Abstract
Primate thalamic action potential bursts associated with low-threshold spikes (LTS) occur during waking sensory and motor activity. We now test the hypothesis that different firing and LTS burst characteristics occur during quiet wakefulness (spontaneous condition) versus mental arithmetic (counting condition). This hypothesis was tested by thalamic recordings during the surgical treatment of tremor. Across all neurons and epochs, preburst interspike intervals (ISIs) were bimodal at median values, consistent with the duration of type A and type B γ-aminobutyric acid inhibitory postsynaptic potentials. Neuronal spike trains (117 neurons) were categorized by joint ISI distributions into those firing as LTS bursts (G, grouped), firing as single spikes (NG, nongrouped), or firing as single spikes with sporadic LTS bursting (I, intermediate). During the spontaneous condition (46 neurons) only I spike trains changed category. Overall, burst rates (BRs) were lower and firing rates (FRs) were higher during the counting versus the spontaneous condition. Spike trains in the G category sometimes changed to I and NG categories at the transition from the spontaneous to the counting condition, whereas those in the I category often changed to NG. Among spike trains that did not change category by condition, G spike trains had lower BRs during counting, whereas NG spike trains had higher FRs. BRs were significantly greater than zero for G and I categories during wakefulness (both conditions). The changes between the spontaneous and counting conditions are most pronounced for the I category, which may be a transitional firing pattern between the bursting (G) and relay modes of thalamic firing (NG).
INTRODUCTION
Thalamic low-threshold spike (LTS) bursts that follow an inhibitory event have long been associated with behavioral states such as slow-wave sleep (Domich et al. 1986; McCarley et al. 1983; Steriade et al. 1997a). More recent reports demonstrate that LTS bursts occur during wakefulness in humans (Jeanmonod et al. 1996; Radhakrishnan et al. 1999; Zirh et al. 1997) and in monkeys (Ramcharan et al. 2000a, 2005). In addition to the association between LTS bursting and state, there is now evidence that thalamic LTS bursting can increase from baseline during sensory stimuli or motor events (Lee et al. 2005; Martinez-Conde et al. 2002; Ramcharan et al. 1998, 2000b, 2001). In monkeys, thalamic LTS bursts occur in neurons in the lateral geniculate nucleus following microscopic eye movements (microsaccades) that occur during visual fixation (Lu et al. 1992; Martinez-Conde et al. 2002). This activity may be transmitted to V1 cortex where bursts of action potentials are likewise related to microsaccades (Martinez-Conde et al. 2000, 2002).
Microstimulation of somatic sensory thalamus with pulse trains like those of LTS bursts will evoke the sensations produced by stimuli that evoke LTS bursts (Lee et al. 2005; Lenz et al. 2004; Patel et al. 2006). Therefore LTS bursts in primates may be related to sensation and to waking behaviors, as well as to behavioral states.
The effect of cognitive tasks on spike-train characteristics of primate thalamic neurons has not previously been studied, to our knowledge. In rabbits, random increases in the level of vigilance are associated with decreased thalamic neuronal burst rates and decreased responses of thalamic neurons to peripheral stimuli (Bezdudnaya et al. 2006). We now test the hypothesis that different firing and LTS burst characteristics occur during quiet wakefulness (spontaneous condition) versus mental arithmetic (counting condition).
The results demonstrate the presence of three types of spike trains including those with frequent LTS burst firing (G grouped category) or with single spike firing (NG nongrouped category). The third spike train category (I intermediate) was characterized by bursts interspersed between single spike firing with variable interspike intervals (ISIs). The I category was more likely than the others to change category during both the spontaneous condition and the transition between the spontaneous and the counting conditions. Among neurons that did not change their category between conditions increases in FRs and decreases in BRs were ascribed to changes in the activity of NG and G neurons, respectively. The characteristic differences between neurons that do or do not change categories suggest that several discrete neuronal mechanisms contribute to the transition between the spontaneous and counting conditions.
METHODS
These studies were carried out at the Johns Hopkins Hospital (2003–2007) during the physiologic exploration of the thalamus, which preceded implantation of deep-brain stimulating (DBS) electrodes in ventral intermediate (Vim) thalamic nuclei for treatment of essential tremor (ET) (Koller et al. 2001). ET is a monosymptomatic illness characterized by a 4- to 7-Hz postural or action tremor, which is often familial and which can be ameliorated by ingestion of alcohol (Deuschl et al. 1998). In this study, exclusions included patients who had neurologic symptoms in addition to the tremor that is characteristic of ET. The exclusions included patients with both essential tremor and Parkinson's tremor or patients with the tremor characteristic of ET in addition to torticollis, or a cerebellar syndrome (Jankovic 2002). A neurologist specializing in movement disorders confirmed the diagnosis of ET and identified patients to be excluded. The protocol was reviewed and approved annually by the Institutional Review Board of the Johns Hopkins University. All patients signed an informed consent prior to participation in these studies. All methods used here have been previously described (Lee et al. 2005; Ohara et al. 2007).
Intraoperative procedures
Prior to the procedure patients had been off all tremor medications for 18 h. The thalamic exploration was performed as a stereotactic procedure using the Leksell frame. First, the frame coordinates of the anterior (AC) and posterior (PC) commissures were measured by magnetic resonance imaging or computed tomography. These coordinates were then used to estimate the nuclear locations. Physiological corroboration of nuclear location was then performed under local anesthesia (i.e., subject fully conscious) by both single-unit recording and stimulation with a microelectrode. The microelectrode was advanced through a burr hole 2.5 cm off the midline just anterior to the coronal suture. The initial trajectories were always focused on the ventral caudal (Vc) thalamic nuclei because the response of neurons in this area to somatosensory stimulation provides the most reliable landmark to guide the procedure (Garonzik et al. 2002; Lenz et al. 1995). Along these trajectories, stimulation sites with thresholds <25 μA were correlated with the presence of neurons with tactile receptive fields, which are found in Vc (Lenz et al. 1988b, 1994). Therefore stimulation confirms the location of the anterior border of Vc as determined by recordings. Surface electromyographic (EMG) signals were recorded from flexors and extensors of the wrist and elbow. The signal from a foot pedal was used to indicate the timing of sensory stimuli or verbal commands.
The counting condition involved mental arithmetic during which the patient carried out a serial subtraction task. The patient first subtracted the number 7 repeatedly from 100 (i.e., 100, 93, 86, etc.). If the patient was unable to carry out the serial subtraction task with the number 7, then a task based on 6 was attempted and so on through the series 7, 6, 4, 3, 2, and 1 until the patient's error rate was ≲10%. This is a complex cognitive task that engages attention, working memory, and calculation (Lezak 1995).
The protocol for each cell began with an epoch of the spontaneous period lasting between 60 and 180 s (average ∼80 s), followed by a counting epoch of approximately the same duration, followed by another spontaneous epoch, and so on to a maximum of eight epochs. Therefore a series of eight epochs would have a duration of about 640 s. The analysis of spontaneous periods was carried out between epochs separated by a counting epoch. Analysis of firing and bursting activity for a single cell, including stationarity, was carried out not only between these spontaneous epochs but also between spontaneous and counting epochs in a complete spike train record.
The behavioral state is well known to change thalamic bursting activity so that LTS bursting increases during drowsiness or sleep (Domich et al. 1986; Steriade et al. 1997b). Therefore we observed the subjects' behavior and measured scalp electroencephalograms or thalamic local field potentials (LFPs) or both intraoperatively during spontaneous activity and multiple cognitive tasks, in addition to the present task. Any change in these measures of state from wakefulness to drowsiness or sleep is very rare in our experience (Lenz et al. 1993, 1994; Zirh et al. 1997). This may be due to the stress of an awake operation and to regular stimulation by the anesthesiologist, the surgeon, the monitoring nurses, and the monitoring devices (blood pressure cuff and the pulse oximeter).
Study of thalamic activity
When a neuron was first isolated, spontaneous activity was recorded briefly. The activity of that neuron was then studied to identify neurons responding to light touch, tapping, or pressure to skin (cutaneous sensory neurons), as well as deep stimuli such as pressure to muscles or ligaments and passive joint movement (deep sensory neurons). These neurons were characterized by a neuronal receptive field (RF). The activity of neurons was also examined as patients carried out movements, such as making a fist, flexing or extending the wrist and elbow, and pointing. We carried out microstimulation along each trajectory.
As previously described, the anterior and inferior borders of Vc were identified by the most anterior and the most inferior deep or cutaneous neuron in the region where the majority of neurons responded to either deep or cutaneous stimuli (Lee et al. 2005; Ohara and Lenz 2003). These points and the location of the AC–PC line were used to overlay the map of the recording data with a standard, sagittal atlas map (Schaltenbrand and Bailey 1959). The nuclear location of any recorded neuron was determined from this overlay. All neurons were located in one of the following thalamic nuclei: ventral oral (Vo), ventral intermediate (Vim), ventral caudal (Vc), and lateral dorsal (LD).
Data collection and analysis
The signals recorded on a digital system (Neuroguide, Alpha-Omega, Nazareth, Israel) during the procedure included: the foot pedal indicating events during the examination, the microelectrode signal, electromyograms (EMGs), and the audio channel describing instructions to the patient, application of stimuli, and so forth. Digitally recorded microelectrode signals were analyzed using Spike2 software (CED, Cambridge, UK) by template matching of action potential waveforms. Subsequent analyses of spike characteristics such as burst detection, firing rates, and duration of identified ISIs were carried out using MATLAB (The MathWorks, Natick, MA). Analysis of bursting was carried out on spontaneous activity with eyes open and with the arm at rest. The absence of tremor was confirmed by visual inspection of both the limbs and the EMG signals.
