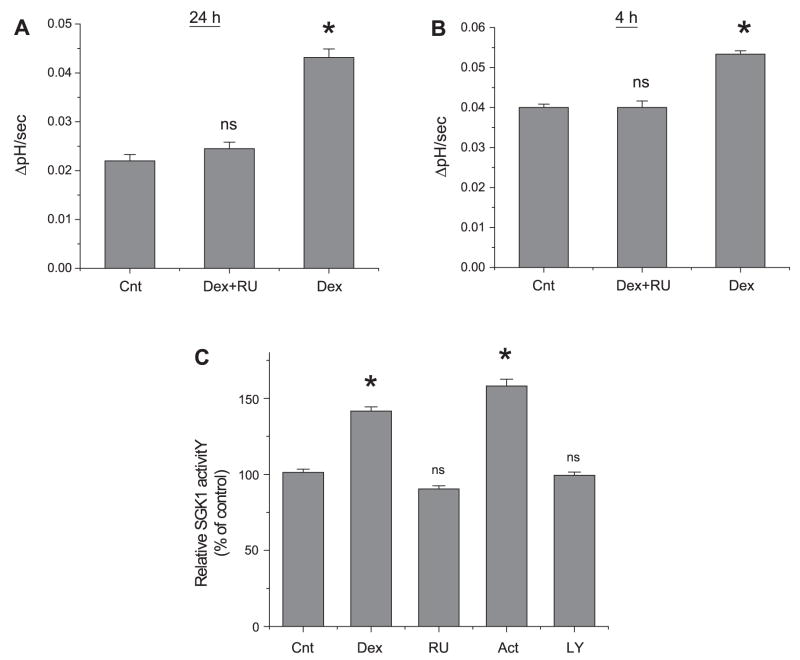
Glucocorticoids exert their biological effects by binding to an intracellular receptor, which translocates to the cell nucleus upon its activation and binds to specific DNA sequences (45). On the other hand, the presence of additional corticosteroid binding sites, such as receptors in the plasma membrane, has been suggested (30, 31). Therefore, we determined the effect of RU486 on dexamethasone-induced activation of NHE3. RU486 preferentially binds to the nuclear receptors, but has a low affinity for the membrane receptor (28, 30). Consistent with the effects of actinomycin D and cycloheximide, RU486 (1 μM) completely abrogated the effect of 24 h dexamethasone treatment on NHE3 activity (Fig. 7A), which further confirmed the importance of genomic regulation. On the other hand, despite the lack of effect by actinomycin D and cycloheximide, RU486 also inhibited the acute regulation of NHE3 (Fig. 7B), indicating that the acute effect is also dependent on the presence of intracellular glucocorticoid receptor (GR). Similar results were obtained from Caco-2 cells (data not shown).
Fig. 7.
The effect of RU486 on NHE3 activity. OK/NHERF2 cells were treated with dexamethasone for 24 h (A) or 4 h (B) in the presence of RU486 and the NHE3 activity was determined as described earlier. C: cells were treated with dexamethasone for 2 h in the absence of inhibitor (Cnt), in the presence of RU486 (RU), actinomycin (Act), and LY-294002 (LY). SGK1 activity was determined as described in MATERIALS AND METHODS. Results are presented as means ± SE; n = 5. *P < 0.01, compared with controls.
To determine whether the activation of SGK1 also requires a functional GR, SGK1 activity was determined in Caco-2 cells, which were treated with dexamethasone for 2 h in the presence or absence of RU486. Figure 7C shows that RU486 abrogated the dexamethasone-induced stimulation of SGK1 activity. On the contrary, actinomycin D showed no effect, consistent with the results shown in Fig. 6 that the acute regulation does not rely on de novo synthesis. Activation of SGK1 requires phosphorylation at Thr256 and Ser422 by 3-phosphoinositide-dependent protein kinase I and II, respectively, which are downstream targets of phosphoinositide 3-kinase (PI3K) (19). Consistently, preincubation of the cells with the PI3K inhibitor, LY-294002 (50 μM), blocked the effect of dexamethasone. A previous study has shown that inhibition of PI3K kinase by LY-294002 completely obliterates the effect of dexamethasone on NHE3 activity in agreement with the effect of LY-294002 on SGK1 activity (46, 47).
