Abstract
Background:
The use of antiretroviral drugs (ARV) to prevent mother-to-child HIV transmission (PMTCT) promises to be effective. However, limited data on the adverse effects of ARV among pregnant women and pregnancy outcomes have been reported in clinical practice.
Objectives:
This study aimed to assess adverse effects and outcomes among pregnant HIV-infected women receiving antiretroviral drugs for either antiretroviral therapy (ART) or PMTCT.
Study Design:
This cohort study was at Chonburi Hospital, Thailand, in 2002-2006.
Results:
A total of 246 pregnant HIV-infected women with the median age (range) of 27 (16-41) years were included in this study. ART was initiated in 16.3% for treatment during ANC, 66.7% for PMTCT during ANC, and 17.1% for PMTCT in labor. Adverse effects, especially anemia, were significantly associated with continuing combined ART in pregnancy (p<0.001). 88.9% delivered normal-term neonates. The prevalence of pre-term delivery was 10.2%. Overall, 24 adverse events from 21 pregnant women (8.5%) were noted. A significantly higher prevalence of pre-term delivery was noted in the groups continuing combined ART, or initiating of PMTCT during labor rather than ANC (p=0.02). The incidence of low Apgar scores was 3.6%, and these were associated with initiation of PMTCT during labor (p=0.004).
Conclusion:
Adverse ARV events were more numerous among the pregnant women who needed ART than PMTCT. ANC is beneficial and strongly recommended for all pregnant HIV-infected women for better pregnancy outcomes.
Keywords: HIV, Adverse effects, antiretroviral drugs, pregnancy, PMTCT.
INTRODUCTION
In Thailand, the prevalence of HIV among pregnant women reached a peak of 2.35% in 1995, and was 0.9% in 2005, with a cumulative 12,213 HIV-infected children via mother-to-child transmission (MTCT) up to 2006. Zidovudine (AZT), one of the nucleoside reverse transcriptase (NRTI), reduced the risk of HIV mother-to-child transmission by nearly 70% in 1994 [1], and had been used in most Thai hospitals since 1999. The efficacy of adding a single dose of nevirapine (NVP) to oral AZT prophylaxis for pregnant women in labor, with or without a dose for the infant, to reduce vertical HIV transmission, was studied in Thailand, in 2001-2003. The observed 80% reduction led to the early interruption of the placebo-placebo group enrollment. On the basis of these findings [2], and for other logistical reasons, the Thai Ministry of Public Health has recommended NVP administration for both mother and infant. Increased routine use of antiretroviral therapy (ART) has led to a dramatic and sustained decrease in mortality and morbidity in HIV infections, and the risk of MTCT. The national prevention of mother-to-child transmission (PMTCT) program estimated the rate of perinatal HIV transmission in Thailand was 0.87% in 2005.
It is currently recommended that HIV infected pregnant women receive similar HAART regimens to non-pregnant women, other than for considerations of potential adverse effects on the fetus [3]. Almost 80% of pregnant women experienced one or more typical adverse effects of antiretroviral drugs (ARV), such as anemia, nausea/vomiting, aminotransferase elevation, or hyperglycemia [2]. Concerns were raised following liver failure with maternal death reported in a randomized study of NVP use in pregnancy with CD4 count > 250 cells/μL, by an AIDS Clinical Trials Group (ACTG) [4]. In addition, the data are conflicting, as to whether combination ART during pregnancy is associated with adverse pregnancy outcomes, such as pre-term delivery [5]. Knowledge of the potential adverse effects of ART, specifically for pregnant women and their fetuses, is very important when considering antiretroviral regimens, especially now a variety of regimens are widely prescribed for pregnant HIV-infected women. This study was conducted to review, retrospectively and prospectively, the adverse effect of antiretroviral therapy among pregnant HIV-infected women and their pregnancy outcomes in a clinical setting.
METHODOLOGY
The cohort study was conducted at Chonburi Hospital, near the famous tourist city of Pattaya, Thailand. The study was approved by the Ethics Committees of Chonburi Hospital and the Faculty of Tropical Medicine, Mahidol University. The study cohort comprised pregnant HIV-infected women who attended an antenatal care clinic (ANC), delivered, or terminated pregnancy at Chonburi Hospital, and received ART for either continued treatment or the initiation of PMTCT, between 1 January 2001 - 31 December 2006. The inclusion criteria were age ≥ 15 years, asymptomatic HIV infection with accepted baseline conditions (i.e. hemoglobin level >8.0 g/dL, alanine aminotransferase (ALT) level <35 U/L) and no severe concomitant disease (e.g. malignancy), which would complicate an assessment of adverse effects during pregnancy. All patients were encouraged to attend hospital ANC regularly. Their clinical status was assessed in compliance with the national PMTCT program, which included intrapartum, perinatal, and neonatal ART.