Analysis of burst activity
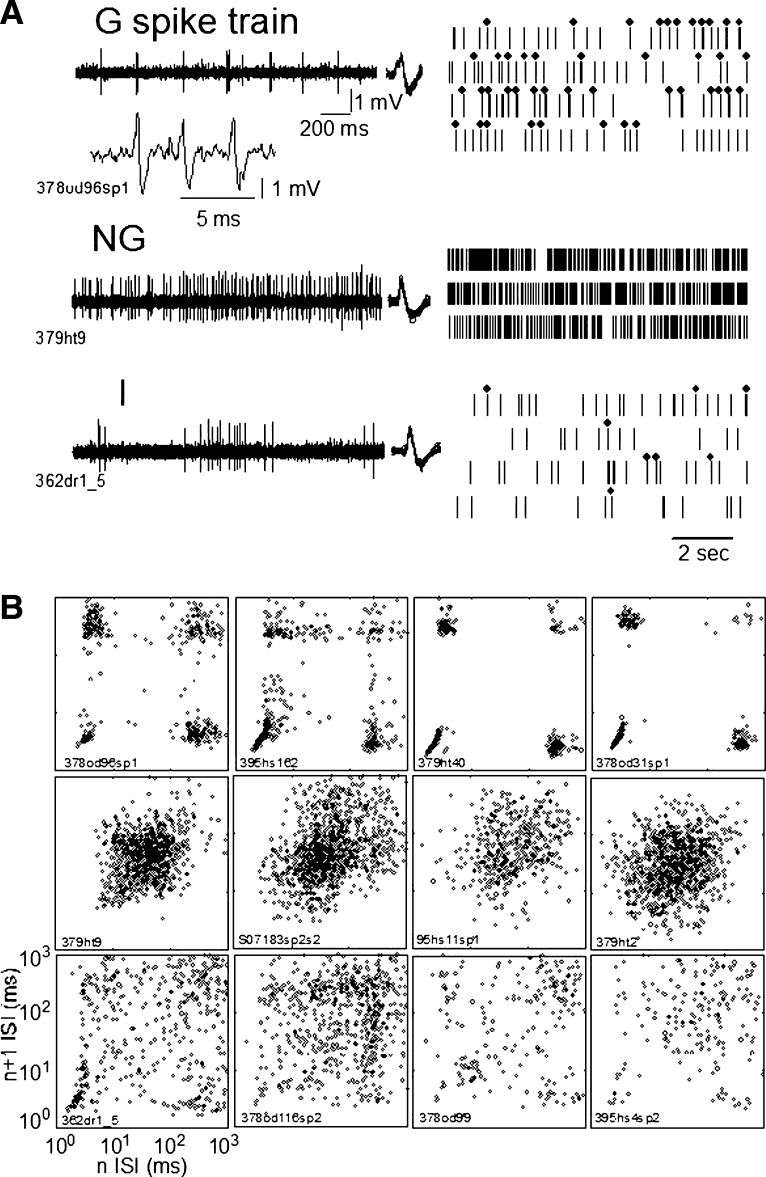
As a bias-free measure of neuronal bursting activity, we constructed plots of the ISI before an action potential in a spike train (n) versus the ISI after (n + 1), designated as an n versus n + 1 plot. This plot provides a graphical display of the characteristics of the spike train overall as reflected by clusters of points in the plot. In the n versus n + 1 plot the cluster at the bottom right indicates a long ISI (∼300 to 400 ms; see Fig. 1 B, top) followed by a short ISI (≲5 ms), the defining characteristic of the first spike in an LTS burst. The cluster at the top left indicates a short ISI (≲16 ms) followed by a long ISI (∼100 ms), so that the short ISI is the final ISI in an LTS burst. The bottom left and top right clusters indicate the ISIs within and between bursts, respectively. This plot indicates spike-train characteristics overall; it does not allow the identification of individual LTS bursts, an example of which is shown in the inset of Fig. 1A (G spike train).
FIG. 1.
Examples of spike trains recorded during the spontaneous condition. A: spike trains of spontaneous activity are shown as the raw microelectrode signal on the left and digitized spike trains on the right. In A, row G the inset is a raw signal showing an LTS-related burst of action potentials. Dots above the digitized spike train indicate the occurrence of LTS bursts as identified by the 50–6–15 criteria (see methods, Analysis of burst activity). These data are shown in A for one each of Grouped (G), Nongrouped (NG), and Intermediate (I) categories as labeled from the top down. B: the n vs. n + 1 plot for the G spike train in A (top row) is the left plot the top row in B; similarly the n vs. n + 1 plot for NG spike train in A is shown at the left end of the middle row of B, and so on for I. From the top down these rows show 4 examples each of the G, NG, and I spike trains in the same order as in A. An alphanumeric identifier is shown with the results from each neuron.
Individual LTS bursts in the spike train were identified by criteria used in studies of awake cynomologous monkeys that have been confirmed by intracellular studies in thalamic slices from the same species (Ramcharan et al. 2000a,b). The criteria (50–6–16) to select these bursts from the spike train were as follows: the ISI preceding the first action potential in the burst had a duration >50 ms, the ISI following the first action potential in the burst had a duration of <6 ms, and all following action potentials are considered as part of the burst if their ISI increased by no more than 2 ms for each succeeding action potential, up to a maximum ISI of 16 ms. All bursts meeting these criteria, including bursts composed of two action potentials, were included in the burst rate. Although the n versus n + 1 plot is a bias-free way to assess a long spike train, it is less accurate than the template method for identification of individual bursts. Therefore the template method is used to determine burst parameters, but not to classify neuronal spike trains. We have repeatedly validated, illustrated, and published these techniques and these burst criteria (Lee et al. 2005; Lenz et al. 1994, 1998).
We also measured the primary event rate (PR), which includes all spikes occurring outside bursts plus the first spike in each burst defined by the above-cited criteria. Therefore the PR is a measure of the rate of spikes occurring between bursts (Cox and Lewis 1966; McCarley et al. 1983). We calculated burst rate/primary event rate (BR/PR) as an indicator of the relationship between burst firing and ongoing neuronal firing, as reflected by the PR. If the difference in burst rates between two neuron types is lost when the ratio of BR/PR is calculated, then the difference in bursting is dependent on the PR and, perhaps, on the degree of membrane depolarization. A measure of neuronal firing that is related to the BR/PR is the percentage of action potentials occurring in bursts (BP). The number of action potentials occurring in bursts included bursts of two action potentials and all action potentials in a burst, including the first and last action potentials.
As indicators of the preburst inhibition, we measured the preburst interspike interval (PBISI) and the ratio of the PBISI/PRISI (inverse of the primary event rate). The PBISI is an indicator of the duration of the inhibitory event preceding a burst, whereas the ratio is an indicator of the strength of the preburst inhibition. All firing, burst, and inhibitory indexes were compared by locations in the thalamus and between spontaneous and counting conditions.
Statistical analysis
Parametric measures of the spike train were compared between different groups and categories by an ANOVA, with post hoc testing including a correction for multiple comparisons (Tukey's honestly significant difference [HSD]). Parametric data that were not normally distributed were tested by Mann–Whitney or Kruskal–Wallis and descriptive statistics were expressed as the median and 5th to 95th percentiles of the distribution (confidence interval of the median [CI]). Paired analysis was carried out using the nonparametric Wilcoxon matched-pair test (Snedecor and Cochran 1967). Differences between proportions were tested with a chi-square or Fisher test, as appropriate. The null hypothesis was rejected at P < 0.05 in all statistical tests.
As a test of the reliability of visual classification of neuronal spike train categories, the Caliński statistic was used to estimate the number of clusters (k) for a given spike train (Weingessel et al. 1999). For any number of clusters (k) large values of the Caliński statistic are evidence for that number of clusters, where this statistic is similar to an F statistic in an ANOVA. The value of the k statistic is measured for a number of values of k and the largest k value indicates the number of clusters.
RESULTS
Thalamic neuronal firing patterns
These results were obtained in 28 patients (12 women and 16 men, between 41 and 79 yr old). Surgery was carried out on the left thalamus in 18 patients; the right, in 10. For each patient, between one and six trajectories were explored (mean 3.3). A total of 117 neurons (LD 13, Vo 36, Vim 42, Vc 26) were studied during periods of ≤749 s.
Each row in Fig. 1A shows examples of the firing of one neuron in each category, as labeled. Each row in Fig. 1B shows four examples of n versus n + 1 plots for each of the three categories, with rows corresponding to categories as labeled in Fig. 1A. The left n versus n + 1 plot in each row of Fig. 1B is derived from the spike train shown in the corresponding row of Fig. 1A. Neuronal spike trains were classified by the category of the first spontaneous epoch.
In Fig. 1A, note that the dots above the digitized G and I category spike trains indicate the occurrence of an LTS burst as identified by the 50–6–16 criteria (see methods). In Fig. 1B (top row), the cluster of dots in the G category at the bottom right of the n versus n + 1 plot indicate a long interval (>100 ms) followed by a short interval (<6 ms), the defining characteristic of the first spike in an LTS burst. The cluster at the top left of the n versus n + 1 plot (Fig. 1B, top row) indicates a short ISI (<16 ms) followed by a long ISI (>100 ms), characteristic of the final ISI in an LTS burst. The bottom left and top right clusters of the n versus n + 1 plot (Fig. 1B, top row) indicate the ISIs within and between bursts, respectively. Therefore spike trains in the G category (Fig. 1, A and B, top row) were identified from n versus n + 1 plots by the presence of well-defined clusters at the four corners of the n versus n + 1 plot.
