Abstract
Current practice guidelines for the treatment of COPD recommend the use of combined inhaled corticosteroids and long-acting bronchodilators in severe and very severe patients (GOLD stages III and IV). The aim of this study was to evaluate, through a simulation model, the economic consequences of this recommendation in Italy. We developed a cost-effectiveness analysis (CEA) on five alternative therapeutic strategies (salmeterol/fluticasone, SF; formoterol/budesonide, FB; salmeterol alone, S; fluticasone alone, F; control, C). Published data on the Italian COPD population and efficacy data from international reference trials were fitted in a disease progression model based on a Markov chain representing severity stages and death. The yearly total direct costs of treating COPD patients in Italy was estimated at approximately €7 billion, with a mean cost per patient per year of around €2450. Mean survival of the cohort is 11.5 years. The C and F strategies were dominated (ie, are associated with worse outcomes and higher costs) by all alternatives. SF and FB were the most effective strategies, with a slight clinical superiority of SF, but they were also marginally more expensive than S. Incremental cost-effectiveness of SF vs S was €679.5 per avoided exacerbation and €3.3 per symptom-free day. Compared with current practice, the recommended use of combined inhaled corticosteroids and long-acting bronchodilators for severe and very severe COPD patients has the potential for improving clinical outcomes without increasing healthcare costs.
Keywords: severe COPD, pharmacoeconomics, Markov chain, inhaled corticosteroids, inhaled bronchodilators, cost-effectiveness
Introduction
Chronic obstructive pulmonary disease (COPD) is the fourth leading cause of death in the world (WHO 2000) and it is ranked among the first causes of disability in developed countries like Italy. COPD in Italy is a disease with substantial social costs as it ranks 7th by number of hospital admissions and 4th by number of hospital days.
Exacerbation is the main cause of hospital admission in COPD patients. Exacerbation implies a substantial impairment of patient respiratory ability and quality of life and can even lead to death.
It has been demonstrated that only smoking cessation can produce a relative slowdown in the chronic course of the disease, while pharmaceutical therapies aim to improve patients’ quality of life by reducing exacerbation frequency and severity.
The Global Initiative for Chronic Lung Disease (GOLD) was established in 1997. Its goals are to increase awareness of COPD and decrease mortality and morbidity from the disease.
The GOLD 2004 guideline (GOLD 2004) classifies disease severity in 4 stages based on chronic symptoms, forced expiratory volume in 1 second (FEV1), and forced vital capacity (FVC).
This pragmatic staging approach aims to simplify practical patient management and offer some general indications for pharmacotherapeutic choice. Risk factor avoidance and influenza vaccination, which can prevent serious illness and complication onset, are baseline recommendations in all stages of patient treatment. At stage I, bronchodilators are generally prescribed on an as-needed basis for relief of persistent, or worsening, symptoms. The most commonly used bronchodilator drugs include ß2-agonists, anticholinergics, and methylxanthines. At stage II, GOLD guidelines recommend the addition of pulmonary rehabilitation and regular treatment with one or more long-acting bronchodilators. Pulmonary rehabilitation aims at resolving a range of nonpulmonary problems including social isolation, altered mood states (especially depression), muscle wasting, and weight loss. The addition of regular treatment with inhaled glucocorticosteroids is appropriate for symptomatic COPD patients with an FEV1 <50% predicted (stage III and stage IV). Combined inhaled glucocorticosteroids and long-acting ß2-agonists are more effective than the individual components; according with GOLD judgments, combining drugs with different mechanisms and durations of action might increase the degree of bronchodilation with equivalent or fewer side-effects. Long-term oxygen therapy is generally added in stage IV, while other pharmacological treatments (such as antioxidants and mucolytic agents) are frequently used as adjuvant therapy.
The economic consequences of the adoption of the GOLD strategy, especially the use of inhaled glucocorticosteroids combined with long-acting ß2-agonists, for the whole Italian COPD patient population have not been assessed yet.
In the absence of “real” data from specific trial or observational studies, it is a quite well established practice to build analytical models that simulate disease dynamics and infer clinical and economic outcomes of competing treatment strategies. In particular, for chronic diseases such as COPD, where the horizon of the analysis spans over the whole patient’s lifetime, a modeling approach for economic evaluation could easily be the only choice. Trials of the necessary size and duration would be unrealistic.
