Abstract
Sixty vestibular nuclei neurons antidromically activated by electrical stimulation of the ventroposterior thalamus were recorded in two alert squirrel monkeys. The majority of these neurons were monosynaptically activated by vestibular nerve electrical stimulation. Forty-seven neurons responded to animal rotations around the earth-vertical axis; 16 of them also responded to translations in the horizontal plane. The mean sensitivity to 0.5-Hz rotations of 80°/s velocity was 0.40 ± 0.31 spikes·s−1·deg−1·s−1. Rotational responses were in phase with stimulus velocity. Sensitivities to 0.5-Hz translations of 0.1 g acceleration varied from 92.2 to 359 spikes·s−1·g−1 and response phases varied from 10.1° lead to −98° lag. The firing behavior in 28 neurons was studied during rotation of the whole animal, of the trunk, and voluntary and involuntary rotations of the head. Two classes of vestibulothalamic neurons were distinguished. One class of neurons generated signals related to movement of the head that were similar either when the head and trunk move together or when the head moves on the stationary trunk. A fraction of these neurons fired during involuntary head movements only. A second class of neurons generated signals related to movement of the trunk. They responded when the trunk moved alone or simultaneously with the head, but did not respond to head rotations while the trunk was stationary.
INTRODUCTION
Already at the dawn of vestibular research it was suggested that the vestibular system provides for perception of self-motion in three-dimensional space (Cyon 1908). Until now, however, the neuronal substrate for vestibular perception of self-motion in space has not been well understood.
Cognitive perception of sensory events of any modality is associated primarily with neuronal activity in specific areas of the cerebral cortex. The representation of vestibular signals, as opposed to other sensory systems, could be found in several cortical regions. In primates these regions include the frontal (Ödkvist et al. 1974), retroinsular (Grüsser et al. 1990), parietal (Bremmer et al. 2002; Büttner and Buettner 1978), and temporal (Fetsch et al. 2007; Gu et al. 2006) cortices.
In the thalamus no distinct nucleus dedicated to forwarding vestibular signals to the cortex has been revealed; instead, neurons responsive to motion stimuli were found distributed over a wide area of the thalamus (Büttner and Henn 1976; Liedgren et al. 1976; Marlinski and McCrea 2008a; Meng et al. 2007). There are indications that in the ventroposterior thalamus these neurons are concentrated in clusters on the borders of lateral and medial ventroposterior nuclei (Marlinski and McCrea 2008a). One of these clusters, surrounded by the posterior border of nucleus ventroposterior lateralis (VPL), the anterior border of lateral pulvinar, and the superior border of the medial geniculate body (MG), contains neurons receiving disynaptic input from the vestibular nerve (Marlinski and McCrea 2008a). Location of this cluster corresponds to the area identified as a primary source of thalamic projections to the parietoinsular vestibular cortex (PIVC)—a “core vestibular cortical area” in primates (Akbarian et al. 1992).
Vestibular nuclei neurons that form ascending projections to the thalamus have been studied primarily with neuroanatomical techniques. These neurons were found sparsely scattered across all vestibular nuclei; their projections to the thalamus were for the most part contralateral (Condé and Condé 1978; Kotchabhakdi et al. 1980; Lang et al. 1979; Shiroyama et al. 1999). A neurophysiological study of a small number of neurons diffusely distributed within a several cubic millimeter area is challenging and therefore only a limited number of reports are available that contain results of recordings from vestibulothalamic neurons. In these studies that were carried out in anesthetized animals it was found that more than half of the vestibulothalamic neurons identified antidromically responded with monosynaptic latencies to electrical stimulation of the vestibular nerve (Isu et al. 1989; Matsuo et al. 1994; Meng et al. 2001).
Neurons located within the vestibular nuclei are primarily responsive to changes of the head position in space detected by labyrinthine end organs, but also sensitive to optokinetic (Beraneck and Cullen 2007; Waespe and Henn 1977), oculomotor (Miles 1974; Scudder and Fuchs 1992), somatosensory (Brink et al. 1980; Gdowski and McCrea 2000; Marlinsky 1995), and visceral stimuli (Jian et al. 2002).
Due to convergence of different inputs, the firing behavior of vestibular nuclei neurons can undergo substantial transformation depending on the context of the motion of the animal or the surrounding visual field. The responses of vestibular nuclei neurons to optokinetic or neck proprioceptive signals could be of the same magnitude as those to vestibular stimuli, but of the opposite directional sensitivity. As a result, neuronal responses to the simultaneous presentation of these stimuli might cancel each other and vestibular nuclei neurons would generate no signal of motion (Gdowski and McCrea 2000; Waespe and Henn 1977). A cancellation of the motion signal could also result from feeding the efferent copy of command governing head movement into the vestibular nuclei neurons (McCrea et al. 1999; Roy and Cullen 2001).
In this work we focused on the first stage of the ascending vestibulo-thalamo-cortical pathways that underlie perception of spatial self-motion and analyzed the firing behavior of antidromically identified vestibulothalamic neurons in alert squirrel monkeys. Of particular interest was the sensitivity of these neurons to head movements that were produced by yaw rotation of the whole animal and by voluntary or involuntary rotation of the head alone, as well as to rotation of the trunk with the head stationary. On the basis of their firing behavior in the course of those movements two functional classes of vestibulothalamic neurons were distinguished: one class of neurons generated similar signals to movement of the head either when the head and the trunk move together or the head moves on the stationary trunk. A fraction of these neurons responded only during involuntary movements of the head. A second class of neurons generated signals related primarily to movement of the trunk. They responded when the trunk moved alone or simultaneously with the head, but did not respond to rotation of the head while the trunk was stationary.
METHODS
Experiments were conducted in two alert squirrel monkeys (Saimiri sciureus) prepared for chronic recording from single neurons. Protocols were approved by the University of Chicago Institutional Animal Care and Use Committee and were in compliance with the National Institutes of Health guidelines for the care and use of animals in research.
Surgical preparation
Details of anesthesia and surgical preparation have been described previously (Marlinski and McCrea 2008a; McCrea et al. 1999). Surgical procedures were carried out under sterile conditions in a surgical suite with the assistance of local veterinarians. Anesthesia was initiated with ketamine (10 mg/kg, administered intramuscularly), maintained with isoflurane inhalation (∼1%) and intravenous injection of remifentanil (0.025–0.075 μg·kg−1·min−1). Analgesics and antibiotics were administered postoperatively.
Surgical preparation included cranial attachment of a head-holding acrylic frame. In the course of surgical preparation, when animal's head was held with a stereotaxic instrument, the frame was oriented in parallel to the cranial horizontal plane (Emmers and Akert 1963). A reference pin was secured to the frame and stereotaxic coordinates of the pin were measured. During experiments the anterior–posterior and medial–lateral coordinates, as well as vertical position of the recording electrode were calculated relative to the pin. An aluminum ring was connected to the frame to permit fine adjustment of the head position and support the recording microelectrode positioner. A coil of Teflon-coated stainless steel wire (Cooner) was implanted in one eye to monitor eye positions with the magnetic search coil technique. Labyrinthine stimulating electrodes of Teflon-coated silver wire (Medwire) were implanted in both temporal bones (Minor and Goldberg 1991).
Experimental setup
During experiments the monkey was seated on a perch placed on a revolving platform that was positioned on an actuator for rotational and translational movements. The animal's head was attached to a vertical rod mounted within a ball-bearing assembly fixed to table structures. The animal's body movements were restrained by a jacket tethered to table structures, whereas the limbs were free to grasp hand and foot rails. Electromagnetic field coils for recording eye and head movements were mounted on the platform.
The actuator for rotational and translational movements consisted of a linear sled (T3D, Trilogy Systems) and two rotary motors (Kollmorgen DDR). The translational axis of the sled was in the horizontal plane. The sled was mounted on top of a rotary motor positioned on the floor. Another rotary motor was mounted on the ceiling. The axes of both rotary motors were in line and aligned with the earth vertical. The bottom motor was used to produce yaw rotation of the whole animal and rotation of the trunk while the head was held stationary. During whole animal rotation the head was secured to the structure mounted on the platform and moved together with the trunk. To rotate the trunk separately the head was anchored in space by the head-holding frame connected to the ceiling motor that remained stationary. A safety electromagnetic clutch (Placid Industries), with a torque threshold of 0.23 Nm, was placed in the junction between the head-holder and the ceiling motor. The ceiling motor was used for producing involuntary head rotations while the trunk was kept stationary.