Data were collected using questionnaires/case record forms to gather general demographic information, pregnancy and medical history, and self-assessments of any adverse events during pregnancy. Antenatal care information, including concomitant drugs, adverse effects and laboratory data (i.e. CD4+ cells count, hematology, liver function tests, kidney function, urine analysis, etc.) were collected with clinical-oriented decisions in clinical practice. Regularly, complete blood count and liver function tests were monitored every 2 and 3 months, respectively during ANC. All toxicities associated with ART use were graded according to the National Institute of Allergy and Infectious Disease Division of AIDS Table for Grading the Severity of Adult and Pediatric Adverse Events, a descriptive scale used in all clinical trials developed and/or sponsored by the ACTG to standardize reporting of adverse events. The scale ranges from grade 1- mild, grade 2- moderate, grade 3- severe, to grade 4- potentially life threatening [6]. The outcomes of pregnancy--abortion, stillbirth, pre-term delivery, birthweight, Apgar score, and birth defects--were assessed. All data were entered and analyzed using the statistical software package Epi Info 2002, revision 2. Categorical variables were compared using Pearson χ2 test. Quantitative variables were compared using the t-test for independent samples or the Mann-Whitney U test, according to the distribution characteristics (symmetrical or skewed, respectively). A p-value <0.05 was considered statistically significant.
RESULTS
246 pregnant HIV-infected women were divided into 3 groups: 1) receiving or continuing treatment with combined ARV, 2) ARV initiated for PMTCT, as scheduled or during ANC, 3) ARV only in labor due to lack of, or inadequate ANC. The maternal characteristics of the 246 pregnant HIV-infected women are shown in Table 1. Their age range was 16-41 years (median 27 years). The majority (61.8%) were in the age group 20-29 years, followed by 30-39 years (29.3%). The rate of teenage pregnancy was 8.1%. The majority of the pregnant women were currently in spousal relationships (60.16%), while 7.3% were separated and 1.2% widowed. 0.4% reported having multiple sex partners. Among the women in spousal relationships, 54% of their current spouses were also HIV seropositive whereas 46% were not. 29.3% had no income and most of the pregnant women had an average monthly income <10,000 Baht. Regarding HIV seroconversion, 73.2% were firstly detected when attending ANC, 15% prior to the current pregnancy, and 11.8% during labour.
Table 1.
Maternal Characteristics of Pregnant HIV-Infected Women, Antenatal Care and HIV History (n=246)
| Characteristics | No. of Pregnant Women (%) |
P value | ||
|---|---|---|---|---|
| Combined ART (N = 40) | PMTCT During ANC (N = 164) | PMTCT in Labor (N = 42) | ||
| Age: median (min- max) | 30 (19 - 41) | 26 (17 - 39) | 27 (16 - 38) | 0.015 |
| Occupation: | ||||
| Laborer | 31 (77.5%) | 143 (87.2%) | 31 (73.8%) | 0.064 |
| Housewife (not working) | 9 (22.5%) | 21 (12.8%) | 11 (26.2%) | |
| Marital status: Married | 29 (72.5%) | 103 (62.8%) | 16 (38.1%) | 0.003 |
| Number of spouse(s) ever: | ||||
| median (min- max) | 1 (1 - 3) | 1 (1 - 4) | 1 (1 - 2) | 0.17 |
| Firstly detected HIV in the current pregnancy | 52.5% | 89.6% | 86.7% | 0.00 |
| Years of known HIV status: | ||||
| median (min-max) | 2 (1 - 13) | 1 (1 - 9) | 1 (1 - 5) | 0.00 |
| CDC classification | ||||
| A | 37 (92.5%) | 163 (99.4%) | 40 (95.2%) | 0.06 |
| B | 2 (5.0%) | 0 | 1 (2.4%) | |
| C | 1 (2.5%) | 1 (0.6%) | 1 (2.4%) | |
| Median CD4 count | 240 | 361 | 298 | 0.00 |
