Abstract
Active sensing requires the brain to distinguish signals produced by external inputs from those generated by the animal's own movements. Because the rodent whisker musculature lacks proprioceptors, we asked whether trigeminal ganglion neurons encode the kinematics of the rat's own whisker movements in air. By examining the role of kinematics, we have extended previous findings showing that many neurons that respond during such movements do not do so consistently. Nevertheless, the majority (∼70%) of trigeminal ganglion neurons display significant correlations between firing rate and a kinematic parameter, and a subset, ∼30%, represent kinematics with high reliability. Preferential firing to movement direction was observed but was strongly modulated by movement amplitude and speed. However, in contrast to the precise time-locking that occurs in response to active whisker contacts, whisker movements in air generate temporally dispersed responses that are not time-locked to the onset of either protractions or retractions.
INTRODUCTION
Active sensing, a perceptual strategy employed by all sensorimotor systems, requires the brain to distinguish signals produced by external inputs (exafference) from those generated by the animal's own movements (reafference). In some systems (e.g., electrolocation, oculomotor saccades, somatosensation), such disambiguation could be mediated by a “corollary discharge” from motor to sensory regions, which has the effect of canceling the reafference generated during the movement (Cullen 2004). Here we address the question of how the rat distinguishes movements produced during contact with objects in the environment from its own whisker movements in air. A first step is to determine whether and how reafferent information about movement kinematics is encoded during whisking in air, and how such encoding differs from that of active whisker contacts.
The rodent vibrissa system has served as an excellent model for studies of active sensing (Kleinfeld et al. 2006; Sachdev et al. 2001). A mobile subset of the whiskers function as an active sensor; their rhythmic oscillations are used for dynamic exploration of the environment. Sensory input from the vibrissae contributes both to orienting behavior (Vincent 1912) and tactile discriminations (Carvell and Simons 1990; Harvey et al. 2001; Mehta et al. 2007). Moreover, during tactile discriminations, rats can modulate whisker movement parameters such as amplitude, speed, and frequency, to meet task demands (Carvell and Simons 1990; Harvey et al. 2001).
Active whisking typically includes both rhythmic whisker movements in air (“whisking”) and whisker contacts with objects in the environment. Both behaviors result in deformation of vibrissa mechanoreceptors that generate neural signals along the trigeminal sensory pathway. Such signals could provide the brain with information about the position of the whisker in air and/or textural properties of the contacted object. However, for neural representations of contacts or self-generated whisker movements to be useful, kinematics must be reliably encoded by neurons of the rat trigeminal system.
In anesthetized animals, whisker-related neurons throughout the brain can reliably encode several parameters of the movements generated passively by whisker contact, including direction, amplitude, and speed (Jones et al. 2004a,b; Pinto et al. 2000; Shipley 1973; Simons 1978; Waite 1973; Zucker and Welker 1969). However, we have little information about the encoding of signals generated during active whisker movements in air.
In the absence of evidence for proprioceptors in whisker musculature (Bowden and Mahran 1956), whisker-related neurons in the rodent trigeminal ganglion (TG) could initially generate the signals on which subsequent processing of movement-related input is based. Many studies have demonstrated that ganglion neurons in anesthetized animals encode responses to passive vibrissa displacement (Jones et al. 2004a,b; Lichtenstein et al. 1990; Shoykhet et al. 2000; Stüttgen et al. 2006; Zucker and Welker 1969). These studies indicate that the receptive field of each ganglion neuron consists of a single vibrissa and that neurons are readily activated by vibrissal displacement. However, the ability of a specific whisker deflection to evoke a neuronal response varies with the neuron's particular amplitude threshold, speed threshold, and directional preference (Lichtenstein et al. 1990; Shoykhet et al. 2000; Stüttgen et al. 2006; Zucker and Welker 1969).
Encoding of whisker movement in the absence of contact was first demonstrated in anesthetized animals using electrical stimulation of the facial motor nerve (Zucker and Welker 1969). Approximately 50% of the sampled neurons responded during electrically evoked vibrissa movements in air, but the number significantly increased if the movement terminated in contact. An “electrical whisking “ paradigm has been used in anesthetized animals by Ahissar and colleagues to examine the response properties of neurons during whisker movements with and without contact (Szwed et al. 2003;Yu et al. 2006). They concluded that, “whisking-responsive neurons fired at specific deflection angles, reporting the actual whiskers' position with high precision” (Szwed et al. 2003; p.1). A subsequent study from the Ahissar lab reported that some neurons in the lemniscal ventral posterior medial thalamic nucleus (VPM), and all neurons in the paralemniscal thalamic nucleus posteromedial (POm), respond strongly to electrically elicited whisker movements in the absence of contact (Yu et al. 2006). However, it is not clear whether responses to electrical whisking reliably model voluntary, active whisking responses. Indeed, we have recently reported that voluntary whisking in awake rats fails to evoke firing in POm neurons (Masri et al. 2008).
Leiser and Moxon (2007) recorded ganglion neuron responses in freely moving rats as they explored a small chamber. They found that all TG neurons responded with relatively low firing rates during whisker movements in air. Moreover, many neurons which did respond during such movements did so in an inconsistent manner: (1) only some movements were accompanied by spikes (2) spikes were not triggered reliably by movement onsets. All neurons increased their frequency of firing when the whisker movements terminated in contact, and no units responded exclusively during whisker movements in air. The fact that some, but not all whisker movements evoked neuronal activity could reflect differences in their movement kinematics, such as speed or amplitude. To address this possibility it is necessary to obtain correlated data on both neuronal activity and movement kinematics, data that were not available in previous studies. These correlated data are necessary also to determine whether the kinematics of self-generated movements in air are reliably encoded by ganglion cells in awake animals. To provide such data, we have simultaneously recorded ganglion cell activity and movement kinematics in awake, behaving rats during operantly conditioned (voluntary) whisker movements in air.
METHODS
All procedures were in accordance with National Institutes of Health guidelines for the use and care of experimental animals and were approved by an institutional animal use and care committee.
Animal preparation
Data were obtained from seven adult female Long Evans rats (200–300 g). Prior to behavioral testing, subjects were anesthetized with isofluorane and fitted with a dental cement headmount, and a stainless-steel ground screw was inserted into the skull. A small window was left in the dental cement at the stereotaxic coordinates of the ganglion to provide access for electrophysiological recordings. Following adaptation to head fixation and consistent performance on the operant task described in the following text, the animals were re-anesthetized, a 1.5 × 1.5-mm opening was made in the skull, and the opening was covered with moist cotton and a thin layer of dental acrylic.
Monitoring of movements and behavioral training
Whisker movements were monitored with high spatiotemporal precision (7 μm, 500 Hz) in head-fixed rats with all whiskers intact, using an optoelectronic monitoring system described in detail elsewhere (Bermejo et al. 1998). Bermejo et al. (2005; Fig. 2) have shown that during whisking in air, vibrissae in different rows and columns (e.g., B1 and C3) on one side of the face move synchronously, with amplitudes differing by <1°. Note that independent movements of adjacent whiskers have only been observed during a whisker contact task (Sachdev et al. 2002). Therefore in the present study, kinematic data tracked in the anterior-posterior plane for a single whisker (C2), served as a surrogate for the kinematics of all surrounding whiskers. Our neuronal data set includes all neurons that responded unambiguously to manual deflection. A list of the whiskers for each ganglion neuron is given in Table 1. Because all data were gathered in awake “whisking” animals, we could not determine whether a neuron was rapidly adapting or slowly adapting.
