Abstract
Respiratory disease has never received priority in relation to its impact on health. Estimated DALYs lost in 2002 were 12% globally (similar for industrialized and developing countries). Chronic airflow limitation (due mainly to asthma and COPD) alone affects more than 100 million persons in the world and the majority of them live in developing countries. International guidelines for management of asthma (GINA) and COPD (GOLD) have been adopted and their cost-effectiveness demonstrated in industrialized countries. As resources are scarce in developing countries, adaptation of these guidelines using only essential drugs is required. It remains for governments to set priorities. To make these choices, a set of criteria have been proposed. It is vital that the results of scientific investigations are presented in these terms to facilitate their use by decision-makers. To respond to this emerging public health problem in developing countries, WHO has developed 2 initiatives: “Practical Approach to Lung Health (PAL)” and the Global Alliance Against Chronic Respiratory Diseases (GARD)”, and the International Union Against Tuberculosis and Lung Diseases (The Union) has launched a new initiative to increase affordability of essential asthma drugs for patients in developing countries termed the “Asthma Drug Facility” (ADF), which could facilitate the care of patients living in these parts of the world.
Keywords: air flow limitation, asthma, COPD, intervention, cost-effectiveness, developing countries
Introduction
Chronic respiratory diseases have never, in any area of the world, been accorded a priority relative to their extent and impact. No political jurisdiction (rich or poor) proportionally commits resources to chronic respiratory diseases equivalent to the burden they represent in the community, whether for research, prevention, or clinical services. It was only in 2005, that the World Health Organisation (WHO) released a report highlighting the high burden of chronic diseases particularly in developing countries and the need for urgent action in the prevention and control of chronic diseases including chronic respiratory diseases (WHO 2005). The reasons for this neglect are unclear. Several possible explanations can be offered.
These diseases have traditionally been stigmatized. It has been virtually impossible to mobilize either patients or society to address them as has been done for HIV/AIDS (Mawar et al 2006; Vanable et al 2006), cardiovascular diseases, or cancer (Chapple et al 2004). With conditions related to tobacco smoke exposure (lung cancer and chronic obstructive pulmonary disease) there has been a ‘blame the victim’ approach which promotes stigmatization (Bayer and Stuber 2006). In addition, the main burden of conditions such as tuberculosis and pneumonia in children are highly associated with poverty and inequity which in turn is fraught with a sense of ‘powerlessness’ which mitigates against social mobilization (Anyangwe et al 2006).
Unlike cancer or cardiovascular diseases, the various respiratory conditions have been ‘partitioned’ to other ‘categories’ (even though they are generally managed within a single subspecialty of medicine) and are thus not ‘counted’ as a single entity, to be compared with other groups. Examples include lung cancer (counted as ‘cancer’), acute respiratory illnesses (especially pneumonia), and tuberculosis (often managed by lung specialists but ‘counted’ with infectious diseases).
Finally, these diseases (and especially chronic airflow limitation) can be ‘silent’. Even where there is extensive knowledge and expertise concerning them, they are frequently unrecognized. For example, if one reviews the medical records of a sample of patients on an adult medical ward in industrialized countries, the frequency with which chronic airflow limitation is mentioned in the clinical record is usually much less than the prevalence of the disease in the community; the frequency of the condition among hospitalized patients in a medical ward is most certainly substantially higher.
This article seeks to address the issue of chronic airflow limitation; a major contributor to the burden of chronic respiratory disease. It will focus on the situation in developing countries where the majority of the world’s population lives. It will outline what is known about the burden of disease and available interventions and will propose an approach to setting priority for action. For the purposes of this article, chronic airflow limitation refers to diseases that cause reduced pulmonary function related to disease of the airways and includes both fixed airflow limitation (as in chronic obstructive pulmonary disease (COPD), bronchiectasis, and the sequelae of tuberculosis) and variable airflow limitation (as in asthma).
Burden of disease
Calculation of the burden of all respiratory diseases combined can be made from recent published reports (Lopez et al 2006) and indicates that respiratory diseases account for 15% of deaths in low-middle-income countries and 14% in high-income countries, second only to cardiovascular diseases which account for 21% and 27% respectively. Viewed in terms of premature deaths (years of life lost), this is even more dramatic: respiratory diseases account for 17%, compared with 15% for other communicable diseases and 14% for cardiovascular diseases. Among the respiratory diseases, lower respiratory tract infections account for the greatest proportion of years of life lost (48% of those due to respiratory diseases) with chronic airflow limitation accounting for a lower proportion (17%).
As part of an effort to prioritize health needs by quantifying the global burden of disease, health economists working in conjunction with the World Bank developed “Disability-Adjusted Life Years” (DALYs); a statistical measurement for account for both death and disability (World Bank 1993). From the World Health Report 2004 (WHO 2004), the estimated DALYs lost due to all respiratory diseases in 2002 was 184 million (12% of the total) compared with 21% for other infectious and parasitic diseases, 13% for neuropsychiatric diseases, and 10% for cardiovascular diseases. Among the respiratory diseases, lower respiratory tract infections accounted for the greatest proportion (50%), with chronic airflow limitation accounting for 23%.
The prevalence of chronic airflow limitation is relatively high. The prevalence of asthma in adults (Table 1) has been demonstrated between 1% and 10% with a median estimate around 2%–3% (ECRHS 1996). Among these cases, it is likely that a minority (less than 30%) have chronic airflow limitation (Aït-Khaled and Enarson 2005), yielding a community prevalence of asthma with chronic airflow limitation of 0.5 to 1.0%. The World Health Report 2004 estimates a range of COPD up to 1.7%, with the global median at just over 1.0%. These are in contrast to prevalence studies of COPD in the general population (confirmed by spirometry) among adults (Table 2) in Europe, America, and Latin America (Mueller et al 1971; Gulsvik 1979; Lange et al 1989; Bakke et al 1991; Marco-Jordan et al 1998; Mannino et al 2000; Viegi et al 2000; Von Hertzen et al 2000; Menezes et al 2005), where the prevalence varied from 4% to 27%. The PLATINO study (Menezes et al 2005) describes the epidemiology of COPD in five major Latin American cities: Sao Paulo (Brazil), Santiago (Chile), Mexico City (Mexico), Montevideo (Uruguay), and Caracas (Venezuela). The same methodology was used and COPD defined as a ratio less than 0.7 of postbronchodilator forced expiratory volume in the first second (FEV1) divided by forced vital capacity (FVC). Crude rates of COPD ranged from 7.8% (95% CI 5.9–9.7) in Mexico City to 19.7% (95% CI 17.2–22.2) in Montevideo. After adjustment for key risk factors, the prevalence of COPD in Mexico City remained significantly lower than that in other cities. Altitude may explain part of the difference in prevalence. These results suggest that COPD is a greater health problem in Latin America than previously realised and suggests of a high prevalence of COPD in developing countries and particularly in middle income countries which are in similar epidemiologic transition (North and South Africa, Asia).
