Abstract
In humans, non-rapid eye movement (NREM) sleep slow waves occur not only spontaneously but can also be induced by transcranial magnetic stimulation. Here we investigated whether slow waves can also be induced by intracortical electrical stimulation during sleep in rats. Intracortical local field potential (LFP) recordings were obtained from several cortical locations while the frontal or the parietal area was stimulated intracortically with brief (0.1 ms) electrical pulses. Recordings were performed in early sleep (1st 2–3 h after light onset) and late sleep (6–8 h after light onset). The stimuli reliably triggered LFP potentials that were visually indistinguishable from naturally occurring slow waves. The induced slow waves shared the following features with spontaneous slow waves: they were followed by spindling activity in the same frequency range (∼15 Hz) as spontaneously occurring sleep spindles; they propagated through the neocortex from the area of the stimulation; and compared with late sleep, waves triggered during early sleep were larger, had steeper slopes and fewer multipeaks. Peristimulus background spontaneous activity had a profound influence on the amplitude of the induced slow waves: they were virtually absent if the stimulus was delivered immediately after the spontaneous slow wave. These results show that in the rat a volley of electrical activity that is sufficiently strong to excite and recruit a large cortical neuronal population is capable of inducing slow waves during natural sleep.
INTRODUCTION
The most distinctive feature of the electroencephalogram (EEG) during non-rapid eye movement (NREM) sleep is the near-synchronous occurrence of slow waves in all or most cortical areas. The fundamental cellular phenomenon underlying the EEG slow waves is the cortical slow oscillation, which consists of an up state, characterized by sustained neuronal depolarization and irregular firing, followed by a hyperpolarized down state, during which every cortical cell ceases firing (Amzica and Steriade 1998; Destexhe et al. 1999; Steriade et al. 1993, 2001). Slow oscillations (<1 Hz) have also been observed in the EEG under anesthesia and in naturally sleeping cats and humans (Achermann and Borbely 1997; Steriade and Amzica 1998).
It has been shown in humans that slow waves recorded with scalp EEG can be induced reliably by transcranial magnetic stimulation (TMS) during sleep (Massimini et al. 2007). Specifically, the TMS-induced slow waves were morphologically similar to the spontaneous sleep slow waves, were followed by a spindle, and propagated across the cortex as a traveling wave (Massimini et al. 2004, 2007). Moreover, TMS stimulation resulted in a deepening of sleep as reflected by an increase in EEG slow-wave activity (SWA, 0.5–4.0 Hz), which is a measure of sleep intensity and is homeostatically regulated (Borbély and Achermann 2005).
In this study, we took advantage of an animal model to address several questions that have so far received little attention. Local stimulation and recording with intracortical local field potentials (LFPs) can provide an important advantage because, unlike human scalp EEG, bipolar intracortical LFP recordings are not affected by volume conduction and reflect primarily local signals. First, it is unknown whether local electrical intracortical stimulation during sleep in rats can lead to the occurrence of full-fledged sleep slow waves and, if so, whether evoked waves show similarities with spontaneous slow waves. Second, it is unknown whether such induced slow waves would be followed by a spindle, as has been shown in humans (Massimini et al. 2007). Third, it is unknown whether, once elicited by local electrical stimulation, slow waves would propagate across cortical areas, as has been shown in vitro, in anesthetized animals, and in humans (MacLean et al. 2005; Massimini et al. 2007; Richardson et al. 2005; Rigas and Castro-Alamancos 2007; Sanchez-Vives and McCormick 2000; Timofeev et al. 2000.
Furthermore, this approach might help to understand the homeostatic function of the cortical slow oscillations and sleep slow waves. While it is well known that high-amplitude slow waves are abundant in early sleep and decrease across the sleep period, neither their function nor the mechanisms underlying their local and global occurrence are clear. We have shown recently that the changes in the amplitude and slopes of slow waves occurring as a function of sleep/wake history might arise from the variations in cortical synchrony due to changes in synaptic strength (Esser et al. 2007; Massimini et al. 2007; Vyazovskiy et al. 2007b, 2008). We expected that the induced slow waves would show similar changes. Moreover, it is possible that cortical responsiveness varies as a function of sleep depth or depending on changes in spontaneous activity, as has been suggested by in vitro and human studies (Massimini et al. 2003; Sanchez-Vives and McCormick 2000; Watson et al. 2008).
The first aim of this study was therefore to investigate whether it is possible to induce slow waves by local intracortical electrical stimulation in sleeping rats. The second aim was to characterize such induced slow waves and to investigate their topography and effects of preceding sleep/wake history and of their background spontaneous activity.
METHODS
Surgery and chronic recording of sleep and wakefulness
Male WKY rats (Harlan, 11–12 wk old at time of surgery) were used. Under deep isoflurane anesthesia (1.5–2% volume), rats were implanted bilaterally in the frontal cortex (B: +2–3 mm, L: 2–3 mm) with bipolar concentric LFP electrodes (outer electrode: length: 1.5 mm, diameter: 0.8 mm; inner electrode projection: 1 mm, diameter: 0.28 mm; PlasticsOne) for stimulation and chronic recordings. In a subset of animals, additional electrodes of the same type (bipolar concentric, PlasticsOne, same dimensions, see preceding text) were implanted bilaterally in the parietal (B: −2.5 mm, L: 5.5 mm) and the occipital areas (B: −7 mm, L: 3.5 mm). Electrodes were fixed to the skull with dental cement. Two stainless steel wires (diameter: 0.4 mm) inserted into the neck muscles served to record the electromyogram (EMG). After surgery all rats were housed individually in transparent Plexiglas cages (36.5 × 25 × 46 cm) and kept in sound-proof recording boxes for the duration of the experiment. Lighting and temperature were kept constant (LD 12:12, light on at 10:00 a.m., 23 ± 1°C; food and water were available ad libitum and replaced daily at 10:00 a.m.). Seven to 10 days were allowed for recovery after surgery, and experiments were started only after the sleep/waking cycle had fully normalized. Rats were connected by means of a flexible cable to a commutator (Airflyte, Bayonne, NJ) and recorded continuously for 2-4- wk using a Grass electroencephalograph (mod. 15LT, Astro-Med.,West Warwick, RI). Video recordings were performed continuously with infrared cameras (OptiView Technologies) and stored in real-time (AVerMedia Technologies).
One group of animals (n = 8) was recorded during several consecutive sessions of cortical stimulation (see following text; each lasting 1–2 h) in the light period. LFP and EMG signals were amplified (amplification factor: 2,000), and filtered [attenuation 50% amplitude (−6 dB)] as follows: EEG: high-pass filter at 0.1 Hz; low-pass filter at 1 kHz; EMG: high-pass filter at 10 Hz; low-pass filter at 100 Hz. All signals were sampled and stored at 5-kHz resolution. Subsequently the LFP signal was downsampled to 128 Hz for some analyses.
Another group of animals (n = 14) (same as in Vyazovskiy et al. 2007b) was recorded undisturbed during 24 h (see Vyazovskiy et al. 2007b for details) and the frontal LFP signal was analyzed to assess time intervals between two consecutive high-amplitude slow waves.
A third group of animals (n = 6) was recorded during an undisturbed 24-h period, and one consolidated sleep episode was selected for the traveling slow waves analysis (see following text) within the first 2 h after light onset (4.4–10.9 min long; mean 8.6 ± 1.3 min). In those animals, LFP electrodes were implanted bilaterally in the frontal (B: +2–3 mm, L: 2–3 mm), parietal (B: −2.5 mm, L: 5.5 mm) and the occipital areas (B: −7 mm, L: 3.5 mm).
Scoring vigilance states
Sleep stages were scored off-line by visual inspection of 4-s epochs (SleepSign, Kissei). Waking was characterized by a low-voltage, high-frequency EEG pattern and phasic EMG-activity. NREM sleep was characterized by the occurrence of high-amplitude slow waves, spindles and low tonic EMG activity. During REM sleep, instead, the EEG was similar to that during waking, but only heart beats and occasional twitches were evident in the EMG signal. Epochs containing artifacts, predominantly during active waking, were excluded from spectral analysis. Vigilance state could always be determined. NREM sleep episodes were defined as periods of NREM sleep lasting for >16 s, preceded or followed by waking or REM sleep, (Vyazovskiy et al. 2007a).