The aim of the present study is to evaluate, through a simulation model, the clinical and economic consequences of the implementation of GOLD 2004 guidelines for severe and very severe COPD patients in Italy.
Material and methods
We developed a cost-effectiveness analysis (CEA) on the use of combined inhaled glucocorticosteroids and long-acting ß2-agonists to evaluate the relative pharmacoeconomic performance of the two available products. We then compared a combined treatment with a single inhaled corticosteroid and with a single long-acting bronchodilator. The control in each case was a standard treatment.
The CEA is the typical economic evaluation that should be performed when comparing 2 or more therapeutic alternatives whose clinic efficacy is not equivalent (Drummond et al 1997). In this analysis, both the costs and the health consequences of the alternatives are examined. The direct comparison between two alternatives is obtained through the incremental cost effectiveness ratio (ICER). Comparing strategy A with strategy B, the ICER value represents the relative increment of cost at which a relative unitary increment of benefit could be obtained. If we indicate the cost of the two alternatives by CA and CB, and the benefits (for instance, life years saved, hospitalization avoided, …) by BA and BB this gives Eq. (1)
| (1) |
In this study the costs and benefits were evaluated with a simulation model. This was designed as a Markov chain which simulates the disease course and consequences under a specific treatment for a given cohort of patients. All calculations were run in MS Excel.
The five therapeutic alternatives considered were:
Control (C): no change with respect to the standard therapy already in use by the patient;
Salmeterol/fluticasone (SF): use of combined salmeterol/fluticasone 50/500 μg bid in GOLD stages III and IV patients in addition to the standard therapy already in use;
Formoterol/budesonide (FB): use of combined formoterol/budesonide 2 × 160/4.5 μg bid in GOLD stages III and IV patients in addition to the standard therapy already in use;
Fluticasone (F): use of fluticasone 500 μg bid in GOLD stages III and IV patients in addition to the standard therapy already in use;
Salmeterol (S): use of salmeterol 50 μg bid in GOLD stages III and IV patients in addition to the standard therapy already in use.
Model structure
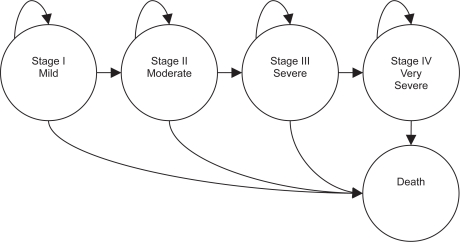
The Markov chain comprised 5 health states, representing 4 COPD severity stages – stage I mild, stage II moderate, stage III severe, and stage IV very severe – and death (Figure 1).
Figure 1.
Markov chain structure. Arrows represent the possible pathways of the disease course.
The simulation cycle lasts 1 year. During every cycle each patient in the cohort has the possibility of progressing to the next worse health state, remaining in the current state, or dying. This progression is regulated by the transition probabilities among states.
Due to the choice of a relatively short cycle duration no direct transition to further worse states (ie, from stage I to stage III) is allowed. This means that we have only transitions between adjacent severity states. Moreover, no regression of the disease (ie, leftward transition) is allowed.
Initial population
In our study the cohort that populates the model at the beginning of the simulation is defined to represent the whole Italian population of COPD patients. Several epidemiological studies have attempted to measure the prevalence of respiratory symptoms and COPD among the general Italian population (Viegi et al 1988, 2001; Stang et al 2000; ISTAT 2001; De Marco et al 2004). An international study (De Marco et al 2004) reported a 1.3% COPD prevalence in Italy for people aged 20–44 years. An Italian study (Viegi et al 2001) reported a 6% prevalence in the population aged 46–55 and 11% in the population aged 56–65 years. A multi-purpose study from the Italian Statistics Institute (ISTAT 2001) reported a 14.1% prevalence in the elderly population (over 65 years).
