Abstract
Basal ganglia circuits are organized as parallel loops that have been proposed to compete in a winner-take-all fashion to determine the appropriate behavioral outcome. However, limited experimental support for strong lateral inhibition mechanisms within striatal regions questions this model. Here, stimulation of the prefrontal cortex (PFC) using naturally occurring bursty patterns inhibited firing in most nucleus accumbens (NA) projection neurons. When an excitatory response was observed for one stimulation site, neighboring PFC sites evoked inhibition in the same neuron. Furthermore, PFC stimulation activated interneurons, and PFC-evoked inhibition was blocked by GABAA antagonists in corticoaccumbens slice preparations. Thus bursting PFC activity recruits local inhibition in the NA, shaping responses of projection neurons with a topographical arrangement that allows inhibition among parallel corticoaccumbens channels. The data indicate a high order of information processing within striatal circuits that should be considered in models of basal ganglia function and disease.
INTRODUCTION
Classical models of basal ganglia loops emphasize the highly parallel nature of corticostriatal projections and suggest the presence of competition among channels of information. Anatomical data on glutamatergic projections from frontal cortical regions to the medial and ventral striatum in primates (Haber et al. 1995; Selemon 1985; Yeterian and Van Hoesen 1978) and rodents (Levesque and Parent 1998; Voorn et al. 2004) are in general consistent with segregated parallel circuits linking cortex and basal ganglia (Alexander et al. 1990). As cortical inputs do contact and activate local interneurons (Bennett and Bolam 1994; Mallet et al. 2005), and striatal medium spiny neurons (MSNs) exhibit extensive collaterals synapsing on other MSNs (O'Donnell and Grace 1993; Somogyi et al. 1981), lateral inhibition could also play a role in defining MSN activity (Blackwell et al. 2003; Carrillo-Reid et al. 2008; Koos and Tepper 1999; Mallet et al. 2005) and output selection in the basal ganglia (Groves 1983). Direct electrophysiological evidence of lateral inhibition, however, is missing (Tepper et al. 2004). We hypothesized that lateral inhibition in the NA is effective only on activation of its cortical afferents with naturally occurring bursty patterns.
In vivo electrophysiological responses of MSNs to single-pulse electrical stimulation of cortical inputs have been extensively studied in the dorsal (Wickens and Wilson 1998; Wilson et al. 1983) and ventral (Brady and O'Donnell 2004; O'Donnell and Grace 1995) striatum. In anesthetized rats, single-pulse PFC stimulation rarely evokes action potentials in NA MSNs unless it follows activation of hippocampal afferents or is delivered during hippocampal-dependent NA up states, suggesting that hippocampal afferents provide a requisite contextual input for gating PFC information through the NA (O'Donnell and Grace 1995). However, PFC neurons typically fire brief bursts of action potentials (≤30–50 Hz) during tasks requiring PFC activation in awake animals (Chafee and Goldman-Rakic 1998; Chang et al. 2002; Peters et al. 2005). The manner in which MSNs integrate cortical bursting activity in vivo is not known. As bursting stimulation of corticostriatal and -accumbens afferents drives prolonged depolarizations and evokes inhibitory processes in slice preparations (Lape and Dani 2004; Vergara et al. 2003), it is possible that PFC activation with trains of electrical stimulation evokes different NA responses than single pulse stimulation in vivo, including recruitment of local inhibitory responses. Here we explored NA neuron membrane potential and immediate early gene responses to stimulation of multiple PFC sites with a pattern that mimics natural bursting, focusing on the effects on MSN action potential firing and on activation of fast-spiking interneurons (FSIs).
METHODS
In vivo recordings
In vivo intracellular recordings were conducted in 13 male adult male Sprague-Dawley rats (270–430 g). All experiments were conducted in accordance with the United States Public Health Service Guide for the Use and Care of Animals, and all procedures were approved by the University of Maryland School of Medicine Institutional Animal Care and Use Committee. Rats were anesthetized with chloral hydrate (400 mg/kg) and placed on a stereotaxic apparatus. Intracellular electrodes were made from glass micropipettes (37–98 MΩ) filled with 3 M potassium acetate and 2% Neurobiotin and lowered into the medial core region of the NA (1.3–1.7 mm rostral from bregma; 1.2–1.4 mm lateral from midline; and 5.5–8.0 mm ventral from brain surface). Intracellular signals were amplified, low-pass filtered at 2 kHz, sampled at 10 kHz, and recorded to hard drive. Only neurons showing a resting membrane potential more negative than −60 mV, action potential amplitude measured from threshold of ≥40 mV, and input resistance >35 MΩ were included in the study. An array of electrodes was implanted in the right prelimbic cortex (centroid of array tips with respect to bregma: 3.2 mm rostral and 0.6 mm lateral; depth: 4.3 mm ventral from skull; 30° angle toward midline). Electrode arrays consisted of three to six pairs of monopolar electrodes made from 115 μm diam Teflon-coated tungsten wire glued together with an approximate tip separation of 200 μm. Electrode pairs were mounted such that they were separated by ∼500–750 μm in the sagittal plane. Electrical stimulation consisted of 50- or 62-Hz trains of 10 pulses delivered every 20 s. Pulses were 150 μs in duration and with a maximal current of 0.2–1.0 mA. The stimulation current was chosen at the beginning of each recording session to be slightly greater than the minimal current needed to evoke up states for each MSN and was maintained at this level throughout the session. At the end of the experiments, the rats were deeply anesthetized by a lethal dose of pentobarbital and killed. Brains were removed and processed for histological identification of stimulating and recording locations. All coordinates of recording and stimulation were based on the rat brain atlas by Paxinos and Watson (2005).
In vitro recordings
Parasagittal brain slices containing the NA were obtained from developmentally mature (postnatal day 52–63; n = 9) male Sprague-Dawley rats. Rats were anesthetized with chloral hydrate (400 mg/kg ip) and perfused with ice-cold artificial cerebrospinal fluid (ACSF) containing (in mM) 125 NaCl, 25 NaHCO3, 10 glucose, 3.5 KCl, 1.25 NaH2PO4, 0.1 CaCl2, 3 MgCl2, pH 7.40; osmolarity 285–295 mosM. Brains were removed, and parasagittal slices (350 μm thick, 1.2–2.0 mm lateral from midline) containing PFC fibers projecting to the NA were cut on a Vibratome in ice-cold ACSF and immediately transferred and incubated in warm (35°C) ACSF solution constantly oxygenated with 95% O2–5% CO2 for ≥70 min before recording. Following incubation, slices were transferred to a recording chamber in which ACSF was perfused (2 ml/min). For the recording ACSF, CaCl2 was increased to 2 mM and MgCl2 was decreased to 1 mM. All experiments were conducted at 33–35°C. Whole cell recordings were performed from MSN in the NA core, identified under visual guidance using infrared differential interference contrast video microscopy. Electrical stimulation (50 Hz, 0.2–0.9 mA) was applied through an electrode consisting of a twisted pair of Teflon-coated tungsten wire. The stimulating electrode was placed near the forceps minor and whole cell recordings were made 700–1,000 μm caudally along intact corticostriatal fibers originating near the electrode site. Patch pipettes (10–16 MΩ) were filled with (in mM) 115 K-gluconate, 10 HEPES, 2 MgCl2, 20 KCl, 2 MgATP, 2Na2-ATP, 0.3 GTP, pH 7.3; 285–295 mOsm. The recording pipette also contained 5 mM QX314 to block Na+ and K+ channels, which eliminated action potentials and facilitated visualization of excitatory and inhibitory components of electrically evoked responses. All bath-applied drugs [picrotoxin and 6-cyano-7-nitroquinoxalene-2,3-dione (CNQX)] were dissolved in ACSF with 0.005% DMSO and applied in the recording solution in known concentrations. Both control and drug-containing ACSF were oxygenated continuously throughout the experiments. The liquid junction potential (calculated to be +10.9 mV) was corrected post hoc.
