Abstract
Certain mutations of the dfna5 gene result in a form of autosomal deafness that holds special interest because its phenotype resembles the hearing loss often seen during aging. Little is known of the function or regulation of dfna5 or its encoded protein. However dfna5 has recently been shown to be induced by p53. It also is epigenetically repressed in gastric cancer. We have discovered that dfna5 can be induced by glucocorticoids (GCs) and that this regulation is influenced by crosstalk with the protein kinase A (PKA) system. We show that GCs induce dfna5 mRNA and that its expression appears to be repressed in the basal state. Induction of dfna5 mRNA correlates with GC-dependent apoptosis of CEM cells, though dfna5 expression alone is not sufficient for apoptosis.
Keywords: dfna5, glucocorticoids, PKA, autosomal deafness, apoptosis, leukemia
1. Introduction
Non-syndromic autosomal deafness can be caused by certain mutations in dfna5, a gene originally identified from studies of a Dutch family and mapped to chromosome 7p15 [1–3]. Cloning of the gene allowed determination of its molecular anatomy and the complex mutation that was associated with the condition [3]. Subsequently, two other families have been reported with mutations in dfna5 that lead to autosomal deafness [4,5]. The gene contains 10 exons that encode a protein of 496 amino acids. Though quite different from one another, each of the three identified mutations results in altered transcript splicing such that the transcript of exon 8 is lost from the final mRNA. Loss of exon 8 therefore seems to be a requisite for the loss-of-hearing phenotype. Subsequently, the homologous gene has been identified in yeast, mouse, rat, horse and zebra fish [6,7]. Limited human tissue surveys for expression at the mRNA level noted expression in placenta, heart, brain and kidney of the eight tissues reported [3]. In the mouse, the homologous gene was found to be expressed in cochlear regions [3].
A limited region of the protein DFNA5 bears homology to the Mem10 family of DNA replication proteins [6]. The use of increasingly powerful databases and search systems led to the discovery that DFNA5 is part of the gasdermin family of proteins [8]. Gasdermin itself is expressed in the upper GI tract and the skin [9]. Its expression is lost in human gastric cancer cells by an epigenetic mechanism [10]. As yet, no specific physiological function is known for dfna5, nor is its regulation understood. Its relevance to human disease obviously extends beyond its role in deafness. Further evidence of the importance of dfna5 for all regulation is found in the fact that it is regulated by p53 and consequently in the response to DNA damage [11]. We now show that dfna5 expression can be regulated by GC.
Glucocorticoid receptors are present in cells throughout the inner ear [12–14] and GCs regulate gene expression in the inner ear [15,16]. Presumably this is the basis for the beneficial effects of corticoids used to increase intrinsic excitability of neurons after vestibular deafferentiation [17] or to protect function after acoustic trauma [18,19]. On the other hand, prenatal GCs in excess decrease the susceptibility of the inner ear to acoustic trauma in the adult [20].
In the course of analyzing the effects of the GC dexamethasone (Dex) on clones of the human acute lymphoblastic leukemia cell line CEM, we discovered that dfna5 mRNA increases upon exposure of the cells to the steroid [21]. The increase only occurred in clones which undergo apoptosis in response to the corticoids, not in a resistant clone. Herein we show that the increase in dfna5 mRNA by corticoid treatment requires RNA synthesis, whereas blocking protein synthesis raises the mRNA level. Hence, the gene appears to be an object of primary transcription induction by GCs and under control of protein-dependent repression. The time course of dfna5 mRNA increase following Dex also is consistent with primary transcriptional induction. Inducibility of dfna5 mRNA can be restored to Dex-resistant cells by using forskolin (FSK) to activate the PKA cAMP pathway. These results show that dfna5 expression can be regulated by GCs in lymphoid cells and that the effect is modulated by crosstalk with the cAMP-driven pathway. Though its induction correlates with apoptosis, knock-down of dfna5 mRNA in these cells does not prevent Dex-dependent apoptosis.
2. Materials and Methods
2.1 Reagents
Dex and other reagent grade chemicals were purchased from Sigma-Aldrich Chemical Company (St. Louis, MO). Cortivazol was kindly donated by Roussel-UCLAF, Paris, FR). The RNeasy total RNA isolation kit was from Qiagen (Santa Clara, CA).
2.2 Cell culture
All cells were carefully maintained in logarithmic growth in Cellgro RPMI 1640 tissue culture medium with L-glutamine (Mediatech, Herndon, VA) supplemented with heat-inactivated 5% fetal bovine serum from Atlanta Biologicals (Norcross, GA) at 37°C in a humidified 5% CO2/95% air incubator.