Table 1.
Estimated prevalence of asthma symptoms
| Region | Estimated prevalence |
|---|---|
| Children 13–14 years * | |
| Oceania | 25.9% |
| North America | 16.5% |
| Latin America | 13.4% |
| Western Europe | 13.0% |
| Eastern Mediterranean | 10.7% |
| Africa | 10.4% |
| Pacific Asia | 9.4% |
| South-East Asia | 4.5% |
| Eastern Europe | 4.4% |
| Adults aged 20–44 years ** | |
| Australia and New Zealand | 6.8–9.7% |
| USA and Northen Europe | >5.0% |
| Western Europe and Mediterranean | 1.0–4.0% |
| Alger (Algeria) | 2.4% |
| Bombay (India) | 2.6% |
Note: * The International Study of Asthma and Allergies in Childhood (ISAAC);
The European Community Respiratory Health Survey (ECRHS)
Table 2.
Prevalence in general population of airway obstruction by spirometry (FEV1/FVC <70%)
| Regions | Countries | Year | Age group |
Prevalence of air way obstruction (%) |
Authors | ||
|---|---|---|---|---|---|---|---|
| Overall | Male | Females | |||||
| Europe | Danemark | 1989 | 20–90 | 3.7 | Lange | ||
| Finland | 2000 | ≥30 | 11 | 5.2 | Von Hertzen | ||
| Italy | 2000 | ≥25 | 11 | 12.5 | 11.8 | Viegy | |
| Norway | 1979 | 16–69 | 4.1 | 3.7 | 4.6 | Gulsvik | |
| 1991 | 18–70 | 4.5 | 4.8 | 4.2 | Bakke | ||
| Spain | 2000 | 40–69 | 9.1 | 14.3 | 3.9 | Maro-Jordan | |
| America | USA | 1971 | 20–69 | 13 | 2 | Mueller | |
| 2000 | ≥17 | 6.8 | Mannino | ||||
| Latin America | Brazil (Sao Paulo) | 2002 | >40 | 18 | 14 | Menezes | |
| Chili (Santiago) | 23.3 | 12.8 | |||||
| Mexico | 11 | 5.5 | |||||
| Uruguay (Montevideo) | 27.1 | 14.5 | |||||
| Venezuela (Caracas) | 15.7 | 10.2 | |||||
Abbreviations: FEV1, forced expiratory volume in one second; FVC, forced vital capacity.
The figures are based on very limited information. There are virtually no community-based surveys of the prevalence of chronic airflow limitation in low-income countries (such as the countries of Africa). More extensive information is available for asthma where the International Study of Asthma and Allergy in Childhood (ISAAC) has led the way in providing comparative results from across the world (Beasley et al 1998). However, this initiative providing a worldwide map of prevalence of asthma symptoms (Table 1) has not yet provided results of lung function and is thus not able to give estimates of chronic airflow limitation among asthma patients. Moreover, its focus is children, which means that population estimates will under represent the situation. More recent initiatives, such as the Burden of Obstructive Lung Disease (BOLD) study, will hopefully improve our knowledge, however this very costly survey will be difficult to conduct in low and in the majority of middle countries. It is lamentable that information on a condition as widespread and as devastating as chronic airflow limitation is so lacking and underscores the point that these patients are greatly underserved.
Based on the available data, the combined community prevalence of airflow limitation due to the two conditions could be estimated in excess of 100 million persons (possibly substantially higher). Estimated DALYs lost in 2002 can be compared among regions (WHO 2004). For comparative purposes and to illustrate the situation in low-income countries, those for Africa can be compared with the global estimates. DALYs lost due to all respiratory diseases were 14% for Africa compared with 12% globally. Among the respiratory diseases, lower respiratory tract infections accounted for a much greater proportion in Africa (68% compared with 50%), COPD accounted for just over 2% compared with 15% and asthma for just over 4% compared with 8% globally. Among patients seeking care in the health services of various developing countries implementing the “Practical Approach to Lung Health” asthma and COPD represent more than 1% of all consultations (Table 3).
Table 3.
Frequency of various conditions for which patients consulted health services in various countries implementing the Practical Approach to Lung Health
| Country |
All |
Respiratory conditions |
Other |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
ARI |
COPD |
Asthma |
Other* |
|||||||||
| No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | |
| Bolivia | 4,911 | 100.0% | 1,045 | 21.3% | 59 | 1.2% | 11 | 0.2% | 39 | 0.8% | 3,757 | 76.5% |
| Kyrgystan | 2,772 | 100.0% | 859 | 31.0% | 76 | 2.7% | 26 | 0.9% | 31 | 1.1% | 1,780 | 64.2% |
| Morocco | 15,911 | 100.0% | 2,876 | 18.1% | 20 | 0.1% | 188 | 1.2% | 268 | 1.7% | 12,559 | 78.9% |
| Tunisia | 4,747 | 100.0% | 1,301 | 27.4% | 27 | 0.6% | 64 | 1.3% | 83 | 1.7% | 3,272 | 68.9% |
Note: * includes tuberculosis cases: 5 from Bolivia, 1 from Kyrgystan, 6 from Morocco, and 1 from Tunisia.
Abbreviations: ARI, ; COPD, chronic obstructive pulmonary disease.