TABLE 1.
Whisker location and number of whisker movements
| Cell Number | Number of Analyzed Whisks | Whisker |
|---|---|---|
| 1 | 494 | C2 |
| 2 | 151 | * |
| 3 | 248 | A2 |
| 4 | 10 | C1 |
| 5 | 17 | B3 |
| 6 | 247 | C1 |
| 7 | 261 | β |
| 8 | 307 | D1 |
| 9 | 248 | B1 |
| 10 | 162 | Y |
| 11 | 53 | C2 |
| 12 | 17 | Y |
| 13 | 325 | C3 |
| 14 | 97 | D1 |
| 15 | 107 | D4 |
| 16 | 27 | B2 |
| 17 | 18 | B1 |
| 18 | 23 | * |
| 19 | 11 | * |
| 20 | 68 | E3 |
| 21 | 25 | C3 |
Because of high levels of background activity, the associated whisker could not be precisely determined; however, all were clearly within the ganglion representation and responded strongly to manual whisker deflection.
An operant conditioning procedure was employed to generate voluntary whisking behavior (Gao et al. 2003). Whisker movements were monitored in real-time and reinforced on a fixed ratio (FR) schedule contingent on protraction amplitudes ≥5 mm. Whisker movements exceeding the criterion amplitude triggered a brief visual signal. A series of 10 such movements (FR 10) was followed by delivery of the reinforcer (Yoo-hoo, a commercially available chocolate drink).
Kinematic analysis
For kinematic analyses, the 500 Hz movement record was smoothed using a 4-ms moving window. The nth bin of the record was replaced with the average of bins N and n + 1. To enable pooling of data across rats, a calibration procedure (Bermejo et al. 1998) for each rat was used to convert micrometer measurements on the sensor to degrees of displacement of the whisker, which are independent of the distance of the CCD device from the whisker shaft. To avoid errors of measurement (see Knutsen et al. 2005) the laser curtain of the optoelectric CCD array was always between 13 and 20 mm away from the face and calibrated for each animal in such a way that it was a constant throughout the different experimental sessions for the animal. Our measurement of a whisker's angle is relative to the face with 90° being orthogonal to the pad. In all figures displaying whisker trajectories, increases of whisker angle correspond to protractions, and decreases correspond to retractions.
Individual whisker movements were identified using a computer algorithm that defines three critical points: protraction onset, peak amplitude, and end of retraction. Protraction onset is identified by locating an increase between consecutive position measurements (every 2 ms) that is >0.14°. Similarly, the end of a retraction was defined as the first time point, after the peak, where the decrease was <0.14°. To minimize the possibility of including movements produced passively during other behaviors (e.g., jaw opening) only whisker movements with well-defined protractions and retractions, and with minimum amplitudes of 4°, were included in the analysis. Due to the limited range of our CCD device, the monitored whisker would sometimes move out of range. Individual movements were only analyzed if the whisker was in range throughout the movement. The largest detectable movements ranged from 80 to 110°, varying with the individual calibration of each rat. Because “in-range” movements >80° were rare, they were not included in our analyses.
Electrophysiological recording
Following head-fixation, the moist cotton and dental acrylic atop the opening in the skull were removed and neuronal activity from single TG neurons was recorded while rats generated whisker movements in the operant task. Using a manual stereotaxic microdrive, an electrode (FHC, stainless steel, 3–5 MΩ, 250-μm shank) was inserted through the dura and slowly lowered down to the TG. Electrophysiological activity was band-pass-filtered from 1 to 10 kHz and then acquired with a sampling rate of 20 kHz. Entry into the ganglion (>9 mm in depth) was usually preceded by a brief period of silence. The whisker representation within the ganglion was identified by manual stimulation of the vibrissa array, the receptive fields of individual whiskers were defined by audiovisual isolation, and neuronal responses to self-generated whisker movements were recorded. Off-line spike sorting was performed using principal component analysis with the Plexon off-line sorter (Dallas, TX). Activity generated during body movements was eliminated from the analysis as were periods of licking, which were monitored by connecting the metal sipper tube to the input of a standard A/D converter, and connecting the rat to ground (see Hayar et al. 2005). Contact between the rat's tongue and the spout produced a junction potential that could be identified in the electrophysiological recording.
Data analysis
Note that all analyses were performed on individual whisker movements (“whisks”) except for the decimation and coherence analyses, which were performed on the entire movement record collected on the C2 whisker for each neuron.
DISCRIMINATION OF STATIONARY FROM MOVING WHISKERS.
For each neuron, we calculated firing rates for periods of whisker movement (protractions and retractions) and periods during which whisker position did not change. The significance of differences in firing rates between the two periods was assessed using Student's t-test (P < 0.05).
DECIMATION PROCEDURE.
If a neuron's activity reliably represents changes in whisker-movement trajectory, there should be significant correlations between its firing rate and movement kinematics. To determine whether and with what resolution a neuron's firing rate could be used to reconstruct whisker-movement trajectories, we used a decimation analysis. The entire movement record, originally acquired at 500 Hz, was down-sampled to frequencies from 250 to 12.5 Hz. The decimated movement records were then interpolated back to 500 Hz so that the position, amplitude, and speed of the movement traces could be correlated with instantaneous firing rates calculated in 2-ms bins.
The kinematic data were not band-pass filtered to avoid possible artifacts produced by edge effects. Firing rate was then correlated with position, amplitude, and speed, using a nonparametric Spearman's rho, which has several advantages over a Pearson's correlation coefficient. Spearman's rho does not assume that the variables have a Gaussian distribution, and the variables can be related in any monotonic manner, not just linearly. Use of rho is justified for whisker kinematics since the distributions were skewed; smaller and slower movements are more common than larger and faster movements. The calculation of rho is identical to the more traditionally used Pearson correlation coefficient with the exception that the former transforms x and y values to ranks.
We have chosen the decimation analysis over spike-triggered covariance because the former can readily handle the fact that rats do not produce a normal distribution of whisker-movement amplitudes and speeds. The method of spike-triggered covariance method may not be valid for non-Gaussian variables (Schwartz et al. 2006).
COHERENCE ANALYSIS.
We also assessed the relation between spike activity and kinematic data using a coherence analysis available on-line in the Chronux toolbox for Matlab (www.chronux.org). Whisker-movement bouts ≥1 s in duration were selected, and the multitaper technique was used to estimate spectra of bouts and their corresponding spiking activity (bandwidth = 2; tapers = 3). Coherence between the movement and spike data were computed for the peak movement frequency between 3 and 20 Hz. Coherence was considered significant if it was in excess of the value of the 95% jackknife error bar.
FIRST-SPIKE LATENCY.