| (min-max) | (2 - 566) | (25 - 996) | (211 - 376) | |
| Median gestational age at first visit | 19.5 | 19.5 | 38 | 0.00 |
| (weeks)(min-max) | (6 - 36) | (5 - 40) | (8 - 41) | |
Among the 37 pregnant women who had known their HIV serostatus before the current pregnancy, 19 (51.4%) had experience with ARV; 16 continued combined ART, 2 had already withdrawn from ART, and one had used AZT plus a single dose of NVP once for PMTCT in the previous pregnancy. The other 18 (48.6%) pregnant women had no experience of ARV. Regarding the ART regimens used by 16 pregnant HIV-infected women, 2NRTIs + NVP (21.6%), 2NRTIs + efavirenz (EFV) (13.5%) and 2NRTIs + boosted indinavir (IDV/r) (8.1%) were noted. EFV was stopped and substituted with NVP among 4 pregnant women at 8, 11, 23, and 25 weeks' gestation; however, one did not stop taking EFV because of late presentation at ANC (35 weeks' gestation). Among the 180 pregnant women whose HIV-seroconversion was firstly detected during ANC, the median gestational age was 19.5 weeks; 83.9% were detected before or at 28 weeks' gestation. 98.2% were in WHO clinical stage [7] 1 (asymptomatic), or CDC classification [8] A. Median CD4 count was 325.5 cells/µL; 13.3% of these were <200 cells/µL. One pregnant woman with a CD4 count of 2 (0%) cells/µl, was classified as WHO clinical stage 3 (or CDC stage B), with perianal abscess and herpes labialis. Five (2.0%) pregnant women had late latent syphilis, and 15 (6.1%) of pregnant women had HBV coinfections.
Of the 246 pregnant women in ANC, 40 (16.3%) received combined ART, 164 (66.7%) PMTCT regimens, and 42 only PMTCT in labor, because of irregular or no ANC. Of those receiving PMTCT during ANC, only 76 (46.3%) started PMTCT before or at 28 weeks; 88 (53.7%) started after 32 weeks' gestation. The common combined ART regimen used in this study was AZT+ lamivudine (3TC)+ NVP (50%) followed by AZT+3TC+IDV/r (20%). Other regimens included stavudine (d4T)+3TC +NVP, d4T+3TC+IDV/r and didanosine (ddI)+3TC+ NVP. Among 164 pregnant HIV-infected women receiving PMTCT during ANC, 118 (72%) received AZT + single dose NVP; 36 (22%) received AZT + single dose NVP with tailed end of AZT+3TC or AZT+ ddI extending for 2-4 weeks' postpartum, to prevent NNRTI resistance mutation; the remaining 6% received AZT alone. Of 42 pregnant women who initiated PMTCT in labor, 38 (90.5%) received AZT + single dose NVP. Two cases received AZT alone, and another 2 received single-dose NVP in labor.
Adverse ARV Events During Pregnancy
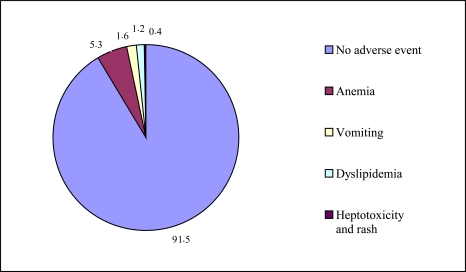
Overall, 24 adverse ARV related events were documented from 21 (8.5%, 95%CI: 5.4%-12.8%) pregnant HIV-infected women. The prevalence of each adverse event is shown in Fig. (1); 13 (5.3%) pregnant women developed anemia, 4 (1.6%) nausea and vomiting, 3 (1.2%) dyslipidemia (1 also reported gestational DM), and 1 (0.4%) hepatotoxicity and rash. No pregnant HIV-infected women initiating PMTCT in labor reported an adverse event. The prevalence of adverse events among pregnant women receiving combined ART (32.5%), and those starting PMTCT during ANC (4.9%), was significantly different (P value < 0.001). Table 2 compares adverse events among 40 pregnant HIV-infected women receiving combined ART vs 164 pregnant HIV-infected women starting PMTCT during ANC. Table 3 compares adverse events among 161 pregnant HIV-infected women receiving ARV grouped by CD4-cells counts during ANC of < 200 vs ≥ 200 cells/μL. Anemia was significantly more common among pregnant women with CD4 cells counts < 200 cells/μL (17.2%) than those with counts ≥ 200 cells/μL (P value =0.04).