These estimates, however, must be viewed with extreme caution due to the severe limitation in data upon which they are based and the prevalence of chronic airflow limitation may be substantially higher than currently believed (as was the case in the studies in Latin America by Menezes and colleagues [2005]). Nevertheless, even with the limited information available, we must conclude that chronic airflow limitation does account for a substantial burden of disease, even in the poorest countries where life expectancy is drastically lower and the effects of such chronic diseases would be expected to be consequently less. That so little attention is given to respiratory diseases in general, and to chronic airflow limitation in particular, in this setting is a travesty.
The financial consequences of chronic respiratory diseases are high and underscore the need for more investment on research and policy. The cost of asthma has increased in recent years in industrialised countries. For example, in 1998, asthma in USA (Weiss and Sullivan 2001) accounted for an estimated US$12.7 billions annually (more than 2 times the estimated cost for 1990). Data on the direct (cost of health-care resources) and indirect (cost of the consequences of the disability) costs of COPD are also available only from industrialised countries. For example in 1993, the total annual economic burden of COPD in USA was estimated at US$23.9 billions (NHLB 1998). These data demonstrate that the total cost for COPD is greater than for other common respiratory disease such as asthma, influenza, pneumonia and tuberculosis.
Tobacco is the main etiological agent for chronic airflow limitation worldwide, although exposure to combustion products of biomass fuel is an important and underestimated risk factor in developing countries (Baris and Ezzati 2004).
Interventions to improve prevention and management
During the last two decades, guidelines for standard case management of asthma and COPD have been adopted in most industrialized countries. They began with the Global Initiative of Asthma (GINA) (GINA 1995), updated in 2005. These guidelines recommend standardised management for asthma attacks and emphasize the crucial need of long-term treatment of asthma to alleviate disease severity, improve quality of life and to prevent exacerbations and attacks. Prevention aims to limit environmental factors that act as triggering factors (essentially allergens and tobacco). The technical measures proposed are based on the pathophysiology of asthma as a chronic inflammatory disorder of the airways (explaining the need of regular use of inhaled steroids) that lead to recurrent episodes of symptoms linked to airflow obstruction (explaining the need of bronchodilators). The characteristic variability of airflow limitation in asthma highlights the importance of tools to measure and monitor lung function (spirometers and peak expiratory flow meters) to confirm the diagnosis and document the grade of severity. The following principles of GINA have been adopted in national guidelines, with regional or local adaptations:
Clear objectives for case management;
Diagnosis based on clinical history and lung function;
Classification of severity based on history and lung function;
Long-term treatment adapted to the severity grade;
Patient education and strong partnership with care providers to improve adherence to treatment and to promote self-management;
Routine follow-up for evaluation and adaptation of treatment.
Following a similar framework, the Global Initiative for Chronic Obstructive Lung Disease (GOLD), elaborated guidelines for case management, and prevention of COPD (GOLD 1998), updated in 2005. They have been adopted by most professional societies. The recommendations are based on a definition of COPD as “a disease state characterised by airflow limitation that is not fully reversible. The airflow limitation is usually both progressive and associated with an abnormal inflammatory reaction of the lungs to noxious particles or gases”. The airflow obstruction is distinguished from that of asthma by being permanent, progressively increasing, with no, or very little, variability.
Severity grade is determined by the level of airflow limitation after bronchodilator test demonstrating no, or incomplete (<15%), reversibility. The principles of case management are the following:
Diagnosis of air flow obstruction based on spirometry: FEV/FVC <70%;
- Severity grade based on level of FEV1:
- Stage 0: At risk – Normal spirometry; chronic symptoms (cough and sputum production).
- Stage I: Mild COPD – FEV1/FVC < 70%, FEV1 ≥ 80% predicted and usually but not always chronic symptoms (cough, sputum production).
- Stage II: Moderate COPD – FEV1/FVC < 70%, 50% ≤ FEV1 < 80% predicted and chronic symptoms (cough, sputum production) usually with shortness of breath.
- Stage III: Severe COPD – FEV1/FVC < 70%, 30% ≤ FEV1 < 50% predicted and chronic symptoms (cough, sputum production, dyspnoea)
- Stage IV : Very severe COPD – FEV1/FVC < 70%, FEV1 < 30% or FEV1 < 50% predicted plus chronic respiratory failure with clinical signs of right heart failure;
- Secondary prevention is based on:
- smoking cessation
- avoidance of other exacerbating factors (indoor, work place pollution) and
- influenza vaccination;
Stepwise treatment based on inhaled bronchodilators (short-acting beta 2 and/or anticholinergics) and long-acting beta agonists and inhaled steroids for moderate and severe cases with repeated exacerbations;
Rehabilitation;
Long term oxygen therapy for hypoxemia or heart failure.
Efficacy of interventions
Cochrane systematic reviews have confirmed both efficacy and safety of inhaled steroids in the management of asthma for adults and children (Adams et al 2001, 2005a, 2005b). All inhaled steroids demonstrate a dose-response relationship for efficacy, but most of the benefit is in mild to moderate disease with low-moderate dose range of each drug. In patients with severe disease who are dependant of oral steroids, there may be a benefit in reducing oral steroids by using high-dose of inhaled steroids (Adams and Jones 2006). Long-acting beta agonists provide additional improvement in lung function and in symptoms and could reduce the rate of exacerbations requiring oral steroids without additional serious adverse reactions (Ni Chroinin et al 2004, 2005).
Cochrane reviews of treatment of COPD have also been reported. Regular long-term use of ipratropium bromide, short-acting beta 2 agonist therapy or their combination show little benefit in stable COPD in improving lung function, symptoms, or exercise tolerance. Patients can choose the short-acting bronchodilator that gives the most improvement of their symptoms (Appleton et al 2006). Long-acting beta agonists give small increases of FEV1 not associated with improvement in quality of life or reduction in breathlessness (Appleton et al 2001). Long-acting anticholinergics reduce COPD exacerbations and hospitalizations.
Evaluation of effectiveness
The effectiveness of asthma guidelines has been demonstrated in several studies, in particular the Gaining Optimal Asthma Control (GOAL) study (Bateman et al 2004). The cost-effectiveness of inhaled steroids in management of persistent asthma has been consistently demonstrated, even in developing countries (Perera 1995). The number of hospital days and emergency visits can be reduced by 50% to 80% for asthma patients requiring inhaled steroids. Substantial savings in healthcare costs have also been demonstrated (Price and Briggs 2002; Castro et al 2003; Adams et al 2001 Schelledy et al 2005: Simonella et al 2006).