Experimental protocol
Motion profile commands were constructed using Spike2 (CED 1401) software, which controlled input to the servomotors. Rotations of the bottom and ceiling rotary motors were sinusoids of 0.25–2.0 Hz with a peak angular velocity of 5–80°/s. In some experiments the ceiling motor was controlled by a waveform stimulus command that reproduced previously recorded voluntary head rotations. Linear motor translations were 0.5- to 4.0-Hz sinusoids of 0.05–0.2 g (0.491–1.944 m/s2) peak acceleration. Rotational or translational stimuli of different frequencies and velocities/accelerations were randomly presented.
Identification of vestibulothalamic neurons
Vestibular nuclei neurons projecting to the thalamus were identified by their antidromic responses to electrical stimulation of the ventroposterior thalamus. Neurons were considered to be activated antidromically if spikes were evoked at constant latencies and collided with spontaneously occurring spikes. Six platinum Teflon-coated wires (A-M Systems), 0.125 mm in diameter with approximately 0.5-mm uninsulated tips, were implanted bilaterally for thalamus stimulation. The wires were positioned stereotaxically in the brain using guide-tube arrays. The tips of the implanted wires were targeted to the area of the thalamus identified as containing neurons receiving vestibular input. Stimulating current could be delivered to any chosen pair of unilaterally located electrodes that allow selection of a subset effective for cell identification. Vestibular nerve (VIIIn) input to vestibular nuclei neurons was identified with labyrinthine stimulating electrodes. Electric shocks for stimulation of the thalamus and the VIIIn were 0.1-ms monophasic pulses with a 30- to 300-μA amplitude.
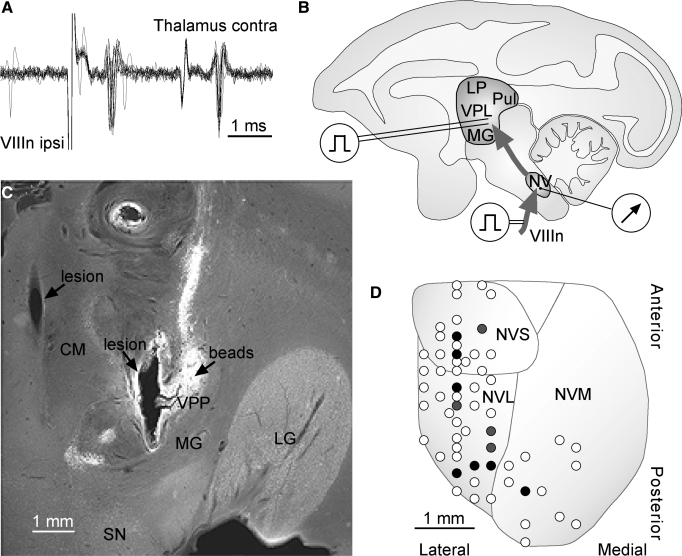
Vestibular-sensitive thalamic neurons recorded extracellularly were recognized on the basis of their responses to rotation and/or translation of the whole animal in darkness (Marlinski and McCrea 2008a). Their location was marked with green fluorescent beads (LumaFluor). On completion of the recordings the microelectrode was removed, while the guide tube used for penetration into the brain was kept in place. The microelectrode positioner was replaced with the micromanipulator bearing the Hamilton microsyringe filled with an aqueous suspension of beads. The microsyringe needle was advanced to the recording area through the guide tube and 2 μl of the suspension injected. The location of injected beads and the stimulating electrode positions, which are seen as lesions of the brain from multiple applications of current, are shown in the frontal section of the thalamus in Fig. 1C.
FIG. 1.
Identification of vestibular nuclei neurons projecting to the thalamus. Recordings from a neuron responding monosynaptically to ipsilateral VIIIn stimulation and activated antidromically by contralateral ventroposterior thalamus stimulation (A). Superposition of 12 recordings. Diagram of experimental setup that included chronically implanted stimulating electrodes in the thalamus and the temporal bone and an electrode for recordings from the vestibular nuclei neurons (B). Coronal section of the right ventroposterior thalamus (C). Fluorescent beads were deposited in the thalamic area where vestibular sensitive neurons were recorded. Beads are seen as a bright spherical area with vertically oriented tail; spherical area is the location of bead deposits; and the tail is a marker spread along the puncture. After beads were deposited, an array of stimulating electrodes was implanted stereotaxically, with the most lateral electrode targeting the labeled area. Brain lesions correspond to positions of 2 stimulating electrodes. Map of electrode tracks in the left vestibular nuclei of one animal (D). Open circles represent tracks where monosynaptic field potentials evoked by electrical stimulation of the VIIIn were observed. Black circles represent tracks where antidromically identified vestibulothalamic neurons with monosynaptic VIIIn input were recorded. Gray circles represent tracks where antidromically identified vestibulothalamic neurons with polysynaptic VIIIn input were recorded. The map superimposed on a diagram of vestibular nuclei projection on the basic horizontal plane of the animal's brain. CM, centrum medianum; MG, medial geniculate body; LG, lateral geniculate body; LP, nucleus lateralis posterior; NV, nuclei vestibularis; NVL, nucleus vestibularis lateralis; NVM, nuclei vestibularis medialis; NVS, nuclei vestibularis superior; Pul, pulvinar; SN, substantia nigra; VPL, nucleus ventroposterior lateralis; VPP, nucleus ventroposterior pars posterior; VIIIn, vestibulo-cochlear nerve.
Recording techniques
Neuronal activity was recorded extracellularly with 4- to 7-MΩ epoxy-insulated tungsten microelectrodes (A-M Systems) advanced to the brain through the guide tube (25-gauge). Microelectrodes were attached to a motorized micropositioner (FHC) secured to the head-holding frame during experiments. Microelectrode recordings were amplified and fed to an acquisition/processor module (AMP-1, FHC), where spikes were discriminated and trigger pulses generated. Trigger pulses were sent to the event channel of the CED 1401 device. In addition, unfiltered microelectrode recordings were sampled at the rate of 55.6 kHz and used for field potential analysis and checking the fidelity of spike discrimination.
Movements of the eye with the chronically implanted coil and movements of the head with another coil attached to the head-holder during experiments were monitored using a magnetic search device (Angle-Meter NT, Primelec). The platform rotational velocity was recorded with an angular velocity sensor (Watson). Linear translations of the platform along two orthogonal axes were recorded with a dual-axis accelerometer (Kionix). Analog signals were low-pass filtered with six-pole 7-kHz Butterworth filters (Frequency Devices), digitized at 992 Hz with CED Power 1401 and saved on a computer for off-line analysis.
Data analysis
Data analysis used routines written for the IGOR Pro (WaveMetrics) and Excel (Microsoft) software environment. The instantaneous discharge rate of the neuron was evaluated for each A/D sample (binwidth 2–5 ms) using a time-symmetric algorithm in which the firing rate was computed from the occurrence of spikes immediately before, after, and during the sample (McCrea et al. 1999). To determine the neuronal sensitivity to the angular velocity or the linear acceleration, the discharge rate of a neuron was averaged over ≥12 rotational or translational sinusoids. The averages of motion stimulus and discharge rate were fitted with a sinusoidal function and the amplitude and phase shift of the fits were estimated. In neurons that could be silenced during an off-direction of rotation, sinusoidal fitting was restricted to epochs where a linear relationship was observed between discharge rate and angular velocity. Response nonlinearities due to inhibitory saturation were eliminated by removing the parts of the response that deviated from linearity (Chen-Huang and McCrea 1998). The fit coefficients were obtained after exclusion of the nonlinear parts. The ratio of the response amplitude to the peak angular velocity gave an estimate of the rotational sensitivity or gainR of a neuron (spikes·s−1deg−1·s−1). The ratio of the response amplitude to the peak linear acceleration gave an estimate of translational sensitivity or gainT of a neuron (spikes·s−1·g−1). The difference between stimulus and response phases gave an estimate of the response phase shift relative to the angular velocity or linear translation of the animal.