Fig. (1).
All adverse events among 246 pregnant HIV-infected women receiving antiretroviral drugs for either combined antiretroviral therapy and prevention of mother to child HIV transmission.
Table 2.
Adverse ARV Events in Pregnant Women Receiving Antiretroviral Drugs During Antenatal Care (n=204)
| Adverse Events | (%) (n=204) | Antiretroviral Drugs | P Value | |
|---|---|---|---|---|
| Combined ART (%) (n=40) | PMTCT During ANC (%) (n=164) | |||
| Anemia | 13 (6.4) | 6 (15.0) | 7 (4.3) | 0.02 |
| Vomiting | 4 (2.0) | 3 (7.5) | 1 (0.6) | 0.02 |
| Dyslipidemia | 3 (1.5) | 3 (7.5) | 0 (0) | 0.01 |
| Hepatotoxicity and rash | 1 (0.5) | 1 (2.5) | 0 (0) | 0.20 |
| Total | 21 (10.4) | 13 (32.5) | 8 (4.9) | < 0.001 |
Table 3.
CD4 Count Level Associated with Adverse ARV Events Among Pregnant Women (n=161)
| Adverse Events | (%) (n=161) | CD4 Count (cells/µl) | P Value | |
|---|---|---|---|---|
| Less than 200 (%) (n=29) | Above or Equal 200 (%) (n=132) | |||
| Anemia | 12 (7.5) | 5 (17.2) | 7 (5.3) | 0.04 |
| Vomiting | 4 (2.5) | 1 (3.4) | 3 (2.3) | 0.55 |
| Dyslipidemia | 3 (1.9) | 1 (3.4) | 2 (1.5) | 0.45 |
| Hepatotoxicity and rash | 1 (0.6) | 1 (3.4) | 0 (0) | 0.18 |
| Total | 20(12.4) | 8 (27.6) | 12 (9.1) | 0.01 |
Anemia
Twenty-two of 204 (10.8%) developed anemia (cut-off haemoglobin level ≤ 9.4 g/dl; ACTG grading; 13 (60%) of these developed anemia after 4 weeks' ARV exposure. The cause of anemia among the other 9 was not identified, and occurred before ART. The median red cell mean corpuscle volume (MCV) among 13 pregnant women with ARV-related anemia was slightly increased, from 81.5 to 86.7%, whereas among the pregnant women with anemia of unidentified cause, it was lower, and decreased slightly from 71.9 to 68.8%. Of the women with ARV-related anemia, 10 were classified as ACTG severity grade 1, 2 were grade 2, and 1 was grade 4. The median duration from AZT initiation to anemia detection was 5 (range: 4-9) weeks. Regarding management of anemia, 3 were given blood transfusions; 2 were given reduced AZT dosage, and 2 were changed to d4T instead of AZT.
Other Adverse Events
Nausea and vomiting were documented in only 4 (1.6%) of 246 pregnant women after taking ARV, of which AZT, ddI, and EFV, were suspected culprits in 2, 1, and 1 cases, respectively. Three pregnant women treated with a protease inhibitor (PI) based regimen developed dyslipidemia during pregnancy. EFV was switched to IDV/r in 2 cases at 8 and 12 weeks' gestation, and dyslipidemia was detected after taking IDV/r for 25 and 13 weeks, respectively. For the other, the ART was changed regimen from AZT+3TC+NVP to boosted saquinavir and indinavir (SQV+ IDV/r) at 25 weeks' gestation due to treatment failure; her dyslipidemia was detected after 13 weeks of the new regimen.
Pregnancy Outcomes
The modes of delivery among the 246 pregnant women were normal labor (72.0%), caesarean section (19.0%), vacuum extraction (2.8%), and forceps extraction (0.8%). Among the 49 women who delivered by caesarean section, indications included previous caesarean section (30.6%), cephalo-pelvic disproportion (18.4%), and breech presentation (10.2%). Four had elective caesareans section due to low CD4 count; 208 (84.6%) had full-term deliveries, 25 (10.2%) pre-term, and one stillbirth; 209 (85.0%) had no delivery-related complication, 17 (6.9%) had pre-term labor pain, 4 (1.6%) premature membrane rupture, and 4 (1.6%) pre-eclampsia. Pre-term delivery was significantly higher among pregnant women on combined ART and among those who started PMTCT in labor because of no ANC (19.4% and 19%, respectively), than among those receiving PMTCT during ANC (6.9%, p=0.02). The incidence of pre-term labor pain among the pregnant women who initiated PMTCT during ANC was significantly lower than the other two groups (p = 0.01). However, when factors affecting maternal status--elderly pregnancy, multigravida, low CD4 count, and anemia--were examined by multivariate analysis, only pregnant women who started PMTCT in labor had a significant association with pre-term delivery (p value = 0.01).