The application of these data to the general Italian population (from ISTAT 1999–2000) leads to an estimate of about 2.9 million COPD patients. This value well compares with the results of a smoking rates-based disease model (Stang et al 2000) which estimated that in Italy 2.6 million men and women aged 45–70 years suffer from diagnosed or undiagnosed COPD. In order to populate the health states, the whole cohort had to be split into severity stages. Unfortunately, no study presenting COPD prevalence by GOLD severity stages is currently available for Italy. The closest pertinent data available on published literature are related to US and UK COPD patients, as reported in the 2004 ERS Congress (Price et al 2004) (Table 1). We used the values published in this study to arrange the initial model population (42% stage I, 41% stage II, 13% stage III, and 4% stage IV).
Table 1.
Model initial population characteristics
| I: Mild | II: Moderate | III: Severe | IV: Very severe | Total | Source | |
|---|---|---|---|---|---|---|
| % population | 42% | 41% | 13% | 4% | 100% | Price et al 2004 |
| Initial population | 1,216,444 | 1,187,481 | 376,518 | 115,852 | 2,896,296 | |
| FEV1 (% predicted) | 89.80% | 63.70% | 40.00% | 26.20% | Antonelli-Incalzi et al 2003 | |
| Exacerbations/patient/year | 0 | 0.8 | 1.8 | 2.6 | Authors’ estimate from ISOLDE (Hankinson et al 1999; Burge and Wedzicha 2003) |
Model health states were characterized by an average FEV1 value and an average exacerbation per patient per year value. The set of FEV1 values by state was obtained from an Italian population study (SaRA – Salute Respiratoria nell’Anziano) (Antonelli-Incalzi et al 2003). The number of exacerbations by severity stage is a set of values hard to find in the literature, mainly because the definition of exacerbation itself is not standard among authors. We made an estimate via an adaptation of data reported in the ISOLDE (Inhaled Steroids in Obstructive Lung Disease in Europe) (Burge and Wezicha 2003) study to GOLD severity stages, with the use of the NHANES/Hankinson equation (Hankinson et al 1999) for predicted FEV1.
Transition probabilities
For each disease stage, the probability of progressing to the next worse health state was calculated from the average pulmonary capacity reduction of COPD patients (Fletcher et al 1976) and predicted values of FEV1 (Crapo and Morris 1981; Crap et al 1981, 1982).
The transition probability to the death state was obtained by applying a stage-specific relative risk (RR) to the mortality rates of the general Italian population (ISTAT 2003). These RRs were calculated in order to fit the 4-year survival ratios presented for each disease severity stage in a 2002 study (Domingo-Salvany et al 2002), which showed a strong relationship between mortality and FEV1.
Outcomes
Treatment efficacy for the SF, S, and F alternatives was derived from the reference trial (Calverley et al 2003) of the salmeterol/fluticasone combination (which compared the combination with the two single components and placebo). The efficacy for the FB alternative was derived from the reference trial (Szafranski et al 2003) of the formoterol/budesonide combination. The two international randomized controlled trials (RCTs) have a substantially overlapping protocol.
Another international RCT (Calverley et al 2003) with similar results, but with a slight protocol difference due to the presence of a run-in phase, was discarded in order to obtain the maximum possible source homogeneity.
The application of international RCT findings to an Italian setting was validated by a smaller Italian study (Dal Negro et al 2003a) which shows very similar results.
All these studies confirm the judgment expressed by the GOLD Panel that none of the existing medications for COPD can modify the long-term decline in lung function. Therefore the main aim of COPD pharmacotherapy is to control symptoms and exacerbations severity and frequency.
Consequently the annual average exacerbation number and percentage of symptom-free days per patient were considered as outcomes for CEA.
Costs and cost perspective
Model costs were calculated via an adaptation of the ICE (Italian Costs for Exacerbations in COPD) study (Lucioni et al 2005) and from Dal Negro et al (2003b), and are classified as direct and indirect costs for each disease stage.
Direct costs take into account hospitalizations, medical visits, and examinations, pharmaceutical treatments (different from SF, FB, S, or F), oxygen therapy, lung ventilation, and rehabilitative therapy.
Indirect costs account for lost productivity of the patient and first degree relatives.
The ICE study classified both direct and indirect costs in two parts, one caused directly by exacerbations and one independent of them.
This classification let us calculate the cost per exacerbation for each disease stage (Table 3)
Table 3.