To examine neuronal responses quantitatively and compare responses to rotations of any velocity profile the firing rate (FR) was modeled with a linear function FR = K0 + K1 × Hs + K2 × Hs + K3 × Ht + K4 × Ht + K5 × Ht, where K0 is the cell's background discharge rate, K1 is the cell's sensitivity to the head angular velocity in space (Hs), K2 is the cell's sensitivity to the angular head acceleration in space (Hs), K3 is the cell's sensitivity to the head position relative to the trunk (Ht), K4 is the cell's sensitivity to the head angular velocity relative to the trunk (Ht), and K5 is the cell's sensitivity to the head angular acceleration relative to the trunk (Ht). To analyze the neuronal responses during voluntary head movements the fragments of recordings of similar duration with peak angular velocities (varying within the range of 20–120°/s, which on average were close to 40°/s) were selected.
The quantitative estimates of the responses of various neuronal groups are presented throughout the text as an average ± SD. Statistical differences between neuronal subgroup averages were verified using one-way ANOVA. The fit accuracy was assessed by the determination coefficient of correlation (R2) between the regression model and experimental data.
RESULTS
In two alert squirrel monkeys 60 vestibular nuclei neurons were recorded that had been activated antidromically by electrical stimulation of the contralateral ventroposterior thalamus with the latency of 0.9 ± 0.2 ms (Fig. 1A). Two of them had been activated with the same latency by electrical stimulation of the ipsilateral ventroposterior thalamus. These neurons included 22 cells from left vestibular nuclei of one animal and 18 cells from left and 20 cells from the right vestibular nuclei of another animal. Vestibular nuclei neurons projecting to the thalamus comprised a small fraction of the neurons (60 of 734, 8%) that we recorded in the area where monosynaptic field potential evoked by electrical stimulation of the VIIIn was observed. The neurons were distributed along the anterior–posterior axis mostly within the lateral and superior and, to a lesser extent, in the lateral part of the medial vestibular nuclei (Fig. 1D). More than half of vestibulothalamic neurons (35 of 60, 58%) were activated by ipsilateral VIIIn stimulation with a monosynaptic latency of 1.1 ± 0.2 ms on average (Fig. 1A). Mean background discharge rate of these neurons was 58 ± 26.5 spikes/s. Sensitivity to rotational or translational stimuli was tested in 50 neurons. Recordings from the other 10 neurons lasted only long enough to detect their antidromic responses. Of specific interest in this study were the vestibular nuclei neurons sensitive to yaw rotation in the horizontal plane (47 cells). Thirty-one cells (66%) were sensitive to rotation only—further called canal neurons—and 16 cells (34%) responded to both rotation and translation, called canal-otolith neurons. Three neurons of our sample were sensitive to translation only and thus considered to be otolith neurons.
Rotation-sensitive neurons
With regard to their directional sensitivity, neurons responsive to rotation were evenly divided into type I (22 of 47, 47%), which increased their discharge rate with ipsilateral stimulus velocity, and type II (25 of 47, 53%), which increased the discharge rate with contralateral stimulus velocity (Duensing and Schaefer 1958) classification. Canal neurons comprised 13 cells of type I and 18 cells of type II; among canal-otolith neurons there were 9 cells of type I and 7 cells of type II.
The vast majority of type I canal neurons (11 of 13) received monosynaptic input from the vestibular nerve, whereas type II canal neurons were almost evenly divided between cells receiving monosynaptic (10 of 18) and polysynaptic (8 of 18) VIIIn inputs. All canal-otolith neurons of type I received monosynaptic input from the VIIIn, whereas all type II neurons received polysynaptic VIIIn input.
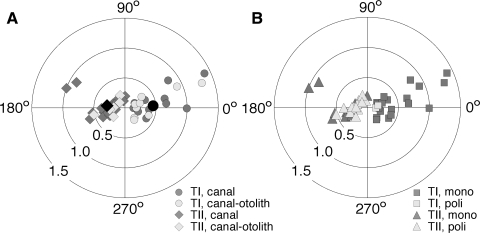
The sensitivity and phase shifts of the responses of neurons sensitive to yaw rotation in the horizontal plane are presented in Table 1 and in the polar graphs of Fig. 2. In Fig. 2A the neurons of type I and type II are classified as canal and canal-otolith units. Rotational sensitivity of neurons was tested with 0.5-Hz sinusoidal stimuli of 80°/s peak velocity. The average rotational sensitivities of canal and canal-otolith neurons of both type I and type II were similar: 0.48 ± 0.38 and 0.33 ± 0.22 spikes·s−1·deg−1·s−1, respectively [the difference between averages was insignificant, F(1,45) = 2.76, P = 0.11]. The population response of type I and type II neurons was calculated as a mean population vector in polar coordinates (Batschelet 1981). The population sensitivity of type I neurons was 0.45 ± 0.42 spikes·s−1·deg−1·s−1 and the phase shift of the population response was 4.6 ± 24.4° relative to ipsilateral velocity (Fig. 2A, black dot). The population sensitivity of type II neurons was 0.29 ± 0.27 spikes·s−1·deg−1·s−1 and the population response phase shift with respect to contralateral velocity was −7.7 ± 33.4° (Fig. 2A, black diamond).
TABLE 1.
Rotational responses
| Neuron Type | n | GainR Average | Phase Average |
Vector Sum |
Bias Average | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GainR | Phase | |||||||||||
| A. Canal neurons | ||||||||||||
| T I mono | 11 | 0.57 ± 0.39 | 7.3 ± 19.2 | 0.54 ± 0.42 | 7.4 ± 19.3 | 69.1 ± 17.8 | ||||||
| T I poly | 2 | 0.16 ± 0.03 | 5.3 ± 8.7 | 0.16 ± 0.04 | 5.3 ± 8.8 | 66.3 ± 4.1 | ||||||
| T II mono | 10 | 0.47 ± 0.28 | −4.0 ± 36.5 | 0.42 ± 0.36 | −1.9 ± 36.3 | 55.7 ± 22.3 | ||||||
| T II poly | 8 | 0.22 ± 0.08 | −14.2 ± 31.2 | 0.20 ± 0.13 | −13.6 ± 0.9 | 53.7 ± 22.3 | ||||||
| T I + II mono | 21 | 0.52 ± 0.34 | 1.9 ± 28.6 | 62.7 ± 20.8 | ||||||||
| T I + II poly | 10 | 0.21 ± 0.08 | −10.3 ± 28.8 | 49.7 ± 20.2 | ||||||||
| All | 31 | 0.42 ± 0.32 | −2.0 ± 28.8 | 60.6 ± 20.5 | ||||||||
| B. Canal-otolith neurons | ||||||||||||
| T I mono | 9 | 0.44 ± 0.39 | −0.4 ± 32.2 | 0.40 ± 0.44 | 0.8 ± 32.3 | 61.5 ± 24.2 | ||||||
| T II poly | 7 | 0.26 ± 0.13 | −11.5 ± 39.1 | 0.22 ± 0.20 | −11.6 ± 36.0 | 44.3 ± 10.4 | ||||||
| All | 16 | 0.37 ± 0.32 | −4.8 ± 34.2 | 54.6 ± 21.2 | ||||||||
| C. Total sample | ||||||||||||
| T I | 22 | 0.48 ± 0.38 | 4.0 ± 24.3 | 65.7 ± 19.7 | ||||||||
| T II | 25 | 0.33 ± 0.22 | −8.7 ± 33.6 | 53.3 ± 20.2 | ||||||||
| Mono | 30 | 0.47 ± 0.32 | 0.6 ± 29.5 | 61.4 ± 21.2 | ||||||||
| Poly | 17 | 0.23 ± 0.10 | −11.3 ± 32.8 | 52.5 ± 18.3 | ||||||||
| All | 47 | 0.40 ± 0.31 | −2.8 ± 30.0 | 59.1 ± 20.7 | ||||||||
Values are averages±SD. GainR, rotational sensitivity, spikes·s−1·deg−1·s−1. Response phase is estimated with respect to effective angular velocity: ipsilateral for type I and contralateral for type II neurons, deg. Bias, background discharge rate, spikes/s.