2.3 RNA extraction
For each GeneChip experiment, 50 ml cultures of cells were grown to a density of 4×105 cells/ml and treated with either ethanol or DMSO (for cortivazol - CVZ) vehicle (≤% final concentration) or 1 μM Dex in vehicle for 2, 8, 12, or 20 hours or 0.1 μM CVZ for 20 hours. Cells were harvested, washed once with chilled phosphate buffered saline, pH 7.4 (Cellgro) and resuspended in lysis buffer (RNeasy kit).
The cell lysate was passed through a QIAshredder column (Qiagen) and processed for total RNA isolation as per the protocol provided (RNeasy kit). RNA samples were then stored at −70°C in ethanol until used for GeneChip analysis. An aliquot fraction of the cells in each experiment was kept in culture for several days, to confirm the cells apoptotic or resistant behavior.
2.3 Target labeling, hybridization and data analysis of microarrays
Target labeling, hybridization to Affymetrix HG_U95 Av2 microarrays and data analysis were as described [21,22] except for the following modification. For each gene or probe set, there are 16–20 probe pairs comprised of short oligonucleotides (~25-mer). Each probe pair has a Perfect Match (PM) oligo and a Mismatch (MM) oligo acting as a hydridization control to monitor background levels. Affymetrix software uses these PM and MM values to assign a Discrimination score, that is then used in a one-sided Wilcoxon’s signed rank test versus a user-defined threshold to determine a p-value. The p-value is the determining factor in calling a gene “present” or “absent”.
2.4 Cloning of DFNA5 into mammalian expression plasmid
A plasmid containing the DFNA5 cDNA was purchased from American Type Culture Collection (ATCC, Manassas, VA). DFNA5 was cloned into pEGFP-C1 (Clontech Laboratories, Mountain View, CA) using restriction sites EcoR1 and BamH I to create a fusion GFP-DFNA5 product. Plasmid DNA was sequenced to verify insert was in frame with the GFP-coding region and no mutations were accidentally introduced during the PCR process.
2.5 Overexpression of DFNA5 into CEM C1-15 cells
To introduce plasmids via electroporation, cells in mid-logarithmic growth were centrifuged at 1000 rpm for 20 minutes at 25°C. Cells were washed once in 10 ml warm Cellgro DPBS without calcium and magnesium (Mediatech) and then centrifuged at 1000 rpm for 10 minutes at 25°C. Cells were then resuspended to 1×107 cells/ml in 37°C serum-free RPMI 1640 medium supplemented with 1.25% DMSO. Aliquots of 400 μl were added to 0.4 cm electrode gap Gene Pulser Cuvettes (Bio Rad Laboratories, Hercules, CA) in addition to 20 μg plasmid DNA per cuvette (either pEGFP or pEGFP-DFNA5). Cuvettes were incubated at 25°C for 10 minutes before being electroporated in a Gene Pulser II (Bio Rad) with settings of 975 μF and 270 V. Each sample was then resuspended in a 4 ml standard growth medium (RPMI 1640 + 5% FBS) supplemented with 1.25% DMSO. Cells were then incubated 24 hours in a 37°C humidified 5% CO2/95% air incubator before being counted with a Vi-CELL cell viability analyzer (Beckman Coulter, Fullerton, CA). Cells were resuspended to be 2×105 cells/ml in standard growth medium and then divided equally and treated with either vehicle (ethanol) or 1 μM Dex and then counted on the Vi-CELL after 24, 48, and 72 hours. Under these electroporation conditions, we typically obtain transfection efficiencies 50–60% using GFP as a reporter and counting with FACSCanto (Becton-Dickinson, Franklin Lakes, NJ).
2.6 Reduction of DFNA5 in CEM C7-14 cells
Electroporation conditions were same as above except for the following modifications. After initial electroporation, cells were resuspended in 6 ml and incubated for 48 hours. After this, cells were counted and underwent a second round of electroporation. Each cuvette received a final concentration of 250 nM siRNA (Dharmacon siGENOME SMARTpool specific for DFNA5 or control Non-Targeting siRNA pool). After the second electroporation, cells were allowed to recover for 2 hours, counted and resuspended to 1.5×105 cells/ml, divided and treated +/− Dex. Cells were then counted as above. Using a non-specific fluorescent siRNA and counting with FACSCanto, we typically get transfection efficiencies 75–85%.
2.7 Real-Time PCR reactions
Mid-logarithmic growing cells were resuspended to 2×105 cells/ml and treated under the following conditions; C7-14 cells – vehicle control, 1 μM Dex, 10 μg/ml cycloheximide (CHX), 1 μg/ml actinomycin D (AD), 40 μM dichlorobenzimidazole riboside (DRB) and combinations of blockers + Dex; C1-15 cells – vehicle control, 1 μM Dex, 10 μM forskolin (FSK) and FSK + Dex. CHX, AD and DRB were all added 30 minutes prior to the addition of Dex. Upon adding Dex, cells were incubated as above for either 16 or 24 hours (16 hours when AD was used due to its toxic effects). Cells were then collected and RNA extracted as noted above. After quantifying RNA concentration, 1 μg of each sample was then sent to the Real-Time PCR Core Facility where the assays were performed using an ABI Prism 7000 Sequence Detection System and its related software (Applied Biosystems, Foster City, CA).
