Abstract
Hemi-gap-junction (HGJ) channels of retinal horizontal cells (HCs) function as transmembrane ion channels that are modulated by voltage and calcium. As an endogenous retinal neuromodulator, zinc, which is coreleased with glutamate at photoreceptor synapses, plays an important role in shaping visual signals by acting on postsynaptic HCs in vivo. To understand more fully the regulation and function of HC HGJ channels, we examined the effect of Zn2+ on HGJ channel currents in bass retinal HCs. Hemichannel currents elicited by depolarization in Ca2+-free medium and in 1 mM Ca2+ medium were significantly inhibited by extracellular Zn2+. The inhibition by Zn2+ of hemichannel currents was dose dependent with a half-maximum inhibitory concentration of 37 μM. Compared with other divalent cations, Zn2+ exhibited higher inhibitory potency, with the order being Zn2+ > Cd2+ ≈ Co2+ > Ca2+ > Ba2+ > Mg2+. Zn2+ and Ca2+ were found to modulate HGJ channels independently in additivity experiments. Modification of histidine residues with N-bromosuccinimide suppressed the inhibitory action of Zn2+, whereas modification of cysteine residues had no significant effect on Zn2+ inhibition. Taken together, these results suggest that zinc acts on HGJ channels in a calcium-independent way and that histidine residues on the extracellular domain of HGJ channels mediate the inhibitory action of zinc.
INTRODUCTION
Horizontal cells (HCs) are second-order neurons in the vertebrate retina that receive excitatory synaptic input from, and provide inhibitory feedback to, photoreceptors (Baylor et al. 1971; Naka 1972; Wu 1992). HCs are extensively coupled via cell-to-cell gap junction channels (Baldridge et al. 1987; McMahon et al. 1989; Witkovsky et al. 1983; Zhang and McMahon 2000) and also express unapposed hemi-gap-junction (HGJ) channels in their nonjunctional membranes (DeVries and Schwartz 1992; Goodenough and Paul 2003; Malchow et al. 1993; Saez et al. 2005; Zhang and McMahon 2001). HC HGJ channels function as transmembrane ion channels (DeVries and Schwartz 1992; Malchow et al. 1993; Zhang and McMahon 2001) and have been proposed to participate in ephaptic feedback from HCs to cones (Kamermans and Fahrenfort 2004; Kamermans et al. 2001). Previous studies have demonstrated that HGJ channels are modulated by transmembrane voltage, extracellular Ca2+, and light-adapting retinal neurotransmitters, such as dopamine, nitric oxide, and retinoic acid (DeVries and Schwartz 1992; Retamal et al. 2006; Ripps et al. 2002; Zhang and McMahon 2000, 2001; Zhang et al. 2008). In this study, we have examined whether zinc modulates HGJ channels of HCs.
Zinc, a trace metal highly concentrated in the retina, plays a pivotal role in maintaining normal visual function (Grahn et al. 2001; Ugarte and Osborne 2001), whereas zinc deficiency results in a variety of ocular diseases (Huber and Gershoff 1975; Morrison et al. 1978). Zinc is concentrated in photoreceptor cell bodies and synaptic terminals (Lee et al. 2008; Wu et al. 1993) and is released from photoreceptors onto HCs and bipolar cells (Akagi et al. 2001; Redenti et al. 2007; Ugarte and Osborne 2001; Zhang et al. 2002). Convergent evidence indicates that zinc functions as a neuromodulator of synaptic transmission in the retina and in the rest of the brain by regulating a variety of voltage-gated and ligand-gated channels in postsynaptic neurons (Akagi et al. 2001; Dong and Werblin 1996; Han and Wu 1999; Huang 1997; Wu et al. 1993; Zhang et al. 2002, 2006). In the retina, zinc has been shown to modulate γ-aminobutyric acid (GABA) receptors, glutamate receptors, and K+ channels on HCs, glycine receptors on ganglion cells, and GABA receptors on bipolar cells (Dong and Werblin 1996; Han and Wu 1999; Qian et al. 1997; Wu et al. 1993; Zhang et al. 2002, 2006).
In this study, we investigated the effects of extracellular Zn2+ on HGJ channels in isolated retinal HCs using whole cell patch-clamp techniques. Our results demonstrate that micromolar Zn2+ partially suppresses HC HGJ channel currents, that HGJ channels are modulated by zinc at physiological concentrations of extracellular calcium, that zinc modulates HGJ channels in a Ca2+-independent way, and that the likely binding sites of Zn2+ are to extracellular histidine residues.
METHODS
Cell culture
Dark-adapted adult hybrid striped bass (Roccus chrysops × Roccus saxitalis) were killed in accordance with National Institutes of Health guidelines for animal use. Retinas were removed under dim red light and then incubated in L-15 medium (GIBCO) containing 20 U/ml papain (Worthington) activated with 0.3 mg/ml cysteine. Penicillin:streptomycin (1:100, Sigma) was routinely added to the medium to reduce contamination. The retinas were incubated in L-15/papain solution for 30 min, followed by six changes of fresh L-15 medium, and then dissociated by repeated passage through a serological pipette. Isolated cells were plated onto plastic 35-mm dishes containing fresh L-15 medium. Cultures were maintained at 20°C and cells were used following 1–4 days in culture.
Solutions and chemicals
The normal Ca2+ extracellular solution contained (in mM): 137 NaCl, 2.5 KCl, 2.5 MgCl2, 2.5 CaCl2, 10 HEPES, 10 glucose, and 1 mg/ml bovine serum albumin (BSA; Sigma, Fraction VII) (pH was adjusted to 7.4 using NaOH). The Ca2+-free extracellular solution contained (in mM): 114.5 NaCl, 30 CsCl, 2.5 KCl, 1 MgCl2, 10 HEPES, 1 Na-pyruvate, 10 glucose, and 1 mg/ml BSA (pH was adjusted to 7.4 with NaOH). To block potassium channels, K+ in the normal pipette solution was replaced by Cs+ and tetraethylammonium chloride (TEA) was added. The pipette solution contained (in mM): 124 CsCl, 1 CaCl2, 11 EGTA, 10 HEPES, 1 Mg-ATP, 0.1 Na-GTP, and 10 TEA (pH to 7.4 with CsOH). Solution changes during recording were performed with a perfusion system and were completed within 30 s.