Effects of dexamethasone on SGK2 and SGK3
SGK2 and SGK3 are closely related to SGK1, but their expression is not regulated by glucocorticoids in fibroblasts (20). A recent study has shown that the glucocorticoid-mediated effect on intestinal NHE3 in SGK1−/− mice is only partially compromised, indicating the presence of a SGK1-independent mechanism (14). Because the effect of glucocorticoids on SGK2 and SGK3 expression in epithelial cells has not been documented, we examined the mRNA expression of SGK2 and SGK3 in Caco-2 cells. Unlike in fibroblasts (20), the SGK2 mRNA level was significantly increased at 2 h and remained elevated for the following 24 h (Fig. 8). The expression level of SGK3, however, was low and its expression was not altered by dexamethasone.
Fig. 8.
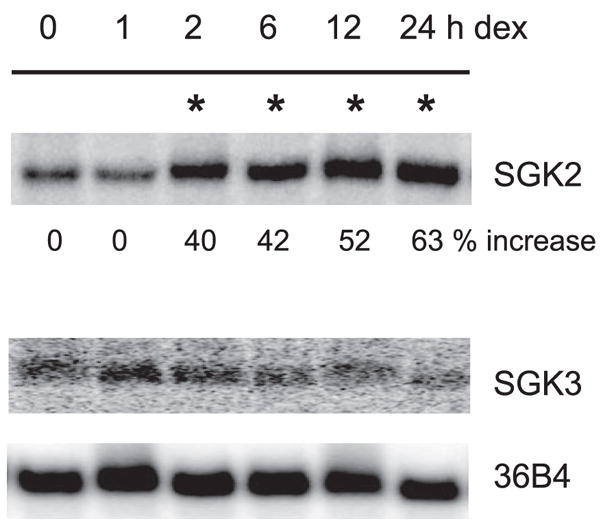
Dexamethasone stimulates SGK2. Caco-2 cells were serum starved and treated with 1 μM dexamethasone. Total RNA was isolated and the expression of SGK2 and SGK3 mRNA was detected by Northern blot hybridization using human SGK2 and SGK3 cDNA. Phosphoprotein 36B4 was used as a loading control. Representative data from 3 separate experiments are shown. Samples with a significant increase in SGK2 mRNA expression over the basal level are marked with asterisk. No significant change in SGK3 mRNA was observed.
DISCUSSION
A robust increase in NHE3 mRNA in response to glucocorticoids occurs in the intestine and renal proximal tubules (2, 9,49). Baum et al. (3) initially reported that incubation of renal proximal tubules with dexamethasone resulted in NHE3 activation in the absence of change in NHE3 mRNA level, suggesting that gene regulation cannot account for all the effects on NHE3. This observation has recently been affirmed using cell model systems (6, 39, 48). To better understand the sequences of multiple changes elicited by glucocorticoids leading to the activation of NHE3, herein we (47) determined the time course of the changes in mRNA, protein, and activity induced by dexamethasone. Upon chronic exposure (24 h) to dexamethasone, there was an increase in NHE3 protein abundance that correlates with the activation of NHE3 transport activity. Although dexamethasone rapidly stimulated NHE3 mRNA, a change in NHE3 protein abundance was not observed until 12 h after the exposure to dexamethasone. The delayed increase in NHE3 protein abundance is in agreement with a recent report (6), but is inconsistent with the acute activation of NHE3 activity observed at 4 h. Compared with the effect on NHE3, the activation of SGK1 activity was rapid and a significant increase in the kinase activity was first noted at 2 h. Hence, there appears to be a general pattern of the changes incurred by dexamethasone, in which the activation of SGK1 precedes that of NHE3. The preceding of the SGK1-related changes is in accordance with a previous study showing that phosphorylation of NHE3 by SGK1 parallels the increases in NHE3 abundance at the cell surface and NHE3 activity (39). It is thought that phosphorylation of NHE3 triggers yet-undefined events that target NHE3 proteins to the cell surface. This conjecture is supported by a recent study (6) showing that dexamethasone increases the rate of exocytic insertion of NHE3 into the plasma membrane in the absence of de novo synthesis of NHE3 protein. Together, these studies suggest that activation of SGK1, which translocates NHE3 from an intracellular pool to the cell surface, comprises part of the mechanism underlying the acute regulation of NHE3. However, because this activation takes hours rather than minutes, the presence of additional steps in this signaling cascade is suggested, perhaps involving induction of other regulatory proteins by dexamethasone.