The latency of the first spike as a function of speed was determined for both protractions and retractions. For this analysis, spikes were not binned. “First-spike” latency is the time of the spike's occurrence relative to movement onset. Speed was computed by determining the slope from 20 to 80% of the peak amplitude. Additionally, the temporal jitter of a first spike was computed by calculating the variance (σ2) of its time of occurrence. Visual and quantitative comparisons of linear and power-law regressions revealed that in the few cells that did display a relationship between speed and first-spike latency, power law functions best captured the relationship. Thus for all neurons, power law functions were used in the regression between speed and first-spike latency. Five speed bins were sufficient to capture the observed power-law relationships between speed and first-spike latency. A minimum of 5 whisks was required in each bin. The bins spanned the range of observed speeds. For similar analyses, see Heil and Irvine (1997) and Shoykhet et al. (2000).
CORRELATIONS BETWEEN FIRING RATE AND WHISKER-MOVEMENT KINEMATICS.
Neural activity was quantified for each neuron by determining the firing rate during the protraction and retraction portion of each movement. This is different from the coherence and decimination analyses, for which, firing rate was binned at 2 ms. Firing rate was then correlated with each of the four kinematic measures independently of set-point (protraction amplitude, protraction speed, retraction amplitude, and retraction speed), using Spearman's rho.
RECEIVER OPERATING CHARACTERISTIC (ROC) ANALYSIS.
For each neuron, we quantified the accuracy with which a movement kinematic could be predicted using firing rate. Because all significant firing rate-correlations were positively monotonic, (i.e., large firing rates were indicative of larger or faster movements), we determined whether a higher firing rate indicated that a movement's actual class was relatively large or fast. For both amplitude and speed parameters, movements were assigned to one of three classes that encompassed the entire range of observed kinematic values (see Table 2). The kinematic values for the three amplitude classes were: <20°, 20–40°, and 40–60°; for protraction speed: <430°/s, 430–760°/s, and 760–1,100°/s. Since retraction speeds tend to be faster, different kinematic values were employed: <1,090°/s, 1,090–1,920°/s, and 1,920–2,750°/s. For each binary comparison of classes (e.g., <20 vs. 20–40°), an ROC curve was constructed. The ROC curve displays the rate of true positives versus the rate of false positives as the criterion firing rate for assignment to the larger or faster movement class is decreased. The area under the curve was used to determine the percentage of accurate classifications. The statistical significance of the calculated area was determined by comparing it with the results of a bootstrap analysis (1,000 repetitions) with randomly shuffled firing rates and kinematic measurements. Calculated ROC areas for individual neurons were considered significant if not contained within the 95% confidence interval of the bootstrap-obtained ROC areas. The 95% confidence interval of the upper and lower bounds of the bootstrapped ROC values were always ∼0.5.
TABLE 2.
Average whisking kinematics (Mean + SD): Freely moving versus head-fixed rats
Information theory and ROC curves from ideal observer analysis are common and complementary methods by which one can evaluate the ability of a neural code to “predict” stimuli. We have chosen to use the ROC curve from the ideal observer analysis because it directly translates to performance (e.g., 70% correct), whereas interpreting the bits from information theory is less straightforward.
RESULTS
In rats operantly conditioned to generate whisking in air, we recorded the responses of 21 isolated TG neurons while simultaneously monitoring large numbers of associated whisker movements (median = 98, see Table 1) with high spatiotemporal resolution. All 21 TG neurons responded to manual deflections of the whiskers, and their receptive fields were distributed throughout the whisker array (see Table 1). There was no apparent relationship between whisker length or location and any aspect of neural responsiveness. A statistical comparison of whisker-movement kinematics in our head-fixed animals and those reported for exploratory and discriminative behaviors in freely moving rats (Carvell and Simons 1990; Jin et al. 2004; Towal and Hartmann 2008) indicates that, while there are some differences for both protraction and retraction movements, the 95% confidence intervals for the two populations clearly overlap (see Table 2).
Figure 1 shows whisker movements and associated unit activity for three neurons and illustrates the range of responsiveness exhibited during self-generated whisker movements in air. The movements shown are variable in amplitude and speed as is typical of exploratory whisking (Bermejo et al. 2002; Towal and Hartmann 2008). One group of neurons (Fig. 1, top) rarely fired during the rat's own whisker movements, but, like all neurons in our sample, could be reliably driven by manual deflections of the whisker. Other neurons emitted spikes during some, but not all, self-generated whisker movements in air, even when the movements were of similar amplitude or speed (Fig. 1, middle). At the other end of the continuum were neurons that fire during almost all whisker movements, producing at least one spike for most movements (Fig. 1, bottom).
FIG. 1.
Trigeminal ganglion activity during whisker movements in air. Each panel shows the spikes (red ticks) generated by a trigeminal ganglion neuron during a 2-s episode of whisking. Right: spike waveforms. The arrangement of the panels from top to bottom reflects increasing correlations between movement kinematics and spiking probability. Top: a ganglion neuron that fired consistently in response to passive deflections but rarely fired during self-generated whisker movements. Even movements as large as 40° failed to evoke a spike in this neuron. Middle: a neuron that responded during some but not all whisker movements. Bottom: a neuron that responded consistently throughout the episode.
Temporal relationship between movement features and spike activity
To determine whether, and with what temporal resolution, a TG neuron's firing rate tracks movement trajectories, we correlated instantaneous firing rate (2-ms bins) with the entire movement record (recorded at 500 Hz). Based on findings of an average response latency of 2–3 ms in the anesthetized rat (Minnery and Simons 2003) the spike record was shifted 2 ms backward. There were no strong correlations between instantaneous firing rate and position, amplitude, or speed, for any of the neurons (rho's < 0.08, P < 0.0001 to < 0.05). It is possible, however, that temporal integration of trigeminal activity is necessary to encode movement trajectories; that is, movement trajectories might be encoded at a lower temporal resolution. To address this possibility, we correlated the firing rates with movement records sampled with progressively coarser temporal resolution (downsampled from 500 to 12.5 Hz, see methods). Regardless of the movement sampling frequency, firing rate did not reliably correlate with position, speed, or acceleration (mean rho across neurons did not exceed 0.08). An additional analysis carried out using an “unrealistic” 0-ms latency (no shift of spike times) produced identical results. This is likely due to the relative smooth trajectory of movements; adjacent segments of the whisker-movement trace will have highly similar positions, speeds, and accelerations.
The decimation analysis examined the ability of TG neurons to track the ongoing trajectory of whisker movements. To determine whether TG neurons could be entrained by the dominant frequency within a bout of whisker movements, we also performed a coherence analysis (see methods for details). Only bouts ≥1 s in duration were used for this analysis: 18 neurons qualified for this analysis (median = 27 bout/neuron). The percentage of bouts per neuron with significant coherence ranged from 0 to only 47.5% (mean = 18%). Thus neither the decimation nor the coherence analyses support the hypothesis that TG spiking activity provides a reliable representation of the kinematics of self-generated whisker-movement trajectories in air.
To evaluate the ability of a TG neuron to phase-lock to particular movement features, individual whisker movements were aligned at three critical points: protraction onset, retraction onset, and retraction end. For this purpose, the movements were rescaled in amplitude and matched in phase at the critical points. Individual whisker movements (whisks) are consistently characterized by a protraction followed by a retraction. The three components that define these whisks (protraction onset, peak protraction, and end of retraction) were obtained using computer algorithms (see methods). In this way, a collection of individual whisks was available for further analysis of where, within each whisk, the neuron produces spikes. The assignment of the retraction to begin at 2/3 of the whisk has been done only for graphical purposes and to facilitate visual identification of phase-locking. As Fig. 2 indicates, some neurons did appear to fire preferentially during certain phases of the movements. For example, cell 2 fired preferentially during retractions, although it responded only during a small proportion of the movements. Cell 4 fired preferentially near the peak amplitude or start of retraction. In other cells, spikes were dispersed throughout the movement. However, in none of the cells were spikes time-locked to the onset of either protraction or retraction movements.