The histidine-modifying reagent N-bromosuccinimide (N-BrSuc, MP Biomedicals) was dissolved in extracellular solution in 1 mM concentration. The membrane-impermeant cysteine modifier, methanethiosulfonate-ethyltrimethylammonium (MTSET, Anatrace), was prepared as a 10 mM stock in extracellular solution, set on ice for ≤1 h, then diluted to a 1 mM working concentration in extracellular solution at room temperature immediately before incubation in cell culture. The noncharged membrane-permeant cysteine modifier N-ethylmaleimide (NEM, Sigma–Aldrich) was prepared as a 100 mM stock in water and then diluted to a 1 mM working concentration in extracellular solution.
Patch-clamp recording
Recordings from solitary HCs were performed using conventional whole cell patch-clamp configuration. Patch pipettes were pulled from Corning 7052 glass (AM Systems) and fire-polished to 4–-6 MΩ resistance. The pipette series resistance and capacitance were compensated by 80%. The offset potential between the pipette and bath solutions was zeroed prior to seal formation. Voltage commands and data analysis were performed by pCLAMP 9.0 software.
Data analysis
Dose–inhibition curves were obtained by calculating the inhibition percentage of Zn2+ at different concentrations and were fitted with the Hill equation, Imem = (Imax × An)/(IC50 + An), where Imem is the cell membrane current elicited by a given antagonist concentration (A), IC50 is the antagonist concentration that elicits a half-maximal response, Imax is the measured maximal response, and n is the Hill coefficient; the normalized percentage inhibition of zinc was calculated by the equation: [(Icontrol − IZn)/Icontrol] × 100%. Here, the inhibition percentage was calculated by measuring the reduction of the current following application of Zn2+ (or other cations) and expressing this as a fraction of the peak current amplitude in the control medium without Zn2+ (or other cations).
The data are presented as means ± SE. P values stated were calculated using either the t-test or the paired t-test. Statistical analyses were performed with Sigmaplot 10.0.
RESULTS
Zinc inhibits HGJ channel currents
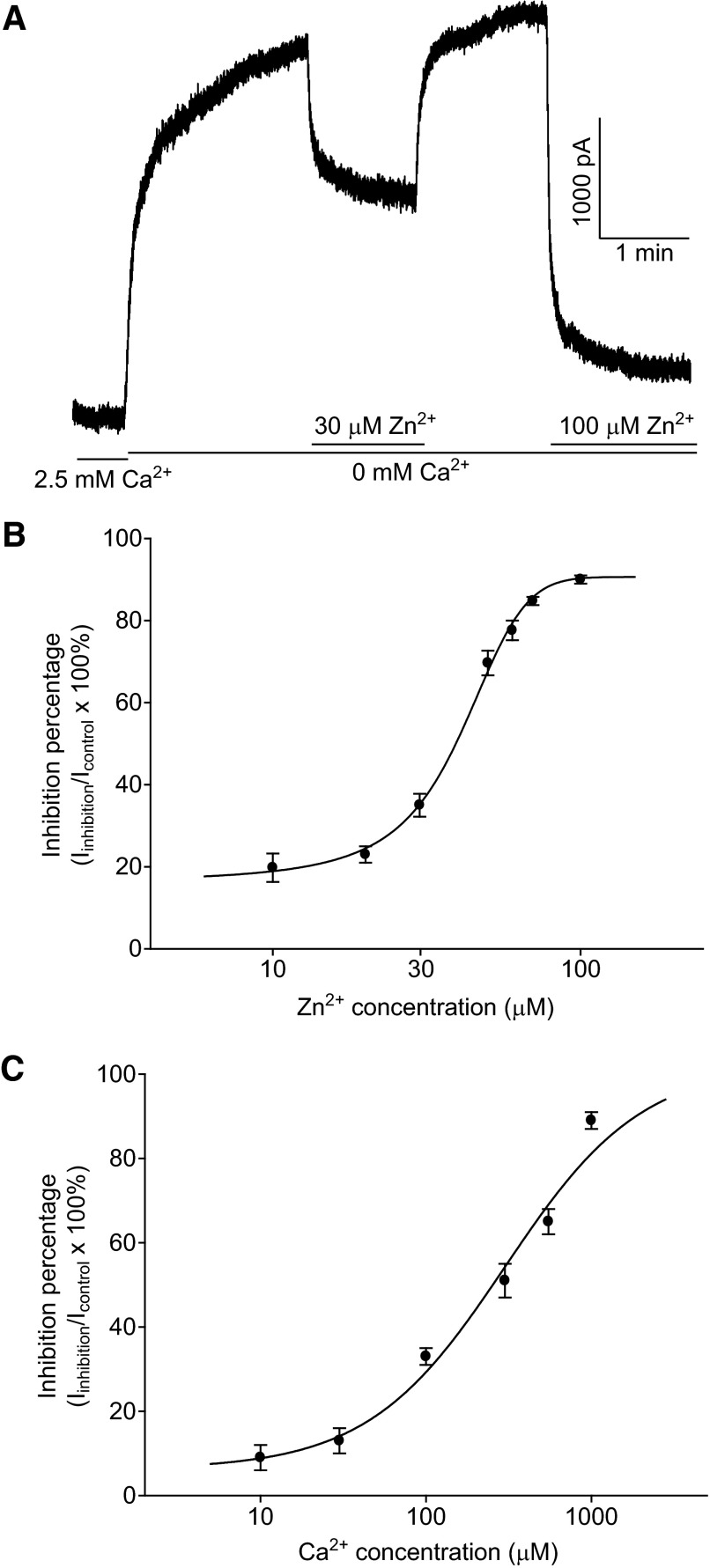
To examine the effect of Zn2+ on HGJ channel currents, isolated solitary HCs were recorded using whole cell voltage clamp, and outward HGJ channel currents were elicited by superfusion with Ca2+-free medium and cell membrane depolarization, while blocking K+ channels with Cs+, and TEA. As shown in Fig. 1A, at a membrane potential of +40 mV, outward HGJ channel current was increased by switching the extracellular solution from 2.5 mM Ca2+ medium to Ca2+-free medium. Application of 30 μM Zn2+ suppressed this hemichannel current from 3,040 to 1,860 pA. The action of 30 μM Zn2+ on HGJ channel currents was reversible on washout and the following application of 100 μM Zn2+ decreased the current further to 390 pA. The dose–inhibition curve for Zn2+ is shown in Fig. 1B and, when fit with the Hill equation, yielded a half-maximum inhibitory concentration (IC50) of 37 μM. We also tested the effect of 5 μM Zn2+ on HGJ channel currents; no potentiation of currents was observed, suggesting that Zn2+ inhibits HGJ channel currents in a monophasic manner. In contrast, Ca2+, another divalent cation modulator for HGJ channel currents (Zhang and McMahon 2001), exhibited an IC50 value of 245 μM, which is close to sevenfold higher than that of zinc (Fig. 1C).