2.8 Statistical analysis
Other than the determination of “absent” or “present” by the Affymetrix 5.0 software, all data were analyzed using Spotfire DecisionSite, version 8.1 (Spotfire Inc., Cambridge, MA). Fold changes were determined and paired T-tests employed to determine level of significance. Paired T-tests were performed on data from three independent experiments with time-matched controls for each time point. These algorithms can be found in the Spotfire DecisionSite for Functional Genomics user’s guide. Combined data from all 12 experiments was evaluated using MS Excel (See Table 1).
3. Results
3.1 Time course of DFNA5 mRNA expression following Dex exposure in CEM cell clones
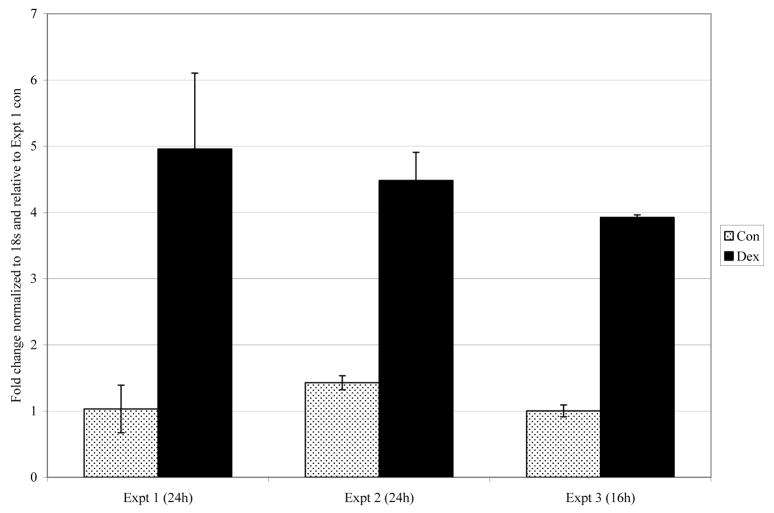
Cells were incubated for 2, 8, 12, or 20 hours in medium containing 1 μM Dex after which RNA samples were prepared and analyzed for gene expression on gene mirochips. We had shown previously that after 20 hours in Dex, dfna5 mRNA had increased by 3 and 4.7 fold in CEM clones C1-6 and C7-14, but was below threshold for detection by this method in the Dex-resistant clone C1-15. The first two clones underwent Dex-dependent apoptosis starting at >20 hours in Dex; clone C1-15 did not and in fact was strongly resistant to corticoid-dependent apoptosis. The time course of dfna5 mRNA expression showed a consistent increase in response to Dex in the sensitive clones, such that by 8–12 hours, mRNA levels had increased several fold. The resistant clone showed only random fluctuations in fluorescence intensity for dfna5 mRNA, always below the levels considered “present” by the Affymetrix statistical software analysis. When data were averaged over all time points for clones C7-14 and C1-6, each showed a highly significant increase in response to Dex. Treatment of C7-14 and C1-15 cells for 20 hours with the more potent corticoid, CVZ, at 0.1 μM resulted in about a 5-fold induction in the sensitive clone, but did not raise the level in C1-15 cells above background (Table 1) and did not cause apoptosis. The increase in dfna5 mRNA in response to Dex was confirmed in clone C7-14 by real-time PCR assays. These showed a consistent 4–5 fold increase in the mRNA after 16 or 24 hours treatment with steroid (Fig. 1).
Fig. 1.
Dex treatment increases dfna5 mRNA in CEM C7-14 cells.
Cells were treated with ethanol vehicle (Con) or 1 μM Dex for designated time, RNA extracted, and analyzed using real-time PCR. Standard deviation bars represent estimates of technical variability within each experiment.