FIG. 2.
Gain and phase of rotational responses of type I (circles) and type II (diamonds) canal and canal-otolith neurons (A). Dark gray symbols represent neurons sensitive to rotation only; light gray symbols, neurons sensitive to both rotation and translation. Black circle represents population response of type I neurons and black diamond the population response of type II neurons. Gain and phase of rotational responses of type I neurons (squares) and type II neurons (triangles) with mono- and polysynaptic VIIIn inputs (B). Dark gray symbols represent neurons responding monosynaptically to ipsilateral VIIIn stimulation; light gray symbols, neurons with polysynaptic response. Plot radius: rotational gainR; angle: response phase shift relative to stimulus velocity.
In Fig. 2B the neurons of type I and type II are classified into cells receiving mono- and polysynaptic input from the vestibular nerve. Both type I and type II neurons receiving monosynaptic VIIIn input were of similar sensitivity to rotational stimuli: 0.51 ± 0.38 and 0.47 ± 0.28, respectively [F(1,28) = 0.09, P = 0.76]. Also similar were the rotational sensitivities of type I and type II neurons receiving polysynaptic VIIIn input: 0.16 ± 0.03 and 0.23 ± 0.10, respectively [F(1,15) = 1.23, P = 0.28]. To compare the rotational sensitivities of neurons with monosynaptic and polysynaptic VIIIn, inputs of both type I and type II were pooled together. The rotational sensitivity of neurons with monosynaptic input was significantly higher than that of neurons with polysynaptic input. The rotational sensitivities of neurons receiving monosynaptic and polysynaptic input from the VIIIn were on average 0.50 ± 0.32 and 0.23 ± 0.10 spikes·s−1·deg−1·s−1, respectively [F(1,45) = 9.6, P < 0.01].
The phase shifts of responses to rotational stimuli were similar among the groups of neurons classified as type I or type II, as well as among neurons receiving mono- or polysynaptic VIIIn input (Table 1). In type I neurons the responses tended to lead in phase ipsilateral stimulus velocity, whereas the opposite was the case in type II: their responses tended to lag behind contralateral stimulus velocity, although statistically the difference between them was insignificant [F(1,45) = 1.23, P = 0.15]. The response phase shifts varied considerably among neurons from a lag of −89° to a lead of 38° with respect to effective sinusoidal stimulus velocity. The average phase shift of responses in all neurons pooled together was −2.8 ± 30.0°, indicating that the rotational response was generally in phase with stimulus velocity.
Responses to 0.5-Hz sinusoidal rotations with angular velocities in the range of 10–100°/s that were recorded in a subset of neurons suggest inverse proportionality between rotational sensitivity and angular velocity (for details see Supplemental text and Fig. S1).1
Canal-otolith neuron response to translation
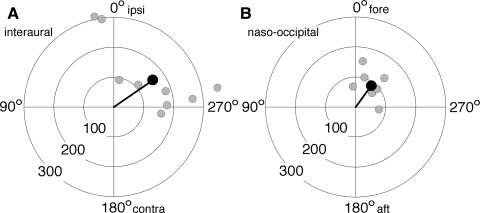
Neuronal responses to translation were tested along only two cardinal axes of the horizontal plane: interaural and naso-occipital. The sample of canal-otolith neurons was divided almost evenly between units responsive to translation along the interaural axis (9 of 16), and the naso-occipital axis (7 of 16). The response characteristics of each of these neurons are represented in polar plots as gray dots, the radial coordinate of which corresponds to translational sensitivity and the angular coordinate to response phase (Fig. 3, A and B). The neuronal sensitivity to sinusoidal translation along the interaural axis with 0.5-Hz frequency and 0.1 g peak acceleration varied from 92.2 to 359 spikes·s−1·g−1; the phase shift of the response varied from 10.1° lead to −98° phase lag relative to linear acceleration. The neuronal sensitivity to similar translations along the naso-occipital axis varied from 68.7 to 135 spikes·s−1·g−1, with response phase shifts from 7.4° lead to −96° lag.
FIG. 3.
Gain and phase of responses to linear translation along the interaural (A) and naso-occipital (B) axes. Light gray circles represent responses of individual neurons. Black circles represent population responses. Plot radius: translational gainT; angle: response phase shift relative to stimulus acceleration.
To evaluate the sensitivity and phase shift of population translational response it was calculated in polar coordinates of a mean vector. The characteristics of population translational responses are represented in Fig. 3, A and B as black dots. The mean population sensitivity to interaural translation was 158.9 ± 108.1 spikes·s−1·g−1 and to naso-occipital translation 88.2 ± 33.1 spikes·s−1·g−1. Population responses to both interaural and naso-occipital translations lagged behind stimulus acceleration by −55.0 ± 44.0 and −36.2 ± 34.4°, respectively.
Responses to 0.5-Hz sinusoidal translation with peak accelerations in the range of 0.05–0.4 g recorded in a subset of neurons suggest that translational sensitivity was inversely proportional to the square root of linear acceleration (for details see Supplemental text and Fig. S2).
Coding self-motion signals
The recordings from 28 neurons were prolonged and stable enough to test responses to the rotation of the whole animal, rotation of the trunk, and both voluntary and involuntary movements of the head. On the basis of their responses to different rotational stimuli these neurons could be divided into distinct groups.
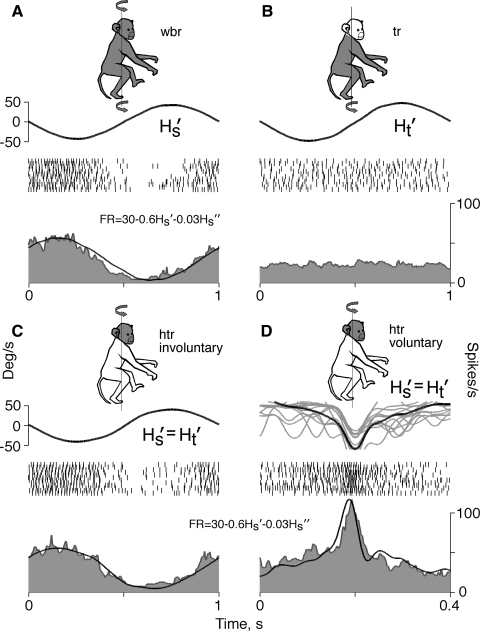
The largest group (16 of 28, 57%) consisted of neurons that responded similarly to rotation of the whole animal and to both voluntary and involuntary movements of the head on a stationary trunk. These neurons were insensitive to rotation of the trunk while the head was kept stationary. The group consisted of almost equal numbers of type I (7 of 16, 44%) and type II (9 of 16, 56%) neurons. Representative neuron firing behavior in the course of different stimulations is illustrated in Fig. 4. Rotation of the whole animal (Fig. 4A) and involuntary rotation of the head (Fig. 4C), both of 1-Hz frequency and 40°/s peak angular velocity, elicited similar responses. The firing rate of the neuron during these two rotations could be modeled quite precisely with the linear equation FR = 30 − 0.6Hs − 0.03Hs (R2 = 0.91 and R2 = 0.93, respectively). This model also accurately approximated the firing rate of the neuron (R2 = 0.81) during voluntary head movements with angular velocities that were in the range of involuntary rotational velocities of the head (Fig. 4D). Rotations of the trunk with frequencies and amplitudes similar to those of involuntary head rotation were not accompanied by changes in the neuronal firing rate (Fig. 4B). These neurons could be classified as cells that receive only vestibular input and code head movement.
FIG. 4.