FIG. 1.
Zinc inhibits hemi-gap-junction (HGJ) channel currents in bass horizontal cells (HCs). A: at a holding potential of +40 mV, outward hemichannel currents were induced by switching the extracellular solution from 2.5 mM Ca2+ medium to Ca2+-free medium. External application of 30 μM Zn2+ or 100 μM Zn2+ suppressed the currents to different degrees. B: normalized dose–inhibition relationships for Zn2+ in Ca2+-free medium. The reduction of currents by Zn2+ was normalized to the initial response obtained in Ca2+-free medium. C: normalized dose–inhibition relationships for Ca2+. The reduction of currents by Ca2+ was normalized to the initial response obtained in Ca2+-free medium. Each point is an average derived from 4 to 5 cells and dose–inhibition curves were fit with the Hill equation.
As shown in Fig. 1C, about 10% of the hemichannel current remained at 1 mM Ca2+, a concentration similar to that estimated in the intact retina (Dearry and Burnside 1984). To test whether Zn2+ inhibits HC hemichannel currents at physiological Ca2+ concentrations, we examined Zn2+ action on hemichannel currents in the presence of 1 mM Ca2+. Zn2+ (10 μM) significantly reduced the Ca2+-resistant hemichannel current to 19 ± 4% of control (P < 0.05, n = 5). These data indicate that Zn2+ can modulate HGJ channel currents at physiological Ca2+ concentrations.
Zinc is a potent modulator of HGJ channel currents
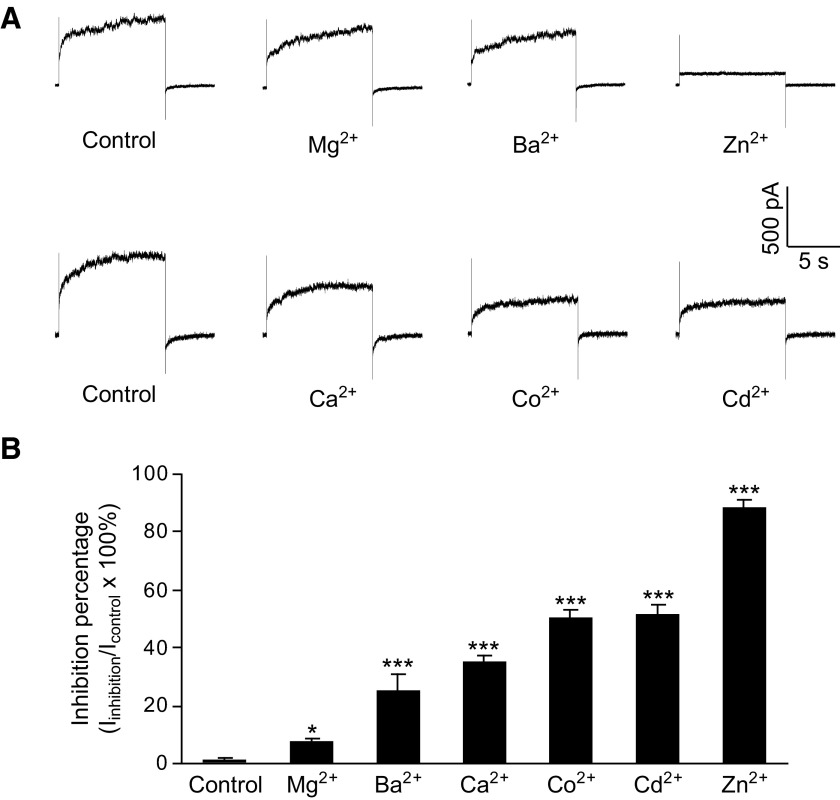
HGJ channels possess a high sensitivity to extracellular divalent cations, which is rarely observed on cell–cell gap junction channels (Spray et al. 2006; Srinivas et al. 2006). To compare the inhibitory potency of Zn2+ with other divalent cations, we added 100 μM of different cations to the Ca2+-free medium and determined their inhibition of HGJ channel currents (Fig. 2A). HGJ channel currents were induced under Ca2+-free medium by stepping membrane potential for 10 s from 0 to +40 mV. The inhibitory potency of these cations was in the order of: Zn2+ > Cd2+ ≈ Co2+ > Ca2+ > Ba2+ > Mg2+ (Fig. 2B).
FIG. 2.
Inhibitory potencies of different divalent cations on HGJ currents. A: representative current traces of the currents induced in control medium and in medium containing 100 μM Mg2+, Ba2+, Zn2+ (top traces), or 100 μM Ca2+, Co2+, Cd2+ (bottom traces). B: average inhibition by the different divalent cations. Asterisks indicate values significantly different from control, paired t-test: *P < 0.05 and ***P < 0.001 (n = 5).
Zinc modulation is independent of calcium
Ca2+ and retinoic acid interact in gating HGJ channels in bass retinal HCs (Zhang and McMahon 2001) and Co2+ and Ni2+ share the same binding site with Ca2+ on connexin hemichannels (Srinivas et al. 2006). Next, we examined the interaction between Zn2+ and Ca2+ on HGJ channel currents.
