Abstract
Chronic obstructive pulmonary disease is characterized by a rapid decline in lung function due to small airway fibrosis, mucus hypersecretion and emphysema. The major causative factor for COPD is cigarette smoking that drives an inflammatory process that gives rise to leukocyte recruitment, imbalance in protease levels and consequently matrix remodeling resulting in small airway fibrosis and loss of alveolar tissue. Current drug treatment improves symptoms but do not alter the underlying progression of this disease. The failure of anti-inflammatory drugs like glucocorticosteroids to have a major impact in this disease has hastened the need to develop novel therapeutic strategies. Phosphodiesterase (PDE)4 inhibitors are novel anti-inflammatory drugs that have recently been show to document clinical efficacy in this disease, although their utility is hampered by class related side-effects of nausea, emesis and diarrhea. Whilst it is not yet clear whether such drugs will prevent emphysema, this is a distinct possibility provided experimental observations from preclinical studies translate to man. This review will discuss the current standing of PDE4 inhibitors like roflumilast as novel treatments for COPD and the potential for developing nonemetic anti-inflammatory drugs.
Keywords: COPD, neutrophil, roflumilast, cilomilast, PDE4, inflammation
Introduction
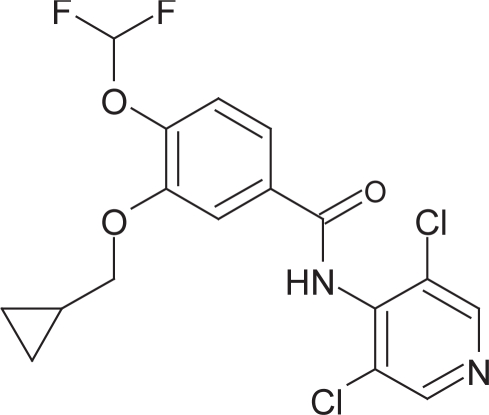
Chronic Obstructive Pulmonary Disease (COPD) is a respiratory condition associated with emphysema and chronic bronchitis. The major risk factor for the development of this disease is tobacco smoking and the most effective way to prevent the development of the disease is not to smoke (Lenfant and Khaltaev 2005; Bergeron and Boulet 2006). COPD is currently diagnosed in 4% of men and 2% of women and if these trends continue is predicted to be the sixth leading cause of death worldwide by 2020 as the number of people who smoke worldwide continues to rise, although other factors including exposure to smoke from biomass fuels particularly in developing countries may also contribute toward disease morbidity (Pauwels et al 2005; Lenfant and Khaltaev 2005). The direct economic cost of COPD in the United Kingdom based on 1996 figures has been estimated at approximately £846 million and the disease accounts for a large proportion of long-term NHS hospitalizations (Pauwels et al 2005; Lenfant and Khaltaev 2005; SRBS 2006). The drugs used in the treatment of COPD have not appreciably changed in the last 25 years and as a result there is clearly a need for novel therapeutic agents to reduce disease progression and possibly reverse the decline in lung function seen with this disease (Barnes and Stockley 2005). Phosphodiesterase (PDE)4 inhibitors are a novel class of drug in development for the treatment of COPD and to date the most promising compound to arise from preclinical and clinical development is Roflumilast (Daxas®; Figure 1) and this review will focus on the clinical experience with this drug for the treatment of COPD.
Figure 1.
Structure of roflumilast.
Chronic obstructive pulmonary disease
Although the diagnosis and mortality rate of COPD continues to increase there are currently no drugs available which can treat the inflammation and emphysematous change underlying this chronic disease. In addition, the majority of the drugs used to treat COPD were not specifically designed solely to treat this condition but were developed to treat asthmatics and therefore may not be ideal therapeutics. Drugs frequently used in the management of COPD as recommended by the World Health Authority (WHO) and GOLD include β2-agonists such as salbutamol and salmeterol, anti-cholinergic agents including ipatropium bromide and tiotropium bromide, methylxanthines and inhaled glucocorticosteroids (Pauwels et al 2005; Lenfant and Khaltaev 2005). Although these drugs have proved to reduce exacerbations and reduce symptoms there is little evidence to suggest they can reduce the progression of this disease. Other pharmacologic interventions frequently used in the management of COPD include antibiotics, mucolytic agents, antioxidants, immunoregulators, anti-tussives, respiratory stimulants and vasodilators. Therefore, there is a real requirement for drugs which can prevent the further damage to and loss of alveoli by targeting the inflammatory processes which are activated in the COPD lung.
The major genetic cause of COPD is α1 anti-trypsin deficiency, which can lead to early onset emphysema particularly in individuals who also smoke. However, this deficiency accounted for a relatively small proportion of diagnosed cases (Stoller and Aboussouan 2005) and the major environmental risk factor underlying the development of COPD is cigarette smoking. It has been estimated that between 15%–20% of smokers will develop this disease (Pauwels et al 2005). Inhaled cigarette smoke activates resident cells in the lungs including epithelial cells and alveolar macrophages which have the capacity to release cytokines, chemokines and lipid mediators including TNFα, IL-8, TGF-β, and LTB4 resulting in the recruitment and activation of inflammatory cells which subsequently release a cocktail of proteases into the matrix compartment that can provoke a complex remodelling process leading to alveolar wall destruction, mucus hypersecretion and peribronchiolar fibrosis (Barnes et al 2003; Hogg 2004; Barnes and Stockley 2005; Bergeron and Boulet 2006). Over time, perpetuation of the inflammatory response by continual exposure to cigarette smoke in combination with impaired repair processes can manifest in the pathology and clinical symptoms of COPD. In addition, it is evident that exposure to tobacco smoke can induce an oxidant/antioxidant imbalance in favor of oxidative stress. There is also evidence that although smoking cessation can be beneficial, inflammation can perpetuate in patients with COPD long after they stop smoking (Retamales et al 2001; Shapiro 2001). Therefore, targeting the inflammation and remodeling processes associated with COPD may slow down disease progression and potentially reverse the decline in lung function in these patients.