Firing behavior of a neuron responding with similar sensitivity to whole body and head on trunk rotations. A: whole body rotation (wbr). B: trunk rotation with head stationary (tr). C: involuntary head on trunk rotation (htr involuntary). D: voluntary head on trunk rotation (htr voluntary). Shaded area of the monkey graphic indicates a part of a body in motion and curved arrows indicate rotational motion of that body part. In each panel the top curve is the angular velocity of the head (Hs) or the trunk (Ht). In D the gray lines represent individual head rotations, whereas the black line is their average. Rasters in the middle represent spikes generated during the corresponding period of time. The bottom histogram represents the average discharge rate. A curve superposed on each histogram is a linear model of the firing rate, of which the equation is shown above the histogram. Left ordinate, angular velocity, deg/s; right ordinate, unit discharge rate, spikes/s; abscissa, time, s.
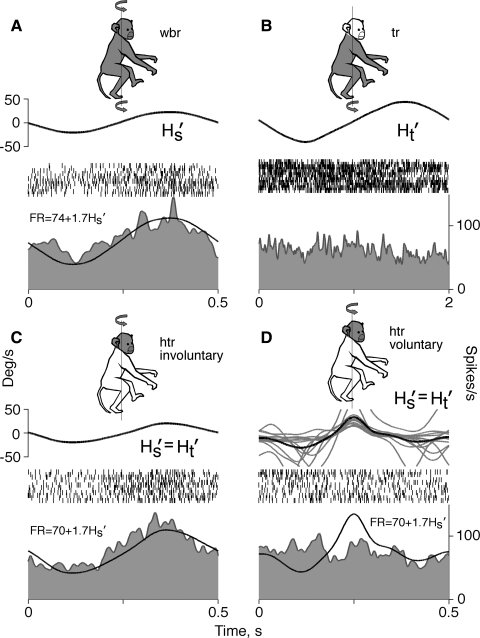
The smallest group of neurons (4 of 28, 14%) responded to rotation of the whole animal, but, at variance to cells of the first group, responded only to involuntary head movement. Half of these neurons were type I and half type II. The firing behavior of one of those neurons is illustrated in the Fig. 5. Whole animal (Fig. 5A) and involuntary head-on-trunk (Fig. 5C) rotation of 0.5-Hz frequency and 20°/s peak velocity evoked similar responses in the neuron. Responses to these two rotations could be modeled satisfactorily with the equation FR = 70 − 1.7Hs − 0.03Hs (R2 = 0.72 and R2 = 0.76). When the animal moved the head voluntarily, the neuron was insensitive to changes in angular head velocity. As seen in Fig. 5D, the actual firing behavior of the neuron during voluntary head movement was very different from that anticipated from the sensitivity to involuntary rotation. The firing rate of the neuron, similar to cells of the first group, was not altered during rotation of the trunk with the head held stationary (Fig. 5B). Neurons of this type could be classified as cells that receive vestibular input, but code only involuntary movement of the head.
FIG. 5.
Firing behavior of a neuron sensitive to involuntary head spatial motion only. A: whole body rotation. B: trunk rotation with the stationary head. C: involuntary head on trunk rotation. D: voluntary head on trunk rotation. Traces, symbols, ordinates, and abscissa are the same as in Fig. 4.
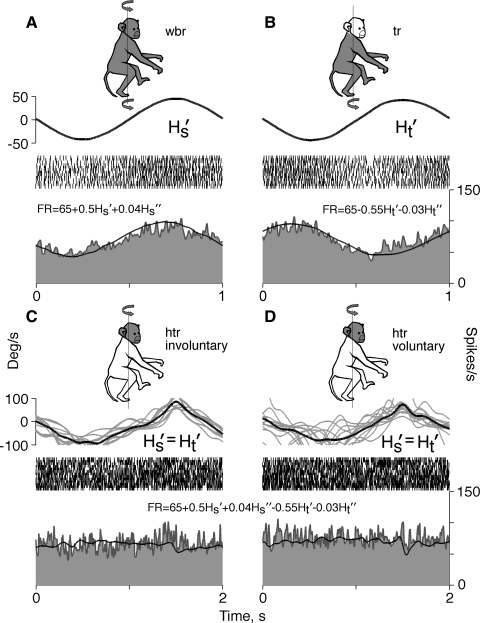
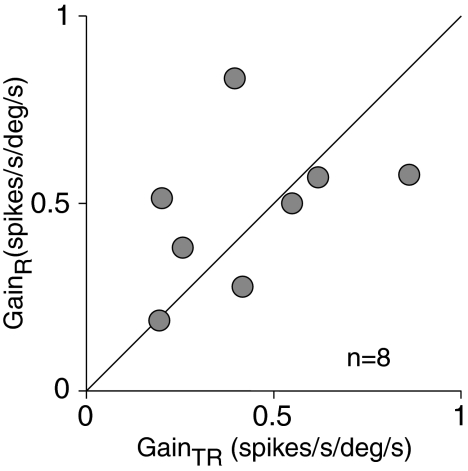
Another group (8 of 28, 29%) consisted of neurons that responded to the rotation of the whole animal and, in contrast to both groups described earlier, were sensitive to rotational movements of the trunk while the head was stationary. Three neurons of this group (38%) were of type I and 5 (62%) of type II. The firing behavior of a representative neuron is shown in Fig. 6. During whole animal rotation, with the head and trunk moving together, an increase in the firing rate followed rightward angular velocity (Fig. 6A). This response could be approximated adequately with the model: FR = 65 + 0.5Hs + 0.04Hs (R2 = 0.81). During rotation of the trunk alone, an increase in the firing rate followed leftward angular velocity (Fig. 6B). The response to trunk rotation could be modeled satisfactorily with the equation: FR = 6 − 0.55Hs − 0.03Hs (R2 = 0.77). The neuron sensitivities to these two different rotational stimuli were equal in magnitude, but opposite in phase. The reciprocity in directional sensitivities had a predictable consequence for neuronal firing behavior during head movement on a stationary trunk. Regardless of whether head movement was involuntary (Fig. 6C) or voluntary (Fig. 6D) the firing rate did not alter. Neurons of this type most likely received neck proprioceptive input in addition to vestibular input. Sensitivities to vestibular and neck proprioceptive inputs varied between neurons of this sample. There were cells with similar sensitivities to both inputs and also cells whose sensitivities to these inputs differed. A plot of neuronal sensitivity to rotations of the whole animal (GainR) versus sensitivity to rotations of the trunk (GainTR) did not reveal correlation between them [F(1,14) = 0.15, P = 0.7] (Fig. 7). Despite variability, average sensitivities to both signals were similar in a population of these neurons: average vestibular sensitivity was 0.48 ± 0.20 spikes·s−1·deg−1·s−1 and neck proprioceptive sensitivity was 0.44 ± 0.23 spikes·s−1·deg−1·s−1. Neuronal responses to vestibular and neck proprioceptive stimuli were in opposite phases, although there was distinction in their response phase shifts relative to angular stimulus velocity. Population vestibular response led angular stimulus velocity by 14.5 ± 25.5°, whereas neck proprioceptive response lagged behind stimulus velocity by −25.4 ± 38.8°; the difference between averages was significant [F(1,14) = 5.92, P = 0.03]. The data indicate that during head-on-trunk rotation the population of these neurons does not signal head motion. As a result, the population response of these neurons follows up only those movements of the head, which are accompanied by movements of the trunk. At the same time, the population of these neurons signals trunk movements that occurred either solely or simultaneously with the head. Because of this selectivity to distinct motion signals, it is suggested that neurons of this type code motion of the trunk, rather than motion of the head.
FIG. 6.
Firing behavior of a neuron responsive to rotations of the whole body (A) and to rotations of the trunk (B). No changes in the discharge rate were seen during involuntary (C) and voluntary (D) head rotations on the stationary trunk. Traces, symbols, ordinates, and abscissa are the same as in Fig. 4.
FIG. 7.
Neuronal sensitivity to rotational movement of the whole body (GainR) plotted as a function of sensitivity to rotation of the trunk only (GainTR). The diagonal line represents identical neuronal sensitivity to both inputs.