FIG. 2.
Spikes of trigeminal ganglion (TG) neurons are not reliably triggered by the onset of protraction or retraction. For each of the 4 cells, 100 whisker movements are aligned to protraction onset, retraction onset, and end. Spike rasters are shown above the movements.
In a previous study using controlled whisker deflections in anesthetized rats, Shoykhet et al. (2000) found that the variances (and means) of TG neuron first-spike latencies decreased as deflection speed was increased. We reasoned therefore that time-locking might occur preferentially in response to fast deflections. To assess the relationship between first-spike latency and the speed of self-generated whisker movements, we assigned movements to one of five classes that spanned the range of observed speeds. Speed was defined as the slope from 20 to 80% of the peak amplitude. For each neuron, a minimum of five movements in each class was required (see methods for details). For each class, first-spike latency means and variances (jitter) were computed. Seventeen TG neurons qualified for this analysis. Power functions were fit to plots of speed versus mean and variance of the first-spike latency. In only 1 of the 17 cells, and only for retraction, was there a negative correlation between speed and first-spike latency (P < 0.05). Decreases in first-spike jitter were displayed by just two neurons for increases in retraction speed.
Taken together, the results of our first-spike latency, decimation, and coherence analyses indicate that spikes were not time-locked to movement onsets and could not track the trajectory of movements. Thus all subsequent analyses examine firing rate during time windows that encompass the duration (∼50–100 ms) of protractions and retractions, i.e., forward and backward movements.
Can TG neurons distinguish between stationary and moving whiskers?
Firing rates were computed separately for individual protractions and retractions, and rates during movement were compared with those observed when the whisker was stationary. As Fig. 3 shows, no TG neurons generated lower firing rates during periods of movement, while 11 of the 21 (∼52%) produced higher firing rates during movements (t-test, P < 0.05). Thus 50% of the TG neurons recorded can signal the occurrence of whisker movements.
FIG. 3.
Comparison of spiking activity for stationary and moving whiskers. Relative to nonwhisking, 10 of 21 (∼48%) TG neurons produce higher firing rates (P < 0.05) during protractions (A) and 7 of 21 (∼33%) do so during retractions (B). •, significant differences.
How reliably do TG neurons encode the kinematics of self generated whisker movements?
The question was motivated by the fact that individual TG neurons encode the kinematics of passive whisker deflections in anesthetized rats (Shoykhet et al. 2000; Stüttgen et al. 2006). To answer this question, we carried out two complementary analyses. For both analyses, individual whisker movements were separated into protraction and retraction components and four kinematic parameters were calculated (protraction amplitude, protraction speed, retraction amplitude, and retraction speed). In the first analysis, we computed Spearman's rho to determine whether there was a significant association between each neuron's firing rate and one or more of the four kinematic parameters.
CORRELATING FIRING RATE AND KINEMATICS.
We sampled 21 TG neurons, all of which could be readily driven by manual whisker deflections. Seven did not show any correlations between firing rate and any of the four kinematic variables (protraction amplitude, protraction speed, retraction amplitude, and retraction speed), even though movements covered a range of ∼70° in amplitude and ∼3,000°/s in speed. The majority of neurons (14/21) did display significant correlations (P < 0.0001 to < 0.05) between firing rate and the magnitude of one or more kinematic variables (see Fig. 4 and Table 3), and are hereafter referred to as “kinematic-responsive neurons.” Only 3 of the 14 neurons displayed a P value for just one of the four kinematics that was >0.025 (the Bonferoni corrected value for a criterion P value of 0.05; each firing rate was used twice, once for amplitude and again for speed). Unless otherwise specified, all subsequent analyses were performed on this subset of our sample. Note that the data plotted in Fig. 2 are from neurons with significant kinematic correlations. Some neurons could not differentiate whisker movements from stationary periods when all whisker movements were considered, but they did display significant correlations because larger or faster movements evoked more spikes than slower or smaller movements.
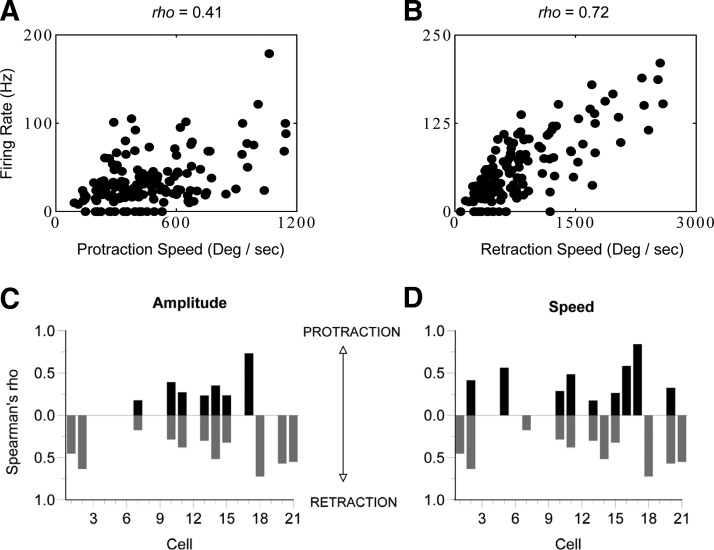
FIG. 4.
Correlations between kinematic parameters and firing rates of TG neurons. A: a typical significant (P < 0.05) relationship between firing rate and a kinematic parameter for cell 2 from D. B: an example of a less common, strong relationship (cell 18 from D). Significant correlations for amplitude (C) and speed (D) in our sample of TG cells. Absence of a bar indicates that none of the correlations were significant. For a given parameter, correlations could be significant in 1 direction but not in the other. 14 of 21 neurons displayed significant correlations with ≥1 movement parameters.
TABLE 3.
Significant correlations between kinematics and firing rate
| Kinematic | Number of Cells (%) | Mean rho | Median rho |
|---|---|---|---|
| Protraction amplitude | 7 (33%) | 0.34 ± 0.07 | 0.27 |
| Protraction speed | 9 (43%) | 0.44 ± 0.07 | 0.41 |
| Retraction amplitude | 11 (52%) | 0.44 ± 0.05 | 0.45 |
| Retraction speed | 11 (52%) | 0.45 ± 0.05 | 0.46 |
Figure 4, A and B, presents two examples that illustrate the range of correlations exhibited by kinematic-responsive neurons. Not only was there considerable variability in the magnitude of calculated rho's (see Fig. 4, C and D), but for both amplitude and speed parameters, cells with significant kinematic correlations could be highly variable in their responses during movements of similar amplitudes or speeds (see Fig. 4A). That is, even kinematic-responsive neurons responded to some movements within a bout but not to other movements of similar magnitude (note failures in Fig. 4, A and B). Nevertheless, for each movement parameter, some neurons displayed strong correlations (Fig. 4B).
RECEIVER OPERATING CHARACTERISTIC ANALYSIS.