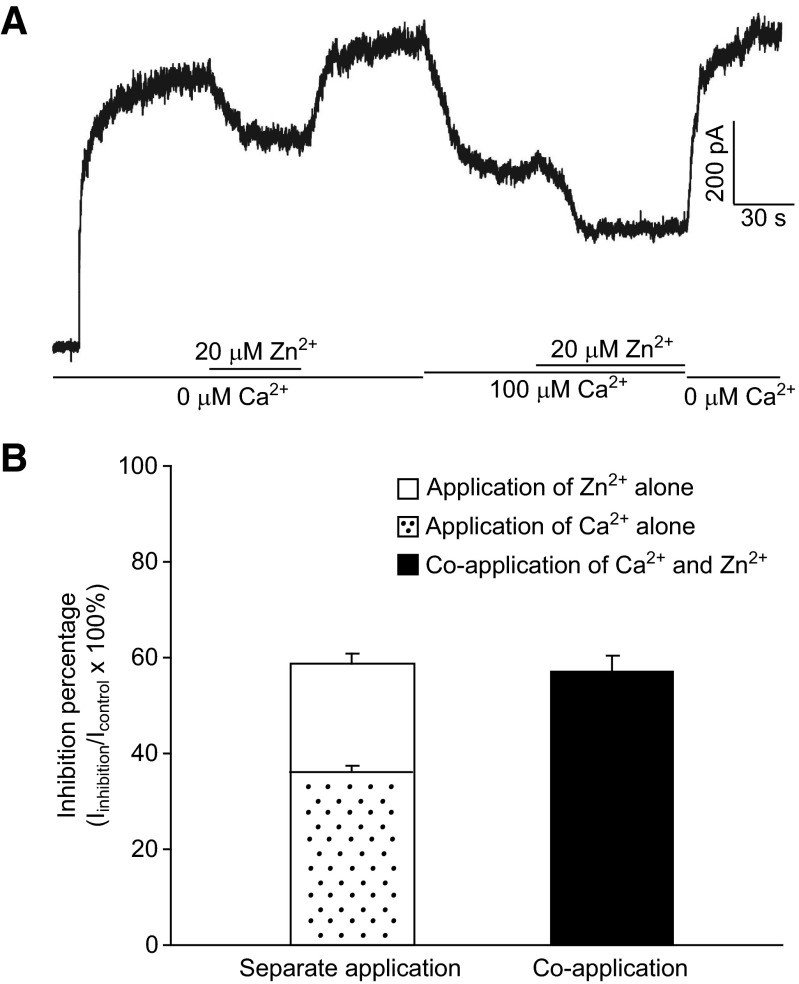
If Zn2+ and Ca2+ act on HGJ channels independently, their inhibitory effects would be expected to be additive when both are present at low concentrations (individual inhibition percentage <50%), provided that there are sufficient HGJ channel targets for both Zn2+ and Ca2+. However, if there is overlap in the binding sites of Zn2+ and Ca2+, one would expect a significantly lower total inhibition instead of simple addition in the presence of both cations. As shown in Fig. 3A, HGJ channel currents were inhibited by 24% (from 705 to 537 pA) by application of 20 μM Zn2+ alone, whereas application of 100 μM Ca2+ alone reduced the current by 40% (from 790 to 471 pA). The coapplication of 20 μM Zn2+ and 100 μM Ca2+ (Ca2+ added before Zn2+) produced a total reduction of 62% (from 790 to 490 pA), which is close to the sum of their separate inhibitory effects (64%). Statistical analyses (Fig. 3B) show that there is no significant difference between the inhibition by coapplication (58 ± 3%) versus the sum (60 ± 4%) of separate inhibitions (t-test, P > 0.05, n = 4). Similar results were also obtained when Zn2+ was added before Ca2+ during coapplication experiments (data not shown). These data suggested that Zn2+ and Ca2+ modulate HGJ channel currents independently, at least at low concentrations.
FIG. 3.
At low concentrations, Zn2+ and Ca2+ act on HGJ channels additively. A: in Ca2+-free medium, HGJ channel currents were evoked by stepping the holding potential from 0 to +40 mV; 20 μM Zn2+ alone produced a 23.8% inhibition from 705 to 537 pA; 100 μM Ca2+ followed by 20 μM Zn2+ were perfused onto the cell. B: average inhibition percentages resulting from the sum of individual application of Zn2+ (white bar) or Ca2+ (dotted bar) and resulting from the coapplication of Zn2+ and Ca2+ (solid bar). Data were collected from 4 cells.
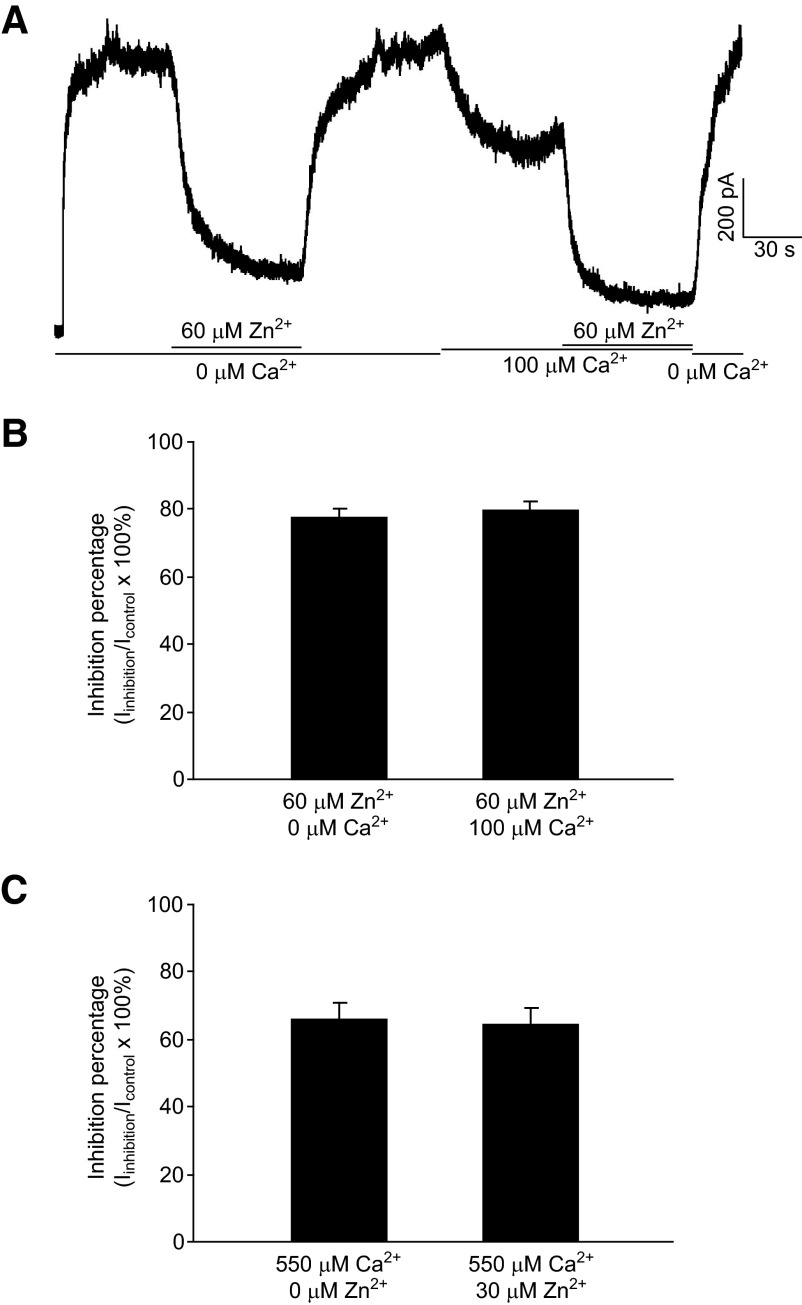
At high concentrations of Zn2+ and Ca2+, especially those at which the sum of separate inhibition exerted by Zn2+ or Ca2+ alone would exceed 100%, it becomes impossible to examine the action mode between Zn2+ and Ca2+ by comparing the inhibition of coapplication with the sum of separate inhibitions. However, in this case, if Zn2+ and Ca2+ modulate HGJ channels independently, the fraction of inhibited channels by Zn2+ in Ca2+-free medium should remain the same as that in the presence of background Ca2+. To test this hypothesis, we examined inhibitions by 60 μM Zn2+ (78 ± 3% inhibition in Fig. 1B) on HGJ currents in the absence and in the presence of 100 μM Ca2+ (34 ± 2% inhibition in Fig. 1C). As shown in Fig. 4A, application of 60 μM Zn2+ reduced the HGJ channel current from 1,000 to 200 pA, resulting in an 80% inhibition in Ca2+-free medium. After washout with Ca2+-free medium, 100 μM background Ca2+ was applied and the current decreased to 640 pA. In the presence of the 100 μM Ca2+, additional application of 60 μM Zn2+ further reduced the currents from 640 to 110 pA, producing an 83% inhibition, which is similar to that observed in Ca2+-free medium. Again, statistical