Whilst smoking cessation and abstinence provide the best approach for the treatment of this disease the pharmaceutical industry are developing molecules which target a number of mediators including LTB4, IL-8, TNF-α, and proteases which are known to contribute to the lung damage within this disease (Barnes and Stockley 2005), however, the clinical utility of targeting a single mediator is unlikely to be of any therapeutic benefit due to the overlapping roles played by the various mediators released within the inflammatory milieu. Anti-inflammatory drugs like glucocorticosteroids which are know to inhibit the activity of inflammatory cells and production of cytokines and chemokines might be expected to be of benefit in this disease. However, the clinical experience with this particular drug class in COPD has been disappointing and it has been suggested that the lack of efficacy of these drugs in this disease is attributable to a molecular defect in histone deacetylase activity (Ito et al 2005). As a consequence, other anti-inflammatory drugs are currently being developed for the treatment of COPD which include the PDE4 inhibitors. The advantage of this approach is that drugs of this nature have the ability of suppressing the release of a wide range of inflammatory mediators, cytokines, chemokines, and proteases from cells implicated in this disease and therefore, offer significant advantages over drugs that target single mediators or single biochemical pathways.
PDE4: relevance to COPD
Phosphodiesterases are ubiquitous enzymes which hydrolyse the cyclic nucleotides; cyclic adenosine-3,5- monophosphate (cAMP) and cyclic guanosine -3,5- monophosphate (cGMP), to their inactive 5′nucleotide monophosphate, 5′ AMP and 5′ GMP respectively (Essayan 2001). A great deal is now known about the phosphodiesterase enzymes and eleven families (1–11) have now been identified based on substrate specificity, enzyme kinetics and distribution and inhibitor potency (Essayan 2001). PDE4 is a cAMP specific phosphodiesterase which is located predominantly to inflammatory cells, including cells important to the pathogenesis of COPD such as neutrophils, macrophages, lymphocytes, fibroblasts and airway smooth muscle. PDE4 is encoded by 4 genes PDE4A, PDE4B, PDE4C, and PDE4D each of which has unique cellular distribution and function (Houslay 2001). Elevation of cAMP in structural and inflammatory cells within the lung can provide an anti-inflammatory action in the context of COPD and this can be achieved by inhibiting PDE4 in target cells (Sanz et al 2005). For example, PDE4 inhibitors like roflumilast (Figure 1) suppressed a variety of functions ascribed to neutrophils including protease (neutrophil elastase, matrix metalloprotease 9) and myeloperoxidase release (Jones et al 2005); oxygen radical production (Hatzelmann and Schudt 2001); and adhesion to endothelial cells (Jones et al 2005) by inhibiting the expression of β2-integrins in these cells (Derian et al 1995). Similarly, PDE4 inhibitors including roflumilast suppress the proliferation and cytokine release from CD4+, CD8+ lymphocytes (Hatzelmann and Schudt 2001; Landells et al 2001; Smith et al 2004) that may involve inhibition of several intracellular signalling pathways including activation of NF-κB a well known pro-inflammatory transcription factor (Kwak et al 2005). Additionally, cytokine release from mononuclear cells, including that of the pro-inflammatory cytokine TNF-α, which is elevated in sputum from patients with COPD (Keatings et al 1996), can be inhibited by PDE4 inhibitors (Hatzelmann and Schudt 2001). Together these studies indicate that inflammatory cell recruitment and activity can be inhibited by PDE4 inhibitors.
The effect of PDE4 inhibitors upon the activity of resident cells within the lung including, fibroblasts, macrophages, and epithelial cells, which are relevant to COPD has also been investigated. Whilst macrophages do express PDE4 it appears that their function is poorly inhibited by PDE4 inhibitors, although the efficacy of these drugs is enhanced when used in combination with agents that raise intracellular levels of cyclic AMP (eg, prostaglandin E2) or if there is concurrent inhibition of PDE3 and PDE7 (Hatzelmann and Schudt 2001; Smith et al 2004). The expression of PDE3 and PDE7 is increased during differentiation of monocytes to macrophages and thereby play a greater role in regulating intracellular levels of cyclic AMP in this cell type (Hatzelmann and Schudt 2001; Smith et al 2004). An important implication of these experimental observations is that PDE4 inhibitors may only be partially effective in COPD in view of the proposed role of macrophages in this disease (Barnes and Stockley 2005) and there is therefore a case for the potential development of dual PDE inhibitors in the context of COPD (Smith et al 2004; Boswell-Smith et al 2006).
The proposed role of macrophage derived MMP12 in the emphysematous changes to the lung in chronic smoking models in mice (Shapiro et al 2003) would suggest that PDE4 inhibitors will be ineffective at inhibiting emphysematous changes to the lung. However, this is not consistent with the findings from a number of preclinical models indicating that PDE4 inhibitors can prevent the development of emphysema in these models (Martorana et al 2005; Leclerc et al 2006) and whilst the exact molecular target was not identified, the suppression of TNFα production and downstream signalling by this cytokine is inferred, in view of the important role this cytokine plays in the development of emphysema in this model (Churg et al 2004). Further studies are required to determine the precise role of PDE4 in modulating MMP12 synthesis and release from alveolar macrophages. Other studies have demonstrated that PDE4 inhibitors can suppress fibroblast chemotaxis and contraction of native collagen gels, a measure of fibroblast activity (Kohyama et al 2002) and the release of two matrix metalloproteases, pro-MMP-1 and 2, from activated isolated human lung fibroblasts (Martin-Chouly et al 2004) which is of particular interest in view of the potential role of MMP’s in the development of small airway fibrosis and emphysema (Barnes et al 2003). Together, these studies demonstrate that PDE4 inhibitors have the potential to modulate the structural changes induced by these proteases in the lung by preventing their release from the appropriate recruited and or resident cell.