The presence of a significant correlation between firing rate and a kinematic parameter, while indicative of an association between the two variables, is not informative as to the reliability with which the parameter is encoded. We used a receiver operating analysis (ROC) to address this issue. An ROC analysis allows one to determine the accuracy with which a variable can be classified while systematically varying the decision criteria but making no assumptions about underlying distributions.
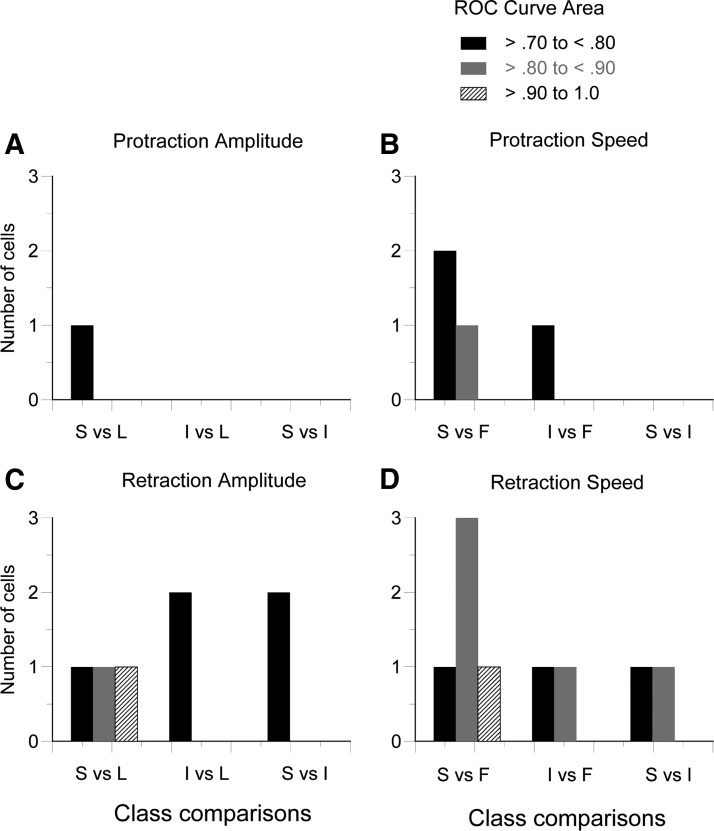
In the preceding section, we reported that for all firing rate-kinematic correlations, the firing rate increased with movement amplitude or speed. In the ROC analysis, we asked whether a higher firing rate indicated that a movement's actual class was relatively large or fast. For both amplitude (small, intermediate, large) and speed (slow, intermediate, fast) movements were assigned to one of three classes. Fourteen of our 21 neurons qualified for this analysis by having at least nine whisks per class. All possible binary movement class comparisons were made (see Fig. 5 for details). The integral of the ROC curve for each comparison was used to determine each neuron's percentage of correct classifications. By shuffling the data and by randomly matching firing rates and kinematic measures, we determined with a 95% confidence interval (CI) whether the calculated areas differed significantly from chance (0.50). Note that in Fig. 5 all reported ROC values differed from chance, and some neurons are represented in more than one movement type. Across all four movement components (protraction amplitude, protraction speed, retraction amplitude, retraction speed), five different TG neurons (5/14, 36%) were able to distinguish the smallest and largest or slowest and fastest movements with ≥70% accuracy. Some neurons could also make the finer discriminations between the intermediate and other classes. Thus a subpopulation of TG neurons can reliably encode whisker-movement kinematics.
FIG. 5.
Receiver operating characteristic (ROC) analysis for each movement kinematic. For amplitude, movements were sorted into 3 classes: small (S), intermediate (I), and large (L) movements. For speed, the classes were slow (S), intermediate (I), and fast (F). We plot those neurons for which ROC curves had areas ≥0.7 and thus could reliably encode movement kinematics.
Do trigeminal neurons encode whisker position or movement direction?
Here, we ask whether cells preferentially respond to protractions or retractions, whether these directional preferences are influenced by either the amplitudes or speeds of the movements, and whether there is a neural correlate of whisker location relative to the face, i.e., a “position” code.
We first asked whether ganglion cells have a preference for either forward or backward movements. An example of such direction-specific firing is shown in Fig. 6A for a neuron which fires preferentially during backward movements. For each of the 14 kinematic-responsive cells we computed a measure of the cell's directional tuning by determining a 95% CI for the difference in firing rate between the protraction and retraction components of individual whisker movements. Preferences for protraction or retraction are indicated, respectively, by values greater or less than zero. Figure 6B plots the mean difference in firing rate between protractions and retractions for cells in which the 95% CI of which differed from zero. Four cells display preferences for protractions and six prefer retractions. Thus 10/21 cells have significant directional preferences.
FIG. 6.
Evaluating the ability of kinematic-responsive neurons to differentiate protractions from retractions. A: directional response difference (Hz) as a function of relative speed (protraction minus retraction) for cell 2 in B–D. Directional response difference is computed for each whisk with protraction firing rate minus retraction firing rate. Similarly, relative speed for each whisk, is protraction speed minus retraction speed. B: mean difference in firing rate for protractions vs. retractions is plotted for all movements. Bars are only shown for those cells (10/14, ∼70%) for which the difference is significant (P < 0.05). C: R2 for the extent to which relative amplitudes and speeds modulate direction preferences. Speed has a stronger effect than amplitude in modulating the amount of directional tuning. D: the presence of a significant y intercept indicates a directional preference persisting after contributions from amplitude or speed are removed. Eight of the 10 cells displayed preferences after eliminating the influence of amplitude differences, and 5 of them responded more during protractions. Six of the 10 originally direction-selective cells continued to differentiate directions after accounting for speed, and they all preferred protractions.
Because protractions and retractions can vary in amplitude and/or speed, we next determined whether the observed direction preference was influenced by these kinematic parameters. To do so, we linearly regressed the amplitude or speed difference between the protraction and retraction of an individual whisker movement against the corresponding firing rate difference. The coefficient of determination (R2) quantifies the ability of speed or amplitude to modulate a directional preference. With the y intercept from the regression analysis, we determine whether the observed direction preference is maintained even when protractions and retractions are of equal amplitude or speed. Data for a sample cell are shown in Fig. 6A. The directional response difference (protraction minus retraction) for each individual whisker movement is plotted as function of relative speed. Across all movements, there is a stronger response for retractions (the plot in Fig. 6A is for cell 2 in B–D). However, as protractions become more similar to retractions in speed, the preference for retractions decreases. Relative speed has a stronger effect than amplitude on modulating direction preferences as indicated by the higher R2 values (Fig. 6C). Eight of 14 cells (∼60%) displayed a significant R2 for speed, whereas only 3 of 14 (∼20%) did so for amplitude. Additionally, the R2 values for speed were larger than those for amplitude (Mann-Whitney test, P = 0.01). For seven of the eight cells with significant speed correlations, the regression slopes are positive, indicating that the direction preference is biased toward the faster movement. Therefore direction preferences are modulated by both movement speed and amplitude.
Some TG neurons continue to display a direction preference even when the effects of amplitude and speed are removed. Figure 6D plots the mean firing rate difference when the amplitude or speed difference is fixed at zero, by using the y intercept (P < 0.0001 to < 0.05). The y intercept corresponds to the direction preference of a cell when the influences of amplitude and speed are removed. Cell 2 (shown in Fig. 6A) continues to respond preferentially to retractions even when the effect of amplitude is removed but loses a directional preference when speed differences are eliminated.