Immunohistochemistry
Rats were anesthetized with chloral hydrate, and stimulation was applied through a single bipolar electrode implanted in the same coordinates as for in vivo recordings (0.7 mA). Trains were delivered every 15 s for 120 min. For control rats, electrodes were implanted, but no stimulation was applied. Animals were then killed by ip injection of chloral hydrate and transcardially perfused with cold buffered paraformaldehyde solution (4%; pH = 7.4). Brains were removed, postfixed, immersed in cryoprotectant (30% sucrose) solution for 72 h, and sliced (50 μm) in the coronal plane on a freezing microtome. Sections through the PFC were Nissl stained for verification of electrode placement. Sections through the NA were immunolabeled with rabbit anti-c-Fos (1:2500 dilution, Cell Signaling Technology, Danvers, MA) and mouse anti-parvalbumin (1:2500, Sigma) using standard laboratory techniques (Martins et al. 2007). Appropriate fluorescently conjugated (Cy2 and Cy3) secondary antibodies (Jackson Immunoresearch) were diluted 1:6,000. Imaging was performed on an Olympus Fluoview microscope. Sections from at least three rats from each group were evaluated.
RESULTS
Cortically evoked up states and suppression of action potential firing in NA MSNs
First, we tested whether PFC activation with trains of stimuli evoked depolarized up states in NA MSNs. In vivo intracellular recordings were obtained from 16 MSNs in the ventromedial striatum, primarily the NA core, of adult rats. Consistent with previous reports (Brady and O'Donnell 2004; O'Donnell and Grace 1995), most MSNs (11/16) exhibited the characteristic spontaneous fluctuations between a hyperpolarized resting potential (down state; −83.1 ± 3.8 mV) and plateau depolarizations (up state; −66.2 ± 2.1 mV) from which action potentials were generated. To mimic natural firing patterns (Chafee and Goldman-Rakic 1998; Chang et al. 2002; Peters et al. 2005), the PFC was stimulated with trains of 10 pulses at 50 or 62 Hz through pairs of electrodes placed within the prelimbic region of the medial PFC (Fig. 1A). The spatial organization of cortico-NA responses was assessed by alternating identical stimulation among several different electrode pairs within the medial PFC, up to three separate locations in 11 cells for a total of 21 unique stimulus-cell pairs. Stimulus trains were highly effective in driving sustained depolarizations from baseline (–84.9 ± 4.9 mV) to the up state (–58.1 ± 7.0 mV) across the population of recorded NA neurons and multiple PFC stimulation locations (Fig. 1, B and C). Thus although spontaneous up states in NA neurons may depend on the integrity of hippocampal afferents (O'Donnell and Grace 1995), up states can be driven by trains of pulses that mimic naturally occurring activity bursts in the PFC.
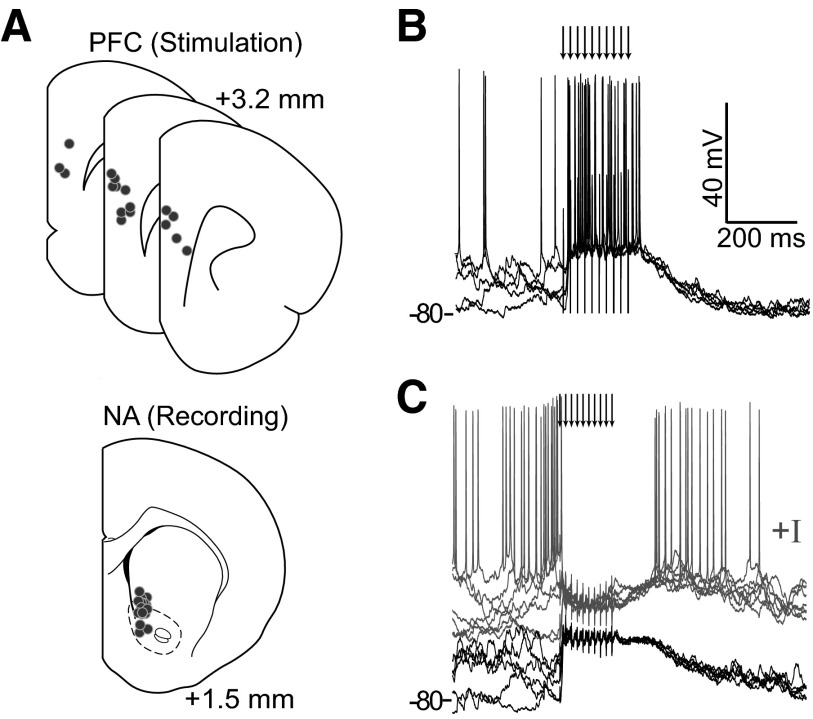
FIG. 1.
In vivo intracellular recordings of prefrontal cortex (PFC)-evoked nucleus accumbens (NA) medium spiny neuron (MSN) responses. A: illustration of stimulating and recording locations shown as dots. Some neurons were dorsal to the classic NA boundary but within the region receiving afferents from the medial PFC (Voorn et al. 2004). B: overlay of voltage traces from a neuron showing a bursting response evoked by PFC train stimulation. Arrows indicate stimulation pulses here and in subsequent figures. C: overlay of traces from a different neuron showing depolarization and lack of firing with undisturbed membrane potential (dark traces), and a suppression of firing (gray traces) evoked by PFC train stimulation when the neuron was depolarized with constant current. The overlays in B and C include traces recorded with stimuli occurring during up and down states, revealing the independence of responses on the membrane potential state at the time of stimulation.