DISCUSSION
The vestibulothalamic nuclei neurons recorded in this study were found distributed over a wide area of the vestibular nuclei complex. Based on reconstructions of the recording sites these neurons were located primarily in the lateral and superior nuclei and partly in the lateral part of the medial nuclei. This observation is in good agreement with an anatomical study of vestibulothalamic neurons in the rhesus monkey, in which labeled cells were found sparsely scattered bilaterally within the superior, lateral, and medial vestibular nuclei following injections of horseradish peroxidase and wheat germ agglutinin into the rostral part of VPL (Lang et al. 1979). More numerous data on the location of these neurons in carnivores (cat) and rodents (rat) resemble in general those of the primate study, despite certain differences between the reports (Condé and Condé 1978; Kotchabhakdi et al. 1980; Shiroyama et al. 1999). From the data available, it can be assumed that vestibulothalamic neurons are scattered across all vestibular nuclei and project bilaterally to the thalamus, although predominantly contralaterally.
In this study, the majority of vestibular nuclei neurons projecting to the thalamus received monosynaptic input from the vestibular nerve. This is analogous to the data of experiments in anesthetized cats, showing that more than half of the vestibulothalamic neurons responded monosynaptically to vestibular nerve stimulation (Isu et al. 1989; Meng et al. 2001). A large number of direct connections between vestibular nerve afferents and vestibular nuclei neurons with ascending projections to the thalamus could ensure rapid processing of self-motion signals in the vestibulo-thalamo-cortical pathways. This is supported by findings in the ventroposterior thalamus, in the area bordering the posterior VPL and the superioxr MG, the neurons activated with disynaptic latency by electrical VIII nerve stimulation (Marlinski and McCrea 2008a).
This study focused on vestibulothalamic neurons responsive to yaw rotation in the horizontal plane, although their response to translational movement in the horizontal plane was also tested. One third of these neurons were responsive to both rotation and translation. This resembles the number of vestibular nuclei neurons receiving input from both semicircular canals and otolith organs in other studies: convergence neurons comprised about one fifth of the neurons recorded in decerebrated cats (Zhang et al. 2001), one third recorded in alert gerbils (Kaufman et al. 2000), and half of the neurons in alert rhesus monkeys (Dickman and Angelaki 2002). There was no difference in rotational sensitivity between vestibulothalamic neurons responsive to rotations only and neurons responsive to both rotation and translation, which is in line with the data from vestibular nuclei neurons in the rhesus monkey (Dickman and Angelaki 2004).
The estimates of rotational sensitivity in the vestibular nuclei neurons of this work are seemingly lower than those of similar cells reported elsewhere (e.g., Cullen and McCrea 1993; Dickman and Angelaki 2004; Gdowski and McCrea 2000; McCrea et al. 1999; Yakushin et al. 2006). The difference can be explained by the fact that in our experiments the animal's head was not set in a position aligning the horizontal semicircular canal plane with the earth-horizontal plane, but rather the basic horizontal plane of the animal's skull was aligned with the earth-horizontal plane, according to the stereotaxic atlas of the squirrel monkey brain (Emmers and Akert 1963). This resulted in 15 to 20° deviation in the horizontal canal plane from the earth-horizontal plane (Blanks et al. 1985). As a consequence, the rotational sensitivity of neurons receiving input from the horizontal canal was attenuated. Some of the neurons responding to yaw rotation could receive an input from the vertical semicircular canals, of which the plane was not orthogonal to the plane of rotation. These neurons would respond to rotation in the horizontal plane with a sensitivity of about 20–30% of maximum (Yakushin et al. 2006). It seems very likely that our neurons with the lowest rotational sensitivity were such cells.
In the neuronal sample of the present study the responses to rotational sinusoids were on average in phase with stimulus velocity. This observation matches estimates for response phase shifts of vestibular nuclei neurons reported elsewhere (Buettner et al. 1978; Cullen and McCrea 1993; Dickman and Angelaki 2004; Melvill Jones and Milsum 1970). Comparing the data on rotational responses of vestibular nerve afferents, vestibulothalamic neurons, and vestibular-sensitive thalamic and cortical neurons shows that in general the responses in all these neurons follow the velocity of motion stimuli (Buettner et al. 1978; Dickman and Angelaki 2004; Grüsser et al. 1990; Marlinski and McCrea 2008a; McCrea et al. 1999; Meng et al. 2007); this work).
During recordings from neurons responsive to linear translation in the horizontal plane the optimal direction of movement eliciting a maximal response was not assessed. The neuronal sensitivity to translation along only two cardinal axes was calculated and thus sensitivity estimates were most likely below maximal. Indeed, our estimates were lower than those obtained in experiments with accurately established optimal direction of translation (Dickman and Angelaki 2002a). At the same time, the phase of neuronal responses was not affected by a deviation in the translational axis from the optimal translational direction. Population responses to translation lagged behind the linear acceleration similarly to other study estimates (Angelaki and Dickman 2000).
In our previous report on ventroposterior thalamus neurons it was proposed that ascending vestibulo-thalamo-cortical pathways contained two functionally distinct channels conveying signals of spatial movement of the head and of the trunk: cephalokinetic and somatokinetic, respectively (Marlinski and McCrea 2008b). In the present work an initial stage of these channels was studied: vestibular nuclei neurons that form ascending projections to the thalamus. The focus was on the firing behavior of the vestibulothalamic neurons during head and trunk rotational motion around the earth-vertical axis. This allows for an analysis of the neuronal sensitivity to signals that originate in the peripheral vestibular organs and proprioceptors of the neck.
The majority of vestibulothalamic neurons found generated cephalokinetic signals. These neurons responded similarly to spatial movement of the head, either when the head moved simultaneously with the trunk or when the head moved alone and the trunk was stationary. Rotation of the trunk with a stationary head position did not elicit responses in these neurons. Thus such neurons received vestibular input, but not neck proprioceptive input. Two populations could be distinguished within this class of neurons. This distinction became apparent when the neuronal firing behavior during voluntary and involuntary movements of the head on stationary trunk was compared. The largest population consisted of neurons equally sensitive to both voluntary and involuntary head movements. Another small population contained neurons sensitive only to involuntary head movements. These cells were similar to the vestibular nuclei neurons insensitive to voluntary head rotations that were described by McCrea and colleagues (1999) and Roy and Cullen (2001). They proposed that the vestibular response in these neurons was cancelled by the efference copy of the command governing head motion in space, as could be predicted by the concept of von Holst and Mittelstaedt (1950). The difference between data of this study and previously published results is the quantitative estimate of neurons receiving an efference copy of the motor command. In the present study these neurons constituted only a small fraction, whereas McCrea and colleagues (1999), for example, reported a decrease in sensitivity during voluntary head rotations in almost all vestibular nuclei neurons and suggested that this decrease was due to the effects of the efference copy of the motor command. A reduction in sensitivity to voluntary head rotations with angular velocities sufficiently exceeding the velocity of reference rotation of the whole animal could result from the dynamic properties of neurons transmitting vestibular signal; as discussed in the supplement, the rotational sensitivity decreased with an increase in angular stimulus velocity. In this report only those neurons were considered that received an efference copy of the motor command, by which the vestibular response during voluntary head rotation was not just attenuated, but cancelled. It is known that human subjective perception of spatial self-motion depends on behavioral context: perception of self-generated voluntary movement is augmented in comparison to similar, but involuntary movement caused by externally applied force (Jürgens et al. 1999; Marlinsky 1999; Stevens and Earhart 2006). Our data indicate that differences in processing signals for voluntary and involuntary movements, which underlie their perceptual distinction, occur already at the first stage of vestibulo-thalamo-cortical pathways, in the vestibular nuclei neurons projecting to the ventroposterior thalamus.