Four infants (1.6%) had congenital defects (stillbirth with hydrocephalus, sensorineural hearing loss, gastroscrisis, and polydactyly). Only 2 (0.8%) had congenital pneumonia. 51 infants showed the results of perinatal HIV infection; the perinatal HIV infection rate was 3.9%. None of the infants born to pregnant women who received combined ART had perinatal HIV infections. Overall, the incidence of low birthweight was high, at 49 (20.0%), comprising 39 low and 10 very low. Infants born to pregnant women who started PMTCT in labor had a higher incidence of both low birthweight (8; 19.0%), and very low birthweight (5; 11.9%) than those receiving PMTCT during ANC (p=0.065). The incidence of low Apgar score was 3.6%; of whom 55.6% were low and the rest very low, including one stillbirth (Agar score = 0). Low Apgar scores were significantly higher among infants born to pregnant women who started PMTCT in labor (11.9%; p=0.004).
DISCUSSION
Despite the positive impact of antiretroviral drugs on HIV-related morbidity and mortality, increased duration of ART has been associated with complications. In the PMTCT scenario, many studies have reported achievement in HIV transmission risk reduction [9]. However, a number of pregnant women experienced the typical adverse effects of ARV such as anemia, nausea and vomiting, aminotransferase elevation, or hyperglycemia. Reported data have conflicted on whether combined ART during pregnancy is associated with adverse pregnancy outcomes, such as pre-term delivery [10]. Additionally, limited data on the potential teratogenicity of antiretroviral drugs in human have been reported [11]. In this study, 40 (16.3%) of 246 pregnant women received combined ART, of which none showed perinatal HIV infection. PMTCT reduced the perinatal HIV-infection rate of 3.9% in this study, consistent with the findings of previous studies [12, 13]. Unfortunately, combined ART was significantly associated with adverse events, especially anemia in pregnancy. The incidence of adverse events in this study was lower than the 15% reported in a previous study in Thailand [14]. This finding might be explained by the clinical setting, at which only clinically oriented adverse events were investigated further. However, when adjusting the sample size by exclusion of women not used AZT, the incidence of adverse events among AZT-based regimens was up to 10.4% (95%CI: 6.5% - 15.3%).
Most of the pregnant women (89.2%) received AZT for PMTCT or combined regimens. Therefore, anemia and nausea/vomiting were expected to be common. 3.8% -38% of pregnant women in general population will develop anemia during pregnancy, depending on nutritional status and trimester [15]. CD4 cell count < 200 cells/μL was significantly associated with anemia, but not nausea/vomiting, dyslipidemia, or hepatotoxicity. Even though > 50% of the combined ART in this study contained NVP, only 1 case of hepatotoxicity was reported, which could be explained by no CD4 cell count cutoff associated with an increased risk of NVP hepatotoxicity in women [16] and fewer HBV coinfected cases in this study. Anemia can also be affected by maternal HIV status. In pregnancy, in the general population, haemoglobin levels usually drop early in the first trimester of gestation, and reverse after the second to third trimesters [15]. In contrast, in this study, the median haemoglobin level of the pregnant women in AZT-related anemia was clear early in the second trimester after the initiation of AZT, and was sustained at a lower level until delivery. Median hematocrit in anemia showed a similar decreasing pattern in all, other than a rapid rise in hematocrit at delivery among the pregnant women with adverse AZT-related anemia. This increment might be explained by the anemia management interventions pre-delivery, reduced AZT dosage in 2 cases, substitution with d4T in 2 cases, and blood transfusion in 3 cases. In the pregnant women receiving AZT for PMTCT, routine complete blood counts should be performed as early as 4 weeks after taking AZT. Anemia should be corrected promptly, to prevent intrauterine growth retardation of the infant.