Cortical stimulation
Intracortical bipolar stimulation was achieved by stimulating the deep part of the electrode (bipolar concentric electrode, PlasticsOne, see preceding text) located in the right frontal or the right parietal cortex, while cortical responses were recorded either in the contralateral frontal cortex or, in addition, in the parietal and the occipital cortices bilaterally. The superficial electrode served as reference for both bipolar stimulation and bipolar recording. In an attempt to stimulate primarily the bodies of cortical pyramidal cells, the electrical pulse was applied to the deep part of the bipolar electrode, located in the layer V, and was referenced to the superficial part of the bipolar electrode. Both the deep and the superficial part of the electrode were located presumably within the same cortical column. Therefore the current flow was oriented in a plane perpendicular to the surface of the cortex, approximating the flow along the cell body and the apical dendrites. Prior to the experiment, input-output tests were performed in each rat. For the final analysis, we chose a stimulation intensity high enough to reliably induce slow waves visually indistinguishable from naturally occurring slow waves but still too low to elicit any noticeable motor output or arousal. S88 Dual Output Square Pulse Stimulator and stimulus isolating unit (PSIU-6) (Grass-Telefactor, AstroMed) were used for electrical stimulation, which consisted of monophasic squared pulse of 0.1-ms duration.
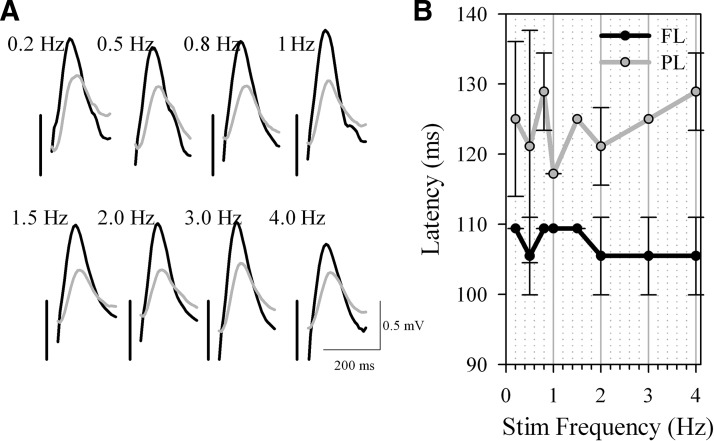
In a pilot study (n = 2 rats), stimuli to the right frontal cortex were delivered automatically at several frequencies (0.2, 0.5, 0.8, 1.0, 2.0, 3.0, and 4.0 Hz) during NREM sleep under constant visual observation, while the evoked slow waves were recorded in the contralateral frontal and parietal area. The amplitude of the evoked slow waves did not significantly change across frequencies, nor was the phase relationship between the derivations noticeably altered (Fig. 1). To investigate the influence of background spontaneous activity on the shape of the evoked slow wave, in all remaining experiments stimuli were delivered continuously during NREM sleep at 0.2 Hz (2,590.7 ± 622.7 waves).
FIG. 1.
Local intracortical electrical stimulation of the right frontal cortex triggers contralateral slow waves. In n = 2 animals, electrical pulses were delivered at various frequencies between 0.2 and 4.0 Hz during spontaneous nonrapid-eye-movement (NREM) sleep. A: the evoked slow waves recorded from the frontal left (black traces) and the parietal left (gray traces) cortical area. Traces are averages of ∼30 trials. Positivity –upward. Vertical bars indicate the timing of the trigger. B: mean ± SD (n = 2) latency to the peak of the frontal (FL) and parietal (PL) slow waves. Note that the parietal waves occurred with a consistent delay compared with the frontal waves.
Detection and analysis of spontaneous and induced slow waves
Detection of individual slow waves was performed as previously described (Vyazovskiy et al. 2007b) on the spontaneous LFP signal and during the slow wave induction protocol. First, band-pass filtering (0.5–4 Hz, stopband edge frequencies: 0.1–10 Hz) with MATLAB filtfilt function exploiting a Chebyshev Type II filter design (MATLAB, The Math Works, Natick, MA) was used (Achermann and Borbely 1997; Vyazovskiy et al. 2007b). Filter settings were optimized visually to obtain the maximal resolution of wave shape, as well as the least intrusion of fast (e.g., spindle) activities. Slow waves were detected as positive deflections of the LFP signal between two consecutive negative deflections below the zero-crossing. Peak amplitude, slopes (mean 1st derivative of the 1st and 2nd segment), and number of peaks within a single wave were computed for each individual spontaneous and evoked wave during NREM sleep. Multipeak waves were defined as waves with more than one positive peak between two consecutive negative deflections below the zero crossing (Vyazovskiy et al. 2007b).
To investigate spontaneous traveling waves, as a first step slow waves were detected in the right frontal and the right parietal derivation (“origin” derivations). Small slow waves (<median amplitude) were initially removed from this analysis. Two approaches were then used to compute the delays in the other derivations. First, we used as a reference the negative peak (slow wave start) prior to the positive slow wave detection in the origin locations. A slow wave was considered to be propagating if all other five derivations also revealed a negative peak within the following 50 ms and the corresponding following positive waves were at least 1/2 of the amplitude of the origin wave. As a second approach, and to enable comparison to the human data (Massimini et al. 2004; Riedner et al. 2007), we used the peak (maximal amplitude) of the slow wave as the reference point, and slow waves were considered to be propagating from the origin if a slow wave of at least 1/2 of the amplitude of the origin wave was detected within the next 50 ms in all other five derivations. Finally, we investigated whether the size of the origin waves affects the speed of slow wave propagation. For this analysis, waves were subdivided into “high-amplitude” (<median amplitude) and “low-amplitude” (>median amplitude), and delays were computed from the start or the peak of the “origin” slow wave to the subsequent starts or peaks, respectively, in each of the five derivations.
Data analysis and statistics
The software package MATLAB (The Math Works) was used for signal and data analysis and statistics. Differences were tested with paired t-test.
Histological verification
On completion of the experiments the position of the LFP electrodes was verified by histology in all animals. Perfusion was performed under deep isoflurane anesthesia (3% in oxygen), with saline followed by a 4% solution of paraformaldehyde (PFA) in 0.1 M sodium phosphate, pH 7.2. Brains were postfixed overnight in 4% PFA, cryoprotected in increasing concentration of sucrose (15, 20, 30%) in PBS, rapidly frozen on dry ice, cut into 50 μm serial coronal sections, and subjected to cresyl-violet (Nissl) staining. In all cases the deep LFP electrode was located within layer V, whereas the superficial LFP electrode was in layers I-II.
Animal protocols followed the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were in accordance with institutional guidelines.
RESULTS
Electrical pulses induce slow waves that are visually indistinguishable from spontaneous slow waves
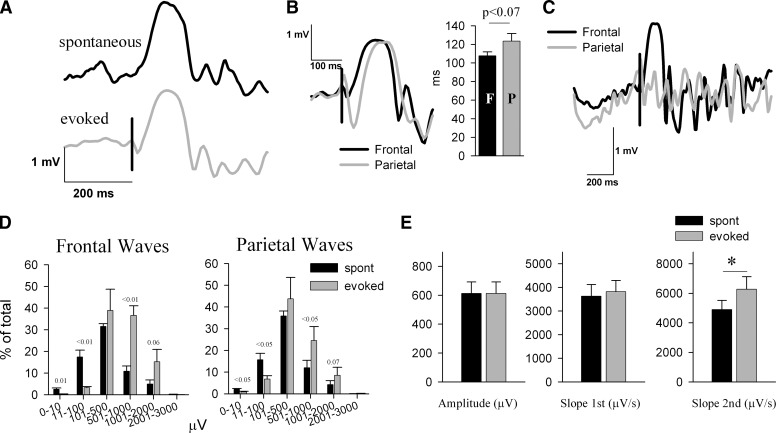
Cortical stimulation during NREM sleep did not elicit notable changes in behavior, muscle twitches, eye blinks, or whisker twitches. The most consistent result of the stimulation of the frontal cortex was the occurrence, in the contralateral frontal derivation and ipsilateral parietal derivation, of large positive waves visually indistinguishable from spontaneously occurring slow waves. Individual traces of evoked and spontaneous NREM sleep slow waves are shown in Fig. 2A. Similar to the spontaneous slow waves recorded from the frontal or parietal LFP electrodes (Vyazovskiy et al. 2007b), evoked slow waves consisted of a rising phase and a falling phase (1st and 2nd segments, respectively). Of note, and consistent with our earlier results (Vyazovskiy et al. 2007b), the slope of the second segment was steeper compared with the first segment (see different scales for y axes in Fig. 2E; both spontaneous and evoked waves: P < 0.01).
FIG. 2.