Standard case management of COPD has been shown to improve the quality of life of patients and to decrease exacerbations and hospitalisations (Paggiaro et al 1998; Friedman et al 1999; Calverley et al 2003a, 2003b; Oostenbrink.et al 2004), although long-term studies are lacking (Barr et al 2005). Use of bronchodilators, steroids, and rehabilitation are recognized to be cost-effective (Rea et al 2004; Halpin 2006) in industrialized countries. Management of COPD using the combination of all technical measures is usually costly (Wouters 2003; Oostenbrink and Rutten-van Molken 2004; Fournier et al 2005) and is not able to arrest, or decrease the progression in, the decline of lung function. A meta-analysis systematically conducted reviewed the efficacy, effectiveness, and safety on inhaled steroids in patient with COPD, and concluded that the risk-benefit ratio appears to favor inhaled steroids in patients with moderate to severe COPD (Gartlehner et al 2006), and has estimated that unplanned use of health services can be reduced by one-third. However, existing evidence does not indicate a treatment benefit for patients with mild COPD.
Relevance for developing countries
Most developing countries have no standard guidelines for assessing and managing chronic noncommunicable respiratory disease. Such services as exist in many low-income countries do not reach large parts of the population, particularly the poorest. A substantial proportion of this group of the population dies before age 40 (before they have the ‘possibility’ of developing chronic airflow limitation) and have limited or no access to health services. They represent 15% of the population in Latin America, 34% in the Middle East, and more than 40% in South-East Asia and Sub-Saharan Africa (UNDP 1997). A clear priority must be to enhance access to health services in general if these patients are to benefit by the care they require.
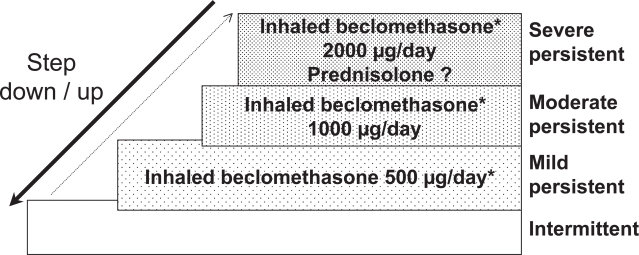
Some guidelines have been published for the management of COPD in developing countries. They include early recognition of disease by questionnaire, confirmation of diagnosis, and assessment of disease severity by clinical evaluation and spirometry (SAPS 1998; MHM and AMMMTS 1999). A standard case management approach for COPD for Asia and Africa has been proposed (Chan-Yeung et al 2004). The long term management recommended is a step-wise approach according to the disease severity (Figure 1) using only inexpensive bronchodilatators: inhaled beta 2 short-action and/or inhaled ipratropium bromide with or without low dosage of slow release theophylline. Inhaled steroids are reserved for those where a clear response to a standardized trial of steroids has been demonstrated. Long-term oxygen therapy and rehabilitation programmes are usually not available in low-income countries.
Figure 1.
Step-wise approach to treatment for COPD in developing countries.
Note: * Add theophylline low dosage in noncontrolled patients and inhaled steroids if positive response to corticosteroid trial.
Although the publication and distribution of international consensus reports are important advances, the benefits have not yet reached patients in many developing countries. Results achieved are not the same as in clinical trials, even in industrialized countries (Rabe et al 2000, 2004; Lai et al 2003; Neffen et al 2005). Such guidelines are usually developed by professional societies and/or specialists and rarely involve service providers at the primary care level (Lalloo and Mclvor 2006). Additional bottlenecks in developing countries are: the low priority accorded to chronic diseases as compared with infectious diseases; lack of organization of follow-up; cultural barriers; poor education of health workers; lack of spirometry in low-income countries; and lack of access to, and the high cost of necessary drugs (Sterk et al 1999; Enarson and Aït-Khaled 1999; Aït-Khaled et al 2000; Gelders et al 2006; Wan and Aït-Khaled 2006).
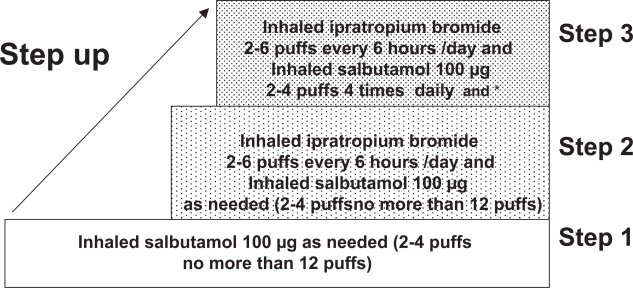
The Union Asthma guide published in 1996 and revised in 2005 (Aït-Khaled and Enarson 2005) proposes a technical package for asthma management, implemented within general health services, and appropriate for services where resources are extremely limited. The standard case management approach recommends the use of two drugs, both of which are included in the WHO essential drugs list: inhaled beclomethasone 250 μg/puff and inhaled salbutamol 100 μg/puff (Figure 2). A standardized information system is recommended, including a patient register in which each new patient with persistent asthma is registered, with information on initial status and follow-up. Routine analysis of case notification and of outcome of management is based on the register. Evaluation of this approach in several low- and middle-income countries demonstrated its feasibility (Aït-Khaled et al 2006a) and its efficiency estimated after one year of follow-up (Aït-Khaled et al 2006b). Despite a high proportion of patients (31%) who did not continue treatment for at least one year, 51% of patients in the cohort had a decrease in their severity of disease (Aït-Khaled et al 2006b); emergency visits and hospitalizations were dramatically reduced with substantial cost-saving to the health services.
Figure 2.
Step-wise approach to treatment:The Union Asthma Guide.
Note: *And inhaled salbutamol 100 μg on demand less than 4 times/day at all stages of severity.