Spike trains in the NG category showed high-frequency spontaneous firing during the spike train (Fig. 1A, middle row), resulting in n versus n + 1 plots characterized by a central cluster (Fig. 1B, middle row). This distribution is consistent with the lack of LTS bursts, as indicated by the small number of data points at the four corners of the n versus n + 1 plot, particularly at the bottom right (Fig. 1A, right middle row). Nevertheless, some of these plots do show data points at the bottom right consistent with doublet LTS bursts (e.g., Fig. 1B, row 2, third and fourth plots from the left).
Spike trains in the I category had irregular spike firing and common LTS bursts (Fig. 1A, bottom row). Spike trains in the I category were identified by two or more clusters (Fig. 1B, bottom row) that were less well defined than, but consistent with, those found in the G category (Fig. 1B, top row). These clusters in the I category (Fig. 1B, bottom row) were often in the bottom right, or the bottom left, or both, approximately consistent with the clusters of the G category. The I category spike trains (Fig. 1B, bottom row) often had a central cluster, consistent with that found for the NG category (Fig. 1B, middle row). Neuronal spike trains in the G and NG categories always maintained their category across all epochs of spontaneous activity (see results, Spike train properties during the spontaneous condition), whereas I category spike trains often changed category between spontaneous epochs (Fig. 4).
In categorizing these results we relied on visual assessment of the n versus n + 1 plots. This assessment was checked against the results of cluster analysis by using the Caliński statistic (see methods, Statistical analysis). Six representative neuronal spike trains, identified visually as being in the G category, were studied by cluster analysis. Of these, five were found to have four clusters. One of these neuronal spike trains had approximately equal likelihood of four or five clusters (spike train: 490hl65newsp2), with the possible fifth cluster in the center of the four clusters characteristic of the G category. The BP for this cell was 39%, which is greater than the BP for 95% of I cells but is at the median value for G cells, confirming visual identification of this spike train in the G category.
Four I spike trains were studied by cluster analysis and were found to have three or four clusters, including a central cluster with two or three peripheral clusters. These were usually located at the bottom right and left of the n versus n + 1 plot.
The Caliński statistic was calculated for four distributions in which there were possible second and third clusters just peripheral to a central cluster. The central cluster was in a location similar to the G category (spike train: XS148013). In none of these cases was the visual classification changed by cluster analysis. Overall, cluster analysis of a sample of spike trains in this data set confirmed the results of visual classification.
Distributions of burst parameters
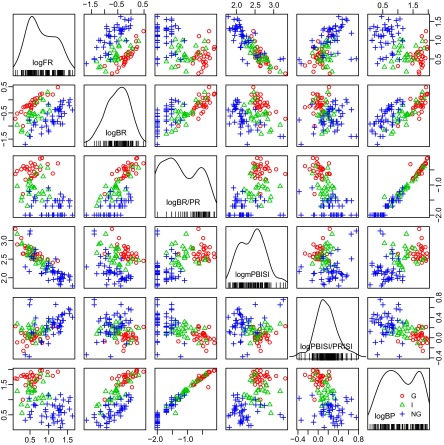
To study the properties of individual bursts we identified the LTS bursts by the 50-6-15 criteria during the spontaneous condition (117 neurons) (methods, Analysis of burst activity). Along the diagonal in Fig. 2 are histograms of burst parameters expressed as logarithms to the base e (natural logarithms, log). These histograms show the histogram of log values for the measures of firing (FR, BP), the bursting (BR, BR/PR), and inhibitory events (PBISI, PBISI/PRISI). Note that the distributions seem to be bimodal for the log FR, log BR/PR, log PBISI, and log BP, whereas the distributions seem to be unimodal for log BR and log PBISI/PRISI.
FIG. 2.
Logarithms (log, base e) of different variables of bursts identified by 50–6–15 criteria for spike trains of the G, NG, and I categories. These variables are described in detail in the methods (Analysis of burst activity) and include variables describing firing (firing rate [FR]), bursting (burst rate [BR], burst rate/primary event rate [BR/PR], burst percentage [BP]), and inhibitory events (including preburst interspike interval [PBISI] and PBISI/primary event interspike interval [PBISI/PRISI]). Histograms for these variables are given along the diagonal, as labeled. The vertical and horizontal axes of each graph are labeled along the corresponding axis of the whole figure. For example, the 4th graph from the left (top row) plots log FR on the vertical axis vs. log PBISI along the horizontal axis. Each red ○ indicates the variable for a G spike train, whereas blue + and green ▵ indicate NG and I spike trains, respectively (inset right bottom graph).
To test the possibility that these distributions were best described by more than one peak, we used the technique of the Bayesian information criterion (BIC) (Schwarz 1978). BIC analysis was used to identify whether these distributions are actually a mixture of two different components. We considered the possibility that these variables were described by a single distribution (one-component model), a mixture of two distributions (two-component model), a mixture of three distributions, and so on. For any two estimated models, the one with the highest value of the BIC is identified as the optimal number of components contributing to the histogram. The analysis provides the estimated mean (and SDs) for each identified component. For distributions with two components these measures indicate the probability that the two components are separate and that a single observation belongs to the first group or the second group.
This analysis was carried out on all epochs from all neurons and demonstrated that the distribution of log (mPBISI) had two peaks (abbreviations defined in methods, Analysis of burst activity). The two peak values of the PBISIs were 97 and 403 ms. These are approximately consistent with the duration of type A and type B γ-aminobutyric acid (GABAA and GABAB) inhibitory postsynaptic potentials (IPSPs) for thalamic relay neurons (Andersen and Sears 1964; Roy et al. 1984; Sherman and Guillery 2001; Soltesz et al. 1989).
Graphs of each variable against the others are shown for individual neurons in the G category (red circles), NG category (blue crosses), and I category (green triangles) along the diagonal in Fig. 2. The G and I categories had long log PBISIs that were associated with the high BRs (Fig. 2, second row from the top, fourth graph from the left). The long log PBISIs were also associated with high percentages of ISIs in bursts (BP), which is characteristic of the G and I categories (Fig. 2, fourth row from the top, right plot).
Two peaks were identified for the log FR with mean (SD) frequencies of the two peaks being at 1.29 and 2.73/s (Fig. 2, left top histogram, Table 1). For the PR two peaks were apparent in the BIC analysis at log PR at 1.00 and 2.71/s. The lower FRs are associated with longer log PBISIs (Fig. 2, top row, fourth graph from the left) and are consistent with the properties of G and I spike trains (Table 1).
TABLE 1.
Optimal number of peaks, as characterized by the mean value (SD), that describe the underlying distribution for each of the variables expressed as the base of the natural logarithms
| Variable | Optimal Number of Normal Distributions | Mean (SD) |
||
|---|---|---|---|---|
| Distribution 1 | Distribution 2 | Distribution 3 | ||
| ln (mPBISI) | 2 | 4.57 (0.29) | 6.00 (0.58) | |
| 96.5 ms | 403 ms | |||
| ln (FR) | 2 | 1.29 (0.51) | 2.73 (0.518) | |
| 3.63/s | 1.53/s | |||
| ln (PR) | 2 | 1.00 (0.52) | 2.71 (0.52) | |
| 2.72/s | 15.0/s | |||
| BP | 3 | 1.48% (1.32) | 8.55% (4.77) | 47.05% (21.64) |
The antiln value of the mean is below the mean (units as indicated). We used a model selection criterion known as the Bayesian Information Coefficient (BIC).
The log BR was unimodal and had substantial overlap of the distributions for the different categories (Fig. 2). A unimodal distribution with overlap of categories was also observed for the ratio of log PBISI/PRISI. These results demonstrate that the rate of inhibitory events (LTS burst rate) and the intensity of preburst inhibition (ratio PBISI/PRISI) consist of a single distribution for the different categories of spike trains.
LTS bursting by 50–6–15 criteria in different categories
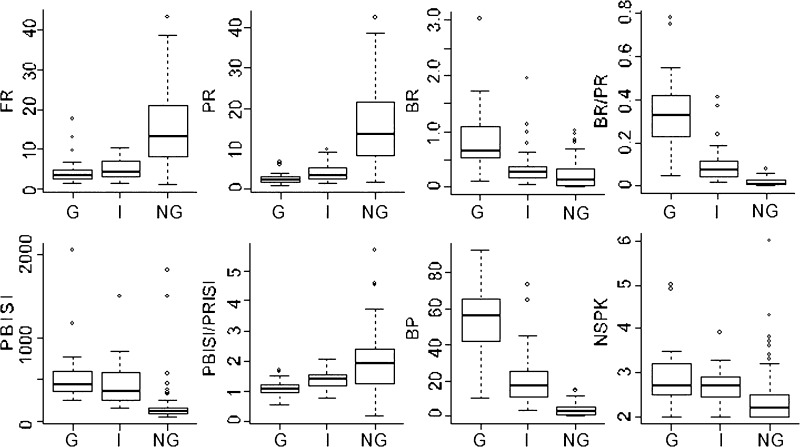
In this analysis, bursts identified by the 50–6–15 criteria were compared statistically between the three categories in the same set of neurons (n = 117) as described earlier. This analysis confirms the presence of high burst rates in the G and I categories. In addition, some spike trains in the NG category have bursts of two action potentials, which is reflected in this analysis. As shown in Fig. 3, FRs were significantly higher in the NG category than those in either the G or the I category (both P < 0.00001, Kruskal–Wallis corrected for multiple comparisons) and the PR, which was also higher for the NG category than that for the G and I categories (both P < 0.00001).
FIG. 3.
Box and whisker plots for rates, bursting, and inhibitory measures of spike trains. The heavy horizontal line is the median value, whereas the bottom and top of the box indicate the 25th and 75th percentiles and the whiskers indicate the 5th and 95th percentiles. Dots indicate values outside the 95th percentile.