The classic mode of glucocorticoids action involves binding of glucocorticoids to intracellular receptors, which then migrate into the nuclei to initiate synthesis of the specific mRNAs. Hence, as expected, RU486, cycloheximide, and actinomycin D completely obliterated the chronic effect of dexamethasone, suggesting that the genomic regulation is the major underlying mechanism. Although we did not determine the time course of actinomycin D effects, in general actinomycin D inhibits transcription within 30 min. Specifically, a previous study showed that actinomycin D inhibited dexamethasone-mediated SGK1 mRNA synthesis in lung epithelia cells within 2 h (18). However, whether the regulation of the NHE3 gene is the sole determinant for the chronic effect is not clear, as these inhibitors are expected to block the synthesis of other gene products that may be involved in this regulation. Glucocorticoids and other steroid hormones have been shown repeatedly to act independently from gene transcription. Direct binding of glucocorticoids on the cell membrane has suggested the presence of glucocorticoid-binding membrane receptors, although the identity of the membrane GRs at the molecular level is unknown (26, 30). Hence, it was anticipated that RU486 showed no effect on the acute regulation of NHE3 by dexamethasone. On the contrary, the effect of RU486 on the acute regulation was unexpected. The inhibitory effect of RU486 suggests that a functional GR is necessary for the regulation of NHE3, and, despite the inhibition by RU486, we conclude that the acute regulation is independent of gene regulation because it was not blocked by actinomycin D or cycloheximide. The necessity for a functional GR and the absence of an effect by actinomycin D do not contradict each other. Studies (25, 36, 42) have shown that the binding of ligands to the intracellular GR can activate signaling cascades that involve mitogen-activated protein kinases, PI3K, or protein kinase C in a nongenomic manner. In particular, GR has been shown to associate with the regulatory subunit of PI3K, p85α, and results in activation of PI3K (16). A potential role of PI3K in the dexamethasone-mediated activation of NHE3 has previously been demonstrated where inhibition of PI3K with LY-294002 obliterates the activation of NHE3 by dexamethasone (47). Consistently, our data showed that inhibition of PI3K obliterated the activation of SGK1 by dexamethasone. Hence, it remains plausible that the binding of glucocorticoids to the intracellular receptors activate PI3K, which phosphorylates SGK1, resulting in increased SGK1 activity without altering its expression.
Rapid activation of SGK1 transcription by steroid hormones has previously been demonstrated. However, there is no consensus on the effect of steroids on SGK1 protein abundance. Dexamethasone acutely increased SGK1 protein expression in mouse mammary epithelial cells (24), but a similar increase was not detected in Caco-2 or OK cells. Similarly, no apparent change in SGK1 expression was reported in rat kidney and intestine (1, 11). Even in the presence of MG132, we could not detect a significant change in SGK1 protein abundance. This also suggests that the degradation of SGK1 protein is not mediated by a proteasome-dependent pathway in Caco-2 and OK cells. Alternatively, the rate of synthesis is extremely low in these cells so that genomic activation is not a major mechanism of SGK1 activation. Instead, we observed an increase in SGK1 activity in a time-dependent manner.