analyses showed no significant difference (Fig. 4B) between the fraction inhibited by 60 μM Zn2+ in the absence (78 ± 3%) and in the presence (80 ± 3%) of 100 μM Ca2+ (t-test, P > 0.05, n = 4). Similar results were obtained when 550 μM Ca2+ was examined using 20 μM Zn2+ as background. As shown in Fig. 4C, the inhibition of 550 μM Ca2+ without Zn2+ (65 ± 2%) remains the same as that in the presence of 20 μM Zn2+ (64 ± 3%, t-test, P > 0.05, n = 4). These data indicate that at high concentrations, Zn2+ and Ca2+ modulate HGJ channel currents independently.
FIG. 4.
At high concentrations, Zn2+ or Ca2+ inhibits a constant proportion of current in coapplication. A: in Ca2+-free medium, outward HGJ channel currents were evoked by stepping from 0 to +40 mV; 60 μM Zn2+, then 100 μM Ca2+, and additional application of 60 μM Zn2+ were perfused onto the cell. B: average inhibition percentages of 60 μM Zn2+ in Ca2+-free medium (left bar) and of 60 μM Zn2+ in the presence of 100 μM Ca2+ (right bar). C: average inhibition percentages of 550 μM Ca2+ in Zn2+-free medium (left bar) and of 550 μM Ca2+ in the presence of 30 μM Ca2+ (right bar). Data were collected from 4 cells.
In addition, we also recorded HGJ channel currents at varying concentrations of Zn2+ in the presence of 100 μM Ca2+. The dose–response curve of Zn2+ in the presence of 100 μM Ca2+ exhibited an IC50 of 32 μM (data not shown), which is similar to the IC50 of Zn2+ in Ca2+-free medium (37 μM), further supporting that Zn2+ and Ca2+ act on HGJ channels independently.
Zinc inhibits HGJ channel currents via histidine not cysteine residues
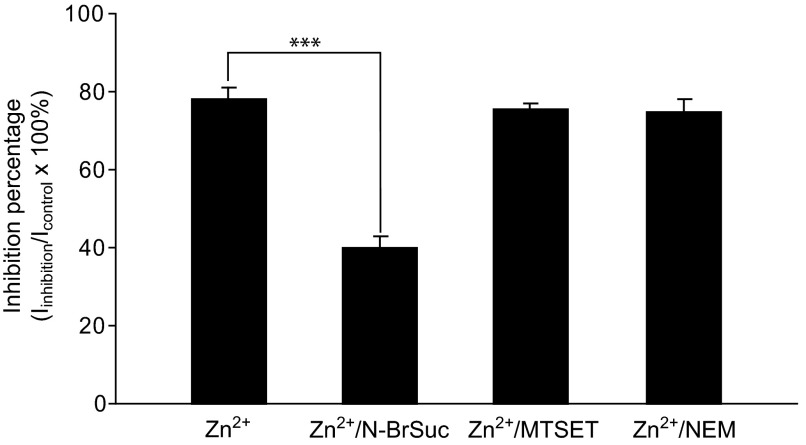
The action of Zn2+ on proteins is generally mediated by complexing zinc ions with histidine or cysteine residues (Connolly and Wafford 2004; Nevin et al. 2003; Norregaard et al. 1998; Seebungkert and Lynch 2001). To test the hypothesis that the inhibition of connexin hemichannels by Zn2+ is mediated by binding to histidine/cysteine residues, the inhibition exerted by 50 μM Zn2+ was compared in the presence or the absence of histidine or cysteine residue modifying agents. In control experiments, with no pretreatment, 50 μM Zn2+ resulted in a 78 ± 2% inhibition of HGJ channel currents (n = 5, Fig. 5). When the brominating agent N-BrSuc was used to modify the external histidine residues by preincubating HCs with 1 mM N-BrSuc for 30 min, the inhibitory effect exerted by 50 μM Zn2+ was significantly reduced to only 40 ± 2% inhibition (t-test, P < 0.001, n = 5, Fig. 5). We then tested whether cysteine residues are involved in mediating the action of Zn2+ on hemichannels. When HCs were preincubated with the fast-reacting, lipid-insoluble cysteine-modifier MTSET, no apparent effect was observed on the inhibition produced by 50 μM Zn2+ (75 ± 3% inhibition, n = 5, Fig. 5), indicating that MTSET-accessible extracellular cysteines are not involved in Zn2+ action. To further confirm the lack of cysteine involvement, a noncharged, membrane-permeant cysteine modifier, NEM, was tested and similar results were obtained (74 ± 3%, n = 5, Fig. 5).