Only two cells lose a direction preference after the role of amplitude is eliminated. However, of the six cells that had retraction preferences across all whisking movements, five do not show a preference when the speed difference is set to zero in the regression, and one cell's preference actually changes to protractions. Therefore only preferences for protraction remain after removing the effect of speed.
In our experimental paradigm, the rat's “whisking space” is determined by the length of our CCD array, and it covers the area from 30 to 140° (90° being perpendicular to the face). Position coding within the space was examined by dividing it into 10° equal bins. For each of the 21 units, we determined whether the spikes are uniformly distributed throughout that space. Nine of the 21 neurons displayed a significantly nonuniform distribution (Kruskall-Wallis test, P < 0.05). To determine the preferred location with respect to the sampled range of locations for each neuron, we divided the bin with maximal response by the range of possible locations to derive a location index. A location index >0.5 indicates that the cell preferred relatively protracted positions. The mean location index for the nine cells with significant preferred locations is 0.78 ± 0.10, indicating that most of these cells responded preferentially at protracted positions.
DISCUSSION
We evaluated the ability of TG neurons in awake rats to reliably encode the kinematics of self-generated whisker movements in air. While the majority (∼70%) of TG neurons displayed significant correlations between firing rate and some kinematic parameter, only a subset, ∼30%, represented kinematics with high reliability. Moreover, neurons that did respond during movements did not do so consistently. Preferential firing to movement direction was observed, but it was strongly modulated by movement amplitude and speed. Finally, in contrast to the precise time-locking reported in previous studies of active whisker contacts, movements in air generated temporally dispersed responses that were not time-locked to the onset of either protractions or retractions. These findings are consistent with observations in awake, behaving animals (Leiser and Moxon 2007). Our results differ from those in a report based on “electrical whisking” in anesthetized animals, which concluded that trigeminal neurons report “the actual whiskers' position with high precision” (Szwed et al. 2003). Because of this inconsistency and because our findings address the question of whether and how behaving rats know the position of their whiskers, we consider first the limitations of our study and the possibility of other interpretations of the data.
Methodological considerations and alternative interpretations
Could the variability in neuronal responses to whisking movements in air be due to differences between the kinematics of our representative whisker (C2) and those of the other whiskers? Bermejo et al. (2005: see Fig. 2) have shown that whiskers in different rows and columns (e.g., B1/C3) exhibit highly synchronous movements, differing in amplitude by less than a degree. Thus it seems unlikely that the relation between neural activity and whisker motion might have been obscured by slight differences in kinematics of different whiskers. Moreover it seems equally unlikely that our optoelectronic monitoring system lacked sufficient spatiotemporal resolution. As for spatial resolution, it is true that ganglion neurons in anesthetized animals are sensitive to inputs <1 μm and frequencies as high as 1 kHz (Gibson and Welker 1983), but it is hard to imagine how movements <7 μm—that is, below the resolution of our detector—could be significant to a ganglion neuron when much larger movements (ranging from 4 to ∼80°) did not always evoke spikes, even in neurons that did display significant correlations between firing rates and kinematics. Additionally, movement of the whisker pad itself and the activity of its three related extrinsic muscles (Hill et al. 2008), could be contributing to the responses of our TG neurons for position. Because the pad moves with the whisker, we do not believe this is likely to be affecting the neural activity for changes in whisker position as measured by amplitude and speed. Regarding temporal resolution, while we can make no statements about the encoding by TG neurons of frequencies exceeding our maximum resolution of 250 Hz, the available data (see Fig. 4 of Wolfe et al. 2008), suggest that frequencies >200 Hz are not normally produced during whisking in air.
Although we could not classify our neurons as either RA or SA, several pieces of evidence suggest that our conclusions apply to both classes. First, Leiser and Moxon (2007) found identical correlations between the frequency within a bout of whisking and its corresponding firing rate for both RA and SA neurons (r = 0.52). Second, for “pink noise” stimuli covering a range of several hundred hertz, the spike trains of RA and SA neurons were identical in their ability to reconstruct stimuli (Jones et al. 2004a). Third, whether or not a neuron is classified as RA or SA varies with the direction of whisker stimulation (Jones et al. 2004b). Nonetheless, both SA and RA neurons, when driven by ramp-and-hold whisker deflections, respond primarily to movement onsets and offsets (Lichtenstein et al. 1990). However because we could not operationally distinguish between the two classes, the extent to which our findings may be generalized to both remains unclear.
Biomechanical considerations
The failure of TG neurons to respond consistently and reliably during whisking in air is surprising because these neurons are driven reliably by passive deflections (contacts) of similar amplitude and speeds (Jones et al. 2004a,b; Shoykhet et al. 2000; Stüttgen et al. 2006). Such differences suggest that follicle-whisker biomechanical interactions contribute critically to the probability of evoking spikes in TG neurons. One possibility is that both manual deflections and self-generated contacts produce relative motion between the whisker and its follicle, and this relative motion is critical for driving TG neurons. Self-generated whisker movements in air may generate less relative motion and, consequently, be less likely to drive some TG neurons. Our observed responses to whisking could be due to whisker-related muscles actually squeezing the follicle during whisker movements. Alternatively, contact may produce larger forces or torques at the whisker base (Birdwell et al. 2007), whereas whisking in air may produce smaller forces or torques. Future studies will be required to distinguish between these possibilities and to clarify the manner in which the kinematics of a whisker movement and its biomechanical consequence interact with the response thresholds of individual ganglion cells.
Encoding of the spatiotemporal properties of “whisking” movements in air
In our awake animals, responses during whisking in air were not time-locked to the three analyzed temporal features of the whisking cycle (protraction onset, retraction onset, or end). This finding differs with the observation by Szwed et al. (2003) that ganglion responses during “electrical whisking” in anesthetized rats are time-locked to movement onset during the initial portion of the protraction. The observed time-locking may reflect the presence of a timing signal in the repetitive facial nerve stimulation used to evoke the movements. The absence of precise time locking seen in our awake animals is also in contrast to findings in studies of the effects of passive vibrissae deflections (contacts) throughout the trigeminal neuraxis. For example, Jones et al. (2004a) demonstrated in anesthetized rats that TG neurons respond reliably and in a time-locked fashion to whisker deflections at frequencies as high as 312 Hz. Also in anesthetized rats, the initial firing rate of a TG neuron in the first 2 ms after deflection onset increases with stimulus speed but not amplitude (Shoykhet et al. 2000). In the ventroposterior thalamus, speed is encoded by the average firing rate across the thalamic population within 2–7 ms after the onset of population activity, and this component of the thalamic population response strongly predicts the response magnitude of individual layer 4 excitatory neurons in barrel cortex (Pinto et al. 2000). Precisely time-locked onset responses to self-generated contacts have also been reported for barrel cortex neurons in awake rats (Hentschke et al. 2006).