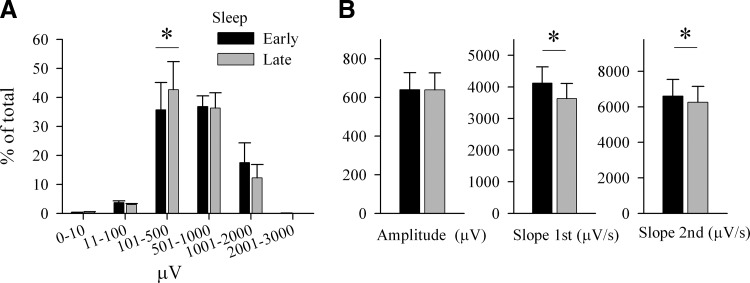
Comparison between evoked and spontaneous sleep slow waves. A: individual traces depicting typical spontaneous (black) and evoked (gray) slow waves from 1 representative rat. Positivity –upward. Vertical bar denotes the timing of the trigger. B: individual traces of the evoked slow waves recorded from the contralateral frontal (black traces) and the ipsilateral parietal (gray traces) cortical area. Bar plot refers to mean ± SE values (n = 6) of the latency (in ms) from the trigger to the peak of the evoked slow waves. C: intracortical electrical stimulation of the right frontal cortex triggers in some cases a local contralateral slow wave. Individual traces from the contralateral frontal (black) and ipsilateral parietal (gray traces) cortical area, where no definable slow wave was elicited. Vertical bar denotes the timing of the trigger. D: distribution of the amplitude of spontaneous and evoked slow waves. The number of waves was computed for groups with logarithmically increasing amplitude. Mean ± SE values (n = 6) are plotted as percentage of the total number of waves. The numbers above the bars denote significance or a tendency level of statistical difference between spontaneous and evoked slow waves (paired t-test). E: mean ± SE values (n = 6) of peak amplitude, and slopes of the 1st and 2nd segments of spontaneous and evoked frontal slow waves. Evoked and spontaneous slow waves were matched by their amplitude. (asterisk, P < 0.05, paired t-test).
When the frontal area was stimulated, the evoked slow wave was invariably observed first in the contralateral frontal cortex, followed by waves in the contralateral and ipsilateral parietal cortex ∼16 ms later (Figs. 2B, 1, and 4). The stimulus sometimes induced a local slow wave only in the contralateral frontal cortex (Fig. 2C). There were, however, some differences between the evoked and spontaneous slow waves. First, computation of the histogram of the slow wave amplitudes revealed that the evoked slow waves were on average larger than spontaneous slow waves (Fig. 2D). Specifically, the amplitude of the top 1% highest waves was significantly higher than the amplitude of the top 1% of spontaneous slow waves (spontaneous: 1,403.1 ± 206.81; evoked: 1,701.0 ± 229.05 μV, P < 0.01). Furthermore, after equating evoked and spontaneous slow waves by their amplitude, we found that the slope of the second segment was significantly steeper in the evoked slow waves (Fig. 2E). Moreover, there were fewer multipeak evoked waves compared with the spontaneous waves (average peaks per wave: frontal, spontaneous: 1.383 ± 0.03; evoked: 1.296 ± 0.02, P < 0.01, ∼7%; parietal, spontaneous: 1.395 ± 0.05; evoked: 1.36 ± 0.04, P < 0.1, ∼3%).
Induced slow waves are associated with high-amplitude spindles and increased sigma power
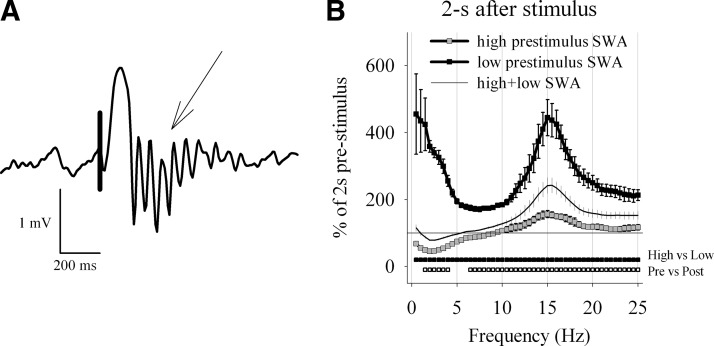
An important further feature of spontaneous slow waves is that they are frequently followed by thalamically generated spindles that propagate to the neocortex (Contreras et al. 1996). Therefore we expected that the evoked slow waves should also be followed by a spindle (Massimini et al. 2007). Figure 3A illustrates an example of the evoked slow wave that was followed by a burst of spindle activity (10–15 Hz), as it is the case for spontaneous slow waves (see also Vyazovskiy et al. 2007b). To determine whether the frequency range of these spindles is similar to that of spontaneously occurring sleep spindles, we computed EEG power spectra of the LFP signal immediately before and after the stimulus. The poststimulus spectrum was characterized by a significant increase of EEG power ≤25 Hz, with a pronounced peak at 15 Hz (Fig. 3B). High-amplitude evoked slow waves led to an even higher increase of sigma power compared with low-amplitude evoked slow waves (not shown). The values of sigma activity (10–15 Hz) computed over the first 2-s window after the trigger were significantly higher as compared with the preceding 2-s window throughout the NREM sleep episode (not shown).
FIG. 3.
Evoked slow waves are associated with spindles. A: individual trace depicting a typical evoked slow wave followed by a prominent spindle (↓). Positivity –upward. B: relative electroencephalographic (EEG) power density (0.5-Hz resolution) in the 2-s intervals immediately following the trigger, represented as percentage of the EEG power during the 2 s immediately preceding the trigger. The curves represent mean values of EEG power (±SE, n = 6). The spectra are computed separately for those trials that occurred on the background of the LFP signal with low slow wave activity (SWA; lowest 25%, = “low,” ▪) and high SWA (top 25% = “high,” ). Thin line depicts the average spectrum over all trials. ▪ below the curves depict those frequency bins where poststimulus EEG power was significantly different between high and low prestimulus SWA (paired t-test, P < 0.05). below the curves depict those frequency bins where EEG power was significantly different from prestimulus values (paired t-test, P < 0.05).
In contrast, in the first 2 s after the stimulus SWA was significantly suppressed between 1.5 and 4.0 Hz compared with prestimulus values (Fig. 3B). The background SWA, however, had a profound influence on the poststimulus spectra. Thus if the stimulus fell on a background of relatively superficial sleep with low SWA (lowest 25%), a dramatic increase in both SWA and spindle-activity was elicited (Fig. 3B). However, those stimuli that occurred during an epoch with intense sleep characterized by high SWA (highest 25%), led an only moderate effect on spindle-activity, whereas the poststimulus SWA was even suppressed.
Spontaneous and evoked slow waves travel across the cortical areas
Another feature of spontaneous slow waves is their near-synchronous occurrence between distant cortical areas as a result of their propagation from the site of origin (Massimini et al. 2004; Riedner et al. 2007). To investigate this aspect with the LFP recordings in the rat, we determined origins of high-amplitude spontaneous slow waves and assessed their relationship to the occurrence of slow waves in other cortical sites.
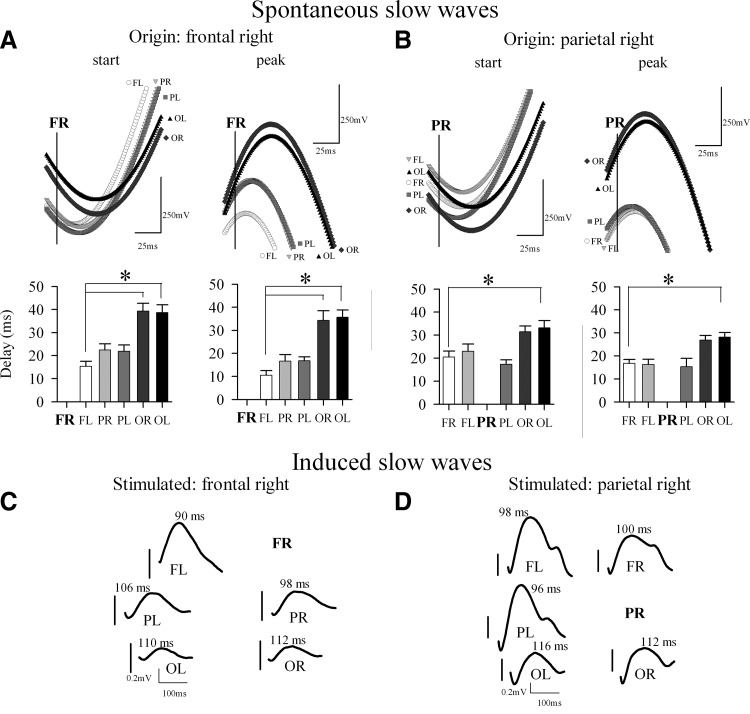
We used two complementary approaches. First, to approximate the approach used by Massimini and colleagues, we computed the sequential involvement of cortical areas based on the positive peak of the LFP slow wave, which corresponds closely to the negativity in the surface EEG. Second, we computed slow wave propagation based on their starting point. The analysis revealed that slow waves could originate in any cortical derivation. Notably, when either the start or the peak of the right frontal slow wave was chosen as origin, the delay was shortest for the slow wave in the contralateral frontal cortex, and longest for the most posterior occipital derivations (Fig. 4A). Similarly, if the origin was located in the right parietal derivation, the shortest delays were observed in the contralateral parietal derivation and in the frontal sites, and the longest—backward in the occipital sites (Fig. 4B).