Setting priorities for management of chronic airflow limitation
In developing countries, the objective of chronic respiratory disease prevention and management is to decrease the burden of illness, prevent avoidable deaths, and increase the quality of life of patients. This is achieved by adaptation of international guidelines to the particular context of developing countries. Such efforts have as objectives to (Aït-Khaled et al 2001, 2002; Bousquet et al 2003):
Reduce tobacco smoking in the whole population;
Encourage smoking cessation for patients who access services;
Improve quality of services by standard case management;
Promote cost-effective approaches to treatment;
Enhance access to affordable essential medications;
Avoid ineffective and costly services.
The tobacco industry has increasingly targeted developing countries (Amos 1996) and tobacco smoking has increased dramatically in many developing countries during the last two decades. The World Bank estimates that a 10% increase in economic level in middle income countries brings about an increase in tobacco consumption of 7%, whereas the same increase in the poorest countries leads to an increase in tobacco consumption of 13% (Yach 1996).
This problem requires firm political action directed to endorsing and implementing the Framework Convention on Tobacco Control and, particularly, to increase legislation to reduce tobacco consumption (WHO 1999). Secondary prevention is essential. This includes smoking cessation programmes, the simplest and most appropriate of which is an intervention called ‘brief advice’ (Slama et al 1995; Slama 1998; Fiore et al 2000). In many countries, efforts to reduce the effects of exposure to combustion products of biomass fuel are important (Baris and Ezzati 2004). For COPD, a number of interventions are not justifiable and should not be used, including periodic courses of antibiotics, long-term oral steroids, and mucolytics.
For asthma, a Cochrane review concluded that there is insufficient evidence to support initiating therapy with a combination of inhaled glucocorticoids and long-acting beta2-agonist rather than inhaled glucocorticoids alone as first line therapy for persistent asthma in steroid-naïve adults (Ni Chroinin et al 2004). Costly investigations not recommended for use in developing countries include allergy skin tests, measurement of total and specific IGE, and nonspecific bronchial challenge. Immunotherapy is not recommended in low income countries (Sterk et al 1999; Bousquet et al 2001) because, in addition to its very high cost and limited indications, many allergens are not well identified in these developing countries, and rare side effects might be severe.
International guidelines for management of COPD may be very difficult to implement in low-income countries as they require redirecting existing resources from other priority activities. This may be possible in some middle-income countries where the resources are greater and quality of services better and in some particular settings in low income countries (private hospitals or universities).
As resources are not limitless, it remains for governments to choose some interventions over others. To make these choices, a set of criteria has been proposed by the WHO, in collaboration with the Public Health Agency of Canada. In acknowledging the growing problem of chronic diseases in developing countries and its relative neglect, they have recommended the following definition of categories of cost-effectiveness (WHO 2005):
Very cost-effective: interventions that avert each DALY at a cost less than gross domestic product per head;
Cost-effective: interventions that avert each DALY at a cost between one and three times gross domestic product per head;
Not cost-effective: interventions that avert each DALY at a cost higher than three times gross domestic product per head.
Clearly, it is vital that researchers present the results of their investigations in these terms if decision-makers are to make use of them.
International responses
WHO has responded to the needs in two ways. A comprehensive integrated package for the standardized management for respiratory disease, Practical Approach to Lung health (PAL) (Ottmani et al 2005), has been developed and implemented in several developing countries. More recently a new WHO initiative, Global Alliance for Respiratory Chronic Diseases (GARD), has been launched. The Union contributes to these initiatives through:
Creation of an Asthma Drug Facility to improve availability and affordability of essential asthma drugs (Aït-Khaled 2006; Billo 2004, 2006)
Implementation of an integrated package for improvement of quality of care at the first level of referral in developing countries. (Enarson 1998)
Developing countries have been left behind while our understandings of respiratory disease, and the interventions to deal with it, have improved in the past decade. The key obstacle to further action is the lack of comprehensive information on the burden of disease and the effects in intervention in relation to return on investment, the limited access to health services among the poor, and the insufficient adaptation and testing of standard case management. These key issues need to be addressed if we are to facilitate the care of more than one hundred million who suffer from chronic airflow limitation.
References
- Adams RJ, Fuhlbrigge A, Finkenelstein JA, et al. Impact of inhaled anti-inflammatory therapy on hospitalisations and emergency department visits for children with asthma. Pediatrics. 2001;107:706–11. doi: 10.1542/peds.107.4.706. [DOI] [PubMed] [Google Scholar]