The release of the pro-inflammatory mediators PGE2, IL-8 and 15-HETE from isolated epithelial cells was unaffected by the PDE4 inhibitors rolipram and roflumilast (Dent et al 1998; Fuhrmann et al 1999). However, rolipram suppressed bacterial-induced epithelial damage of the bronchial mucosa and cilomilast suppressed TNF-α release from epithelial cells taken from COPD patients (Profita et al 2003). In addition roflumilast attenuated epithelial growth factor-induced MUC5AC mRNA and protein expression in isolated human airway epithelial cells providing evidence that PDE4 inhibitors may suppress mucus secretion in airways disease (Mata et al 2005). Collectively, these data demonstrate that in isolated cell populations these drugs demonstrate potent anti-cellular activity.
With respect to airways smooth muscle, there is little evidence that PDE4 inhibitors including roflumilast are potent relaxant agonists. Human bronchial preparations gain tone spontaneously and this is mediated by the contractile effects of endogenously liberated leukotrienes and histamine (Ellis and Undem 1994). However, PDE4 inhibitors only weakly suppressed spontaneously generated tone, or contractions of isolated human airways smooth muscle in response to allergen, LTC4, and histamine, although this functional antagonism is significant when combined with a PDE3 inhibitor (Schmidt et al 2000). These data suggest that PDE4 inhibitors would not be expected to have significant bronchodilator efficacy when administered alone (Engelstatter et al 2005) and in allergic asthma, roflumilast demonstrated very modest activity in preventing acute bronchoconstriction to allergen challenge (Van Schalkwyk et al 2005) which is in stark contrast to the well known bronchoprotective activity of β2-adrenoceptor agonists.
There are no satisfactory animal models of COPD and so there is a general reliance on using models that measure the effect of anti-inflammatory drugs on neutrophil recruitment to the airways in response to a given stimulus (eg, LPS) although it is clear that this particular stimulus may not be discriminatory for the development of anti-inflammatory drugs for the treatment of COPD (Leclerc et al 2006). The effect of drugs on matrix turnover and tissue damage is being used to assess the potential utility of novel agents against emphysematous changes seen with following cigarette smoke exposure (Churg et al 2002; Leclerc et al 2006), although very few studies attempt to measure changes in pulmonary mechanics (eg, lung compliance) as an index of any physiological change of lung function to the various insults directed against the lung. Notwithstanding these challenges, a number of preclinical studies have shown that PDE4 inhibitors have the capacity to suppress the recruitment of neutrophils (Bundschuh et al 2001; Spond et al 2001; Leclerc et al 2006), and mucus hypersecretion (Sturton and Fitzgerald 2002) in rodents challenged with LPS; and reverse sub epithelial fibrosis and hypertrophy in a murine model of asthma (Kumar et al 2003). Furthermore, PDE4 inhibitors including roflumilast prevented parenchymal damage and suppressed indices of inflammation in mice exposed to cigarette smoke indicating that these drugs could prevent further emphysematous changes to the lung in COPD (Martorana et al 2005; Leclerc et al 2006).
Despite these very positive preclinical findings, the clinical development of PDE4 inhibitors has progressed slowly due to the dose limiting side effects associated with this drug class. Cilomilast is 10-fold more selective for PDE4D compared with other PDE4 subtypes, and might explain its propensity to cause gastrointestinal adverse events that include nausea and diarrhoea (Rennard et al 2006). Similarly, whilst roflumilast does not demonstrate any PDE4 subtype selectivity and is two orders of magnitude more potent than cilomilast (Hatzelmann and Schudt 2001), COPD patients treated with this drug also experience side-effects like nausea and diarrhea (Rabe et al 2005). Interestingly, the adverse events experienced by individuals appeared to diminish over the course of the study and the mechanistic basis of this observation remains to be established (Rabe et al 2005). The presence of PDE4 in parietal cells and emetic centres (area postrema, nucleus tractus solitaris) provide an explanation for the incidence of these side effects following administration of this drug class. There has been some attempt to ascertain whether specific PDE4 subtypes exist in these regions and therefore offer the possibility of developing nonemetic subtype selective PDE4 inhibitors. Both PDE4B and PDE4D are found within the nucleus tractis solitaris in man and rodents (Takahashi et al 1999; Cherry and Davis 1999; Perez-Torres et al 2000) whilst studies in the squirrel monkey have only demonstrated PDE4D in the area postrema with many neurones also positive for substance P (Lamontagne et al 2001). Since PDE4B and not PDE4D appear to play an important role in neutrophilic inflammation (Jin and Conti 2002), suggests that PDE4 inhibitors with low selectivity for PDE4D could prove to be novel nonemetic anti-inflammatory drugs (Lamontagne et al 2001). It remains to be established however, to what extent PDE4B plays a role in nausea and emesis given its presence within the nucleus tractus solitaris (Perez-Torres et al 2000; Lamontagne et al 2001) and this issue will only be resolved by examining the effect of PDE4 subtype selective inhibitors in models of emesis.
Clinical findings with roflumilast
Two potent PDE4 inhibitors cilomilast (Ariflo®) and roflumilast (Daxas®) and have progressed farthest in development and are in late Phase III clinical trials (Lipworth 2005; Giembycz 2006) but only the findings for roflumilast will be considered in this review (Table 1). Roflumilast has undergone wide-scale clinical investigation in patients with COPD. It is an orally-active compound (bioavailability 79%) and can be administered once daily due to its favorable pharmacokinetics. Roflumilast is metabolized to its active metabolite roflumilast N-oxide which is only 2–3-fold less potent than the parent compound, and whose plasma levels remain elevated for up to 24 hours (David et al 2004). Roflumilast is safe and well tolerated and no significant interactions with other drugs routinely prescribed to COPD patients including salbutamol (Weimar et al 2002), budesonide (Hunnemeyer et al 2002a) and warfarin (Hauns et al 2003) have been reported. Furthermore, roflumilast has no reported interactions with cigarette smoke, which makes it safe for smokers to use (Hunnemeyer et al 2002b).