The same dataset was analyzed to determine the impact of these PFC stimulation patterns on NA cell firing. Despite the stimulation driving NA MSNs to membrane potentials in the range of spontaneous up states, few neurons showed sustained activation (i.e., >3 action potentials) during the stimulus trains (2/21 stimulus-cell pairs in 2/11 neurons; Fig. 1B). The majority of NA neurons failed to generate action potentials during the stimulation period (19/21 stimulus-cell pairs in 11/11 neurons) even though these stimulations drove neurons to similarly depolarized potentials (−60.4 ± 6.5 mV; Fig. 1C). To assess whether this failure reflected insufficient excitatory input, some neurons were depolarized by current injection through the recording electrode to drive spontaneous action potential firing, and stimulation trials were repeated. Even under depolarization, many neurons showed a pronounced suppression of firing during PFC stimulation (11/14 stimulus-cell pairs in 6/8 neurons; Fig. 1C). The data show that PFC stimulation with naturally occurring bursts inhibits most NA neurons.
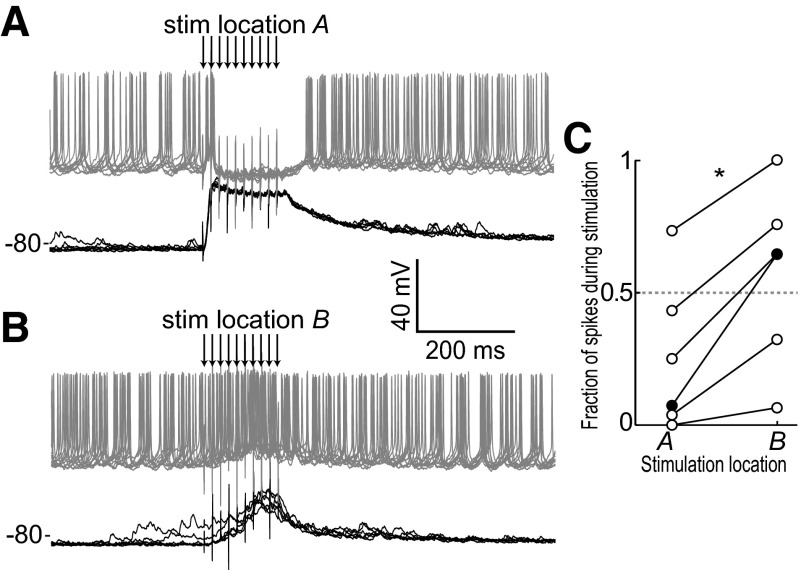
PFC-evoked suppression of NA MSN firing was dependent on the spatial location of PFC stimulation. Identical patterns and intensities of stimulation delivered to separate locations in the PFC (∼200–750 μm apart) could evoke dramatically different responses in the same NA neuron. In some cases, stimulation in one location evoked a strong suppression of firing while stimulation of a nearby site led to an increase in firing (Fig. 2, A and B). To quantify the impact of stimulation site on firing response patterns, we computed the ratio of firing during stimulation (from the 1st pulse to 200 ms later) to firing during and after the stimulation (from the 1st pulse to 400 ms later) while depolarizing the NA neurons to induce spontaneous firing. Evoked firing patterns changed significantly with the location of cortical stimulation (Δ ratio = 0.32 ± 0.15; P = 0.031 by 2-sided Wilcoxon rank-sum test; n = 6; Fig. 2C). In some neurons (n = 3), this ratio switched from <0.5 (indicating firing suppression) to >0.5 (indicating enhanced firing) when stimulation sites were exchanged. PFC stimulation bursts were effective in driving cortico-accumbens fibers, as EPSPs can be observed following each pulse in the train (Fig. 3). These data reveal that burst PFC stimulation, despite evoking depolarized up states, can suppress or enhance action potential firing in NA MSNs in a spatially dependent manner.
FIG. 2.
Individual neurons can show different responses to 2 adjacent cortical sites. A and B: evoked response of an MSN showing a suppression of firing for stimulation in 1 PFC location (A) but an enhancement of firing for stimulation in a different PFC location (B). Black traces were recorded without current injection; gray traces were recorded with constant intracellular current injection that caused spontaneous firing. C: ratio of firing during stimulation (from 1st pulse to 200 ms later) to firing during and after stimulation (from 1st pulse to 400 ms later) for 6 neurons showing that response pattern depends on stimulation location.
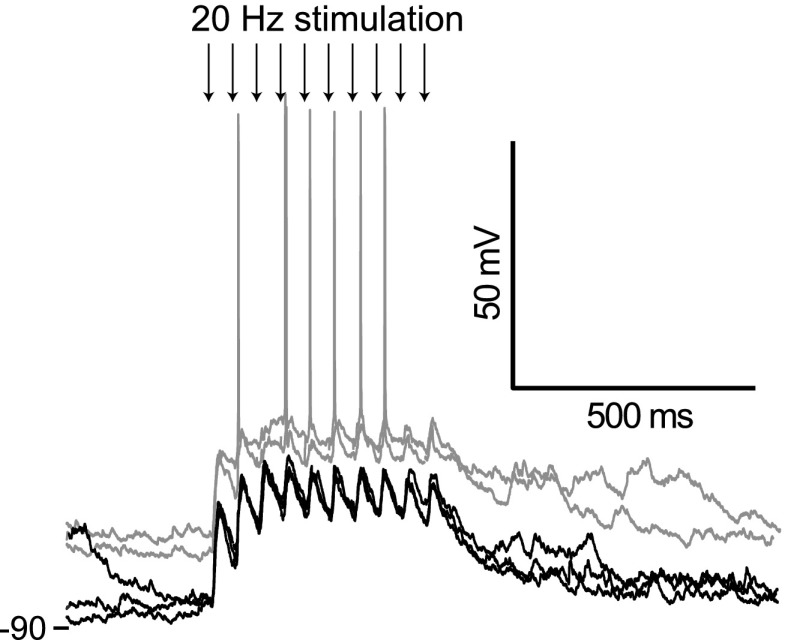
FIG. 3.
Corticoaccumbens excitatory postsynaptic potentials (EPSPs) are observed throughout the burst stimulation. Overlay of traces showing responses in a NA neuron to PFC burst stimulation at a lower frequency (20 Hz), showing EPSPs in response to every stimulus in the train (arrows point to the stimuli). The gray traces were obtained from the same neuron when depolarized by intrasomatic current injection, revealing action potential firing evoked by some stimuli.