- Adams NP, Bestall JB, Malouf R, et al. Beclomethasone versus placebo for chronic asthma. Cochrane Database Syst Rev. 2005a;1:CD002738. doi: 10.1002/14651858.CD002738.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams NP, Bestall JM, Lasserson TJ, et al. Fluticasone versus beclomethasone or budesonide for chronic asthma in adults and children. Cochrane Database Syst Rev. 2005b;2:CD002310. doi: 10.1002/14651858.CD002310.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams NP, Jones PW. The dos-response characteristics of inhaled corticosteroids when used to treat asthma: An overview of Cochrane systematic reviews. Respir Med. 2006;100:1297–306. doi: 10.1016/j.rmed.2006.04.015. [DOI] [PubMed] [Google Scholar]
- Aït-Khaled N, Auregan G, Bencharif N, et al. Affordability of inhaled corticosteroids as a potential barrier to treatment of asthma in some developing countries. Int J Tuberc Lung Dis. 2000;4:268–71. [PubMed] [Google Scholar]
- Aït-Khaled N, Enarson DA, Bousquet J. Chronic respiratory diseases in developing countries: the burden and strategies for prevention and management. Bull World Health Organ. 2001;79:971–9. [PMC free article] [PubMed] [Google Scholar]
- Ait- Khaled N, Chaulet P, Enarson DA, et al. Clinical management of clinical obstructive pulmonary disease. In: Similowski T, Derenne P, editors. Epidemiology and management of stable chronic obstructive pulmonary disease in Africa. Vol. 43. New York: Marcel Dekker; 2002. pp. 1007–30. [Google Scholar]
- Aït-Khaled N, Enarson DA.2005Management of asthma A guide to the essentials of good clinical practice 2nd edParis, France: International Union Against Tuberculosis and Lung Disease; URL: http//:www.iuatld.org [PubMed] [Google Scholar]
- Aït-Khaled N, Enarson DA, Bencharif N, et al. Implementation of asthma guidelines in health centres of several developing countries. Int J Tuberc Lung Dis. 2006a;10:104–9. [PubMed] [Google Scholar]
- Aït-Khaled N. Favoriser l’accessibilité aux médicaments essentiels de l’asthme. Rev Mal Resp. 2006;23:10S76–10S79. [PubMed] [Google Scholar]
- Aït-Khaled N, Enarson DA, Bencharif N, et al. Treatment outcome of asthma after one year follow-up in health centres of several developing countries. Int J Tuberc Lung Dis. 2006b;10:911–16. [PubMed] [Google Scholar]
- Amos A. Women and smoking: a global issue. World Health Stat Q. 1996;49:127–33. [Google Scholar]
- Anyangwe SC, Mtonga C, Chirwa B. Health inequities, environmental insecurity and the attainments of the millennium development goals in sub-Saharan Africa: The case study of Zambia. Int J Environ Res Public Health. 2006;3:217–27. doi: 10.3390/ijerph2006030026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appleton S, Poole P, Smith B, et al. Long-acting beta2-agonists for chronic obstructive pulmonary disease patients with poorly reversible airflow limitation. Cochrane Database Syst Rev. 2001;4:CD001104. doi: 10.1002/14651858.CD001104. [DOI] [PubMed] [Google Scholar]
- Appleton S, Jones T, Poole P, et al. Ipratropium bromide versus short acting beta-2 agonists for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2006;2:CD001387. doi: 10.1002/14651858.CD001387.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr RG, Bourbeau J, Camargo CA, et al. Tiotropium for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2005;2:CD002876. doi: 10.1002/14651858.CD002876.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer R, Stuber J. Tobacco control, stigma, and public health : rethinking the relations. Am J Public Health. 2006;96:47–50. doi: 10.2105/AJPH.2005.071886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beasley R, Keil U, Von Mutius E, et al. ISAAC Steering Committee Worldwide variation in the prevalence of asthma, allergic rhinoconjonctivitis and atopic eczema symptoms: the International Study of Asthma and Allergies in Childhood (ISAAC) Lancet. 1998;351:1225–32. [PubMed] [Google Scholar]
- Bakke S, Baste V, Hanoa R, et al. Prevalence of obstructive lung disease in a general population: relation to occupational title and exposure to some airborne agents. Thorax. 1991;46:863–70. doi: 10.1136/thx.46.12.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baris E, Ezzati M. Should interventions to reduce respirable pollutants be linked to tuberculosis control programmes? BMJ. 2004;329:1090–3. doi: 10.1136/bmj.329.7474.1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateman ED, Boushey HA, Bousquet J, et al. GOAL Investigators Group Can guideline-defined asthma control be achieved? The Gaining Optimal Asthma Control Study. Am J Respir Crit Care Med. 2004;170:836–44. doi: 10.1164/rccm.200401-033OC. [DOI] [PubMed] [Google Scholar]
- Billo N. Do we need an asthma drug facility? Int J Tuberc Lung Dis. 2004;8:391. [PubMed] [Google Scholar]
- Billo N. ADF from concept to reality. Int J Tuber Lung Dis. 2006;10:709. [PubMed] [Google Scholar]
- Bousquet J, Ndiaye M, Aït-Khaled N, et al. Management of chronic respiratory and allergic diseases in developing countries. Focus on sub-Saharan Africa. Allergy. 2003;58:265–83. doi: 10.1034/j.1398-9995.2003.02005.x. [DOI] [PubMed] [Google Scholar]
- Bousquet J, Van Cauwenberg P, Khaltaev N, et al. ARIA workshop report in collaboration with WHO. J Allergy Clin Immunol. 2001;108(5 Suppl):S147–334. doi: 10.1067/mai.2001.118891. [DOI] [PubMed] [Google Scholar]
- Calverley PM, Pauwels R, Vestbo J, et al. Trial of Inhaled steroids and long acting beta 2 agonists. study group Combined salmeterol and fluticasone in the treatment of chronic obstructive pulmonary disease: a randomised controlled trial. Lancet. 2003a;361:449–56. doi: 10.1016/S0140-6736(03)12459-2. [DOI] [PubMed] [Google Scholar]
- Calverley PM, Boonsawat W, Cseke Z, et al. Maintenance therapy with budesonide and fomoterol in chronic obstructive disease. Eur Respir J. 2003b;22:912–19. doi: 10.1183/09031936.03.00027003. [DOI] [PubMed] [Google Scholar]
- Castro M, Zimmerman NA, Crocker S, et al. Asthma intervention program prevents readmissions in high healthcare users. Am J Respir Crit Care. 2003;168:1095–9. doi: 10.1164/rccm.200208-877OC. [DOI] [PubMed] [Google Scholar]
- Chan-Yeung M, Aït-Khaled N, White N, et al. Management of COPD in Asia and Africa. Int Union Tuberc Lung Dis. 2004;8:159–70. [PubMed] [Google Scholar]