Table 1.
Summary of the clinical findings with roflumilast in COPD
| Study | Trial design | Primary outcome measurements | Findings | Reference |
|---|---|---|---|---|
| RECORD (1411 subjects) | Post-bronchodilator FEV1 (30–80%predicted value)
Roflumilast (250 μg, 500 μg) versus placebo 24 weeks |
Post-bronchodilator FEV1 St Georges respiratory Questionnaire scores (SGRQ) scores |
Post-bronchodilator FEV1 significantly improved compared with placebo
(250 μg: 74 ml [SD 18] and 500 μg 97 ml [SD18]) SGRQ improved though no difference between groups (Roflumilast 250 μg; −3.4 units; 500 μg; −3.5 units; placebo −1.8 units) |
Rabe et al 2005 |
| RATIO/M2-112 (1513 subjects) | Severe and very severe COPD
Roflumilast (500 μg), 52 weeks |
Post-bronchodilator FEV1 Frequency of moderate and severe exacerbations |
Significant improvement in FEV1 7% reduction in total exacerbations (P > 0.05) 18% Reduction in moderate exacerbations (P < 0.0.0147) |
Altana 2005 |
| Bronchodilator activity (15 subjects) | Mild to moderate asthmatics
Roflumilast (500 μg; 1000 μg versus placebo) 3 period (one day each) placebo controlled cross over study (7–14 day washout period) |
FEV1 measured for up to 6 h post treatment | Neither dose of roflumilast significantly altered FEV1 vs placebo
No evidence of direct bronchodilator activity |
Engelstatter et al 2005 |
| Sputum Analysis (38 subjects) | Post-bronchodilator FEV1 61% predicted
4 week crossover study 500 μg roflumilast placebo |
Sputum sample cell counts IL-8 and Neutrophil Elastase levels | Reduction in number of: neutrophils (35%) eosinophils (50%)
Reduction in the levels of IL8 (25.9%) neutrophil elastase (30.6%) |
Grootendorst et al 2005 |
| Withdrawal Study (581 subjects) | FEV1 35%–75% predicted
Roflumilast (500 μg) versus placebo for 12 weeks |
Post-bronchodilator FEV1 | In patients withdrawing from roflumilast FEV1 slowly declined over weeks but remained above placebo levels | Boszormenyi-Nagy et al 2005 |
| Safety (397 subjects) | Post-bronchodilator FEV1 (35%–75% predicted)
Roflumilast (500 μg), 52 weeks |
Adverse events | 49% during double blind period (same frequency all treatment groups) 41% during open label study.
Most mild to moderate and transient in nature >90% unrelated to study drug 10 patients (2.5%) experienced 13 adverse events which were definitely related to study drug. 3 lead to study discontinuation |
Bateman et al 2004 |
Abbreviations: COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in one second; IL, interleukin; SD, standard deviation.
The efficacy of roflumilast was assessed by monitoring forced expiratory volume in one second (FEV1), forced vital capacity (FVC), morning peak expiratory flow (PEF) and exacerbation rates in a double-blind, placebo controlled trial of 516 mild-moderate COPD subjects (post bronchodilator FEV1 between 35 and 75%) given either placebo, or roflumilast (250 μg or 500 μg) once daily for 26 weeks. Roflumilast dose-dependently improved lung function and exacerbation rates (48% reduction in 500 μg group) (Leichtl et al 2002a). Importantly, roflumilast was safe and well tolerated with the most frequently described adverse events being nausea (2%), headache (2%) and diarrhoea, which were mostly mild – moderate in nature (Leichtl et al 2002b).
In a larger multi-centre 24 week trial (OPUS trial), 1411 patients were administered either roflumilast (250 μg or 500 μg) or placebo once daily. Outcome measurements in this study included lung function, health related quality of life using the St George’s Respiratory Questionnaire (SGRQ) and exacerbation rate. There was a significant improvement in post-bronchodilator FEV1 in the treatment groups compared with the control groups. Furthermore, quality of life scores also improved in the roflumilast-treated patients compared with patients treated with placebo; although this was not found to be significant. Comparison of the mean exacerbation rates demonstrated this was 34% lower in the roflumilast (500 μg) group compared with the placebo treated group (Rabe et al 2005). Recent results released by Altana (2005) in a press release from another Phase III clinical trial (RATIO study) of 1513 patients with severe and very severe COPD were also encouraging. Roflumilast (500 μg) treatment significantly improved post-bronchodilator FEV1 in these more severe patients and induced significant reduced moderate exacerbations of COPD although it did not significantly affect the overall exacerbation rate (Altana 2005).
In a further placebo controlled study, 581 subjects with COPD were given roflumilast (500 μg) or placebo for the full 24 weeks of study or roflumilast for the first 12 weeks followed by placebo for the remaining 12 weeks. Roflumilast significantly improved post-bronchodilator FEV1 compared with placebo (p = 0.0003) which were maintained throughout the 24 weeks of study. In the patients where roflumilast was withdrawn after 12 weeks, there was a slow decline in FEV1 but it still remained above placebo levels by the end of the study (Boszormenyi-Nagy et al 2005).