3.2 RNA synthesis is required for dfna5 induction by Dex, but blocking protein synthesis induces dfna5 mRNA
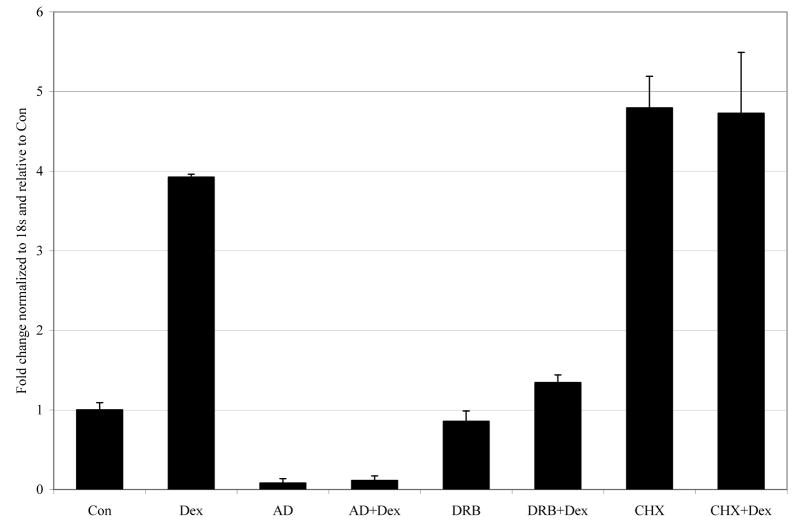
Two inhibitors of RNA synthesis with differing mechanisms of action were used to test the need for de novo RNA synthesis during the Dex-dependent increase of dfna5 mRNA. Both AD and DRB prevented the increase (Fig. 2). Thus, the Dex-evoked increase in dfna5 mRNA requires de novo RNA synthesis. When CHX was used to block protein synthesis, an increase in the mRNA was observed, similar to that caused by Dex alone or by Dex preceded by CHX for thirty minutes (Fig. 2, CHX vs Dex, CHX + Dex). Thus, inhibition of protein synthesis at the translation step allows as great an accumulation of dfna5 mRNA as is seen after induction by Dex.
Fig. 2.
Induction of CEM C7-14 dfna5 levels by Dex requires RNA synthesis, whereas inhibition of protein synthesis induces dfna5 mRNA.
Cells were treated with vehicle alone (Con), 1 μM Dex, 1 μg/ml AD, 40 μM DRB, 10 μg/ml CHX or combinations thereof. Total RNA was extracted and analyzed using real-time PCR. Standard deviation bars represent 1 standard deviation between triplicate samples within the experiment.
3.3 Induction of dfna5 mRNA correlates with sensitivity to Dex-dependent apoptosis
We have previously shown that FSK and Dex synergize to cause apoptosis in both sensitive and resistant CEM clones [23]. FSK alone slows cell growth without causing apoptosis, but this adenyl cyclase activator aids the apoptotic response to Dex. We therefore tested C1-15 cells, a subclone of resistant clone C1, to see whether treatment with FSK had a permissive effect on the Dex-inducibility of the dfna5 gene. Cells were incubated with FSK with or without Dex, and after 24 hours cell extracts were assayed for increased dfna5 mRNA by real time PCR. FSK alone caused an increase of several fold in the RNA, and the combination of Dex and FSK demonstrated a strong synergy, with >25 fold induction, to levels well above those seen after Dex alone in sensitive clone C7-14 (Fig. 3).
Fig. 3.
FSK markedly synergizes with Dex to induce dfna5 mRNA.
CEM C7-14 dfna5 mRNA levels compared to those of C1-15 cells. Cells were treated with vehicle alone (Con), 1 μM Dex, 10 μM FSK, or FSK+Dex for 24 hours. RNA was extracted and analyzed using real-time PCR. Standard deviation bars are from internal triplicates derived from 2 biological replicates.
3.4 Altering dfna5 mRNA levels alone is not sufficient to alter the Dex-sensitive or –resistant phenotype of CEM cells
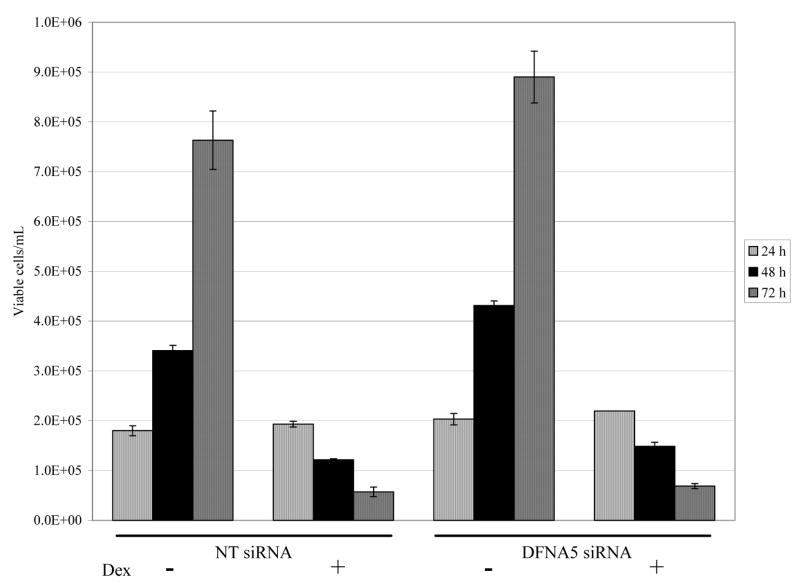
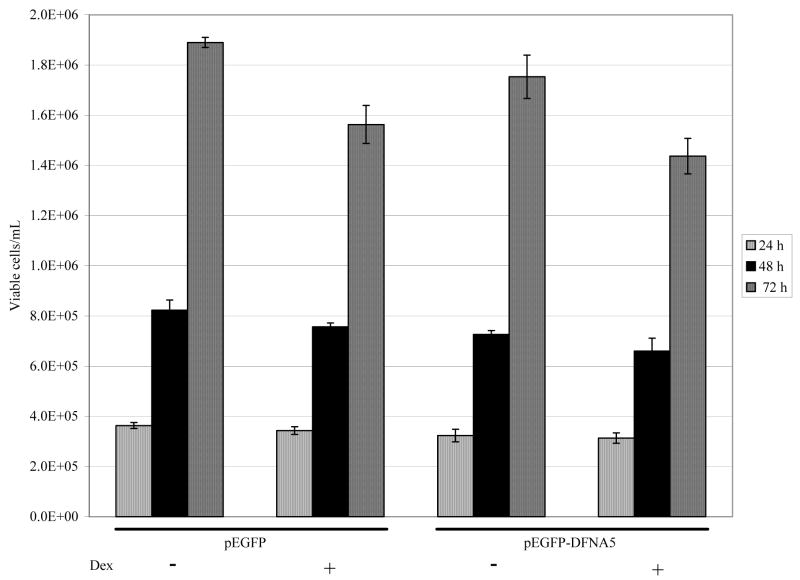
dfna5 mRNA levels were diminished in C7-14 cells by 60% (24 hours) or 75% (48 hours) following transfection of dfna5 siRNA (data not shown). Therefore, cells were transfected with dfna5 siRNA and assayed for sensitivity to Dex-dependent apoptosis over a 72 hour period. No effect on cellular sensitivity to Dex-dependent apoptosis was observed (Fig. 4). To see whether dfna5 mRNA would prove pro-apoptotic in resistant C1-15 cells, they were transfected with an dfna5 expression plasmid and followed for growth and apoptosis with or without Dex treatment. Cell growth and viability was unaffected by the transfection, and there was no change in cellular resistance to Dex (Fig. 5). Propidium iodine staining coupled with flow cytometry confirmed this result (data not shown).
Fig. 4.
Reduction of dfna5 mRNA does not prevent Dex-dependent apoptosis of sensitive CEM cells.