FIG. 4.
Spontaneous and induced slow waves during NREM sleep propagate across multiple cortical locations. A, top: the start or the peak of the average spontaneous slow waves recorded from the left frontal (FL), right and left parietal (PR and PL, respectively) and occipital (OR and OL, respectively) derivations aligned relative to the start or the peak of the origin slow wave in the right frontal derivation (FR), indicated with a vertical line. Bottom panels: Average delays from the start or the peak of the origin slow wave relative to the start or the peak, respectively, of the next slow wave within the 1st 50 ms in other 5 cortical derivations. Mean ± SE values, n = 6. B: same as in A but for the origin slow wave in the right parietal derivation (PR). The gray shadings of the symbols in the top panels correspond to the fill colors of the bar plots in the bottom panels. C and D: local intracortical electrical stimulation of the right frontal cortex (C) or the right parietal cortex (D) triggers slow waves in the remaining five locations (abbreviations as above). Average traces of one individual rat. Note different y axis in C and D. Vertical bars indicate the timing of the trigger for each individual location. Positivity –upward. Numbers above the traces denote the latency of the peak (in ms) in relation to the trigger. Note the sequential propagation of the evoked slow waves across cortical regions.
To investigate whether evoked slow waves behave in a similar manner, we investigated the propagation of induced slow waves after stimulating the right frontal or the right parietal cortex. The stimulation of the right frontal cortex (FR) evoked the largest slow wave in the contralateral frontal derivation but also elicited a clear-cut slow wave time-locked to the trigger in the parietal and occipital areas of both sides (Fig. 4C). The latency from the trigger to the peak was shortest in the contralateral frontal area, and delayed in other leads, showing a clear sequential propagation from the frontal left derivation, where the peak of the evoked slow wave was encountered 90 ms after the trigger, through the occipital right derivation, where the peak of the slow wave occurred 22 ms later. When the right parietal area (PR) was stimulated, the slow wave again occurred first in the contralateral area (parietal left, 96 ms after the trigger), and then a gradual involvement of other cortical areas was observed starting with the frontal derivations and reaching at last the left occipital derivation 20 ms later (Fig. 4C). Thus the pattern of slow wave propagation across cortical areas was highly consistent between spontaneous and induced slow waves. This suggests that electrical local cortical stimulation triggers a wave of recruitment across neocortex involving a large population of neurons in the same cycle of the slow oscillation.
We observed that when spontaneous slow waves occurring in the right frontal derivation were considered the origins, the average delay to the contralateral site was ∼10–15 ms and not significantly different from both parietal leads. By contrast, after electrical stimulation of the right frontal site, the latencies to the right and left parietal derivation were different. We hypothesized therefore that the size of the origin slow wave (a measure of the strength of the recruitment wave) could affect the speed of the involvement of other areas. To directly test this hypothesis, we compared the delays to other derivations between the high- and low-amplitude “origin” waves. We found indeed that high-amplitude slow waves were followed by waves that, in most other derivations, had significantly shorter delays compared with those that followed by low-amplitude slow waves (not shown).
Effects of sleep pressure on evoked slow waves
If the evoked slow waves share the same substrates and mechanisms with spontaneous slow waves, they should also show similar changes when sleep pressure is increased after a period of waking (Vyazovskiy et al. 2007b). Therefore the next question we addressed was whether the parameters of the evoked slow waves differ during early and late sleep. A first finding was that even under low sleep pressure it was still possible to induce slow waves of the largest amplitude, indicating that a powerful external or internal trigger can recruit a sufficiently large neuronal population. Thus the maximal amplitude of the evoked slow wave that could be achieved in early and late sleep was similar (mean over top 2%: early: 1,346.7 ± 183.96 μV, late: 1,273.8 ± 158.75 μV, P > 0.1, paired t-test). The amplitude of the evoked slow waves showed in general a similar distribution between early and late sleep (Fig. 5A).
FIG. 5.
Effect of sleep/wake history on the parameters of evoked slow waves. A: distribution of the amplitude of evoked slow waves. The number of waves was computed for groups with logarithmically increasing amplitude. Mean ± SE values (n = 6) are plotted as percentage of the total number of waves within a condition (early sleep, late sleep). * above the bars depicts significant difference (P < 0.05, paired t-test) between early and late sleep (paired t-test). B: mean ± SE values (n = 6) of peak amplitude, and slopes of the 1st and 2nd segments of evoked slow waves during early and late sleep. Waves in early and late sleep are matched by their amplitude (*, P < 0.05, paired t-test).
However, when we matched the evoked slow waves in early and late sleep by their amplitude, we found that their slopes were consistently higher during early sleep (Fig. 5B). Thus across the day the slope of the first segment decreased by 13.8 ± 1.82% (P < 0.01) while the slope of the down-going swing was 5.4 ± 1.85% lower than during early sleep (P < 0.05). Moreover, consistent with our earlier finding in spontaneous slow waves (Vyazovskiy et al. 2007b), the evoked slow waves tended to have more peaks after sleep pressure had dissipated (mean values peaks per wave: high: 1.26 ± 0.01, late: 1.34 ± 0.04, P < 0.05, paired t-test). Similar changes were observed also in the evoked parietal slow waves with an exception of the down-going slope that did not show a significant change (not shown).
Background activity influences the amplitude of evoked slow waves
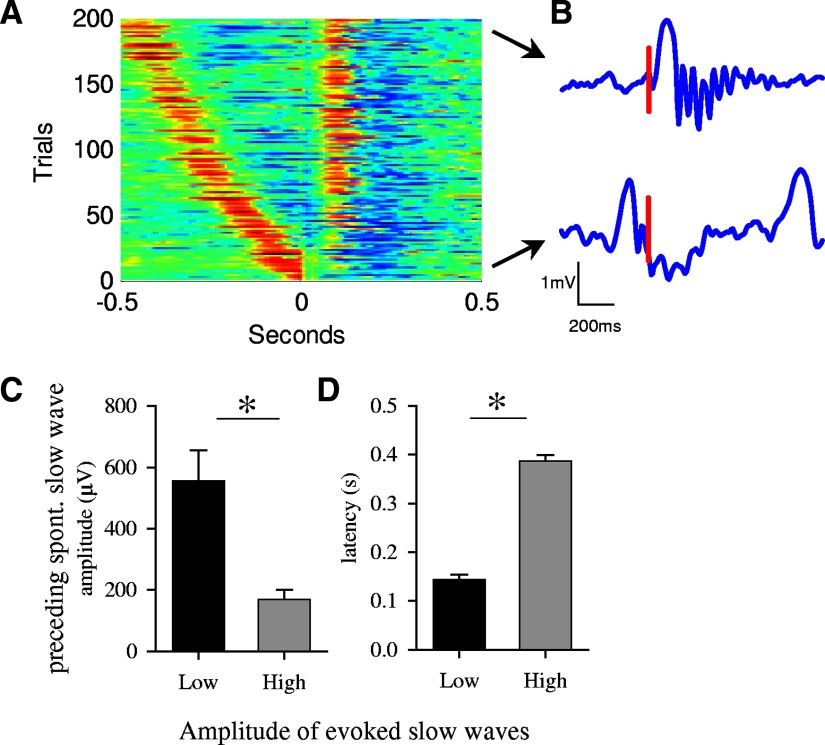
As it is the case for spontaneous slow waves, evoked slow waves were of variable amplitude (Fig. 2D). Closer examination revealed that the amplitude of evoked slow waves was determined largely by the peristimulus background LFP activity during the time interval between the trigger and the preceding spontaneous slow wave. Figure 6B depicts an example of two frontal waves, one triggered on a background of low-amplitude activity and another evoked immediately after a spontaneous high-amplitude slow wave. In the second case, the evoked slow wave was virtually absent. This relationship was further highlighted by plotting the amplitude of the evoked slow waves as a function of the timing of the immediately preceding high-amplitude slow wave (200 trials of 1 representative animal, Fig. 6A). It is apparent that when the trigger fell right after the spontaneous slow wave, on its descending leg, the amplitude of the evoked wave was drastically diminished.
FIG. 6.