Since PDE4 inhibitors are weak bronchoprotective drugs it seems highly probable that these small improvements in FEV1 can be attributed to the anti-inflammatory properties of these drugs. In a study specifically investigating evidence of anti-inflammatory activity by roflumilast, 38 patients with COPD were treated with roflumilast (500 μg od) or placebo for 4 weeks. Samples of sputum were collected at week 0, 2 and 4 with assessment of inflammatory cell count, IL-8 levels and neutrophil elastase levels assessed at each time point. By week 4 roflumilast had significantly reduced the number of neutrophils and eosinophils (38% and 50%, respectively) and furthermore, there was a concomitant reduction in both neutrophil elastase and IL-8 levels (Grootendorst et al 2005). This data supports the anti-inflammatory mode of action of PDE4 inhibitors observed in preclinical studies and is also consistent with a study which examined sputum inflammatory cell counts and inflammatory cell infiltration into airway tissue in biopsies from patients administered cilomilast (15 mg) twice daily for 12 weeks. Although cilomilast did not affect sputum cell numbers it significantly reduced CD8+ lymphocyte and macrophage infiltration into the airways confirming the anti-inflammatory nature of this drug class (Gamble et al 2003) and this appears to correlate with the reported beneficial effect of cilomilast in COPD (Rennard et al 2006) indicating a class effect.
In the clinical studies described above (summarized in Table 1) there was a relatively low incidence of adverse events and the side effects reported were mostly mild and transient in nature. Examination of the data from a long term safety study conducted in patients with COPD showed there was no significant difference in the incidence of adverse effects between the placebo control and drug treatment group (49% during the first 26 weeks, 41% thereafter). Greater than 90% of these adverse drug events were deemed to be unrelated to drug treatment, although this still suggests that nearly 10% of adverse events were a result of roflumilast treatment. During the open label extension 10 patients (2.5%) experienced adverse side effects which were definitely related to roflumilast and 3 of these patients dropped out of the study (Bateman et al 2004). Nevertheless, this still represents a small fraction of the total patients on roflumilast and demonstrates that although there is still an incidence of class associated side effects, in general administration of roflumilast for up to 52 weeks is safe and well tolerated. One further concern with long-term administration of PDE4 inhibitors is the development of arteriopathy. Arteriopathy is a condition associated with arterial necrosis and has been observed in rats after treatment with large doses of many vasodilators including PDE3 and PDE4 inhibitors, adenosine agonists, endothelin receptor antagonists and potassium channel openers (Spina 2004). Nevertheless, the mechanisms by which these agents induce arteriopathy in rats remain to be established, as is whether rats are more susceptible to this condition that other species. However, 3 month treatment with rising, high doses of the PDE4 inhibitor N-(3,5-Dichloro-1-oxido-4-pyridinyl)-8-methoxy-2-(trifluoromethyl)-5-quinoline carboxamide (SCH 351591; 12, 24, and 48 mg/kg) to cynomolgus monkeys was also associated with inflammation of the arterioles in many organs and tissues including the mesentery (Losco et al 2004). These data suggest that arteriopathy resulting from PDE4 inhibitors is not peculiar to the rat and can also be observed in nonhuman primates, previously thought resistant to such toxicity.
These effects observed in animals, are not anticipated to be observed in clinical trials as the dose used in humans is likely to be a fraction of that used in these toxicity studies. Furthermore, theophylline, which has long been used to treat asthma and COPD for over half a century, can also induce arteriopathy in rats, with no evidence of this occurring in humans. Nonetheless, there remains the concern that PDE4 inhibitors may induce mesenteric inflammation in patients with asthma and COPD, particularly as gastrointestinal disturbances are the predominant adverse effect see with these drugs, although there was no evidence of arteriopathy in patients treated with cilomilast (Giembycz 2006).
The data presented in these studies shows that roflumilast is eliciting a moderate anti-inflammatory effect in patients with COPD. However, the dose which can be administered to patients is limited by the side effects associated with the drug and thus the full potential of this drug may not be realized. Nonetheless, roflumilast is a new type of treatment which specifically targets the underlying inflammation, mucus secretion, and exacerbations associated with COPD. Furthermore quality of life scores, as assessed by the St George’s Healthcare Questionnaire improved in patients taking roflumilast, whereas they declined in patients administered placebo. The improvements in post-bronchodilator FEV are less impressive, but given that COPD is associated with irreversible airways obstruction, this is not surprising given that unlike beta2-adrenoceptor agonists PDE4 inhibitors are poor bronchoprotective agents. The difficulty with any anti-inflammatory drug development programme for COPD is the choice of a suitable clinical outcome measure. This is evident from the recent correspondence in the Lancet concerning the utility of roflumilast in treating COPD where it has (Rabe et al 2005). Nevertheless, if roflumilast can attenuate inflammation and suppress mucus secretion and prevent perpetuation of the decline in lung function in COPD patients then it could be deemed a successful drug. An application for regulatory approval was submitted for Daxas® in the treatment of asthma and COPD to the European Medicines Agency (EMEA) in March 2005. However, in November 2005 this application was withdrawn until further clinical trials are completed and so it is not clear when this drug will be licensed.
Conclusion
It is clear from the few biopsy studies that have been undertaken that PDE4 inhibitors induce modest changes in a number of important biochemical markers of this disease (eg, IL8, neutrophil elastase) that correlated with improvements in exacerbation rates, lung function and quality of life, however, it remains to be established whether this drug class can prevent small airway fibrosis and emphysema. Nonetheless, the clinical experience with roflumilast suggests that PDE4 inhibitors with greater tolerability can be developed with improved clinical efficacy for the treatment of COPD.
References
- [SRBS] A statistics Report from the British Society The burden of lung disease [online] 2006 Accessed September 20, 2006. URL: http://www.brit-thoracic.org.uk/BurdenofLungDisease2.html.