BRs were significantly higher in G than those in NG (P < 0.0000001) or I categories (P < 0.04). Analysis of the BR/PR did not lead to any change in the rank in categories with respect to BRs or the statistical significance for G versus NG or I categories. Therefore the BRs were not dependent on a high FR, which could lead to identification of bursts resulting from random clustering of short ISIs. The 5th percentile of the CI of the median of the BR was greater than zero for the G and I categories, but not for the NG category (Fig. 3), which indicates that BRs were significantly greater than zero for G and I categories but not for the NG category.
PBISIs were significantly longer in G and I categories than in the NG category (both P < 0.0000001), although bursts did not commonly occur in the latter category (see results, Thalamic neuronal firing patterns). PBISIs were not significantly different between the G and I categories (P > 0.05). The duration of PBISIs was >100 ms for all categories: G (362–500 ms, 5-95% CI of the median), NG (267-519), and I (99-136), consistent with latencies required to deinactivate the LTS (Sherman and Guillery 2001; Steriade et al. 1990).
The longer PBISIs might reflect slower firing rates between bursts rather than preburst inhibition. This possibility was assessed by examining the ratio of the PBISI/PRISI, which will be >1 if there is preburst inhibition. The CI of the ratio was >1 for I and NG neurons, suggesting that the LTS bursts are the result of preburst inhibitory events. The ratio was significantly greater in the NG than that in the G category (P < 0.0000001) or the I category (P < 0.03), which indicates that the occasional bursts during NG spike trains clearly follow an inhibitory event. The median of the CI of this ratio was >1 for G spike trains, which have a very low FR. In this situation, bursting may result from tonic inhibitory drive or inhibitory events occurring in the presence of a relatively hyperpolarized resting membrane potential (Fig. 3).
LTS properties of neurons located in different thalamic nuclei
The properties of spike trains in different nuclei were studied during the spontaneous condition for 117 neurons. The 5% CI of the median demonstrated that neurons in all nuclei had burst rates significantly greater than zero, i.e., all 5th percentile CIs >0 (Table 2). The number of spike trains in the G category was significantly higher in the case of neurons in Vc (13/26) than in Vim (5/42, P < 0.002, Fisher) or Vo (7/36, P < 0.001), but not LD (4/13, P = 0.31).
TABLE 2.
Firing, burst, and preburst parameters for neurons in four different thalamic nuclei
| Nucleus | Firing Rate, s−1 | Primary Rate, s−1 | Burst Rate, s−1 | Burst Rate/Primary Rate | Preburst ISI, ms | Ratio of PBISI/Inverse PR |
|---|---|---|---|---|---|---|
| LD (n = 13) | 4.2, 3.2–8.0 | 3.5, 2.6–10.2 | 0.34, 0.15–0.46 | 0.085, 0.03–0.21 | 435, 192–586 | 1.53, 1.23–1.82 |
| Vo (n = 36) | 4.4, 3.0–9.4 | 3.2, 2.5–8.9 | 0.12, 0.05–0.20 | 0.035, 0.01–0.09 | 323, 131–433 | 1.19, 1.12–1.57 |
| Vim (n = 42) | 12.3, 8.7–18.7 | 12.3, 7.1–8.4 | 0.21, 0.14–0.34 | 0.020, 0.01–0.03 | 148, 119–219 | 1.99, 1.53–2.28 |
| Vc (n = 26) | 5.2, 3.7–9.5 | 2.9, 2.2–5.7 | 0.67, 0.47–0.86 | 0.260, 0.08–0.41 | 327, 222–422 | 0.96, 0.84–1.35 |
Each cube includes the median and 5th to 95th percentile confidence interval (CI) of the median. Units of each parameter are given in the table headings. Bold and underlined variables for nuclei with median and CI are outliers that are significantly different from nuclei with median and CI shown in bold; e.g., burst rates in Vc are higher than those in Vo and Vim.
The number of spike trains in the NG category was significantly lower in the case of neurons in Vc (7/26) than in Vim (32/42, P < 0.002, Fisher) or Vo (20/36, P = 0.033), but not LD (5/13, P = 0.49, Fisher). The number of spike trains in the I category was not significantly different (P > 0.31, Fisher or chi-square) in the case of neurons in Vc (6/26) than in Vim (5/42) or Vo (9/36), and LD (3/13). Therefore the majority of neuronal spike trains in Vc were category G, whereas the majority in the Vim and Vo were category NG and spike trains in category I were equally as common in Vc, Vim, and Vo.
Neurons in the G category had lower FRs and PRs than those in the NG or I category. Therefore it is not surprising that FRs were significantly higher in Vim than those in LD (P < 0.04, Fisher), Vo (P < 0.001), and Vc (P < 0.005), with more spike trains in the G category. PRs were significantly higher in Vim than those in Vo (P < 0.005) and Vc (P < 0.0001). The FRs in Vo were uniformly <50/s (cf. Smith and Sherman 2002).
Neurons in Vc had higher burst rates that those in Vo (P < 0.0001, Kruskal–Wallis) and Vim (P = 0.044) but not LD (P = 0.54). Neurons in Vc had burst rate/primary event rate ratios that were significantly higher than those in Vim and Vo (P < 0.005 in both cases). The PBISIs were significantly (P < 0.05) shorter in Vim than those in Vc, perhaps consistent with the higher firing rates in Vim. The PBISI was short enough to be in the GABAA range for Vim (148 ms), whereas the PBISI was in the GABAB range for the rest of the nuclei (range 323–435 ms) (Andersen and Sears 1964; Roy et al. 1984; Sherman and Guillery 2001; Soltesz et al. 1989).
The PBISI/PRISI was smaller in Vim than that in Vc (P < 0.05). The CI of the median demonstrated that the PBISI was significantly >100 ms for neurons in all nuclei, which is consistent with the length of the inhibition required to deinactivate the LTS (Jahnsen and Llinás 1984). The ratio of PBISI/PRISI was significantly higher in Vim than that in Vc. The median of the CI was >1 for the majority of neuronal trains in all nuclei, which indicates the presence of a significant preburst inhibition.
Differences in the number of ISIs within a burst (NSPK) were not significant between nuclei (P = 1 for all comparisons between nuclei). The greatest number of ISIs per burst was found in Vc (2.6, 2.3–2.9: median: 5th to 95th percentile) and the least in Vim (2.5, 2.2–2.7). The number of action potentials in an LTS burst is an indicator of the size of the LTS and the magnitude of the preburst inhibition (Steriade et al. 1990).
The number of neurons in the G category among nuclei in the lateral group containing a majority of cells with receptive fields (Vc) is uniformly greater than that in the nuclei in which the majority of cells do not have a receptive field (Vim and Vo) (Lenz et al. 1988a). Among spike trains of all neurons with receptive fields the proportion of spike trains in the NG category (14/32 Fisher) was not significantly different from the proportion in the I category (2/7, P = 0.44, Fisher) and G category (2/14, P = 0.092, Fisher) (see Neurons that did not change categories between conditions). Therefore among cells with RFs 14 were in the NG category and 2 were in each of the G and I categories. Since these results are obtained during spontaneous activity, these results suggest that the G pattern predominates in sensory nuclei and neurons in the absence of stimulation (Lee et al. 2005; Ramcharan et al. 2000b). Furthermore, prior studies demonstrate that somatic simulation uniformly results in increased firing and bursting rates (Lee et al. 2005).
Stationary behavior of spike train during the spontaneous condition
In all, 46 neurons in 12 subjects were studied during multiple epochs of the spontaneous and counting task, so that each epoch had a duration of >80 s. Analysis of stationarity of LTS burst rates by median runs tests was carried out on a subset of 28 G and I spike trains during spontaneous and counting tasks (Snedecor and Cochran 1967). NG spike trains were not included in analysis of stationarity of the burst rate because they rarely fire in LTS bursts. The spike trains during the spontaneous condition were nonstationary for burst rates over 10-s intervals in a distinct minority of spike trains (5/28, P = 0.0005, b3inomial). The number of nonstationary burst rates within spike trains was significantly less during epochs composed of spontaneous tasks exclusively (5/28, P < 0.05, Fischer) than in those composed of both the spontaneous and counting tasks (13/28).
The stationarity of FRs over 5-s intervals was tested among all three categories. This analysis demonstrated that spike trains were commonly stationary (24/38) across spontaneous epochs. All nonstationary epochs in both FR and BR variables were characterized by fewer transitions from below to above the median, or vice versa, than expected at random. We next examined changes in category during the spontaneous condition among the spike trains with the greatest duration.