Increasing evidence has accrued delineating an important role of SGK1 in regulation of multiple transporters and channels (23). We have initially suggested a potential role of SGK1 by using a dominant-negative SGK1, which significantly blocked the effect of dexamethasone on NHE3 activity (39, 46, 47). Dexamethasone treatment of wild-type mice for 4 days greatly increased NHE3 protein abundance in the brush border membrane, but not in SGK1−/− mice. This study suggested that SGK1 is important in the trafficking of NHE3 protein into the surface membrane. Despite the significant aberration imposed by the absence of SGK1, the residual activation indicates that there is an alternative mechanism for the activation. It is possible that the increase in NHE3 protein is responsible for the residual activation in SGK1−/− mice. Nonetheless, we speculate that one potential candidate is SGK2, a close isoform of SGK1 (20). Although SGK2 and SGK3 expression is not induced by glucocorticoids in fibroblasts, we found that SGK2 mRNA is induced in Caco-2 cells. Although we have not determined the effect of dexamethasone on SGK2 protein expression, it is conceivable that SGK2 may show an increase, parallel with the increase in transcript. SGK3, on the other hand, was not induced by dexamethasone, and compound SGK1/SGK3−/− mice display a moderate impairment in renal Na retention similar to SGK−/− mice, indicating a minor role, if any, of SGK3 in Na transport (13, 17, 44).
In summary, dexamethasone-mediated activation of NHE3 is biphasic. The chronic activation is dependent on the genomic activity of GR, which activates both NHE3 and SGK1. Functional GRs are also required for the acute regulation of NHE3, but this regulation is independent of the activation of NHE3 gene as NHE3 activity is clearly stimulated without a change in NHE3 protein abundance. The changes in SGK1 preceded those of NHE3 suggesting SGK1 is an important component in this regulation).
Acknowledgments
We thank Drs. Daniel Biemesderfer at Yale University and Fayez Ghishan at the University of Arizona for antibodies to NHE3. Caco-2 cells were provided by the Emory Epithelial Pathobiology Research Development Center.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-061418. The Emory Epithelial Pathobiology Research Development Center is supported by Grant DK-064399.
References
- 1.Alvarez de la Rosa D, Coric T, Todorovic N, Shao D, Wang T, Canessa CM. Distribution and regulation of expression of serum- and glucocorticoid-induced kinase-1 in the rat kidney. J Physiol. 2003;551:455–466. doi: 10.1113/jphysiol.2003.042903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baum M, Biemesderfer D, Gentry D, Aronson PS. Ontogeny of rabbit renal cortical NHE3 and NHE1: effect of glucocorticoids. Am J Physiol Renal Fluid Electrolyte Physiol. 1995;268:F815–F820. doi: 10.1152/ajprenal.1995.268.5.F815. [DOI] [PubMed] [Google Scholar]
- 3.Baum M, Cano A, Alpern RJ. Glucocorticoids stimulate Na+/H+ anti-porter in OKP cells. Am J Physiol Renal Fluid Electrolyte Physiol. 1993;264:F1027–F1031. doi: 10.1152/ajprenal.1993.264.6.F1027. [DOI] [PubMed] [Google Scholar]