- Altana 2005. Top line results of first one year study with Daxas [online]. Accessed September 20, 2006. URL: http://www.altana.com/root/index.php?page_id=202&cms_press_id=355
- Barnes PJ, Shapiro SD, Pauwels RA. Chronic obstructive pulmonary disease: molecular and cellular mechanisms. Eur Respir J. 2003;4:672–88. doi: 10.1183/09031936.03.00040703. [DOI] [PubMed] [Google Scholar]
- Barnes PJ, Stockley RA. COPD: current therapeutic interventions and future approaches. Eur Respir J. 2005;6:1084–106. doi: 10.1183/09031936.05.00139104. [DOI] [PubMed] [Google Scholar]
- Bateman ED, Holmes M, Muir JF, et al. Safety profile of roflumilast, a novel, selective phosphodiesterase4 inhibitor, in patients with moderate to severe COPD. Am J Respir Crit Care Med. 2004:A596. [Google Scholar]
- Bergeron C, Boulet LP. Structural changes in airway diseases: characteristics, mechanisms, consequences, and pharmacologic modulation. Chest. 2006;4:1068–87. doi: 10.1378/chest.129.4.1068. [DOI] [PubMed] [Google Scholar]
- Boswell-Smith V, Spina D, Oxford AW, et al. The pharmacology of two novel long-acting phosphodiesterase 3/4 inhibitors, RPL554 [9,10-dimethoxy-2(2,4,6-trimethylphenylimino)-3-(n-carbamoyl-2-aminoethyl) -3,4,6,7-tetrahydro-2H-pyrimido[6,1-a]isoquinolin-4-one] and RPL565 [6,7-dihydro-2-(2,6-diisopropylphenoxy)-9,10-dimethoxy-4H-pyrimido[6,1-a]i soquinolin-4-one] J Pharmacol Exp Ther. 2006;2:840–8. doi: 10.1124/jpet.105.099192. [DOI] [PubMed] [Google Scholar]
- Boszormenyi-Nagy G, Pieters WR, Steffen H, et al. The effect of roflumilast treatment and subsequent withdrawal in patients with COPD. Proc Am Thorac Soc. 2005:A544. [Google Scholar]
- Bundschuh DS, Eltze M, Barsig J, et al. In vivo efficacy in airway disease models of roflumilast, a novel orally active PDE4 inhibitor. J Pharmacol Exp Ther. 2001;1:280–90. [PubMed] [Google Scholar]
- Cherry JA, Davis RL. Cyclic AMP phosphodiesterases are localized in regions of the mouse brain associated with reinforcement, movement and affect. J Comp Neurol. 1999;407:287–301. [PubMed] [Google Scholar]
- Churg A, Wang RD, Tai H, et al. Tumor necrosis factor-alpha drives 70% of cigarette smoke-induced emphysema in the mouse. Am J Respir Crit Care Med. 2004;5:492–8. doi: 10.1164/rccm.200404-511OC. [DOI] [PubMed] [Google Scholar]
- Churg A, Zay K, Shay S, et al. Acute cigarette smoke-induced connective tissue breakdown requires both neutrophils and macrophage metalloelastase in mice. Am J Respir Cell Mol Biol. 2002;3:368–74. doi: 10.1165/rcmb.4791. [DOI] [PubMed] [Google Scholar]
- David ME, Zech K, Seiberling A, et al. Roflumilast, a novel, oral, selective PDE4 inhibitor, shows high absolute bioavalability. J Allergy Clin Immunol. 2004;2:780. [Google Scholar]
- Dent G, White SR, Tenor H, et al. Cyclic nucleotide phosphodiesterase in human bronchial epithelial cells: characterization of isoenzymes and functional effects of PDE inhibitors. Pulm Pharmacol Ther. 1998;1:47–56. doi: 10.1006/pupt.1998.0115. [DOI] [PubMed] [Google Scholar]
- Derian CK, Santulli RJ, Rao PE, et al. Inhibition of chemotactic peptide-induced neutrophil adhesion to vascular endothelium by cAMP modulators. J Immunol. 1995;1:308–17. [PubMed] [Google Scholar]
- Ellis JL, Undem BJ. Role of cysteinyl-leukotrienes and histamine in mediating intrinsic tone in isolated human bronchi. Am J Respir Crit Care Med. 1994;1:118–22. doi: 10.1164/ajrccm.149.1.8111568. [DOI] [PubMed] [Google Scholar]
- Engelstatter R, Wingertzahn M, Schmid-Wirlitsch C, et al. Roflumilast, an oral, once-daily phosphodiesterase 4 (PDE4) inhibitor, does not exhibit bronchodilatory activity. Ann Allergy Asthma Immunol. 2005;1:159. [Google Scholar]
- Essayan DM. Cyclic nucleotide phosphodiesterases. J Allergy Clin Immunol. 2001;5:671–80. doi: 10.1067/mai.2001.119555. [DOI] [PubMed] [Google Scholar]
- Fuhrmann M, Jahn HU, Seybold J, et al. Identification and function of cyclic nucleotide phosphodiesterase isoenzymes in airway epithelial cells. Am J Respir Cell Mol Biol. 1999;2:292–302. doi: 10.1165/ajrcmb.20.2.3140. [DOI] [PubMed] [Google Scholar]
- Gamble E, Grootendorst DC, Brightling CE, et al. Antiinflammatory effects of the phosphodiesterase-4 inhibitor cilomilast (Ariflo) in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2003;8:976–82. doi: 10.1164/rccm.200212-1490OC. [DOI] [PubMed] [Google Scholar]
- Giembycz MA. An update and appraisal of the cilomilast Phase III clinical development programme for chronic obstructive pulmonary disease. Br J Clin Pharmacol. 2006;2:138–52. doi: 10.1111/j.1365-2125.2006.02640.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grootendorst DC, Gauw SA, Sterk PJ, et al. Treatment with PDE4 Inhibitor Roflumilast Reduces Sputum Neutrophil and Eosinophil Numbers in Patients with COPD. Proc Am Thorac Soc. 2005:A543. doi: 10.1136/thx.2006.075937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatzelmann A, Schudt C. Anti-inflammatory and immunomodulatory potential of the novel PDE4 inhibitor roflumilast in vitro. J Pharmacol Exp Ther. 2001;1:267–79. [PubMed] [Google Scholar]