About one third of the neurons projecting to the thalamus were found to be sensitive to rotational movement of the trunk while the head did not move. Thus these neurons receive vestibular input as well as neck proprioceptive input. Neck afferent projections to the vestibular nuclei neurons are well documented with anatomical, electrophysiological, and behavioral methods (Bankoul and Neuhuber 1990; Brink et al. 1980; Gdowski and McCrea 2000; Mergner et al. 1991; Schweigart et al. 2002). The integration of vestibular and neck proprioceptive input during rotation of the head on the trunk, which is the most common and frequent movement in primates, was described in vestibular nuclei neurons by Gdowski and McCrea (2000). Similar observations were made in the present study: the responses of vestibulothalamic neurons to both inputs were comparable in magnitude, but opposite in phase. During rotation of the head on the trunk both vestibular and neck proprioceptive inputs were active, but cancelled each other. As a result, these neurons did not signal motion of the head while the trunk is stationary. On the other hand, these neurons always generated signals when motion in space involved movement of the trunk, whether the trunk moved alone or simultaneously with the head. It seems probable that the signal generated by these neurons codes neither head motion in space nor head motion withn respect to the trunk; instead, this signal is transformed into one that codes motion of the trunk in space. This suggestion is supported by the findings of vestibular-sensitive cerebellar nuclei neurons that code motion in a trunk-centered, rather than in a head-centered, coordinate frame (Kleine et al. 2004; Shaikh et al. 2004). It is known from psychophysical studies that blindfolded humans, rotated around the earth-vertical axis, can perceive spatial movement of the trunk that is distinct from head movement in space (Mergner et al. 1991; Schweigart et al. 2002). No specific sensory organs sensitive to motion in space are situated in the trunk; those organs are located in the temporal bones of the skull. Therefore perception of trunk motion in space cannot be an immediate result of activation of specific peripheral sensory organs, but is rather a product of a new signal constructed within the brain. It is suggested that this new somatokinetic signal is built in the vestibulothalamic neurons receiving both vestibular and neck proprioceptive inputs.
GRANTS
This research was supported by National Institute on Deafness and Other Communication Disorders Grant DC-05056.
Supplementary Material
Acknowledgments
We thank J. Nurrish-Weiß for editing the manuscript.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The online version of this article contains supplemental data.
REFERENCES
- Akbarian et al. 1992.Akbarian S, Grüsser OJ, Guldin WO. Thalamic connections of the vestibular cortical fields in the squirrel monkey (Saimiri sciureus). J Comp Neurol 326: 423–441, 1992. [DOI] [PubMed] [Google Scholar]
- Angelaki and Dickman 2000.Angelaki DE, Dickman JD. Spatiotemporal processing of linear acceleration: primary afferent and central vestibular neuron responses. J Neurophysiol 84: 2113–2132, 2000. [DOI] [PubMed] [Google Scholar]
- Bankoul and Neuhuber 1990.Bankoul S, Neuhuber WL. A cervical primary afferent input to vestibular nuclei as demonstrated by retrograde transport of wheat germ agglutinin-horseradish peroxidase in the rat. Exp Brain Res 79: 405–411, 1990. [DOI] [PubMed] [Google Scholar]
- Batschelet 1981.Batschelet E Circular Statistics in Biology. London: Academic Press, 1981, p. 371.
- Beraneck and Cullen 2007.Beraneck M, Cullen KE. Activity of vestibular nuclei neurons during vestibular and optokinetic stimulation in the alert mouse. J Neurophysiol 98: 1549–1565, 2007. [DOI] [PubMed] [Google Scholar]
- Blanks et al. 1985.Blanks RH, Curthoys IS, Bennett ML, Markham CH. Planar relationships of the semicircular canals in rhesus and squirrel monkeys. Brain Res 340: 315–324, 1985. [DOI] [PubMed] [Google Scholar]
- Bremmer et al. 2002.Bremmer F, Klam F, Duhamel JR, Ben Hamed S, Graf W. Visual-vestibular interactive responses in the macaque ventral intraparietal area (VIP). Eur J Neurosci 16: 1569–1586, 2002. [DOI] [PubMed] [Google Scholar]
- Brink et al. 1980.Brink EE, Hirai N, Wilson VJ. Influence of neck afferents on vestibulospinal neurons. Exp Brain Res 38: 285–292, 1980. [DOI] [PubMed] [Google Scholar]
- Buettner et al. 1978.Buettner UW, Büttner U, Henn V. Transfer characteristics of neurons in vestibular nuclei of the alert monkey. J Neurophysiol 41: 1614–1628, 1978. [DOI] [PubMed] [Google Scholar]
- Büttner and Buettner 1978.Büttner U, Buettner UW. Parietal cortex (2v) neuronal activity in the alert monkey during natural vestibular and optokinetic stimulation. Brain Res 153: 392–397, 1978. [DOI] [PubMed] [Google Scholar]
- Büttner and Henn 1976.Büttner U, Henn V. Thalamic unit activity in the alert monkey during natural vestibular stimulation. Brain Res 103: 127–132, 1976. [DOI] [PubMed] [Google Scholar]
- Chen-Huang and McCrea 1998.Chen-Huang C, McCrea RA. Contribution of vestibular nerve irregular afferents to viewing distance-related changes in the vestibulo-ocular reflex. Exp Brain Res 119: 116–130, 1998. [DOI] [PubMed] [Google Scholar]
- Condé and Condé 1978.Condé F, Condé H. Thalamic projections of the vestibular nuclei in the cat as revealed by retrograde transport of horseradish peroxidase. Neurosci Lett 9: 141–148, 1978. [DOI] [PubMed] [Google Scholar]
- Cullen and McCrea 1993.Cullen KE, McCrea RA. Firing behavior of brain stem neurons during voluntary cancellation of the horizontal vestibuloocular reflex. I. Secondary vestibular neurons. J Neurophysiol 70: 828–843, 1993. [DOI] [PubMed] [Google Scholar]
- Cyon 1908.Cyon E Das Ohrlabyrinth als Organ der mathematischen Sinne für Raum und Zeit. Berlin: Springer-Verlag, 1908, p. 432.
- Dickman and Angelaki 2002a.Dickman JD, Angelaki DE. Vestibular convergence patterns in vestibular nuclei neurons of alert primates. J Neurophysiol 88: 3518–3533, 2002a. [DOI] [PubMed] [Google Scholar]
- Dickman and Angelaki 2004.Dickman JD, Angelaki DE. Dynamics of vestibular neurons during rotational motion in alert rhesus monkeys. Exp Brain Res 155: 91–101, 2004. [DOI] [PubMed] [Google Scholar]
- Duensing and Schaefer 1958.Duensing F, Schaefer KP. Die Aktivität einzelner Neurone im Bereich der Vestibulariskerne bei Horizontalbeschleungungen unter besonderer Berücksichtgung des vestiulären Nystagmus. Arch Psychiat Nervenkr 198: 225–252, 1958. [DOI] [PubMed] [Google Scholar]
- Emmers and Akert 1963.Emmers R, Akert K. A Stereotaxic Atlas of the Brain of the Squirrel Monkey (Saimiri sciureus). Madison, WI: The Univ. of Wisconsin Press, 1963, p. 102.