Intra-accumbens inhibition
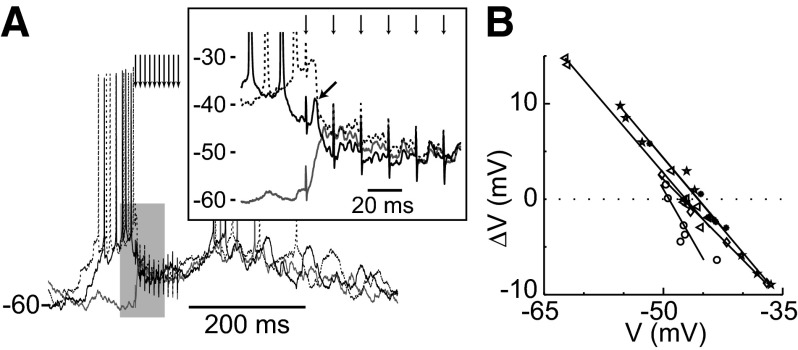
We next sought to determine whether local inhibition was involved in the PFC-evoked suppression of NA MSN firing. Evoked responses of suppressed neurons presented multiple components. Typically, a fast early depolarization evoked by the first stimulation pulse reversed at a highly depolarized membrane potential (Fig. 4A), indicative of a glutamatergic synaptic response. With somatic current injection, these initial depolarizations were accompanied by an action potential (spike probability = 0.91 ± 0.16). For those neurons that showed a suppression of firing, the responses to the second and subsequent pulses reversed at −47.1 ± 1.5 mV as determined by linear regression of evoked membrane potential changes with respect to the value prior to stimulation (Fig. 4B). Thus the late components in MSN response to PFC burst stimulation include conductances that reverse at negative membrane potentials (i.e., Cl−) that can counteract depolarizing glutamatergic currents. This result suggests that PFC stimulation can recruit local inhibitory mechanisms in the NA.
FIG. 4.
Reversal of evoked in vivo responses. A: overlay of traces from a representative cell showing responses to PFC train stimulation under constant artificial depolarization by current injection. Variation in membrane potential prior to stimulation is due to spontaneous activity. An expanded view (inset) of the gray shaded region shows an early component with a highly depolarized reversal (arrow) followed by a convergence of the response at an intermediate potential. B: linear regression (lines) of evoked reversals for inhibited cells (symbols) shows a mean reversal of −47.1 ± 1.5 mV.
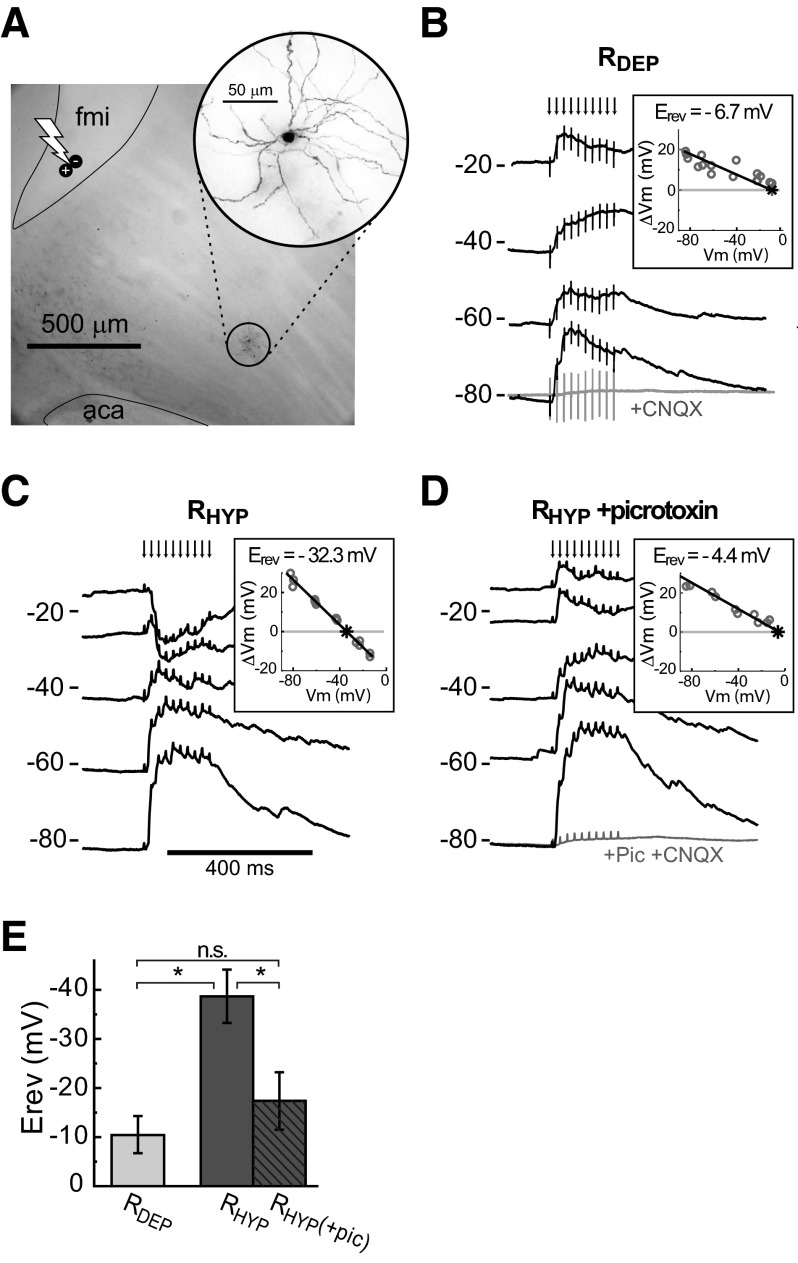
Multiple sources of inhibition can affect MSN firing. These include GABAergic inputs from striatal (Koos et al. 2004) and extrastriatal (Groenewegen et al. 1993; Van Bockstaele and Pickel 1995) sources, as well as intrinsic membrane conductances (Vilchis et al. 2000). To explore the contribution of intra-NA sources, we performed whole cell recordings from MSNs in parasagittal slices that preserved cortico-accumbens fibers but excluded nonstriatal structures (O'Donnell and Grace 1994) (Fig. 5A). The Na+/K+ channel blocker QX314 was added to the intracellular solution to eliminate action potentials and the associated afterhyperpolarization that can obscure synaptic membrane potential changes at depolarized membrane potentials. Cortico-accumbens stimulation with a train of pulses similar to that used in the in vivo recordings (10 pulses at 50 Hz, 0.54 ± 0.19 mA) evoked robust depolarizing responses (Fig. 5B) in MSNs located 0.5–1 mm away from the stimulation site along visualized fibers connecting stimulation and recording sites. Bath application of the AMPA antagonist CNQX (50 μM) greatly attenuated the evoked responses, confirming them to be synaptic in origin (n = 9 of 9). Evoked responses reversed at depolarized potentials in most NA MSNs (RDEP neurons; −10.4 ± 6.9 mV by linear regression; n = 17 of 25; Fig. 5B). However, evoked responses reversed at more hyperpolarized potentials in some MSNs (RHYP cells; −39 ± 6.0 mV; n = 8 of 25; Fig. 5C), suggesting that in some cases a Cl− component could be evoked by PFC afferents. The evoked responses in RHYP cells are consistent with the negative reversal observed in vivo in the responses to the late stimulation pulses of a PFC burst in NA neurons in which PFC stimulation suppressed firing. Bath application of the GABA antagonist picrotoxin (10 μM) shifted the evoked reversal potential of all RHYP neurons tested to a depolarized potential closely resembling that of RDEP cells (−17 ± 8.5 mV; n = 6 of 6; P = 0.11 by ANOVA; Fig. 5, D and E), confirming the inward conductance to be mediated by GABAA receptors. Thus burst activation of cortico-accumbens fibers can evoke GABA release from striatal sources that influence MSN evoked firing.