CEM C7-14 cells were electroporated with 250 nM Non-Targeting (NT) control or dfna5 siRNA followed by treatment (−/+ Dex). Viable cell numbers were then counted 24, 48 and 72 hours on a Vi-CELL cell viability analyzer. Non-specific siRNA (NT siRNA) at left, siRNA specific for dfna5 (DFNA5 siRNA) at the right.
Fig. 5.
Transfection of Dex-resistant CEM C1-15 cells with an expression plasmid for dfna5 (pEGFP-DFNA5) does not cause apoptosis or restore Dex sensitivity.
Cells were transfected and incubated 24 hours to allow expression of the DFNA5 protein, then resuspended to 2×105 cells/ml, divided and treated with or without 1 μM Dex. Viable cells were then counted 24, 48, and 72 hours later.
4. Discussion
The role of dfna5 in autosomal deafness is not understood. In part this is due to lack of an animal or cellular model in which its regulation can be studied conveniently. No deaf mouse mutants have been mapped to the mouse chromosome 6 region that encodes dfna5h, the mouse homolog [3]. Several leads to possible functions have been suggested, however. An effect of dfna5 on apoptosis was suggested by studies in melanoma cells, wherein resistance to etoposide-drive apoptosis was correlated with loss of dfna5 expression [24]. Re-expression of dfna5 by transfection into cells resistant to etoposide partially restored sensitivity. In breast cancer cell lines and patient samples, dfna5 (in this study termed ICERE-1, subsequently identified as dfna5) expression was found to be inversely correlated with estrogen receptor expression [25]. In gastric cancer, epigenetic repression of dfna5 has been documented [10], and the gene is part of the gasdermin family [8]. A yeast model for study of dfna5 was launched with notice that the gene contains a zinc-finger-like motif found in the protein encoded by Mcm10/dna43, a gene involved in DNA replication [6]. Otherwise, Mcm10 and dfna5 are dissimilar. It was noted that the insertion/deletion mutation in dfna5 that leads to deafness results in loss of the shared motif. Transfection and expression studies in yeast suggested that mutant DFNA5 caused a G1/S cell cycle block and influenced the function of the Mcm10 homolog, mutant yeast gene cdc23. Normal DFNA5 could not complement the mutant [4]. A direct intranuclear function of DFNA5 or the mutant DFNA5 seems unlikely, since both proteins showed cytoplasmic localization when labeled with green fluorescent protein and expressed in mammalian cells [26]. The authors hypothesized that the mutant DFNA5 lacking the information in exon 8 is a deleterious gain-of-function mutant. Transfection of the mutant gene into HEK293T cells doubled cell death (considered to be necrotic in nature), compared to that seen in cells transfected with the gene for normal DFNA5. This is a particularly confusing result in light of the above-mentioned results in melanoma cells, in which normal DFNA5 enhanced an apoptotic response to etoposide. Furthermore, our results in CEM cells show an increase in dfna5 mRNA following GC treatment of cells destined for apoptosis [21,22]. While this manuscript was in revision, it was reported that dfna5 is under the control of p53, a protein critical for cellular responses to DNA damage [11]. In response to repairable levels of damage, p53 causes a pause in the cell replicative cycle until the damage is repaired. After excessive DNA damage, p53 expression directs the cell to apoptosis. The connection between p53 and dfna5, coupled with our findings here, point to its potential importance for basic cell controls over DNA repair and apoptosis.