Direct and indirect costs. Derived from Lucioni et al (2005) and Dal Negro et al (2003b)
|
Costs (€/patient/year) | ||||
|---|---|---|---|---|
| Stage I | Stage II | Stage III | Stage IV | |
| Direct cost independent of exacerbation | 527.48 | 918.38 | 1592.59 | 3586.18 |
| Direct cost per exacerbation | 1219.46 | 1475.87 | 2637.33 | |
| Indirect cost independent of exacerbation | 15.77 | 21.97 | 43.71 | |
| Indirect cost per exacerbation | 27.81 | 38.76 | 77.10 | |
The pharmaceutical cost for the active treatment (SF, FB, S, or F) should be added to the direct exacerbation-independent cost. In Italy this cost is paid by the National Health Service.
Different cost perspectives are adopted in CEA:
Patient perspective: in this case only indirect costs were taken into account;
Italian National Health Service perspective: only direct costs were taken into account;
Societal perspective: direct plus indirect costs were taken into account.
Results
The time horizon of the simulation was 1, 5, 10 years, and lifelong, which means that the model iteration was stopped when the whole cohort reaches the death state. At the end of the simulation the average cohort survival was 11.5 years (Table 4).
Table 4.
Model outcomes and costs (€) at the end of a life-long simulation (average values per patient)
| Strategy | Exacerbation number | Symptom-free days | Direct cost | Indirect cost | Direct + Indirect cost |
|---|---|---|---|---|---|
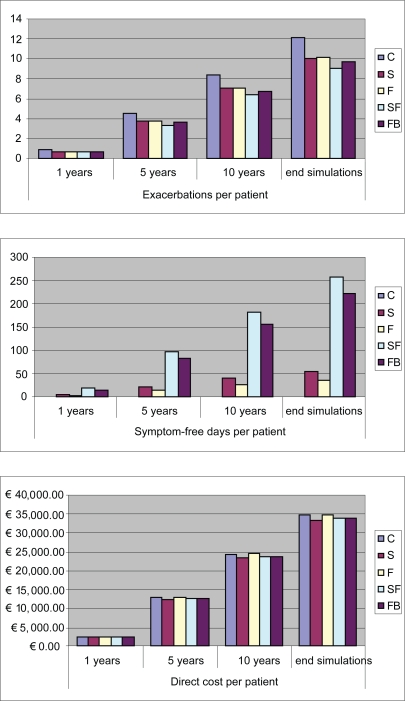
| C | 12.04 | 0 | 34,632.09 | 715.74 | 35,347.83 |
| S | 10.07 | 55 | 33,369.28 | 619.87 | 33,989.15 |
| F | 10.14 | 37 | 34,754.38 | 623.55 | 35,377.93 |
| SF | 9.09 | 257 | 34,037.71 | 571.93 | 34,609.64 |
| FB | 9.66 | 220 | 33,944.51 | 599.73 | 34,544.25 |
Abbreviations: C, control; F, fluticasone alone; FB, formoterol/budesonide; S, salmeterol alone; SF, salmeterol/fluticasone.
The total yearly direct costs of managing COPD patients in Italy were estimated at approximately €7 billion, with a mean cost per patient per year of around €2450.
The indirect costs results were scarcely significant compared with the direct costs, as they represent on average less than 2% of the total. Therefore CEA is performed only from the perspective of the Italian National Health Service, in which only direct costs are taken into account.
All strategies appeared to be dominant (ie, give a better outcome at a smaller cost) with respect to C, except for S which was slightly more costly. SF and FB were the most effective strategies, with SF having a slight clinical advantage, but they were also marginally more expensive than S (Figure 2).
Figure 2.
Cost and outcomes resulting from 1, 5, and 10 years, and life-long time horizon simulation.
Abbreviations: C, control; F, fluticasone alone; FB, formoterol/budesonide; S, salmeterol alone; SF, salmeterol/fluticasone.
The ICER was calculated for these two alternatives with respect to S. The ICER value here represents the amount that should be paid to obtain a unit increase in outcome (ie, one exacerbation avoided or a symptom-free day gained) with the adoption of a more efficient and more expensive strategy (SF or FB vs S) (Table 5).
Table 5.