Regarding delivery outcomes and complications, the incidence of pre-term delivery was 10.7%, which was similar to a previous study in Thailand [14]. When the groups were compared by antiretroviral regimen used in pregnancy, pregnant women on combined ART had a significantly higher rate of pre-term delivery than those starting PMTCT during pregnancy (19.4% vs 6.9%, p=0.02), consistent with the findings of previous studies [2, 12]. Pre-term delivery was also higher among the pregnant women initiating PMTCT in labor than among those initiating during pregnancy (19.0% vs 6.9%, p=0.02). This might reflect the effect of inadequate antenatal care. A stillborn infant was born to a mother starting AZT at 31 weeks’ gestation; hydrocephalus, detected simultaneously, was likely the consequence of congenital infection rather than an adverse AZT-related event. The incidence of pre-eclampsia was 1.7%, quite similar to the rate in the general population (2.8%) [3]. The incidence of pre-eclampsia among pregnant women receiving combined ART was 3.2%, which was contrast to a previous study [3].
Regarding the infants, low birthweight was high (20%), compared with 7.9% - 9.3% in a previous study in Thailand [14], and 5.9% - 9.2% in another study [17]. The pregnant women who started PMTCT in labor had a high percentage of low-birthweight infants. Inadequate ANC for pregnant women might be the cause of the low birthweight noted in our study. However, no significant association between low birthweight and time to initiate antiretroviral drug, maternal CD4 cell count, or gestational age at first ANC visit was found. The rate of low Apgar scores was 3.6%, which was significantly higher among the pregnant women who started ARV during labor, and the women who visited an ANC clinic for the first time at > 28 weeks’ gestation (p=0.004 and p=0.01, respectively). These differences might also be the effect of no or inadequate ANC. Low Apgar scores should be detected among neonates born to pregnant HIV-infected women without any history of PMTCT during ANC. No birth defect was suspected to be related to ART. Five pregnant women who received EFV delivered normal babies. An infant with sensorineural hearing loss was born to a pregnant woman without history of ANC as a consequence of preterm status. There was no evidence to suggest that ART increased the risk of congenital abnormality [18, 19].
In conclusion, antiretroviral therapy for PMTCT or treatment for a pregnant HIV-infected woman is beneficial and outweighs the risk of adverse effects or the costs of the drugs. However, it cannot replace good ANC, which should be emphasized for all pregnant HIV-infected women, to maximise early and regular ANC visits.
ACKNOWLEDGEMENTS
The authors thank all staff at the HIV Clinic, the Chonburi Hospital for their hospitality, the Faculty of Tropical Medicine, Mahidol University, Thailand for funding the costs of publication, and Mr. Paul Adams for English-language corrections.
REFERENCES
- 1.Connor EM, Sperling RS, Gelber R, et al. Reduction of maternal-infant transmission of human immunodeficiency virus type 1 with zidovudine treatment. N Engl J Med. 1994;331:1173–80. doi: 10.1056/NEJM199411033311801. [DOI] [PubMed] [Google Scholar]
- 2.Lorenzi P, Spicher VM, Laubereau B, et al. Antiretroviral therapies in pregnancy: maternal, fetal and neonatal effects Swiss HIV Cohort Study, the Swiss Collaborative HIV and Pregnancy Study, and the Swiss Neonatal HIV Study. AIDS. 1998;12:F241–7. doi: 10.1097/00002030-199818000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Suy A, Martínez E, Coll O, et al. Increased risk of pre-eclampsia and fetal death in HIV-infected pregnant women receiving highly active antiretroviral therapy. AIDS. 2006;20:59–66. doi: 10.1097/01.aids.0000198090.70325.bd. [DOI] [PubMed] [Google Scholar]
- 4.Hitti J, Frenkel LM, Stek AM, et al. Maternal toxicity with continuous nevirapine in pregnancy results from PACTG 1022. J Acquir Immune Defic Syndr. 2004;36:772–6. doi: 10.1097/00126334-200407010-00002. [DOI] [PubMed] [Google Scholar]
- 5.Perinatal HIV Guidelines Working Group, USA Public Health Service Task Force. Recommendations for use of antiretroviral drugs in pregnant HIV-1 infected women for maternal health and interventions to reduce perinatal HIV-1 transmission in the United States [monograph on the internet] [cited 2008 Sep 6];Department of Health and Human Services; Available from: http://aidsinfo.nih.gov/contentfiles/PerinatalGL.pdf . 2006
- 6.Division of Acquired Immunodeficiency Syndrome, USA. Division of AIDS Table for Grading the Severity of Adult and Pediatric Adverse Events [monograph on the internet] [cited 2008 Apr 14];National Institute of Allergy and Infectious Disease, National Institute of Health (US); Available from: http://www.doh.gov.za/ docs/factsheets/guidelines/artguidelines04/sec4.pdf . 2004
- 7.World Health Organization. Interim WHO clinical staging of HIV/AIDS and HIV/AIDS case definition for surveillance: African region [monograph on the internet] [cited 2008 Apr 14];Geneva: World Health Organization WHO/HIV/2005.02. Available from: http://www.who.int/hiv/pub/guidelines/clinicalstaging.pdf .