- Chapple A, Ziebland S, McPherson A. Stigma, shame, and blame experienced by patients with lung cancer: qualitative study. BMJ. 2004;328:1470. doi: 10.1136/bmj.38111.639734.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enarson DA, Aït-Khaled N. Cultural barriers to asthma management. Pediatr Pulmonol. 1999;28:297–300. doi: 10.1002/(sici)1099-0496(199910)28:4<297::aid-ppul9>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Enarson DA. Lung health and the International Union Against Tuberculosis and Lung Disease. Int J Tuberc Lung Dis. 1998;2:969–70. [PubMed] [Google Scholar]
- [ECRHS] European Community Respiratory Health Survey Variations in the prevalence of respiratory symptoms, self-reported asthma attacks, and use of asthma medications in the European Community Respiratory Health Survey (ECRHS) Eur Respir J. 1996;9:687–95. doi: 10.1183/09031936.96.09040687. [DOI] [PubMed] [Google Scholar]
- Fiore MC, Bailey WC, Cohen SJ, et al. Treating tobacco use and dependance Clinical Practice Guideline. Rockville, MD: U.S. Department of Health and Human Services; 2000. [Google Scholar]
- Friedman M, Serby CW, Hilleman DE, et al. Pharmacoeconomic Evaluation of a combination of Ipratropium Plus Albuterol Compared With Ipratropium and albuterol alone in COPD. Chest. 1999;115:635–41. doi: 10.1378/chest.115.3.635. [DOI] [PubMed] [Google Scholar]
- Fournier M, Tonnel AB, Housset B, et al. Economic burden of COPD in France: The Scope Study [French] Rev Mal Respir. 2005;22:247–55. doi: 10.1016/s0761-8425(05)85478-6. [DOI] [PubMed] [Google Scholar]
- Gartlehner G, Hansen RA, Carson SS, et al. Efficacy and safety of inhaled corticosteroids in patients with COPD. A systematic review and meta-analysis of health outcomes. Ann Fam Med. 2006;4:253–62. doi: 10.1370/afm.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelders S, Ewen M, Noguchi N, et al. 2006. Price, availability and affordability. An international comparison of chronic disease medicines [online]. Accessed on February 2, 2007. Geneva: WHO, Health Action International. URL: http://mednet3.who.int/medprices/CHRONIC.pdf
- [GINA] Global Initiative for Asthma 2005. Global strategy for asthma management and prevention. NHLBI/WHO workshop report [online]. Accessed on February 2, 2007. NIH publication number 95–3659. Revision 2005. URL: http://www.gina.com/
- [GOLD] Global Initiative for Chronic Obstructive Lung Disease 2005. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease [online]. Accessed on February 2, 2007. NIH, NHLBI publication Number 2701, April 2001. Updated 2005. URL: http://www.goldcopd.org/
- Gulsvik A. Prevalence and manifestations of obstructive lung disease in the city of Oslo. Scand J Respir Dis. 1979;60:286–96. [PubMed] [Google Scholar]
- Halpin DM. Heath economics of chronic obstructive pulmonary disease. Proc Am Thorac. 2006;3:227–33. doi: 10.1513/pats.200507-072SF. [DOI] [PubMed] [Google Scholar]
- Lai CKW, De Guia TS, Kim YY, et al. Asthma control in Asia-Pacific Study. J Allergy Clin Immunol. 2003;111:263. doi: 10.1067/mai.2003.30. [DOI] [PubMed] [Google Scholar]
- Lalloo UG, Mclvor RA. Management of chronic asthma in adults in diverse regions of the world. Int Union Tuberc Lung Dis. 2006;10:474–83. [PubMed] [Google Scholar]
- Lange P, Groth S, Nyboe J, et al. Chronic obstructive lung disease in Copenhagen: cross-sectional, epidemiological aspects. J Intern Med. 1989;226:25–32. doi: 10.1111/j.1365-2796.1989.tb01349.x. [DOI] [PubMed] [Google Scholar]
- Lopez AD, Mathers CD, Ezzati M, et al. Global and regional burden of diseases and risk factors, 2001: systematic analysis of population health data. Lancet. 2006;367:1747–57. doi: 10.1016/S0140-6736(06)68770-9. [DOI] [PubMed] [Google Scholar]
- Mannino DM, Gagnon RC, Petty TL, et al. Obstructive lung disease and low lung function in adults in the United States: data from the National Health and Nutrition Examination Survey, 1988–1994. Arch Intern Med. 2000;160:1683–9. doi: 10.1001/archinte.160.11.1683. [DOI] [PubMed] [Google Scholar]
- Marco-Jordan L, Martin-Berra J, Inigo MC, et al. Enfermedad pulmonar obstructiva cronica en la poblacion general: estudio epidemiologico realizado en Guipuzcoa. Arch Bronconeumol. 1998;34:23–7. [PubMed] [Google Scholar]
- Mawar N, Saha S, Pandit A, et al. The third phase of HIV pandemic: social consequences of HIV/AIDS stigma and discrimination and future needs. Indian J Med Resp. 2005;122:471–84. [PubMed] [Google Scholar]
- Menezes AM, Perez-Padilla R, Jardim JB, et al. Chronic obstructive pulmonary disease in five Latin American cities (the PLATINO study): a prevalence study. Lancet. 2005;366:1875–81. doi: 10.1016/S0140-6736(05)67632-5. [DOI] [PubMed] [Google Scholar]
- [MHM and AMMMTS] Ministry of Health of Malaysia, Academy of Medicine of Malaysia and Malaysian Thoracic Society Guidelines in the management of chronic obstructive pulmonary disease. A consensus statement. Med J Malaysia. 1999;54:387–401. [PubMed] [Google Scholar]
- Mueller RE, Keble DL, Plummer J, et al. The prevalence of chronic bronchitis, chronic airway obstruction, and respiratory symptoms in a Colorado city. Am Rev Respir Dis. 1971;103:209–28. doi: 10.1164/arrd.1971.103.2.209. [DOI] [PubMed] [Google Scholar]
- [MHLBI] National Heart, Lung and Blood Institute . Morbidity and mortality: 1998 Chartbook on cardiovascular, lung, and blood diseases. Bethesda MD: US Department, of Health and Human Services, Public Health Service, National Institutes of Health; 1998. [Google Scholar]
- Neffen H, Fritsher C, Schacht FC, et al. AIRLA Survey Group Asthma control in Latin America: the Asthma Insights and Reality in Latin America (AIRLA) survey. Rev Panam Salud Publica. 2005;17:191–7. doi: 10.1590/s1020-49892005000300007. [DOI] [PubMed] [Google Scholar]
- Ni Chroinin M, Greenstone IR, Ducharme FM. Addition of inhaled long-acting beta2-agonists to inhaled steroids as first line therapy for persistent asthma in steroid-naive adults. Cochrane Database Syst Rev. 2004;4:CD005307. doi: 10.1002/14651858.CD005307. [DOI] [PubMed] [Google Scholar]