Peristimulus background activity has an influence on the amplitude of evoked slow waves. A: triggered single-trial responses in the left frontal derivation after the right frontal derivation was stimulated (200 trials from one representative rat) sorted as a function of the latency to the preceding spontaneous high-amplitude slow wave (> mean +1 SD). Voltage is color-coded (red, positive; blue, negative). Note that the proximity of the preceding spontaneous slow wave to the trigger resulted in a failure of the electrical pulse to trigger slow waves in ∼40 cases. B: typical individual traces depicting slow waves triggered on the background of low-amplitude activity (top trace) and triggered immediately after spontaneous slow wave (bottom trace) from 1 representative animal. Positivity - upward. Vertical bar denotes the timing of the trigger. C and D: mean amplitude of spontaneous slow waves, preceding the trigger, and their latency prior to the trigger, were computed separately for those cases where full-fledged high-amplitude evoked slow waves was induced, and for those cases where the induction was not successful. Specifically, for this analysis all individual trials were sorted based on the maximal value of the signal within the 1st 250 ms after the trigger. Subsequently, the mean amplitude and the latency of the closest immediately preceding spontaneous slow wave were computed for the top and bottom 100 trials, corresponding to the “biggest” and “smallest” 100 evoked slow waves (mean ± SE, n = 6; *, P < 0.05, paired t-test).
Next we sorted all the evoked slow waves as a function of their amplitude and compared the latency to the preceding spontaneous slow waves and their amplitude for the first and last 100 trials, corresponding to the largest and smallest evoked slow waves (Fig. 6C). This analysis revealed that the smallest evoked slow waves were consistently preceded by a large spontaneous slow wave occurring within the previous 100–200 ms. By contrast, large evoked slow waves were not immediately preceded by any slow wave (mean latency was almost 400 ms), and in the few cases when this was the case, the preceding slow wave was small (Fig. 6C). This was the case also for the evoked parietal slow waves (not shown).
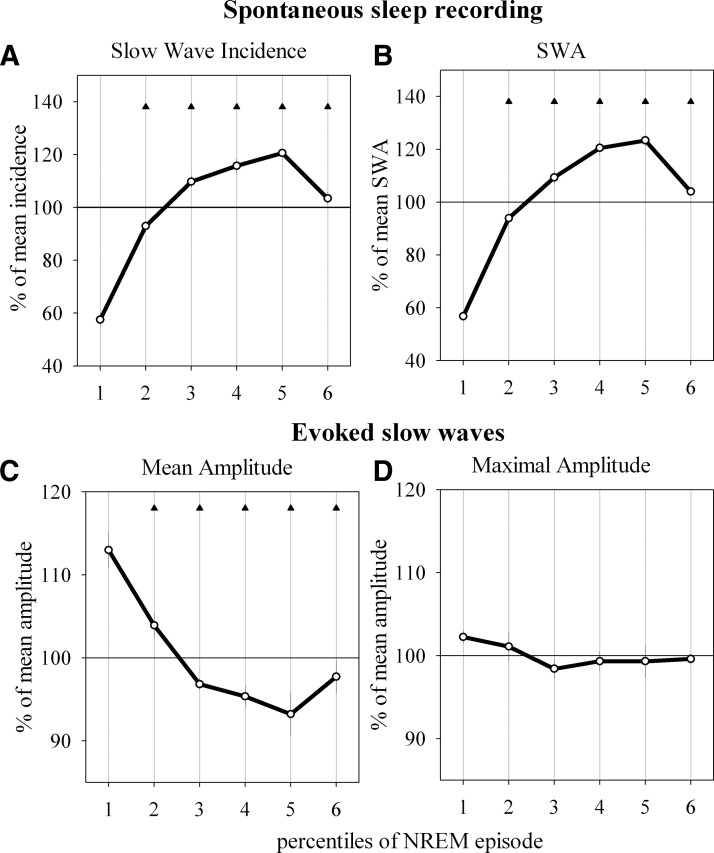
The effect of background activity on the amplitude of the evoked slow waves was especially apparent when we compared the beginning of NREM sleep episodes, when only few spontaneous slow waves are present, with the episode midpoint, when slow waves are abundant (Fig. 7, A and B). As shown in Fig. 7C, the average amplitude of evoked slow waves was largest at the beginning of NREM sleep episode and declined gradually by almost 20% toward the middle of the episode. Note, however, that the largest evoked waves were just as large at the beginning of the episode as during the middle of the episode (frontal waves: Fig. 7D, parietal waves: not shown). Thus a full-fledged slow wave can be elicited even during the deepest portions of NREM sleep.
FIG. 7.
Time course of evoked and spontaneous slow waves and SWA within NREM sleep episodes (only episodes lasting >3min are included). Each individual episode is subdivided into 6 percentiles of equal duration. A: time course of the incidence of spontaneous high-amplitude slow waves, defined as waves > mean +SD and represented as percentage of mean incidence within the corresponding NREM episode (means ± SE, n = 14 rats). B: SWA for each percentile represented as percentage of the mean SWA for the entire NREM sleep episode (mean values ± SE, n = 14). C: mean amplitude of evoked slow waves within each percentile, represented as percentage of the mean amplitude of all evoked slow waves within the corresponding NREM sleep episode (mean ± SE, n = 6). D: mean maximal amplitude of evoked slow waves encountered within each percentile of NREM sleep episodes represented as percentage of the mean between all percentiles of the corresponding episode (mean ± SE, n = 6). Triangles indicate significant differences from the first percentile (P < 0.05, Dunn-Sidak test).
Spontaneously occurring slow waves depend on the timing of preceding events
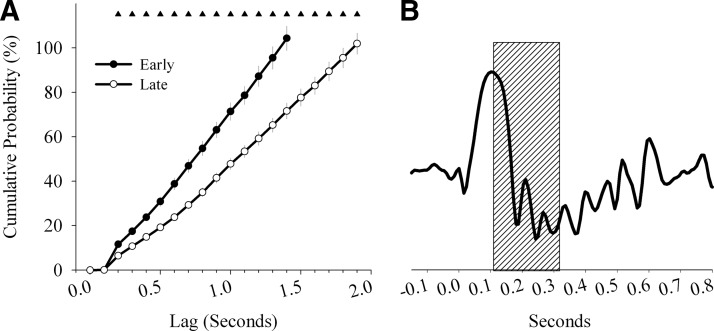
The observed interference exerted by spontaneous background activity on the evoked slow waves raises the question whether a similar relationship also exists among spontaneously occurring individual slow waves. In other words, does the occurrence of a high-amplitude slow wave preclude the immediate emergence of another spontaneous high-amplitude slow wave? This question is relevant because although high sleep pressure is always characterized by the occurrence of many high-amplitude slow waves, it is unclear what triggers them spontaneously and why at that particular rate. Therefore we computed the probability of the occurrence of high-amplitude slow waves during natural undisturbed NREM sleep as a function of the time lag from the preceding high-amplitude slow wave. In Fig. 8A the cumulative probability for the occurrence of the high-amplitude slow wave recorded in the frontal derivation is plotted as a function of the time lag starting from the peak of the preceding wave. The probability was close to 0% within the first ∼200 ms and then gradually increased until reaching ∼100% for time lags of 1.5 s. As expected, during late sleep the probability curve was shifted to the right, reaching 100% probability only after ∼2 s. This result indicates that there is a refractory period right after the spontaneous slow wave, lasting ∼200 ms (Fig. 8B), where initiation of the spontaneous slow wave is unlikely even under the highest physiological pressure for sleep.
FIG. 8.
A: cumulative probability of the occurrence of a high-amplitude spontaneous sleep slow wave (> mean + SD) recorded in the frontal derivation plotted as a function of the time lag starting from the peak of the preceding spontaneous sleep slow wave (500 slow waves were included in this analysis for each of the two conditions: early sleep (hours 1–2 of the light period), late sleep (hours 7–8 of the light period)). The probability is computed as a proportion of trials where ≥1 slow wave was detected within a given time lag. Note that in no case a slow wave was initiated within the 1st 200 ms from the preceding peak. 100% probability was observed with time lags of ∼1.5 and 2 s for early sleep and late sleep, respectively (means ± SE, n = 14). Triangles indicate significant differences between early sleep and late sleep (P < 0.05, paired t-test). B: schematic depiction of a typical slow wave and the time window (gray box) when evoked or spontaneous slow waves cannot occur.
DISCUSSION
The principal finding of the present study is that local electrical stimulation of the cortex in the rat during natural sleep is able to reliably induce slow waves that are similar to the spontaneous slow waves of NREM sleep. This result provides support for our earlier human study, where sleep slow waves were induced in the scalp EEG by transcranial magnetic stimulation (Massimini et al. 2007). Here we took advantage of an intracortical local stimulation and recording that is not affected by volume conduction. The evoked slow waves resembled morphologically the spontaneous slow waves, were followed by a spindle, were similarly able to propagate across the cortex and had steeper slopes under increased physiological sleep pressure.