Therefore the temporal dispersion of responses we observed raises doubts as to the utility of ganglion activity during whisking in air for the downstream encoding of whisking kinematics. To derive useful kinematic information from such dispersed ganglion responses, downstream neurons in whisker-related brain stem structures would need to integrate those inputs over a relatively long time window that matches the duration of whisker movements (∼50–100 ms). While individual TG neurons do not emit reproducible spike trains during whisking in air, our ROC analysis indicates that ∼1/3 of the neurons are able to accurately discriminate kinematics with ≥70% accuracy. As each whisker is innervated by 100–150 ganglion neurons (Lee and Woolsey 1975), it is possible that the population activity of a subset of TG neurons might transmit reliable kinematic information to downstream structures. Because downstream neurons receive converging sensory inputs, they could represent kinematics more reliably than their presynaptic inputs, due to an increase in the signal-to-noise ratio (e.g., the receptive fields of superior colliculus neurons include ∼14.5 vibrissae) (Hemelt and Keller 2007). For example, in the both the auditory and the electrosensory systems, the ability to track ongoing input changes is greater in neurons of downstream structures than in the responses of single primary afferents (Carlson and Kawasaki 2008; Hoffman et al. 2008; Joris et al. 1994; Louage et al. 2005).
Encoding of whisker position or direction of movement in awake animals
Fee et al. (1997) were the first to report that barrel cortex neurons in awake animals fired at different and specific phase angles of a whisker movement in air. The responses were eliminated by facial nerve inactivation, suggesting an origin in movement-generated, sensory inputs. Recently, a cortical signal correlated with whisker angle has been reported for intracellular recordings from mouse barrel cortex (Poulet and Petersen 2007). A sensory origin for this signal is suggested because it is eliminated by whisker deafferentation.
Leiser and Moxon (2007) reported that TG cells responded preferentially either to forward movements (protractions) or backward movements (retractions). Our observations are consistent with this finding as we found that cells fired preferentially at particular positions and differentially according to movement direction. However, because a whisker can arrive at the same angle during a protraction or a retraction, responses to movement amplitude and angular position may be confounded. This concern is supported by our observation that such directional “preferences” are strongly modulated by amplitude and speed, such that some neurons lose their directional preferences when these effects are removed. Those neurons that continue to exhibit preferences tend to respond preferentially during forward movements, an observation consistent with our finding that cells respond preferentially at relatively protracted angles.
Conclusions
Our findings demonstrate that during whisking in air, some whisker-associated TG neurons encode information about the amplitude, direction, or speed of whisker movements. Neural encoding of such features could be used by the rat to adaptively modulate its motor output (Carvell and Simons 1990; Harvey et al. 2001) and, in conjunction with contact signals, to provide information about object location (Mehta et al. 2007; Szwed et al. 2003). Moreover, the absence of time-locked responses to whisker movements in air and their previously reported presence for whisker contacts could help to differentiate the two types of movement signals. However, we also found that only ∼30% of ganglion neurons exhibit reliable kinematic encoding and that preferential responsiveness to movement direction is strongly modulated by movement amplitude and speed. Thus the utility of these signals for processing by downstream structures remains to be determined by recording experiments throughout the trigeminal neuraxis in awake, actively whisking animals.
GRANTS
This work was supported by National Institute of Neurological Disorders and Stroke Grants NS-048937 to H. P. Zeigler, NS-51799 and NS-35360 to A. Keller, and NS-058758 to J. C. Brumberg and City University of New York Collaborative Grant 80209 to H. P. Zeigler and J. C. Brumberg. Infrastructure support for the Zeigler lab comes from RCMI Grant RR-03037.
Acknowledgments
The authors thank V. Lawson for preparing the animals and assisting in behavioral training and W. Friedman for comments on the manuscript and assistance with the coherence analysis.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- Bermejo et al. 2005.Bermejo R, Friedman W, Zeigler HP. Topography of whisking. II. Interaction of whisker and pad. Somatosens Mot Res 22: 213–220, 2005. [DOI] [PubMed] [Google Scholar]
- Bermejo et al. 1998.Bermejo R, Houben D, Zeigler HP. Optoelectronic monitoring of individual whisker movements in rats. J Neurosci Methods 83: 89–96, 1998. [DOI] [PubMed] [Google Scholar]
- Birdwell et al. 2007.Birdwell JA, Solomon JH, Thajchayapong M, Taylor MA, Cheely M, Towal RB, Conradt J, Hartmann MJZ. Biomechanical models for radial distance determination by the rat vibrissal system. J Neurophysiol 98: 2439–2455, 2007. [DOI] [PubMed] [Google Scholar]
- Bowden and Mahran 1956.Bowden RE, Mahran ZY. The functional significance of the pattern of innervation of the muscle quadratus labii superioris of the rabbit, cat, and rat. J Anat 90: 217–227, 1956. [PMC free article] [PubMed] [Google Scholar]
- Carlson and Kawasaki 2008.Carlson BA, Kawasaki M. From stimulus estimation to combination sensitivity: encoding and processing of amplitude and timing information in parallel, convergent sensory pathways. J Comput Neurosci. doi: 10.1007/jcomputneurosci.18027-007-0062-6.2008. [DOI] [PMC free article] [PubMed]
- Carvell and Simons 1990.Carvell GE, Simons DJ. Biometric analyses of vibrissal tactile discrimination in the rat. J Neurosci 10: 2638–2648, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen 2004.Cullen KE Sensory signals during active versus passive movement. Curr Opin Neurobiol 14: 698–706, 2004. [DOI] [PubMed] [Google Scholar]
- Fee et al. 1997.Fee MS, Mitra PP, Kleinfeld D. Central versus peripheral determinants of patterned spike activity in rat vibrissa cortex during whisking. J Neurophysiol 78: 1144–1149, 1997. [DOI] [PubMed] [Google Scholar]
- Gao et al. 2003.Gao P, Ploog BO, Zeigler HP. Whisking as a “voluntary” response: operant control of whisking parameters and effects of whisker denervation. Somatosens Mot Res 20: 179–189, 2003. [DOI] [PubMed] [Google Scholar]
- Gibson and Welker 1983.Gibson JM, Welker WI. Quantitative studies of stimulus coding in first-order vibrissa afferents of rats. I. Receptive field properties and threshold distributions. Somatosens Res 1: 51–67, 1983. [DOI] [PubMed] [Google Scholar]
- Harvey et al. 2001.Harvey MA, Bermejo R, Zeigler HP. Discriminative whisking in the head-fixed rat: optoelectronic monitoring during tactile detection and discrimination tasks. Somatosens Mot Res 18: 211–222, 2001. [DOI] [PubMed] [Google Scholar]