- Hauns B, et al. Investigation of pharmacokinetics of roflumilast and roflumilast N-oxide in healthy subjects after a single morning or evening oral administration of 500æg roflumilast in healthy subjects-an open, randomised, two-period crossover study. Am J Respir Crit Care Med. 2003;7:A92. [Google Scholar]
- Hogg JC. Pathophysiology of airflow limitation in chronic obstructive pulmonary disease. Lancet. 2004;9435:709–21. doi: 10.1016/S0140-6736(04)16900-6. [DOI] [PubMed] [Google Scholar]
- Houslay MD. PDE4 cAMP-specific phosphodiesterases. Prog Nucleic Acid Res Mol Biol. 2001:249–315. doi: 10.1016/s0079-6603(01)69049-4. [DOI] [PubMed] [Google Scholar]
- Hunnemeyer A, et al. No interaction of roflumilast and its active metabolite, roflumilast N-oxide, with inhaled budesonide. Am J Respir Crit Care Med. 2002a;8 [Google Scholar]
- Hunnemeyer A, Hauns B, Drollman A, et al. Pharmacokinetics of roflumilast and its active metabolite roflumilast N-oxide is not influenced by smoking. Am J Respir Crit Care Med. 2002b;8:A594. [Google Scholar]
- Ito K, Ito M, Elliott WM, et al. Decreased histone deacetylase activity in chronic obstructive pulmonary disease. N Engl J Med. 2005;19:1967–76. doi: 10.1056/NEJMoa041892. [DOI] [PubMed] [Google Scholar]
- Jin SL, Conti M. Induction of the cyclic nucleotide phosphodiesterase PDE4B is essential for LPS-activated TNF-alpha responses. Proc Natl Acad Sci U S A. 2002;11:7628–33. doi: 10.1073/pnas.122041599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones NA, Boswell-Smith V, Lever R, et al. The effect of selective phosphodiesterase isoenzyme inhibition on neutrophil function in vitro. Pulm Pharmacol Ther. 2005;2:93–101. doi: 10.1016/j.pupt.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Keatings VM, Collins PD, Scott DM, et al. Differences in interleukin-8 and tumor necrosis factor-alpha in induced sputum from patients with chronic obstructive pulmonary disease or asthma. Am J Respir Crit Care Med. 1996;2:530–4. doi: 10.1164/ajrccm.153.2.8564092. [DOI] [PubMed] [Google Scholar]
- Kohyama T, Liu X, Zhu YK, et al. Phosphodiesterase 4 inhibitor cilomilast inhibits fibroblast-mediated collagen gel degradation induced by tumor necrosis factor-alpha and neutrophil elastase. Am J Respir Cell Mol Biol. 2002;4:487–94. doi: 10.1165/rcmb.4818. [DOI] [PubMed] [Google Scholar]
- Kumar RK, Herbert C, Thomas PS, et al. Inhibition of inflammation and remodeling by roflumilast and dexamethasone in murine chronic asthma. J Pharmacol Exp Ther. 2003;1:349–55. doi: 10.1124/jpet.103.053819. [DOI] [PubMed] [Google Scholar]
- Kwak HJ, Song JS, Heo JY, et al. Roflumilast inhibits lipo-polysaccharide-induced inflammatory mediators via suppression of nuclear factor-kappaB, p38 mitogen-activated protein kinase, and c-Jun NH2-terminal kinase activation. J Pharmacol Exp Ther. 2005;3:1188–95. doi: 10.1124/jpet.105.092056. [DOI] [PubMed] [Google Scholar]
- Lamontagne S, Meadows E, Luk P, et al. Localization of phosphodi-esterase-4 isoforms in the medulla and nodose ganglion of the squirrel monkey. Brain Res. 2001:1–2. 84–96. doi: 10.1016/s0006-8993(01)03023-2. [DOI] [PubMed] [Google Scholar]
- Landells LJ, Szilagy CM, Jones NA, et al. Identification and quantification of phosphodiesterase 4 subtypes in CD4 and CD8 lymphocytes from healthy and asthmatic subjects. Br J Pharmacol. 2001;5:722–9. doi: 10.1038/sj.bjp.0704120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leclerc O, Lagente V, Planquois JM, et al. Involvement of MMP-12 and phosphodiesterase type 4 in cigarette smoke-induced inflammation in mice. Eur Respir J. 2006;6:1102–9. doi: 10.1183/09031936.06.00076905. [DOI] [PubMed] [Google Scholar]
- Leichtl S, et al. Efficacy of once-daily roflumilast, a new, orally active selective phosphodiesterase 4 inhibitor, in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2002a:A229. [Google Scholar]
- Leichtl S, et al. Roflumilast, a new, orally active, selective phosphodiesterase 4 inhibitor, is safe and well tolerated in patients with Chronic Obstructive Pulmonary Disease. Eur Respir J. 2002b;(Suppl38):P303s. [Google Scholar]
- Lenfant C, Khaltaev N.2005Workshop report: Global strategy for the diagnosis, management, and prevention of COPD [online]Accessed September 20, 2006. URL: http://www.goldcopd.org/Guidelineitem.asp?l1=2&l2=1&intId=989
- Lipworth BJ. Phosphodiesterase-4 inhibitors for asthma and chronic obstructive pulmonary disease. Lancet. 2005;9454:167–75. doi: 10.1016/S0140-6736(05)17708-3. [DOI] [PubMed] [Google Scholar]
- Losco PE, Evans EW, Barat SA, et al. The toxicity of SCH 351591, a novel phosphodiesterase-4 inhibitor, in Cynomolgus monkeys. Toxicol Pathol. 2004;3:295–308. doi: 10.1080/01926230490431493. [DOI] [PubMed] [Google Scholar]
- Martin-Chouly CA, Astier A, Jacob C, et al. Modulation of matrix metalloproteinase production from human lung fibroblasts by type 4 phosphodiesterase inhibitors. Life Sci. 2004;7:823–40. doi: 10.1016/j.lfs.2004.01.021. [DOI] [PubMed] [Google Scholar]