FIG. 5.
Cortically activated inhibition in cortico-accumbens slices. A: photomontage of a corticoaccumbens slice and a Neurobiotin-stained neuron (inset) illustrating the relative placement of stimulating electrode (bolt symbol) and recorded neuron with respect to the forceps minor (fmi) and anterior commissure (aca). B: overlay of voltage traces in current clamp from a MSN showing depolarization evoked by train stimulation of corticostriatal afferents and attenuation of evoked responses by bath application of 6-cyano-7-nitroquinoxalene-2,3-dione (CNQX) (50 μM; bottom trace). Current was injected to depolarize the neuron to explore the reversal potential (Erev) of the evoked response, which was –6.7 mV as determined by linear regression (inset). Neurons with such depolarized reversals are classified as RDEP. C: overlay of voltage traces from an RHYP MSN that showed a more hyperpolarized reversal of the evoked response at –32.3 mV. D: overlay of voltage traces from the same neuron as in C during bath application of the GABA antagonist picrotoxin (10 μm), which shifted the reversal of the evoked response to a less negative value. E: the addition of picrotoxin shifts the reversal of RHYP neurons to potentials similar to those observed in RDEP neurons.
PFC stimulation activates parvalbumin-expressing NA interneurons
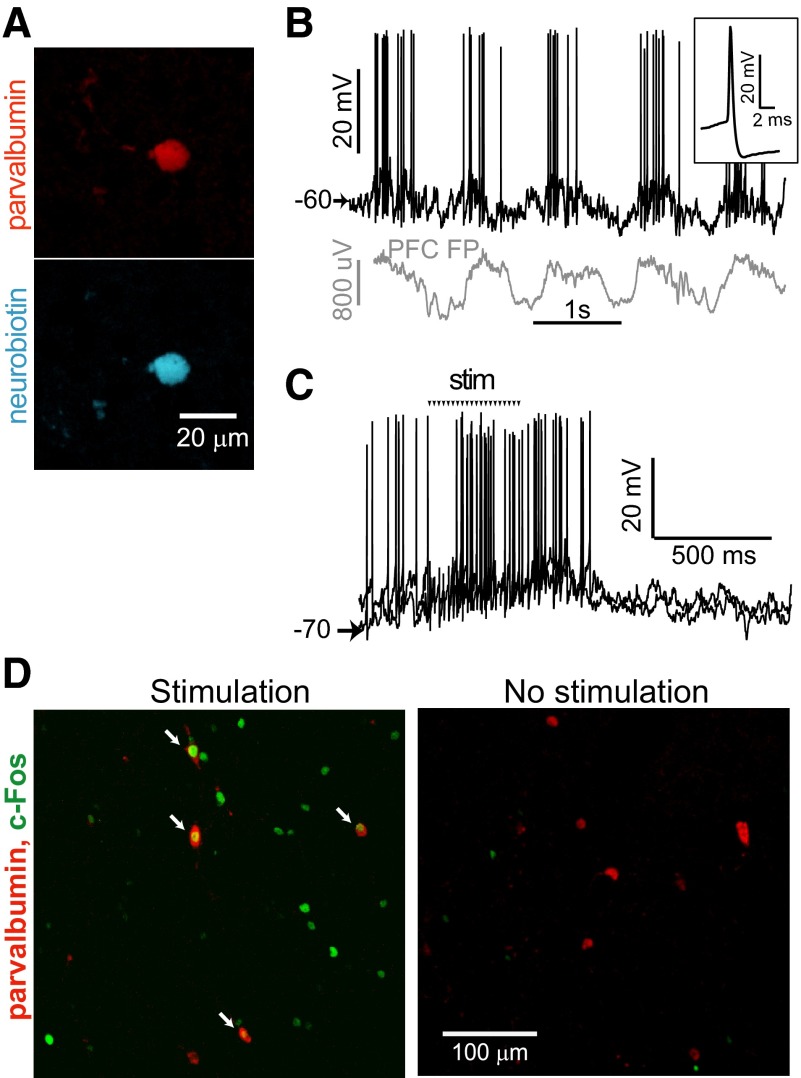
A likely source of intra-NA GABA that impacts somatic membrane potential are GABAergic interneurons (Koos et al. 2004). Cortical stimulation in vivo activates parvalbumin (PV)-containing GABAergic interneurons in the dorsal striatum (Mallet et al. 2005; Parthasarathy and Graybiel 1997). We explored whether this was also the case in the NA with in vivo intracellular recordings from two putative interneurons, one of which was confirmed as an interneuron by PV immunostaining (Fig. 6A). Both neurons exhibited electrophysiological properties typically associated with interneurons (Kawaguchi 1993): depolarized resting membrane potential, narrow spikes and large afterhyperpolarization (Fig. 6B). These neurons showed membrane potential fluctuations with a time course similar to that of cortical field potentials, and both neurons were activated by electrical PFC stimulation. The responses to brief stimulation trains were difficult to discriminate because the spontaneous activity of these neurons had a burst-like pattern. Stimulation with longer trains (>10 pulses; Fig. 6C) revealed clear excitatory responses to cortical stimulation. The data suggest interneurons can be driven by burst PFC stimulation.
FIG. 6.
Activation of NA interneurons by PFC train stimulation in vivo. A: parvalbumin (red) and Neurobiotin (blue) co-labeling of a fast spiking interneuron recorded intracellularly in vivo in the NA. B: recording from the neuron in a (dark trace) and a simultaneously recorded PFC field potential (PFC FP, gray trace—the polarity was inverted to make it easier to visualize the synchronization) showing spontaneous fluctuations. Inset shows the action potential waveform. C: overlay of traces from the same neuron in response to PFC stimulation. Arrows show timing of the electrical pulses, and stimulus artifacts have been removed for clarity. D: confocal image of a NA section labeled for c-Fos (green) and parvalbumin (red) following in vivo train stimulation (left). The presence of co-labeling (arrows) indicates that PFC train stimulation can activate NA interneurons. A control section from an unstimulated brain (right) shows very little c-Fos immunoreactivity. Scale is the same in both panels.
To more reliably determine whether NA interneurons were activated by PFC stimulation, we examined immediate early gene expression in a separate group of rats (n = 3) that received identical PFC train stimulation (10 pulses, 62 Hz, 0.7 mA delivered every 15 s for 120 min) and in the same PFC region as in the electrophysiological experiments. Immunohistochemical analysis of c-Fos expression demonstrated more active NA cells in rats that had received PFC stimulation than in rats that did not (3,819 ± 2,379 cells per mm3 in stimulated; 556 ± 374 cells per mm3 in nonstimulated rats; P = 0.039; Student's t-test). Co-labeling for PV, a calcium binding protein specific to FSI, revealed that some FSI were activated by the bursting cortical stimulation (208 ± 49 cells per mm3), but no activated FSI could be observed in the NA from control rats (P = 0.036; Wilcoxon rank sum test; Fig. 6D). These data show that NA interneurons are activated by bursting PFC activation.