FIG. 5.
Effects of histidine and cysteine modification on zinc inhibition. Histogram shows 50 μM zinc inhibition of HGJ channels with or without pretreating with histidine/cysteine modifying reagents. Asterisks indicate values significantly different from the control, t-test: ***P < 0.001 (n = 5).
DISCUSSION
We have characterized the effects of Zn2+ on HGJ channels of solitary retinal HCs and our principal findings are as follows: 1) Zn2+ inhibits HGJ channel currents at micromolar concentrations in a monophasic manner. 2) Zn2+ and Ca2+ modulate HGJ currents independently. 3) The inhibition of Zn2+ is mediated through histidine residues on the extracellular domain of hemichannels, whereas the contribution of cysteines is limited. Taken together, our data suggest that Zn2+ is a potent regulator of native HGJ channel currents.
Zinc acts monophasically and extracellularly
Previous studies have shown that extracellular Zn2+ modulates perch Cx35 and human Cx26 HGJ channels expressed on Xenopus oocytes in a biphasic pattern, with low micromolar concentrations of Zn2+ enhancing HGJ currents and high concentrations suppressing them (Chappell et al. 2003, 2004). This biphasic action of Zn2+ has also been reported for other Zn2+-binding proteins (Han and Wu 1999). In contrast, in this study of native HC hemichannels, we found that Zn2+ suppressed HGJ channel currents at both low and high micromolar concentrations. This monophasic inhibitory effect of Zn2+ indicates that the Zn2+ modulation of bass HGJ channels is different from the biphasic model, which has two metal ion-binding sites with different affinities. It is interesting that a similar monotonic Zn2+ inhibition of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA)-type glutamate receptors has also been reported in bass retinal HCs (Zhang et al. 2002).
The presence of 1 mM external Ca2+ did not affect the Zn2+ inhibition of HGJ currents, indicating that Zn2+ acts on HGJ channels via specific binding interactions, not due to nonspecific cationic surface charge actions. We also found that Zn2+ suppressed HGJ currents at both positive and negative potentials. This voltage independence of Zn2+ modulation further suggests that the binding sites for Zn2+ are unlikely to be located within the pore of HGJ channels. Furthermore, we observed no significant effect of extracellular Zn2+ on cell-to-cell coupling of bass HCs (data not shown), indicating that the binding sites for Zn2+ are located on the extracellular surfaces exposed on unapposed HGJ channels. Taken together, these results suggest that the modulation of HGJ channels by Zn2+ occurs at specific binding sites on the extracellular surface, as has been suggested for Cx46 hemichannels (Verselis and Srinivas 2008). The distinct regulatory pattern and potency of Zn2+ observed on Cx35 and Cx26 HGJ channels heterologously expressed in oocytes (Chappell et al. 2003, 2004) may be due either to sequence differences in those connexin proteins compared with whatever bass HC connexin protein that forms the hemichannels we recorded or to differences in zinc regulation of native HC versus heterologously expressed non-HC connexins.
Zinc acts independently of calcium
Previous studies have shown that Ca2+ has interactions with retinoic acid (Zhang and McMahon 2001) and shares the same binding sites with Co2+ and Ni2+ in regulating HGJ channel currents (Srinivas et al. 2006). In this study, we found that at low concentrations of both Zn2+ and Ca2+, their actions were additive, whereas at high concentrations the fraction of current inhibited by one cation remained the same in the presence of the other. These results indicate that Zn2+ regulates HGJ channels by coordinating with binding sites that are distinct from those for Ca2+. Furthermore, the IC50 of Zn2+ does not change significantly in the presence of Ca2+, suggesting that the affinity of HGJ channels for Zn2+ remains the same under those conditions. All these data suggest that Zn2+ regulates HGJ channel currents in a Ca2+-independent manner.
Zinc acts on histidine residues
Histidines and cysteines are the most common coordinating residues at Zn2+-binding sites (Connolly and Wafford 2004; Horenstein and Akabas 1998; Katz and Luong 1999; Nevin et al. 2003; Norregaard et al. 1998; Seebungkert and Lynch 2001). Although the specific sequences of the bass HC connexins mediating hemichannel currents have not been identified, connexin proteins possess conserved cysteine residues on the extracellular loops that stabilize the folding and cell–cell interactions of connexin proteins (Foote et al. 1998; Richard et al. 1998; Toyofuku et al. 1998). Histidine residues have also been found conserved in many different connexins (Beahm and Hall 2002; Stergiopoulos et al. 1999). Our data show that Zn2+ inhibition was significantly attenuated by pretreating cells with a histidine modifier, N-BrSuc, a common brominating agent used to modify histidines in different proteins (Mancini et al. 1992; Poe et al. 1979), indicating that the Zn2+ inhibition involves binding to one or more exposed histidine residues. To assess the contribution of cysteine residues to the Zn2+ inhibition, the effects of two cysteine modifiers, a membrane-impermeant agent MTSET and a membrane-permeant agent NEM, were also examined and neither exerted significant influence on Zn2+ modulation of HGJ currents. Overall, these results suggest that histidines are the major residues for coordinating Zn2+, whereas cysteine residues contribute little to this inhibition. Interestingly, conserved histidine residues on HGJ channels have been identified on the extracellular domains of connexin proteins (Beahm and Hall 2002). However, since the modification of histidine residues did not block the Zn2+ inhibition completely, our data do not exclude the possibility that other residues might also contribute to the coordination of Zn2+.
Physiological relevance of retinal zinc
Endogenous Zn2+ has been shown to be highly concentrated in the synaptic terminals of photoreceptors (Lee et al. 2008; Wu et al. 1993). Recently, both intracellular redistribution of Zn2+ and depolarization-induced Zn2+ release have been reported in isolated zebrafish photoreceptors (Redenti et al. 2007). Furthermore, Zn2+ has also been shown to modulate GABAC and AMPA receptors in teleost retinal HCs, indicating that Zn2+ can potentially regulate visual signal processing within retinal circuits (Dong and Werblin 1996; Zhang et al. 2002). The experiments with isolated HCs reported here, which allow direct measurement of native hemichannel currents not possible in HCs in intact retinas, have established novel aspects of HC HGJ currents and their modulation by zinc and suggest the potential for these mechanisms to contribute to the regulation of HC function.