Incremental cost effectiveness ratio (ICER) calculation with respect to the less expensive strategy (S)
| Exacerbations per patient | Symptom-free days per patient | Direct cost per patient (€) | ||
|---|---|---|---|---|
| SF vs S | Difference | 0.98 | 202 | 668.43 |
| ICER | 679.55 (€/exacerbation) | 3.31 (€/symptom-free day) | ||
| FB vs S | Difference | 0.41 | 165 | 575.23 |
| ICER | 1392.38(€/exacerbation) | 3.48(€/symptom-free day) |
Abbreviations: C, control; F, fluticasone alone; FB, formoterol/budesonide; RR, relative risk; S, salmeterol alone; SF, salmeterol/fluticasone.
Sensitivity analysis
One-way sensitivity analyses on the main model parameters were performed in order to assess the robustness of the results obtained from the model. The SF vs C case (with exacerbation reduction as an outcome) is presented here. All other possible combinations show a similar behavior.
Dominancy of the SF strategy could be lost mainly due to:
Reduction of the average initial number of exacerbations per patient per year;
Reduction of treatment efficacy in terms of exacerbations reduction;
Reduction of hospitalization costs;
Increase of treatment pharmaceutical cost.
This means that as a consequence of one of these variations the SF strategy could become more costly than C. The threshold value gives the point where the differential cost between SF and C strategies equals zero (Table 6).
Table 6.
Base case and threshold values of model parameters that can cause strategy dominancy loss more rapidly
| Parameter | Base value | Threshold value |
|---|---|---|
| Average initial population number of exacerbations/patient/year | 0.67 | 0.59 |
| Relative risk exacerbations | 0.700 | 0.734 |
| Hospitalization costs | €1862.00 | €1584.00 |
| Treatment pharmaceutical cost | €81.43 | €91.28 |
Discussion
Attention to COPD is constantly increasing worldwide because its high prevalence, morbidity, and mortality represent a challenging problem for all healthcare systems. The burden of COPD, measured as its impact on patients’ symptoms and quality of life and the corresponding use of healthcare resources, is still a major aspect of the disease.
For these reasons healthcare decision makers, before deciding which strategies should be preferred, need to improve their understanding of the concept of good value for money, in order to control the disease and reduce the huge costs required to meet patients’ needs.
It is now well established that the main proportion of COPD costs depends on the clinically uncontrolled disease and on its high exacerbation rate, frequently leading to the patients’ hospitalization.
Recommendations to treat COPD according to the most accepted guidelines have been disseminated over recent years even though COPD still remains under-diagnosed and under-treated worldwide. Obviously, more severe degrees of COPD have received most attention both in terms of monitoring of clinical outcomes and in assessing the economic value of therapeutic interventions, although the effects of guideline recommendations have been investigated in terms of pharmacoeconomic convenience only episodically.
The main scope of this study was to evaluate the clinical and economic consequences of implementation of GOLD guidelines for severe and very severe COPD patients in Italy. The affordability of its widespread application, as well as the relative pharmacoeconomic performance of the available options for severe and very severe COPD, were investigated. Although the clinical efficacy of the suggested approach is widely accepted, the economic consequences and National Health Service budget impact have not yet been well assessed. To contribute to the understanding of this point, we designed a pharmacoeconomic analytical model which combines clinical data from international RCTs with local economic studies and demographic data. The main limit of this study is the model itself. Analytical models necessarily are a simplification and schematization of the real process they represent. But modeling is often the only viable approach to economic evaluation, because for chronic disease trials of the necessary size and duration would be unrealistic. We should point out the frequent need in our model for assumptions mainly because of the heterogeneity of the different sources of information and the substantial scarcity of Italian data.
There is a good general consensus that combining medications of different pharmacological classes represents a much more convenient strategy in COPD, particularly for severe or very severe disease. Additional effects have in fact been proven both in functional and in clinical terms under these conditions. In particular, health status, quality of life, and exacerbations represent the most affected outcomes in more severe COPD (basal FEV1 <50% predicted) when treated with combined long-acting β2-agonists and inhaled corticosteroids over time. These data highlighted the favorable therapeutic performance of this particular strategy, thus suggesting that healthcare costs would be also affected positively.