Changes in category during the spontaneous condition
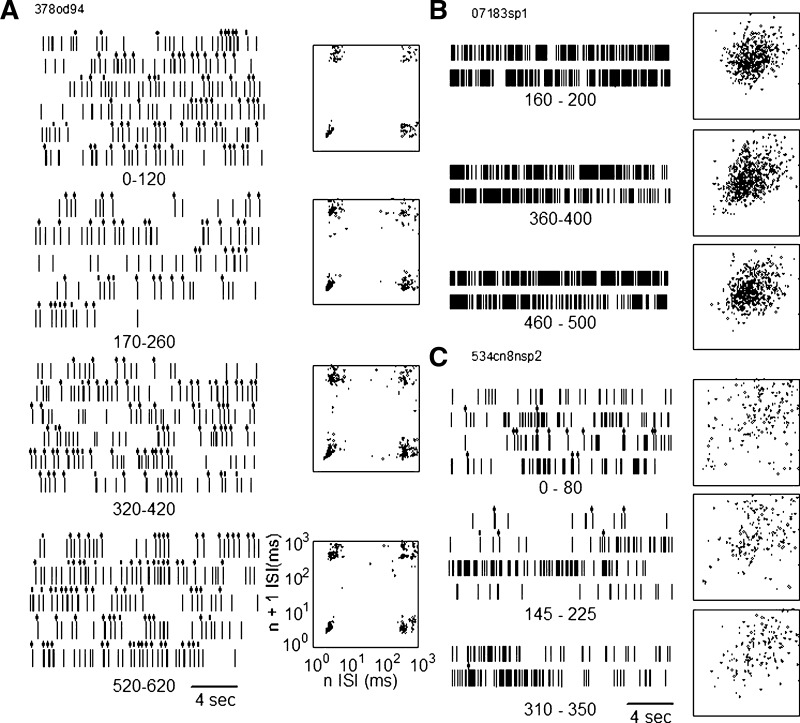
The n versus n + 1 plots of spontaneous neuronal activity were studied over multiple epochs of ≥40 s each, extracted from 21 spike trains recorded over periods of 6 to 12 min (369 to 749 s), which contained both spontaneous and counting conditions. This is a subset of the 46 neuronal spike trains (12 patients) that were studied during the spontaneous and counting conditions over intervals of >80 s combined. In the G category, none of the eight spike trains changed its category from that in the first spontaneous epoch. Five of these eight neurons showed constant n versus n + 1 plots across the sampling period for spontaneous activity as in Fig. 4, left. Three neurons showed variability in the dispersion of the top right cluster, corresponding to variability within intraburst ISIs (Fig. 4A, second and third from the top). The four clusters were seen clearly in all samples for each neuron. Therefore the spike trains in the G category had the same category during quiet wakefulness over intervals, spanning both conditions, of ≤12 min.
FIG. 4.
Spike trains and n vs. n + 1 distributions measured from spike trains over long samples of spontaneous activity. The spike train in the G category is shown in the left column, the NG in the top right column, and I in the bottom right column. Intervals below each raster indicate the duration of the epoch of the spontaneous epoch from which the raster was taken.
In the NG category, nine neuronal spike trains were studied, all of which were classified in the NG category over all epochs of the spontaneous task across the complete spike train. For five of these eight neuronal spike trains the n versus n + 1 plot remained constant. In three, the large central cluster migrated toward the origin (Fig. 4B, middle and bottom) and then away from the origin of the plot. These changes were consistent (Fig. 4C, top) with changes in the mean firing rate (neurons 7929.2, 7929.1, 6734).
Four neurons were classified in the I category, based on the first epoch of the spontaneous condition of clusters in bottom left and right corners of the n versus n + 1 (Fig. 4C, top and middle) (see Thalamic neuronal firing patterns). This pattern was found in all spontaneous across the complete spike train for two neurons (378od11b.2, 540sz11.1). For the other two spike trains in the I category, the bottom left and right clusters (Fig. 4, top and bottom) disappeared so that the distribution assumed the appearance of the NG category (neuron 534cn8.2; see Fig. 4C, bottom, and 540sz11.2) (Lee et al. 2007). These changes were correlated with the numbers of bursts in the spike trains, which decreased in Fig. 4B from the top panel to the middle and the bottom panels. Therefore two of the I neuronal spike trains maintained their category and had a constant FR and BR, whereas two others changed their category.
The incidence of changes in spike train category was significantly higher for the I category (2/4) versus the G and NG categories combined (0/17, P = 0.0286, Fisher). Therefore the I category had greater variability of the firing activity than did NG or G categories, as measured by median runs tests on FR and BR, and by changes in category between spontaneous categories in the complete spike train.
Changes in firing and burst rates at the transition between spontaneous and counting conditions overall
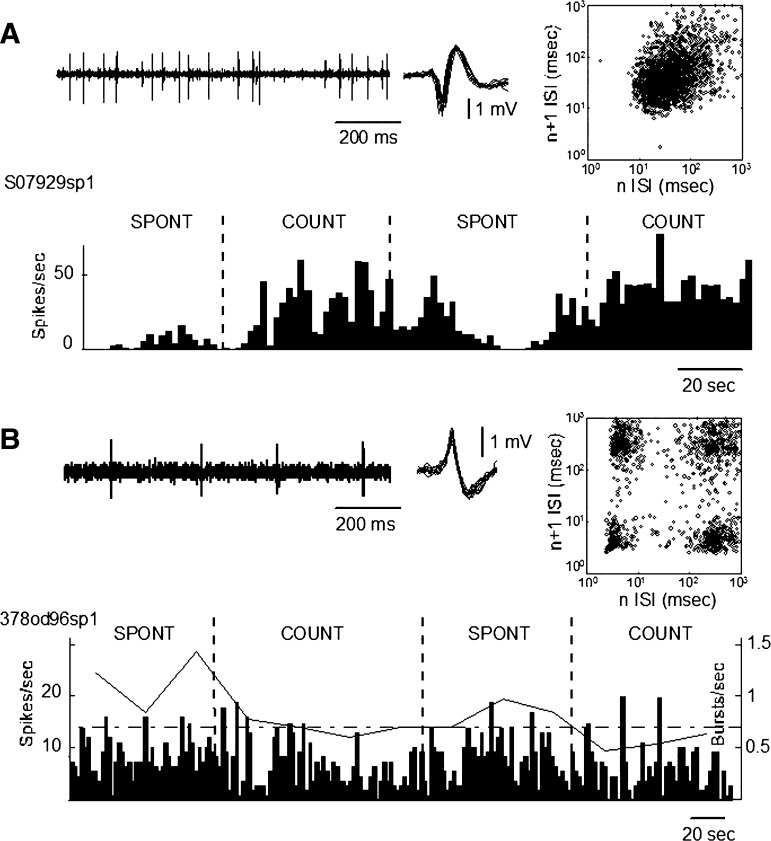
Spontaneous condition versus counting condition was studied in the same 46 neuronal spike trains as described earlier. Figure 5 shows examples of spike trains recorded over epochs including both spontaneous and counting periods. The neuronal spike train shown in the top panel was categorized as NG based on a single central cluster on the n versus n + 1 plot. The bottom panel in Fig. 5 shows an example of a neuron categorized G based on the presence of the four clusters, one at each corner of the plot (Fig. 1). After epochs had been classified into categories based on n versus n + 1 plots, the burst parameters were calculated for bursts as identified by the 50–6–16 template. Note that the burst rate of the G spike-train category decreased during counting and the firing rate of neurons in the NG category increased during counting. We first examined these differences in LTS burst indexes among neurons overall.
FIG. 5.
Spike trains during epochs containing both spontaneous and counting conditions, as labeled. A, top: the spike train of NG neuron (left) as the raw spike train (inset, shape of the discriminated action potential), and the n vs. n + 1 plot (right). The bottom panel shows the histogram of the neuronal firing rate. B: the results for a spike train in the G category. Conventions are as in A except that the burst rate is plotted above the firing rate histogram.
Differences between LTS burst indexes were tested by a nonparametric analysis between spontaneous and counting conditions (Wilcoxon matched-pair test) (Snedecor and Cochran 1967). The results demonstrate that the burst rate and percentage of ISIs occurring in bursts (or BR/PR) were less during the spontaneous conditions by approximately half (P < 0.004, Table 3). The increase in firing rate of approximately one quarter during the counting condition showed a trend toward significance (P = 0.09) overall. There were no significant changes in indices of inhibitory LTS-associated events (PBISI and PBISI/PRISI). We next examined changes in the spikes train within neurons that changed their spike train category with the condition.
TABLE 3.
Thalamic spike train characteristics during spontaneous and counting conditions
| Parameter | Spontaneous Task | Count Task | Probability |
|---|---|---|---|
| Firing rate, s−1 | 4.24 (3.37–5.31) | 5.33 (3.36–6.33) | 0.090 |
| Primary-event rate, 1/s | 2.96 (2.55–3.67) | 3.23 (2.59–4.96) | 0.671 |
| Burst rate, s−1 | 0.23 (0.16–0.35) | 0.13 (0.08–0.20) | 0.004 |
| Burst rate/primary-event rate | 0.12 (0.05–0.19) | 0.06 (0.03–0.10) | 0.004 |
| PBISI, ms | 372.10 (318.1–450.8) | 338.90 (269.0–433.5) | 0.886 |
| PBISI/inverse of primary-event rate | 1.19 (1.12–1.28) | 1.16 (1.04–1.29) | 0.975 |
| Burst percentage, % | 13.70 (6.35–27.35) | 6.70 (2.47–18.41) | 0.011 |
The units of the indices are shown in the first column. Each cube gives the median and the 5th to 95th percentile confidence interval of the median. The units of each row are given in the first column.
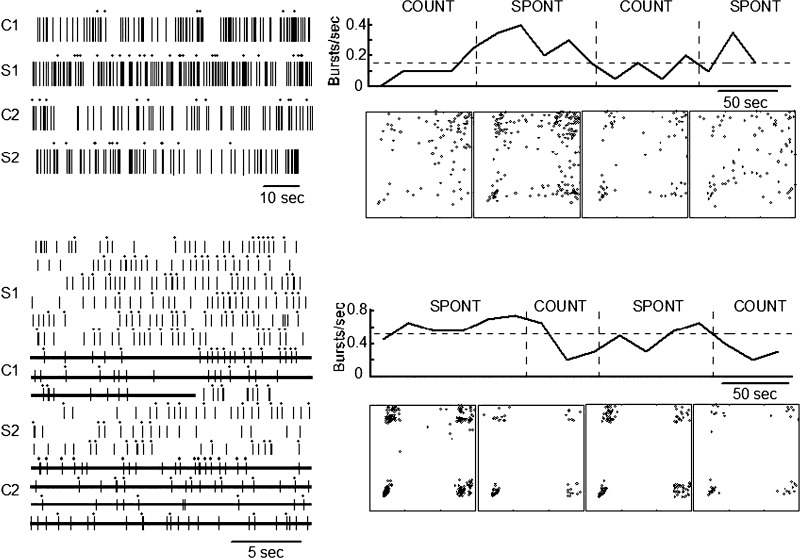
Spike trains that changed categories at the transition between conditions
The top panel of Fig. 6 shows an example of a neuronal spike train categorized as I during the first counting epoch, based on poorly defined clusters including an absence of a bottom right cluster on the n versus n + 1 plot. This spike train changed to the G category for the first spontaneous period and the subsequent counting task period. The evidence of this change is based on the presence of well-defined clusters at the four corners of the n versus n + 1 plot and, particularly, on the emergence of a bottom right cluster (see Thalamic neuronal firing patterns). In the second epoch of the spontaneous condition the peripheral clusters were less defined and a poorly defined central cluster emerged, leading to classification of this epoch in the I category.