Intracortical stimulation induces physiological sleep slow waves
The induced slow waves were visually virtually indistinguishable from the spontaneous LFP slow waves typical for sleep. The electrical pulses seemed to serve as powerful enough triggers for induction of physiological slow waves, possibly by creating a massive excitatory volley that suffused the cortical surface by recruiting a distributed neuronal population. Such a volley can possibly mimic the burst of activity that characterizes the up state of the slow oscillation (Sanchez-Vives and McCormick 2000). Like the induced slow waves, spontaneous slow waves showed positive polarity, their down-going slope was steeper than the up-going slope (Vyazovskiy et al. 2007b), and they occurred near-synchronously between distant cortical areas (see following text). In most cases, the induced slow wave was also followed by a distinct spindle, as is the case for spontaneous slow waves (Mölle et al. 2002; Steriade 2006; Steriade et al. 1993, 2001). The induced spindles occurred in the frequency range typical for spindling in rats (10–15 Hz). Moreover, consistent with the data in humans (Massimini et al. 2007), the stimuli also increased EEG power in faster frequencies. In mice, the occurrence of spindles is associated with higher fast frequencies ≤25 Hz (Vyazovskiy et al. 2004). Moreover, the modulation of sigma (12–15 Hz) and beta (15–25 Hz) frequencies by the cortical slow oscillation in humans also showed similar dynamics (Mölle et al. 2002). Thus both RMS of spindle- and beta-activity were increased in association with slow positive half-waves and were suppressed during slow negative half-waves (Mölle et al. 2002).
In humans, TMS led to an increase in SWA of up to eight times around the stimulation site and to a twofold increase over the rest of the scalp (Massimini et al. 2007). We observed an up to fourfold augmentation of SWA in the first 2 s after the stimulus as compared with the prestimulus SWA. Importantly, a necessary condition for such an effect was the falling of stimulus on a background of a relatively superficial sleep with low SWA (see following text). However, we found that it was still possible to induce very large slow waves not only at the beginning of NREM episode, when the spontaneous occurrence of large slow waves is rare, but also in the middle of a NREM sleep episode when large spontaneous slow waves are abundant. These observations suggest that high-amplitude slow waves can be evoked irrespectively of the level of neuromodulators or of brain temperature, factors that may change in the course of a NREM sleep episode. Instead as soon as an excitatory volley recruits a sufficiently large neuronal population, the latter can transition synchronously into the down state.
A novel aspect of this study was that the incidence of high-amplitude slow waves (>median amplitude) progressively increases during the course of a sleep episode. Increased incidence of high-amplitude slow waves necessarily entails shortening of the intervals between them. Such changes closely parallel the intra-episodic time course of SWA, indicating that SWA and high-amplitude slow waves reflect both the deepening of sleep within a single sleep episode (on a time scale of minutes) as well as the homeostatic sleep pressure (on a time scale of hours). It was recently suggested that longer sleep episodes might have “higher recovery value” because they are characterized by higher SWA compared with short sleep episodes (Vyazovskiy et al. 2007a).
It remains to be investigated whether the induced slow waves fulfill the physiological role of spontaneous slow waves. It has been shown that inducing slow oscillation-like potential fields by transcranial application of oscillating potentials during NREM sleep enhances the retention of hippocampus-dependent declarative memories in humans (Marshall et al. 2006). Further experiments are necessary to investigate whether a power nap rich of evoked high-amplitude slow waves has the same recuperative value of spontaneous deep sleep (Massimini et al. 2007).
Spontaneous and evoked slow waves propagate across the cortical areas in a sequential order
A novel finding of this study was that spontaneous LFP slow waves in naturally sleeping rats propagate across the cortical areas. Earlier data suggested such a possibility in various preparations (Calvet et al. 1973; Hughes 1995; Massimini et al. 2004; Riedner et al. 2007; Sanchez-Vives and McCormick 2000; Timofeev et al. 2000; Verzeano and Negishi 1960). In humans both spontaneously occurring and TMS-triggered sleep slow waves propagate across the cortex (Massimini et al. 2004, 2007). We investigated whether this is also the case for spontaneous slow waves recorded with intracortical local field potential electrodes in the rat. Previous studies (Massimini et al. 2004; Riedner et al. 2007) demonstrated slow wave propagation based on the negative peak of surface EEG slow wave. We now confirm this result based on the positive peak of the LFP slow wave recorded in the depth of the cortex as well as based on the slow wave onset. First, we found that most waves had a specific site of origin. Second, those waves that were observed first in the frontal derivations were consistently followed by parietal and only then by occipital waves. At the same time, slow waves that started at one of the parietal derivations were first followed by slow waves in the contralateral parietal derivation and with a short delay in the frontal leads, and only ∼10 ms later reached the occipital derivations. An interesting observation was that the size of the slow waves was an important factor affecting the speed of the slow wave propagation. Our recent data suggested that large slow waves arise from a synchronous and fast recruitment of a large neuronal population (Esser et al. 2007). It is plausible to assume that the synchronous involvement of a large neuronal pool in the same up state would likely generate a more powerful volley of activation; this in turn would lead to a more efficient recruitment of neurons in the next cycle. Consistent with this prediction, slow waves induced by electrical stimulation had steeper slopes of the second segment, compared with spontaneous slow waves. This observation suggests that high-amplitude slow waves are more likely followed by another large slow wave.
We found than that the evoked slow waves, once elicited locally, also propagate throughout the neocortex, involving sequentially other areas (Figs. 1, 2, and 4). Again, as was the case for spontaneous slow waves, both for the frontally and parietally induced waves the shortest latency was observed for the corresponding contralateral site with subsequent involvement of other areas. Invariably, the occipital sites were reached last. Direct cortico-cortical connections are likely to play an important role in such propagation as the latencies were shortest to the contralateral homotopic areas connected to the origins by the direct callosal excitatory fibers. It is known that the activity of cortical neurons is driven not only by a local intracortical network but also by neurons from homotopic regions of the contralateral hemisphere (Cisse et al. 2003). Moreover, synchronization of the slow oscillation requires integrity of cortico-cortical connections, as demonstrated with pharmacological and surgical tools (Amzica and Steriade 1995). The functional significance of slow wave propagation remains unclear. It was suggested that up states propagate by spreading from a specific focus (Massimini et al. 2004; Sanchez-Vives and McCormick 2000) or by synchronization of weak activity, originating at multiple locations (Massimini et al. 2004; Timofeev et al. 2000; Volgushev et al. 2006). It is possible that the traveling of the slow oscillation represents a wave of recruitment of cortical areas into the same cycle of slow oscillation. The efficiency of such recruitment may be determined by the strength of synaptic cortico-cortical connections, which changes as a result of the sleep/wake history (Esser et al. 2007; Vyazovskiy et al. 2007b).
Background peristimulus activity has a profound influence on the amplitude of the evoked slow waves
The ability to induce slow waves was strongly influenced by the proximity to the preceding spontaneous slow wave. Specifically, evoked slow waves were virtually absent if the stimulus was delivered immediately after a large spontaneous slow wave. Further analysis indicated that this was also the case for spontaneous slow waves. Specifically, we observed that during the ∼200 ms immediately following the crest of a spontaneous slow wave, presumably corresponding to the up state of the slow oscillation, the initiation of either the next spontaneous slow wave or of an evoked slow wave was extremely unlikely even under the highest sleep pressure.
This forbidden or refractory period within the first ∼200 ms after the onset of the up state may be related to the cellular mechanisms thought to underlie the generation of the slow oscillation. Experimental work as well as computer simulations indicate that the bistability of the membrane potential during sleep is brought about by neuromodulatory changes that lead to an increase in potassium leak conductance on transition from waking to sleep, and to concomitant changes in synaptic release and intrinsic currents (reviewed in (Hill and Tononi 2005)). Specifically, a short-term synaptic depression mechanism associated with the progressive depletion of presynaptic vesicle pools, in conjunction with the progressive increase in a depolarization-activated potassium current during the up state, are involved in the eventual termination of the up state and the precipitation of a down state (Compte et al. 2003; Hill and Tononi 2005; Massimini and Amzica 2001; Timofeev et al. 2000). It is possible that it may take ≤200 ms of an up state before the vesicle depletion and/or the increase of the depolarization-activated potassium current are sufficient to bring about a down state. The refractory period for the induction of slow oscillations may have also a functional significance. For example, it may favor the unidirectional propagation of slow waves and ensure that up states have a sufficient minimal duration to fulfill their hypothesized cellular functions, such as in mediating plastic changes (Marshall et al. 2006; Massimini et al. 2004; Tononi and Cirelli 2006).