In agreement with the GOLD recommendation, the therapeutic strategies investigated the use of two different combined inhaled corticosteroids and long-acting bronchodilators in comparison with a single inhaled corticosteroid, a single long-acting bronchodilator, and control.
Results from the model showed that the recommended use of combined inhaled corticosteroids and long-acting bronchodilators for severe and very severe COPD patient treatment, compared with current practice, had the potential to improve clinical outcomes, and consequently patients quality of life, without increasing healthcare costs. The same conclusion was drawn in recent studies (Gagnon et al 2005; Lofdahl et al 2005; Spencer et al 2005), based on a similar modeling approach, developed for the US, Canadian, and Swedish healthcare systems, respectively.
This conclusion seems relevant not only from the patient’s perspective (such as improvement of clinical conditions) but also from a societal perspective. These data confirm that it is possible to improve substantially the health status of severe and very severe COPD patients without further increasing social costs.
Table 2.
Main outcomes. Derived from Calverley et al (2003) and Szafransky et al (2003)
| C | SF (Calverley) | FB (Szafranski) | S (Calverley) | F (Calverley) | |
|---|---|---|---|---|---|
| Exacerbation RR (vs placebo) | 0.70 | 0.76 | 0.80 | 0.81 | |
| Symptom-free days | 0% | 14% | 12% | 3% | 2% |
Abbreviations: C, control; F, fluticasone alone; FB, formoterol/budesonide; RR, relative risk; S, salmeterol alone; SF, salmeterol/fluticasone.
Acknowledgments
This study was funded by GlaxoSmithKline Italia.
References
- Antonelli-Incalzi R, Imperiale C, Bellia V, et al. Do GOLD stages of COPD severity really correspond to differences in health status? Eur Respir J. 2003;22:444–9. doi: 10.1183/09031936.03.00101203. [DOI] [PubMed] [Google Scholar]
- Burge S, Wedzicha JA. COPD exacerbations: definitions and classifications. Eur Respir J. 2003;21(Suppl 41):46s–53s. doi: 10.1183/09031936.03.00078002. [DOI] [PubMed] [Google Scholar]
- Calverley PM, Boonsawat W, Cseke Z, et al. Maintenance therapy with budesonide and formoterol in chronic obstructive pulmonary disease. Eur Respir J. 2003;22:912–19. doi: 10.1183/09031936.03.00027003. [DOI] [PubMed] [Google Scholar]
- Calverley P, Pauwels R, Vestbo J, et al. Combined salmeterol and fluticasone in the treatment of chronic obstructive pulmonary disease: a randomised controlled trial. The Lancet. 2003;361:449–56. doi: 10.1016/S0140-6736(03)12459-2. [DOI] [PubMed] [Google Scholar]
- Crapo RO, Morris AH, Gardner RM. Reference spirometric values using techniques and equpment that meet ATS recommendations. Am Rev Respir Dis. 1981;123:659–64. doi: 10.1164/arrd.1981.123.6.659. [DOI] [PubMed] [Google Scholar]
- Crapo RO, Morris AH. Standardized single-breath normal values for carbon monoxide diffusing capacity. Am Rev Respir Dis. 1981;123:185–9. doi: 10.1164/arrd.1981.123.2.185. [DOI] [PubMed] [Google Scholar]
- Crapo RO, Morris AH, Clayton PD, et al. Lung volumes in healthy nonsmoking adults. Bull Europ Physiopathol Respir. 1982;18:419–25. [PubMed] [Google Scholar]
- Dal Negro RW, Pomari C, Tognella S, et al. Salmeterol and fluticasone 50 μg/250 μg bid in combination provides a better long-term control than salmeterol 50 μg bid alone and placebo in COPD patients already treated with theophylline. Pulm Pharmacol Ther. 2003a;16:241–6. doi: 10.1016/s1094-5539(03)00065-8. [DOI] [PubMed] [Google Scholar]
- Dal Negro RW, Rossi A, Cerveri I. The burden of COPD in Italy: results from the Confronting COPD survey. Respir Med. 2003b;97(Suppl C):S43–S50. doi: 10.1016/s0954-6111(03)80024-0. [DOI] [PubMed] [Google Scholar]