FIG. 6.
Spike-train epochs initially in the I (top) and G (bottom) categories across epochs including the spontaneous and counting conditions as labeled. Dots above the spike train indicate spikes forming a burst (left). Burst rates are shown in the graph above the corresponding n vs. n + 1 plots. In the bottom panel lines through the raster indicate the epochs of the counting condition.
The numbers of neuronal spike trains changing their category by the condition were significantly lower among neuronal spike trains categorized during the spontaneous condition as NG (0/18) than in those categorized as G (6/22, P = 0.0243, Fisher) and I (4/6, P < 0.002). Differences were not significant between spike trains categorized as G and those categorized as I (P = 0.147, Fisher).
Among neuronal spike trains initially categorized as G (n = 22) during the spontaneous condition, two changed to the NG category and four changed to the I category at the transition to the counting condition. Some of the neuronal spike trains categorized as I during the spontaneous condition changed to the NG category (4/6). In contrast, none of the spike trains in the NG category changed its category (0/18). Therefore all changes in the category of spike-train activity between the two conditions were in the direction of categories with lower BRs and higher FRs (e.g., G changes to NG or I). Assuming that either rate during the counting condition will be greater than that during the spontaneous condition with a P = 0.5, the odds that the FR will always go up and the BR will always go down for any change in category P = 0.25. Therefore uniformity of these changes across neurons in these rates is much greater than expected at random (10/10, P = 0.000001, binomial).
The neuronal spike trains that changed category between conditions tended to be different between nuclei. Neuronal spike trains of any category that changed category at the transition from the spontaneous condition to the counting condition tended to be less common in Vo (3/30, Fisher) than in other nuclei including: Vc (2/4, P = 0.093), Vim (3/7, P = 0.068), but not LD (2/5, P = 0.14). Similarly, there was no difference among neuronal spike trains that changed their category from I category or from G category at the transition to the counting task. We next focused on the remaining spike trains that did not change their category with condition.
Neurons that did not change categories between conditions
Figure 5 and the bottom panel in Fig. 6 show examples of neuronal spike trains that did not change category by condition. Among the 22 such spike trains categorized as NG there were significant increases in FR (P < 0.001, Wilcoxon matched-pair test) and PR from spontaneous to counting task periods (Table 4, P = 0.003). Burst rates were lower for the counting condition overall (P = 0.004, Table 3). Among the G category that did not change at the transition between conditions there was a strong trend toward decreased BRs during the counting task versus the spontaneous task (P = 0.054, Table 4). None of the other firing, burst, or inhibitory indexes was different between spontaneous and counting conditions for neurons in the G and NG categories. Only two spike trains in the I category did not change their category by condition, so that spike-train measures could not be studied in this category. Sensory properties of neurons were not related to spike-train categories during spontaneous condition, or counting condition, or at the transition between spontaneous and counting.
TABLE 4.
Firing and burst indices during spontaneous and counting conditions for G and NG cells that did not change their spike train category between tasks
| G Spontaneous | G Counting | NG Spontaneous | NG Counting | |
|---|---|---|---|---|
| FR | 3.30 (2.6–4.3) | 3.00 (2.2–3.9) | 9.10 (6.5–13)** | 15.00 (10–18) |
| PR | 2.20 (1.8–2.8) | 2.10 (1.6–2.8) | 10.00 (6.5–16)** | 14.00 (9.2–17) |
| BR | 0.61 (0.45–0.85)* | 0.44 (0.36–0.62) | 0.01 (0.00–0.04) | 0.04 (0.02–0.08) |
| BR/PR | 0.33 (0.22–0.36) | 0.26 (0.17–0.31) | 0.01 (0.00–0.011) | 0.01 (0.00–0.01) |
| mPBISI | 461.00 (422–637) | 469.00 (380–600) | 97.00 (80–125) | 100.00 (85–120) |
| PBISI/PRISI | 1.10 (1.0–1.2) | 1.10 (1.0–1.2) | 1.30 (0.97–1.8) | 1.50 (0.89–2.0) |
| BP | 49.00 (37–61) | 48.00 (48–55) | 0.30 (0.0–1.3) | 0.80 (0.40–1.40) |
Each result is given as median (5th to 95th percentile of the median). Results with significant differences between conditions for any variable are underlined and the level of significance is indicated by ** (P < 0.003) or a trend toward significance (P = 0.054, Wilcoxon paired) is indicated by *. The I category was not included because only two neurons did not change category.
DISCUSSION
We have tested the hypotheses that human thalamic activity changes between quiet wakefulness versus the condition produced by mental arithmetic. The results demonstrate changes during the counting condition versus the spontaneous condition in spike-train category or in firing and burst rates within a category. Overall, the counting condition is characterized by higher FRs and lower BRs. The G spike trains that did not change category between conditions had lower BRs during the counting condition, whereas the FRs and PRs did not change between these spike trains (Table 4). In the case of spike trains that changed between conditions, the G category spike trains changed to I or NG (Fig. 3), and the I spike trains changed to NG. The parameters of inhibitory events were not different between conditions for these G and NG neurons (PBISI and PBISI/PBISI) (Table 4). The constancy of the FR and PR in the G spike trains between conditions suggests that the resting membrane potential is unchanged, whereas the decrease in the BR suggests that there is a decrease in the rate of inhibitory events in the counting condition. These results suggest that thalamic activity can be dramatically altered by a cognitive task and that the mechanisms governing human thalamic activity during cognitive tasks engage many different neuronal processes.
Methodologic concerns
In identifying LTS burst activity we have used the n versus n + 1 plot, a bias-free method that demonstrates the presence of LTS bursts in our data. However, to be able to quantify the indices that describe LTS burst, it is necessary to identify individual bursts. Therefore we adopted the spike-burst template (50–06–16; see methods, Analysis of burst activity), which has been used to identify LTS bursting in studies of behaving monkeys (Ramcharan et al. 2000a,b). In one of these reports, a parallel study in monkey thalamic slice demonstrated the validity of this template in identifying the burst pattern associated with primate LTS (Ramcharan et al. 2000a). This template was used in the present recent study because there are no human thalamic intracellular studies (Lee et al. 2005).
It is possible that an LTS can produce one spike without a burst, which is defined by two or more action potentials following a long ISI (see Fig. 1 in Ramcharan et al. 2000a). Some of the points in the top right of the n versus n + 1 plots may represent such events. It is also possible that these extracellular recordings and analysis will identify bursts that are not associated with underlying LTS, although the burst fits the template. We cannot identify either of these two potential sources of error without intracellular recordings and thus we account for these potential errors by increased variance within the statistical analysis.
The present study is complicated by the demonstration that the rate of LTS bursting is increased in a number of neurologic diagnoses and symptoms including chronic pain (Jeanmonod et al. 1996; Lenz et al. 1994), Parkinson's tremor, dystonia, tinnitus, and some psychiatric diagnoses (Jeanmonod et al. 1996). The presence of LTS bursts during normal wakefulness is suggested by its presence in all these diagnoses, since it seems unlikely that the same pathology would be observed in these very different conditions. The normal presence of LTS bursts during waking is also consistent with the present results in two waking conditions and with studies in healthy monkeys (Lee et al. 2005; Martinez-Conde et al. 2002; Ramcharan et al. 1998, 2000a,b, 2001, 2005).
Although thalamic surgery is often carried out for treatment of ET (Koller et al. 2001), to our knowledge LTS bursting has not been reported to be altered in ET. We studied subjects with ET because it can be characterized as a monosymptomatic illness without cognitive symptoms and without the complex physiologic/clinical abnormalities of other neurologic diseases treated with thalamic surgery, such as Parkinson's disease (see methods and Deuschl et al. 1998). Finally, these results focus on differences between spike trains recorded from the single neurons during both spontaneous and counting conditions, so that disease-related effects are unlikely to account for the differences between conditions.
Differences in thalamic LTS bursting between nuclei
These results suggest that LTS bursting and significant preburst inhibition occur in these first-order (Vc, Vim, Vo) and higher-order thalamic nuclei (LD), which is consistent with studies of awake rhesus monkeys (Ramcharan et al. 2005). The FRs and PRs are higher in Vim than those in any other nucleus studied, whereas the LTS burst rate, the burst rate/primary event rate, and the proportion of G category spike trains are higher in Vc. The PBISI is shorter in the Vim (148 ms) versus Vc (327 ms), which is approximately compatible with the duration of IPSPs mediated by GABAA versus GABAB receptors, respectively (Andersen and Sears 1964; Roy et al. 1984; Soltesz et al. 1989; Steriade et al. 1997b). These results suggest that inhibitory events in Vc are more frequent and more intense than those in Vim. Although Vo receives inhibitory (pallidal) inputs it has a significantly lower rate of LTS bursts than the other nuclei and the FR in Vo is lower to the point that neurons in Vo may not respond to inhibitory inputs (Smith and Sherman 2002).