- 4.Baum M, Quigley R. Glucocorticoids stimulate rabbit proximal convoluted tubule acidification. J Clin Invest. 1993;91:110–114. doi: 10.1172/JCI116158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhargava A, Fullerton MJ, Myles K, Purdy TM, Funder JW, Pearce D, Cole TJ. The serum- and glucocorticoid-induced kinase is a physiological mediator of aldosterone action. Endocrinology. 2001;142:1587–1594. doi: 10.1210/endo.142.4.8095. [DOI] [PubMed] [Google Scholar]
- 6.Bobulescu IA, Dwarakanath V, Zou L, Zhang J, Baum M, Moe OW. Glucocorticoids acutely increase cell surface Na+/H+ exchanger-3 (NHE3) by activation of NHE3 exocytosis. Am J Physiol Renal Physiol. 2005;289:F685–F691. doi: 10.1152/ajprenal.00447.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brennan FE, Fuller PJ. Rapid upregulation of serum and glucocorticoid-regulated kinase (sgk) gene expression by corticosteroids in vivo. Mol Cell Endocrinol. 2000;166:129–136. doi: 10.1016/s0303-7207(00)00274-4. [DOI] [PubMed] [Google Scholar]
- 8.Brickley DR, Mikosz CA, Hagan CR, Conzen SD. Ubiquitin modification of serum and glucocorticoid-induced protein kinase-1 (SGK-1) J Biol Chem. 2002;277:43064–43070. doi: 10.1074/jbc.M207604200. [DOI] [PubMed] [Google Scholar]
- 9.Cho JH, Musch MW, DePaoli AM, Bookstein CM, Xie Y, Burant CF, Rao M, Chang EB. Glucocorticoids regulate Na/H exchange expression and activity in region- and tissue-specific manner. Am J Physiol Cell Physiol. 1994;267:C796–C803. doi: 10.1152/ajpcell.1994.267.3.C796. [DOI] [PubMed] [Google Scholar]
- 10.Collins JF, Xu H, Kiela PR, Zeng J, Ghishan FK. Functional and molecular characterization of NHE3 expression during ontogeny in rat jejunal epithelium. Am J Physiol Cell Physiol. 1997;273:C1937–C1946. doi: 10.1152/ajpcell.1997.273.6.C1937. [DOI] [PubMed] [Google Scholar]
- 11.Coric T, Hernandez N, De La Rosa DA, Shao D, Wang T, Canessa CM. Expression of ENaC and serum- and glucocorticoid-induced kinase 1 in the rat intestinal epithelium. Am J Physiol Gastrointest Liver Physiol. 2004;286:G663–G670. doi: 10.1152/ajpgi.00364.2003. [DOI] [PubMed] [Google Scholar]
- 12.Dallman MF, la Fleur SE, Pecoraro NC, Gomez F, Houshyar H, Akana SF. Minireview: glucocorticoids–food intake, abdominal obesity, and wealthy nations in 2004. Endocrinology. 2004;145:2633–2638. doi: 10.1210/en.2004-0037. [DOI] [PubMed] [Google Scholar]
- 13.Grahammer F, Artunc F, Sandulache D, Rexhepaj R, Friedrich B, Risler T, McCormick JA, Dawson K, Wang J, Pearce D, Wulff P, Kuhl D, Lang F. Renal function of gene-targeted mice lacking both SGK1 and SGK3. Am J Physiol Regul Integr Comp Physiol. 2006;290:R945–R950. doi: 10.1152/ajpregu.00484.2005. [DOI] [PubMed] [Google Scholar]
- 14.Grahammer F, Henke G, Sandu C, Rexhepaj R, Hussain A, Friedrich B, Risler T, Just L, Skutella T, Wulff P, Kuhl D, Lang F. Intestinal function of gene targeted mice lacking the serum and glucocorticoid inducible kinase SGK1. Am J Physiol Gastrointest Liver Physiol. 2006;290:G1114–G1123. doi: 10.1152/ajpgi.00231.2005. [DOI] [PubMed] [Google Scholar]
- 15.Grasset EM, Pinto M, Dessaulx E, Zweibaum A, Desjeux JF. Epithelial properties of human carcinoma cell line Caco-2: electrical parameters. Am J Physiol Cell Physiol. 1984;247:C260–C267. doi: 10.1152/ajpcell.1984.247.3.C260. [DOI] [PubMed] [Google Scholar]