- De Marco R, Accordini S, Cerveri I, et al. An international survey of chronic obstructive pulmonary disease in young adults according to GOLD stages. Thorax. 2004;59:120–5. doi: 10.1136/thorax.2003.011163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domingo-Salvany A, Lamarca R, Ferrer M, et al. Health-related quality of life and mortality in male patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2002;166:680–5. doi: 10.1164/rccm.2112043. [DOI] [PubMed] [Google Scholar]
- Drummond MF, O’Brien B, Stoddart GL, et al. Methods for the economic evaluation of health care programmes. 2nd ed. Oxford: Oxford University Press; 1997. [Google Scholar]
- Fletcher CM, Peto R, Tinker CM, et al. The natural history of chronic bronchitis and emphysema. Oxford: Oxford University Press; 1976. [Google Scholar]
- Gagnon YM, Levy AR, Spencer MD, et al. Economic evaluation of treating chronic obstructive pulmonary disease with inhaled corticosteroids and long-acting beta2-agonists in a health maintenance organization. Respir Med. 2005;99:1534–45. doi: 10.1016/j.rmed.2005.03.018. [DOI] [PubMed] [Google Scholar]
- [GOLD] Global Initiative for Chronic Obstructive Lung Disease Global Strategy for the Diagnosis, Management and Prevention of Chronic Obstructive Pulmonary Disease. Updated. 2004. Executive summary. Based on April 1998 NHLBI/WHO Workshop.
- Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. Population. Am J Respir Crit Care Med. 1999;159:179–87. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- [ISTAT] Italian National Statistic Institute . Indagine Multiscopo sulle famiglie: condizioni di salute e ricorso ai servizi sanitari anno 1999–2000. Rome: ISTAT; 2001. [Google Scholar]
- [ISTAT] Italian National Statistic Institute . Annuario statistico italiano 2003. Rome: ISTAT; 2003. [Google Scholar]
- Lofdahl CG, Ericsson A, Svensson K, et al. Cost effectiveness of budesonide/formoterol in a single inhaler for COPD compared with each monocomponent used alone. Pharmacoeconomics. 2005;23:365–75. doi: 10.2165/00019053-200523040-00006. [DOI] [PubMed] [Google Scholar]
- Lucioni C, Donner CF, De Benedetto F, et al. I costi della broncopneumopatia cronica ostruttiva: la fase prospettica dello studio ICE (Italian Costs for Exacerbations in COPD) Pharmacoeconomics. Italian research articles. 2005;7:119–34. [Google Scholar]
- Price D, Tinkelman DG, Nordyke RJ, et al. 2004Severity distribution of COPD in primary care [abstract] Proceedings of the European Respiratory Society CongressAbstract ERS4L1_2562. [Google Scholar]
- Stang P, Lydick E, Silberman C, et al. The prevalence of COPD. Using smoking rates to estimate disease frequency in the general population. Chest. 2000;117:354s–9s. doi: 10.1378/chest.117.5_suppl_2.354s. [DOI] [PubMed] [Google Scholar]
- Spencer M, Briggs AH, Grossman RF, et al. Development of an economic model to assess the cost effectiveness of treatment interventions for chronic obstructive pulmonary disease. Pharmacoeconomics. 2005;23:619–37. doi: 10.2165/00019053-200523060-00008. [DOI] [PubMed] [Google Scholar]
- Szafranski W, Cukier A, Ramirez A, et al. Efficacy and safety of budesonide/formoterol in the management of chronic ostructive pulmonary disease. Eur Respir J. 2003;21:74–81. doi: 10.1183/09031936.03.00031402. [DOI] [PubMed] [Google Scholar]
- Viegi G, Paolitti P, Prediletto R, et al. Prevalence of respiratory symptoms in an unpolluted area of Northern Italy. Eur Respir J. 1988;I:311–18. [PubMed] [Google Scholar]
- Viegi G, Scognamiglio A, Baldacci S, et al. Epidemiology of chronic obstructive pulmonary disease. Respiration 2001. 2001;68:4–19. doi: 10.1159/000050456. [DOI] [PubMed] [Google Scholar]
- [WHO] World Health Organization . World health report. Geneva: WHO; 2000. [Google Scholar]