- Ni Chroinin M, Greenstone IR, Danish A, et al. Long-acting beta2-agonists versus placebo in addition to inhaled corticosteroids in children and adults with chronic asthma. Cochrane Database Syst Rev. 2005;4:CD005535. doi: 10.1002/14651858.CD005535. [DOI] [PubMed] [Google Scholar]
- Oostenbrink JB, Rutten-van Mölken MP, Noord JA, et al. One-year cost-effectiveness of tiotropium versus ipratropium to treat chronic obstructive pulmonay disease. Eur Respir J. 2004;23:241–9. doi: 10.1183/09031936.03.00083703. [DOI] [PubMed] [Google Scholar]
- Oostenbrink JB, Rutten-van Molken MP. Resource use and risks factors in high-cost exacerbations of COPD. Respir Med. 2004;98(9):883–91. doi: 10.1016/j.rmed.2004.02.013. [DOI] [PubMed] [Google Scholar]
- Ottmani SE, Sherpbier R, Pio A, et al. 2005Practical Approach to Lung Health (PAL): A primary health care strategy for the integrated management of respiratory conditions in people five years of age and over Geneva: World Health Organization; WHO/HTM/TB/2005.351. [Google Scholar]
- Paggiaro PL, Dahle R, Bakran I, et al. Multicentre randomised placebo-controlled trial of inhaled fluticasone in patients with COPD. International COPD study group. Lancet. 1998;14:773–80. doi: 10.1016/s0140-6736(97)03471-5. [DOI] [PubMed] [Google Scholar]
- Perera BJ. Efficacy and cost effectiveness of inhaled steroids in asthma in developing countries. Arch Dis Child. 1995;72:312–16. doi: 10.1136/adc.72.4.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price MJ, Briggs AH. Development of an economic model to assess the cost effectiveness of asthma management strategies. Pharmaconeconomics. 2002;20:183–94. doi: 10.2165/00019053-200220030-00004. [DOI] [PubMed] [Google Scholar]
- Rabe KF, Vermeire PA, Soriano JB, et al. Clinical management of asthma in 1999: the Asthma Insights and Reality in Europe (AIRE) study. Eur Respir J. 2000;16:802. doi: 10.1183/09031936.00.16580200. [DOI] [PubMed] [Google Scholar]
- Rabe KF, Adachi M, Lai CKW, et al. Worldwide severity and control of asthma in children and adults: the Global Asthma Insights and Reality Surveys. J Allergy Clin Immunol. 2004;114:40–7. doi: 10.1016/j.jaci.2004.04.042. [DOI] [PubMed] [Google Scholar]
- Rea H, McAuley S, Stewart A, et al. A chronic disease management programme can reduce days hospital for patients with COPD. Intern Med J. 2004;34:608–14. doi: 10.1111/j.1445-5994.2004.00672.x. [DOI] [PubMed] [Google Scholar]
- [SAPS] Working Group of the South African Pulmonary Society Guidelines for the management of chronic obstructive pulmonary disease. South African Med J. 1998;88:8. [PubMed] [Google Scholar]
- Schelledy DC, McCormick SR, LeGrand TS, et al. The effect of a pediatric asthma management program provided by respiratory therapists and patient outcomes and costs. Heart Lung. 2005;34:423–8. doi: 10.1016/j.hrtlng.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Simonella L, Marks G, Sanderson K. Cost-effectiveness of current and optimal treatment for adult asthma. Intern Med J. 2006;36:244. doi: 10.1111/j.1445-5994.2006.01054.x. [DOI] [PubMed] [Google Scholar]
- Slama K, Karsenty S, Hirsch A. Effectiveness of minimal intervention by general practitioners with their smoking patients: a randomised, controlled trial in France. Tob Control. 1995;4:162–9. [Google Scholar]
- Slama K.1998. Tobacco control and prevention. A guide for low income countries. Paris: International Union against Tuberculosis and. Lung Disease. URL at http://www.iuatld.org/
- Sterk PJ, Buist SA, Woolcock AJ, Marks GB, et al. The message from the 1998 World Asthma Meeting. Eur Respir J. 1999;14:1435–53. doi: 10.1183/09031936.99.14614359. [DOI] [PubMed] [Google Scholar]
- [UNDP] United Nations Development Programme . Human development report 1997. Human development to eradicate poverty [French] Paris: Editions Economica; 1997. [Google Scholar]
- Vanable PA, Carey MP, Blair DC, et al. Impact of HIV-related stigma on health behaviors and psychological adjustment among HIV-positive men and women. AIDS Behav. 2006;10:473–82. doi: 10.1007/s10461-006-9099-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viegi G, Pedreschi M, Pistelli F, et al. Prevalence of airways obstruction in a general population: European Respiratory Society vs American Thoracic Society definition. Chest. 2000;117(5 Suppl 2):339S–45S. doi: 10.1378/chest.117.5_suppl_2.339s. [DOI] [PubMed] [Google Scholar]
- Von Hertzen L, Reunanen A, Impivaara O, et al. Airway obstruction in relation to symptoms in chronic respiratory disease—a nationally representative population study. Respir Med. 2000;94:356–63. doi: 10.1053/rmed.1999.0715. [DOI] [PubMed] [Google Scholar]
- Wan, Aït-Khaled N. Dissemination and implementation of Asthma Guidelines. State of the Art. Inter J Tuberc Lung Dis. 2006;10:710–16. [PubMed] [Google Scholar]
- Weiss KB, Sullivan SD. The health economics of asthma and rhinitis. Assessing the economic impact. J Allergy Clin Immunol. 2001;107:3–8. doi: 10.1067/mai.2001.112262. [DOI] [PubMed] [Google Scholar]
- World Bank 1993. World Development Report 1993: Investing in Health. World Bank Publication.
- [WHO] World Health Organization 1999. Framework convention on tobacco control. Technical Briefing Series. WHO/NCD/TF1/99.1–7 [Google Scholar]
- [WHO] World Health Organization . The World Health Report 2004: changing history. Statistical Annex 127–131. Geneva: World Health Organization; 2004. [Google Scholar]
- [WHO] World Health Organization . Preventing chronic diseases: a vital investment [online] Geneva: World Health Organization; 2005. Accessed on November 8, 2006. URL: http://www.who.int/chp/chronic_disease_report/contents/en/index.html. [Google Scholar]
- Wouters EF. Economic analysis of the confronting COPD survey: an overview of results. Respir Med. 2003;97(suppl C):S3–14. doi: 10.1016/s0954-6111(03)80020-3. [DOI] [PubMed] [Google Scholar]
- Yach D. Le tabac en Afrique. Forum Mondial de la Santé. 1996;17:30–8. [Google Scholar]