DISCUSSION
High-frequency PFC stimulation in vivo not only drove NA MSNs to depolarized up states but often suppressed action potential firing. Stimulation at different PFC locations could evoke firing for one site and decrease firing for another in some NA neurons. Whole cell recordings in cortico-accumbens slices revealed a GABAA receptor-mediated component evoked by a similar pattern of PFC burst stimulation. Furthermore, FSI in the NA expressed c-Fos and increased firing following in vivo PFC burst stimulation. Thus local inhibition by NA interneurons is a common and important component of NA MSN responses to cortical activation with naturally occurring and behaviorally relevant bursting patterns.
High-frequency cortical stimulation depolarized MSNs in vivo while suppressing firing during the stimulation period in many cases. Although the silencing of neighboring cortical regions caused by single-pulse electrical cortical stimulation induces down states and eliminates MSN firing following stimulation (Kasanetz et al. 2006; Wilson et al. 1983), several pieces of information suggest the presence of an active local inhibitory mechanism being recruited by PFC train stimulation. As the cortex was stimulated repeatedly, any disfacilitation caused by the initial pulse would be overridden by subsequent pulses during the stimulation period. In fact, low-frequency stimulus trains allowed visualization of excitatory postsynaptic potentials (EPSPs) in response to every pulse in the train, suggesting active cortical afferents. In addition, c-Fos immunoreactivity revealed strong c-Fos induction throughout the PFC in stimulated brains (data not shown) rather than lack of expression that would suggest stimulation-induced cortical disfacilitation. Furthermore, the reversal of the evoked response around −47 mV for those neurons that did not generate spikes during the stimulation suggests that a chloride current was activated by cortical inputs. This reversal potential of evoked responses is more depolarized than the resting potential but is more polarized than the threshold for action potential generation in these neurons. Such difference would allow for a shunting inhibition mechanism and can account for our finding that PFC stimulation could suppress firing even though responses were depolarizing from baseline. Slice recordings revealed MSN responses with similarly negative reversal potentials, which were changed to reversal potentials more consistent with a glutamatergic response (i.e., more depolarized) by a GABAA antagonist. The data indicate that in some cases, the inhibitory mechanism driven by cortical stimulation depends on intra-NA GABA release.
A variety of sources could account for cortically induced GABA release in the NA. First, NA MSNs exhibit extensive axon collaterals (O'Donnell and Grace 1993; Somogyi et al. 1981). Collaterals from other MSN have been recently identified to provide a very small extent of feedback inhibition (Koos et al. 2004; Plenz 2003; Tunstall et al. 2002). As these GABA synapses would occur on distal dendrites (Koos et al. 2004), they would have minimal, if any, effect on somatic membrane potential but could effectively shunt corticoaccumbens synaptic responses in the dendritic arbor and could conceivably contribute to the reduced firing observed during burst stimulation. On the other hand, although GABAergic interneurons comprise a small proportion of striatal neurons (Kita 1993), they can exert a strong influence on MSNs (Koos and Tepper 1999; Koos et al. 2004). The presence of c-Fos in PV expressing neurons following stimulation indicates that PFC train stimulation activates this interneuron subpopulation in the ventral striatum, similar to observations in the dorsal striatum (Parthasarathy and Graybiel 1997). More direct evidence for cortical activation of NA FSI was provided by our recordings from putative interneurons in vivo. A third potential source of GABA release by cortical afferent stimulation could be antidromic activation of mesocortical GABA fibers. This is unlikely, however, as these fibers are seen in both areas but not with a trajectory that would remain intact in our slices (Carr and Sesack 2000). Thus PFC-driven inhibition in the NA observed in slices and in vivo is likely to involve activation of NA interneurons.
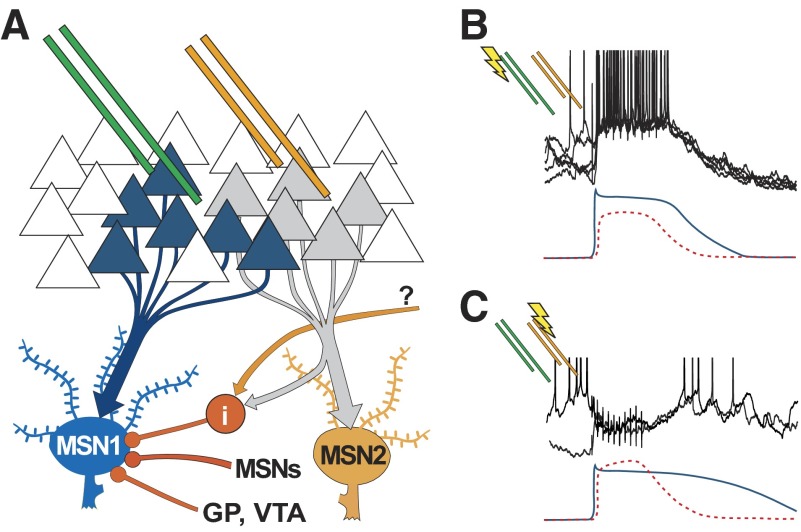
A novel and important aspect of the present data is the pronounced dependence of MSN evoked firing on the spatial location of cortical stimulation in vivo. Individual MSNs can display a bursting response to stimulation of a given PFC region and a complete firing suppression when an adjacent PFC region is stimulated. This finding could be interpreted as the recordings capturing lateral inhibition mechanisms within the NA. Indeed local glutamate administration in the dorsal striatum of rats enhances activity of nearby neurons while suppressing activity far from the injection site (Rebec and Curtis 1988), and an analysis of c-Fos expression in monkeys has shown that burst stimulation of sensorimotor cortex activates a wide network of parvalbumin-containing GABAergic interneurons throughout the dorsal striatum, whereas MSNs are activated in a few focal clusters (Parthasarathy and Graybiel 1997). Thus the variations in response to different stimulation sites in vivo may correspond to stimulating and recording electrodes being placed in matching ends of hardwired channels of information (yielding increased firing with cortical activation) or a mismatch that could pick up more effectively the feed-forward inhibition (yielding reduced NA firing with PFC stimulation; Fig. 7). Although the different stimulation sites were likely to generate activity with different cortical loci, it is also possible that these could differentially activate cortical laminae and thus alter corticostriatal output in complex ways that may vary in similarity to relevant patterns in the awake state. The recruitment of fast-spiking interneurons could tune spatial NA activity in a distributed assembly of neurons (Carrillo-Reid et al. 2008; O'Donnell 2003), allowing an effective filtering of irrelevant corticostriatal information.
FIG. 7.