GRANTS
This work was supported by National Eye Institute Grant R01 EY-09256 to D. G. McMahon.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- Akagi et al. 2001.Akagi T, Kaneda M, Ishii K, Hashikawa T. Differential subcellular localization of zinc in the rat retina. J Histochem Cytochem 49: 87–96, 2001. [DOI] [PubMed] [Google Scholar]
- Baldridge et al. 1987.Baldridge WH, Ball AK, Miller RG. Dopaminergic regulation of horizontal cell gap junction particle density in goldfish retina. J Comp Neurol 265: 428–436, 1987. [DOI] [PubMed] [Google Scholar]
- Baylor et al. 1971.Baylor DA, Fuortes MG, O'Bryan PM. Receptive fields of cones in the retina of the turtle. J Physiol 214: 265–294, 1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beahm and Hall 2002.Beahm DL, Hall JE. Hemichannel and junctional properties of connexin 50. Biophys J 82: 2016–2031, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chappell et al. 2004.Chappell RL, Qian H, Zakevicius J, Ripps H. Histidine suppresses zinc modulation of connexin hemichannels. Biol Bull 207: 188–190, 2004. [DOI] [PubMed] [Google Scholar]
- Chappell et al. 2003.Chappell RL, Zakevicius J, Ripps H. Zinc modulation of hemichannel currents in Xenopus oocytes. Biol Bull 205: 209–211, 2003. [DOI] [PubMed] [Google Scholar]
- Connolly and Wafford 2004.Connolly CN, Wafford KA. The Cys-loop superfamily of ligand-gated ion channels: the impact of receptor structure on function. Biochem Soc Trans 32: 529–534, 2004. [DOI] [PubMed] [Google Scholar]
- Dearry and Burnside 1984.Dearry A, Burnside B. Effects of extracellular Ca++, K+, and Na+ on cone and retinal pigment epithelium retinomotor movements in isolated teleost retinas. J Gen Physiol 83: 589–611, 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVries and Schwartz 1992.DeVries SH, Schwartz EA. Hemi-gap-junction channels in solitary horizontal cells of the catfish retina. J Physiol 445: 201–230, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong and Werblin 1996.Dong CJ, Werblin FS. Use-dependent and use-independent blocking actions of picrotoxin and zinc at the GABAC receptor in retinal horizontal cells. Vision Res 36: 3997–4005, 1996. [DOI] [PubMed] [Google Scholar]
- Foote et al. 1998.Foote CI, Zhou L, Zhu X, Nicholson BJ. The pattern of disulfide linkages in the extracellular loop regions of connexin 32 suggests a model for the docking interface of gap junctions. J Cell Biol 140: 1187–1197, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodenough and Paul 2003.Goodenough DA, Paul DL. Beyond the gap: functions of unpaired connexon channels. Nat Rev Mol Cell Biol 4: 285–294, 2003. [DOI] [PubMed] [Google Scholar]
- Grahn et al. 2001.Grahn BH, Paterson PG, Gottschall-Pass KT, Zhang Z. Zinc and the eye. J Am Coll Nutr 20: 106–118, 2001. [DOI] [PubMed] [Google Scholar]
- Han and Wu 1999.Han Y, Wu SM. Modulation of glycine receptors in retinal ganglion cells by zinc. Proc Natl Acad Sci USA 96: 3234–3238, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horenstein and Akabas 1998.Horenstein J, Akabas MH. Location of a high affinity Zn2+ binding site in the channel of alpha1beta1 gamma-aminobutyric acidA receptors. Mol Pharmacol 53: 870–877, 1998. [PubMed] [Google Scholar]
- Huang 1997.Huang EP Metal ions and synaptic transmission: think zinc. Proc Natl Acad Sci USA 94: 13386–13387, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber and Gershoff 1975.Huber AM, Gershoff SN. Effects of zinc deficiency on the oxidation of retinol and ethanol in rats. J Nutr 105: 1486–1490, 1975. [DOI] [PubMed] [Google Scholar]
- Kamermans and Fahrenfort 2004.Kamermans M, Fahrenfort I. Ephaptic interactions within a chemical synapse: hemichannel-mediated ephaptic inhibition in the retina. Curr Opin Neurobiol 14: 531–541, 2004. [DOI] [PubMed] [Google Scholar]
- Kamermans et al. 2001.Kamermans M, Fahrenfort I, Schultz K, Janssen-Bienhold U, Sjoerdsma T, Weiler R. Hemichannel-mediated inhibition in the outer retina. Science 292: 1178–1180, 2001. [DOI] [PubMed] [Google Scholar]
- Katz and Luong 1999.Katz BA, Luong C. Recruiting Zn2+ to mediate potent, specific inhibition of serine proteases. J Mol Biol 292: 669–684, 1999. [DOI] [PubMed] [Google Scholar]
- Lee et al. 2008.Lee SC, Zhong YM, Li RX, Yu Z, Yang XL. Localization of zinc in the outer retina of carp: a light- and electron-microscopic study. Synapse 62: 352–357, 2008. [DOI] [PubMed] [Google Scholar]
- Malchow et al. 1993.Malchow RP, Qian H, Ripps H. Evidence for hemi-gap junctional channels in isolated horizontal cells of the skate retina. J Neurosci Res 35: 237–245, 1993. [DOI] [PubMed] [Google Scholar]
- Mancini et al. 1992.Mancini GM, Beerens CE, Galjaard H, Verheijen FW. Functional reconstitution of the lysosomal sialic acid carrier into proteoliposomes. Proc Natl Acad Sci USA 89: 6609–6613, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon et al. 1989.McMahon DG, Knapp AG, Dowling JE. Horizontal cell gap junctions: single-channel conductance and modulation by dopamine. Proc Natl Acad Sci USA 86: 7639–7643, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison et al. 1978.Morrison SA, Russell RM, Carney EA, Oaks EV. Zinc deficiency: a cause of abnormal dark adaptation in cirrhotics. Am J Clin Nutr 31: 276–281, 1978. [DOI] [PubMed] [Google Scholar]
- Naka 1972.Naka KI The horizontal cells. Vision Res 12: 573–588, 1972. [DOI] [PubMed] [Google Scholar]