- 16.Hafezi-Moghadam A, Simoncini T, Yang Z, Limbourg FP, Plumier JC, Rebsamen MC, Hsieh CM, Chui DS, Thomas KL, Prorock AJ, Laubach VE, Moskowitz MA, French BA, Ley K, Liao JK. Acute cardiovascular protective effects of corticosteroids are mediated by non-transcriptional activation of endothelial nitric oxide synthase. Nat Med. 2002;8:473–479. doi: 10.1038/nm0502-473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang DY, Wulff P, Volkl H, Loffing J, Richter K, Kuhl D, Lang F, Vallon V. Impaired regulation of renal K+ elimination in the sgk1-knockout mouse. J Am Soc Nephrol. 2004;15:885–891. doi: 10.1097/01.asn.0000120368.59693.a8. [DOI] [PubMed] [Google Scholar]
- 18.Itani OA, Liu KZ, Cornish KL, Campbell JR, Thomas CP. Glucocorticoids stimulate human sgk1 gene expression by activation of a GRE in its 5′-flanking region. Am J Physiol Endocrinol Metab. 2002;283:E971–E979. doi: 10.1152/ajpendo.00021.2002. [DOI] [PubMed] [Google Scholar]
- 19.Kobayashi T, Cohen P. Activation of serum- and glucocorticoid-regulated protein kinase by agonists that activate phosphatidylinositide 3-kinase is mediated by 3-phosphoinositide-dependent protein kinase-1 (PDK1) and PDK2. Biochem J. 1999;339:319–328. [PMC free article] [PubMed] [Google Scholar]
- 20.Kobayashi T, Deak M, Morrice N, Cohen P. Characterization of the structure and regulation of two novel isoforms of serum- and glucocorticoid-induced protein kinase. Biochem J. 1999;344:189–197. [PMC free article] [PubMed] [Google Scholar]
- 21.Laborda J. 36B4 cDNA used as an estradiol-independent mRNA control is the cDNA for human acidic ribosomal phosphoprotein PO. Nucleic Acids Res. 1991;19:3998. doi: 10.1093/nar/19.14.3998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lamprecht G, Weinman EJ, Yun CC. The role of NHERF and E3KARP in the cAMP-mediated inhibition of NHE3. J Biol Chem. 1998;273:29972–29978. doi: 10.1074/jbc.273.45.29972. [DOI] [PubMed] [Google Scholar]
- 23.Lang F, Henke G, Embark HM, Waldegger S, Palmada M, Bohmer C, Vallon V. Regulation of channels by the serum and glucocorticoid-inducible kinase–implications for transport, excitability and cell proliferation. Cell Physiol Biochem. 2003;13:41–50. doi: 10.1159/000070248. [DOI] [PubMed] [Google Scholar]
- 24.Leong ML, Maiyar AC, Kim B, O’Keeffe BA, Firestone GL. Expression of the serum- and glucocorticoid-inducible protein kinase, Sgk, is a cell survival response to multiple types of environmental stress stimuli in mammary epithelial cells. J Biol Chem. 2003;278:5871–5882. doi: 10.1074/jbc.M211649200. [DOI] [PubMed] [Google Scholar]
- 25.Losel R, Wehling M. Nongenomic actions of steroid hormones. Nature Rev Mol Cell Biol. 2003;4:46–55. doi: 10.1038/nrm1009. [DOI] [PubMed] [Google Scholar]
- 26.Maier C, Runzler D, Schindelar J, Grabner G, Waldhausl W, Kohler G, Luger A. G-protein-coupled glucocorticoid receptors on the pituitary cell membrane. J Cell Sci. 2005;118:3353–3361. doi: 10.1242/jcs.02462. [DOI] [PubMed] [Google Scholar]
- 27.Moe OW. Acute regulation of proximal tubule apical membrane Na/H exchanger NHE-3: role of phosphorylation, protein trafficking, and regulatory factors. J Am Soc Nephrol. 1999;10:2412–2425. doi: 10.1681/ASN.V10112412. [DOI] [PubMed] [Google Scholar]