Sleep/wake history affects the parameters of the evoked slow waves in a manner similar to the spontaneous sleep slow waves
A novel finding of this study was that the parameters of evoked slow waves change as a function of the homeostatic sleep pressure. We have shown previously that the incidence of high-amplitude slow waves increases after a period of waking (Vyazovskiy et al. 2007b). However, even when sleep pressure was the lowest, some waves of the largest amplitude were still observed, albeit at a much lower rate. Similarly, we have shown here that it was still possible to induce very large slow waves by electrical stimulation not only under low sleep pressure, when the spontaneous occurrence of large slow waves is rare, but also in the middle of a NREM sleep episode when spontaneous slow waves are abundant. Apparently, as long as a spontaneous excitatory volley recruits a sufficiently large neuronal population, the latter can transition synchronously into the down state.
Between early and late sleep, the slope of spontaneous slow waves decreases, while the number of multipeak waves increases (Riedner et al. 2007; Vyazovskiy et al. 2007b). Consistent with the results obtained with spontaneous slow waves, we found that the slope of evoked slow waves was steeper under high sleep pressure, while the number of evoked multipeak waves was lower. Modeling and human studies indicate that multipeak waves originate from independent sources (Esser et al. 2007; Riedner et al. 2007). Therefore under high sleep pressure, the electrical trigger is likely to have efficiently entrained virtually the entire available neuronal population, reducing the possibility of the occurrence of an independent slow wave at a distant source. The many similarities between evoked and spontaneous slow waves may be due to the fact that, during the transition to the up state, spontaneous slow waves also behave as a hypersynchronous volley of excitation traveling within the cortex (Vyazovskiy et al. 2007b).
The slope of slow waves is likely to reflect, at least in part, the strength of the recurrent excitatory connections that drive and maintain neurons in the up state (Sanchez-Vives and McCormick 2000). In modeling studies, stronger synaptic connections cause an increase in the slope of slow waves, which is associated with an increased recruitment of neurons into the up state, and an increased decruitment into the down state (Esser et al. 2007). Indeed the homeostatic changes in the slope of evoked slow waves during sleep are also consistent with the changes observed in the slopes of the early monosynaptic component of the cortical evoked response recorded during wakefulness (Vyazovskiy et al. 2008). Thus the slope of cortical evoked responses elicited by direct cortical stimulation appears to reflect sleep homeostasis both during wakefulness and during sleep.
Concluding remarks
Triggering slow waves during sleep in the rat by intracortical electrical stimulation appears to be a promising tool to investigate dynamic changes in the responsiveness of cortical networks. Our results support and extend previous studies in which sleep slow waves were induced in humans with TMS. Both techniques led to a successful induction of sleep slow waves that were similar to spontaneous slow waves, were followed by spindles, and propagated through the neocortex from the area of the stimulation. In addition, induced slow waves were virtually absent if the stimulus was delivered immediately after the spontaneous slow wave. Whether the similarities between the effects observed with TMS and intracortical electrical stimulation reflect the involvement of the same neuronal compartment, however, remains unclear. On one hand, both techniques lead to the induction of a current flow in a circumscribed cortical region, either indirectly (TMS) or directly (intracortical electrical stimulation) (Kesner and Patterson 1981; Pascual-Leone 2002). This eventually leads to neuronal depolarization and generation of spikes and delayed postsynaptic responses of local and distant cells. On the other hand, bipolar intracortical electrical stimulation induces a vertically oriented flow of current, presumably within a single cortical column, whereas TMS pulses elicit a horizontally flowing current (see Kesner and Patterson 1981; Pascual-Leone 2002 for details). This difference may be significant because the ability of fibers, their terminations, and cell bodies to be stimulated strongly depends on their orientation relative to the magnetic field or the current flow (Abdeen and Stuchly 1994; Maccabee et al. 1993; Ranck 1975). Human TMS studies have already established the optimal stimulus strength and specific cortical sites where the stimulation is most efficient (Massimini et al. 2007), while future studies in animals are needed to clarify which specific cortical layers should be stimulated to produce slow waves most reliably.
The approach used in this study is a way to probe the ability of the cortex in vivo to generate sleep slow waves and thus may help understanding the mechanisms underlying sleep homeostasis. While it is well known that high-amplitude slow waves are abundant in early sleep and decrease across the sleep period (Bergmann et al. 1987; Feinberg et al. 1978; Mistlberger et al. 1987; Riedner et al. 2007; Vyazovskiy et al. 2007b), the mechanisms underlying their occurrence are unclear. It was suggested recently that sleep SWA may reflect the average strength of cortical synapses, which would increase during wakefulness as a result of plastic processes and decrease during sleep due to a sleep-dependent mechanism of synaptic downscaling (Tononi and Cirelli 2003, 2006). Consistent with this hypothesis computer simulations showed that a net decrease in cortical synaptic strength leads to a marked decrease in network synchronization, which in turn results in a reduction of SWA in the simulated LFP (Esser et al. 2007). Moreover, decreased homeostatic sleep pressure was associated with a decline of large slow waves in vivo and a decrease of their slopes (Riedner et al. 2007; Vyazovskiy et al. 2007b). The present results suggest that high-amplitude slow waves in the cortex are generated as a result of a strong volley of electrical activity. During spontaneous sleep in vivo, such a volley may be effective only when synaptic connections between individual neurons are strong enough to allow efficient recruitment of a large cortical population in synchronous activation. Notably, as it was the case for spontaneous sleep slow waves (Riedner et al. 2007; Vyazovskiy et al. 2007b) and in agreement with the synaptic homeostasis hypothesis (Tononi and Cirelli 2003, 2006), we found here that waves triggered during early sleep had steeper slopes compared with late sleep, suggesting increased readiness of the cortical networks to engage synchronously in the global slow oscillation. Future experiments are necessary to investigate in detail how the dynamic changes in the cortical network, arising from the bistability of individual neurons and from their short- and long-range interactions, change as sleep deepens or as homeostatic sleep pressure dissipates. Such changes should be reflected in the incidence and other parameters of high-amplitude slow waves, thus providing a direct link between cellular substrates of sleep homeostasis and EEG SWA.
GRANTS
This study was supported by National Institutes of Health Director's Pioneer Award to G. Tononi and Grant P20 MH-077967 to C. Cirelli and Swiss National Science Foundation Grant PBZHB-106264 to V. V. Vyazovskiy.
Acknowledgments
We thank Drs. M. Massimini and S.K. Esser for helpful comments.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- Abdeen and Stuchly 1994.Abdeen MA, Stuchly MA. Modeling of magnetic field stimulation of bent neurons. IEEE Trans Biomed Eng 41: 1092–1095, 1994. [DOI] [PubMed] [Google Scholar]
- Achermann and Borbely 1997.Achermann P, Borbely AA. Low-frequency (< 1 Hz) oscillations in the human sleep electroencephalogram. Neuroscience 81: 213–222, 1997. [DOI] [PubMed] [Google Scholar]
- Amzica and Steriade 1995.Amzica F, Steriade M. Disconnection of intracortical synaptic linkages disrupts synchronization of a slow oscillation. J Neurosci 15: 4658–4677, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amzica and Steriade 1998.Amzica F, Steriade M. Electrophysiological correlates of sleep delta waves. Electroencephalogr Clin Neurophysiol 107: 69–83, 1998. [DOI] [PubMed] [Google Scholar]
- Bergmann et al. 1987.Bergmann BM, Mistlberger RE, Rechtschaffen A. Period-amplitude analysis of rat electroencephalogram: stage and diurnal variations and effects of suprachiasmatic nuclei lesions. Sleep 10: 523–536, 1987. [PubMed] [Google Scholar]
- Borbély and Achermann 2005.Borbély AA, Achermann P. Sleep homeostasis and models of sleep regulation. In: Principles and Practice of Sleep Medicine edited by Kryger MH, Roth T, and Dement WC. Philadelphia: W. B. Saunders, 2005, p. 405–417.