- 8.Centers for Disease Control and Prevention. 1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. [Accessed 14 April 2008];MMWR Recomm Rep Internet: www.cdc.gov/mmwr/pre-view/mmwrhtml/00018871.htm . 1992 41(RR-17):1–19. [PubMed] [Google Scholar]
- 9.Volmink J, Siegfried NL, van der Merwe L, Brocklehurst P. Antiretrovirals for reducing the risk of mother-to-child transmission of HIV infection. Cochrane Database Syst Rev. 2007;24:CD003510. doi: 10.1002/14651858.CD003510.pub2. [DOI] [PubMed] [Google Scholar]
- 10.Thorne C, Newell ML. Safety of agents used to prevent mother-to-child transmission of HIV: is there any cause for concern? Drug Saf. 2007;30:203–13. doi: 10.2165/00002018-200730030-00004. [DOI] [PubMed] [Google Scholar]
- 11.Watts DH. Teratogenicity risk of antiretroviral therapy in pregnancy. Curr HIV/AIDS Rep. 2007;4:135–40. doi: 10.1007/s11904-007-0020-y. [DOI] [PubMed] [Google Scholar]
- 12.Townsend CL, Cortina-Borja M, Peckham CS, Tookey PA. Antiretroviral therapy and premature delivery in diagnosed HIV-infected women in the United Kingdom and Ireland. AIDS. 2007;21:1019–26. doi: 10.1097/QAD.0b013e328133884b. [DOI] [PubMed] [Google Scholar]
- 13.Harris NS, Fowler MG, Sansom SL, Ruffo N, Lampe MA. Use of enhanced perinatal human immunodeficiency virus surveillance methods to assess antiretroviral use and perinatal human immunodeficiency virus transmission in the United States 1999-2001. Am J Obstet Gynecol. 2007;197(3 Suppl):S33–S41. doi: 10.1016/j.ajog.2007.03.081. [DOI] [PubMed] [Google Scholar]
- 14.Lallemant M, Jourdain G, Le Coeur S, et al. Single-dose perinatal nevirapine plus standard zidovudine to prevent mother-to-child transmission of HIV-1 in Thailand. N Engl J Med. 2004;351:217–28. doi: 10.1056/NEJMoa033500. [DOI] [PubMed] [Google Scholar]
- 15.Center of Disease Control and Prevention. Anemia during pregnancy in low-income women--United States, 1987. Morb Mortal Wkly Rep. 1990;39:73–6. [PubMed] [Google Scholar]
- 16.Stern JO, Robinson PA, Love J, Lanes S, Imperiale MS, Mayers DL. A comprehensive hepatic safety analysis of nevirapine in different populations of HIV infected patients. J Acquir Immune Defic Syndr. 2003;34(Suppl 1):S21–33. doi: 10.1097/00126334-200309011-00005. [DOI] [PubMed] [Google Scholar]
- 17.Shaffer N, Bulterys M, Simonds RJ. Short courses of zidovudine and perinatal transmission of HIV. N Engl J Med. 1999;340:1042–3. [PubMed] [Google Scholar]
- 18.Patel D, Thorne C, Fiore S, Newell ML. European Collaborative Study Does Highly Active Antiretroviral Therapy Increase the Risk of Congenital Abnormalities in HIV-Infected Women? J Acquir Immune Defic Syndr. 2005;40:116–8. doi: 10.1097/01.qai.0000156854.99769.a5. [DOI] [PubMed] [Google Scholar]
- 19.Antiretroviral Pregnancy Registry Steering Committee. Antiretroviral pregnancy registry interim report for 1st January 1989 to 31st January 2003[monograph on the internet] [cited 2008 Aug 5];Geneva: World Health Organization; Available from http: // www.apregistry.com/who.htm . 2005