Neurons in the thalamic sensory nuclei have demonstrated that prolonged hyperpolarization in the range of 0.5 s results from inhibition-evoked phosphorylation of T channels and results in increased LTS burst firing (Leresche et al. 2004). The latency of this effect is similar to the median of PBISIs in Vc (Table 2), suggesting that this effect might explain the characteristics of preburst inhibition and LTS bursting in this sensory nucleus.
Spike trains in the spontaneous condition
Results of this study (Figs. 4 and 5, and results, Stationary behavior of spike train during the spontaneous condition) show that the FRs and BRs are relatively constant during spontaneous conditions, even though the epochs of spontaneous condition were interrupted by epochs of the counting condition in the complete spike train. The majority of spike trains were characterized by the stationary BR and FR during epochs of the spontaneous condition. This result suggests that the timing of action potentials is random, as measured by FRs and BRs during the spontaneous condition. By the median runs test, all nonstationary epochs were characterized by fewer transitions in FR and BR than expected at random. In the spontaneous condition, spike trains initially categorized the G and NG category never changed category. Therefore there was relatively little variability of spontaneous neuronal activity in these two categories.
On the other hand, spike trains in the I category frequently changed category during the spontaneous period. In this category, all spike trains are composed of both single spikes and LTS bursts, unlike the G and NG category spike trains, which are largely composed of LTS bursts and single action potentials, respectively (Figs. 1 and 4). These characteristics of the I category are compatible with high variability of membrane conductance, as suggested by modeling studies and studies of a thalamic slice (Debay et al. 2004; Wolfart et al. 2005). These proposed changes in conductance could result from corticothalamic connections, which provide a dense excitatory input both to the distal dendrites of thalamocortical neurons and to the inhibitory interneurons projecting to thalamocortical neurons (Ahlsen et al. 1982; Contreras and Steriade 1995; Liu et al. 1995; Steriade et al. 1997b). During the spontaneous condition and at the transition to the counting condition, spike trains in the I category have a significantly greater propensity to change categories than the G and NG categories. These changes suggest that the I category may be a transitional firing pattern between the thalamic burst mode (G category) and the relay mode (NG).
Spike trains in the spontaneous versus the counting condition
Among G neuronal spike trains that did not change category with the counting condition, it seems likely that decreased BRs result from decreased rates of inhibitory events. The spike trains that changed from the G category to I or NG will also result in fewer inhibitory events, as reflected in the decreased BRs. NG spike trains have shorter PBISIs than those of I and G spike trains (Fig. 3 and Table 4) so that the inhibitory input may switch from GABAB to GABAA receptors at the transition from G or I to NG. This change in inhibitory processes could be the result of anatomic distribution of receptors or of activity changes in pathways innervating the neuron (Fig. 3) (Bal et al. 1995a; Roy et al. 1984; Sherman and Guillery 2001; Soltesz et al. 1989).
It seems likely that some of these changes in inhibitory processes during the cognitive task are mediated through inputs to the thalamus from the cortex. Inhibition of thalamic relay neurons may be mediated through cortical inputs to the thalamic reticular nucleus (TRN) or local interneurons or both. For example, excitatory inputs to the thalamic reticular nucleus are highly correlated with LTS bursts in thalamocortical neurons, probably as a result of inhibitory input from the TRN to thalamocortical neurons, which is mediated through GABAA and GABAB receptors (Bal et al. 1995b; Jones 2002).
In summary, BRs were significantly greater than zero for G and I categories during both (waking) conditions. Decreased BR of G spike trains during the counting condition versus the spontaneous condition may be due to decreases in corticothalamic inputs to TRN or local interneurons that lead to IPSPs in thalamocortical neurons. The increased FRs of NG spike trains in the absence of burst firing may be due to depolarization mediated through excitatory cortical input to thalamocortical neurons. Increased variance of membrane conductance, leading eventually to membrane depolarization, may explain the transformation from G category spike trains, first to I, and then to NG category spike trains, so that the I category is a transitional state between the thalamic bursting (G category) and relay modes (NG category) (Debay et al. 2004; Wolfart et al. 2005). Since bursting leads to increased efficacy of thalamocortical synapses, to expanded cortical activation, and to altered thalamocortical responses to peripheral stimuli, the spike trains in the I category may facilitate cortical plasticity (Beierlein et al. 2002; Guido et al. 1995; Swadlow and Gusev 2001fs).
GRANTS
This work was supported by an Eli Lilly Corporation grant and National Institute of Neurological Disorders and Stroke Grants NS-383493 and NS-40059 to F. A. Lenz.
Acknowledgments
We thank L. Rowland for excellent technical assistance.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- Ahlsen et al. 1982.Ahlsen G, Grant K, Lindstrom S. Monosynaptic excitation of principal cells in the lateral geniculate nucleus by corticofugal fibers. Brain Res 234: 454–458, 1982. [DOI] [PubMed] [Google Scholar]
- Andersen and Sears 1964.Andersen P, Sears TA. The role of inhibition in the phasing of spontaneous thalamocortical discharge. J Physiol 173: 459–480, 1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bal et al. 1995a.Bal T, von Krosigk M, McCormick DA. Role of the ferret perigeniculate nucleus in the generation of synchronized oscillations in vitro. J Physiol 483: 665–685, 1995a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bal et al. 1995b.Bal T, von Krosigk M, McCormick DA. Synaptic and membrane mechanisms underlying synchronized oscillations in the ferret lateral geniculate nucleus in vitro. J Physiol 483: 641–663,1995b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beierlein et al. 2002.Beierlein M, Fall CP, Rinzel J, Yuste R. Thalamocortical bursts trigger recurrent activity in neocortical networks: layer 4 as a frequency-dependent gate. J Neurosci 22: 9885–9894, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezdudnaya et al. 2006.Bezdudnaya T, Cano M, Bereshpolova Y, Stoelzel CR, Alonso JM, Swadlow HA. Thalamic burst mode and inattention in the awake LGNd. Neuron 49: 421–432, 2006. [DOI] [PubMed] [Google Scholar]
- Contreras and Steriade 1995.Contreras D, Steriade M. Cellular basis of EEG slow rhythms: a study of dynamic corticothalamic relationships. J Neurosci 15: 604–622, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox and Lewis 1966.Cox DR, Lewis PAW. The Statistical Analysis of Series of Events. London: Chapman & Hall, 1966.
- Debay et al. 2004.Debay D, Wolfart J, Le FY, Le MG, Bal T. Exploring spike transfer through the thalamus using hybrid artificial-biological neuronal networks. J Physiol (Paris) 98: 540–558, 2004. [DOI] [PubMed] [Google Scholar]
- Deuschl et al. 1998.Deuschl G, Bain P, Brin M, Ad Hoc Scientific Committee. Consensus statement of the Movement Disorder Society on Tremor. Mov Disord 13, Suppl. 3: 2–23, 1998. [DOI] [PubMed] [Google Scholar]
- Domich et al. 1986.Domich L, Oakson G, Steriade M. Thalamic burst patterns in the naturally sleeping cat: a comparison between cortically-projecting and reticularis neurones. J Physiol 379: 429–449, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garonzik et al. 2002.Garonzik IM, Hua SE, Ohara S, Lenz FA. Intraoperative microelectrode and semi-microelectrode recording during the physiological localization of the thalamic nucleus ventral intermediate. Mov Disord 17, Suppl. 3: S135–S144, 2002. [DOI] [PubMed] [Google Scholar]
- Guido et al. 1995.Guido W, Lu SM, Vaughan JW, Godwin DW, Sherman SM. Receiver operating characteristic (ROC) analysis of neurons in the cat's lateral geniculate nucleus during tonic and burst response mode. Vis Neurosci 12: 723–741, 1995. [DOI] [PubMed] [Google Scholar]
- Jahnsen and Llinás 1984.Jahnsen H, Llinás R. Ionic basis for the electro-responsiveness and oscillatory properties of guinea-pig thalamic neurones in vitro. J Physiol 349: 227–247, 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankovic 2002.Jankovic J Essential tremor: a heterogenous disorder. Mov Disord 17: 638–644, 2002. [DOI] [PubMed] [Google Scholar]
- Jeanmonod et al. 1996.Jeanmonod D, Magnin M, Morel A. Low-threshold calcium spike bursts in the human thalamus. Common physiopathology for sensory, motor and limbic positive symptoms. Brain 119: 363–375, 1996. [DOI] [PubMed] [Google Scholar]
- Koller et al. 2001.Koller WC, Lyons KE, Wilkinson SB, Troster AI, Pahwa R. Long-term safety and efficacy of unilateral deep brain stimulation of the thalamus in essential tremor. Mov Disord 16: 464–468, 2001. [DOI] [PubMed] [Google Scholar]
- Lee et al. 2005.Lee JI, Ohara S, Dougherty PM, Lenz FA. Pain and temperature encoding in the human thalamic somatic sensory nucleus (ventral caudal): inhibition-related bursting evoked by somatic stimuli. J Neurophysiol 94: 1676–1687, 2005. [DOI] [PubMed] [Google Scholar]
- Lee et al. 2007.Lee JI, Verhagen ML, Ohara S, Dougherty PM, Kim JH, Lenz FA. Internal pallidal neuronal activity during mild drug-related dyskinesias in Parkinson's disease: decreased firing rates and altered firing patterns. J Neurophysiol 97: 2627–2641, 2007. [DOI] [PubMed] [Google Scholar]
- Lenz et al. 1988a.Lenz FA, Dostrovsky JO, Tasker RR, Yamashiro K, Kwan HC, Murphy JT. Single-unit analysis of the human ventral thalamic nuclear group: somatosensory responses. J Neurophysiol 59: 299–316, 1988a. [DOI] [PubMed] [Google Scholar]
- Lenz et al. 1998.Lenz FA, Garonzik IM, Zirh TA, Dougherty PM. Neuronal activity in the region of the thalamic principal sensory nucleus (ventralis caudalis) in patients with pain following amputations. Neuroscience 86: 1065–1081, 1998. [DOI] [PubMed] [Google Scholar]
- Lenz et al. 1994.Lenz FA, Kwan HC, Martin R, Tasker R, Richardson RT, Dostrovsky JO. Characteristics of somatotopic organization and spontaneous neuronal activity in the region of the thalamic principal sensory nucleus in patients with spinal cord transection. J Neurophysiol 72: 1570–1587, 1994. [DOI] [PubMed] [Google Scholar]
- Lenz et al. 1995.Lenz FA, Normand SL, Kwan HC, Andrews D, Rowland LH, Jones MW, Seike M, Lin YC, Tasker RR, Dostrovsky JO. Statistical prediction of the optimal site for thalamotomy in parkinsonian tremor. Mov Disord 10: 318–328, 1995. [DOI] [PubMed] [Google Scholar]
- Lenz et al. 2004.Lenz FA, Ohara S, Gracely RH, Dougherty PM, Patel SH. Pain encoding in the human forebrain: binary and analog exteroceptive channels. J Neurosci 24: 6540–6544, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenz et al. 1993.Lenz FA, Seike M, Richardson RT, Lin YC, Baker FH, Khoja I, Jaeger CJ, Gracely RH. Thermal and pain sensations evoked by microstimulation in the area of human ventrocaudal nucleus. J Neurophysiol 70: 200–212, 1993. [DOI] [PubMed] [Google Scholar]
- Lenz et al. 1988b.Lenz FA, Tasker RR, Kwan HC, Schnider S, Kwong R, Murayama Y, Dostrovsky JO, Murphy JT. Single unit analysis of the human ventral thalamic nuclear group: correlation of thalamic “tremor cells” with the 3–6 Hz component of parkinsonian tremor. J Neurosci 8: 754–764, 1988b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leresche et al. 2004.Leresche N, Hering J, Lambert RC. Paradoxical potentiation of neuronal T-type Ca2+ current by ATP at resting membrane potential. J Neurosci 24: 5592–5602, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lezak 1995.Lezak MD Neuropsychological Assessment. New York: Oxford Univ. Press, 1995.