- Calvet et al. 1973.Calvet J, Fourment A, Thiefry M. Electrical activity in neocortical projection and association areas during slow wave sleep. Brain Res 52: 173–187, 1973. [DOI] [PubMed] [Google Scholar]
- Cisse et al. 2003.Cisse Y, Grenier F, Timofeev I, Steriade M. Electrophysiological properties and input-output organization of callosal neurons in cat association cortex. J Neurophysiol 89: 1402–1413, 2003. [DOI] [PubMed] [Google Scholar]
- Compte et al. 2003.Compte A, Sanchez-Vives MV, McCormick DA, Wang XJ. Cellular and network mechanisms of slow oscillatory activity (<1 Hz) and wave propagations in a cortical network model. J Neurophysiol 89: 2707–2725, 2003. [DOI] [PubMed] [Google Scholar]
- Contreras et al. 1996.Contreras D, Destexhe A, Sejnowski TJ, Steriade M. Control of spatiotemporal coherence of a thalamic oscillation by corticothalamic feedback. Science 274: 771–774, 1996. [DOI] [PubMed] [Google Scholar]
- Destexhe et al. 1999.Destexhe A, Contreras D, Steriade M. Spatiotemporal analysis of local field potentials and unit discharges in cat cerebral cortex during natural wake and sleep states. J Neurosci 19: 4595–4608, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esser et al. 2007.Esser SK, Hill SL, Tononi G. Sleep homeostasis and cortical synchronization. I. Modeling the effects of synaptic strength on sleep slow waves. Sleep 30: 1617–1630, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg et al. 1978.Feinberg I, March JD, Fein G, Floyd TC, Walker JM, Price L. Period and amplitude analysis of 0.5–3 c/sec activity in NREM sleep of young adults. Electroencephalogr Clin Neurophysiol 44: 202–213, 1978. [DOI] [PubMed] [Google Scholar]
- Hill and Tononi 2005.Hill S, Tononi G. Modeling sleep and wakefulness in the thalamocortical system. J Neurophysiol 93: 1671–1698, 2005. [DOI] [PubMed] [Google Scholar]
- Hughes 1995.Hughes JR The phenomenon of travelling waves: a review. Clin Electroencephalogr 26: 1–6, 1995. [DOI] [PubMed] [Google Scholar]
- Kesner and Patterson 1981.Kesner RP, Patterson MM. Electrical Stimulation Research Techniques. New York: Academic, 1981.
- Maccabee et al. 1993.Maccabee PJ, Amassian VE, Eberle LP, Cracco RQ. Magnetic coil stimulation of straight and bent amphibian and mammalian peripheral nerve in vitro: locus of excitation. J Physiol 460: 201–219, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLean et al. 2005.MacLean JN, Watson BO, Aaron GB, Yuste R. Internal dynamics determine the cortical response to thalamic stimulation. Neuron 48: 811–823, 2005. [DOI] [PubMed] [Google Scholar]
- Marshall et al. 2006.Marshall L, Helgadottir H, Mölle M, Born J. Boosting slow oscillations during sleep potentiates memory. Nature 444: 610–613, 2006. [DOI] [PubMed] [Google Scholar]
- Massimini and Amzica 2001.Massimini M, Amzica F. Extracellular calcium fluctuations and intracellular potentials in the cortex during the slow sleep oscillation. J Neurophysiol 85: 1346–1350, 2001. [DOI] [PubMed] [Google Scholar]
- Massimini et al. 2007.Massimini M, Ferrarelli F, Esser SK, Riedner BA, Huber R, Murphy M, Peterson MJ, Tononi G. Triggering sleep slow waves by transcranial magnetic stimulation. Proc Natl Acad Sci USA 104: 8496–8501, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massimini et al. 2004.Massimini M, Huber R, Ferrarelli F, Hill S, Tononi G. The sleep slow oscillation as a traveling wave. J Neurosci 24: 6862–6870, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massimini et al. 2003.Massimini M, Rosanova M, Mariotti M. EEG slow (approximately 1 Hz) waves are associated with nonstationarity of thalamo-cortical sensory processing in the sleeping human. J Neurophysiol 89: 1205–1213, 2003. [DOI] [PubMed] [Google Scholar]
- Mistlberger et al. 1987.Mistlberger R, Bergmann B, Rechtschaffen A. Period-amplitude analysis of rat electroencephalogram: effects of sleep deprivation and exercise. Sleep 10: 508–522, 1987. [PubMed] [Google Scholar]
- Mölle et al. 2002.Mölle M, Marshall L, Gais S, Born J. Grouping of spindle activity during slow oscillations in human non-rapid eye movement sleep. J Neurosci 22: 10941–10947, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual-Leone 2002.Pascual-Leone A Handbook of Transcranial Magnetic Stimulation. London: Arnold, 2002.
- Ranck 1975.Ranck JB Which elements are excited in electrical stimulation of mammalian central nervous system: a review. Brain Res 98: 417–440, 1975. [DOI] [PubMed] [Google Scholar]
- Richardson et al. 2005.Richardson KA, Schiff SJ, Gluckman BJ. Control of traveling waves in the mammalian cortex. Phys Rev Lett 94:028103, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedner et al. 2007.Riedner BA, Vyazovskiy VV, Huber R, Massimini M, Esser S, Murphy M, Tononi G. Sleep homeostasis and cortical synchronization. III. A high-density EEG study of sleep slow waves in humans. Sleep 30: 1643–1657, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigas and Castro-Alamancos 2007.Rigas P, Castro-Alamancos MA. Thalamocortical up states: differential effects of intrinsic and extrinsic cortical inputs on persistent activity. J Neurosci 27: 4261–4272, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Vives and McCormick 2000.Sanchez-Vives MV, McCormick DA. Cellular and network mechanisms of rhythmic recurrent activity in neocortex. Nat Neurosci 3: 1027–1034, 2000. [DOI] [PubMed] [Google Scholar]
- Steriade 2006.Steriade M Grouping of brain rhythms in corticothalamic systems. Neuroscience 137: 1087–1106, 2006. [DOI] [PubMed] [Google Scholar]
- Steriade and Amzica 1998.Steriade M, Amzica F. Slow sleep oscillation, rhythmic K-complexes, and their paroxysmal developments. J Sleep Res 7 Suppl 1: 30–35, 1998. [DOI] [PubMed] [Google Scholar]
- Steriade et al. 1993.Steriade M, Nunez A, Amzica F. A novel slow (< 1 Hz) oscillation of neocortical neurons in vivo: depolarizing and hyperpolarizing components. J Neurosci 13: 3252–3265, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade et al. 2001.Steriade M, Timofeev I, Grenier F. Natural waking and sleep states: a view from inside neocortical neurons. J Neurophysiol 85: 1969–1985, 2001. [DOI] [PubMed] [Google Scholar]
- Timofeev et al. 2000.Timofeev I, Grenier F, Bazhenov M, Sejnowski TJ, Steriade M. Origin of slow cortical oscillations in deafferented cortical slabs. Cereb Cortex 10: 1185–1199, 2000. [DOI] [PubMed] [Google Scholar]
- Tononi and Cirelli 2003.Tononi G, Cirelli C. Sleep and synaptic homeostasis: a hypothesis. Brain Res Bull 62: 143–150, 2003. [DOI] [PubMed] [Google Scholar]
- Tononi and Cirelli 2006.Tononi G, Cirelli C. Sleep function and synaptic homeostasis. Sleep Med Rev 10: 49–62, 2006. [DOI] [PubMed] [Google Scholar]
- Verzeano and and Negishi 1960.Verzeano M, and Negishi K. Neuronal activity in cortical and thalamic networks. J Gen Physiol 43 Suppl 6: 177–195, 1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volgushev et al. 2006.Volgushev M, Chauvette S, Mukovski M, Timofeev I. Precise long-range synchronization of activity and silence in neocortical neurons during slow-wave oscillations [corrected]. J Neurosci 26: 5665–5672, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyazovskiy et al. 2004.Vyazovskiy VV, Achermann P, Borbely AA, Tobler I. The dynamics of spindles and EEG slow-wave activity in NREM sleep in mice.Arch Ital Biol 142: 511–523, 2004. [PubMed] [Google Scholar]
- Vyazovskiy et al. 2007a.Vyazovskiy VV, Achermann P, Tobler I. Sleep homeostasis in the rat in the light and dark period. Brain Res Bull 74: 37–44, 2007a. [DOI] [PubMed] [Google Scholar]
- Vyazovskiy et al. 2008.Vyazovskiy VV, Cirelli C, Pfister-Genskow M, Faraguna U, Tononi G. Molecular and electrophysiological evidence for net synaptic potentiation in wake and depression in sleep. Nat Neurosci 11: 200–208, 2008. [DOI] [PubMed] [Google Scholar]
- Vyazovskiy et al. 2007b.Vyazovskiy VV, Riedner BA, Cirelli C, Tononi G. Sleep homeostasis and cortical synchronization. II. A local field potential study of sleep slow waves in the rat. Sleep 30: 1631–1642, 2007b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson et al. 2008.Watson BO, MacLean JN, Yuste R. UP states protect ongoing cortical activity from thalamic inputs. PLoS ONE 3: e3971, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]