Model of cortico-accumbens processing. A: diagram illustrating the hypothetical organization of cortico-accumbens projections in which electrode placement can activate cortical units directly projecting to 1 MSN (MSN1; blue) from 1 position (green pair) while another placement could activate a different cortico-NA channel (orange electrodes and MSN2), driving lateral inhibition via interneurons (i— red) and potentially also via collaterals from other MSNs. Other inputs to the NA could also activate interneurons (question mark). B and C: illustrations of the magnitude and time course of hypothetical excitatory (solid) and inhibitory (dotted) somatic components are shown under previously shown data traces. MSN1 receives a predominantly excitatory input with stimulation via the left (green) electrodes (B) and produces a bursting response. Stimulation via the right (orange) electrodes (C) activates feedforward and feedback GABAergic pathways, resulting in a strong shunting inhibitory input to MSN1 during the stimulation.
The functional role of accumbens inhibitory interneurons remains an open question. Although networks of interconnected GABAergic interneurons are important for synchronizing activity in hippocampus and cortex in part through shunting inhibition (Bartos et al. 2007), such a synchronization has not been reported in striatum. Rather striatal interneurons activate asynchronously in slice preparations (Carrillo-Reid et al. 2008) and in behaving animals (Berke 2008), suggesting interneurons do not play a role in synchronizing activity across the striatum. Our data, however, indicate that NA interneurons can be strongly activated by bursty cortical stimulation, and in vivo studies in the dorsal striatum have also shown strong responses of fast-spiking interneurons to cortical stimulation, which in turn can drive feed-forward suppression of MSN firing (Carrillo-Reid et al. 2008; Gustafson et al. 2006; Koos and Tepper 1999; Mallet et al. 2005). Thus in addition to local collaterals from other MSN affecting EPSPs in distal dendrites (but being ineffective in controlling somatic membrane potential), interneurons activated by strong cortical inputs can exert feed-forward inhibition of firing via strong shunting inhibition, as occurs in dorsal striatum during strong spike-and-wave discharges in a rodent model of absence epilepsy (Slaght et al. 2004).
NA neuron activation by the PFC is more complex than simple EPSPs. Bursts of inputs mimicking natural firing patterns observed during PFC activation in awake animals engage NA mechanisms yielding persistent depolarizations and recruit inhibitory mechanisms (likely local) that provide temporal and spatial constrains to NA responses. In this way, only strongly activated, behaviorally relevant inputs could be enhanced over potentially competing NA neural assemblies, probably yielding a pattern of activity appropriate for the selection of a correct behavioral response. The robustness of inhibition during the stimulation suggests that local circuit processing is essential for regulating ensemble coding in the NA and must be taken into account when considering the integration of motivational and sensory information in the ventral striatum for guiding goal-directed behaviors (Christakou et al. 2004; Floresco et al. 1999).
GRANTS
This work was supported by National Institue of Mental Health Grant MH-60131 to P. O'Donnell and a Tourette Syndrome Association award to A. J. Gruber.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- Alexander 1990.Alexander GE, Crutcher MD, DeLong MR. Basal ganglia-thalamocortical circuits: parallel substrates for motor, oculomotor, “prefrontal” and “limbic” functions. Prog Brain Res 85: 119–146, 1990. [PubMed] [Google Scholar]
- Bartos 2007.Bartos M, Vida I, Jonas P. Synaptic mechanisms of synchronized gamma oscillations in inhibitory interneuron networks. Nat Rev Neurosci 8: 45–56, 2007. [DOI] [PubMed] [Google Scholar]
- Bennett 1994.Bennett BD, Bolam JP. Synaptic input and output of parvalbumin-immunoreactive neurons in the neostriatum of the rat. Neuroscience 62: 707–719, 1994. [DOI] [PubMed] [Google Scholar]
- Berke 2008.Berke JD Uncoordinated firing rate changes of striatal fast-spiking interneurons during behavioral task performance. J Neurosci 28: 10075–10080, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwell 2003.Blackwell KT, Czubayko U, Plenz D. Quantitative estimate of synaptic inputs to striatal neurons during up and down states in vitro. J Neurosci 23: 9123–9132, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady 2004.Brady AM, O'Donnell P. Dopaminergic modulation of prefrontal cortical input to nucleus accumbens neurons in vivo. J Neurosci 24: 1040–1049, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr 2000.Carr DB, Sesack SR. GABA-containing neurons in the rat ventral tegmental area project to the prefrontal cortex. Synapse 38: 114–123, 2000. [DOI] [PubMed] [Google Scholar]
- Carrillo-Reid 2008.Carrillo-Reid L, Tecuapetla F, Tapia D, Hernandez-Cruz A, Galarraga E, Drucker-Colin R, Bargas J. Encoding network states by striatal cell assemblies. J Neurophysiol 99: 1435–1450, 2008. [DOI] [PubMed] [Google Scholar]
- Chafee 1998.Chafee MV, Goldman-Rakic PS. Matching patterns of activity in primate prefrontal area 8a and pariental area 7ip neurons during a spatial working memory task. J Neurophysiol 79: 2919–2940, 1998. [DOI] [PubMed] [Google Scholar]
- Chang 2002.Chang JY, Chen L, Luo F, Shi LH, Woodward DJ. Neuronal responses in the frontal cortico-basal ganglia system during delayed matching-to-sample task: ensemble recording in freely moving rats. Exp Brain Res 142: 67–80, 2002. [DOI] [PubMed] [Google Scholar]
- Christakou 2004.Christakou A, Robbins TW, Everitt BJ. Prefrontal cortical-ventral striatal interactions involved in affective modulation of attentional performance: implications for corticostriatal circuit function. J Neurosci 24: 773–780, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco 1999.Floresco SB, Braaksma DN, Phillips AG. Thalamic-cortical-striatal circuitry subserves working memory during delayed responding on a radial arm maze. J Neurosci 19: 11061–11071, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groenewegen 1993.Groenewegen HJ, Berendse HW, Haber SN. Organization of the output of the ventral striatopallidal system in the rat: ventral pallidal efferents. Neuroscience 57: 113–142, 1993. [DOI] [PubMed] [Google Scholar]
- Groves 1983.Groves PM A theory of the functional organization of the neostriatum and the neostriatal control of voluntary movement. Brain Res 286: 109–132, 1983. [DOI] [PubMed] [Google Scholar]