- Liu et al. 1995.Liu XB, Honda CN, Jones EG. Distribution of four types of synapse on physiologically identified relay neurons in the ventral posterior thalamic nucleus of the cat. J Comp Neurol 352: 69–91, 1995. [DOI] [PubMed] [Google Scholar]
- Lu et al. 1992.Lu SM, Guido W, Sherman SM. Effects of membrane voltage on receptive field properties of lateral geniculate neurons in the cat: contributions of the low-threshold Ca2+ conductance. J Neurophysiol 68: 2185–2198, 1992. [DOI] [PubMed] [Google Scholar]
- Martinez-Conde et al. 2000.Martinez-Conde S, Macknik SL, Hubel DH. Microsaccadic eye movements and firing of single cells in the striate cortex of macaque monkeys. Nat Neurosci 3: 251–258, 2000. [DOI] [PubMed] [Google Scholar]
- Martinez-Conde et al. 2002.Martinez-Conde S, Macknik SL, Hubel DH. The function of bursts of spikes during visual fixation in the awake primate lateral geniculate nucleus and primary visual cortex. Proc Natl Acad Sci USA 99: 13920–13925, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarley et al. 1983.McCarley RW, Benoit O, Barrionuevo G. Lateral geniculate nucleus unitary discharge in sleep and waking: state and rate specific aspects. J Neurophysiol 50: 798–818, 1983. [DOI] [PubMed] [Google Scholar]
- Ohara and Lenz 2003.Ohara S, Lenz FA. Medial lateral extent of thermal and pain sensations evoked by microstimulation in somatic sensory nuclei of human thalamus. J Neurophysiol 90: 2367–2377, 2003. [DOI] [PubMed] [Google Scholar]
- Ohara et al. 2007.Ohara S, Taghva A, Kim JH, Lenz FA. Spontaneous low threshold spike bursting in awake humans is different in different lateral thalamic nuclei. Exp Brain Res 180: 280–281, 2007. [DOI] [PubMed] [Google Scholar]
- Patel et al. 2006.Patel S, Ohara S, Dougherty PM, Gracely RH, Lenz FA. Psychophysical elements of place and modality specificity in the thalamic somatic sensory nucleus (ventral caudal, vc) of awake humans. J Neurophysiol 95: 646–659, 2006. [DOI] [PubMed] [Google Scholar]
- Radhakrishnan et al. 1999.Radhakrishnan V, Tsoukatos J, Davis KD, Tasker RR, Lozano AM, Dostrovsky JO. A comparison of the burst activity of lateral thalamic neurons in chronic pain and non-pain patients. Pain 80: 567–575, 1999. [DOI] [PubMed] [Google Scholar]
- Ramcharan et al. 2000a.Ramcharan EJ, Cox CL, Zhan XJ, Sherman SM, Gnadt JW. Cellular mechanisms underlying activity patterns in the monkey thalamus during visual behavior. J Neurophysiol 84: 1982–1987, 2000a. [DOI] [PubMed] [Google Scholar]
- Ramcharan et al. 1998.Ramcharan EJ, Gnadt JW, Sherman SM. State dependent changes in the firing pattern of relay neurons in the monkey LGN. Soc Neurosci Abstr 24: 139, 1998. [Google Scholar]
- Ramcharan et al. 2000b.Ramcharan EJ, Gnadt JW, Sherman SM. Burst and tonic firing in thalamic cells of unanesthetized, behaving monkeys. Vis Neurosci 17: 55–62, 2000b. [DOI] [PubMed] [Google Scholar]
- Ramcharan et al. 2001.Ramcharan EJ, Gnadt JW, Sherman SM. The effects of saccadic eye movements on the activity of geniculate relay neurons in the monkey. Vis Neurosci 18: 253–258, 2001. [DOI] [PubMed] [Google Scholar]
- Ramcharan et al. 2005.Ramcharan EJ, Gnadt JW, Sherman SM. Higher-order thalamic relays burst more than first-order relays. Proc Natl Acad Sci USA 102: 12236–12241, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy et al. 1984.Roy JP, Clercq M, Steriade M, Deschênes M. Electrophysiology of neurons of lateral thalamic nuclei in cat: mechanisms of long-lasting hyperpolarizations. J Neurophysiol 51: 1220–1235, 1984. [DOI] [PubMed] [Google Scholar]
- Schaltenbrand and Bailey 1959.Schaltenbrand G, Bailey P. Introduction to Stereotaxis with an Atlas of the Human Brain. Stuttgart, Germany: Thieme, 1959.
- Schwarz 1978.Schwarz G Estimating the dimension of a model. Anal Stat 6: 461–464, 1978. [Google Scholar]
- Sherman and Guillery 2001.Sherman SM, Guillery RW. Exploring the Thalamus and Its Role in Cortical Function. New York: Oxford Univ. Press, 2001.
- Smith and Sherman 2002.Smith GD, Sherman SM. Detectability of excitatory versus inhibitory drive in an integrate-and-fire-or-burst thalamocortical relay neuron model. J Neurosci 22: 10242–10250, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snedecor and Cochran 1967.Snedecor GW, Cochran WG. Statistical Methods. Ames, IA: Iowa State Univ. Press, 1967.
- Soltesz et al. 1989.Soltesz I, Lightowler S, Leresche N, Crunelli V. On the properties and origin of the GABAB inhibitory postsynaptic potential recorded in morphologically identified projection cells of the cat dorsal lateral geniculate nucleus. Neuroscience 33: 23–33, 1989. [DOI] [PubMed] [Google Scholar]
- Steriade et al. 1990.Steriade M, Jones EG, Llinás RR. Thalamic Oscillations and Signaling. New York: Wiley, 1990.
- Steriade et al. 1997a.Steriade M, Jones EG, McCormick DA. Thalamic, organization and chemical neuroanatomy. In: Thalamus. Amsterdam: Elsevier, 1997a, vol. 1, p. 269–338.
- Steriade et al. 1997b.Steriade M, Jones EG, McCormick DA. Thalamus Organisation and Function. Amsterdam: Elsevier, 1997b.
- Swadlow and Gusev 2001.Swadlow HA, Gusev AG. The impact of “bursting” thalamic impulses at a neocortical synapse. Nat Neurosci 4: 402–408, 2001. [DOI] [PubMed] [Google Scholar]
- Weingessel et al. 1999.Weingessel A, Dimitriadou A, Dolnicar S. An Examination of Indexes for Determining the Number of Clusters in Binary Data Sets (Working Paper Series No. 29). Vienna, Austria: Vienna Univ. of Economics and Business Administration, 1999, p. 1–19.
- Wolfart et al. 2005.Wolfart J, Debay D, Le MG, Destexhe A, Bal T. Synaptic background activity controls spike transfer from thalamus to cortex. Nat Neurosci 8: 1760–1767, 2005. [DOI] [PubMed] [Google Scholar]
- Zirh et al. 1997.Zirh AT, Lenz FA, Reich SG, Dougherty PM. Patterns of bursting occurring in thalamic cells during parkinsonian tremor. Neuroscience 83: 107–121, 1997. [DOI] [PubMed] [Google Scholar]