A particularly interesting recent finding in zebra fish embryos showed a connection between the fish homolog of dfna5, the production of hyaluronic acid (HA), and ear malformations [7]. HA is known to be important for ear development [27]. The gene was expressed ubiquitously quite early; then its expression became localized to the intermediate cell mass and certain brain or pre-brain centers. Later, expression was particularly high in brain and ear. Use of morpholino antisense constructs designed to block dfna5 expression or only to cause splicing out of exon 8 resulted in ear and jaw defects in the developing fish embryos. Pharyngeal cartilage development and inner ear epithelial cell columns were affected adversely, and this was traced to a reduction in HA levels. The dfna5 morphants showed a marked reduction in the enzyme uridine 5-diphosphate glucose dehydrogenase, upon which HA synthesis depends [28]. This finding potentially links the signaling functions of HA in the extracellular matrix to dfna5. The results also show that excess exon 8-deleted dfna5 expression can cause a reduction in ugdh mRNA levels. In short, DFNA5 may regulate the biosynthetic pathway for HA.
We have discovered that the dfna5 gene is expressed in leukemic lymphoid cells, in which it is induced by GCs in two clones sensitive to Dex-dependent apoptosis, but not in a resistant sister clone. We show by use of inhibitors of macromolecular synthesis that this induction probably is at the transcriptional level. The fact that blocking protein synthesis increases dfna5 mRNA suggests that either the gene is under transcriptional repression or that its mRNA stability is controlled by a protein that turns over relatively rapidly. In the Dex-resistant cell clone C1-15, FSK alone raises dfna5 mRNA to levels reached after treatment with the steroid in Dex-sensitive C7-14 cells. However, FSK alone does not cause apoptosis in C1-15 cells. Adding Dex with FSK greatly boosts the dfna5 mRNA level and results in apoptosis, as we have documented [23]. In our previous work, we have shown that FSK treatment activates PKA in our cell system and that non-hydrolysable analogs of cAMP also synergize with Dex to cause apoptosis [23]. We have ruled out involvement of EPAC, the alternative cAMP response pathway (unpublished results). We therefore hypothesize that the synergistic induction of dfna5 by FSK and Dex proceeds through activation of PKA. Reduction of dfna5 mRNA levels in sensitive cells does not block Dex-dependent apoptosis. Collectively, these data suggest that dfna5 expression may be involved in apoptosis when the Dex/glucocorticoid receptor pathway is affected by a complex of changes brought about by FSK treatment, through a PKA pathway that renders the cells Dex sensitive [23]. These results are of interest in that while dfna5 alone is not sufficient, it may be part of the complex machinery that leads to Dex-dependent apoptosis in leukemic lymphoid cells.
Our data show for the first time that dfna5 is a steroid and PKA sensitive gene. Inspection of the 5′ regulatory region, up to 2kb from the transcription start site of the dfna5 gene, shows that it contains a partial GC response element in proximity to AP-1 and Oct binding sites. Our results suggest that studies exploring the possibility of hormonal control of dfna5 in central nervous system cells pertinent to the autosomal deafness syndrome would be of value.