- 28.Moguilewsky M, Philibert D. RU 38486: potent antiglucocorticoid activity correlated with strong binding to the cytosolic glucocorticoid receptor followed by an impaired activation. J Steroid Biochem. 1984;20:271–276. doi: 10.1016/0022-4731(84)90216-4. [DOI] [PubMed] [Google Scholar]
- 29.Naray-Fejes-Toth A, Fejes-Toth G, Volk KA, Stokes JB. SGK is a primary glucocorticoid-induced gene in the human. J Steroid Biochem Mol Biol. 2000;75:51–56. doi: 10.1016/s0960-0760(00)00136-9. [DOI] [PubMed] [Google Scholar]
- 30.Orchinik M, Matthews L, Gasser PJ. Distinct specificity for corticosteroid binding sites in amphibian cytosol, neuronal membranes, and plasma. Gen Comp Endocrinol. 2000;118:284–301. doi: 10.1006/gcen.2000.7462. [DOI] [PubMed] [Google Scholar]
- 31.Orchinik M, Moore FL, Rose JD. Mechanistic and functional studies of rapid corticosteroid actions. Ann NY Acad Sci. 1994;746:101–112. doi: 10.1111/j.1749-6632.1994.tb39219.x. [DOI] [PubMed] [Google Scholar]
- 32.Perrotti N, He RA, Phillips SA, Haft CR, Taylor SI. Activation of serum- and glucocorticoid-induced protein kinase (Sgk) by cyclic AMP and insulin. J Biol Chem. 2001;276:9406–9412. doi: 10.1074/jbc.M007052200. [DOI] [PubMed] [Google Scholar]
- 33.Schacke H, Docke WD, Asadullah K. Mechanisms involved in the side effects of glucocorticoids. Pharmacol Ther. 2002;96:23–43. doi: 10.1016/s0163-7258(02)00297-8. [DOI] [PubMed] [Google Scholar]
- 34.Schultheis PJ, Clarke LL, Meneton P, Miller ML, Soleimani M, Gawenis LR, Riddle TM, Duffy JJ, Doetschman T, Wang T, Giebisch G, Aronson PS, Lorenz JN, Shull GE. Renal and intestinal absorptive defects in mice lacking the NHE3 Na+/H+ exchanger. Nat Genet. 1998;19:282–285. doi: 10.1038/969. [DOI] [PubMed] [Google Scholar]
- 35.Sellin JH, Field M. Physiologic and pharmacologic effects of glucorcorticoids on ion transport across rabbit ileal mucosa in vitro. J Clin Invest. 1981;67:770–778. doi: 10.1172/JCI110094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Simoncini T, Hafezi-Moghadam A, Brazil DP, Ley K, Chin WW, Liao JK. Interaction of oestrogen receptor with the regulatory subunit of phosphatidylinositol-3-OH kinase. Nature. 2000;407:538–541. doi: 10.1038/35035131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Verriere VA, Hynes D, Faherty S, Devaney J, Bousquet J, Harvey BJ, Urbach V. Rapid effects of dexamethasone on intracellular pH and Na+/H+exchanger activity in human bronchial epithelial cells. J Biol Chem. 2005;280:35807–35814. doi: 10.1074/jbc.M506584200. [DOI] [PubMed] [Google Scholar]
- 38.Wang D, Lee HJ, Cooper DS, Cebotaro L, Walden PD, Choi I, Yun CC. Coexpression of MAST205 inhibits the activity of Na+/H+ exchanger NHE3. Am J Physiol Renal Physiol. 2006;290:F428–F437. doi: 10.1152/ajprenal.00161.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang D, Sun H, Lang F, Yun CC. Activation of NHE3 by dexamethasone requires phosphorylation of NHE3 at Ser663 by SGK1. Am J Physiol Cell Physiol. 2005;289:C802–C810. doi: 10.1152/ajpcell.00597.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Webster MK, Goya L, Firestone GL. Immediate-early transcriptional regulation and rapid mRNA turnover of a putative serine/threonine protein kinase. J Biol Chem. 1993;268:11482–11485. [PubMed] [Google Scholar]