- Fetsch et al. 2007.Fetsch CR, Wang S, Gu Y, Deangelis GC, Angelaki DE. Spatial reference frames of visual, vestibular, and multimodal heading signals in the dorsal subdivision of the medial superior temporal area. J Neurosci 27: 700–712, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs and Kimm 1975.Fuchs AF, Kimm J. Unit activity in vestibular nucleus of the alert monkey during horizontal angular acceleration and eye movement. J Neurophysiol 38: 1140–1161, 1975. [DOI] [PubMed] [Google Scholar]
- Gdowski and McCrea 2000.Gdowski GT, McCrea RA. Neck proprioceptive inputs to primate vestibular nucleus neurons. Exp Brain Res 135: 511–526, 2000. [DOI] [PubMed] [Google Scholar]
- Grüsser et al. 1990.Grüsser OJ, Pause M, Schreiter U. Localization and responses of neurones in the parieto-insular vestibular cortex of awake monkeys (Macaca fascicularis). J Physiol 430: 537–557, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu et al. 2006.Gu Y, Watkins PV, Angelaki DE, DeAngelis GC. Visual and nonvisual contributions to three-dimensional heading selectivity in the medial superior temporal area. J Neurosci 26: 73–85, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isu et al. 1989.Isu N, Sakuma A, Kitahara M, Ichikawa T, Watanabe S, Uchino Y. Extracellular recording of vestibulo-thalamic neurons projecting to the spinal cord in the cat. Neurosci Lett 104: 25–30, 1989. [DOI] [PubMed] [Google Scholar]
- Jian et al. 2002.Jian BJ, Shintani T, Emanuel BA, Yates BJ. Convergence of limb, visceral, and vertical semicircular canal or otolith inputs onto vestibular nucleus neurons. Exp Brain Res 144: 247–257, 2002. [DOI] [PubMed] [Google Scholar]
- Jürgens et al. 1999.Jürgens R, Boss T, Becker W. Estimation of self-turning in the dark: comparison between active and passive rotation. Exp Brain Res 128: 491–504, 1999. [DOI] [PubMed] [Google Scholar]
- Kaufman et al. 2000.Kaufman GD, Shinder ME, Perachio AA. Convergent properties of vestibular-related brain stem neurons in the gerbil. J Neurophysiol 83: 1958–1971, 2000. [DOI] [PubMed] [Google Scholar]
- Kleine et al. 2004.Kleine JF, Guan Y, Kipiani E, Glonti L, Hoshi M, Büttner U. Trunk position influences vestibular responses of fastigial nucleus neurons in the alert monkey. J Neurophysiol 91: 2090–2100, 2004. [DOI] [PubMed] [Google Scholar]
- Kotchabhakdi et al. 1980.Kotchabhakdi N, Rinvik E, Walberg F, Yingchareon K. The vestibulothalamic projections in the cat studied by retrograde axonal transport of horseradish peroxidase. Exp Brain Res 40: 405–418, 1980. [DOI] [PubMed] [Google Scholar]
- Lang et al. 1979.Lang W, Büttner-Ennever JA, Büttner U. Vestibular projections to the monkey thalamus: an autoradiographic study. Brain Res 177: 3–17, 1979. [DOI] [PubMed] [Google Scholar]
- Liedgren et al. 1976.Liedgren SR, Milne AC, Schwarz DW, Tomlinson RD. Representation of vestibular afferents in somatosensory thalamic nuclei of the squirrel monkey (Saimiri sciureus). J Neurophysiol 39: 601–612, 1976. [DOI] [PubMed] [Google Scholar]
- Marlinski and McCrea 2008a.Marlinski V, McCrea RA. Activity of ventro-posterior thalamus neurons during rotation and translation in the horizontal plane in the alert squirrel monkey. J Neurophysiol 99: 2533–2545, 2008a. [DOI] [PubMed] [Google Scholar]
- Marlinski and McCrea 2008b.Marlinski V, McCrea RA. Coding of self-motion signals in ventro-posterior thalamus neurons in the alert squirrel monkey. Exp Brain Res 189: 463–472, 2008b. [DOI] [PubMed] [Google Scholar]
- Marlinsky 1995.Marlinsky VV The effect of somatosensory stimulation on second-order and efferent vestibular neurons in the decerebrate decerebellate guinea-pig. Neuroscience 69: 661–669, 1995. [DOI] [PubMed] [Google Scholar]
- Marlinsky 1999.Marlinsky VV Vestibular and vestibulo-proprioceptive perception of motion in the horizontal plane in blindfolded man—I. Estimations of linear displacement. Neuroscience 90: 389–394, 1999. [DOI] [PubMed] [Google Scholar]
- Matsuo et al. 1994.Matsuo S, Hosogai M, Nakao S. Ascending projections of posterior canal-activated excitatory and inhibitory secondary vestibular neurons to the mesodiencephalon in cats. Exp Brain Res 100: 7–17, 1994. [DOI] [PubMed] [Google Scholar]
- McCrea et al. 1999.McCrea RA, Gdowski GT, Boyle R, Belton T. Firing behavior of vestibular neurons during active and passive head movements: vestibulo-spinal and other non-eye-movement related neurons. J Neurophysiol 82: 416–428, 1999. [DOI] [PubMed] [Google Scholar]
- Melvill and Milsum Jones 1970.Melvill Jones G, Milsum JH. Characteristics of neural transmission from the semicircular canal to the vestibular nuclei of cats. J Physiol 209: 295–316, 1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng et al. 2001.Meng H, Bai RS, Sato H, Imagawa M, Sasaki M, Uchino Y. Otolith-activated vestibulothalamic neurons in cats. Exp Brain Res 141: 415–424, 2001. [DOI] [PubMed] [Google Scholar]
- Meng et al. 2007.Meng H, May PJ, Dickman JD, Angelaki DE. Vestibular signals in primate thalamus: properties and origins. J Neurosci 27: 13590–13602, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mergner et al. 1991.Mergner T, Siebold C, Schweigart G, Becker W. Human perception of horizontal trunk and head rotation in space during vestibular and neck stimulation. Exp Brain Res 85: 389–404, 1991. [DOI] [PubMed] [Google Scholar]
- Miles 1974.Miles FA Single unit firing patterns in the vestibular nuclei related to voluntary eye movements and passive body rotation in conscious monkeys. Brain Res 71: 215–224, 1974. [DOI] [PubMed] [Google Scholar]
- Minor and Goldberg 1991.Minor LB, Goldberg JM. Vestibular-nerve inputs to the vestibulo-ocular reflex: a functional-ablation study in the squirrel monkey. J Neurosci 11: 1636–1648, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ödkvist et al. 1974.Ödkvist LM, Schwarz DW, Fredrickson JM, Hassler R. Projection of the vestibular nerve to the area 3a arm field in the squirrel monkey (Saimiri sciureus). Exp Brain Res 21: 97–105, 1974. [DOI] [PubMed] [Google Scholar]
- Roy 2001.Roy JE, Cullen KE. Selective processing of vestibular reafference during self-generated head motion. J Neurosci 21: 2131–2142, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweigart et al. 2002.Schweigart G, Chien RD, Mergner T. Neck proprioception compensates for age-related deterioration of vestibular self-motion perception. Exp Brain Res 147: 89–97, 2002. [DOI] [PubMed] [Google Scholar]
- Scudder and Fuchs 1992.Scudder CA, Fuchs AF. Physiological and behavioral identification of vestibular nucleus neurons mediating the horizontal vestibuloocular reflex in trained rhesus monkeys. J Neurophysiol 68: 244–264, 1992. [DOI] [PubMed] [Google Scholar]
- Shaikh et al. 2004.Shaikh AG, Meng H, Angelaki DE. Multiple reference frames for motion in the primate cerebellum. J Neurosci 24: 4491–4497, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiroyama et al. 1999.Shiroyama T, Kayahara T, Yasui Y, Nomura J, Nakano K. Projections of the vestibular nuclei to the thalamus in the rat: a Phaseolus vulgaris leucoagglutinin study. J Comp Neurol 407: 318–332, 1999. [PubMed] [Google Scholar]
- Stevens and Earhart 2006.Stevens ES, Earhart GM. Changes in perception of active but not passive turning following stepping on the rotating treadmill. Exp Brain Res 171: 340–346, 2006. [DOI] [PubMed] [Google Scholar]
- von Holst and Mittelstaedt 1950.von Holst E, Mittelstaedt H. Das Reafferenzprinzip. Wechselwirkungen zwischen Zentralnervensystem und Peripherie. Naturwissenschaften 37: 464–476, 1950. [Google Scholar]
- Waespe and Henn 1977.Waespe W, Henn V. Neuronal activity in the vestibular nuclei of the alert monkey during vestibular and optokinetic stimulation. Exp Brain Res 27: 523–538, 1977. [DOI] [PubMed] [Google Scholar]
- Yakushin et al. 2006.Yakushin SB, Raphan T, Cohen B. Spatial properties of central vestibular neurons. J Neurophysiol 95: 464–478, 2006. [DOI] [PubMed] [Google Scholar]
- Zhang et al. 2001.Zhang X, Zakir M, Meng H, Sato H, Uchino Y. Convergence of the horizontal semicircular canal and otolith afferents on cat single vestibular neurons. Exp Brain Res 140: 1–11, 2001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.