- Heil and Irvine 1997.Heil P, Irvine DR. First-spike timing of auditory-nerve fibers and comparison with auditory cortex. J Neurophysiol 78: 2438–2454, 1997. [DOI] [PubMed] [Google Scholar]
- Hemelt and Keller 2007.Hemelt ME, Keller A. Superior sensation: superior colliculus participation in rat vibrissa system. BMC Neurosci 31: 8–12, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hentschke et al. 2006.Hentschke H, Haiss F, Schwarz C. Central signals rapidly switch tactile processing in rat barrel cortex during whisker movements. Cereb Cortex 8: 1142–1156, 2006. [DOI] [PubMed] [Google Scholar]
- Hill et al. 2008.Hill DN, Bermejo R, Zeigler HP, Kleinfeld D. Biomechanics of the vibrissal motor plant in the rat: rhythmic whisking consists of triphasic neuromuscular activity. J Neurosci 28: 3438–3455, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofman et al. 2008.Hofman MH, Jung SN, Siebenaller U, Preiβner M, Chagnaud BP, Wilkens LA. Response properties of electrosensory units in the midbrain tectum of the paddlefish (Polyodon spathula Walbaum). J Exp Biol 211: 773–779, 2008. [DOI] [PubMed] [Google Scholar]
- Jin et al. 2004.Jin T, Witzemann V, Brecht M. Fiber types of the intrinsic whisker muscle and whisking behavior. J Neurosci 24: 3386–3393, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones et al. 2004a.Jones LM, Depireux DA, Simons DJ, Keller A. Robust temporal coding in the trigeminal system. Science 304: 1986–1989, 2004a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones et al. 2004b.Jones LM, Lee S, Trageser JC, Simons DJ, Keller A. Precise temporal responses in whisker trigeminal neurons. J Neurophysiol 92: 665–668, 2004b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joris et al. 1994.Joris PX, Carney LH, Smith PH, Yin TC. Enhancement of neuronal synchronization in the anteroventral cochlear nucleus. I. Responses to tones at the characteristic frequency. J Neurophysiol 71: 1022–1036, 1994. [DOI] [PubMed] [Google Scholar]
- Kleinfeld et al. 2006.Kleinfeld D, Ahissar E, Diamond ME. Active sensation: insights from the rodent vibrissa sensorimotor system. Curr Opin Neurobiol 16: 435–444, 2006. [DOI] [PubMed] [Google Scholar]
- Knutsen et al. 2005.Knutsen PM, Derdikman D, Ahissar E. Tracking whisker and head movements in unrestrained behaving rodents. J Neurophysiol 93: 2294–2301, 2005. [DOI] [PubMed] [Google Scholar]
- Lee and Woolsey 1975.Lee KJ, Woolsey TA. A proportional relationship between peripheral innervation density and cortical neuron number in the somatosensory system of the mouse. Brain Res 99: 349–353, 1975. [DOI] [PubMed] [Google Scholar]
- Leiser and Moxon 2007.Leiser SC, Moxon KA. Responses of trigeminal ganglion neurons during natural whisking behaviors in the awake rat. Neuron 53: 117–133, 2007. [DOI] [PubMed] [Google Scholar]
- Lichtenstein et al. 1990.Lichtenstein SH, Carvell GE, Simons DJ. Reponses of rat trigeminal ganglion neurons to movements of vibrissae in different directions. Somatosens Mot Res 7: 47–65, 1990. [DOI] [PubMed] [Google Scholar]
- Louage et al. 2005.Louage DH, van der Heijden M, Joris PX. Enhanced temporal response properties of anteroventral cochlear nucleus neurons to broadband noise. J Neurosci 25: 1560–1570, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masri et al. 2008.Masri R, Bezdudnaya T, Trageser JC, Keller A. Encoding of stimulus frequency and sensor motion in the posterior medial thalamic nucleus.J Neurophysiol (Jan 30, 2008). doi: 10.1152/jn.01322.2007. [DOI] [PMC free article] [PubMed]
- Mehta et al. 2007.Mehta SB, Whitmer D, Figueroa R, Williams BA, Kleinfeld D. Active spatial perception in the vibrissa scanning sensorimotor system. PLoS Biol 5:e15, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minnery and Simons 2003.Minnery BS, Simons DJ. Response properties of whisker-associated trigeminothalamic neurons in rat nucleus principalis. J Neurophysiol 89: 40–56, 2003. [DOI] [PubMed] [Google Scholar]
- Pinto et al. 2000.Pinto DJ, Brumberg JC, Simons DJ. Circuit dynamics and coding strategies in rodent somatosensory cortex. J Neurophysiol 83: 1158–1166, 2000. [DOI] [PubMed] [Google Scholar]
- Poulet and Petersen 2007.Poulet JF, Petersen CCH. Single and dual whole-cell recordings from the barrel cortex of awake mice during quantified whisking behavior. Soc Neurosci Abstr 402.21, 2007. [Google Scholar]
- Sachdev et al. 2002.Sachdev R, Sato T, Ebner FF. Divergent movement of adjacent whiskers. J Neurophysiol 87: 1440–1448, 2002. [DOI] [PubMed] [Google Scholar]
- Sachdev et al. 2001.Sachdev R, Jenkinson E, Zeigler HP, Ebner FF. Sensorimotor plasticity in the rodent vibrissa system. In: The Mutable Brain: Dynamic and Plastic Features of the Developing and Mature Brain, edited by Kaas JH. Amsterdam: Harwood Academic Publishers, 2001, p. 91–100.
- Schwartz et al. 2006.Schwartz O, Pillow JW, Rust NC, Simonocelli EP. Spike triggered neural characterization. J Vision 6: 484–507, 2006. [DOI] [PubMed] [Google Scholar]
- Shipley 1973.Shipley MT Response characteristics of single units in the rat's trigeminal nuclei to vibrissa displacements. J Neurophysiol 37: 73–90, 1973. [DOI] [PubMed] [Google Scholar]
- Shoykhet et al. 2000.Shoykhet M, Doherty D, Simons DJ. Coding of deflection velocity and amplitude by whisker primary afferent neurons: implications for higher level processing. Somatosens Mot Res 17: 171–180, 2000. [DOI] [PubMed] [Google Scholar]
- Simons 1978.Simons DJ Response properties of vibrissa units in the rat SI somatosensory cortex. J Neurophysiol 41: 798–820, 1978. [DOI] [PubMed] [Google Scholar]
- Stüttgen et al. 2006.Stüttgen MC, Rüter J, Schwarz C. Two psychophysical channels of whisker deflection in rats align with two neuronal classes of primary afferents. J Neurosci 26: 7933–7941, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szwed et al. 2003.Szwed M, Bagdasarian K, Ahissar E. Encoding of vibrissal active touch. Neuron 40: 621–630, 2003. [DOI] [PubMed] [Google Scholar]
- Towal and Hartmann 2008.Towal RB, Hartmann MJZ. Variability in velocity profiles during free air whisking behavior of unrestrained rats. J Neurophysiol 100: 740–752, 2008. [DOI] [PubMed] [Google Scholar]
- Vincent 1912.Vincent SB The function of the vibrissae in the behavior of the white rat. Behav Monographs 1: 7–85, 1912. [Google Scholar]
- Waite 1973.Waite PME The responses of cells in the rat thalamus to mechanical movements of the whiskers. J Physiol 228: 541–561, 1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe et al. 2008.Wolfe J, Hill DN, Pahlavan S, Drew PJ, Kleinfeld D, Feldman DE. Texture coding in the rat whisker system: slip-stick versus differential resonance. PLoS Biol 6: e215. doi: 10.1371/journal.pbio.0060215. 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu et al. 2006.Yu C, Derdikman D, Haidarliu S, Ahissar E. Parallel thalamic pathways for whisking and touch signals in the rat. PLoS Biol 4: e124, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker and Welker 1969.Zucker E, Welker WI. Coding of somatic sensory input by vibrissae neurons in the rat's trigeminal ganglion. Brain Res 12: 138–156, 1969. [DOI] [PubMed] [Google Scholar]