- Gustafson 2006.Gustafson N, Gireesh-Dharmaraj E, Czubayko U, Blackwell KT, Plenz D. A comparative voltage and current-clamp analysis of feedback and feedforward synaptic transmission in the striatal microcircuit in vitro. J Neurophysiol 95: 737–752, 2006. [DOI] [PubMed] [Google Scholar]
- Haber 1995.Haber SN, Kunishio K, Mizobuchi M, Lynd-Balta E. The orbital and medial prefrontal circuit through the primate basal ganglia. J Neurosci 15: 4851–4867, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasanetz 2006.Kasanetz F, Riquelme LA, O'Donnell P, Murer MG. Turning off cortical ensembles stops striatal Up states and elicits phase perturbations in cortical and striatal slow oscillations in rat in vivo. J Physiol 577: 97–113, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi 1993.Kawaguchi Y Physiological, morphological, and histochemical characterization of three classes of interneurons in rat neostriatum. J Neurosci 13: 4908–4923, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kita 1993.Kita H GABAergic circuits of the striatum. Prog Brain Res 99: 51–72, 1993. [DOI] [PubMed] [Google Scholar]
- Koos 1999.Koos T, Tepper JM. Inhibitory control of neostriatal projection neurons by GABAergic interneurons. Nat Neurosci 2: 467–472, 1999. [DOI] [PubMed] [Google Scholar]
- Koos 2004.Koos T, Tepper JM, Wilson CJ. Comparison of IPSCs evoked by spiny and fast-spiking neurons in the neostriatum. J Neurosci 24: 7916–7922, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lape 2004.Lape R, Dani JA. Complex response to afferent excitatory bursts by nucleus accumbens medium spiny projection neurons. J Neurophysiol 92: 1276–1284, 2004. [DOI] [PubMed] [Google Scholar]
- Levesque 1998.Levesque M, Parent A. Axonal arborization of corticostriatal and corticothalamic fibers arising from prelimbic cortex in the rat. Cereb Cortex 8: 602–613, 1998. [DOI] [PubMed] [Google Scholar]
- Mallet 2005.Mallet N, Le Moine C, Charpier S, Gonon F. Feedforward inhibition of projection neurons by fast-spiking GABA interneurons in the rat striatum in vivo. J Neurosci 25: 3857–3869, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins 2007.Martins GJ, Plachez C, Powell EM. Loss of embryonic MET signaling alters profiles of hippocampal interneurons. Dev Neurosci 29: 143–158, 2007. [DOI] [PubMed] [Google Scholar]
- O'Donnell 2003.O'Donnell P Dopamine gating of forebrain neural ensembles. Eur J Neurosci 17: 429–435, 2003. [DOI] [PubMed] [Google Scholar]
- O'Donnell 1993.O'Donnell P, Grace AA. Physiological and morphological properties of accumbens core and shell neurons recorded in vitro. Synapse 13: 135–160, 1993. [DOI] [PubMed] [Google Scholar]
- O'Donnell 1994.O'Donnell P, Grace AA. Tonic D2-mediated attenuation of cortical excitation in nucleus accumbens neurons recorded in vitro. Brain Res 634: 105–112, 1994. [DOI] [PubMed] [Google Scholar]
- O'Donnell 1995.O'Donnell P, Grace AA. Synaptic interactions among excitatory afferents to nucleus accumbens neurons: hippocampal gating of prefrontal cortical input. J Neurosci 15: 3622–3639, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parthasarathy 1997.Parthasarathy HB, Graybiel AM. Cortically driven immediate-early gene expression reflects modular influence of sensorimotor cortex on identified striatal neurons in the squirrel monkey. J Neurosci 17: 2477–2491, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos 2005.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates (5th ed.). San Diego: Academic, 2005.
- Peters 2005.Peters YM, O'Donnell P, Carelli RM. Prefrontal cortical cell firing during maintenance, extinction, and reinstatement of goal-directed behavior for natural reward. Synapse 56: 74–83, 2005. [DOI] [PubMed] [Google Scholar]
- Plenz 2003.Plenz D When inhibition goes incognito: feedback interaction between spiny projection neurons in striatal function. Trends Neurosci 26: 436–443, 2003. [DOI] [PubMed] [Google Scholar]
- Rebec 1988.Rebec GV, Curtis SD. Reciprocal zones of excitation and inhibition in the neostriatum. Synapse 2: 633–635, 1988. [DOI] [PubMed] [Google Scholar]
- Selemon 1985.Selemon LD Longitudinal topography and interdigitation of corticostriatal projections in the rhesus monkey. J Neurosci 5: 776–794, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slaght 2004.Slaght SJ, Paz T, Chavez M, Deniau JM, Mahon S, Charpier S. On the activity of the corticostriatal networks during spike-and-wave discharges in a genetic model of absence epilepsy. J Neurosci 24: 6816–6825, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somogyi 1981.Somogyi P, Bolam JP, Smith AD. Monosynaptic cortical input and local axon collaterals of identified striatonigral neurons. A light and electron microscopic study using the Golgi-peroxidase transport-degeneration procedure. J Comp Neurol 195: 567–584, 1981. [DOI] [PubMed] [Google Scholar]
- Tepper 2004.Tepper JM, Koos T, Wilson CJ. GABAergic microcircuits in the neostriatum. Trends Neurosci 27: 662–669, 2004. [DOI] [PubMed] [Google Scholar]
- Tunstall 2002.Tunstall MJ, Oorschot DE, Kean A, Wickens JR. Inhibitory interactions between spiny projection neurons in the rat striatum. J Neurophysiol 88: 1263–1269, 2002. [DOI] [PubMed] [Google Scholar]
- Van Bockstaele 1995.Van Bockstaele EJ, Pickel VM. GABA-containing neurons in the ventral tegmental area project to the nucleus accumbens in rat brain. Brain Res 682: 215–221, 1995. [DOI] [PubMed] [Google Scholar]
- Vergara 2003.Vergara R, Rick C, Hernandez-Lopez S, Laville JA, Guzman JN, Galarraga E, Surmeier DJ, Bargas J. Spontaneous voltage oscillations in striatal projection neurons in a rat corticostriatal slice. J Physiol 553: 169–182, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilchis 2000.Vilchis C, Bargas J, Ayala GX, Galvâan E, Galarraga E. Ca2+ channels that activate Ca2+-dependent K+ currents in neostriatal neurons. Neuroscience 95: 745–752, 2000. [DOI] [PubMed] [Google Scholar]
- Voorn 2004.Voorn P, Vanderschuren LJ, Groenewegen HJ, Robbins TW, Pennartz CM. Putting a spin on the dorsal-ventral divide of the striatum. Trends Neurosci 27: 468–474, 2004. [DOI] [PubMed] [Google Scholar]
- Wickens 1998.Wickens JR, Wilson CJ. Regulation of action-potential firing in spiny neurons of the rat neostriatum in vivo. J Neurophysiol 79: 2358–2364, 1998. [DOI] [PubMed] [Google Scholar]
- Wilson 1983.Wilson CJ, Chang HT, Kitai ST. Disfacilitation and long-lasting inhibition of neostriatal neurons in the rat. Exp Brain Res 51: 227–235, 1983. [DOI] [PubMed] [Google Scholar]
- Yeterian 1978.Yeterian EH, Van Hoesen GW. Cortico-striate projections in the rhesus monkey: the organization of certain cortico-caudate connections. Brain Res 139: 43–63, 1978. [DOI] [PubMed] [Google Scholar]