Acknowledgments
The authors express their appreciation to Rosemary Roque and Rhoda Thompson for their assistance in preparing this paper for submission. This work was supported by a grant CA41407 to E. Brad Thompson.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Van Camp G, Couke P, Balemans W, van Velzen D, van de Bilt B, van Laer L, Smith RJ, Fukushima K, Padberg GW, Frants RR. Localization of a gene for non-syndromic hearing loss (DFNA5) to chromosome 7p15. Hum Mol Genet. 1995;4:2159–2163. doi: 10.1093/hmg/4.11.2159. [DOI] [PubMed] [Google Scholar]
- 2.Van Laer L, can Camp G, van Zuijlen D, Green ED, Verstreken M, Schatteman I, van de Heyning P, Balemans W, Couke P, Greinwald JH, Smith RJ, Huizing E, Willems P. Refined mapping of a gene for autosomal dominant progressive sensorineural hearing loss (DFNA5) to a 2-cM region, and exclusion of a candidate gene that is expressed in the cochlea. Eur J Hum Genet. 1997;5:397–405. [PubMed] [Google Scholar]
- 3.Van Laer L, Huizing EH, Verstreken M, van Zuijlen D, Wauters JG, Bossuyt PK, van de Heyning P, McGuirt WT, Smith RJH, Willems P, Legan PK, Richardson GP, van Camp G. Nonsyndromic hearing impairment is associated with a mutation in DFNA5. Nat Genet. 1998;20:194–197. doi: 10.1038/2503. [DOI] [PubMed] [Google Scholar]
- 4.Yu C, Meng X, Zhang S, Zhao G, Hu L, Kong X. A 3-nuecleotide deletion in the polypyrimide tract of intron 7 of the DFNA5 gene causes noncyndromic hearing impairment in a Chinese family. Genomics. 2003;82:575–579. doi: 10.1016/s0888-7543(03)00175-7. [DOI] [PubMed] [Google Scholar]
- 5.Bischoff AM, Luijendijk MW, Huygen PL, van Duijnhoven G, De Leenheer EM, Oudesluijs GG, van Laer L, Cremers FP, Cremers CW, Kremer H. A novel mutation identified in the DFNA5 gene in a Dutch family: a clinical and genetic evaluation. Audiol Neuro-Otol. 2004;9:34–46. doi: 10.1159/000074185. [DOI] [PubMed] [Google Scholar]
- 6.Gregan J, Van Laer L, Lieto LD, Van Camp G, Kearsey SE. A yeast model for the study of human DFNA5, a gene mutated in nonsyndromic hearing impairment. Biochem Biophys Acta. 2003;1638:179–186. doi: 10.1016/s0925-4439(03)00083-8. [DOI] [PubMed] [Google Scholar]
- 7.Busch-Nentwich E, Sollner C, Roehl H, Nicolson T. The deafness gene dfna5 is crucial for ugdh expression and HA production in the developing ear in zebrafish. Development. 2004;131:943–951. doi: 10.1242/dev.00961. [DOI] [PubMed] [Google Scholar]
- 8.Sun Q, Zhang LQ, He FC. Progress of researchers on gene function of GSDMDC family. Yi Chuan. 2006;28(5):596–600. [PubMed] [Google Scholar]
- 9.Saeki N, Kuwahara Y, Sasaki H, Satoh H, Shiroishi T. Gasdermin (Gsdm) localizing to mouse Chromosome 11 is predominately expressed in upper gastrointestinal tract, but significantly suppressed in human gastric cancer cells. Mamm Genome. 2000;11:718–724. doi: 10.1007/s003350010138. [DOI] [PubMed] [Google Scholar]
- 10.Akino K, Toyota M, Suzuki H, Imai T, Maruyama R, Kusano M, Nishikawa N, Watanabe Y, Sasaki Y, Abe T, Yamamoto E, Tarasawa I, Sonoda T, Mori M, Imai K, Shinomura Y, Tokino T. Identification of DFNA5 as a target of epigenetic inactivation in gastric cancer. Cancer Sci. 2006;98:88–95. doi: 10.1111/j.1349-7006.2006.00351.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Masuda Y, Futamura M, Kamino H, Najamura Y, Kitamura N, Ohnishi S, Miyamoto Y, Ichikawa H, Ohta T, Ohki M, Kiyono T, Egami H, Baba H, Arakawa H. The potential role of DFNA5, a hearing impairment gene, in p53-medicated cellular response to DNA damage. Japan Soc Hum Genet Springer-Verlag. 2006;51:652–664. doi: 10.1007/s10038-006-0004-6. [DOI] [PubMed] [Google Scholar]
- 12.Rarey KE, Curtis LM, ten Cate WJ. Tissue specific levels of glucocorticoid receptor within the rat inner ear. Hear Res. 1993;64(2):205–210. doi: 10.1016/0378-5955(93)90007-n. [DOI] [PubMed] [Google Scholar]
- 13.Rarey KE, Curtis LM. Receptors for glucocorticoids in the human inner ear. Otolaryngol Head Neck Surg. 1996;115(1):38–41. doi: 10.1016/S0194-5998(96)70133-X. [DOI] [PubMed] [Google Scholar]