- Martorana PA, Beume R, Lucattelli M, et al. Roflumilast fully prevents emphysema in mice chronically exposed to cigarette smoke. Am J Respir Crit Care Med. 2005;7:848–53. doi: 10.1164/rccm.200411-1549OC. [DOI] [PubMed] [Google Scholar]
- Mata M, Sarria B, Buenestado A, et al. Phosphodiesterase 4 inhibition decreases MUC5AC expression induced by epidermal growth factor in human airway epithelial cells. Thorax. 2005;2:144–52. doi: 10.1136/thx.2004.025692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauwels RA, Buist AS, Calverley PM, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) Workshop summary. Am J Respir Crit Care Med. 2005;5:1256–76. doi: 10.1164/ajrccm.163.5.2101039. [DOI] [PubMed] [Google Scholar]
- Perez-Torres S, Miro X, Palacios JM, et al. Phosphodiesterase type 4 isozymes expression in human brain examined by in situ hybridization histochemistry and[3H]rolipram binding autoradiography. Comparison with monkey and rat brain. J Chem Neuroanat. 2000;3–4:349–74. doi: 10.1016/s0891-0618(00)00097-1. [DOI] [PubMed] [Google Scholar]
- Profita M, Chiappara G, Mirabella F, et al. Effect of cilomilast (Ariflo) on TNF-alpha, IL-8 and GM-CSF release from airway cells of patients with COPD. Thorax. 2003;7:573–9. doi: 10.1136/thorax.58.7.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabe KF, Bateman ED, O’Donnell D, et al. Roflumilast--an oral anti-inflammatory treatment for chronic obstructive pulmonary disease: a randomised controlled trial. Lancet. 2005;9485:563–71. doi: 10.1016/S0140-6736(05)67100-0. [DOI] [PubMed] [Google Scholar]
- Rennard SI, Schachter N, Strek M, et al. Cilomilast for COPD: results of a 6-month, placebo-controlled study of a potent, selective inhibitor of phosphodiesterase 4. Chest. 2006;1:56–66. doi: 10.1378/chest.129.1.56. [DOI] [PubMed] [Google Scholar]
- Retamales I, Elliott WM, Meshi B, et al. Amplification of inflammation in emphysema and its association with latent adenoviral infection. Am J Respir Crit Care Med. 2001;3:469–73. doi: 10.1164/ajrccm.164.3.2007149. [DOI] [PubMed] [Google Scholar]
- Sanz MJ, Cortijo J, Morcillo EJ. PDE4 inhibitors as new anti-inflammatory drugs: Effects on cell trafficking and cell adhesion molecules expression. Pharmacol Ther. 2005;3:269–97. doi: 10.1016/j.pharmthera.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Schmidt DT, Watson N, Dent G, et al. The effect of selective and non-selective phosphodiesterase inhibitors on allergen- and leukotriene C(4)-induced contractions in passively sensitized human airways. Br J Pharmacol. 2000;8:1607–18. doi: 10.1038/sj.bjp.0703725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro SD. End-stage chronic obstructive pulmonary disease: the cigarette is burned out but inflammation rages on. Am J Respir Crit Care Med. 2001;3:339–40. doi: 10.1164/ajrccm.164.3.2105072c. [DOI] [PubMed] [Google Scholar]
- Shapiro SD, Goldstein NM, Houghton AM, et al. Neutrophil elastase contributes to cigarette smoke-induced emphysema in mice. Am J Pathol. 2003;6:2329–35. doi: 10.1016/S0002-9440(10)63589-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SJ, Cieslinski LB, Newton R, et al. Discovery of BRL 50481, a Selective Inhibitor of Phosphodiesterase 7: In vitro Studies in Human Monocytes, Lung Macrophages and CD8+ T-Lymphocytes. Mol Pharmacol. 2004;66:1679–89. doi: 10.1124/mol.104.002246. [DOI] [PubMed] [Google Scholar]
- Spina D. The potential of PDE4 inhibitors in respiratory disease. Curr Drug Targets Inflamm Allergy. 2004;3:231–6. doi: 10.2174/1568010043343822. [DOI] [PubMed] [Google Scholar]
- Spond J, Chapman R, Fine J, et al. Comparison of PDE 4 inhibitors, rolipram and SB 207499 (ariflo), in a rat model of pulmonary neutrophilia. Pulm Pharmacol Ther. 2001;2:157–64. doi: 10.1006/pupt.2001.0291. [DOI] [PubMed] [Google Scholar]
- Stoller JK, Aboussouan LS. Alpha1-antitrypsin deficiency. Lancet. 2005;9478:2225–36. doi: 10.1016/S0140-6736(05)66781-5. [DOI] [PubMed] [Google Scholar]
- Sturton G, Fitzgerald M. Phosphodiesterase 4 inhibitors for the treatment of COPD. Chest. 2002;5(Suppl):192S–6S. doi: 10.1378/chest.121.5_suppl.192s. [DOI] [PubMed] [Google Scholar]
- Takahashi M, Terwilliger R, Lane C, et al. Chronic antidepressant administration increases the expression of cAMP-specific phosphodiesterase 4A and 4B isoforms. J Neurosci. 1999;2:610–8. doi: 10.1523/JNEUROSCI.19-02-00610.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Schalkwyk E, Strydom K, Williams Z, et al. Roflumilast, an oral, once-daily phosphodiesterase 4 inhibitor, attenuates allergen-induced asthmatic reactions. J Allergy Clin Immunol. 2005;2:292–8. doi: 10.1016/j.jaci.2005.04.023. [DOI] [PubMed] [Google Scholar]
- Weimar C, Bethke T, Westphal K, et al. Roflumilast and its active metabolite, roflumilast N-oxide, do not interact with inhaled salbutamol. Am J Respir Crit Care Med. 2002;8:A594. [Google Scholar]