- Nevin et al. 2003.Nevin ST, Cromer BA, Haddrill JL, Morton CJ, Parker MW, Lynch JW. Insights into the structural basis for zinc inhibition of the glycine receptor. J Biol Chem 278: 28985–28992, 2003. [DOI] [PubMed] [Google Scholar]
- Norregaard et al. 1998.Norregaard L, Frederiksen D, Nielsen EO, Gether U. Delineation of an endogenous zinc-binding site in the human dopamine transporter. EMBO J 17: 4266–4273, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poe et al. 1979.Poe M, Hoogsteen K, Matthews DA. Proton magnetic resonance studies on Escherichia coli dihydrofolate reductase. Assignment of histidine C-2 protons in binary complexes with folates on the basis of the crystal structure with methotrexate and on chemical modifications. J Biol Chem 254: 8143–8152, 1979. [PubMed] [Google Scholar]
- Qian et al. 1997.Qian H, Li L, Chappell RL, Ripps H. GABA receptors of bipolar cells from the skate retina: actions of zinc on GABA-mediated membrane currents. J Neurophysiol 78: 2402–2412, 1997. [DOI] [PubMed] [Google Scholar]
- Redenti et al. 2007.Redenti S, Ripps H, Chappell RL. Zinc release at the synaptic terminals of rod photoreceptors. Exp Eye Res 85: 580–584, 2007. [DOI] [PubMed] [Google Scholar]
- Retamal et al. 2006.Retamal MA, Cortes CJ, Reuss L, Bennett MV, Saez JC. S-Nitrosylation and permeation through connexin 43 hemichannels in astrocytes: induction by oxidant stress and reversal by reducing agents. Proc Natl Acad Sci USA 103: 4475–4480, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard et al. 1998.Richard G, Smith LE, Bailey RA, Itin P, Hohl D, Epstein EH Jr, DiGiovanna JJ, Compton JG, Bale SJ. Mutations in the human connexin gene GJB3 cause erythrokeratodermia variabilis. Nat Genet 20: 366–369, 1998. [DOI] [PubMed] [Google Scholar]
- Ripps et al. 2002.Ripps H, Qian H, Zakevicius J. Pharmacological enhancement of hemi-gap-junctional currents in Xenopus oocytes. J Neurosci Methods 121: 81–92, 2002. [DOI] [PubMed] [Google Scholar]
- Saez et al. 2005.Saez JC, Retamal MA, Basilio D, Bukauskas FF, Bennett MV. Connexin-based gap junction hemichannels: gating mechanisms. Biochim Biophys Acta 1711: 215–224, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seebungkert and Lynch 2001.Seebungkert B, Lynch JW. A common inhibitory binding site for zinc and odorants at the voltage-gated K+ channel of rat olfactory receptor neurons. Eur J Neurosci 14: 353–362, 2001. [DOI] [PubMed] [Google Scholar]
- Spray et al. 2006.Spray DC, Ye ZC, Ransom BR. Functional connexin “hemichannels”: a critical appraisal. Glia 54: 758–773, 2006. [DOI] [PubMed] [Google Scholar]
- Srinivas et al. 2006.Srinivas M, Calderon DP, Kronengold J, Verselis VK. Regulation of connexin hemichannels by monovalent cations. J Gen Physiol 127: 67–75, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stergiopoulos et al. 1999.Stergiopoulos K, Alvarado JL, Mastroianni M, Ek-Vitorin JF, Taffet SM, Delmar M. Hetero-domain interactions as a mechanism for the regulation of connexin channels. Circ Res 84: 1144–1155, 1999. [DOI] [PubMed] [Google Scholar]
- Toyofuku et al. 1998.Toyofuku T, Yabuki M, Otsu K, Kuzuya T, Hori M, Tada M. Intercellular calcium signaling via gap junction in connexin-43-transfected cells. J Biol Chem 273: 1519–1528, 1998. [DOI] [PubMed] [Google Scholar]
- Ugarte and Osborne 2001.Ugarte M, Osborne NN. Zinc in the retina. Prog Neurobiol 64: 219–249, 2001. [DOI] [PubMed] [Google Scholar]
- Verselis and Srinivas 2008.Verselis VK, Srinivas M. Divalent cations regulate connexin hemichannels by modulating intrinsic voltage-dependent gating. J Gen Physiol 132: 315–327, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkovsky et al. 1983.Witkovsky P, Owen WG, Woodworth M. Gap junctions among the perikarya, dendrites, and axon terminals of the luminosity-type horizontal cell of the turtle retina. J Comp Neurol 216: 359–368, 1983. [DOI] [PubMed] [Google Scholar]
- Wu 1992.Wu SM Feedback connections and operation of the outer plexiform layer of the retina. Curr Opin Neurobiol 2: 462–468, 1992. [DOI] [PubMed] [Google Scholar]
- Wu et al. 1993.Wu SM, Qiao X, Noebels JL, Yang XL. Localization and modulatory actions of zinc in vertebrate retina. Vision Res 33: 2611–2616, 1993. [DOI] [PubMed] [Google Scholar]
- Zhang and McMahon 2000.Zhang DQ, McMahon DG. Direct gating by retinoic acid of retinal electrical synapses. Proc Natl Acad Sci USA 97: 14754–14759, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang and McMahon 2001.Zhang DQ, McMahon DG. Gating of retinal horizontal cell hemi gap junction channels by voltage, Ca2+, and retinoic acid. Mol Vis 7: 247–252, 2001. [PubMed] [Google Scholar]
- Zhang et al. 2002.Zhang DQ, Ribelayga C, Mangel SC, McMahon DG. Suppression by zinc of AMPA receptor-mediated synaptic transmission in the retina. J Neurophysiol 88: 1245–1251, 2002. [DOI] [PubMed] [Google Scholar]
- Zhang et al. 2006.Zhang DQ, Sun Z, McMahon DG. Modulation of A-type potassium currents in retinal horizontal cells by extracellular calcium and zinc. Vis Neurosci 23: 825–832, 2006. [DOI] [PubMed] [Google Scholar]
- Zhang et al. 2008.Zhang L, Deng T, Sun Y, Liu K, Yang Y, Zheng X. Role for nitric oxide in permeability of hippocampal neuronal hemichannels during oxygen glucose deprivation. J Neurosci Res 86: 2281–2291, 2008. [DOI] [PubMed] [Google Scholar]