- 14.Shimazaki T, Ichimiya I, Suzuki M, Mogi G. Localization of glucocorticoid receptors in the murine inner ear. Ann Otol Rhinol Laryngol. 2002;111(12 Pt 1):1133–1138. doi: 10.1177/000348940211101213. [DOI] [PubMed] [Google Scholar]
- 15.Yao X, Buhi WC, Alvarez IM, Curtis LM, Rarey KE. De novo synthesis of glucocorticoid hormone regulated inner ear proteins in rats. Hear Res. 1995;86(1–2):183–188. doi: 10.1016/0378-5955(95)00069-g. [DOI] [PubMed] [Google Scholar]
- 16.Fukushima M, Kitahara T, Fuse U, Uno Y, Doi K, Kobo T. Changes in aquaporin expression in the inner ear of the rat after i.p. injection of steroids. Acta Oto-Laryngol Suppl. 2004;553:13–18. doi: 10.1080/03655230410017599. [DOI] [PubMed] [Google Scholar]
- 17.Cameron SA, Dutia MB. Lesion-induced plasticity in rat vestibular nucleus neurons dependent on glucocorticoid receptor activation. J Physiol. 1999;518(Pt 1):151–158. doi: 10.1111/j.1469-7793.1999.0151r.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tahera Y, Meltser I, Johansson P, Hansson AC, Canlon B. Glucocorticoid receptor and nuclear factor-kappa B interactions in restraint stress-mediated protection against acoustic trauma. Endocrinology. 2006;147(9):4430–4437. doi: 10.1210/en.2006-0260. [DOI] [PubMed] [Google Scholar]
- 19.Van Wijk F, Staecker H, Keithley E, Lefebvre PP. Local perfusion of the tumor necrosis factor alpha blocker infliximab to the inner ear improves autoimmune neurosensory hearing loss. Audiol Neuro-Otol. 2006;11(6):357–365. doi: 10.1159/000095897. [DOI] [PubMed] [Google Scholar]
- 20.Canlon B, Erichsen S, Nemlander E, Chen M, Hossain A, Celsi G, Ceccatelli S. Alterations in the intrauterine environment by glucocorticoids modifies the developmental programme of the auditory system. Eur J Neurosci. 2003;17(10):2035–2041. doi: 10.1046/j.1460-9568.2003.02641.x. [DOI] [PubMed] [Google Scholar]
- 21.Medh RD, Webb MS, Miller AL, Johnson BH, Fofanov Y, Li T, Wood TG, Luxon BA, Thompson EB. Gene expression profile of human lymphoid CEM cells sensitive and resistant to glucocorticoid-evoked apoptosis. Genomics. 2003;81:543–555. doi: 10.1016/s0888-7543(03)00045-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Webb MS, Miller AL, Johnson BH, Fofanov Y, Li T, Wood TG, Thompson EB. Gene networks in glucocorticoid-evoked apoptosis in leukemic cells. J Steroid Biochem Mol Biol. 2003;85:183–193. doi: 10.1016/s0960-0760(03)00194-8. [DOI] [PubMed] [Google Scholar]
- 23.Medh RD, Saeed MF, Johnson BH, Thompson EB. Resistance of human leukemic CEM-C1 cells is overcome by synergism between glucocorticoid and protein kinase A pathways: correlation with c-Myc suppression. Cancer Res. 1998;58:3684–3693. [PubMed] [Google Scholar]
- 24.Lage H, Helmbach H, Grottke C, Dietel M, Schadendorf D. DFNA5 (ICERE-1) contributes to acquired etoposide resistance in melanoma cells. FEBS Lett. 2001;494:54–59. doi: 10.1016/s0014-5793(01)02304-3. [DOI] [PubMed] [Google Scholar]
- 25.Thompson DA, Weigel RJ. Characterization of a gene that is inversely correlated with estrogen receptor expression (ICERE-1) in breast carcinomas. Eur J Biochem. 1998;252:169–177. doi: 10.1046/j.1432-1327.1998.2520169.x. [DOI] [PubMed] [Google Scholar]
- 26.Van Laer L, Vrijens K, Thys S, van Tendeloo VFI, Smith RJH, van Bockstaele DR, Timmermans JP, van Camp G. DFNA5: hearing impairment exon instead of hearing impairment gene? J Med Genet. 2004;41:401–406. doi: 10.1136/jmg.2003.015073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haddon C, Lewis J. Hyaluronan as a propellant for epithelial movement: the development of semicircular canals in the inner ear of Xenopus. Development. 1991;112:541–550. doi: 10.1242/dev.112.2.541. [DOI] [PubMed] [Google Scholar]
- 28.Roman E. Studies on the role of UDP-glucose dehydrogenase in polysaccharide biosynthesis. Comprehensive Summaries of Uppsala Dissertations from the Faculty of Medicine 13388 Acta Universitatis Upsaliensis Uppsala. 2004 [Google Scholar]