- 41.Webster MK, Goya L, Ge Y, Maiyar AC, Firestone GL. Characterization of sgk, a novel member of the serine/threonine protein kinase gene family which is transcriptionally induced by glucocorticoids and serum. Mol Cell Biol. 1993;13:2031–2040. doi: 10.1128/mcb.13.4.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Winter C, Schulz N, Giebisch G, Geibel JP, Wagner CA. Nongenomic stimulation of vacuolar H+-ATPases in intercalated renal tubule cells by aldosterone. Proc Natl Acad Sci USA. 2004;101:2636–2641. doi: 10.1073/pnas.0307321101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Woo AL, Noonan WT, Schultheis PJ, Neumann JC, Manning PA, Lorenz JN, Shull GE. Renal function in NHE3-deficient mice with transgenic rescue of small intestinal absorptive defect. Am J Physiol Renal Physiol. 2003;284:F1190–F1198. doi: 10.1152/ajprenal.00418.2002. [DOI] [PubMed] [Google Scholar]
- 44.Wulff P, Vallon V, Huang DY, Volkl H, Yu F, Richter K, Jansen M, Schlunz M, Klingel K, Loffing J, Kauselmann G, Bosl MR, Lang F, Kuhl D. Impaired renal Na+ retention in the sgk1-knockout mouse. J Clin Invest. 2002;110:1263–1268. doi: 10.1172/JCI15696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yang YX, Lichtenstein GR. Corticosteroids in Crohn’s disease. Am J Gastroenterol. 2002;97:803–823. doi: 10.1111/j.1572-0241.2002.05596.x. [DOI] [PubMed] [Google Scholar]
- 46.Yun CC. Concerted roles of SGK1 and the Na+/H+ exchanger regulatory factor 2 (NHERF2) in regulation of NHE3. Cell Physiol Biochem. 2003;13:29–40. doi: 10.1159/000070247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yun CC, Chen Y, Lang F. Glucocorticoid activation of Na+/H+ exchanger isoform 3 revisited. The roles of SGK1 and NHERF2. J Biol Chem. 2002;277:7676–7683. doi: 10.1074/jbc.M107768200. [DOI] [PubMed] [Google Scholar]
- 48.Yun CC, Palmada M, Embark HM, Fedorenko O, Feng Y, Henke G, Setiawan I, Boehmer C, Weinman EJ, Sandrasagra S, Korbmacher C, Cohen P, Pearce D, Lang F. The serum and glucocorticoid inducible kinase SGK1 and the Na+/H+ exchange regulating factor NHERF2 synergize to stimulate the renal outer medullary K+ channel ROMK1. J Am Soc Nephrol. 2002;13:2823–2830. doi: 10.1097/01.asn.0000035085.54451.81. [DOI] [PubMed] [Google Scholar]
- 49.Yun CHC, Gurubhagavatula S, Levine SA, Montgomery JLM, Cohen ME, Cragoe EJ, Pouyssegur J, Tse CM, Donowitz M. Glucocorticoid stimulation of ileal Na+ absorptive cell brush border Na/H exchange and association with an increase in message for NHE-3, an epithelial Na/H exchanger isoform. J Biol Chem. 1993;268:206–211. [PubMed] [Google Scholar]
- 50.Yun CHC, Tse CM, Nath SK, Levine SA, Brant SR, Donowitz M. Mammalian Na+/H+ exchanger gene family: structure and function studies. Am J Physiol Gastrointest Liver Physiol. 1995;269:G1–G11. doi: 10.1152/ajpgi.1995.269.1.G1. [DOI] [PubMed] [Google Scholar]
- 51.Zachos NC, Tse M, Donowitz M. Molecular physiology of intestinal Na+/H+ exchange. Annu Rev Physiol. 2005;67:411–443. doi: 10.1146/annurev.physiol.67.031103.153004. [DOI] [PubMed] [Google Scholar]